Abstract
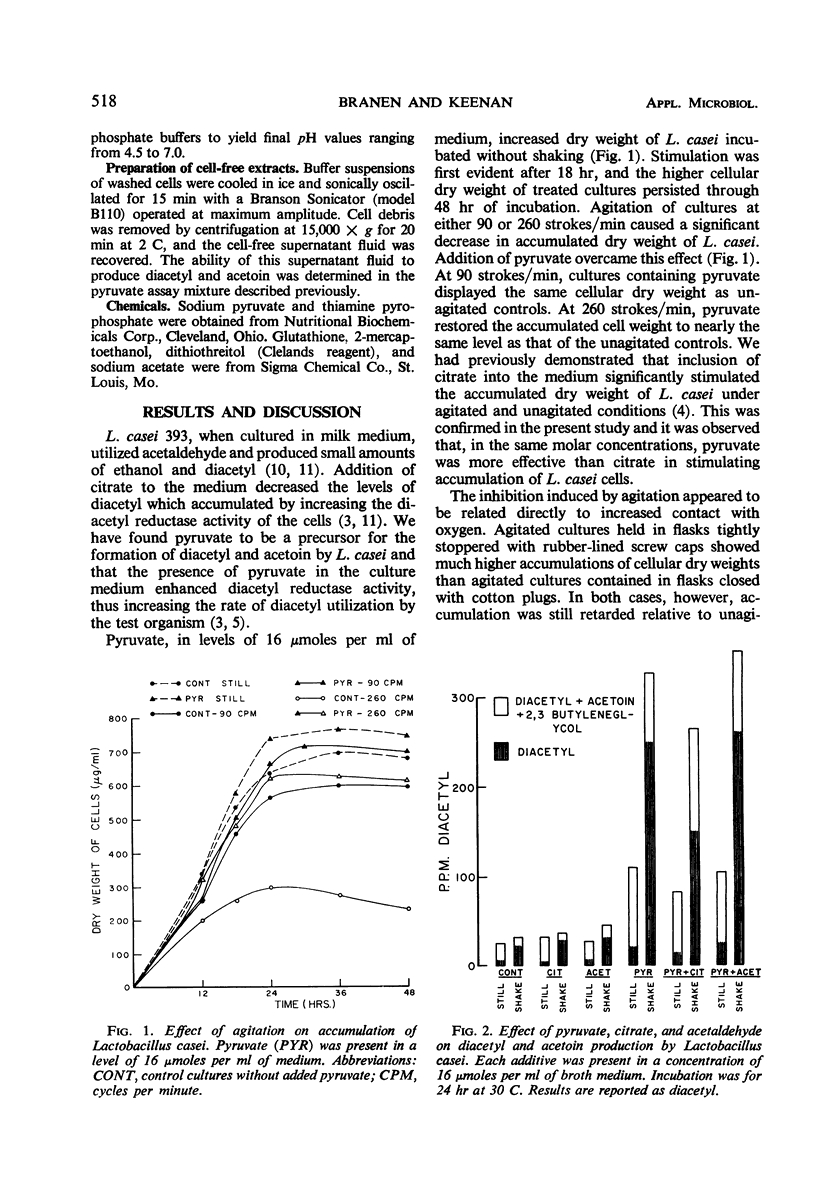
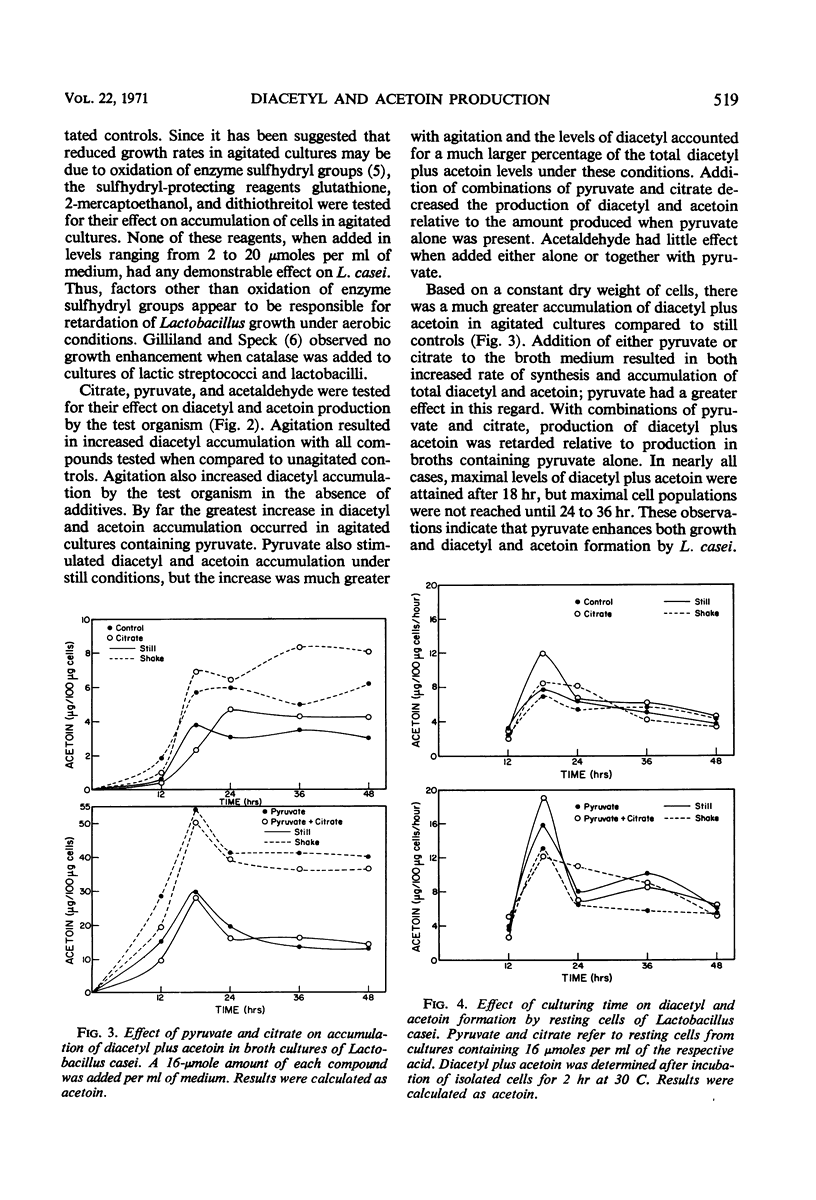
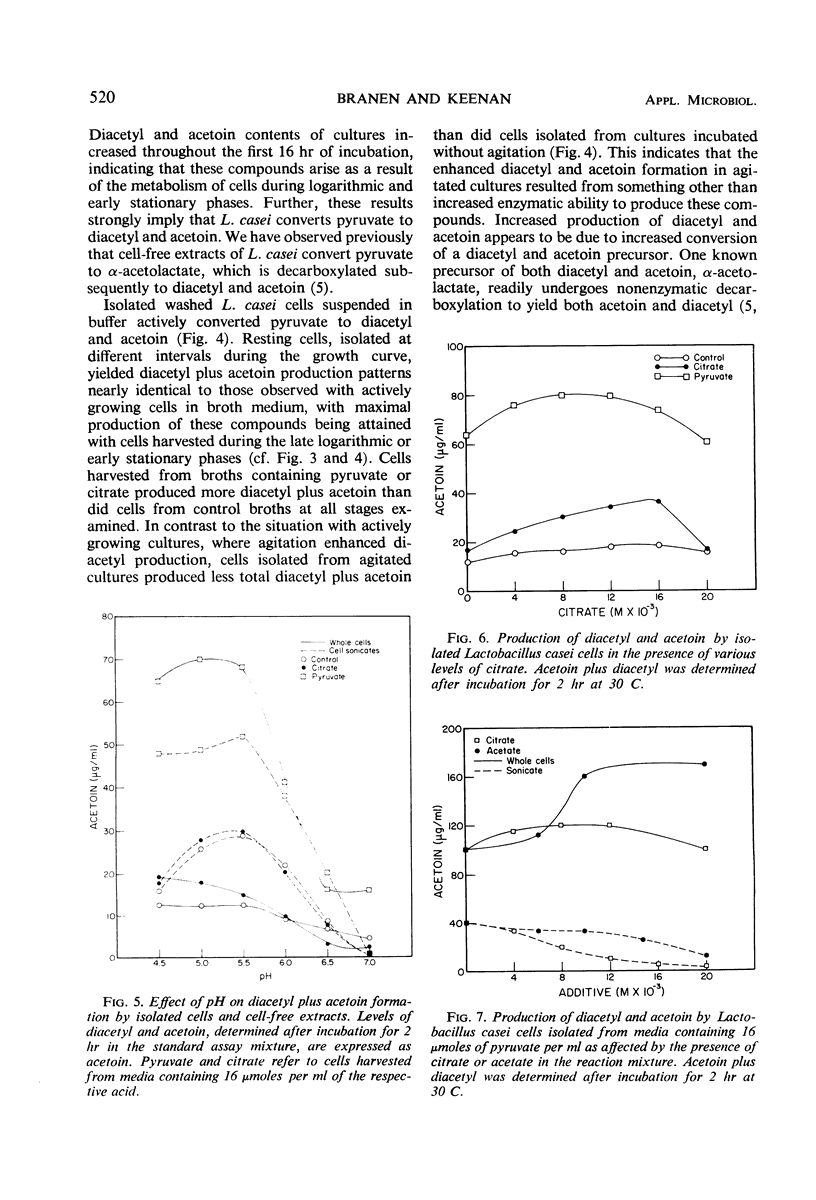
Agitation of broth cultures of Lactobacillus casei retarded cellular dry weight accumulation but enhanced production of both diacetyl and acetoin. Addition of pyruvate overcame this retardation, but addition of sulfhydryl-protecting reagents did not. Both pyruvate and citrate enhanced accumulated dry weight of L. casei incubated without agitation, but only pyruvate increased diacetyl accumulation. Both actively dividing cells and cells suspended in buffer converted pyruvate to diacetyl and acetoin. Maximum production of diacetyl and acetoin occurred during the late logarithmic or early stationary phases. Cells isolated from pyruvate- or citrate-containing cultures showed the greatest ability to convert pyruvate to diacetyl and acetoin. The optimum pH for diacetyl and acetoin formation by whole cells was in the range of 4.5 to 5.5. The presence of citrate or acetate enhanced diacetyl and acetoin formation by L. casei cells in buffer suspension.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassette R., Bawdon R. E., Claydon T. J. Production of volatile materials in milk by some species of bacteria. J Dairy Sci. 1967 Feb;50(2):167–171. doi: 10.3168/jds.S0022-0302(67)87383-1. [DOI] [PubMed] [Google Scholar]
- Branen A. L., Keenan T. W. Diacetyl reductase of Lactobacillus casei. Can J Microbiol. 1970 Oct;16(10):947–951. doi: 10.1139/m70-162. [DOI] [PubMed] [Google Scholar]
- Branen A. L., Keenan T. W. Growth stimulation of Lactobacillus casei by sodium citrate. J Dairy Sci. 1970 May;53(5):593–597. doi: 10.3168/jds.S0022-0302(70)86259-2. [DOI] [PubMed] [Google Scholar]
- Gilliland S. E., Speck M. L. Biological response of lactic streptococci and lactobacilli to catalase. Appl Microbiol. 1969 Jun;17(6):797–800. doi: 10.1128/am.17.6.797-800.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUNI E. Mechanisms of formation of acetoin by bacteria. J Biol Chem. 1952 Apr;195(2):715–726. [PubMed] [Google Scholar]
- Rogosa M., Love L. L. Direct quantitative gas chromatographic separation of C2-C6 fatty acids, methanol, and ethyl alcohol in aqueous microbial fermentation media. Appl Microbiol. 1968 Feb;16(2):285–290. doi: 10.1128/am.16.2.285-290.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speckman R. A., Collins E. B. Diacetyl biosynthesis in Streptococcus diacetilactis and Leuconostoc citrovorum. J Bacteriol. 1968 Jan;95(1):174–180. doi: 10.1128/jb.95.1.174-180.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomalainen H., Ronkainen P. Mechanism of diacetyl formation in yeast fermentation. Nature. 1968 Nov 23;220(5169):792–793. doi: 10.1038/220792a0. [DOI] [PubMed] [Google Scholar]


