Abstract
Background
We have previously reported that cancer incidence for lung, female breast, and colon and rectum for Hispanics decreases with increasing percentage of Hispanics at the census tract. In contrast, cervical cancer incidence increases with increasing percentage of Hispanics at the census tract.
Methods
In this study, we investigate the hypothesis that Hispanics living in census tracts with high percentages of Hispanics are diagnosed with more advanced cancer, with respect to tumor size and stage of diagnosis. Data from the Surveillance, Epidemiology, and End Results registry and the U.S. Census Bureau were used to estimate the odds of diagnosis at a “late” stage (II, III, IV) versus “early” stage (I) and breast cancer tumor size among Hispanics as a function of census tract percent Hispanic. Hispanic ethnicity in the Surveillance, Epidemiology, and End Results registry was identified by medical record review and Hispanic surname lists. The study also used income of Hispanics living in the census tract and controlled for age at diagnosis and gender.
Results
We found that Hispanics living in neighborhoods with higher density of Hispanic populations were more likely to be diagnosed with late-stage breast, cervical, or colorectal cancer, and to have a larger tumor size of breast cancer.
Conclusions
Our findings suggest that the benefits of lower cancer incidence in high tract percent Hispanics are partially offset by poorer access and reduced use of screening in conjunction with lower income, poorer health insurance coverage, and language barriers typical of these communities.
Introduction
Increasing evidence shows widening disparities in incidence and mortality from all cancers and many common cancers by socioeconomic status. Cancer incidence and mortality are now usually higher in socioeconomic status–disadvantaged populations (1, 2). In the United States, the death rate from all cancers combined in 1975 was 2% higher among men in poorer counties compared with more affluent counties; by 1999, it was 13% higher (3). This pattern reflects a reversal of a former pattern of higher cancer incidence and mortality in higher socioeconomic status populations, most notably with respect to lung cancer (3). Current patterns are likely multifactorial and have been attributed to a combination of class differences in understanding and modification of lifestyle risks, occupational and other environmental exposures, physiological responses to disadvantage status, use of screening, and access to definitive therapies after diagnosis.
In the United States, racial disparities generally fit the pattern linking greater disadvantage to higher cancer incidence and mortality. African American race and lower socioeconomic status are independently and in combination associated with higher cancer incidence and mortality (2, 4). For Hispanics, however, the relationship is more complicated. Hispanics have lower incidence and mortality than non-Hispanic Whites for cancers of all sites and of the lung, breast, prostate, and colon and rectum. Hispanics have higher rates of incidence and mortality for cancers of the cervix, stomach, liver, and gallbladder (4–6). For all cancers, excluding nonmelanoma skin carcinomas, the age-adjusted incidence per 100,000 persons was 352 for Hispanics versus 490 for non-Hispanic Whites. Cancer mortality was 138 per 100,000 for Hispanics versus 202 for non-Hispanic Whites (5). This reduction in cancer mortality among Hispanics is a major contributor to the so-called Hispanic epidemiological paradox in which many health indicators for U.S. Hispanics are comparable with or better than those for non-Hispanic Whites, although this population is substantially disadvantaged in income, education, and access to medical care (7–9).
The causes of this inversion of expected relationships among Hispanics has not been fully explained, although there is evidence linking it in part to lower mortality and risk behaviors among immigrants. Cancer mortality rates are lower for Hispanic immigrants compared with Hispanics born in the United States (10–12), a fact that is consistent with the hypothesis of increasing risk profiles associated with acculturation. These changes include increases in smoking (11–14), obesity (11, 12, 15), and alcohol consumption (14, 16, 17) and decreases in dietary quality (14, 16, 18) and physical activity (19). Although cancer incidence and mortality is lower for Hispanics compared with non-Hispanic Whites, other studies have shown that survival after a diagnosis of cancer is poorer among Hispanics than in non-Hispanic Whites (20–25). This poorer survival is explained in part by the fact that some cancers are diagnosed on average at a more advanced stage in Hispanics and perhaps because they experience barriers to access to appropriate cancer care associated with low income, no health insurance, and language barriers.
Numerous recent studies have investigated the relationship between poorer neighborhood socioeconomic status and higher morbidity and mortality (26–28). Concordantly, a smaller literature has reported and investigated a linkage between racial segregation, higher mortality, and poorer health for African Americans (29–32). This latter work draws on a voluminous sociological literature identifying racially exclusionary residential patterns as a primary contributor to racial disparities in the United States across many domains, including health, in part because the hypersegregation typical of most American metropolitan areas is a major contributor to the concentration of extreme poverty in many African American neighborhoods, and with a wide range of resulting deficits in the physical, social, commercial, and service environments in these communities (33–37).
The literature focused on neighborhood residential patterns for Hispanics has generally started from different premises and reported different relationships. For Hispanics, levels of segregation from non-Hispanic Whites have been lower than for African Americans. Hispanics have tended to follow a pattern of increasing concentration spatial assimilation with non-Hispanics in conjunction with economical mobility and acculturation. Partly as a consequence, the literature on concentrated Hispanic “barrios” has viewed them positively as emerging through migration chaining and regional concentration. Neighborhood concentration of Hispanics has consequently sometimes been hypothesized to be a mechanism for maintenance of cultural patterns and social organization that, in the context of the Hispanic mortality advantage, might be advantageous to health.
Concordant with this hypothesis, we have previously reported that the incidence of cancers of the lung, female breast, and colon and rectum for Hispanics follows a cross-sectional pattern of decreased incidence with increasing isolation from non-Hispanic populations; that is, where a larger percentage of census tract residents were Hispanic [tract percent Hispanic (TpH)]. By contrast, the incidence of cancers of the cervix was higher in higher TpH tracts (38). However, if the mechanism produces some health advantages for Hispanics living in high-density Hispanic barrio communities, the hypothesized sociocultural factors affecting the incidence of cancer would not be expected to generalize to patterns of access to medical care, which are more likely to follow patterns expected in conjunction with the greater socioeconomic disadvantage generally experienced in ethnically isolated Hispanic communities.
Screening is widely recommended or practiced for cervical, breast, prostate, and colorectal cancer with the goal of an early diagnosis of those cancers. Hispanics living in socioeconomically disadvantaged communities tend to have lower screening rates, and, consequently, advanced stage at diagnosis of cancer (39–41). For instance, Hispanics living in poor areas are more likely to present with distant stage at diagnosis for cervical, breast, prostate, colorectal (women), and lung (men) cancer than those living in wealthier areas (3, 42). In this study, we investigate the hypothesis that Hispanics living in census tracts with high percentages of Hispanics are diagnosed with more advanced cancer, with respect to tumor size and stage of diagnosis. This relationship is likely because increasing percentage of Hispanics in a census tract is associated with higher poverty rates and lower rates of insurance coverage and therefore with decreased access to medical care (38). In particular, we thought that the detrimental effect would be most apparent for cancers for which effective screening tests exist.
Materials and Methods
Overview
The current study was a descriptive study of cancer stage at diagnosis. Data from the Surveillance, Epidemiology, and End Results (SEER) registry and the U.S. Census Bureau were used to estimate the odds of diagnosis at a “late” stage versus “early” stage as a function of census TpH. Logistic regression models generated odds ratios to compare census tracts with different concentrations of Hispanics in the census tract, with adjustment for age and gender when appropriate.
Source of Data
Data from the SEER tumor registry were used to identify cancer cases (43). The 13 SEER areas (Connecticut, Iowa, New Mexico, Utah, Hawaii, Detroit, Atlanta, San Francisco-Oakland, Seattle-Puget Sound, Alaska, San Jose-Monterey, Los Angeles, and rural Georgia) covered approximately 14% of the U.S. population.
Study Cases
Eligible cancer cases were Hispanics ages 21 to 90 years old, living in a SEER area with a diagnosis of non–in situ breast (women), cervical (women), and colorectal (men and women) carcinomas from 1988 to 2002. There were 32,934 Hispanics diagnosed during the years of study in the SEER areas; excluding those whose first cancer site was not one of the three cancers, the number of cases was reduced to 29,259. Of these, 28,896 had information for TpH with either a 2000 census tract number or a 1990 tract that could be mapped to the 2000 census data. After excluding cases with missing stage at diagnosis (n = 1,360), those younger than 21 years or older than 90 years (n = 478), and those with stage 0 (in situ, n = 6,240) at diagnosis, the final sample included 20,818 patients.
Measures
In the SEER registry, Hispanic ethnicity is identified by a combination of medical record review and matching surnames against lists of Hispanic surnames (43, 44). Also defined at the patient level in this data set are age at diagnosis (0–4, 5–9, 80–84, and 85 years) and gender. Type of cancer was determined by the site recode variable in the SEER data set. Stage at diagnosis was categorized as early (I) or late (II, III, IV) by the American Joint Committee on Cancer staging.
The percentage of population that was Hispanic, or TpH, was calculated as the total number of Hispanic individuals in the tract divided by the total population (Hispanic individuals and non-Hispanic individuals) in the census tract, according to the year of diagnosis of cancer and using the closest census information (1990 or 2000). For each sample of analysis (breast, cervical, or colorectal cancer), there were strong associations (r = −0.62, −0.64, and −0.60) between TpH and percent neighborhood income. However, the maximum variance inflation factors (2.9, 2.9, and 2.5) that were associated with these correlations were well below the threshold (variance inflation factor = 10) that would indicate a problem of multicollinearity in multivariate regression models.
Statistical Analyses
We used the χ2 to test the association between TpH and income categories. We used logistic regression analyses adjusted for age and gender (where relevant) to report the odds ratio of diagnosis at a late stage (II, III, IV) versus the early stage (I) as a function of TpH. In the multivariable models, we used an F test to determine the significance of interactions between TpH and age or gender (where relevant), and reported the odds ratios when the contribution of the set was significant (P < 0.05). In the sample for women with breast cancer, we analyzed the mean tumor size as a function of TpH and income, using ANOVA. Analyses were carried out with SAS for Windows version 9.1 (SAS Institute).
Results
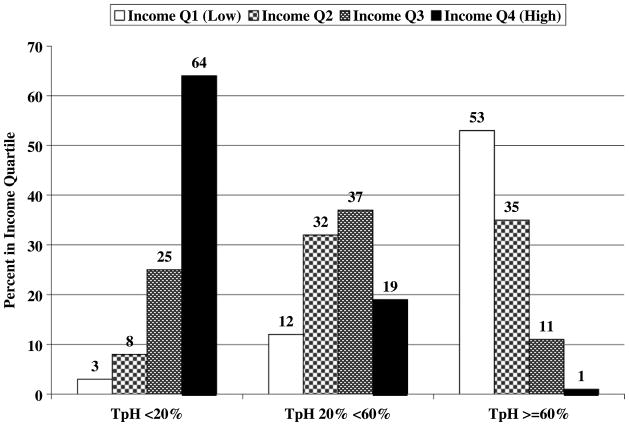
Figure 1 shows the distribution of census tract mean income quartiles by percentage of Hispanics living in the census tract (TpH) for Hispanic women with breast cancer (n = 10,309). The association of these two variables is strong, with a large majority of cases (64%) in the lowest TpH found in the highest income quartile, and a majority of cases in the highest TpH tracts found in the lowest income quartile. The likelihood ratio χ2 is 6,003 with 6 degrees of freedom (P < 0.0001; γ = −0.7933).
Figure 1.
Distribution of census tract income quartiles (Q1 = low to Q4 = high) by percentage of Hispanics living in the census tract (TpH) for Hispanic women with breast cancer, 1988 to 2002 (n = 10,309).
Table 1 shows the odds of diagnosis at late versus early stage of breast, cervical, and colorectal cancer in Hispanic patients as a function of neighborhood percent Hispanic, as a function of income, and as a function of the two jointly. For cancers of all three sites, increasing TpH is significantly associated with a later stage at diagnosis. The interaction effects between TpH-age or TpH-gender were not significant. Increasing income in the census tract is associated with decreased odds of late diagnosis of breast cancer among Hispanics. The interaction effects between income-age or income-gender were not significant. Controlling for both income and TpH, the effect of each measure is reduced, but both remain statistically significant. Tract income is not independently associated with stage for cervical and colorectal cancer, and controlling for this measure does not substantively affect the relationship between higher TpH and probability of a later stage at diagnosis.
Table 1.
Odds of diagnosis at a late versus early stage for breast, cervical, or colorectal cancer in Hispanics as a function of the percentage of Hispanics living in the census tract and tract income (1988–2002)
| Characteristic | OR (95% CI)
|
||
|---|---|---|---|
| Breast cancer (n = 10,309)* | Cervical cancer (n = 932)* | Colorectal cancer (n = 9,577)*, † | |
| TpH | |||
| <20% | 1.0 | 1.0 | 1.0 |
| 20% to <60% | 1.21 (1.10–1.32) | 1.21 (0.85–1.72) | 1.11 (0.99–1.23) |
| ≥60% | 1.38 (1.25–1.53) | 1.58 (1.11–2.24) | 1.14 (1.02–1.29) |
| Tract income | |||
| Q1 (low) | 1.0 | 1.0 | 1.0 |
| Q2 | 0.80 (0.71–0.90) | 1.14 (0.82–1.57) | 1.04 (0.91–1.19) |
| Q3 | 0.75 (0.67–0.84) | 0.81 (0.56–1.15) | 0.98 (0.86–1.11) |
| Q4 (high) | 0.65 (0.58–0.73) | 0.78 (0.52–1.19) | 0.92 (0.81–1.06) |
| TpH and tract income‡ | |||
| TpH <20% | 1.0 | 1.0 | 1.0 |
| TpH 20% to <60% | 1.12 (1.01–1.25) | 1.20 (0.81–1.78) | 1.09 (0.97–1.24) |
| TpH ≥60% | 1.14 (1.00–1.31) | 1.64 (1.04–2.60) | 1.14 (0.98–1.33) |
| Income Q1 (low) | 1.0 | 1.0 | 1.0 |
| Income Q2 | 0.81 (0.72–0.92) | 1.30 (0.92–1.85) | 1.06 (0.92–1.22) |
| Income Q3 | 0.78 (0.68–0.93) | 1.03 (0.68–1.57) | 1.02 (0.88–1.19) |
| Income Q4 (high) | 0.71 (0.62–0.83) | 1.13 (0.66–1.92) | 1.01 (0.85–1.20) |
NOTE: Data are from SEER areas. Late stage is defined as stages II, III, and IV; early stage is defined as stage I.
Abbreviation: OR, odds ratio; 95% CI, 95% confidence interval.
Adjusted for age.
Adjusted for gender.
Both variables were entered in the model.
Table 2 shows the mean tumor size of breast cancer as a function of TpH, of tract income, and of both jointly. Increasing TpH is associated with increased mean tumor size of breast cancer at diagnosis among Hispanic women. Increasing income in the census tract is associated with decreased mean tumor size of breast cancer among Hispanic women. After controlling for income and TpH jointly, the relationship between TpH and tumor size remains positive, but is not statistically significant.
Table 2.
Breast cancer size at diagnosis in Hispanic women as a function of the percentage of Hispanics living in the census tract and also the mean income of residents of the census tract (1988–2002; n = 9,792)
| Adjusted for age | Adjusted for age, TpH, and tract income | |
|---|---|---|
| TpH | Tumor size (±SD), mm | Tumor size (±SD), mm |
| <20% | 23.5 (0.36)* | 24.4 (0.43) |
| 20% to <60% | 24.9 (0.34) | 25.1 (0.35) |
| ≥60% | 26.2 (0.38) | 25.2 (0.44) |
| Census tract income | ||
| Q1 (low) | 27.1 (0.43)* | 26.8 (0.50)* |
| Q2 | 25.0 (0.40) | 24.8 (0.42) |
| Q3 | 24.4 (0.40) | 24.4 (0.42) |
| Q4 (high) | 23.2 (0.38) | 23.5 (0.46) |
NOTE: Mean tumor size was determined by TpH or income categories (ANOVA).
P < 0.0001.
Discussion
In this study, we investigated the relationship between Hispanic neighborhood environment and cancer stage at diagnosis, using SEER data. We found a substantial health disadvantage associated with increasing neighborhood percent Hispanic; specifically, Hispanics living in neighborhoods with higher density Hispanic populations were more likely to be diagnosed with late stage of breast, cervical, and colorectal cancer, and to have a larger tumor size for breast cancer.
Spatial distribution is an important marker of incorporation of immigrants and ethnic populations descended from them. Many immigrant populations, including Hispanics in the United States, follow a trajectory of spatial assimilation in which they initially settle in segregated enclaves, and then, with the passage of time, they and their descendants increasingly intermingle with members of the host society from other ethnic populations (45, 46). This segregation of some Hispanics into barrio enclaves may have contradictory effects on cancer incidence and mortality.
Immigrant populations often have profiles of site-specific cancer incidence that more closely resemble those of the countries from which they came than those of the host population of the country to which they migrate (47–49). The profiles of cancer incidence rates converge with those of the host society with increasing years and generations of residence in the destination country, as behavioral patterns and associated profiles of cancer risks converge with those of the host society (47–49). Spatial distribution of immigrant populations and their descendants may influence the trajectory of change of cancer risk profiles. Segregated barrio populations retain group-specific sociocultural risk profiles that are advantageous with respect to the incidence of cancers of the most common sites (38).
On the other hand, as we have shown, isolated barrio communities are generally economically disadvantaged and may have impaired access to medical care, with negative implications for timely diagnosis, receipt of definitive therapies, and survival. Effective screening tests exist for cancers of the colon and rectum, female breast, and cervix. The use of these tests varies across ethnic and social class groups (21, 50). Stage at diagnosis and tumor size, reflecting in part use of screening, are critical determinants of survival after diagnosis. Our findings that diagnosis is more likely to be at a later stage and larger tumor size with increasing TpH suggests that the benefits of lower incidence in high-TpH Hispanic communities are partially offset by poorer access and reduced use of screening in conjunction with lower income, poorer health insurance coverage, and language barriers typical of these communities (50–52).
However, spatial incorporation along with isolation or marginalization can result from societal circumstances, such as prejudice, institutional racism, and segregational rules or laws, or historical circumstances, such as when a territory is invaded or annexed by another country (53, 54). The link between isolation and increased perceived discrimination (e.g., among Hispanics, directed against their group; ref. 55) or decreased perceived social acceptance (56), along with socioeconomic deprivation, is associated with risky health behaviors and refraining from seeking medical treatment, which may contribute to increased burden of physical or mental disease (57, 58). Thus, a sense of discrimination among Hispanics may decrease the likelihood that they will seek medical care and, in conjunction with other socioeconomic barriers, may contribute to reduced use of cancer screening.
Earlier diagnosis may be related to increased awareness of symptoms and access to medical care. For example, for the four cancer sites for which screening is widely recommended or practiced (cervical, breast, colorectal, and prostate), the proportion of cases diagnosed at localized stage is lower and the proportion diagnosed at distant stage is higher in high-poverty compared with low-poverty census tracts (3, 42, 59). Therefore, our data suggest that a late stage may be linked to less screening in the Hispanic population as it has been shown in other studies, where Hispanics tend to have lower screening (mammography, colonoscopy or fecal occult blood test, and Pap test) rates than non-Hispanic Whites, and subjects less acculturated tend to have lower screening rates than those more acculturated (40, 41, 59).
One limitation of our study is that our findings might be an artifact of variations in cancer surveillance. That is, if diagnosis and/or registration of stage of diagnosis of cancer in tumor registries were less complete in neighborhoods with higher percentages of Hispanic residents, this could produce the pattern of results that we found. This is an unlikely explanation for our results. The SEER registry is generally recognized to be complete, with ascertainment rates higher than 95% (5, 43). A potential limitation is the ascertainment of Hispanic ethnicity. The SEER registry uses an algorithm including information from medical records supplemented by comparison with a list of Hispanic surnames, whereas the census reports ethnicity by self-identification.
Our findings that greater isolation and lower income are associated with larger tumor size and later stage of diagnosis may have implications in other disadvantaged minority populations, where both living in low income and segregated or isolated communities may have influences on access to health care, therefore affecting stage at diagnosis for cancer.
In conclusion, we confirm our hypothesis that Hispanics living in neighborhoods with high percentages of Hispanics tend to be diagnosed at more advanced cancer stage and with larger tumor size. Further research is needed to explore the association between TpH and survival after a diagnosis of cancer.
Acknowledgments
Grant support: National Cancer Institute grant P50 CA10563-02 and Komen Foundation Grant POP9999706 Department of Defense grant W81XWH-06-1-0290 (University of Texas Medical Branch Center for Population Health and Health Disparities).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Mackillop WJ, Zhang-Salomons J, Boyd CJ, Groome PA. Associations between community income and cancer incidence in Canada and the United States. Cancer. 2000;89:901–12. doi: 10.1002/1097-0142(20000815)89:4<901::aid-cncr25>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 2.Albano JD, Ward E, Jemal A, et al. Cancer mortality in the United States by education level and race. J Natl Cancer Inst. 2007;99:1384–94. doi: 10.1093/jnci/djm127. [DOI] [PubMed] [Google Scholar]
- 3.Singh GK, Miller BA, Hankey BF, et al., editors. NIH publication no 03–5417. Bethesda (MD): National Cancer Institute; 2003. Area socioeconomic variations in U S. cancer incidence, mortality, stage, treatment, and survival, 1975–1999. NCI cancer surveillance monograph series, number 4. [Google Scholar]
- 4.Ries LAG, Melbert D, Krapcho M, et al., editors. SEER cancer statistics review, 1975–2004. Bethesda (MD): National Cancer Institute; 2007. [Google Scholar]
- 5.Ries LAG, Eisner MP, Kosary CL, et al., editors. SEER cancer statistics review, 1975–2000. Bethesda (MD): National Cancer Institute; 2003. [Google Scholar]
- 6.Trapido EJ, Burciaga-Valdez R, Obeso JL, Strickman-Stein N, Rotger A, Perez-Stable EJ. Epidemiology of cancer among Hispanics in the United States. J Natl Cancer Inst Monogr. 1995;18:17–28. [PubMed] [Google Scholar]
- 7.Elo IT, Preston SH. Racial and ethnic differences in mortality at older ages. In: Martin LG, Soldo BJ, editors. Racial and ethnic differences in the health of older Americans. Washington (DC): National Academy Press; 1997. pp. 10–42. [Google Scholar]
- 8.Markides KS, Coreil J. The health of Hispanics in the southwestern United States: an epidemiologic paradox. Public Health Rep. 1986;101:253–65. [PMC free article] [PubMed] [Google Scholar]
- 9.Markides KS, Eschbach K. Aging, migration, and mortality: current status of research on the Hispanic paradox. J Gerontol B Psychol Sci Soc Sci. 2005;60:68–75. doi: 10.1093/geronb/60.special_issue_2.s68. [DOI] [PubMed] [Google Scholar]
- 10.Sorlie PD, Backlund E, Johnson NJ, Rogot E. Mortality by Hispanic status in the United States. JAMA. 1993;270:2464–8. [PubMed] [Google Scholar]
- 11.Singh GK, Siahpush M. Ethnic-immigrant differentials in health behaviors, morbidity and cause-specific mortality in the United States: an analysis of two national databases. Hum Biol. 2002;74:83–109. doi: 10.1353/hub.2002.0011. [DOI] [PubMed] [Google Scholar]
- 12.Singh GK, Hiatt RA. Trends and disparities in socioeconomic and behavioural characteristics, life expectancy, and cause-specific mortality of native-born and foreign-born populations in the United States, 1979–2003. Int J Epidemiol. 2006;35:903–19. doi: 10.1093/ije/dyl089. [DOI] [PubMed] [Google Scholar]
- 13.Perez-Stable EJ, Ramirez A, Villareal R, et al. Cigarette smoking behavior among US Latino men and women from different countries of origin. Am J Public Health. 2001;91:1424–30. doi: 10.2105/ajph.91.9.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Otero-Sabogal R, Sabogal F, Pérez-Stable EJ, Hiatt RA. Dietary practices, alcohol consumption, and smoking behavior: ethnic, sex and acculturation differences. J Natl Cancer Inst Monogr. 1995;18:73–82. [PubMed] [Google Scholar]
- 15.Sundquist J, Winkleby M. Country of birth, acculturation status and abdominal obesity in a national sample of Mexican-American women and men. Int J Epidemiol. 2000;29:470–7. [PubMed] [Google Scholar]
- 16.Marks G, Garcia M, Solis JM. Health risk behaviors of Hispanics in the United States: findings from HHANES, 1982–84. Am J Public Health. 1990;80:20–6. doi: 10.2105/ajph.80.suppl.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Markides KS, Ray LA, Stroup-Benham C, Trevino F. Acculturation and alcohol consumption in the Mexican American population of the southwestern United States: findings from the HHANES 1982–1984. Am J Public Health. 1990;80:42–6. doi: 10.2105/ajph.80.suppl.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guendelman S, Abrams B. Dietary intake among Mexican American women: generational differences and a comparison with white non-Hispanic women. Am J Public Health. 1995;85:20–5. doi: 10.2105/ajph.85.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon-Larsen P, Harris KM, Ward DS, Popkin BM. Acculturation and overweight-related behaviors among Hispanic immigrants to the US: the National Longitudinal Study of Adolescent Health. Soc Sci Med. 2003;57:2023–34. doi: 10.1016/s0277-9536(03)00072-8. [DOI] [PubMed] [Google Scholar]
- 20.Samet J, Key C, Hunt WC, Goodwin JS. Survival of American Indian and Hispanic Cancer patients in New Mexico and Arizona, 1969–1982. J Natl Cancer Inst. 1987;79:457–63. [PubMed] [Google Scholar]
- 21.Samet JM, Hunt WC, Lerchen ML, Goodwin JS. Delay in seeking care for cancer symptoms: a population-based study of elderly New Mexicans. J Natl Cancer Inst. 1988;80:432–8. doi: 10.1093/jnci/80.6.432. [DOI] [PubMed] [Google Scholar]
- 22.Goodwin JS, Hunt WC, Samet JM. Determinants of choice of cancer therapy in elderly patients: results from a population-based study. Cancer. 1993;72:594–602. doi: 10.1002/1097-0142(19930715)72:2<594::aid-cncr2820720243>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 23.Goodwin JS, Samet JM, Hunt WC. Determinants of survival in older cancer patients. J Natl Cancer Inst. 1996;88:1031–8. doi: 10.1093/jnci/88.15.1031. [DOI] [PubMed] [Google Scholar]
- 24.Gilliland FD, Hunt WC, Key CR. Trends in the survival of American Indian, Hispanic and non-Hispanic white cancer patients in New Mexico and Arizona, 1969–1983. Cancer. 1988;82:1769–83. doi: 10.1002/(sici)1097-0142(19980501)82:9<1784::aid-cncr26>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 25.Modiano MB, Villar-Werstler P, Meister J, Figueroa-Valles N. Cancer in Hispanics. J Natl Cancer Inst Monogr. 1995;18:35–9. [PubMed] [Google Scholar]
- 26.Bosma H, van de Mheen HD, Borsboom GJJM, Mackenbach JP. Neighborhood socioeconomic status and all cause mortality. Am J Epidemiol. 2001;153:363–71. doi: 10.1093/aje/153.4.363. [DOI] [PubMed] [Google Scholar]
- 27.Stjarne MK, Fritzell J, Ponce De Leon A, Hallqvist J for the SHEEP Study Group. Neighborhood socioeconomic context, individual income and myocardial infarction. Epidemiology. 2006;17:14–23. doi: 10.1097/01.ede.0000187178.51024.a7. [DOI] [PubMed] [Google Scholar]
- 28.Merkin SS, Diez-Roux AV, Coresh J, Fried LF, Jackson SA, Powe NR. Individual and neighborhood socioeconomic status and progressive chronic kidney disease in an elderly population: The Cardiovascular Health Study. Soc Sci Med. 2007;65:809–21. doi: 10.1016/j.socscimed.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 29.LaVeist TA. Racial segregation and longevity among African Americans: an individual-level analysis. Health Serv Res. 2003;38:1719–33. doi: 10.1111/j.1475-6773.2003.00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller DK, Wolinsky FD, Malmstrom TK, Andresen EM, Miller JP. Inner city, middle-aged African Americans have excess frank and subclinical disability. J Gerontol Med Sci. 2005;60A:207–12. doi: 10.1093/gerona/60.2.207. [DOI] [PubMed] [Google Scholar]
- 31.Robert SA, Ruel E. Racial segregation and health disparities between Black and White older adults. J Gerontol B Psychol Sci Soc Sci. 2006;61:S203–11. doi: 10.1093/geronb/61.4.s203. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez RA, Sen S, Mehta K, Moody-Ayers S, Bacchetti P, O’Hare AM. Geography matters: relationships among urban residential segregation, dialysis facilities, and patient outcomes. Ann Intern Med. 2007;146:493–501. doi: 10.7326/0003-4819-146-7-200704030-00005. [DOI] [PubMed] [Google Scholar]
- 33.Massey DS, Denton NA. Hypersegregation in U.S. metropolitan areas: black and Hispanic segregation along five dimensions. Demography. 1989;26:373–91. [PubMed] [Google Scholar]
- 34.Rankin BH, Quane JM. Neighborhood poverty and the social isolation of innercity African American families. Social Forces. 2000;79:139–64. [Google Scholar]
- 35.Williams DR, Collins CA. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. 2001;116:404–16. doi: 10.1093/phr/116.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson SM, Howell F, Wing S, Sobsey M. Environmental injustice and the Mississippi Hog Industry. Environ Health Perspect. 2002;110:195–201. doi: 10.1289/ehp.02110s2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zenk SN, Schulz AJ, Israel BA, James SA, Bao S, Wilson ML. Neighborhood racial composition, neighborhood poverty, and the spatial accessibility of supermarkets in Metropolitan Detroit. Am J Public Health. 2005;95:660–7. doi: 10.2105/AJPH.2004.042150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eschbach K, Mahnken JD, Goodwin JS. Neighborhood composition and incidence of cancer among Hispanics in the United States. Cancer. 2005;103:1036–44. doi: 10.1002/cncr.20885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barret RE, Cho YI, Weaver KE, et al. Neighborhood change and distant metastasis at diagnosis of breast cancer. Ann Epidemiol. 2008;18:43–7. doi: 10.1016/jannepidem.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Schootman M, Jeffe DB, Baker EA, Walker MS. Effect of area poverty rate on cancer screening across US communities. J Epidemiol Community Health. 2006;60:202–7. doi: 10.1136/jech.2005.041020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schootman M, Walker MS, Jeffe DB, Rohrer JE, Baker EA. Breast cancer screening and incidence in communities with high proportion of uninsured. Am J Prev Med. 2007;33:379–86. doi: 10.1016/j.amepre.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh GK, Miller BA, Hankey BF, Edwards BK. Persistent area socioeconomic disparities in U S. Incidence of cervical cancer, mortality, stage and survival, 1975–2000. Cancer. 2004;101:1051–7. doi: 10.1002/cncr.20467. [DOI] [PubMed] [Google Scholar]
- 43.Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence—SEER 9 Regs Public-Use, Nov 2004 Sub (1973–2002) National Cancer Institute DCCPS, Surveillance Research Program, Cancer Statistics Branch; ( http://www.seer.cancer.gov) released April 2005, based on the November 2004 submission. [Google Scholar]
- 44.Stewart SL, Swallen KC, Glaser SL, Horn-Ross PL, West DW. Comparison of methods for classifying Hispanic ethnicity in a population-based cancer registry. Am J Epidemiol. 1999;149:1063–71. doi: 10.1093/oxfordjournals.aje.a009752. [DOI] [PubMed] [Google Scholar]
- 45.Massey DS, Denton NA. Spatial assimilation as a socioeconomic outcome. Am Soc Rev. 1985;50:94–106. [Google Scholar]
- 46.Charles CZ. The dynamics of racial residential segregation. Ann Rev Sociol. 2003;29:167–207. [Google Scholar]
- 47.Parkin DM, Khlat M. Studies of cancer in migrants: rationale and Methodology. Eur J Cancer. 1996;32A:761–71. doi: 10.1016/0959-8049(96)00062-7. [DOI] [PubMed] [Google Scholar]
- 48.Hemminki K, Li XJ, Czene K. Cancer risks in first generation immigrants to Sweden. Int J Cancer. 2002;99:218–28. doi: 10.1002/ijc.10322. [DOI] [PubMed] [Google Scholar]
- 49.Hemminki K, Li XJ. Cancer risks in second generation immigrants to Sweden. Int J Cancer. 2002;99:229–37. doi: 10.1002/ijc.10323. [DOI] [PubMed] [Google Scholar]
- 50.Freeman HP. American Cancer Society, editor. Cancer and socioeconomically disadvantaged. Atlanta (GA): American Cancer Society; 1990. Cancer in the socioeconomically disadvantaged; pp. 4–26. [Google Scholar]
- 51.Friedell GH, Linville LH, Hullet S. Cancer control in rural Appalachia. Cancer. 1998;83:1868–71. [Google Scholar]
- 52.Haynes MA, Smedley BD, editors. An assessment of NIH research and programs for ethnic minorities and the medically underserved. Washington (DC): National Academy Press; 1999. The unequal burden of cancer. [PubMed] [Google Scholar]
- 53.Marin G. Issues in the measurement of acculturation among Hispanics. In: Geisinger KF, editor. Pshychological Testing of Hispanics. Washington (DC): American Psychological Association; pp. 23–51. [Google Scholar]
- 54.Lara M, Gamboa C, Kahramanian MI, Morales LS, Hayes-Bautista DE. Acculturation and Latino Health in the United Status: a review of the literature and its sociopolitical context. Annu Rev Public Health. 2005;26:367–97. doi: 10.1146/annurev.publhealth.26.021304.144615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shorey HS, Cowan G, Sullivan MP. Predicting perceptions of discrimination among Hispanics and Anglos. Hispanic J Behav Sci. 2002;24:3–22. [Google Scholar]
- 56.Arcia E, Skinner M, Bailey D, Correa V. Models of acculturation and health behaviors among Latino immigrants to the US. Soc Sci Med. 2001;53:41–53. doi: 10.1016/s0277-9536(00)00310-5. [DOI] [PubMed] [Google Scholar]
- 57.Williams DR, Neighbors HW, Jackson JS. Racial/ethnic discrimination and health: findings from community studies. Am J Public Health. 2003;93:200–8. doi: 10.2105/ajph.93.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wamala S, Merlo J, Bostrom G, Hogstedt C. Perceived discrimination, socioeconomic disadvantage and refraining from seeking medical treatment in Sweden. J Epidemiol Community Health. 2007;61:409–15. doi: 10.1136/jech.2006.049999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]