Abstract
Background
Primary skeletal muscle fibers form during embryonic development and are characterized as fast or slow fibers based on contractile protein gene expression. Different avian primary muscle fiber types arise from myoblast lineages committed to formation of diverse fiber types. To understand the basis of embryonic muscle fiber type diversity and the distinct myoblast lineages that generate this diversity, gene expression analyses were conducted on differentiated muscle fiber types and their respective myoblast precursor lineages.
Results
Embryonic fast muscle fibers preferentially expressed 718 genes, and embryonic fast/slow muscle fibers differentially expressed 799 genes. Fast and fast/slow myoblast lineages displayed appreciable diversity in their gene expression profiles, indicating diversity of precursor myoblasts. Several genes, including the transcriptional regulator EMX2, were differentially expressed in both fast/slow myoblasts and muscle fibers versus fast myoblasts and muscle fibers. EMX2 was localized to nuclei of fast/slow myoblasts and muscle fibers and was not detected in fast lineage cells. Furthermore, EMX2 overexpression and knockdown studies indicated that EMX2 is a positive transcriptional regulator of the slow myosin heavy chain 2 (MyHC2) gene promoter activity in fast/slow muscle fibers.
Conclusions
These results indicate the presence of distinct molecular signatures that characterize diverse embryonic myoblast lineages before differentiation.
Keywords: Myoblast, Lineage, Fiber Type, Transcription, Gene Expression
Introduction
Adult and developing vertebrate musculature is composed of muscle fibers that vary in contractile and metabolic characteristics. These types of muscle fibers are often categorized as fast or slow, based principally upon the contractile properties and expression of the myosin heavy chain (MyHC) genes that determines the fiber type specific contractile characteristics in both adult and developing muscle (Reiser, et al., 1985; Reiser, et al., 1988). In avian species, muscle fibers are defined as fast, fast/slow, or slow based on expression of genes encoding MyHCs with corresponding ATPase activities. Nearly all avian muscle fibers express one or more fast MyHC isoform genes throughout development and in the adult (Bandman, et al., 1982). Three slow MyHC isoform genes are expressed in chicken development and in the adult. The slow MyHC3 gene is expressed transiently during skeletal muscle development and becomes restricted to the atria as development proceeds (Wang, et al., 1996). Slow MyHC1 and slow MyHC2 are the predominant slow MyHC isoforms in skeletal muscle. The slow MyHC1 gene is expressed in nearly all slow muscle fibers, many of which also express the slow MyHC2 gene. Slow MyHC2 gene expression is restricted to slow muscle fibers and is most characteristic of the slow muscle fiber phenotype (Page, et al., 1992). Therefore, expression of the slow MyHC2 gene defines those avian muscle fibers that are most distinct from fibers that express exclusively fast MyHC isoform genes.
Vertebrate skeletal muscle fiber formation occurs in three distinct stages. The embryonic phase generates primary muscle fibers from the differentiation of embryonic myoblasts. These primary muscle fibers establish the basic anatomic structure of each muscle and presage the general contractile and metabolic characteristics of the muscle as a whole. The following fetal phase of myogenesis yields secondary muscle fibers from fetal myoblasts. Lastly, the adult stage of myogenesis is partly characterized by the presence of mitotically quiescent satellite cells (Stockdale, 1992). Diversity in avian muscle fiber types is readily detectable throughout development at each phase of myogenesis with fiber type specific expression of MyHC isoform genes, including the slow MyHC2 gene (Page et al., 1992).
Although both primary and secondary muscle fibers display similar phenotypic diversity in MyHC gene expression, the mechanisms that control their myogenic precursors and ultimate diversification of fiber types within each phase are quite different (Hutcheson, et al., 2009). The embryonic and fetal myoblast populations that give rise to primary and secondary muscle fibers, respectively, display developmental stage specific differences in response to proliferative cues, differentiation and fusion properties, and morphology (Biressi, et al., 2007a). Moreover, embryonic and fetal myoblasts have unique patterns of genome-wide gene expression, including expression of Nuclear Factor IX (Nfix) that activates expression of fetal stage specific myogenic genes and suppresses embryonic stage specific genes (Biressi, et al., 2007b; Messina, et al., 2010).
Most research on the mechanisms that control muscle fiber types has focused on regulation in adult muscle in response to altered activity, electrical stimulation, and innervation. A number of transcriptional regulators and signaling molecules have been implicated in control of adult skeletal muscle fiber phenotypes. These factors include calcineurin, Nuclear Factor of Activated T cells (NFAT; Calabria, et al., 2009), Myocyte Enhancer Factor (MEF2; Liu, et al., 2005), MusTRD/GTF3 (Calvo, et al., 2001; Polly, et al., 2003) and PGC1α (Lin, et al., 2002). Expression patterns of the Myogenic Regulatory Factors (e.g. MyoD, myogenin) have also been associated with different adult muscle fiber types (Hughes, et al., 1993). The majority of these signaling proteins and transcriptional regulators function in response to activity states and innervation patterns in the adult.
Additional research has focused on the appearance of fiber type diversity during secondary myogenesis. Broadly similar to the regulatory mechanisms in adult muscle, the repertoire of contractile and metabolic genes expressed in diverse fiber types at fetal stages is determined in large part by the specific motor neuron input and activity status of the muscle (Schiaffino, et al., 2007). For example, cross-reinnervation of fast and slow contracting muscles with the accompanying neural input induces a switch in expression of fiber type specific genes and corresponding contractile characteristics of the muscle (Roy, et al., 1996). Yet, restrictions to secondary fiber type diversification and plasticity in response to altered activity and innervation in both mammalian and avian species have been shown by different laboratories (Condon, et al., 1990; DiMario, et al., 1997).
The cellular mechanisms that regulate muscle fiber type diversification during embryonic muscle development are less well understood. Cell autonomous, lineage-dependent differentiation of myoblasts into diverse muscle fiber types in the absence of functional innervation has been reported in avian, rodent, cat, and zebrafish model systems in vivo and in vitro (Page, et al., 1992; Miller and Stockdale, 1986a; Condon, et al., 1990; Roy, et al., 2008; Devoto, et al., 1996). In addition, primary muscle fibers continue to express fiber type specific genes after surgical or functional denervation (Crow and Stockdale, 1986; Fredette and Landmesser, 1991). Furthermore, clonal analysis of embryonic avian myoblasts has shown that individual myoblasts are committed to the formation of specific muscle fiber types both in vitro and in vivo (Miller and Stockdale, 1986b: DiMario, et al., 1993). Therefore initial diversity in muscle fiber types arises from intrinsic embryonic myoblast commitment within specific myoblast lineages.
Only a few clues regarding the transcriptional regulation of embryonic muscle fiber type formation have been garnered. These have been primarily derived from studies in zebrafish, mouse, and avian model systems. Interestingly, many of the signaling and transcriptional regulators that control fiber type specific gene expression in adult and/or fetal stages do not appear to be operative at earlier stages of development. For example, calcineurin is required for the maintenance of adult slow muscle fibers (most of which are derived from fetal myoblasts) in the mouse, but is not required for generation of embryonic slow muscle fibers (Oh, et al., 2005). Similarly, diversification of embryonic muscle fiber types from distinct avian myoblast lineages occurs independently of NFAT and MEF2 transcription factor activities, which are required for expression of muscle fiber type specific genes at later stages of avian and mammalian development (Theobald and DiMario, 2011; Jiang, et al., 2004; Olson and Williams, 2000).
Several factors have been identified that regulate muscle fiber type development in embryonic stages. Six1 and Six4 homeodomain proteins are required for normal hypaxial muscle development and full activation of the fast muscle fiber phenotype in mouse myotomal muscle (Grifone, et al., 2004; Grifone, et al., 2005; Niro, et al., 2010). Six1/Six4 deficient embryos display altered fiber type specific gene expression at fetal (ED18.5) stages of development (Richard, et al., 2011). In zebrafish, Hedgehog signaling induces expression of the u-boot (ubo) gene which encodes the transcription factor Blimp1/PRDM1 (Baxendale, et al., 2004). PRDM1 activates the slow muscle fiber phenotype and represses the fast muscle fiber phenotype in the developing zebrafish myotome (Liew, et al., 2008). PRDM1 also represses Sox6 gene expression during zebrafish myotome development (von Hofsten, et al., 2008). Interestingly, Sox6 gene expression during fetal (E15.5) mouse muscle development contributes to development of fast muscle fibers by repression of the slow fiber phenotype. Sox6 knockout mice display increased slow muscle fibers, indicating that Sox6 functions as a transcriptional repressor of the slow fiber phenotype (Hagiwara, et al., 2007).
EMX1 and EMX2 are vertebrate homologs of the Drosophila empty spiracles (ems) gene. In Drosophila, ems functions as a gap homeobox gene and is required for normal anterior (head) structure specification and development of posterior spiracles (Walldorf and Gehring, 1992). In vertebrates, EMX2 is expressed in a wide variety of developing tissues and is involved in diverse developmental events. It is expressed in the developing cerebral cortex and olfactory bulbs of mice at E9.5 (Simeone, et al., 1992). EMX2 promotes neurogenesis and may contribute to correct neuronal pathfinding by direct transcriptional activation of the teneurin-1 gene (Brancaccio, et al., 2010; Beckmann, et al., 2011). EMX2 is also required for normal development of the mouse urogenital system (Miyamoto, et al., 1997) and hair cell development in the inner ear (Holley, et al., 2010). In vertebrate limb development, EMX2 is required for scapula and ilium formation (Pellegrini, et al. 2001; Malashichev, et al., 2008).
Results
Genome-wide Gene Expression Analysis of Differentiated Fast and Fast/Slow Myogenic Cell Lineages
Embryonic avian myoblasts, isolated from developing limbs during primary muscle fiber formation, are stably committed to the formation of specific muscle fiber types in vitro and in vivo (Miller and Stockdale, 1986a,b; DiMario et al., 1993). For this study, multiple clonal populations of myoblasts were expanded and each clonal population was characterized for its differentiation into muscle fibers that expressed either fast MyHC genes or both fast MyHC and slow MyHC2 genes. Differentiated muscle fibers in vitro formed from clonal myoblasts were immunostained with monoclonal antibodies F59 and S58 to detect fast MyHCs and slow MyHC2, respectively. We have previously reported aggregate data regarding numbers of types of myoblast clones, the similar fusion indices of fast and fast/slow myoblasts, and expression of fast MyHC and slow MyHC2 genes in differentiated clonal cultures (Theobald and DiMario, 2011). For genome-wide gene expression analysis, five fast myogenic clones and four fast/slow myogenic clones were used. The expression of fast MyHC and slow MyHC2 genes in muscle fibers from each clone is shown in Supplement Figure 1A. Myotubes derived from myoblasts committed to the fast fiber fate expressed fast MyHC gene(s) and did not express the slow MyHC2 gene. Myotubes derived from fast/slow myoblasts immunostained with both F59 and S58 antibodies, indicating expression of both fast MyHC gene(s) and the slow MyHC2 gene. Fast and fast/slow myoblast clonal populations selected for gene expression analysis had similar average fusion indices (Supp Fig 1B). RNA was isolated from differentiated muscle fiber cultures of each clonal population. RNAs from the five fast muscle fiber cultures were pooled, as were RNAs from the four fast/slow clonal muscle fiber cultures, to reduce any relative clonal variations (Kendziorski, et al., 2005).
Pooled samples were hybridized to the Affymetrix GeneChip Chicken Genome Array that allows for determination of expression levels of 28,000 transcripts. To validate the results from the microarray hybridization relative to the differentiated phenotypes of the fast and fast/slow muscle fiber clonal populations, expression levels of myosin and myosin-associated protein genes were evaluated (Tables 1 and 2). Genes typically associated with fast muscle fiber types were expressed in differentiated cultures of both fast and fast/slow myoblast types. This is evident by the relative expression levels of fast fiber associated genes in both fast and fast/slow muscle fibers. On average, fast fiber associated genes were expressed 1.32 times greater in fast/slow muscle fibers compared to fast muscle fibers (Table 1). Since all muscle fibers derived from both fast and fast/slow myogenic clones express a fast MyHC gene(s), it is reasonable to anticipate that fast muscle fiber associated genes would be expressed and represented in both fast and fast/slow myogenic clone samples used for microarray analysis. Indeed, the microarray data does not indicate a difference in expression of fast fiber associated genes. In contrast, expression of slow muscle fiber associated genes was on average 6.45 times greater in muscle fibers derived from fast/slow myoblasts versus fast myoblasts (Table 2). Therefore, the microarray analysis identified differential gene expression supporting the existence of myogenic cell clones that differentiate into distinct fast versus fast/slow muscle fiber types.
Table 3.
Genes Preferentially Expressed in Fast Myotubes
| Gene Symbol | Probe Set ID | Fold Change | Gene Title/Comments |
|---|---|---|---|
| Apoptosis | |||
| BAG3 | GgaAffx.12756.1.S1_at | 2.53 | BCL2-associated athanogene 3 |
| CABC1 | Gga.6127.1.S1_at | 2.63 | chaperone, ABC1 activity of bc1 complex homolog (S. pombe) |
| MCL1 | Gga.16560.2.S1_s_at | 2.35 | myeloid cell leukemia sequence 1 (BCL2-related) |
| TNFRSF6B | Gga.5386.1.S1_at | 4.22 | tumor necrosis factor receptor superfamily, member 6b, decoy |
| Cell Adhesion | |||
| ADRM1 | Gga.4135.2.S1_a_at | 2.37 | adhesion regulating molecule 1 |
| ITGB3 | Gga.1039.1.S1_at | 6.89 | integrin, beta 3 (platelet glycoprotein IIIa, antigen CD61) |
| K-CAM | Gga.728.1.S1_a_at | 4.24 | B-cadherin |
| NCAM1 | GgaAffx.22381.3.S1_at | 2.16 | neural cell adhesion molecule 1 |
| Cell Cycle | |||
| ANAPC2 | Gga.7685.3.S1_a_at | 3.35 | anaphase promoting complex subunit 2 |
| CDT1 | Gga.7249.1.S1_at | 2.64 | chromatin licensing and DNA replication factor 1 |
| Chromatin Remodelling | |||
| SMARCD1 | GgaAffx.3872.1.S1_at | 2.03 | SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily d, member 1 |
| SMARCE1 | GgaAffx.11797.1 .S1_at | 2.35 | SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily e, member 1 |
| Cytoskeleton | |||
| CAPZB | Gga.4050.2.S1_a_at | 2.22 | capping protein (actin filament) muscle Z-line, beta |
| DCTN4 | GgaAffx.2799.1.S1_at | 2.51 | dynactin 4 (p62) |
| HIP1 | GgaAffx.22557.1.S1_s_at | 2.04 | huntingtin interacting protein 1 |
| EMILIN3 | GgaAffx.2369.1.S1_at | 2.36 | elastin microfibril interfacer 3 |
| MGP | Gga.540.1.S1_at | 8.37 | matrix Gla protein |
| TUFT1 | Gga.14691.1.S1_at | 4.61 | tuftelin 1 |
| Metabolism | |||
| ACOT7 | Gga.5995.1.S1_at | 2.16 | acyl-CoA thioesterase 7 |
| ASCC3L1 | Gga.9209.1.S1_at | 2.96 | activating signal cointegrator 1 complex subunit 3-like 1 |
| AYTL2 | Gga. 16935.1.S1_at | 2.05 | acyltransferase like 2 |
| B4GALT2 | Gga.2424.2.S1_a_at | 2.70 | UDP-Gal:betaGlcNAc beta 1,4- galactosyltransferase, polypeptide 2 |
| CKB | Gga.2722.1.S1_a_at | 3.48 | creatine kinase, brain |
| CREB3L1 | GgaAffx.5291.1.S1_at | 2.38 | cAMP responsive element binding protein 3-like 1 |
| FBP1 | Gga.5139.1.S1_at | 3.77 | fructose-1,6-bisphosphatase 1 |
| FOXRED1 | Gga.18113.1.S1_at | 2.33 | FAD-dependent oxidoreductase domain containing 1 |
| GALE | Gga. 9722.1.S1_at | 2.02 | UDP-galactose-4-epimerase |
| GALNS | GgaAffx.21893.2.S1_s_at | 2.24 | galactosamine (N-acetyl)-6-sulfate sulfatase (Morquio syndrome, mucopolysaccharidosis type IVA) |
| GALNT5 | GgaAffx.7959.1.S1_at | 2.62 | UDP-N-acetyl-alpha-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase 5 (GalNAc-T5) |
| GALT | GgaAffx.1454.1.S1_at | 2.55 | galactose-1-phosphate uridylyltransferase |
| GCAT | Gga. 16744.1.S1_at | 2.78 | glycine C-acetyltransferase (2-amino-3-ketobutyrate coenzyme A ligase) |
| GCK | Gga.12945.1.S1_at | 3.04 | glucokinase |
| GOT2 | Gga.4425.1.S2_at | 2.60 | glutamic-oxaloacetic transaminase 2, mitochondrial (aspartate aminotransferase 2) |
| GPD2 | Gga.11036.1.S1_s_at | 2.94 | glycerol-3-phosphate dehydrogenase 2 (mitochondrial) |
| GPI | GgaAffx.11394.1.S1_s_at | 2.35 | glucose phosphate isomerase |
| GRHPR | Gga. 7241.1.S1_at | 2.42 | glyoxylate reductase/hydroxypyruvate reductase |
| GSS | Gga. 5371.1.S1_at | 2.10 | glutathione synthetase |
| HMGCL | Gga. 2537.1. S1_at | 2.22 | 3-hydroxymethyl-3-methylglutaryl-Coenzyme A lyase |
| LIPT1 | Gga.11145.1.S1_at | 2.09 | lipoyltransferase 1 |
| NDOR1 | GgaAffx.5614.1.S1_at | 3.48 | NADPH dependent diflavin oxidoreductase 1 |
| NOX4 | GgaAffx.25209.3.S1_s_at | 2.56 | NADPH oxidase 4 |
| PFKL | Gga.2810.2.S1_at | 2.29 | phosphofructokinase, liver |
| PFKM | Gga.2810.1.S1_at | 7.17 | phosphofructokinase, muscle |
| PI4K2A | GgaAffx.3835.1.S1_at | 4.87 | phosphatidylinositol 4-kinase type 2 alpha |
| PIP5K1C | GgaAffx.515.2.S1_s_at | 4.07 | phosphatidylinositol-4-phosphate 5-kinase, type I, gamma |
| PKM2 | Gga.4299.1.S1_at | 2.09 | pyruvate kinase, muscle |
| PYGL | GgaAffx.12722.1.S1_s_at | 3.42 | liver glycogen phosphorylase |
| RRM2B | GgaAffx.10231.1.S1_at | 2.03 | ribonucleotide reductase M2 B (TP53 inducible) |
| SARDH | GgaAffx.1837.1.S1_s_at | 2.02 | sarcosine dehydrogenase |
| TPI1 | Gga.4148.1.S1_at | 2.26 | triosephosphate isomerase 1 |
| UROD | GgaAffx.6433.3.S1_s_at | 2.18 | uroporphyrinogen decarboxylase |
| Signal Transduction | |||
| BMP10 | Gga.9509.1.S1_at | 4.58 | bone morphogenetic protein 10 |
| CHRM4 | GgaAffx.5277.1.S1_at | 3.37 | cholinergic receptor, muscarinic 4 |
| DDR2 | Gga.1162.1.S1_at | 2.62 | discoidin domain receptor family, member 2 |
| EPHB3 | Gga. 3053.1. S1_at | 4.33 | EPH receptor B3 |
| FBXW4 | GgaAffx.22338.1.S1_at | 2.24 | F-box and WD repeat domain containing 4 |
| FGD3 | GgaAffx.26456.1.S1_s_at | 2.25 | FYVE, RhoGEF and PH domain containing 3 |
| FGF13 | Gga.2685.1.S2_at | 6.65 | fibroblast growth factor 13 |
| FGFR3 | Gga. 16413.1.A1_a_at | 8.42 | fibroblast growth factor receptor 3 |
| GPR88 | GgaAffx.26462.1.S1_at | 2.20 | G protein-coupled receptor 88 |
| GRK6 | Gga.19304.1.S1_s_at | 2.71 | G protein-coupled receptor kinase 6 |
| HGS | Gga.7570.1.S1_at | 2.73 | hepatocyte growth factor-regulated tyrosine kinase substrate |
| MAP2K1IP1 | Gga.4355.2.S1_s_at | 2.55 | mitogen-activated protein kinase kinase 1 interacting protein 1 |
| PPP2R2B | GgaAffx.4722.1.S1_s_at | 2.24 | protein phosphatase 2 (formerly 2A), regulatory subunit B, beta isoform |
| PRKAB2 | GgaAffx.1098.1.S1_s_at | 2.91 | protein kinase, AMP-activated, beta 2 non-catalytic subunit |
| RAP2A | GgaAffx.10815.1.S1_at | 2.33 | RAP2A, member of RAS oncogene family |
| RERG | GgaAffx.8303.1.S1_at | 2.71 | RAS-like, estrogen-regulated, growth inhibitor |
| RHOC | Gga. 17535.1.S1_at | 2.10 | ras homolog gene family, member C |
| Structural | |||
| MYL2 | Gga.839.1.S1_at | 2.27 | Myosin light chain 2 (LC2f) |
| MYL | Gga.840.2.S1_a_at | 2.28 | Myosin alkali light chain mRNA, complete cds, clone pG17-1 |
| ACTG2 | Gga.644.1.S1_at | 5.37 | actin, gamma 2, smooth muscle, enteric |
| MYL3 | Gga.4198.2.S1_a_at | 2.32 | myosin, light chain 3, alkali; ventricular, skeletal, slow |
| MYL4 | Gga.2698.1.S1_at | 3.97 | myosin, light chain 4, alkali; atrial, embryonic |
| SYNC1 | GgaAffx.2198.1.S1_at | 3.50 | syncoilin, intermediate filament 1 |
| TLN1 | Gga.4319.1.S1_at | 2.31 | talin 1 |
| TNNC2 | Gga.823.1.S1_at | 5.57 | troponin C type 2 (fast) |
| TNNT3 | Gga.4090.6.S1_a_at | 2.90 | troponin T type 3 (skeletal, fast) |
| TPM1 | Gga.4108.5.S1_x_at | 2.72 | tropomyosin 1 (alpha) |
| TPM3 | Gga.4975.1.S1_a_at | 2.35 | tropomyosin 3 |
| Transcription | |||
| BHLHB2 | GgaAffx.22522.1.S1_at | 2.34 | basic helix-loop-helix domain containing, class B, 2 |
| CBFB | Gga.17908.1.S1_s_at | 2.23 | core-binding factor, beta subunit |
| CEBPB | Gga.4285.1.S1_at | 2.05 | CCAAT/enhancer binding protein (C/EBP), beta |
| DACH1 | Gga.79.1.S1_at | 5.13 | dachshund homolog 1 (Drosophila) |
| ELK4 | GgaAffx.26765.1.S1_at | 2.70 | ELK4, ETS-domain protein (SRF accessory protein 1) |
| ETV5 | Gga.447.1.S1_at | 9.23 | ets variant gene 5 (ets-related molecule) |
| FHL2 | Gga.3108.1.S1_at | 2.80 | four and a half LIM domains 2 |
| FOXC2 | Gga.469.1.S1_at | 5.45 | forkhead box C2 (MFH-1, mesenchyme forkhead 1) |
| HES1 | Gga. 3754.2. S1_at | 2.61 | hairy and enhancer of split 1, (Drosophila) |
| HOXA10 | Gga. 10332.1.S1_at | 3.56 | Homeobox A10 |
| ID1 | Gga.892.1.S1_at | 2.64 | inhibitor of DNA binding 1, dominant negative helix-loop-helix protein |
| ID2 | Gga.3125.1.S2_at | 2.67 | inhibitor of DNA binding 2, dominant negative helix-loop-helix protein |
| IFRD1 | GgaAffx.21710.1.S1_s_at | 2.17 | interferon-related developmental regulator 1 |
| IRF10 | Gga.158.1.S1_a_at | 15.34 | interferon regulatory factor 10 |
| MED16 | GgaAffx.25352.1.S1_s_at | 2.83 | mediator complex subunit 16 |
| MITF | Gga.275.1.S1_at | 2.82 | microphthalmia-associated transcription factor |
| MIZF | Gga.7048.1.S1_at | 2.52 | MBD2-interacting zinc finger |
| NKX-6.1 | Gga.4083.1.S1_at | 2.22 | homeodomain protein |
| SOX8 | Gga.4309.1.S1_at | 2.17 | SRY (sex determining region Y)-box 8 |
| Transport | |||
| ABCA3 | GgaAffx.25344.4.S1_s_at | 2.13 | ATP-binding cassette, sub-family A (ABC1), member 3 |
| AE2 | Gga. 1335.1. S1_at | 2.20 | AE2-1 anion exchanger |
| ATP1B1 | Gga.3301.1.S1_at | 5.10 | ATPase, Na+/K+ transporting, beta 1 polypeptide |
| ATP6V0A1 | Gga. 4672.1.S1_at | 2.09 | ATPase, H+ transporting, lysosomal V0 subunit A1 |
| CACNA1G | GgaAffx.4763.7.S1_at | 2.76 | calcium channel, voltage-dependent, T type, alpha 1G subunit |
| IPO13 | GgaAffx.12959.1.S1_at | 2.15 | importin 13 |
| PITPNC1 | GgaAffx.25933.1.S1_at | 2.08 | phosphatidylinositol transfer protein, cytoplasmic 1 |
| SCAMP4 | Gga. 17554.1.S1_at | 3.10 | secretory carrier membrane protein 4 |
| SLC1A6 | GgaAffx.26346.2.S1_s_at | 2.33 | solute carrier family 1 (high affinity aspartate/glutamate transporter), member 6 |
| SLC37A2 | GgaAffx.25722.2.S1_s _at | 6.73 | solute carrier family 37 (glycerol-3-phosphate transporter), member 2 |
| TMC6 | GgaAffx.4503.1.S1_at | 2.93 | transmembrane channel-like 6 |
| XPO5 | GgaAffx.23205.1 .S1_s_at | 3.87 | exportin 5 |
Table 4.
Genes Preferentially Expressed in Fast/Slow Myotubes
| Gene Symbol | Probe Set ID | Fold Change | Gene Title/Comments |
|---|---|---|---|
| Apoptosis | |||
| API5 | GgaAffx. 11374.1. S1_at | 2.61 | apoptosis inhibitor 5 |
| BFAR | GgaAffx. 25742.1.S1_at | 2.02 | bifunctional apoptosis regulator |
| Cell Adhesion | |||
| CD164 | Gga.7158.1.S1_at | 2.15 | CD164 molecule, sialomucin |
| CDH2 | GgaAffx.21844.1.S1_s_at | 2.08 | cadherin 2, type 1, N-cadherin (neuronal) |
| FN1 | Gga.9772.1.S1_s_at | 2.12 | fibronectin 1 |
| ITGA1 | Gga.566.1.S1_at | 3.27 | integrin, alpha 1 |
| ITGA6 | Gga.2967.1.S1_at | 4.20 | integrin, alpha 6 |
| SDC1 | Gga.6597.1.S1_at | 2.09 | syndecan 1 |
| THBS2 | GgaAffx.21822.1.S1_s_at | 2.19 | thrombospondin 2 |
| TJP1 | Gga.20045.1.S1_s_at | 2.64 | tight junction protein 1 (zona occludens 1) |
| Cell Cycle | |||
| CCAR1 | GgaAffx.11996.1.S1_s_at | 2.07 | cell division cycle and apoptosis regulator 1 |
| CCND1 | Gga.3039.1.S1_at | 2.04 | cyclin D1 |
| CENP-N | GgaAffx.8595.2.S1_s_at | 2.05 | centromere protein N |
| GSPT1 | Gga.9336.1.S1_at | 2.20 | G1 to S phase transition 1 |
| SPIN1 | Gga.4322.1.S1_at | 2.63 | spindlin 1 |
| Chromatin Remodeling | |||
| ARID1B | GgaAffx.24250.1.S1_s_at | 3.32 | AT rich interactive domain 1B (SWI1-like) |
| ATRX | GgaAffx.22386.2.S1_s_at | 3.43 | alpha thalassemia/mental retardation syndrome X-linked |
| BAZ1A | Gga.19082.1.S1_s_at | 4.11 | bromodomain adjacent to zinc finger domain, 1A |
| SMARCA1 | Gga.2597.1.S1_at | 3.29 | SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily a, member 1 |
| SMARCA5 | GgaAffx. 11920.1.S1_s_at | 2.71 | SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily a, member 5 |
| Cytoskeleton and ECM | |||
| CKAP4 | GgaAffx.8020.1.S1_at | 3.09 | cytoskeleton-associated protein 4 (p63) |
| EML4 | GgaAffx.23072.2.S1_s_at | 6.74 | echinoderm microtubule associated protein like 4 |
| NEXN | Gga.13445.1.S1_s_at | 2.36 | nexilin |
| TIMP4 | GgaAffx. 26374.1. S1_at | 2.72 | TIMP metallopeptidase inhibitor 4 |
| Metabolism | |||
| AACS | GgaAffx.1857.1.S1_s_at | 2.18 | acetoacetyl-CoA synthetase |
| AGA | GgaAffx.12577.1.S1_at | 7.58 | aspartylglucosaminidase |
| AGPS | Gga.5897.1.S1_at | 4.02 | alkylglycerone phosphate synthase |
| ALDH1L2 | GgaAffx.8040.1.S1_s_at | 2.97 | aldehyde dehydrogenase 1 family, member L2 |
| AMPD3 | GgaAffx. 26558.1. S1_at | 3.15 | adenosine monophosphate deaminase (isoform E) |
| ARSJ | GgaAffx. 23739.1. S1_at | 2.60 | arylsulfatase family, member J |
| BHMT | GgaAffx.2789.1.S1_at | 6.35 | betaine-homocysteine methyltransferase |
| CASK | Gga.7689.2.S1_x_at | 6.03 | calcium/calmodulin-dependent serine protein kinase (MAGUK family) |
| CDO1 | Gga.6921.1.S1_a_at | 2.16 | cysteine dioxygenase, type I |
| CPT1A | GgaAffx.20100.1.S1_at | 10.00 | carnitine palmitoyltransferase 1A (liver) |
| DDX21 | Gga.5656.1.S1_a_at | 2.32 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 21 |
| GALC | GgaAffx.6700.1.S1_s_at | 4.81 | galactosylceramidase |
| GFPT2 | GgaAffx.8765.3.S1_s_at | 2.02 | glutamine-fructose-6-phosphate transaminase 2 |
| GNPDA2 | GgaAffx.9013.1.S1_at | 2.02 | glucosamine-6-phosphate deaminase 2 |
| GNPTAB | GgaAffx.23993.3.S1_s_at | 2.02 | N-acetylglucosamine-1-phosphate transferase, alpha and beta subunits |
| GPD1L | GgaAffx. 7282.1.S1_at | 2.39 | glycerol-3-phosphate dehydrogenase 1-like |
| GSTT1 | Gga.2437.1.S1_at | 2.90 | glutathione S-transferase theta 1 |
| IDE | GgaAffx.8525.8.S1_s_at | 3.25 | insulin-degrading enzyme |
| IDI1 | Gga.8851.2.S1_a_at | 2.27 | isopentenyl-diphosphate delta isomerase 1 |
| ME1 | Gga.1132.1.S1_at | 2.90 | malic enzyme 1, NADP(+)-dependent, cytosolic |
| PGM5 | GgaAffx.9522.1.S1_at | 4.38 | phosphoglucomutase 5 |
| SOD2 | Gga.937.1.S1_at | 6.63 | superoxide dismutase 2, mitochondrial |
| SOD3 | Gga.1128.2.S1_a_at | 3.11 | superoxide dismutase 3, extracellular |
| Signal Transduction | |||
| CALM2 /// RCJMB04_24e7 | Gga.4454.2.S1_s_at | 2.01 | calmodulin 2 (phosphorylase kinase, delta) /// calmodulin 1 (phosphorylase kinase, delta) |
| CAMK2G | Gga.17610.1.S1_at | 2.22 | calcium/calmodulin-dependent protein kinase (CaM kinase) II gamma |
| AGTR1 | Gga.632.1.S1_at | 5.72 | angiotensin II receptor, type 1 |
| ARFGEF2 | GgaAffx.26281.3.S1_s_at | 3.30 | ADP-ribosylation factor guanine nucleotide-exchange factor 2 (brefeldin A-inhibited) |
| ARHGAP21 | Gga.2743.1.S1_at | 3.03 | Rho GTPase activating protein 21 |
| ASCC3 | GgaAffx.9843.1.S1_s_at | 13.31 | activating signal cointegrator 1 complex subunit 3 |
| EPHA3 | Gga.805.1.S1_at | 12.94 | EPH receptor A3 |
| EPHB1 | Gga.694.1.S1_at | 2.51 | EPH receptor B1 |
| ERBB2IP | GgaAffx.24516.2.S1_s_at | 3.09 | erbb2 interacting protein |
| GDAP2 | Gga.12508.1.S1_at | 2.63 | ganglioside induced differentiation associated protein 2 |
| GRM7 | GgaAffx. 5262.1.S1_at | 2.75 | glutamate receptor, metabotropic 7 |
| IL1R1 | Gga.846.1.S1_at | 4.50 | interleukin 1 receptor, type I |
| INPP5F | Gga. 13374.1.S1_at | 2.30 | inositol polyphosphate-5-phosphatase F |
| LTBP1 | GgaAffx.6607.2.S1_s_at | 3.32 | latent transforming growth factor beta binding protein 1 |
| MAPK9 | Gga.3651.1.S1_at | 2.63 | mitogen-activated protein kinase 9 |
| PDE3A | GgaAffx.24123.1.S1_at | 3.01 | phosphodiesterase 3A, cGMP-inhibited |
| PDGFD | Gga.9675.1.S1_at | 5.61 | platelet derived growth factor D |
| PIK3C2A | GgaAffx.26752.1.S1_s_at | 2.83 | phosphoinositide-3-kinase, class 2, alpha polypeptide |
| PIK3CA | GgaAffx. 5619.1.S1_at | 2.62 | phosphoinositide-3-kinase, catalytic, alpha polypeptide |
| PKIA | Gga.3155.1.S1_at | 2.94 | protein kinase (cAMP-dependent, catalytic) inhibitor alpha |
| PLCD1 | Gga.12980.1.S1_s_at | 2.84 | phospholipase C, delta 1 |
| PRKD3 | GgaAffx.6712.2.S1_s_at | 2.51 | protein kinase D3 |
| RCAN1 | Gga.5465.1.S1_at | 3.80 | regulator of calcineurin 1 |
| RGS9BP | Gga.9490.1.S1_at | 2.69 | regulator of G protein signaling 9 binding protein |
| SGSM2 | GgaAffx.3595.1.S1_s_at | 3.65 | small G protein signaling modulator 2 |
| TOB1 | Gga.1160.1.S1_at | 13.60 | transducer of ERBB2, 1 |
| WISP1 | Gga. 7551.1.S1_at | 3.16 | WNT1 inducible signaling pathway protein 1 |
| Structural | |||
| COL1A2 | Gga.3607.1.S1_a _at | 3.67 | collagen, type I, alpha 2 |
| DMD | Gga.718.2.S1_a_at | 2.09 | dystrophin |
| MYO1B | GgaAffx.22337.2.S1_s_at | 2.25 | myosin IB |
| MYOM3 | GgaAffx.2577.2.S1_s_at | 5.50 | myomesin family, member 3 |
| TMOD3 | GgaAffx.11704.1.S1_s_at | 2.52 | tropomodulin 3 (ubiquitous) |
| TTC8 | GgaAffx. 6738.1.S1_at | 2.68 | tetratricopeptide repeat domain 8 |
| TUBB | Gga.4579.1.S1_x_at | 4.10 | tubulin, beta |
| Transcription | |||
| BCLAF1 | GgaAffx.24308.2.S1_s_at | 2.13 | BCL2-associated transcription factor 1 |
| BRD1 | GgaAffx. 22617.1.S1_at | 2.28 | bromodomain containing 1 |
| BRMS1L | GgaAffx.11818.1.S1_s_at | 2.29 | breast cancer metastasis-suppressor 1-like |
| EBF1 | Gga.276.1.S1_at | 2.79 | early B-cell factor 1 |
| EMX2 | Gga. 7683.1.S1_at | 4.95 | empty spiracles homeobox 2 |
| EYA4 | Gga.420.1.S1_s_at | 3.19 | eyes absent homolog 4 (Drosophila) |
| EZH2 | Gga.20057.1.S1_s_at | 2.26 | enhancer of zeste homolog 2 (Drosophila) |
| FHL5 | Gga.10208.1.S1_a_at | 2.69 | four and a half LIM domains 5 |
| FOXO1A | Gga.3406.1.S1_at | 2.21 | forkhead box O1A |
| FOXO3 | Gga.19700.1.S1_at | 2.35 | forkhead box O3 |
| HOXA7 | Gga.5122.1.S1_at | 2.02 | homeobox A7 |
| HOXD8 | Gga.3187.1.S1_at | 5.80 | homeobox D8 |
| JAZF1 | Gga.7912.1.S1_at | 2.35 | JAZF zinc finger 1 |
| LHX9 | Gga.2348.1.S1_a_at | 6.63 | LIM homeobox 9 |
| MEIS2 | Gga.4046.1.S1_at | 2.91 | Meis homeobox 2 |
| MEOX2 | Gga.90.1.S1_at | 3.95 | mesenchyme homeobox 2 |
| NFATC3 | Gga.19337.1.S1_s_at | 2.14 | nuclear factor of activated T-cells, cytoplasmic, calcineurin-dependent 3 |
| NFIB | Gga.17307.1.S1_at | 3.92 | nuclear factor I/B |
| PITX1 | Gga.13903.1.S1_at | 3.83 | paired-like homeodomain 1 |
| PPARA | Gga.4006.2.S1_a_at | 3.03 | peroxisome proliferator-activated receptor alpha |
| PPARG | Gga.3858.2.S1_a_at | 2.16 | peroxisome proliferator-activated receptor gamma |
| R3HDM1 | GgaAffx.23837.4.S1_s_at | 2.10 | R3H domain containing 1 |
| RAB8B | Gga.13026.1.S1_at | 2.30 | RAB8B, member RAS oncogene family |
| RAI14 | Gga.12606.1.S1_s_at | 3.75 | retinoic acid induced 14 |
| RARB | Gga.2668.2.S1_at | 2.54 | retinoic acid receptor, beta |
| RREB1 | Gga.1491.1.S1_at | 2.45 | ras responsive element binding protein 1 |
| SMARCD3 | GgaAffx.8276.3.S1_s_at | 3.03 | SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily d, member 3 |
| SP3 | Gga.2337.1.S1_s_at | 2.03 | Sp3 transcription factor |
| TBPL1 | Gga.4434.1.S1_at | 2.95 | TBP-like 1 |
| TFDP1 | Gga.3952.1.S1_at | 5.16 | transcription factor Dp-1 |
| TSHZ3 | Gga.15899.1.S1_at | 3.52 | teashirt zinc finger homeobox 3 |
| YAF2 | Gga.1754.1.S1_s_at | 2.51 | YY1 associated factor 2 |
| ZEB1 | Gga.3548.1.S1_at | 2.22 | zinc finger E-box binding homeobox 1 |
| ZFHX4 | GgaAffx.9993.1.S1_at | 4.29 | zinc finger homeobox 4 |
| ZMYND11 | GgaAffx.21984.1.S1_at | 3.07 | zinc finger, MYND domain containing 11 |
| Transport | |||
| ATP6AP1 | GgaAffx.5549.1.S1_at | 2.17 | ATPase, H+ transporting, lysosomal accessory protein 1 |
| BBS5 | Gga.19986.1.S1_at | 2.13 | Bardet-Biedl syndrome 5 |
| BIN1 | GgaAffx.11745.1.S1_s_at | 2.13 | bridging integrator 1 |
| CAST | GgaAffx.9300.1.S1_at | 2.20 | calpastatin |
| COLEC12 | Gga.10960.1.S1_at | 2.82 | collectin sub-family member 12 |
| CYB5 | GgaAffx.21828.1.S1_s_at | 3.58 | cytochrome b-5 |
| FTD | Gga.20.1.S2_at | 9.22 | ferritoid |
| KPNA3 | Gga.1482.1.S1_at | 2.68 | karyopherin alpha 3 (importin alpha 4) |
| OPTN | Gga.4189.1.S1_s_at | 2.45 | optineurin |
| RBP7 | Gga.9386.1.S1_at | 3.12 | retinol binding protein 7, cellular |
| SCFD1 | GgaAffx.6231.1.S1_s_at | 2.11 | sec1 family domain containing 1 |
| SCP2 | Gga.3425.1.S1_at | 2.86 | sterol carrier protein 2 |
| SLC22A16 | GgaAffx.24590.1.S1_s_at | 5.21 | solute carrier family 22 (organic cation transporter), member 16 |
| SLC25A36 | GgaAffx.3298.1.S1_s_at | 2.56 | solute carrier family 25, member 36 |
| SLC30A1 | Gga.10012.1.S1_s_at | 2.60 | solute carrier family 30 (zinc transporter), member 1 |
| SLC45A4 | Gga.5046.1.A1_s_at | 2.30 | Solute carrier family 45, member 4 |
| SNX2 | GgaAffx. 3339.1.S1_at | 3.30 | sorting nexin 2 |
| SRP54 | Gga.1375.3.S1_s_at | 2.57 | signal recognition particle 54kDa |
| STX16 | GgaAffx.12300.1.S1_s_at | 2.05 | syntaxin 16 |
| SYTL2 | GgaAffx.8937.1.S1_at | 4.55 | synaptotagmin-like 2 |
| TMED5 | Gga.3703.1.S1_s_at | 2.14 | transmembrane emp24 protein transport domain containing 5 |
Table 1.
Expression of Fast Muscle Fiber Associated Genes
| Gene Symbol | Probe Set ID | Fold Change | Gene Title/Comments |
|---|---|---|---|
|
| |||
| TPM1 | Gga4108.4.S1.s.at | 1.25 | tropomyosin 1 alpha |
| Gga4108.1.S2.at | 0.28 | ||
| Gga 4108.4.S1.x.at | 1.50 | ||
| Gga4108.1.S1.at | 0.90 | ||
| GgaAffx20738.1.S1.s.at | 0.54 | ||
|
| |||
| TNNT3 | Gga4090.6.S1.a at | 2.90 | troponin T type 3 |
| Gga4090.1.S1.a.at | 2.15 | ||
|
| |||
| TNNI2 | Gga 700.1.S1.at | 1.73 | troponin I type 2 |
|
| |||
| MYBPC2 | Gga4986.1.S1.at | 0.69 | myosin binding protein C |
|
| |||
| MYL1 | Gga18909.1.S1.s.at | 1.53 | myosin light chain 1 |
| Gga18909.1.S1.a.at | 0.98 | ||
| Gga4835.1.S1.a.at | 1.44 | ||
Table 2.
Expression of Fast/Slow Muscle Fiber Associated Genes
| Gene Symbol | Probe Set ID | Fold Change | Gene Title/Comments |
|---|---|---|---|
|
| |||
| TPM3 | Gga4975.1.S1.a.at | 2.35 | tropomyosin 3 |
|
| |||
| TNNI1 | Gga6340.2.S1.a.at | 2.14 | troponin I type 1 |
|
| |||
| TNNC1 | Gga3041.1.S1.at | 1.49 | troponin C type 1 |
|
| |||
| STNT | GgaAffx21770.S1.s.at | 1.59 | slow troponin T |
|
| |||
| MYBPC1 | Gga3063.1.S1.at | 3.09 | myosin binding protein C1 |
| Gga10173.1.S1.at | 54.93 | ||
| Gga10173.1.S1.s.at | 8.59 | ||
| GgaAffx8106.1.S1.s.at | 0.39 | ||
|
| |||
| MYL2 | Gga841.1.S1.at | 1.83 | myosin light chain 2 |
|
| |||
| MYL3 | Gga4198.2.S1.a.at | 2.32 | myosin light chain 3 slow |
|
| |||
| SM1 | Gga16803.1.S1.s.at | 0.79 | slow myosin heavy chain 1 |
|
| |||
| MYO1C | GgaAffx11931.1.S1.s.at | 1.09 | myosin 1C |
|
| |||
| MYH7 | GgaAffx11330.1.S1.at | 0.53 | myosin heavy chain 7 |
|
| |||
| MYH7B | Gga103.1.S1.at | 2.20 | myosin heavy chain 7B |
|
| |||
| AMHC1 | Gga5315.1.S1.s.at | 13.46 | atrial myosin heavy chain 1 |
Embryonic muscle fibers formed from fast and fast/slow myoblast clonal populations exhibited differences in gene expression in a variety of cellular functions. Fast muscle fibers exhibited increased expression of 718 genes, and fast/slow fibers had increased expression of 799 genes. Relative gene expression levels of two fold or greater were included in the data shown in Figure 1. Biological functions of differentially expressed genes were assigned by GO annotation and/or Entrez Gene and Expasy Proteomics Servers. Functional gene categories include metabolism, transcription, signal transduction, etc. Of those genes that were differentially expressed in fast versus fast/slow embryonic muscle fibers, 23.1% and 23.5% of them were genes associated with metabolic function in fast and fast/slow muscle fibers, respectively. Genes associated with transcriptional regulation in fast versus fast/slow muscle fibers comprised 7.2% and 10.4%, respectively, of differentially expressed genes. Signal transduction genes in fast versus fast/slow muscle fibers accounted for 7.4% and 10.3%, respectively, of differentially expressed genes.
Fig. 1.

Relative distribution of genes differentially expressed in fast versus fast/slow myotubes based on function. Genes expressed more than two-fold in fast or fast/slow myotubes were included in the analysis. Gene functions were assigned by GO annotation and Entrez Gene and Expasy Proteomic Servers. Pie charts represent the percentages of genes assigned particular functions (refer to color legend) for genes differentially expressed in fast myotubes (718 total genes) and fast/slow myotubes (799 total genes) from multiple myogenic clones.
Table 3 lists genes of known identity differentially expressed in fast versus fast/slow muscle fibers. Transcriptional regulatory genes differentially expressed in fast fibers included several helix-loop-helix (HLH) regulatory genes (e.g. ID1, ID2, BHLHB2), interferon regulatory genes (e.g. IFRD1 and IRF10), and homeodomain protein genes (e.g. HoxA10 and NKX-6.1). A complete list of the fast muscle fiber identified gene profile is included in Supplement Table 1.
Table 4 lists genes of known identity differentially expressed in fast/slow versus fast embryonic muscle fibers. Transcriptional regulatory genes expressed in fast/slow muscle fibers included several Hox genes (e.g. HoxA7, Meis2, MEOX2), Nuclear Factor of Activated T Cells (NFATC3), peroxisome proliferator-activated receptor genes (PPARA and PPARG), and zinc finger protein genes (e.g. ZEB1 and Sp3). A complete list of identified genes differentially expressed in fast/slow versus fast embryonic muscle fibers is included in Supplement Table 2.
Eight genes were selected for verification of relative expression levels by quantitative RT-PCR. Relative expression of four genes differentially expressed in fast muscle fibers (DACH1, FHL2, FoxC2, and Sox8) and four genes expressed in fast/slow muscle fibers (EYA4, Foxo1A, NFIB, and PPARA) were quantitated (Figure 2). Differentially expressed genes in fast or fast/slow muscle fibers identified by microarray analyses were differentially expressed by 2.3 to 3.8 fold. The qRT-PCR results validated the microarray analyses.
Fig. 2.

Quantitative RT-PCR of select genes. Expression levels of 8 genes was determined by qRT-PCR. Four genes (FHL2, Sox8, FoxC2, and DACH1) were selected from the list of fast myotube associated genes generated from the microarray analysis. Similarly, four genes (Foxo1A, EYA4, NFIB, and PPARA) were selected from the list of fast/slow myotube associated genes. Bars represent relative expression levels of genes. For example, FHL2 is expressed approximately 3 fold higher in fast muscle fibers versus fast/slow fibers.
Fast and fast/slow primary embryonic muscle fibers are derived from myoblasts committed to the fast and fast/slow myogenic cell lineages, respectively. To investigate the basis for differential commitment of fast and fast/slow myoblast lineages to specific embryonic fiber type formation, genome-wide gene expression analysis was conducted on undifferentiated fast and fast/slow myoblasts. Five fast and four fast/slow clonal myoblast populations were pooled according to fiber type commitment (i.e. fast versus fast/slow) and expression of the chicken genome was interrogated. Fast myoblasts differentially expressed 303 genes (Figure 3), and 12% of these genes were associated with transcriptional regulation. Genes encoding the transcription factors BTF3 and PITX2 were among the genes in this functional group expressed in fast myoblasts (Table 5). Genes associated with signal transduction accounted for 16% of genes differentially expressed in fast myoblasts and included FGF13 and GDF10. Transport function was associated with 8% of fast myoblast genes, and 23% were associated with metabolic function. The complete list of genes differentially expressed in fast myoblasts is included in Supplement Table 3. Fast/slow myoblasts differentially expressed 380 genes (Table 6). Genes associated with transcriptional regulation (e.g. MEOX2 and HoxD8) and signal transduction (e.g. FGF4 and IGFBP5) accounted for 10% and 12% of these genes, respectively (Figure 3). Transport and metabolic functions were associated with 7% and 18%, respectively, of genes differentially expressed in fast/slow myoblasts versus fast myoblasts. The complete list of genes differentially expressed in fast/slow myoblasts is included in Supplement Table 4. Collectively, these results indicate that fast and fast/slow myoblasts express unique subsets of genes and further indicate that fast and fast/slow myoblasts are distinct cell types.
Fig. 3.
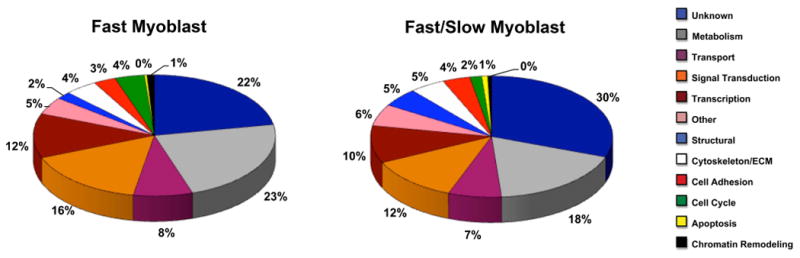
Relative distribution of genes differentially expressed in fast versus fast/slow myoblasts. Genes expressed more than two-fold in fast or fast/slow myoblasts were included in the analysis. Gene functions were assigned by GO annotation and Entrez Gene and Expasy Proteomic Servers. Pie charts represent the percentages of genes assigned particular functions (refer to color legend) for genes differentially expressed in fast myoblasts (303 total genes) and fast/slow myoblasts (380 total genes) from multiple myogenic clones.
Table 5.
Genes Preferentially Expressed in Fast Myoblasts
| Gene Symbol | Probe Set ID | Fold Change | Gene Title/Comments |
|---|---|---|---|
| Cell Adhesion | |||
| ALCAM | Gga.2734.1.S2_at | 7.70 | activated leukocyte cell adhesion molecule |
| ANKK1 | GgaAffx.22381.3.S1_s_at | 2.65 | ankyrin repeat and kinase domain containing 1 |
| ITGB3 | Gga.1039.1.S1_at | 2.31 | integrin, beta 3 (platelet glycoprotein IIIa, antigen CD61) |
| Cell Cycle | |||
| CCNF | GgaAffx.22831.1.S1_at | 2.18 | cyclin F |
| CDKN2C | GgaAffx. 6661.1.S1_at | 2.49 | cyclin-dependent kinase inhibitor 2C (p18, inhibits CDK4) |
| CKS2 | Gga.1958.1.S1_a_at | 3.34 | CDC28 protein kinase regulatory subunit 2 |
| GINS1 | Gga.12208.1.S1_a_at | 3.12 | GINS complex subunit 1 (Psf1 homolog) |
| KNTC1 | GgaAffx.26234.1.S1_s_at | 2.01 | kinetochore associated 1 |
| SEPT2 | GgaAffx.3632.1.S1_at | 2.32 | septin 2 |
| Chromatin Remodeling | |||
| SUZ12 | Gga.19626.1.S1_s_at | 2.11 | suppressor of zeste 12 homolog (Drosophila) |
| Cytoskeleton | |||
| AFAP1 | Gga.185.1.S1_a_at | 2.33 | actin filament associated protein 1 |
| DCTN4 | GgaAffx.2799.1.S1_at | 3.04 | dynactin 4 (p62) |
| DYNLL2 | Gga.17308.1.S1_s_at | 2.07 | dynein, light chain, LC8-type 2 |
| KIF26A | GgaAffx.23603.1.S1_s_at | 2.05 | kinesin family member 26A |
| MAP4 | GgaAffx.21343.1.S1_s_at | 2.04 | microtubule-associated protein 4 |
| Metabolism | |||
| AER61 | GgaAffx.8545.1.S1_s_at | 2.00 | glycosyltransferase |
| ASPH | Gga.11883.4.S1_s_at | 2.35 | Aspartate beta-hydroxylase |
| B4GALT6 | GgaAffx.9633.1.S1_s_at | 3.60 | UDP-Gal:betaGlcNAc beta 1,4- galactosyltransferase, polypeptide 6 |
| COMT | Gga.7199.1.S1_s_at | 3.35 | catechol-O-methyltransferase |
| DHFR | GgaAffx.11934.1.S1_s_at | 2.07 | dihydrofolate reductase |
| GLT25D2 | Gga.3249.1.S1_at | 2.49 | glycosyltransferase 25 domain containing 2 |
| HEXB | Gga.9970.1.S1_at | 2.40 | hexosaminidase B (beta polypeptide) |
| HMGCR | GgaAffx.12414.1.S1_s_at | 2.20 | 3-hydroxy-3-methylglutaryl-Coenzyme A reductase |
| MAN1A1 | Gga.20070.1.S1_at | 3.38 | mannosidase, alpha, class 1A, member 1 |
| NADK | GgaAffx.907.1.S1_at | 2.20 | NAD kinase |
| NAT13 | GgaAffx.9403.1.S1_s_at | 2.17 | N-acetyltransferase 13 |
| PCMT1 | Gga. 16623.2.S1_a_at | 2.08 | protein-L-isoaspartate (D-aspartate) O-methyltransferase |
| PMPCB | Gga.7638.1.A1_at | 2.28 | peptidase (mitochondrial processing) beta |
| PTPN2 | Gga.1107.1 .S1_at | 2.15 | protein tyrosine phosphatase, non-receptor type 2 |
| ROR1 | Gga.9476.1.S1_at | 2.56 | receptor tyrosine kinase-like orphan receptor 1 |
| SENP8 | GgaAffx.1329.1.S1_at | 2.43 | SUMO/sentrin specific peptidase family member 8 |
| ST3GAL1 | Gga. 3672.1.S1_at | 3.26 | ST3 beta-galactoside alpha-2,3-sialyltransferase 1 |
| ST8SIA2 | Gga.19493.2.S1_s_at | 4.92 | ST8 alpha-N-acetyl-neuraminide alpha-2,8-sialyltransferase 2 |
| TXNDC10 | GgaAffx.24266.1 .S1_at | 2.21 | thioredoxin domain containing 10 |
| Signal Transduction | |||
| ADCYAP1R1 | GgaAffx. 3269.1. S1_at | 12.84 | adenylate cyclase activating polypeptide 1 (pituitary) receptor type I |
| ARHGAP12 | GgaAffx.4536.1.S1_s_at | 2.39 | Rho GTPase activating protein 12 |
| BMPR1A | Gga.755.1.S1_at | 2.27 | bone morphogenetic protein receptor, type IA |
| DKK3 | Gga.3573.2.S1_a_at | 4.44 | dickkopf homolog 3 (Xenopus laevis) |
| EDN1 | GgaAffx.8070.1.S1_at | 5.40 | endothelin 1 |
| EPHB3 | Gga.3053.1.S1_at | 2.03 | EPH receptor B3 |
| FGF13 | GgaAffx.21832.1.S1_s_at | 7.36 | fibroblast growth factor 13 |
| FGFR3 | Gga.16413.1.A1_a_at | 5.02 | fibroblast growth factor receptor 3 |
| FLT1 | Gga.150.2.S1_a_at | 2.72 | fms-related tyrosine kinase 1 |
| FRZB | Gga.4955.1.S1_at | 3.08 | frizzled-related protein |
| GDF10 | GgaAffx.3720.1.S1_at | 8.39 | growth differentiation factor 10 |
| GFRA1 | Gga.588.1.S1_at | 3.21 | GDNF family receptor alpha 1 |
| GPR23 | Gga.11466.2.S1_a_at | 6.08 | G protein-coupled receptor 23 |
| IL1R1 | Gga.846.1.S1_at | 2.32 | interleukin 1 receptor, type I |
| ITPR3 | GgaAffx.1993.5.S1_s_at | 2.37 | inositol 1,4,5-triphosphate receptor, type 3 |
| KREMEN1 | GgaAffx.3631.1.S1_at | 2.67 | kringle containing transmembrane protein 1 |
| MRAS | Gga.5500.2.S1_a_at | 2.22 | muscle RAS oncogene homolog |
| RASL11B | Gga.12911.1.S1_at | 2.16 | RAS-like, family 11, member B |
| RGS3 | Gga.8344.2.A1_a_at | 3.47 | regulator of G-protein signalling 3 |
| RPS6KA1 | Gga.9321.1.S1_at | 7.40 | ribosomal protein S6 kinase, 90kDa, polypeptide 1 |
| SOCS1 | Gga.10606.1.S1_at | 2.74 | suppressor of cytokine signaling 1 |
| Structural | |||
| CTXN1 | GgaAffx.210.1.S1_at | 2.84 | cortexin 1 |
| FBLN2 | GgaAffx.3200.1.S1_s_at | 2.18 | fibulin 2 |
| TNNT2 | Gga.4984.1.S1_at | 3.51 | troponin T type 2 (cardiac) |
| Transcription | |||
| BRD8 | GgaAffx.9060.2.S1_s_at | 2.02 | bromodomain containing 8 |
| BTF3 | Gga.11922.1.S1_at | 2.40 | basic transcription factor 3 |
| E2F1 | Gga.3213.1.S1_at | 2.06 | E2F transcription factor 1 |
| EGR1 | GgaAffx.11738.1.S1_s_at | 2.71 | early growth response 1 |
| FOXP1 | GgaAffx.4846.4.S1_s_at | 2.87 | forkhead box P1 |
| HOXA11 | Gga.957.1.S1_at | 2.06 | homeobox A11 |
| PITX2 | Gga.3398.2.S1_a_at | 4.47 | paired-like homeodomain 2 |
| SNAI1 | Gga.3851.1.S1_at | 2.01 | snail homolog 1 (Drosophila) |
| TCF12 | Gga.4007.3.S1_a_at | 2.03 | transcription factor 12 (HTF4, helix-loop-helix transcription factors 4) |
| ZBTB41 | GgaAffx.25447.1.S1_at | 2.31 | zinc finger and BTB domain containing 41 |
| Transport | |||
| ATP2B1 | GgaAffx.23508.1.S1_at | 2.17 | ATPase, Ca++ transporting, plasma membrane 1 |
| KCNK1 | Gga.4356.1.S1_at | 2.25 | potassium channel, subfamily K, member 1 |
| SLC39A10 | GgaAffx.22358.1.S1_s_at | 2.51 | solute carrier family 39 (zinc transporter), member 10 |
| SNX30 | Gga.11940.1.S1_at | 2.27 | sorting nexin family member 30 |
| VLDLR | Gga.679.1.S1_at | 3.64 | very low density lipoprotein receptor |
Table 6.
Genes Preferentially Expressed in Fast/Slow Myoblasts
| Gene Symbol | Probe Set ID | Fold Change | Gene Title/Comments |
|---|---|---|---|
| Cell Adhesion | |||
| ITGA1 | Gga.566.1.S1_at | 10.65 | integrin, alpha 1 |
| LAMA2 | Gga.8352.1.S1_at | 2.81 | similar to laminin alpha 2 subunit precursor; laminin M |
| RELN | Gga.496.1.S1_at | 5.44 | extracellular reelin |
| THBS2 | Gga.1686.1.S1_s_at | 2.50 | thrombospondin 2 |
| TNC | GgaAffx.26374.1.S1_at | 2.87 | tenascin |
| Cell Cycle | |||
| CCNG2 | Gga.15984.1.S1_at | 5.98 | cyclin G2 |
| CDC42 | Gga.4438.1.S1_at | 2.74 | cell division cycle 42 |
| Chromatin Remodeling | |||
| SMARCA1 | GgaAffx.4778.1.S1_s_at | 2.15 | similar to possible global transcription activator SNF2L1 |
| Cytoskeleton | |||
| DCN | Gga.1719.1.S1_at | 3.87 | decorin |
| FBLN5 | Gga.10096.1.S1_at | 3.99 | fibulin 5 |
| KRT75 | Gga.17686.1.S1_at | 6.24 | type II alpha keratin IIB |
| MAP1LC3C | Gga.3183.1.S1_a_at | 8.28 | microtubule-associated protein 1 light chain 3 gamma |
| NEFM | Gga.4179.1.S1_at | 9.43 | neurofilament 3 |
| SDC2 | Gga. 4675.1. S1_at | 2.13 | syndecan 2 |
| Metabolism | |||
| CAMK2D | GgaAffx.12207.1.S1_at | 2.39 | calcium/calmodulin-dependent protein kinase IID |
| CARS | GgaAffx.21941.1.S1_at | 4.10 | cysteinyl-tRNA synthetase |
| CDO1 | Gga.6921.1.S1_a_at | 5.14 | similar to cysteine dioxygenase |
| DPYD | GgaAffx.3458.1.S1_s_at | 6.38 | dihydropyrimidine dehydrogenase |
| DPYSL3 | Gga.9493.1.S1_at | 10.32 | dihydropyrimidinase-like 3 |
| DUSP1 | Gga.4120.1.S1_at | 2.34 | dual specificity phosphatase 1 |
| DUSP5 | Gga.19025.1.S1_at | 2.64 | dual specificity phosphatase 5 |
| FAP | GgaAffx.23453.2.S1_s_at | 3.78 | fibroblast activation protein, alpha |
| FECH | Gga.166.1.S1_at | 2.91 | ferrochelatase |
| FUT8 | GgaAffx.13151.1.S1_at | 2.91 | fucosyltransferase 8 |
| GALNTL4 | Gga.11756.1.S1_at | 10.49 | N-acetylgalactosaminyltransferase-like 4 |
| GFPT2 | GgaAffx.8765.2.S1_at | 3.05 | similar to glutamine:fructose-6-phosphate amidotransferase 2 |
| GSTK1 | Gga.14517.1.S1_s_at | 3.26 | glutathione S-transferase kappa 1 |
| GSTT1 | Gga. 2437.1. S1_at | 2.76 | glutathione S-transferase theta 1 |
| HAS2 | Gga.329.1.S1_at | 2.44 | hyaluronan synthase 2 |
| LYCAT | Gga.7898.1.S1_at | 4.02 | lysocardiolipin acyltransferase |
| MAN2C1 | GgaAffx.1059.1.S1_s_at | 2.05 | similar to alpha-mannosidase 2C1 |
| ME1 | Gga.1132.1.S1_at | 3.32 | malic enzyme 1, NADP(+)-dependent, cytosolic |
| MOXD1 | Gga.969.1.S1_at | 2.43 | monooxygenase, DBH-like 1 |
| MTRR | GgaAffx.24101.1.S1_at | 2.04 | similar to methionine synthase reductase isoform 2 |
| PAPSS1 | GgaAffx.23250.1.S1_s_at | 2.07 | 3′-phosphoadenosine 5′-phosphosufate synthase 1 |
| PGM5 | GgaAffx.9522.1.S1_at | 3.55 | similar to phosphoglucomutase 5 |
| PLK2 | Gga.10660.2.S1_at | 2.21 | similar to polo-like kinase 2 |
| PPAP2B | GgaAffx.23330.1.S1_at | 4.09 | similar to phosphatidic acid phosphatase type 2B |
| SOD2 | Gga.4220.1.S1_a_at | 3.22 | superoxide dismutase 2, mitochondrial |
| SOD3 | Gga.19934.1.S1_at | 3.03 | superoxide dismutase 3, extracellular |
| SULT1B1 | Gga.735.1.S1_at | 4.44 | sulfotransferase family, cytosolic, 1B, member 1 |
| UPP1 | Gga.18724.1 .S1_s_at | 3.71 | uridine phosphorylase 1 |
| Signal Transduction | |||
| CXCL14 | GgaAffx.21581.1.S1_s_at | 4.66 | chemokine ligand 14 |
| DGKH | GgaAffx.10860.2.S1_s_at | 2.16 | similar to A-kinase anchor protein 11 |
| DKK1 | Gga.897.1.S1_at | 4.03 | Dikkopf homolog 1 |
| EPHA3 | Gga.805.1.S1_at | 22.05 | EPH receptor A3 |
| FGF3 | Gga.2701.1.S1_at | 4.25 | fibroblast growth factor 3 |
| FGF4 | GgaAffx.4716.1.S1_at | 49.16 | fibroblast growth factor 4 |
| GTPBP4 | Gga.9844.1.S1_s_at | 2.49 | GTP binding protein 4 |
| IGF2R | Gga.3597.1.S1_at | 2.08 | insulin-like growth factor 2 receptor |
| IGFBP2 | Gga.759.1.S1_at | 3.13 | insulin-like growth factor receptor binding protein 2 |
| IGFBP5 | Gga. 9364.1.S1_at | 4.19 | insulin-like growth factor binding protein 5 |
| IL6 | Gga.2769.1.S1_at | 2.07 | interleukin 6 |
| IL8 | Gga.826.1.S1_s_at | 3.87 | interleukin 8 |
| LSP1 | Gga.16589.1.S1_at | 10.23 | lymphocyte-specific protein 1 |
| LTBP1 | GgaAffx.6607.2.S1_s_at | 2.65 | similar to latent transforming growth factor beta binding protein 1 |
| MAPK13 | GgaAffx.549.1.S1_at | 2.58 | mitogen-activated protein kinase 14 |
| NRG1 | Gga.135.3.S1_a_at | 8.07 | neuregulin 1 |
| PDE3A | GgaAffx.24123.1.S1_at | 4.09 | similar to cyclic nucleotide phosphodiesterase PDE3A |
| PDGFD | Gga. 9675.1.S1_at | 4.07 | platelet derived growth factor D |
| PPP2R3A | GgaAffx.23502.1.S1_at | 2.86 | similar to alpha isoform of regulatory subunit B, protein phosphatase 2, isoform 1 |
| RHOJ | Gga.12598.1.S1_at | 3.05 | ras homolog gene family, member J |
| SH3BGR | Gga.11787.2.S1_s_at | 3.38 | SH3 domain binding glutamic acid-rich protein |
| TGFB3 | GgaAffx.21766.1.S1_s_at | 2.86 | transforming growth factor beta 3 |
| VEGFC | Gga.10930.1.S1_at | 2.11 | similar to vascular endothelial growth factor C |
| WNT9A | GgaAffx.21279.1.S1_at | 2.99 | wingless-type MMTV integration site family , member 9A |
| ZIC1 | Gga.11492.1.S1_at | 2.84 | zic family member 1 |
| Structural | |||
| ACTA1 | Gga. 5962.1.S1_at | 2.32 | A-actin |
| ACTN2 | Gga.4843.2.S1_a_at | 4.36 | actinin, alpha 2 |
| MYH6 | Gga. 2617.1. S1_at | 3.06 | myosin, heavy polypeptide 6 |
| MYL3 | Gga.4198.2.S1_a_at | 3.16 | myosin, light polypeptide 3, alkali; skeletal slow |
| MYOM2 | Gga.4216.1.S1_at | 9.40 | myomesin (M-protein) 2 |
| SHROOM3 | Gga.15872.1.S1_s_at | 2.48 | similar to shroom-related protein |
| TNNC2 | Gga. 1722.1.S1_at | 2.42 | troponin C type 2 |
| TNNI1 | Gga.3818.1.S1_at | 2.27 | troponin I type 1 |
| Transcription | |||
| EBF1 | Gga.276.2.S1_a_at | 12.03 | early B-cell factor 1 |
| EMX2 | Gga. 7683.1. S1_at | 5.35 | empty spiracles homolog 2 |
| EYA2 | Gga.1839.1.S1_at | 3.75 | eyes absent homolog 2 |
| EYA4 | GgaAffx.24324.1.S1_at | 12.58 | eyes absent homolog 4 |
| FOXO1A | Gga. 3406.1. S1_at | 2.49 | forkhead box 01A |
| FOXP2 | GgaAffx.5942.1.S1_at | 33.57 | forkhead box P2 |
| HEY2 | GgaAffx.9430.1.S1_at | 2.26 | similar to hairy/enhancer-of-split related |
| HOXD8 | Gga.3187.1.S1_at | 6.63 | homeobox D8 |
| ID4 | Gga.2070.2.S1_a_at | 3.54 | inhibitor of DNA binding 4 |
| KLF3 | Gga. 12232.1.S1_at | 2.33 | Krüppel-like factor 3 |
| LHX9 | Gga.2348.1.S1_a_at | 10.27 | LIM homeobox 9 |
| MEOX2 | Gga.90.1.S1_at | 16.35 | mesenchme homeobox 2 |
| NFE2L2 | Gga. 3659.1. S1_at | 2.05 | nuclear factor (erythroid-derived 2)-like 2 |
| NFIB | Gga.17307.1.S1_at | 3.49 | nuclear factor I/B |
| PPARA | Gga.4006.1.S1_at | 2.46 | peroxisome proliferative activated receptor, alpha |
| PRRX1 | Gga. 1546.1. S1_at | 5.35 | paired related homeobox 1 |
| SOX4 | Gga.937.1.S1_at | 2.24 | SRY (sex determining region Y)-box 4 |
| Transport | |||
| ATP6V0D1 | Gga.7507.1.S1_at | 2.06 | ATPase, H+ transporting |
| ATP6V1G1 | Gga.4824.1.S1_at | 10.86 | similar to ATP6V1G1-prov protein |
| CYB5A | GgaAffx.21828.1.S1_s_at | 2.26 | cytochrome B-5 |
| FABP4 | Gga.4939.1.S1_s_at | 20.36 | fatty acid binding protein 4 |
| FTD | Gga.20.1.S2_at | 3.29 | ferritoid |
| NXT2 | GgaAffx.11867.1.S1_s_at | 2.49 | nuclear transport factor 2-like export factor 2 |
| ORMDL1 | GgaAffx.25485.1.S1_at | 2.32 | solute carrier family 40 (iron-regulated transporter), member 1 |
| RBP4 | Gga.4126.1.S1_at | 2.28 | retinol binding protein 4, plasma |
| RBP7 | Gga.9386.1.S1_at | 3.97 | retinol binding protein 7, cellular |
A subset of genes was differentially expressed in both fast myoblasts and fast myotubes (Table 7). Of the 15 genes expressed before and after fast myogenic cell clone differentiation, 6 genes were associated with signal transduction and included FGF13 and FGFR3. Three genes were associated with metabolic function. Similarly, three genes encoded proteins of the cytoskeleton. Lastly, only 2 genes were associated with adhesion function, and 1 gene was identified with structural function. No genes encoding transcriptional regulators were identified as genes differentially expressed in both fast myoblasts and myotubes.
Table 7.
Genes Expressed in Both Fast Myoblasts and Myotubes
| Gene Symbol | Gene Title/Comments |
|---|---|
| Cell Adhesion | |
| ITGB3 | integrin, beta 3 (platelet glycoprotein IIIa, antigen CD61) |
| PODXL | podocalyxin-like |
| Cytoskeleton | |
| DCTN4 | dynactin 4 (p62) |
| MGP | matrix Gla protein |
| SMTN | smoothelin |
| Metabolism | |
| CTSD | cathepsin D |
| GALNT5 | UDP-N-acetyl-alpha-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase 5 (GalNAc-T5) |
| GPD2 | glycerol-3-phosphate dehydrogenase 2 (mitochondrial) |
| Signal Transduction | |
| CCNDBP1 | cyclin D-type binding-protein 1 |
| DKK3 | dickkopf homolog 3 (Xenopus laevis) |
| EPHB3 | EPH receptor B3 |
| FGF13 | fibroblast growth factor 13 |
| FGFR3 | fibroblast growth factor receptor 3 |
| FZD2 | frizzled homolog 2 (Drosophila) |
| Structural | |
| FBLN2 | fibulin 2 |
A total of 51 genes were identified as differentially expressed in both fast/slow myoblasts and myotubes versus the fast myogenic cell lineage (Table 8). Genes associated with metabolic function comprised the largest category (41%). Representative genes included SOD2 and SOD3. Signal transduction genes comprised approximately 25% of these genes. Transcriptional regulatory genes accounted for one-third of genes differentially expressed in both fast/slow myoblasts and myotubes versus fast myoblasts and myotubes. Representative genes in this group included TSHZ2, TSHZ3, PPARA, and EMX2.
Table 8.
Genes Expressed in Both Fast Myoblasts and Myotubes
| Gene Symbol | Gene Title/Comments |
|---|---|
| Cell Adhesion | |
| ARVCF | armadillo repeat gene |
| FMN1 | formin |
| ITGA1 | integrin, alpha 1 |
| POSTN | periostin, osteoblast specific factor |
| THBS2 | thrombospondin 2 |
| WTIP | |
| Cell Cycle | |
| CCNG2 | cyclin G2 |
| PPP3CA | protein phosphatase 3, catalytic subunit, alpha isoform |
| Chromatin Remodeling | |
| SMARCA1 | similar to possible global transcription activator SNF2L1 |
| Cytoskeleton | |
| SPARC | |
| TIMP4 | |
| Metabolism | |
| ANXA1 | calcium-dependent membrane binding protein annexin 1 |
| CARS | cysteinyl-tRNA synthetase |
| CASK | similar to CASK |
| CDO1 | similar to cysteine dioxygenase |
| CRISPLD1 | cysteine-rich secretory protein LCCL domain containing 1 |
| CRISPLD2 | |
| FAP | fibroblast activation protein, alpha |
| GFPT2 | similar to glutamine:fructose-6-phosphate amidotransferase 2 |
| GSTT1 | glutathione S-transferase theta 1 |
| HAS2 | hyaluronan synthase 2 |
| HTRA3 | HtrA serine peptidase 3 |
| ME1 | malic enzyme 1, NADP(+)-dependent, cytosolic |
| MTRF1 | mitochondrial translational release factor 1 |
| PGM5 | similar to phosphoglucomutase 5 |
| PLK2 | similar to polo-like kinase 2 |
| PRSS35 | protease, serine, 35 |
| PXDN | |
| RDH10 | similar to retinol dehydrogenase 10 |
| SOD2 | superoxide dismutase 2, mitochondrial |
| SOD3 | superoxide dismutase 3, extracellular |
| UPP1 | uridine phosphorylase 1 |
| Signal Transduction | |
| CAMSAP1L1 | |
| DENND2A | DENN/MADD domain containing 2A |
| EPHA3 | EPH receptor A3 |
| ITSN1 | intersectin 1 |
| LTBP1 | latent transforming growth factor beta binding protein |
| MYO10 | similar to myosin X |
| PDE3A | similar to cyclic nucleotide phosphodiesterase PDE3A |
| PDGFD | platelet derived growth factor D |
| PTGFR | |
| RGS9BP | RGS9-1 anchoring protein R9AP |
| RHOJ | ras homolog gene family, member J |
| WNT9A | wingless-type MMTV integration site family , member 9A |
| ZAK | similar to mixed lineage kinase-related kinase MRK-beta |
| Structural | |
| CDC42EP3 | similar to CDC42 effector protein 3 |
| DMD | dystrophin |
| ECM2 | extracellular matrix protein 2 |
| MID1 | midline 1 |
| MYOM3 | |
| SHROOM3 | similar to shroom-related protein |
| Transcription | |
| ANKRD1 | ankyrin repeat domain 1 |
| EBF1 | early B-cell factor 1 |
| EMX2 | empty spiracles homolog 2 |
| EYA2 | eyes absent homolog 2 |
| EYA4 | eyes absent homolog 4 |
| FOXO1A | forkhead box 01A |
| HOXD8 | homeobox D8 |
| LHX9 | LIM homeobox 9 |
| MEOX2 | mesenchme homeobox 2 |
| MYCBP2 | myc binding protein 2 |
| NFIB | nuclear factor I/B |
| PPARA | peroxisome proliferative activated receptor, alpha |
| PRRX1 | paired related homeobox 1 |
| RARB | retinoic acid receptor, beta |
| TSHZ2 | zinc finger protein 218 |
| TSHZ3 | zinc finger protein 537 |
| YAF2 | YY1 associated factor 2 |
| Transport | |
| BIN1 | bridging integrator 1 |
| COLEC12 | collectin 1 precursor CL-3 |
| CYB5A | cytochrome B-5 |
| FTD | ferritoid |
| RBP7 | retinol binding protein 7, cellular |
| SCP2 | sterol carrier potein-2 |
| SYTL2 | similar to synaptotagmin-like 2 isoform B |
EMX2 Expression in Fast and Fast/Slow Myogenic Clones
The gene encoding the transcriptional regulator EMX2 was identified in the microarray analysis as a gene that was expressed in both fast/slow myoblasts and muscle fibers. To verify that the gene encoding EMX2 was differentially expressed in fast/slow versus fast myogenic cell lineages, RT-PCR was conducted using two fast myoblast clones and two fast/slow myoblast clones (Figure 4). Expression of the EMX2 gene was identified in both fast/slow myoblast clones, and no significant levels of EMX2 gene expression were detected in fast myoblast clones. Similarly, EMX2 cDNA was amplified from RNAs obtained from differentiated cultures of the two fast/slow myogenic clones (Figure 4). EMX2 gene expression was not detected in differentiated cultures of fast myogenic clones. The product of RT-PCR amplification using the EMX2-specific primers was verified as EMX2 cDNA by DNA sequencing.
Fig. 4.

RT-PCR amplification of EMX2 cDNA. RNAs from cultures of undifferentiated myoblasts and differentiated muscle fibers from two fast (Lanes 1 and 2) and two fast/slow (Lanes 3 and 4) myogenic clones were reverse transcribed and amplified using EMX2-specific primers. EMX2 RNA was detected in fast/slow myoblasts and myotubes, but not in fast myoblasts or myotubes. GAPDH cDNA was amplified as a control for all samples.
To detect EMX2 protein in embryonic myoblasts, myoblast clones differentially committed to the formation of fast and fast/slow primary muscle fibers were fixed and incubated with EMX2 antibody (Figure 5). EMX2 was detected in myoblasts that differentiate into fast/slow primary muscle fibers. EMX2 was predominantly localized to nuclei in these cells. EMX2 was not readily detected in myoblasts committed to the formation of fast primary muscle fibers. Similarly, EMX2 protein was detected in muscle fibers derived from fast/slow myogenic clones and was not readily detected in differentiated cultures of fast myogenic clones (Figure 5).
Fig. 5.

Immunodetection of EMX2 protein. Myoblasts and myotubes from fast and fast/slow myogenic clones were immunostained using an EMX2 antibody followed by a FITC-conjugated secondary antibody. EMX2 protein was detected in myoblasts and myotubes of fast/slow myogenic cell origin. EMX2 protein was primarily associated with nuclei. EMX2 was not readily detected in fast myoblasts or myotubes. DAPI staining located all nuclei.
EMX2 is a Positive Regulator of Slow MyHC2 Promoter Activity
To determine whether expression of EMX2 contributes to the embryonic fast/slow muscle fiber phenotype, the effect of EMX2 expression on slow muscle fiber type specific gene promoter activity was measured. The slow MyHC2 gene promoter is regulated by distinct molecular mechanisms in fast/slow embryonic versus fast/slow fetal muscle fibers. The slow MyHC2 promoter in fast/slow fetal muscle fibers is regulated by an innervation and stimulation-dependent transcriptional mechanism involving MEF2, NFAT, and the proximal 1.43kb promoter (Jiang, et al., 2004). However this promoter region does not confer muscle fiber type specific slow MyHC2 gene expression in embryonic muscle fibers. An additional ∼4kb of upstream DNA contained within the promoter-reporter construct, 6150SM2Luc, confers this fiber specificity (Theobald and DiMario, 2011).
Fast and fast/slow myoblast clones were transiently transfected with 6150SM2Luc. Myoblasts were also co-transfected with the EMX2 expression construct, CMVEMX2, or the empty plasmid vector DNA. Myoblasts were allowed to differentiate for 4 days and promoter activities were then measured (Figure 6A). Luciferase activities from the promoterless pGL3Basic vector were unaffected by co-transfection of CMVEMX2. We have previously shown that the slow MyHC2 promoter is specifically activated in fast/slow versus fast embryonic muscle fibers (Theobald and DiMario, 2011). Forced expression of EMX2 in embryonic fast/slow muscle fibers further increased, by approximately 2 fold, slow MyHC2 promoter activity. Interestingly, expression of EMX2 in fast muscle fibers further reduced residual slow MyHC2 promoter activity.
Fig. 6.

EMX2 gene expression regulates slow MyHC2 promoter activity. A: Fast and fast/slow myoblast clones were transiently co-transfected with the full-length slow MyHC2 promoter-luciferase DNA construct, 61050SM2Luc, and the EMX2 expression construct, CMVEMX2 (+EMX2), or empty vector (-EMX2). Alternatively, promoterless pGL3Basic (Basic) was also co-transfected with or without CMVEMX2. Bars are mean fold activation of slow MyHC2 promoter activities by EMX2 expression as measured by luciferase activities and normalized by Renilla luciferase activities from co-transfection of pRLSV40 (mean ± S.E.M.). EMX2 expression significantly increased 6150SM2Luc activity in fast/slow muscle fibers (n = 27; p<0.01) and significantly repressed activity in fast muscle fibers (n = 10; p<0.01). p values were determined by two-tailed Student's T test. B: Transfection of EMX2 siRNAs reduced EMX2 gene expression as determined by RT-PCR. Myoblasts were transfected with EMX2-specific siRNAs (EMX2 siRNA) or siRNAs containing scrambled EMX2 nucleotide sequence (Control siRNA; see Experimental Procedures). RNA was prepared from differentiated myotubes. RNA samples from myotubes transfected with control siRNAs were similarly processed, but without reverse transcriptase (-RT) to access genomic DNA contamination. A representative RT-PCR analysis is shown (n=3). C: Transfection of EMX2 siRNAs versus control siRNAs significantly reduced slow MyHC2 promoter activity in fast/slow myotubes (mean ± S.E.M, p<0.01 as determined by one-tailed Student's T test).
To further investigate the role of EMX2 as a positive regulator of slow MyHC2 gene expression, EMX2 gene expression was knocked down by transfection of EMX2-specific siRNAs. Fast/slow myoblasts were transfected with 6150SM2Luc and either control siRNAs of scrambled nucleotide sequence or EMX2-specific siRNAs. After myogenic differentiation, EMX2 gene expression was assessed by RT-PCR, and slow MyHC2 promoter activities were measured. EMX2-specific siRNAs effectively reduced EMX2 gene expression by 83.3% (Figure 6B). Furthermore, EMX2 siRNAs reduced slow MyHC2 promoter activity in fast/slow myotubes by 41% (Figure 6C). The EMX2 overexpression and knockdown studies indicate that EMX2 functions as a positive regulator of slow MyHC2 gene transcription.
Discussion
Skeletal muscle fiber type diversity arises through different mechanisms at specific developmental stages. Numerous studies in a variety of model systems have demonstrated that skeletal muscle fiber type is dependent on specific neural input or stimulation patterns. However, the studies on muscle fiber type regulation have typically focused on muscle fibers derived from fetal stages of development. Few studies have focused on the mechanism of muscle fiber type diversification during embryonic formation of primary muscle fibers from embryonic myoblasts. Clonal analysis studies, both in vitro and in vivo, have demonstrated that embryonic myoblasts are stably committed to the formation of distinct muscle fiber types and that this commitment is independent of neural input (Miller and Stockdale, 1986a,b; DiMario, et al., 1993). These distinct myoblast cell lineages differentiate into muscle fibers expressing either fast MyHC genes or both fast and slow MyHC genes. The basis of differential expression of fast versus slow fiber type specific genes in embryonic and fetal muscle fibers is also different. For example, slow MyHC2 gene expression in innervated or stimulated fetal avian muscle fibers derived from myoblasts of slow muscle origin is dependent on NFAT transcriptional activity (Jiang, et al., 2004; Crew, et al., 2010). However, slow MyHC2 gene expression in embryonic muscle fibers is not regulated by NFAT in a fiber type specific manner (Theobald and DiMario, 2011).
To investigate the nature of the differences that define fast versus fast/slow embryonic avian muscle fiber types, gene expression profiles of differentiated cultures of fast and fast/slow clonal myoblasts were generated. Fast and fast/slow embryonic muscle fibers displayed a wide array of genes that were differentially expressed. Microarray analysis identified differential expression of 718 genes in fast muscle fibers and 799 genes in fast/slow muscle fibers. The divergent gene expression profiles of fast versus fast/slow embryonic muscle fibers indicate that the muscle fiber diversification extends beyond expression of different myosin genes. The fast and fast/slow muscle fibers displayed significant heterogeneity in gene expression within multiple cellular processes and functions. Of the genes assigned definitive functions, the largest gene categories included metabolism, transport, signal transduction, and transcription.
To developmentally link fast and fast/slow embryonic muscle fibers as distinct differentiated cells to distinct myoblast cell lineages, additional gene expression profiling was conducted. Similar to differentiated fast and fast/slow muscle fibers, the myoblasts committed to formation of these fast and fast/slow muscle fibers also displayed significant heterogeneity in gene expression. Fast myoblasts differentially expressed 303 genes relative to fast/slow myoblasts. Conversely, fast/slow myoblasts differentially expressed 380 genes. Transcriptional regulators accounted for 12% and 10% of these genes, respectively. This heterogeneity in expression of genes that control transcription as well as other cellular functions such as metabolism, transport, and signal transduction further substantiates the existence of inherent differences between myoblast lineages committed to the differentiation of diverse muscle fiber types.
Comparative analysis of the gene expression profiles of the distinct myoblast types in relation to their corresponding differentiated muscle fiber type was also conducted. Within the embryonic fast myogenic lineage, 15 genes were differentially expressed in both myoblasts and muscle fibers, compared to the fast/slow myogenic lineage. Genes functionally related to cell metabolism and signal transduction were expressed in both fast myoblasts and muscle fibers. Interestingly, no genes of known transcriptional regulators were identified in the shared fast myoblast and fast/slow muscle fiber expression profiles. In contrast, 51 genes were differentially expressed in both myoblasts and muscle fibers of the fast/slow myogenic lineage. Genes associated with metabolic function, transport, and signal transduction were identified. Importantly, 17 genes encoding transcriptional regulators were identified as differentially expressed genes in both fast/slow myoblasts and muscle fibers.
Gene expression profiling of embryonic myoblasts committed to the fast/slow muscle fiber fate as well as profiling of fast/slow muscle fibers themselves identified the transcriptional regulator EMX2 as a gene expressed in fast/slow versus fast myogenic cells. Expression of EMX2 in fast/slow myoblasts and muscle fibers was verified by RT-PCR and immunodetection. This is the first known evidence of expression of EMX2 in skeletal muscle cells.
The EMX2 gene was overexpressed in fast and fast/slow muscle fibers to determine the effect on activity of the slow MyHC2 promoter. Forced EMX2 expression significantly increased slow MyHC2 promoter activity in fast/slow muscle fibers. Therefore, EMX2 is a positive regulator in the differentiation of the fast/slow embryonic myogenic lineage. Furthermore, since EMX2 gene expression occurs in both embryonic fast/slow myoblasts and muscle fibers, it is a marker of this myogenic lineage. The role of EMX2 in lineage determination has also been described in development of the central nervous system. In mammalian cerebral cortex, EMX2 functions as a molecular determinant of CNS precursor cell fate (Heins, et al., 2001). Forced expression of EMX2 in embryonic chick telencephalon resulted in a shift of cell specification toward neuroepithelial identity (von Frowein, et al., 2006). It has been reported that EMX2 gene expression is regulated by developmental signaling pathways such as the β-catenin pathway in developing limbs (Hill, et al., 2006). However, expression of the EMX2 gene can also be cell-autonomous (Nakagawa, et al., 1996). The results reported here demonstrate cell autonomous expression of the EMX2 gene in both embryonic fast/slow myoblasts and muscle fibers.
As an autonomously expressed transcriptional regulator in fast/slow myoblasts and muscle fibers, it is reasonable to hypothesize that EMX2 orchestrates the molecular mechanism of myogenic lineage commitment to embryonic fiber type formation as a singular regulatory factor. As such, it may be anticipated that EMX2 gene expression would drive re-specification of the fast myoblast lineage to the fate of fast/slow muscle fibers when forcibly expressed in the fast myogenic lineage. This hypothesis is supported by increased slow MyHC2 promoter activity in fast/slow muscle fibers overexpressing the EMX2 gene. However, to date, we have not been able to demonstrate that EMX2 gene expression in fast myoblasts and muscle fibers results in a fast to fast/slow lineage re-specification or fiber type transition. There are several possibilities to account for these observations. EMX2 may function as a transcriptional regulator that further distinguishes lineage commitment and/or expression of fiber type specific genes, such as the slow MyHC2 gene. These possible outcomes are not necessarily the same, and additional research is required to completely define the role of EMX2 gene expression in these processes. Nevertheless, our studies suggest that EMX2 gene expression does contribute to molecular and phenotypic distinctions between fast and fast/slow muscle fibers by enhancement of slow MyHC2 promoter activity in fast/slow muscle fibers. Another function for EMX2 gene expression may be more directly related to myogenic fiber type lineage commitment. EMX2 may participate in embryonic myoblast commitment of specific fiber type formation but require other transcription factors, either as direct co-regulators within a transcriptional complex or as other transcription factors simultaneously expressed. Further research is required to elucidate these possible mechanisms.
Experimental Procedures
Cell Culture
Embryonic myoblasts were incubated in cell culture medium consisting of 10% horse serum (Hyclone), 5% chick embryo extract, supplemented with 1.32mM CaCl2, 2mM glutamine and 1X antibiotic/antimycotic (Invitrogen) in Ham's F-10 basal medium (Sigma) mixed with an equal volume of the same medium conditioned by incubation for 2 days in cultures of fully differentiated ED13 chicken myotubes. These cells were prepared as previously described (O'Neill and Stockdale, 1972).
Immunostaining and Fusion Indices
To detect MyHC isoforms, myotubes were immunostained with monoclonal antibodies F59 and S58 for fast MyHCs and slow MyHC2, respectively, as previously described (Crow and Stockdale, 1986; Theobald and DiMario, 2011). Texas Red-conjugated anti-mouse IgG (Vector Labs) and fluorescein-conjugated anti-mouse IgA (Southern Biotech) were used to detect F59 and S58 primary antibodies, respectively. EMX2 was detected using an EMX2 antibody (Sigma). Cells were washed with phosphate buffered saline (PBS) and fixed in 3.7% formaldehyde, 0.1% NP-40 in PBS for 10 minutes. Cells were washed with PBS and then incubated in blocking solution (5% horse serum, 2% bovine serum albumin in PBS) for 1 hour at room temperature. Cells were then incubated in EMX2 antibody, diluted 1:100 in blocking solution, for 1 hour at room temperature. Cells were washed as before and then incubated in FITC-conjugated anti-rabbit IgG (Vector Labs), diluted 1:200 in PBS, for 1 hour at room temperature. Cells were then washed as before and viewed by fluorescence microscopy.
To determine fusion indices, differentiated myotube cultures were immunostained with F59 monoclonal antibody to detect all myotubes. All nuclei were stained with 1.2uM 4′6-diamidino-2-phenylindole (DAPI) in PBS. The ratio of myotube nuclei to all nuclei within a microscopic field was determined. Four to six random fields were counted for each myogenic clone. A minimum of 1,000 nuclei was counted for each clone.
Microarray Analysis
Total RNAs were extracted using RNA-STAT 60 reagent (Tel-Test, Inc) from 5 fast myogenic clones and 4 fast/slow myogenic clones. RNAs were obtained from clonal myoblasts and myotubes. An equal amount of RNA (1ug) from each clone was used to generate a pooled sample for the fast and fast/slow myoblasts and myotubes (total 4 samples). RNA integrity was assessed using the Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). The microarray chips used in this study were the GeneChip Chicken Genome Arraychips (Affy part #900590). Briefly, the Chicken Genome Array contains comprehensive coverage of 32,773 transcripts corresponding to over 28,000 chicken genes. Microarray analysis was performed essentially as described (McCarthy, et al., 2007). The pooled RNA samples were used to synthesize cDNAs that were then used as templates to generate biotinylated cRNAs. cRNA was fragmented and hybridized to the Chicken Genome Array chip, washed, scanned and intensity values for each probe set condensed using the GC-RMA algorithm. A total of 4 chips were processed in this manner, and the data files will be available at Gene Expression Omnibus (www.ncbi.nih.gov/geo). A custom-written MATLAB routine (The MathWorks, Inc., Natick, MA) was used to scrub the data by removing probe sets that were considered “not-expressed” in both fast and fast/slow clones. Our criteria for this was to remove all probe sets in which the intensity value for both fast and fast/slow clones was <350. If one of the two samples or both of the samples had a probe set intensity above 350, the probe set was kept in the dataset for analysis. The analysis for differential expression in the dataset compared the fast sample versus the fast/slow sample or the fast/slow sample versus the fast sample. Those probe sets that were expressed ≥ 2-fold higher were kept in the analysis.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
For quantitative real-time PCR, RNA was extracted as above. cDNA synthesis and amplification was conducted using Full Velocity or Brilliant II SYBR Green QRT-PCR Master Mix Kit (Stratagene) and MJ Research Opticon 2 DNA Engine. Gene expression levels were determined using the comparative Ct method. For semiquantitative RT-PCR, total RNA was extracted using RNA-STAT 60 reagent (Tel-Test, Inc.). EMX2 gene specific product was reverse transcribed and amplified using Access RT-PCR reagents (Promega) and the following oligonucleotides: 5′-CCCAAGCGCTGTTTCACCATCG-3′ and 3′-ATCGTCCGACGTGACGTCGATTTCTT-5′. Quail GAPDH RNA was reverse transcribed and amplified using the following DNA primers: Forward Primer: 5′- CGCCATCACTATCTTCCAGGAGC-3′; Reverse Primer: 5′- GCCAAAGTTGTCATGGATGACC-3′. PCR products were resolved in a 1.2% agarose gel. Identities of the amplified products were verified by DNA sequencing.
Promoter Activity Analysis
Either the slow MyHC2 promoter-reporter DNA (6150SM2Luc) or a promoterless pGL3Basic luciferase DNA construct (Promega) (3ug) was transfected into myogenic cell clones in 35mm cell culture plates using Lipofectamine 2000 (Invitrogen). pRL-SV40 (Promega) (2μg) containing Renilla luciferase was co-transfected to normalize for variations in transfection efficiencies. Either the pCMVTAG empty vector (Stratagene) or CMVEMX2 expression construct (1ug/plate) was co-transfected. Cells were transfected in cell culture medium containing the DNAs and without antibiotic for 5 hours at 37°C in a 5% CO2 incubator. Transfection medium was then replaced with normal cell culture medium. Five days following transfection, luciferase activities were measured using the Dual-Glo Luciferase Assay (Promega).
For EMX2 knockdown, fast/slow myoblasts in 35mm cell culture plates were co-transfected with 6150SM2Luc (3ug) and the following EMX2-specific siRNAs and their reverse compliment oligonucleotides (100pM): 5′-AAACUCAGGUAAAAGUAUGGUdTdT-3′, 5′-AAGGGAUCCCUCCACCUUCUAdTdT-3′, 5′-AAGGACAAAGUUCAAGCGGCAdTdT-3′. Control siRNAs were designed by randomization of each EMX2-specific siRNA nucleotide sequence. pRL-SV40 (2μg) was co-transfected to normalize for variations in transfection efficiencies. Myoblasts were allowed to differentiate for 5 days before promoter activity was measured.
Supplementary Material
Key findings.
Embryonic myoblasts are comprised of distinct myogenic cell lineages, each with unique signatures of gene expression and muscle fiber type formation.
EMX2 gene expression is associated with the fast/slow embryonic myoblast lineage.
EMX2 gene expression is required for normal slow MyHC2 promoter activity in myotubes derived from the fast/slow embryonic myoblast lineage.
Acknowledgments
Grant Sponsor: NIH AR058043 to J.X. DiMario
References
- Bandman E, Matsuda R, Strohman RC. Developmental appearance of myosin heavy and light chain isoforms in vivo and in vitro in chicken skeletal muscle. Dev Biol. 1982;93:508–518. doi: 10.1016/0012-1606(82)90138-5. [DOI] [PubMed] [Google Scholar]
- Baxendale S, Davison C, Muxworthy C, Wolff C, Ingham PW, Roy S. The B-cell maturation factor Blimp-1 specifies vertebrate slow-twitch muscle fiber identity in response to Hedgehog signaling. Nat Genet. 2004;36:88–93. doi: 10.1038/ng1280. [DOI] [PubMed] [Google Scholar]
- Beckmann J, Vitobello A, Ferralli J, Broz DK, Rijli FM, Chiquet-Ehrismann R. Human teneurin-1 is a direct target of the homeobox transcription factor EMX2 at a novel alternate promoter. BMC Dev Biol. 2011;11:35. doi: 10.1186/1471-213X-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biressi S, Molinaro M, Cossu G. Cellular heterogeneity during vertebrate skeletal muscle development. Dev Biol. 2007a;308:281–293. doi: 10.1016/j.ydbio.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Biressi S, Tagliafico E, Lamorte G, Monteverde S, Tenedini E, Roncaglia E, Ferrari S, Cusella-DeAngelis MG, Tajbakhsh S, Cossu G. Intrinsic phenotypic diversity of embryonic and fetal myoblasts is revealed by genome-wide gene expression analysis on purified cells. Dev Biol. 2007b;304:633–651. doi: 10.1016/j.ydbio.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Brancaccio M, Pivetta C, Granzotto M, Filippis C, Mallamaci A. Emx2 and Foxg1 inhibit gliogenesis and promote neurogenesis. Stem Cells. 2010;28:1206–1218. doi: 10.1002/stem.443. [DOI] [PubMed] [Google Scholar]
- Calabria E, Ciciliot S, Moretti I, Garcia M, Picard A, Dyar KA, Pallafacchina G, Tothova J, Schiaffino S, Murgia M. NFAT isoforms control activity-dependent muscle fiber type specification. Proc Natl Acad Sci. 2009;106:13335–13340. doi: 10.1073/pnas.0812911106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo S, Vulhorst D, Venepally P, Cheng J, Karavanova I, Buonanno A. Molecular dissection of DNA sequences and factors involved in slow muscle-specific transcription. Mol Cell Biol. 2001;21:8490–8503. doi: 10.1128/MCB.21.24.8490-8503.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condon K, Silberstein L, Blau HM, Thompson WJ. Differentiation of fiber types in aneural musculature of the prenatal rat hindlimb. Dev Biol. 1990;138:275–295. doi: 10.1016/0012-1606(90)90197-q. [DOI] [PubMed] [Google Scholar]
- Crew JR, Falzari K, DiMario JX. Muscle fiber type specific induction of slow myosin heavy chain 2 gene expression by electrical stimulation. Exp Cell Res. 2010;316:1039–1049. doi: 10.1016/j.yexcr.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow MT, Stockdale FE. Myosin expression and specialization among the earliest muscle fibers of the developing avian limb. Dev Biol. 1986;113:238–254. doi: 10.1016/0012-1606(86)90126-0. [DOI] [PubMed] [Google Scholar]
- Devoto SH, Melancon E, Eisen JS, Westerfield M. Identification of separate slow and fast muscle precursor cells in vivo, prior to somite formation. Development. 1996;122:3371–3380. doi: 10.1242/dev.122.11.3371. [DOI] [PubMed] [Google Scholar]
- DiMario JX, Fernyak SE, Stockdale FE. Myoblasts transferred to the limbs of embryos are committed to specific fibre fates. Nature. 1993;362:165–167. doi: 10.1038/362165a0. [DOI] [PubMed] [Google Scholar]
- DiMario JX, Stockdale FE. Both myoblast lineage and innervation determine fiber type and are required for expression of the slow myosin heavy chain 2 gene. Dev Biol. 1997;188:167–180. doi: 10.1006/dbio.1997.8619. [DOI] [PubMed] [Google Scholar]
- Fredette BJ, Landmesser LT. A reevaluation of the role of innervation in primary and secondary myogenesis in developing chick muscle. Dev Biol. 1991;143:19–35. doi: 10.1016/0012-1606(91)90051-4. [DOI] [PubMed] [Google Scholar]
- Grifone R, Demignon J, Houbron C, Souil E, Bertin F, Laclef C, Xu PX, Maire P. Six1 and Six4 homeoproteins are required for Pax3 and MRF expression during myogenesis in the mouse embryo. Development. 2005;132:2235–2249. doi: 10.1242/dev.01773. [DOI] [PubMed] [Google Scholar]
- Grifone R, Laclef C, Spitz F, Lopez S, Demignon J, Guidotti JE, Kawakami K, Xu PX, Kelly R, Petrof BJ, Daegelen D, Concordet JP, Maire P. Six1 and Eya1 expression can reprogram adult muscle from the slow-twitch phenotype into the fast-twitch phenotype. Mol Cell Biol. 2004;24:6253–6267. doi: 10.1128/MCB.24.14.6253-6267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara N, Yeh M, Liu A. Sox6 is required for normal fiber type differentiation of fetal skeletal muscle in mice. Dev Dyn. 2007;236:2062–2076. doi: 10.1002/dvdy.21223. [DOI] [PubMed] [Google Scholar]
- Heins N, Cremisi F, Malatesta P, Gangemi RM, Corte G, Price J, Goudreau G, Gruss P, Götz M. Emx2 promotes symmetric cell divisions and a multipotential fate in precursors from the cerebral cortex. Mol Cell Neurosci. 2001;18:485–502. doi: 10.1006/mcne.2001.1046. [DOI] [PubMed] [Google Scholar]
- Hill TP, Taketo MM, Birchmeier W, Hartmann C. Multiple roles of mesenchymal beta-catenin during murine limb patterning. Development. 2006;133:1219–1229. doi: 10.1242/dev.02298. [DOI] [PubMed] [Google Scholar]
- Holley M, Rhodes C, Kneebone A, Herde MK, Fleming M, Steel KP. Emx2 and early hair cell development in the mouse inner ear. Dev Biol. 2010;340:547–556. doi: 10.1016/j.ydbio.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes SM, Taylor JM, Tapscott SJ, Gurley CM, Carter WJ, Peterson CA. Selective accumulation of MyoD and myogenin mRNAs in fast and slow adult skeletal muscle is controlled by innervation and hormones. Development. 1993;118:1137–1147. doi: 10.1242/dev.118.4.1137. [DOI] [PubMed] [Google Scholar]
- Hutcheson DA, Zhao J, Merrell A, Haldar M, Kardon G. Embryonic and fetal limb myogenic cells are derived from developmentally distinct progenitors and have different requirements for β-catenin. Genes Dev. 2009;23:997–1013. doi: 10.1101/gad.1769009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Jordan T, Li J, Li H, DiMario JX. Innervation-dependent and fiber type-specific transcriptional regulation of the slow myosin heavy chain 2 promoter in avian skeletal muscle fibers. Dev Dyn. 2004;231:292–302. doi: 10.1002/dvdy.20137. [DOI] [PubMed] [Google Scholar]
- Kendziorski C, Irizarry RA, Chen KS, Haag JD, Gould MN. On the utility of pooling biological samples in microarray experiments. Proc Natl Acad Sci. 2005;102:4252–4257. doi: 10.1073/pnas.0500607102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew HP, Choksi SP, Wong KN, Roy S. Specification of vertebrate slow-twitch muscle fiber fate by the transcriptional regulator Blimp1. Dev Biol. 2008;324:226–235. doi: 10.1016/j.ydbio.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- Liu Y, Shen T, Randal WR, Schneider MF. Signaling pathways in activity-dependent fiber type plasticity in adult skeletal muscle. J Muscle Res Cell Motil. 2005;26:13–21. doi: 10.1007/s10974-005-9002-0. [DOI] [PubMed] [Google Scholar]
- Malashichev Y, Christ B, Pröls F. Avian pelvis originates from lateral plate mesoderm and its development requires signals from both ectoderm and paraxial mesoderm. Cell Tissue Res. 2008;331:595–604. doi: 10.1007/s00441-007-0556-6. [DOI] [PubMed] [Google Scholar]
- McCarthy JJ, Andrews JL, McDearmon EL, Campbell KS, Barber BK, Miller BH, Walker JR, Hogenesch JB, Takahashi JS, Esser KA. Identification of the circadian transcriptome in adult mouse skeletal muscle. Physiol Genomics. 2007;31:86–95. doi: 10.1152/physiolgenomics.00066.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina G, Biressi S, Monteverde S, Magli A, Cassano M, Perani L, Roncaglia E, Tagliafico E, Starnes L, Campbell CE, Grossi M, Goldhamer DJ, Gronostajski RM, Cossu G. Nfix regulates fetal-specific transcription in developing skeletal muscle. Cell. 2010;140:554–566. doi: 10.1016/j.cell.2010.01.027. [DOI] [PubMed] [Google Scholar]
- Miller JB, Stockdale FE. Developmental regulation of the multiple myogenic cell lineages of the avian embryo. J Cell Biol. 1986a;103:2197–2208. doi: 10.1083/jcb.103.6.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JB, Stockdale FE. Developmental origins of skeletal muscle fibers: clonal analysis of myogenic cell lineages based on expression of fast and slow myosin heavy chains. Proc Natl Acad Sci. 1986b;83:3860–3864. doi: 10.1073/pnas.83.11.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto N, Yoshida M, Kuratani S, Matsuo I, Aizawa S. Defects of urogenital development in mice lacking Emx2. Development. 1997;124:1653–1664. doi: 10.1242/dev.124.9.1653. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Kaneko T, Ogura T, Suzuki T, Torii M, Kaibuchi K, Arai K, Nakamura S, Nakafuku M. Roles of cell-autonomous mechanisms for differential expression of region-specific transcription factors in neuroepithelial cells. Development. 1996;122:2449–2464. doi: 10.1242/dev.122.8.2449. [DOI] [PubMed] [Google Scholar]
- Niro C, Demignon J, Vincent S, Liu Y, Giordani J, Sgarioto N, Favier M, Guillet-Deniau I, Blais A, Maire P. Six1 and Six4 gene expression is necessary to activate the fast-type muscle gene program in the mouse myotome. Dev Biol. 2010;338:168–182. doi: 10.1016/j.ydbio.2009.11.031. [DOI] [PubMed] [Google Scholar]
- Oh M, Rybkin II, Copeland V, Czubryt MP, Shelton JM, van Rooij E, Richardson JA, Hill JA, De Windt LJ, Bassel-Duby R, Olson EN, Rothermel BA. Calcineurin is necessary for the maintenance but not embryonic development of slow muscle fibers. Mol Cell Biol. 2005;25:6629–6638. doi: 10.1128/MCB.25.15.6629-6638.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill MC, Stockdale FE. A kinetic analysis of myogenesis in vitro. J Cell Biol. 1972;52:52–65. doi: 10.1083/jcb.52.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EN, Williams RS. Remodeling muscles with calcineurin. Bioessays. 2000;22:510–519. doi: 10.1002/1521-1878(200011)22:11<1049::AID-BIES14>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Page S, Miller JB, DiMario JX, Hager EJ, Moser A, Stockdale FE. Developmentally regulated expression of three slow isoforms of myosin heavy chain: diversity among the first fibers to form in avian muscle. Dev Biol. 1992;154:118–128. doi: 10.1016/0012-1606(92)90053-j. [DOI] [PubMed] [Google Scholar]
- Pellegrini M, Pantano S, Fumi MP, Lucchini F, Forabosco A. Agenesis of the scapula in Emx2 homozygous mutants. Dev Biol. 2001;232:149–156. doi: 10.1006/dbio.2001.0159. [DOI] [PubMed] [Google Scholar]
- Polly P, Haddadi LM, Issa LL, Subramaniam N, Palmer SJ, Tay ES, Hardeman EC. hMusTRD1alpha1 represses MEF2 activation of the troponin I slow enhancer. J Biol Chem. 2003;278:36603–36610. doi: 10.1074/jbc.M212814200. [DOI] [PubMed] [Google Scholar]
- Reiser PJ, Moss RL, Giulian GG, Greaser ML. Shortening velocity in single fibers from adult rabbit soleus muscles is correlated with myosin heavy chain composition. J Biol Chem. 1985;260:9077–9080. [PubMed] [Google Scholar]
- Reiser PJ, Greaser ML, Moss RL. Myosin heavy chain composition of single cells from avian slow skeletal muscle is strongly correlated with velocity of shortening during development. Dev Biol. 1988;129:400–407. doi: 10.1016/0012-1606(88)90387-9. [DOI] [PubMed] [Google Scholar]
- Richard AF, Demignon J, Sakakibara I, Pujol J, Favier M, Strochlic L, Le Grand F, Sgarioto N, Guernec A, Schmitt A, Cagnard N, Huang R, Legay C, Guillet-Deniau I, Maire P. Genesis of muscle fiber-type diversity during mouse embryogenesis relies on Six1 and Six4 gene expression. Dev Biol. 2011;359:303–320. doi: 10.1016/j.ydbio.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Roy RR, Eldridge L, Baldwin KM, Edgerton VR. Neural influence on slow muscle properties: inactivity with and without cross-reinnervation. Muscle Nerve. 1996;19:707–714. doi: 10.1002/(SICI)1097-4598(199606)19:6<707::AID-MUS4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Roy RR, Pierotti DJ, Garfinkel A, Zhong H, Baldwin KM, Edgerton VR. Persistence of motor unit and muscle fiber types in the presence of inactivity. J Exp Biol. 2008;211:1041–1049. doi: 10.1242/jeb.013722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino S, Sandri M, Murgia M. Activity-dependent signaling pathways controlling muscle diversity and plasticity. Physiology. 2007;22:269–278. doi: 10.1152/physiol.00009.2007. [DOI] [PubMed] [Google Scholar]
- Simeone A, Gulisano M, Acampora D, Stornaiuolo A, Rambaldi M, Boncinelli E. Two vertebrate homeobox genes related to the Drosophila empty spiracles gene are expressed in the embryonic cerebral cortex. EMBO J. 1992;11:2541–2550. doi: 10.1002/j.1460-2075.1992.tb05319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockdale FE. Myogenic Cell Lineages. Dev Biol. 1992;154:284–298. doi: 10.1016/0012-1606(92)90068-r. [DOI] [PubMed] [Google Scholar]
- Theobald J, DiMario JX. Lineage-based primary muscle fiber type diversification independent of MEF2 and NFAT in chick embryos. J Muscle Res Cell Motil. 2011;31:369–381. doi: 10.1007/s10974-011-9242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Frowein J, Wizenmann A, Götz M. The transcription factors Emx1 and Emx2 suppress choroid plexus development and promote neuroepithelial cell fate. Dev Biol. 2006;296:239–252. doi: 10.1016/j.ydbio.2006.04.461. [DOI] [PubMed] [Google Scholar]
- von Hofsten J, Elworthy S, Gilchrist MJ, Smith JC, Wardle FC, Ingham PW. Prdm1- and Sox6-mediated transcriptional repression specifies muscle fibre type in the zebrafish embryo. EMBO Reports. 2008;9:683–689. doi: 10.1038/embor.2008.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walldorf U, Gehring WJ. Empty spiracles, a gene containing a homeobox involved in Drosophila head development. EMBO J. 1992;11:2247–2259. doi: 10.1002/j.1460-2075.1992.tb05284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GF, Nikovits W, Jr, Schleinitz M, Stockdale FE. Atrial chamber-specific expression of the slow myosin heavy chain 3 gene in the embryonic heart. J Biol Chem. 1996;271:19836–19845. doi: 10.1074/jbc.271.33.19836. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


