Abstract
Objective
To report a single institution experience in surgical stage I-II serous endometrial cancer using combined carboplatin/paclitaxel and intravaginal radiation (IVRT).
Methods
Between 10/00 and 12/06, 25 stage I-II patients with serous endometrial cancer were treated at our institution with surgery, postoperative IVRT, and concurrent chemotherapy (CT).
Results
The mean age was 67 years old (range, 53-80y). Surgery consisted of hysterectomy (TAH/BSO, 64%, LAVH/BSO, 36%), peritoneal washing, omental biopsy, and pelvic lymph-node dissection (median 14 nodes). Para-aortic nodes sampling was done in 88% (median, 6). IVRT median dose was 21 Gy (range, 18-21 Gy, in 3 fractions) and concurrent CT consisted of carboplatin to AUC = 5 and taxol to 175 mg/m2 given every 3 weeks for 6 cycles. CT was well tolerated with 22/25 (88%) receiving 6 cycles. Three patients received ≤5 cycles; 2 owing to physician preference (3 and 4 cycles) and 1 owing to toxicity (5 cycles). Only 1 patient (4%) had grade 3 toxicity (abscess). Grade 2 neurotoxicity was seen in 5 patients (20%). All patients finished their IVRT as scheduled, and there was no grade 3 toxicity. With a median follow-up of 30 months, the 5-year progression-free and overall survival rate was 88%. None of the patients developed vaginal recurrence.
Conclusions
Based on this study, surgical staging followed by IVRT and carboplatin/paclitaxel is well tolerated and effective in stage I-II serous endometrial cancer. Confirmation of these results on a larger number of patients with longer follow-up is still needed.
Introduction
Despite the relative infrequency of serous endometrial cancer, which represents about 5-10% of all uterine cancers [1], there is no shortage of treatment recommendations, including surgery alone [2], whole-abdomen radiation [3], and chemotherapy [4], even for patients with early-stage disease. This range of treatment options reflects some of the uncertainty about how serous endometrial cancer should be viewed. Should it be treated like ovarian cancer owing to similar morphology and to some extent patterns of spread, or like endometrioid endometrial cancer because it has in common the organ of origin. Recent microarray data indicate that the gene-expression profile of serous endometrial cancer is distinct from that of other adenocarcinomas of the uterus, as well as from that of serous carcinoma of the ovary [5,6].
Clearly the optimal treatment for serous endometrial cancer awaits a future targeted therapy based on better understanding of the molecular basis of this disease. Until such molecular understanding is reached, the current emphasis has been for comprehensive surgical staging and adjuvant chemotherapy, along the lines of what is done in ovarian cancer. This is coupled with a move away from whole-abdomen radiation and more toward tumor-directed radiation, as is currently being done in endometrial cancer [7-9]. The extent of surgical staging, the type of chemotherapy used, and mode of radiation delivery varied in many of those studies, reflecting the changes in treatment philosophy over last few decades. The purpose of this study is to report a single-institution experience where surgical staging, chemotherapy consisting of 6 cycles of carboplatin and paclitaxel, and intravaginal radiation (IVRT) were routinely offered to patients with surgical stage I-II serous endometrial cancer.
Methods and Materials
The patient population consisted of 25 patients with pathologic stage IA-IIB serous endometrial cancer who were treated at Memorial Sloan-Kettering Cancer Center (MSKCC) between 10/2000 and 12/2006. Only patients with occult cervical involvement were included in this analysis. All patients had surgical staging, intravaginal radiation (IVRT), and carboplatin/paclitaxel with all components of treatment being delivered at MSKCC. Seven patients were excluded: 2 patients received their chemotherapy outside MSKCC, 4 declined chemotherapy (1/4 agreed to have only carboplatin), and 1 received pelvic radiation instead of intravaginal radiation after chemotherapy because of deep cervical stromal invasion.
Surgery consisted of simple hysterectomy, bilateral salpingo-oophorectomy, peritoneal washing, omental biopsy, pelvic lymph-node dissection, and selective para-aortic lymph node sampling. An MSKCC, gynecologic pathologist reviewed all the pathological materials including initial endometrial biopsy or dilatation and curettage (D&C) specimen as well as the uterine and staging specimens.
Postoperative radiation was given in the form of high dose rate IVRT. Adjuvant chemotherapy consisted of 6 cycles of carboplatin and paclitaxel given every 3 weeks. The dose of paclitaxel was 175 mg/m2 and carboplatin was AUC (area under the curve) = 5. The timing of each IVRT fraction and chemotherapy was structured so that both treatments were not given simultaneously but rather a minimum of 1 week apart. Toxicity was assessed using the Common Terminology Criteria for Adverse Events (CTCAE).
Associations between variables were tested using the chi-square test. Survival rates were calculated using the Kaplan-Meier product-limit method [10]. Comparisons of survival curves were performed using the log-rank test [11]. The follow-up schedule consisted of physical examinations including pelvic examination every 3-4 months in the first 2 years, then every 6 months for the next 3 years, and yearly thereafter. Pap smears were done every 6 months for the first 2 years, then once a year for the next 3 years. CT scans of the abdomen and pelvis were performed as needed.
Results
Patients
The mean age was 67 years (range, 53-80y). Out of 25 patients, 18 (72%) were Caucasian, 6 (24%) were African-American, and 1 (4%) was of other races. The type of hysterectomy was as follows; total-abdominal hysterectomy and bilateral salpingo-oophorectomy (TAH/BSO) in 64% (n = 16) of patients and laparoscopic-assisted vaginal hysterectomy and bilateral salpingo-oophorectomy (LAVH-BSO) in 36% (n=9). The median number of pelvic lymph nodes removed was 14 (range, 2-37). Para-aortic lymph-node sampling was done in 22/25 patients (88%), with a median of 6 nodes removed (range, 1-12). The depth of myometrial invasion was ≥50% in 3 patients (12%), and the cervix was involved with the tumor in 20% (n=5) of the patients. Lympho-vascular invasion (LVI) was identified in 24% (n = 6), as shown in Table 1. When determining the depth of myometrial invasion, only contiguous tumor extension was included but not deep LVI. The distribution according to FIGO staging was as follows: I-A 28% (n=7), I-B 48% (n=12), I-C 4% (n=1), II-A 8% (n=2), and II-B 12% (n=3). All 7 stage I-A patients (method of initial diagnosis: D&C in 4, biopsy in 3) had residual disease at the time of the hysterectomy. The median follow-up time, calculated from the date of surgery, was 30 months (range, 17-79).
Table 1. Patient Characteristics (N=25).
| n | % | |
|---|---|---|
| Age | ||
| < 70 | 15 | 60% |
| ≥ 70 | 10 | 40% |
| Race | ||
| Caucasian | 18 | 72% |
| Other | 7 | 28% |
| Stage | 7 | 28% |
| IA | 12 | 48% |
| IB | 6 | 24% |
| IC-IIB | ||
| LVI | ||
| Yes | 6 | 24% |
| No | 19 | 76% |
| CI | ||
| Yes | 5 | 20% |
| No | 20 | 80% |
| Pure serous | ||
| Yes | 17 | 68% |
| No | 8 | 32% |
LVI: Lympho-Vascular Invasion, CI: Cervical Involvement.
Treatment compliance/toxicity
Of the 25 patients in this study, 22 (88%) were able to complete all 6 cycles of carboplatin/paclitaxel. Three patients had ≤5 cycles; 2 owing to physician preference (3, 4 cycles), and 1 (5 cycles) owing to toxicity. There was 1 patient (4%) who developed grade 3 toxicity in the form of abdominal abscess. Grade 2 neurotoxicity was seen in 8 patients: peripheral neuropathy in 5 (20%), tinnitus in 2 (8%), and smell alteration in 1 (4%). Other grade 2 toxicity included fatigue in 4 (16%), constipation in 1 (4%), dehydration in 1 (4%), and myalgias in 1 (4%). All 25 patients had at least one type of grade 1 toxicity. Dose reduction in chemotherapy dose due to toxicity was required in 5 patients (20%). With regard to IVRT, there was no grade 3 toxicity, 1 patient (4%) had grade 2 bladder toxicity, and 10 (40%) had different types of grade 1 toxicity. No change was required in IVRT dose because of toxicity.
Outcome
With a median follow-up of 30 months, 4 (16%) patients relapsed, and 3/4 expired from their disease (Table 2). All 4 developed distant metastasis to liver, lung, or peritoneum. In 3 out of 4, there was also a para-aortic (PA) recurrence, and the number of PA nodes removed from each patient at the time of initial surgery was 3, 8, and 10. Of interest, 1 of the 4 relapses occurred 5.5 years after the initial diagnosis, and on imaging, there was peritoneal seeding, enlarged pelvic (the only pelvic relapse in the current study) and PA nodes, as well as a pancreatic mass. Fine-needle aspirate of the pancreatic mass revealed adenocarcinoma and was inconclusive but suggestive of endometrial cancer.
Table 2. Characteristics of patients who relapsed.
| Case | Age | Stage | # PLN | # PALN | Pure serous | LVI | Sites of relapse | VS |
|---|---|---|---|---|---|---|---|---|
| 1 | 70 | IA | 23 | 12 | + | - | Peritoneum | A |
| 2 | 73 | IB | 37 | 8 | + | - | PA, liver, mediastinum | D |
| 3 | 54 | IIB | 14 | 10 | + | + | PA, liver, lung | D |
| 4♣ | 73 | IIB | 5 | 3 | + | - | Pelvis, PA, peritoneum, liver | D |
PLN: Pelvic Lymph Nodes, PALN: Para-Aortic Lymph Nodes, PA: Para-Aortic, LVI: Lympho-Vascular Invasion, VS: Vital Status, A: Alive, D: Dead.
Patient relapsed 5 and half years after diagnosis.
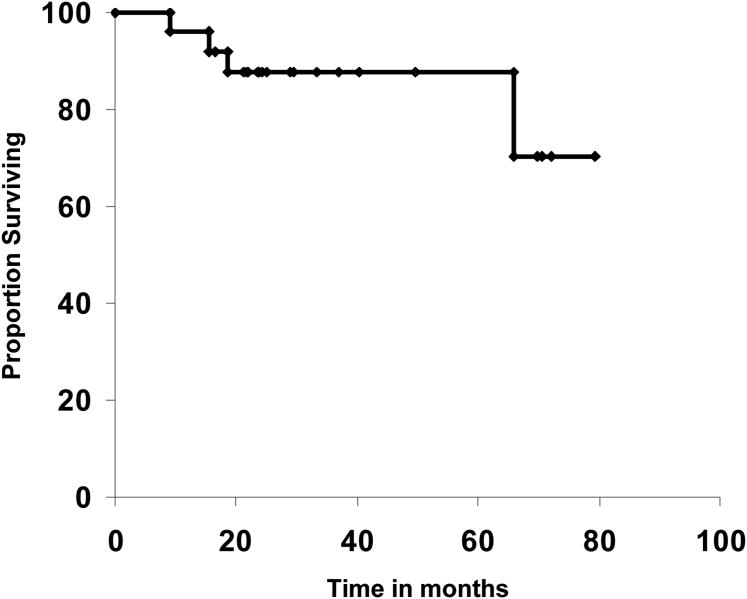
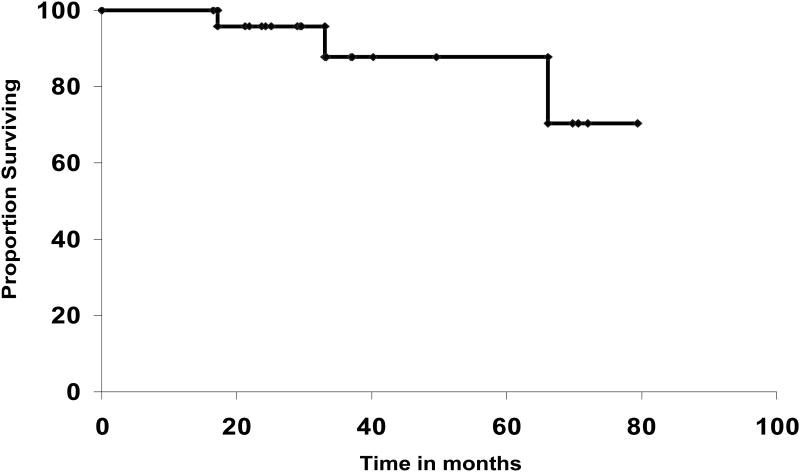
The 5-year disease-free (DFS) and overall survival rates were 88% (95% CI, 75-100) and 88% (95% CI, 71-100), respectively (Figures 1, 2). In patients who were ≥ 70 years old, the 5-year DFS rate was 80 (95% CI, 55-100) compared to 93% (95% CI, 80-100) for those <70 years of age, but that difference was not statistically significant (P=0.29). For patients with stage I-A, the 5-year DFS rate was 83%; for I-B 92%, and for IC-IIB 83%, (P=0.7).
Figure 1. Disease-free survival.
Figure 2. Overall survival.
Discussion
In the current study, 25 patients with stage IA-IIB disease were treated with carboplatin/paclitaxel and IVRT, yielding 88% disease-free and overall survival rate at 5 years. The group at Yale University has been a strong advocate of postoperative platinum-based chemotherapy with vaginal brachytherapy in early-stage serous endometrial cancer [12]. In their most recent analysis of 74 patients with stage I disease reported by Kelly et al. [13], the use of platinum-based chemotherapy (n = 31; 13/31 had paclitaxel/platinum) was associated with improved disease-free survival (P <0.01) and overall survival (P <0.05), and none of the patients who received vaginal brachytherapy (n = 43) had local recurrence. While such results are impressive, there are still some questions about what type of chemotherapy to use and whether radiation is needed. In the most recent reports on the role of adjuvant chemotherapy for serous endometrial cancer, carboplatin/paclitaxel seems to be the preferred regimen [14,15]. A study by the Rare Cancer Network [8] of 137 patients from several institutions found that cisplatin/carboplatin plus paclitaxel was the most commonly used regimen (n = 25, 50% of those receiving chemotherapy).
In the current study, chemotherapy was well tolerated, with 88% (22/25) of patients completing all 6 cycles. Three patients had ≤5 cycles; 2 owing to physician preference (3 and 4 cycles), and 1 to toxicity (5 cycles). Only 1 patient had grade 3 toxicity (abscess), and grade 2 neurotoxicity was seen in 5 patients (20%).
With regard to adjuvant radiation, the suboptimal results of whole-abdomen radiation obtained by the Gynecologic Oncology Group (GOG) study # 94 in patients with stage I-II serous and clear cell carcinoma of the endometrium raised questions about the efficacy of such an approach. Of the 21 patients with stage I-II disease, the 5-year progression-free survival was only 38%, and over half of the relapses were within the radiation field. It is important to point out that the majority of those in-field relapses were in the abdomen, and that there was only 1 case of isolated vaginal recurrence [16]. A similar low rate of vaginal recurrence with adjuvant radiation has been demonstrated by several other investigators. DuBeshter [17] reported only 1 in 16 vaginal recurrence using intravaginal radiation for patients with stage I-II serous endometrial cancer. In the study by the Rare Cancer Network [8], the rate of local recurrence (combined vaginal/pelvic) was 29% for those who did not receive RT compared to 14% for those who did (P=0.047). Havrilesky et al. [18] reported that in patients who received no adjuvant RT, the rate of vaginal recurrence was 10.2 % (5/49), compared with 5.8% (1/17) for those who did. The majority of relapses in patients who had RT (87%) from that study were distant, further indicating the need for combined systemic therapy and RT [18]. In the current study, with a median follow-up of 30 months, none of the 25 patients treated with intravaginal radiation and chemotherapy had vaginal recurrence. There was only 1 pelvic relapse, but it was associated with peritoneal carcinomatosis.
Some of the limitations in this study include the retrospective nature with all its inherent limitations and the relatively small number of patients. Clearly, confirmation of the results from this study by a prospective randomized trial is needed. The number of patients included in this study (n = 25) is small, but even in GOG # 94, which is a multi- institutional study, the number of patients was only 21, reflecting the relative rarity of this disease. One could argue that the excellent results obtained in the present study are due in part to the high proportion of patients with very early-stage disease (28% stage I-A, and 48% I-B). Such stage distribution, however, is similar to what Creasman et al. [19] found in their review of the 25th annual report of FIGO, were 148 patients with stage I serous endometrial cancer were identified, and of those 22% were stage I-A and 45% stage I-B. Kelly et al. [13] also reported that while patients with stage I-A with no cancer in the hysterectomy specimen do well (no relapse in any of the 12 patients with such finding), those with residual disease in the hysterectomy specimen did poorly if no adjuvant treatment was given (6/14 relapsed). In the current study, all 7 patients with I-A disease had residual cancer in their hysterectomy specimen.
In conclusion, the data on intravaginal RT and carboplatin/paclitaxel in early-stage serous endometrial cancer are encouraging. The results compare favorably with those obtained with adjuvant RT alone or chemotherapy alone, but longer follow-up on a larger number of patients is needed to confirm those findings.
References
- 1.Cirisano FD, Robboy SJ, Dodge RK, et al. The outcome of stage I-II clinically and surgically staged papillary serous and clear cell endometrial cancers when compared with endometrioid carcinoma. Gynecol Oncol. 2000;77:55–65. doi: 10.1006/gyno.2000.5737. [DOI] [PubMed] [Google Scholar]
- 2.Huh WK, Powell M, Leath CA, et al. Uterine papillary serous carcinoma: comparisons of outcomes in surgical Stage I patients with and without adjuvant therapy. Gynecol Oncol. 2003;91:470–475. doi: 10.1016/j.ygyno.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 3.Martinez AA, Weiner S, Podratz K, et al. Improved outcome at 10 years for serous-papillary/clear cell or high-risk endometrial cancer patients treated by adjuvant high-dose whole abdomino-pelvic irradiation. Gynecol Oncol. 2003;90:537–46. doi: 10.1016/s0090-8258(03)00199-9. [DOI] [PubMed] [Google Scholar]
- 4.Zanoti KM, Belinson JL, Kennedy AW, et al. The use of paclitaxel and platinum-based chemotherapy in uterine papillary serous carcinoma. Gynecol Oncol. 1999;74:272–7. doi: 10.1006/gyno.1999.5444. [DOI] [PubMed] [Google Scholar]
- 5.Maxell GL, Chanramouli GVR, Dainty L, et al. Microarray analysis of endometrial carcinomas and mixed mullerian tumors reveals distinct gene expression profiles associated with different histologic types of uterine cancer. Clin Cancer Res. 2005;11:4056–66. doi: 10.1158/1078-0432.CCR-04-2001. [DOI] [PubMed] [Google Scholar]
- 6.Zorn KK, Bonome T, Gangi L, et al. Gene expression profiles of serous, endometrioid, and clear cell subtypes of ovarian and endometrial cancer. Clin Cancer Res. 2005;11:6422–30. doi: 10.1158/1078-0432.CCR-05-0508. [DOI] [PubMed] [Google Scholar]
- 7.Bristow RE, Asrari F, Trimble EL, et al. Extended surgical staging for uterine papillary serous carcinoma: survival outcome of locoregional (Stage I-III) disease. Gynecol Oncol. 2001;81:279–286. doi: 10.1006/gyno.2001.6159. [DOI] [PubMed] [Google Scholar]
- 8.Goldgerg H, Miller RC, Abdha-Bortnyak R, et al. Outcome after combined modality treatment for uterine papillary serous carcinoma: a study by the Rare Cancer Network (RCN) Gynecol Oncol. 2008;108:298–305. doi: 10.1016/j.ygyno.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 9.Hoskins PJ, Swenerton KD, Pike JA, et al. Paclitaxel and carboplatin, alone or with irradiation, in advanced or recurrent endometrial cancer: a phase II study. J Clin Oncol. 2001;19:4048–53. doi: 10.1200/JCO.2001.19.20.4048. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assoc. 1958;53:457–481. [Google Scholar]
- 11.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 12.Schwartz PE. The management of serous papillary uterine cancer. Curr Opin Oncol. 2006;18:494–9. doi: 10.1097/01.cco.0000239890.36408.75. [DOI] [PubMed] [Google Scholar]
- 13.Kelly MG, O'Malley D, Hui P, et al. Improved survival in surgical stage I patients with uterine papillary serous carcinoma (UPSC) treated with adjuvant platinum-based chemotherapy. Gynecol Oncol. 2005;98:353–359. doi: 10.1016/j.ygyno.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Vaidya AP, Littell R, Krasner C, Duska LR. Treatment of uterine papillary serous carcinoma with platinum-based chemotherapy and paclitaxel. Int J Gynecol Cancer. 2006;16:267–72. doi: 10.1111/j.1525-1438.2006.00413.x. [DOI] [PubMed] [Google Scholar]
- 15.Bozas GT, Bamias A, Kastritis E, et al. Adjuvant chemotherapy with paclitaxel and carboplatin in non-endometrioid carcinoma of the uterus. Eur J Gynaecol Oncol. 2005;26:627–31. [PubMed] [Google Scholar]
- 16.Sutton G, Axelrod JH, Bundy BN, et al. Adjuvant whole abdominal irradiation in clinical stages I and II papillary serous or clear cell carcinoma of the endometrium: a phase II study of the Gynecologic Oncology Group. Gynecol Oncol. 2006;100:349–54. doi: 10.1016/j.ygyno.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 17.DuBeeshter B, Estler K, Altobelli K, et al. High-dose rate brachytherapy for Stage I/II papillary serous or clear cell endometrial cancer. Gynecol Oncol. 2004;94:383–6. doi: 10.1016/j.ygyno.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Havrilesky LJ, Secord AA, Bae-Jump V, et al. Outcomes in surgical stage I uterine papillary serous carcinoma. Gynecol Oncol. 2007;105:677–82. doi: 10.1016/j.ygyno.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 19.Creasman WT, Kohler MF, Odicino F, et al. Prognosis of papillary serous, clear cell, and grade 3 stage I carcinoma of the endometrium. Gynecol Oncol. 2004;95:593–6. doi: 10.1016/j.ygyno.2004.08.019. [DOI] [PubMed] [Google Scholar]