Abstract
Background
To date, no studies have validated the Medication Adherence Self-Efficacy Scale (MASES) in an independent sample of hypertensive African Americans.
Purpose
The purpose of this study was to revise and assess the validity of the MASES.
Methods
Study sample included 168 African Americans followed in primary care practices. Mean age was 54 (SD = 12.36); 86% was female; and 76% reported high school education or greater. Participants provided demographic information; completed the MASES, self-report and electronic measures of medication adherence for prescribed antihypertensive medications at baseline and three months.
Results
Confirmatory (CFA), exploratory (EFA) factor analyses, and classical test theory (CTT) analyses, suggested that MASES is a unidimensional and internally reliable measure with relatively stable scores over 3 months. Results of item response theory (IRT) analyses led to revision of the scale to a 13-item version: the MASES-R. EFA, CTT, and IRT results for the MASES-R supported its reliability and validity.
Conclusions
Findings suggest that the MASES-R is a brief scale that is quick to administer and can capture useful data on adherence self-efficacy for African Americans. Research examining its psychometric properties in other ethnic groups will improve generalizability of findings and utility of the scale in diverse groups.
Introduction
Hypertension affects over 65 million persons in the United States and remains one of the major chronic diseases contributing to the racial mortality gap between African Americans and whites.1 Compared to whites, African Americans have a higher prevalence of hypertension (HTN), with approximately 40% compared to 28% in whites.2 However, HTN is controlled in only 30% of African Americans compared to 35% of whites.3 Poor medication adherence is a major contributing factor to poor blood pressure (BP) control with an estimated 50–70% of hypertensive patients reporting poor adherence rates.4 African Americans have poorer rates of medication adherence compared to whites and this may account for the disproportionately higher rates of hypertension-related outcomes in this patient population.5–8 Several behavioral models have been proposed to explain adherence behavior, but the one that has received the most attention in the literature is the social cognitive model.9, 10 One component of this model that has been shown to predict initiation and maintenance of recommended health behaviors is Bandura’s construct of self-efficacy. 9, 11
Self-efficacy refers to an individual’s judgment of his or her confidence to carry out a specific task in order to produce a desired outcome.9 The stronger one’s self-efficacy beliefs, the more likely a person will initiate and maintain recommended health behaviors.9 In patients with chronic diseases, positive self-efficacy appraisals have been found to consistently predict the adoption of, and adherence to a variety of health-related behaviors including dietary recommendations, exercise regimens, self-management behaviors, and adherence to antiretroviral therapies.11–17 However, despite evidence documenting the importance of self-efficacy in influencing health behaviors in patients with chronic diseases, little is known about its role in hypertensive African Americans. To address this issue, we developed and evaluated the reliability of a Medication Adherence Self-Efficacy Scale (MASES) in hypertensive African American patients.18 The MASES is a patient derived, self-report measure designed to assess efficacy beliefs regarding adherence to prescribed anti-hypertensive medications. Preliminary support for the internal consistency and test-retest reliability of the MASES was demonstrated in a sample of 72 hypertensive African Amerricans.18
In this study, we conducted further psychometric evaluation and revision of the MASES in a larger independent sample of hypertensive African Americans. The evaluation included an assessment of the unidimensionality of the scale; its internal consistency using classical test theory (CTT); estimates of item parameters and item information functions using item response theory (IRT). In addition, we assessed its predictive and concurrent validity with both self-report and electronic measures of medication adherence.
Methods
Study Design and Participants
Data for this study were collected as part of a larger study designed to evaluate the effects of a behavioral intervention on medication adherence in a group of hypertensive African Americans, followed in a community-based primary care practice. Detailed information on the study design and methods of the larger study are outlined elsewhere.19 Eligible patients were identified via electronic medical records (EMR) using the following criteria: hypertension diagnosis; self-identification as black or African American; age 18 years or older; fluency in English; and taking at least one antihypertensive medication. All patients were approached during their regular clinic visits and asked to participate in the study. They were required to sign informed consent for participation in the trial, which was approved by the institutional ethics review board of Columbia University Medical Center.
Data collection
Upon enrollment into the trial, trained research assistants conducted baseline assessments on all patients. All baseline and three month data were collected prior to randomization and delivery of the behavioral intervention. Study participants completed the MASES questionnaire and a self-report measure of medication adherence at baseline and 3 months. In addition, they were give electronic pill bottle cap fitted on a pill bottle in order to monitor their adherence to prescribed antihypertensive medications for the study duration. Patients were taught to use the electronic pill bottles and asked to put their antihypertensive medications in them. Additional data collected included demographics; insurance status; socioeconomic status.
Measures
Self Efficacy was assessed with the MASES.18 This is a 26-item scale that is used to assess patients’ confidence in their ability to take their antibypertensive medications in a variety of situations. Some examples of situations include “when busy at home,” “while at work,” “when they cause some side effects.” Items are scored from 1 (not at all sure) to 4 (extremely sure) and a total score on the measure is computed by averaging across responses to all items. Higher scores indicate a greater level of self-efficacy. Cronbach’s alpha for the measure was 0.95.
Self-report medication adherence was assessed with the well-validated four-item scale developed by Morisky et al.20 It asks patients to respond “yes” or “no” to the following questions: “Do you ever forget to take your medicine?” “Are you careless at times about taking your medicine?” When you feel better do you sometimes stop taking your medicine?” and “Sometimes if you feel worse when you take the medicine, do you stop taking it?” Respondents were categorized into a dichotomous “adherent” category if they responded “no” to all items, and into the “non-adherent” category if they responded “yes” to one or more items.
Electronic measure of medication adherence was assessed with the Medication Event Monitoring System (MEMS; Aprex, a division of Aardex Corporation, Union City, CA)
This consists of a pill bottle and an electronic cap which utilizes microchip technology to record each time that the pill bottle is opened and closed. Data are downloaded to a computer directly from the cap using Powerview software (Aprex, a division of Aardex Corporation, Union City, CA). Medication adherence was defined as the ratio of number of doses taken to number prescribed for the study duration. The MEMS is currently considered the gold standard for adherence measurement.21–23
Analytic Overview
We first evaluated the unidimensionality of the MASES using both confirmatory (CFA) and exploratory factor (EFA) models. The MASES was hypothesized to have a single factor structure. Thus, the model outlined for the CFA analysis included 25 MASES items that were administered as observed variables, which load on a single latent variable of “Medication Adherence Self-Efficacy”. Goodness-of-fit statistics were calculated as part of the CFA, and as is conventional, several of these were used to evaluate model fit, including the chi-square statistic, Comparative Fit Index (CFI), Tucker Lewis Index (TLI), and the Root Mean Square Error of Approximation (RMSEA).24–26 A well-fitting model is suggested by a chi-square with a p-value > 0.05;24 CFI or TLI values > 0.90;26 and an RMSEA values < 0.05.25, 26 Structural equation modeling (SEM) software was used to evaluate the one-factor CFA model.
We then evaluated the corrected item total correlations, item means and coefficient alpha in a classic test theory (CTT) analysis and finally, we estimated the 2 parameter Item characteristic curves and item information functions using Samejima’s graded response model.27 The assumption of unidimensionality is critical for both CTT and item response theory (IRT) based scale evaluations. Based on these analyses we revised and shortened the MASES by removing items that were found to provide little information about a patients’ adherence self-efficacy. Predictive and concurrent validity of the revised MASES was subsequently evaluated by assessing its correlation with 3-month self-report medication adherence and MEMS.
The Statistical Package for the Social Sciences (SPSS) version 13.0 was used for CTT and EFA analyses,28 M-plus was used for the CFA analysis and the IRT analyses were conducted with MULTILOG.29
Results
Patient characteristics
Characteristics of study participants are shown in Table 1 for the 168 participants that provided both baseline and 3 month data. Their average age was 54 years (SD = 12.36) with 86% female. More than two thirds had either high school or college education.
Table 1.
Participant characteristics (n = 168)
| Characteristic | Value, n (%) |
|---|---|
| Age (+/− SD) | 54 (+/− 12.36) |
| Female | 86% |
| Education | |
| Elementary or Junior High School | 24% |
| High School | 45% |
| Some College or More | 31% |
| Income | |
| Unknown | 29% |
| ≤20K/year | 49% |
| >20K/year | 22% |
| Charlson Comorbidity Score | |
| Unknown | 1% |
| 0 | 19% |
| 1–2 | 32% |
| 3–4 | 17% |
| ≥ 5 | 31% |
| Diastolic Blood Pressure (+/− SD) | 86 (+/− 11.18) |
| Systolic Blood Pressure (+/− SD) | 144 (+/− 18.11) |
CFA and EFA analyses
Results of the CFA indicated that the observed correlations among the items were not well-described by a one-factor (unidimensional) model, χ2275, N = 190 = 942.27, p < .01; CFI = .72; TLI = .69; RMSEA = .11 (90% CI = .10, .12). Subsequently, model modification indices were examined to determine whether changes to the model would improve fit. The analysis did not reveal any meaningful changes, therefore, EFA was used to further examine factor structure of the MASES. The principal components EFA analysis revealed five factors with eigenvalues > 1.0 at both baseline and 3 months. At baseline, 19 items loaded on the first factor, which had the highest eigenvalue and explained the most variance (37.93%). There was a considerable drop in eigenvalue from the first factor with a value of 9.48 to 1.83 for the second factor. The percent variance explained by the first factor as well as the large drop in eigenvalues from first to second factor and beyond meets established criteria for the “essential” unidimensionality of the scale.30 A similar pattern was noted for the 3-month self-report adherence data. These results supported the essential unidimensionality of the MASES and justified proceeding with both CTT and IRT analyses. See Table 2 for eigenvalues and percent variance explained and Table 3 for individual item factor loadings.
Table 2.
Results of MASES EFA: Baseline and 3 month samples
| Factor | Eigenvalue | Variance Explained (%) | ||
|---|---|---|---|---|
|
| ||||
| Baseline | 3 Month | Baseline | 3 Month | |
| 1 | 9.48 | 9.44 | 37.93 | 37.75 |
| 2 | 1.83 | 2.02 | 7.31 | 8.08 |
| 3 | 1.79 | 1.90 | 7.14 | 7.61 |
| 4 | 1.45 | 1.46 | 5.80 | 5.85 |
| 5 | 1.01 | 1.39 | 4.05 | 5.56 |
Table 3.
MASES item loadings and results of IRT analyses
| Items | EFA Analyses | IRT Analyses | |
|---|---|---|---|
|
| |||
| Factor Loadings | Items Retained | ||
|
| |||
| Confidence in taking medications: | Baseline | 3 Month | |
| 1. When you are busy at home | .75 | .66 | * |
| 2. When you are at work | .44 | .54 | |
| 3. When there is no one to remind you | .69 | .68 | * |
| 4. When they cause some side effects | .32 | .35 | |
| 5. When you worry about taking them for the rest of your life | .63 | .59 | * |
| 6. When they cost a lot of money | .53 | .50 | |
| 7. When you come home late from work | .42 | .46 | |
| 8. When you do not have any symptoms | .65 | .67 | * |
| 9. When you are with family members | .75 | .71 | * |
| 10. When you are in a public place | .73 | .71 | * |
| 11. When you are afraid of becoming dependent on them | .50 | .66 | |
| 12. When you are afraid they may affect your sexual performance | .57 | .57 | |
| 13. When the time to take them is between your meals | .75 | .65 | * |
| 14. When you feel you do not need them | .53 | .66 | |
| 15. When you are travelling | .74 | .69 | * |
| 16. When you take them more than once a day | .66 | .82 | * |
| 18. If they sometimes make you tired | .43 | .50 | |
| 19. When you have other medications to take | .77 | .67 | * |
| 20. When you feel well | .73 | .75 | * |
| 21. If they make you want to urinate while away from home | .63 | .58 | * |
| Confidence in ability to carry out the following tasks: | |||
| 22. Get refills for your medications before you run out | .46 | .30 | |
| 23. Make taking your medications part of your routine | .66 | .66 | * |
| 24. Fill your prescriptions whatever they cost | .49 | .44 | |
| 25. Always remember to take your blood pressure medications | .63 | .66 | |
| 26. Take your blood pressure medications for the rest of your life | .61 | .56 | |
Note: Bolded values indicate significant loadings.
CTT Evaluation
Mean scores on individual MASES items are shown in Table 4. Total MASES score for this sample was 3.50 (SD = .46) at baseline and 3.57 (SD = .46) at 3 months. Reliability of the MASES was assessed through evaluation of internal consistency at baseline and 3 months, and its stability over the same time period. Cronbach’s alpha for the measure was .91 at both time points, p < .05, with a test-retest coefficient of 0.56 between baseline and 3 months, p < .05, suggesting moderately stable scores. These findings indicate that the MASES is an internally reliable measure with moderately stable scores over a 3-month period. See Table 4 for results of additional item level analysis, including item-to-total correlations, and alpha coefficient if item deleted.
Table 4.
Item analysis for the MASES: Baseline and 3-month
| Items | MeanB (N = 188) | Mean3-Mo. (N = 168) | SDB | SD3-Mo. | r | ITCB | ITC3-Mo. |
|---|---|---|---|---|---|---|---|
| How confident are you that you can take your blood pressure medications: | |||||||
| 1. When you are busy at home | 3.54 | 3.73 | .70 | .55 | .20 | .68 | .59 |
| 2. When you are at work | 3.58 | 3.80 | .84 | .61 | .15 | .38 | .47 |
| 3. When there is no one to remind you | 3.59 | 3.74 | .69 | .62 | .45 | .59 | .61 |
| 4. When they cause some side effects | 2.60 | 2.80 | 1.18 | 1.19 | .33 | .34 | .37 |
| 5. When you worry about taking them for the rest of your life | 3.57 | 3.60 | .71 | .81 | .28 | .62 | .54 |
| 6. When they cost a lot of money | 2.99 | 3.04 | 1.11 | 1.19 | .45 | .55 | .51 |
| 7. When you come home late from work | 3.64 | 3.69 | .77 | .77 | .30 | .36 | .38 |
| 8. When you do not have any symptoms | 3.62 | 3.67 | .70 | .75 | .31 | .60 | .63 |
| 9. When you are with family members | 3.70 | 3.82 | .56 | .49 | .25 | .68 | .62 |
| 10. When you are in a public place | 3.64 | 3.79 | .71 | .56 | .37 | .67 | .62 |
| 11. When you are afraid of becoming dependent on them | 3.18 | 3.40 | 1.08 | 1.00 | .38 | .50 | .66 |
| 12. When you are afraid they may affect your sexual performance | 3.46 | 3.42 | .88 | .99 | .28 | .55 | .55 |
| 13. When the time to take them is between your meals | 3.72 | 3.76 | .49 | .57 | .19 | .64 | .56 |
| 14. When you feel you do not need them | 3.32 | 3.42 | 1.00 | 1.03 | .22 | .51 | .62 |
| 15. When you are travelling | 3.68 | 3.81 | .63 | .50 | .25 | .67 | .60 |
| 16. When you take them more than once a day | 3.60 | 3.66 | .72 | .73 | .38 | .59 | .77 |
| 18. If they sometimes make you tired | 3.06 | 3.12 | 1.11 | 1.14 | .28 | .45 | .52 |
| 19. When you have other medications to take | 3.67 | 3.74 | .56 | .64 | .19 | .68 | .58 |
| 20. When you feel well | 3.60 | 3.75 | .72 | .65 | .27 | .70 | .68 |
| 21. If they make you want to urinate while away from home | 3.46 | 3.53 | .84 | .92 | .29 | .64 | .55 |
| Please rate how sure you are that you can carry out the following tasks: | |||||||
| 22. Get refills for your medications before you run out | 3.54 | 3.60 | .74 | .74 | .35 | .39 | .26 |
| 23. Make taking your medications part of your routine | 3.62 | 3.80 | .61 | .44 | .35 | .60 | .60 |
| 24. Fill your prescriptions whatever they cost | 3.24 | 3.30 | 1.06 | 1.09 | .48 | .49 | .44 |
| 25. Always remember to take your blood pressure medications | 3.48 | 3.67 | .78 | .63 | .36 | .56 | .59 |
| 26. Take your blood pressure medications for the rest of your life | 3.56 | 3.63 | .80 | .78 | .23 | .59 | .53 |
IRT Evaluation
Item information curves were examined for all items at baseline and 3 months. Examination of the curves allowed for elimination of items that provided little information about the underlying construct of adherence self-efficacy. An item was retained if Item information curves suggested that it performed well at one time point, or both time points. Ten items performed well at both time points, while 3 performed well at one time point. This process resulted in retention of 13 items that make up the shortened revised version of the scale, the MASES-R. See Table 3 for item content.
MASES-R CTT and IRT Evaluation
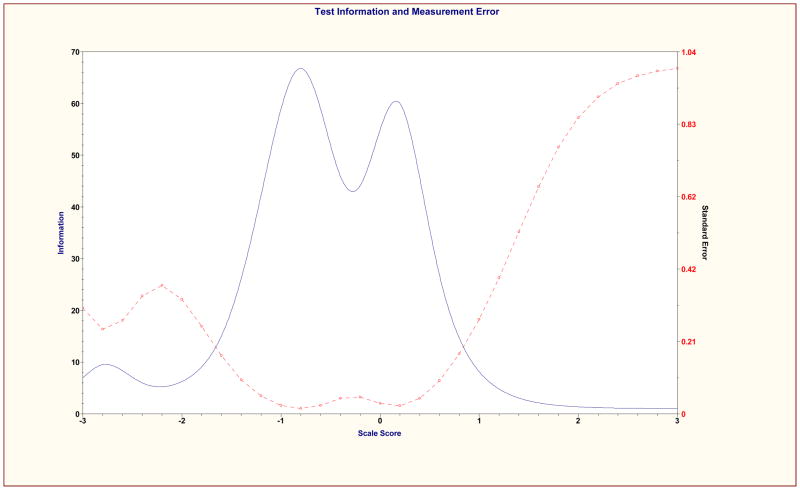
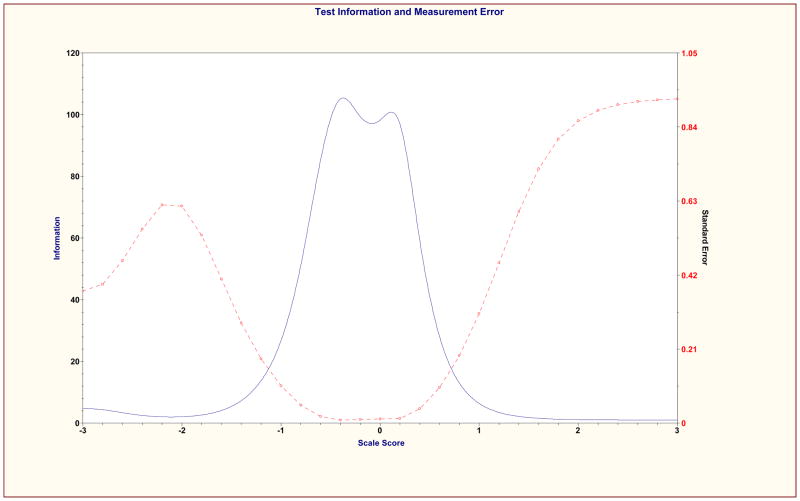
The psychometric properties of the shortened revised MASES-R was then examined using previous CTT and IRT methods. Mean total score on the MASES-R was 3.62 (SD = .48) at baseline and 3.72 (SD = .44) at 3 months. Cronbach’s alpha coefficients were .92 and .90 at baseline and 3 months respectively. Test-retest coefficient for MASES-R was 0.51, p < .001. Results of EFA on the MASES-R were similar to the results of EFA for the 25-item MASES. At baseline, a single factor emerged from the analysis that explained 53.20% of variance and had an eigenvalue of 6.92. All 13 items loaded significantly on this factor with factor loadings of .62 or greater. At 3 months, the solution included two factors. Factor 1 explained 49.87% of variance, and had an eigenvalue of 6.48. Factor 2 explained 12% of variance and had a much smaller eigenvalue of 1.66. All 13 items loaded highest and significantly on Factor 1, with values of at least .52. These results support the unidimensionality of the MASES-R. Item Information Curves were examined for the 13 items retained and Scale Information Curve was examined for the total scale. All items and the total scale performed well at both baseline and 3-month time points. Consistent with the individual item information curves, the scale information curves were most informative about adherence self-efficacy in the moderately low to the middle ranges of the construct. See Figures 1 and 2 for MASES-R Scale Information Curves at both time points.
Figure 1.
MASES-R Scale Information Curve at baseline
Figure 2.
MASES-R Scale Information Curve at 3 months
Concurrent and Predictive Validity of MASES-R
The scores of MASES-R were examined in relation to self-report adherence and MEMS. We hypothesized that the MASES-R scores would correlate positively with both measures of medication adherence. As expected, at baseline MASES-R scores were higher for patients whose self-report categorized them as adherent (M = 3.81, SD = .33) compared to those that were categorized as non-adherent (M = 3.51, SD = .52; t = −4.26, p < .001). These data provide preliminary support for the concurrent validity of the MASES-R. Additional support for the scale’s concurrent validity was provided by significant and positive correlation between 3-month MASES-R and 3-month MEMS (r = .20, p = .02). Similarly, correlation between baseline MASES-R scores and 3-month MEMS adherence rates (r = .19, p = .02) was significant and positive, indicating support for the predictive validity of the MASES-R. These results support the initial validity of MASES-R in relation to medication adherence in this patient sample.
Discussion
The evaluation and revision of the Medication Adherence Self Efficacy Scale was described in this paper. The shortened scale, titled the MASES-R, consists of 13 items that assess an individual’s belief in their confidence to adhere to prescribed anti-hypertensive medications under a variety of challenging situations. Twelve of the items ask about confidence in specific situations (e.g. busy at home, no symptoms, traveling), and one item asks about confidence in ability to make medication adherence a part of daily routine. There are no subscales on the measure, but rather the total scale score ranges from 1 to 4 and is the average score of all 13 items.
Like the original MASES, the 13-item version of the measure demonstrates decent psychometric properties at both time points. The scale has good internal consistency and reliability with excellent alpha coefficients, item to total correlations, test-retest coefficients, and has a unidimensional factor structure. In addition, the study provides evidence of both concurrent and predictive validity of the revised MASES-R in hypertensive African Americans. The MASES-R correlated positively and significantly with electronic medication adherence, suggesting that as adherence self-efficacy increases, medication adherence also increases. When examining self-report adherence data, a similar pattern emerged in that those who self-categorized as adherent also reported greater mean adherence self-efficacy scores.
Of interest, the scale is most informative at distinguishing among those in the middle to lower middle of the scale. This is where both item information curves and scale information curves for the MASES-R provided the most information about adherence self-efficacy. This pattern of findings suggests that clinicians and researchers alike will be able to use the scale to distinguish between those with very low to moderately low self-efficacy. In turn; this information can be useful in the development and implementation of interventions targeting self-efficacy for improved medication adherence. While it is true that the results also suggest that the MASES-R did not distinguish well between those at the higher end of the self-efficacy continuum, this characteristic of the measure is of little practical significance as those with high to very high self-efficacy for adherence are least likely to need intervention.
As noted previously, major strengths of the MASES included use of patient input and feedback for item generation and refinement, and the MASES focus on condition-specific list of situations where self-efficacy may vary.18 For example, when patient is asymptomatic as may be the case in hypertension but may not be the case with other health conditions. Two strengths of the MASES-R deserve mention. First is the expansion of response options in the measure. In the original MASES, we used a 3-point response option compared to the 4-point used in the MASES-R. While our goal with this change was to provide greater variability and improve predictive power of the revised scale, we previously noted that patients had difficulty discriminating between five response choices in the development phase.18 Thus, the new version of the scale includes four choices to indicate level of self efficacy, including: “Not at all sure,” “A little sure,” “Fairly sure,” and “Extremely sure.” Second, two forms of medication adherence data were used in this study for scale validation: self-report adherence and electronic monitoring, which is currently considered the ‘gold-standard’ of adherence assessment. Thus, a limitation that was previously noted in the introduction of the MASES has been addressed in this study. One limitation of the study is that the large proportion of women in the sample (86%) limits the ability to generalize findings to a broader population, including African American men. Future studies on the MASES-R should address this limitation as well as collect data on the MASES-R in multiple ethnic groups, as is currently being done in a separate study of ours.31
In summary, the data presented in this study suggest that the MASES-R is a reliable and valid instrument that is suitable for use in hypertensive African Americans. The MASES-R can provide useful data to both clinicians and researchers who are interested in understanding the role of self-efficacy in medication adherence among hypertensive African Americans.
Acknowledgments
Preparation of this manuscript was supported by Grants R01 HL 69408, R01 HL078566, and R24 HL076857 from the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, USA. Dr. Fernandez was supported by minority supplement to grant R01HL078566-02S1 and the NIH LRP in Health Disparities Research. Dr. Schoenthaler was supported by grant F31HL081926.
We are grateful to David Y. Berger and David Statman for their assistance with data cleaning for this project.
Appendix. MASES-R
Situations come up that make it difficult for people to take their medications as prescribed by their doctors. Below is a list of such situations. We want to know your opinion about taking your blood pressure medication(s) under each of them. Please indicate your response by checking the box that most closely represents your opinion. There are no right or wrong answers.
For each of the situations listed below, please rate how sure you are that you can take your blood pressure medications all of the time.
| Items | Not at all sure | A little sure | Fairly sure | Extremely sure |
|---|---|---|---|---|
| How confident are you that you can take your blood pressure medications: | ||||
| 1. When you are busy at home | ||||
| 2. When there is no one to remind you | ||||
| 3. When you worry about taking them for the rest of your life | ||||
| 4. When you do not have any symptoms | ||||
| 5. When you are with family members | ||||
| 6. When you are in a public place | ||||
| 7. When the time to take them is between your meals | ||||
| 8. When you are travelling | ||||
| 9. When you take them more than once a day | ||||
| 10. When you have other medications to take | ||||
| 11. When you feel well | ||||
| 12. If they make you want to urinate while away from home | ||||
| Please rate how sure you are that you can carry out the following task: | ||||
| 13. Make taking your medications part of your routine |
Contributor Information
Senaida Fernandez, Columbia University, College of Physicians and Surgeons
William Chaplin, St. John’s University
Antoinette Schoenthaler, Columbia University, College of Physicians and Surgeons
Gbenga Ogedegbe, Columbia University, College of Physicians and Surgeons
References
- 1.American Heart Association. High Blood Pressure Statistics. [Accessed March 20, 2006.]. [Google Scholar]
- 2.Centers for Disease Control and Prevention. Racial/ethnic disparities in prevalence, treatment, and control of hypertension: United States, NHANES 1999–2002. MMWR Morbidity & Mortality Weekly Report. 2005;54:57–59. [PubMed] [Google Scholar]
- 3.Hertz RP, Unger AN, Cornell JA, Saunders E. Racial disparities in hypertension prevalence, awareness, and management. Archives of Internal Medicine. 2005;165(18):2098–2104. doi: 10.1001/archinte.165.18.2098. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Adherence to long-term therapies: Evidence for action. Geneva: World Health Organization; 2003. [Google Scholar]
- 5.Bosworth HB, Dudley T, Olsen MK, et al. Racial differences in blood pressure control: potential explanatory factors. American Journal of Medicine. 2006;119(1):70.e9–70.e15. doi: 10.1016/j.amjmed.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 6.Burt VL, Cutler JA, Higgins M, et al. Trends in the prevalence, awareness, treatment, and control of hypertension in the adult US population. Data from the health examination surveys, 1960 to 1991. Hypertension. 1995;26(1):60–69. doi: 10.1161/01.hyp.26.1.60. [DOI] [PubMed] [Google Scholar]
- 7.Kirscht JP, Rosenstock IM. Patient adherence to antihypertensive medical regimens. Journal of Community Health. 1977;3(2):115–124. doi: 10.1007/BF01674233. [DOI] [PubMed] [Google Scholar]
- 8.Monane M, Bohn RL, Gurwitz JH, Glynn RJ, Levin R, Avorn J. Compliance with antihypertensive therapy among elderly Medicaid enrollees: The roles of age, gender, and race. American Journal of Public Health. 1996;86(12):1805–1808. doi: 10.2105/ajph.86.12.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychological Review. 1977;84(2):134–139. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- 10.Bandura A. Self-efficacy: The exercise of control. New York: W.H. Freeman; 1997. [Google Scholar]
- 11.Strecher VJ, DeVellis BM, Becker MH, Rosenstock IM. The role of self-efficacy in achieving health behavior change. Health Education Quarterly. 1986;13(1):73–92. doi: 10.1177/109019818601300108. [DOI] [PubMed] [Google Scholar]
- 12.Allegrante JP, Marks R. Self-efficacy in management of osteoarthritis. Rheumatic Diseases Clinics of North America. 2003;29(4):747–768. vi–vii. doi: 10.1016/s0889-857x(03)00060-7. [DOI] [PubMed] [Google Scholar]
- 13.Catz SL, Kelly JA, Bogart LM, Benotsch EG, McAuliffe TL. Patterns, correlates, and barriers to medication adherence among persons prescribed new treatments for HIV disease. Health Psychology. 2000;19(2):124–133. [PubMed] [Google Scholar]
- 14.Gifford AL, Bormann JE, Shively MJ, Wright BC, Richman DD, Bozzette SA. Predictors of self-reported adherence and plasma HIV concentrations in patients on multidrug antiretroviral regimens. Journal of Acquired Immune Deficiency Syndromes. 2000;23(5):386–395. doi: 10.1097/00126334-200004150-00005. [DOI] [PubMed] [Google Scholar]
- 15.Johnson MO, Chesney MA, Goldstein RB, et al. Positive provider interactions, adherence self-efficacy, and adherence to antiretroviral medications among HIV-infected adults: A mediation model. AIDS Patient Care & STDS. 2006;20(4):258–268. doi: 10.1089/apc.2006.20.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lorig K, Chastain RL, Ung E, Shoor S, Holman HR. Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis. Arthritis & Rheumatism. 1989;32(1):37–44. doi: 10.1002/anr.1780320107. [DOI] [PubMed] [Google Scholar]
- 17.Nakahara R, Yoshiuchi K, Kumano H, Hara Y, Suematsu H, Kuboki T. Prospective study on influence of psychosocial factors on glycemic control in Japanese patients with type 2 diabetes. Psychosomatics. 2006;47(3):240–246. doi: 10.1176/appi.psy.47.3.240. [DOI] [PubMed] [Google Scholar]
- 18.Ogedegbe G, Mancuso CA, Allegrante JP, Charlson ME. Development and evaluation of a medication adherence self-efficacy scale in hypertensive African-American patients. Journal of Clinical Epidemiology. 2003;56(6):520–529. doi: 10.1016/s0895-4356(03)00053-2. [DOI] [PubMed] [Google Scholar]
- 19.Ogedegbe GO, Schoenthaler AM, Richardson T, et al. An RCT of the effect of motivational interviewing on medication adherence in hypertensive African Americans: Rationale and design. Contemporary Clinical Trials. 2007;28:169–181. doi: 10.1016/j.cct.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Medical Care. 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Choo PW, Rand CS, Inui TS, et al. Validation of patient reports, automated pharmacy records, and pill counts with electronic monitoring of adherence to antihypertensive therapy. Medical Care. 1999;37(9):846–857. doi: 10.1097/00005650-199909000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Hugen PWH, Langebeek N, Burger DM, et al. Assessment of adherence to HIV protease inhibitors: Comparison and combination of various methods, including MEMS (electronic monitoring), patient and nurse report, and therapeutic drug monitoring. Journal of Aquired Immune Deficiency Syndromes. 2002;30:324–334. doi: 10.1097/00126334-200207010-00009. [DOI] [PubMed] [Google Scholar]
- 23.Liu H, Golin CE, Miller LG, et al. A comparison study of multiple measures of adherence to HIV protease inhibitors. [see comment][erratum appears in Ann Intern Med 2002 Jan 15;136(2):175] Annals of Internal Medicine. 2001;134(10):968–977. doi: 10.7326/0003-4819-134-10-200105150-00011. [DOI] [PubMed] [Google Scholar]
- 24.Bollen KA. Structural equations with latent variables. New York: Wiley; 1989. [Google Scholar]
- 25.Cudeck R, Browne MW. Constructing a covariance matrix that yields a specified minimizer and a specified minimum discrepancy function value. Psychometrika. 1992;57:357–369. [Google Scholar]
- 26.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- 27.Samejima F. Estimation of latent ability using a response pattern of graded scores. Psychometrika Monographs. 1969;34(4 Pt 2, Whole No 17) [Google Scholar]
- 28.SPSS. Statistical Package for Social Sciences, Version 13.0. 2004. [Google Scholar]
- 29.Thissen D. Multilog User’s Guide. Lincolnwood, IL: Scientific Software International Incorporated; 2003. [Google Scholar]
- 30.Lord FM. Applications of item response theory to practical testing problems. Hillsdale, NJ: Lawrence Erlbaum Associates; 1980. [Google Scholar]
- 31.Gerin W, Tobin JN, Schwartz JE, et al. The medication Adherence and Blood Pressure Control (ABC) trial: A multi-site randomized controlled trial in a hypertensive, multi-cultural, economically disadvantaged population. Contemporary Clinical Trials. 2007 doi: 10.1016/j.cct.2007.01.003. In Press. [DOI] [PubMed] [Google Scholar]