Abstract
Trypanosoma brucei causes human African trypanosomiasis and regularly switches its major surface antigen variant surface glycoprotein (VSG) to evade mammalian host immune responses at the bloodstream form (BF) stage. Monoallelic expression of BF Expression Site (BES)-linked VSGs and silencing of metacyclic VSGs (mVSGs) in BF cells are essential for antigenic variation, whereas silencing of both BES-linked and mVSGs in the procyclic form (PF) cells is important for cell survival in the midgut of its insect vector. We have previously shown that silencing BES-linked VSGs in BF cells depends on TbRAP1. We now show that TbRAP1 silences both BES-linked and mVSGs at both BF and PF stages. The strength of TbRAP1-mediated BES-linked VSG silencing is stronger in the PF cells than that in BF cells. In addition, Formaldehyde-Assisted Isolation of Regulatory Elements analysis and MNase digestion demonstrated that depletion of TbRAP1 in PF cells led to a chromatin structure change, which is significantly stronger at the subtelomeric VSG loci than at chromosome internal loci. On the contrary, no significant chromatin structure changes were detected on depletion of TbRAP1 in BF cells. Our observations indicate that TbRAP1 helps to determine the chromatin structure at the insect stage, which likely contributes to its strong silencing effect on VSGs.
INTRODUCTION
Human African trypanosomiasis is caused by infection of Trypanosoma brucei and is inevitably fatal without treatment. Inside the human host, bloodstream form (BF) T. brucei cells stay in extracellular spaces and express variant surface glycoprotein (VSG) as its major surface antigen that is exposed to the host immune system (1,2). To evade the host’s immune responses, T. brucei cells undergo antigenic variation and regularly switch their VSG coat (3), which is essential for a persistent infection. Although there are >1000 VSG genes and pseudogenes in the T. brucei genome (4), in BF cells, VSGs are expressed exclusively from BF VSG Expression Sites (BESs), which are polycistronically transcribed by RNA Pol I (5). The VSG gene is the last gene in the BES and is located adjacent to the telomere while the BES promoter is usually 40–60 kb upstream (6). The T. brucei 427 strain used in this study has ∼20 nearly identical BESs (7), 14 of which carry distinctive VSG genes (8). However, at any moment, only one BES promoter is fully active, resulting in a single type of VSG being expressed. VSG monoallelic expression ensures the effectiveness of antigenic variation and is essential for T. brucei virulence.
Trypanosoma brucei is transmitted through its insect vector, tsetse (Glossina spp.). After ingestion by tsetse into its midgut, T. brucei differentiates into the procyclic form (PF) and expresses procyclins as surface glycoproteins, of which the C-terminus is resistant to protease cleavage (9). VSG is susceptible to protease degradation, and all VSG genes are silent in PF cells. Therefore, VSG silencing at the insect stage is critical for T. brucei to survive in its environment, but the underlying mechanism is poorly understood. Once T. brucei cells migrate into the salivary glands of tsetse, they differentiate into the metacyclic form, shed procyclins, express metacyclic VSGs (mVSGs) on the cell surface and acquire infectivity again. Each cell expresses one type of mVSG, but the population expresses many different mVSGs (10). This heterogeneity will presumably facilitate the population to establish an infection in mammalian hosts (11). Similar to BF VSGs, mVSGs are also transcribed from subtelomeres by RNA Pol I, but transcription of mVSGs is monocistronic (12,13). mVSG expression is silenced within a few days after the initial infection, allowing subsequent antigenic variation to take place, but the mechanisms of mVSG silencing have not been extensively studied.
Accumulating data have revealed that multiple mechanisms are involved in the regulation of BES-linked VSG monoallelic expression at the BF stage, including limited accessibility to RNA Pol I (14), restricted transcription elongation (15,16), chromatin remodeling (17–22) and telomeric silencing (23). Telomeres, located at the ends of linear chromosomes, are essential for genome stability. In several organisms including yeast, human, mouse, T. brucei and Plasmodium falciparum, telomeres form a heterochromatic structure that suppresses the transcription of subtelomeric genes (24–29). Trypanosoma brucei telomere DNA consists of thousands of duplex TTAGGG repeats (30). We have previously identified TbTRF as the duplex telomere DNA-binding factor (31) and TbRAP1 as a TbTRF-interacting partner (23). Depletion of TbRAP1 led to derepression of all subtelomeric silent BES-linked VSGs and simultaneous expression of multiple VSG proteins on the cell surface at the BF stage (23). However, whether TbRAP1 has a similar VSG silencing function in PF cells and the mechanism of TbRAP1-mediated silencing are not clear.
In this study, we found that depletion of TbRAP1 by RNA interference (RNAi) led to derepression of mVSGs at both the BF and PF stages. Silencing of BES-linked VSGs in PF cells depends on TbRAP1 and is stronger than that in BF cells. Furthermore, removal of TbRAP1 led to a loosened chromatin structure in PF cells, particularly at the subtelomeric VSG loci, but not significantly in BF cells, indicating that TbRAP1-mediated silencing involves modulation of chromatin structure in PF cells.
MATERIALS AND METHODS
Reverse transcription and quantitative reverse transcriptase-PCR
Total RNA was extracted using RNAstat 60 (Tel-Test Inc), treated with DNase I (Qiagen) and purified using RNeasy columns (Qiagen). Reverse transcription, quantitative reverse transcriptase-PCR (qRT-PCR) and quantification were performed as in (23).
Formaldehyde-assisted isolation of regulatory elements
Formaldehyde-assisted isolation of regulatory elements (FAIRE) analyses were performed according to (32). Cells were fixed with formaldehyde before the chromatin was sonicated in a Bioruptor (Diagenode). Free DNA was extracted by phenol chloroform. Input samples were reverse cross-linked before DNA was extracted. The amount of DNA was estimated by quantitative PCR using iTaq SYBR Green Supermix with ROX in an Opticon II (Bio-Rad), and the amount of FAIRE-extracted DNA was normalized with that of the input DNA.
Micrococcal nuclease digestion
In all, 5 × 107 cells per sample were permeabilized with digitonin and treated with 1 unit of Micrococcal nuclease (MNase) (Worthington Biochemicals) for 1, 2.5 or 5 min. DNA was isolated from treated cells, separated by agarose gel electrophoresis, blotted onto a nylon membrane and hybridized with specific probes.
Statistical analysis
P-values of unpaired t-tests were calculated using GraphPad Prism.
RESULTS
TbRAP1 is essential for normal cell growth in PF cells
To examine the functions of TbRAP1 in PF cells, we introduced an inducible TbRAP1 RNAi construct in 29-13 cells that expresses the Tet repressor and the T7 polymerase (33). Several independent clones were obtained that exhibited different growth defects on induction of TbRAP1 RNAi (Supplementary Figure S1A–C). To reduce the phenotypic variations often associated with clonal cell lines, we obtained a pool of TbRAP1 RNAi cells (referred to as ‘PRi-pool’) for subsequent detailed analyses, and critical phenotypes were confirmed in the PRi-C2 clone (Supplementary Figures S1B, D and F and S8B).
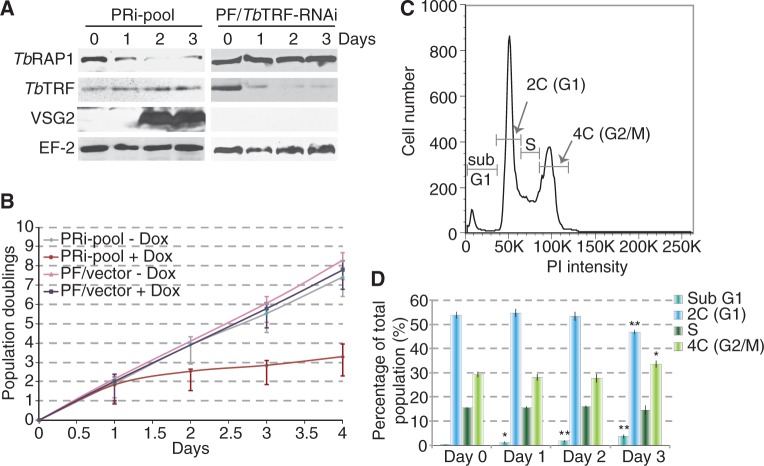
On induction of TbRAP1 RNAi, we observed a decreased TbRAP1 protein level and a severe growth defect by day 2 in PRi-pool cells (Figure 1A and B, Supplementary Figure S1E), indicating that TbRAP1 is essential for cell viability. Similar TbRAP1 knockdown and growth arrest were seen in PRi-C2 cells (Supplementary Figure S1B and F). The control 29-13 cells carrying an empty RNAi vector did not show any growth defects or decrease in the TbRAP1 protein level when treated with the same amount of doxycycline (Figure 1B, Supplementary Figure S1E). Flow cytometry analysis was performed to examine cell cycle profile after depletion of TbRAP1. We observed a mild decrease in the G1 population and a mild increase in the G2/M and the sub G1 populations in PRi-pool cells (Figure 1D, Supplementary Figure S2A), which is similar to what we observed in TbRAP1-depleted BF cells in our current and earlier studies (Supplementary Figure S2B and C) (23).
Figure 1.
Depletion of TbRAP1 led to a cell growth defect and derepression of BES-linked VSG2 in PF cells. (A) Western analysis of various protein levels in PRi-pool (left) and TbTRF RNAi (right) cells. Total cell lysates were prepared at days 0, 1, 2 and 3 after adding doxycycline. (B) Growth curves for PRi-pool and control cells with the empty vector under induced (+ Dox) or un-induced (−Dox) conditions were plotted as Population Doublings versus days after induction. Average Population Doubling values were calculated from six independent experiments. Error bars represent standard deviation. (C) Gating schematic of un-induced PRi-pool cells in the flow cytometry analysis. Sub-G1 cells contain DNA contents less than 2C and often experience DNA degradations. PI, propidium iodide. (D) Quantification of populations of PRi-pool cells at different cell cycle stages before (day 0) and after (days 1, 2 and 3) depletion of TbRAP1. Unpaired t-tests were done to compare day 1, 2 and 3 values with the day 0 value. Significant differences are marked with asterisks. *, 0.01 < P ≤ 0.05; **, 0.001 < P ≤ 0.01.
BES-linked VSG silencing depends on TbRAP1 at the insect stage
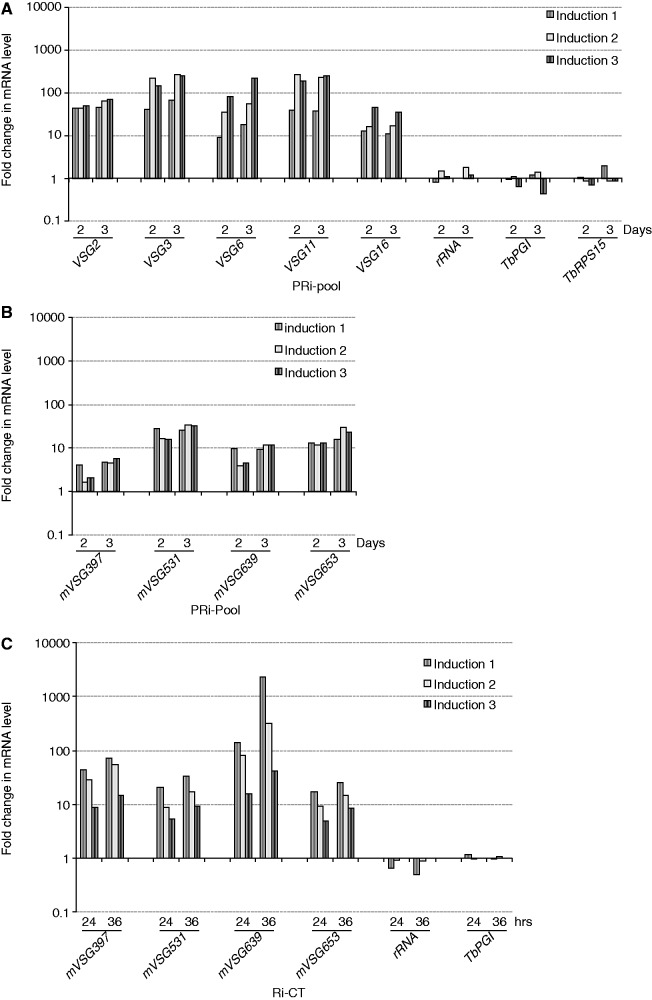
We compared the steady–state mRNA levels of several BES-linked VSGs before and after depletion of TbRAP1 by qRT-PCR. Thirty-five percent of TbRAP1 was still present at day 1 after induction of RNAi, but <10% of TbRAP1 was left by day 2 (Figure 1A). We therefore examined VSG derepression at day 2 and day 3. All tested VSG mRNA levels increased several 10- to several 100-fold after depletion of TbRAP1 (Figure 2A, see Supplementary Table S1 for VSG nomenclature). Western analysis also detected a prominent amount of VSG2 in PRi-pool cells (Figure 1A, Supplementary Figure S1E). Therefore, TbRAP1 is essential for silencing BES-linked VSGs in PF cells. Similar to that in BF cells, expression of RNA Pol II-transcribed TbPGI (a glycolytic protein), TbRPS15 (a ribosomal protein) or RNA Pol I-transcribed rRNA was not affected, indicating that TbRAP1’s effect on VSGs is specific. In addition to BES-linked and mVSGs, VSG genes located on minichromosomes (which mainly consist of internal 177 bp repeats and terminal telomere repeats) are also at subtelomeres but lack upstream promoters. Silencing of a minichromosomal VSG, VSG671, was not affected by depletion of TbRAP1—we did not detect its mRNA in northern blotting (data not shown), although the VSG671 gene was detectable in Southern analysis (Supplementary Figure S3)—indicating that VSG derepression requires a functional upstream promoter. Similar VSG derepression phenotype was also observed in PRi-C2 cells (Supplementary Figure S1D). On the contrary, no VSG derepression was detected in control cells by qRT-PCR (Supplementary Figure S4A) or western blotting (Supplementary Figure S1E). In addition, induction of TbTRF RNAi led to a cell growth arrest within 2 days in PF cells (31), but not VSG2 expression (Figure 1A), indicating that VSG derepression is not a consequence of cell growth arrest. Although TbRAP1 interacts with TbTRF (23), it has not been proved that TbTRF is strictly required for localizing TbRAP1 at telomeres. Therefore, depletion of TbTRF does not necessarily have the same VSG derepression phenotype.
Figure 2.
Depletion of TbRAP1 resulted in derepression of BES-linked VSGs in PF cells (A) and derepression of mVSGs in PF (B) and BF (C) cells. Steady–state mRNA levels for BES-linked VSGs 2, 3, 6, 11, mVSGs 397, 531, 639, 653 and control genes at days 0, 2 and 3 (PF) or at 0, 24 and 36 h (BF) after adding doxycycline in TbRAP1 RNAi cells were estimated using qRT-PCR and normalized against that before induction. Day 0 value is set to 1 but not shown. The fold change in mRNA levels for three independent induction experiments was shown.
TbRAP1 is required for silencing of mVSGs in BF and PF T. brucei cells
mVSGs are normally silent in BF and PF cells, but the silencing mechanisms are poorly understood. In our laboratory strain 427, five mVSG genes have been identified: mVSGs 397, 531, 639, 653 and 1954 (34). qRT-PCR analysis showed that mVSG mRNA levels increased several to several 10-fold on depletion of TbRAP1 (Figure 2B), whereas no significant changes were detected in control cells (Supplementary Figure S4B). We performed similar analyses in BF cells using two cell lines carrying pZJMβ-based (35) TbRAP1 RNAi vectors. One includes the TbRAP1 BRCT fragment (Ri-BRCT) and the other the TbRAP1 C-Terminus (Ri-CT). Both cell lines exhibited growth arrest and derepression of BES-linked VSGs on induction of TbRAP1 RNAi (Supplementary Figure S5), indicating that these cells behaved similarly to the Ri-2 and Ri-9 TbRAP1 RNAi cells studied previously (23). mVSGs were derepressed several to over a 1000-fold on TbRAP1 depletion in Ri-BRCT and Ri-CT cells, but not in control cells (Figure 2C; Supplementary Figure S4C and D). Therefore, TbRAP1 is required to silence mVSGs in both BF and PF cells.
Comparing the TbRAP1-mediated VSG silencing at different life cycle stages
We found that TbRAP1 silences BES-linked and metacyclic VSGs, but the derepression of BES-linked VSGs appeared to be much stronger in PF cells than in BF cells. In our previous study, on TbRAP1 depletion, VSG 3, 6 and 11 were derepressed up to 8-, 23- and 41-fold, respectively, in BF cells (23), whereas the same genes were derepressed up to 270-, 220- and 260-fold, respectively, in PF cells (Figure 2A).
To better quantify VSG derepression, we used qRT-PCR to carefully compare individual BES-linked VSG mRNA levels at the silent state in wild-type (WT) PF and BF cells, at the derepressed state in TbRAP1-depleted PF and BF cells, and at the fully active state in BF cells. The steady state VSG mRNA levels under various conditions were normalized against that in PF WT cells, which was arbitrarily set to 1. Three BES-linked VSGs—VSGs 2, 3 and 9—were analyzed owing to availability of all necessary cell lines in our laboratory (Supplementary Table S2). We found that first, in WT cells, VSGs 2, 3 and 9 mRNA levels were 28-, 16- and 9-fold higher in BF cells than in PF cells, respectively (Figure 3, PF WT and BF WT). This is likely owing to the fact that the VSG mRNAs are less stable in PF cells than in BF cells (36). Second, TbRAP1 was essential for VSG silencing at both stages. Depletion of TbRAP1 elevated the VSG2, 3 and 9 mRNA levels on average 333-, 527- and 217-fold, respectively, in PF cells and on average 22-, 133- and 30-fold, respectively, in BF cells (Figure 3, PF/TbRAP1RNAi −/+ Dox, BF/TbRAP1RNAi −/+ Dox). Third, the derepression of BES-linked VSGs resulting from TbRAP1 depletion was significantly stronger in PF cells (217–527-fold) than that in BF cells (22–133-fold). The P-values were all <0.002 for VSG2, 3 and 9, in unpaired t-tests when comparing changes in BF and PF cells. Therefore, the TbRAP1-mediated BES VSG silencing was stronger in PF cells than in BF cells. We noticed that in un-induced PRi-pool cells, the VSG mRNA level is ∼3-fold lower than that in PF WT cells (Figure 3), possibly reflecting small variations between different cell populations.
Figure 3.
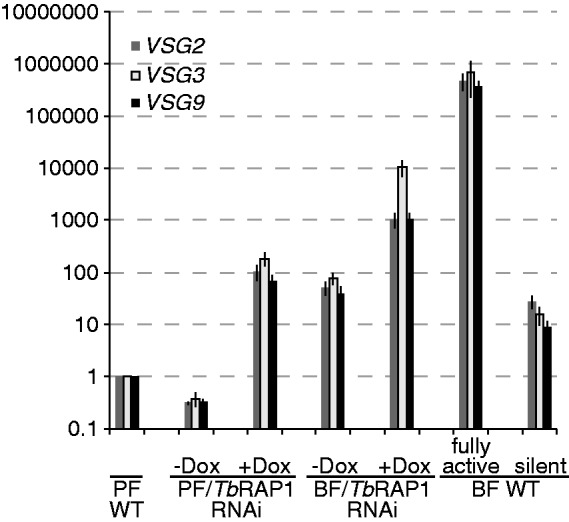
TbRAP1-mediated BES-linked VSG silencing is stronger in PF cells than in BF cells. The relative mRNA levels for the same VSG genes were estimated by qRT-PCR when the VSG is in BF or PF TbRAP1 RNAi cells before (silent) and after (derepressed) TbRAP1 depletion and when the VSG is in the active BES in WT BF cells (fully active) or in the silent state in both PF and BF cells (PF WT and silent BF WT, respectively). The mRNA levels at various states were normalized against that in PF WT silent state, which was arbitrarily set to 1. Average values calculated from five independent experiments were presented for each VSG. Error bars represent standard deviation. VSG derepression in PF cells was analyzed at day 0 and day 2.5 in PRi-pool cells (labeled as PF/TbRAP1 RNAi). At the BF stage, VSG3 and VSG9 derepression was analyzed at 0 h and 36 h in Ri-2 cells (expresses VSG2) (23), and VSG2 derepression was analyzed at the same time points in Ri-9 cells (expresses VSG9) (23), which are labeled as BF/TbRAP1 RNAi. Three BF WT cells were also used: Single Marker (SM) expresses VSG2 (33), pVS3-2/OD1-1 expresses VSG9 (23) and a SM-derived line expresses VSG3. All cell lines are listed in Supplementary Table S1.
In yeast, longer telomeres confer stronger telomeric silencing (37). However, telomeres in our PF and BF TbRAP1 RNAi cells are of similar length (on average ∼15 kb), suggesting that telomere length is not the reason for different TbRAP1-mediated silencing in BF and PF cells. To rule out the possibility that TbRAP1 might not be equally depleted in BF and PF cells, which could contribute to the differential VSG-derepression at the two stages, we examined TbRAP1 protein levels in all TbRAP1 RNAi cells. In Ri-2 and Ri-9 cells, TbRAP1 was depleted as efficiently as described previously (23). At the time points when various TbRAP1 RNAi cells were examined, comparable residual amounts of TbRAP1 were detected (on average, 4% in Ri-2, 14%–11% in Ri-9 and 16% in PRi-pool; Supplementary Figure S6A–C) (23). Therefore, it is unlikely that different levels of VSG derepression in BF and PF cells were due to different levels of TbRAP1 depletion. Another possibility is that TbRAP1 was expressed at different levels in BF and PF cells. However, by northern and western analyses, we found that TbRAP1 mRNA and protein levels were approximately the same at the two life cycle stages (Supplementary Figure S6D and E).
Although TbRAP1 is also required for mVSG silencing, a higher degree of variation in mVSG derepression was observed in independent inductions of TbRAP1 RNAi (Figure 2B and C), and no significant differences were observed between BF and PF cells (P > 0.05), except that derepression of mVSG397 in Ri-BRCT cells is slightly higher than that in PRi-pool cells (P = 0.03).
Depletion of TbRAP1 led to more loosely packed chromatin structures in PF cells
To further investigate whether TbRAP1-mediated VSG silencing involves a chromatin remodeling mechanism, we compared the subtelomeric chromatin structure before and after depletion of TbRAP1 using the FAIRE analysis (32), where DNA fragments free of bound proteins are enriched in the final phenol-chloroform-extracted fraction. We used FAIRE to extract subtelomeric BES-linked VSG DNAs, whose amount was estimated by quantitative PCR, and the fold change was calculated by dividing the post-TbRAP1 depletion amount by the pre-depletion amount.
Depletion of TbRAP1 for 1.5 days did not cause a significant change in FAIRE values in PRi-pool cells (data not shown), probably owing to insufficient TbRAP1 depletion (35% of TbRAP1 protein was left by day 1, Figure 1A). We therefore performed FAIRE at day 2.5 in PRi-pool and found an 8–19-fold increase in BES-linked VSG DNA and a 4–7-fold increase in mVSG DNA enrichment, which were significantly higher than that in the control cells (Figure 4). To determine whether this effect is telomere-specific, we also examined chromatin structure at several random chromosome-internal gene loci, including Tb927.2.2430, Tb927.2.2440 and Tb09.211.1510. Depletion of TbRAP1 in PF cells led to a 3–4-fold increase in FAIRE-extracted DNAs at these loci, which was mildly but significantly higher than that in the control cells (Figure 4), suggesting that TbRAP1 may have a more global effect on chromatin structure than we anticipated. However, the changes at subtelomeric VSG loci were significantly stronger than that at the control loci (P < 0.05, Figure 4), indicating that the effect of TbRAP1 on chromatin structure is more prominent at subtelomeric loci. The BES promoter regions that are 40–60 kb upstream of the telomere were similarly affected by TbRAP1 depletion as the chromosome internal regions (Figure 4).
Figure 4.
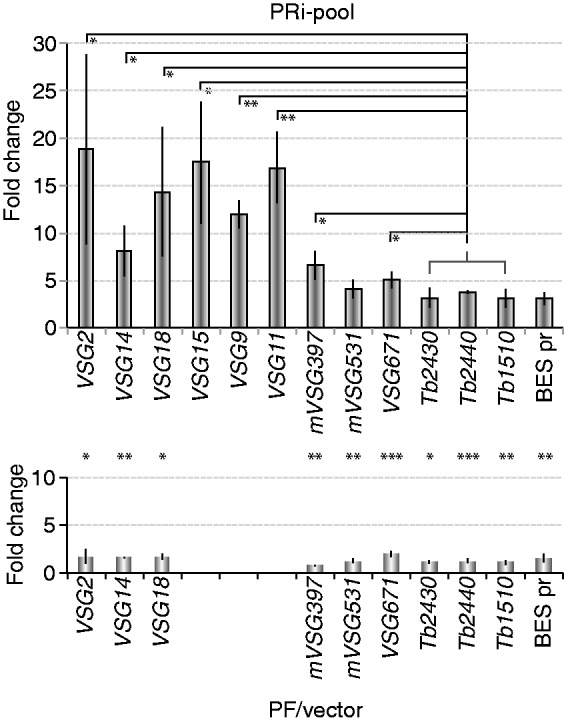
Depletion of TbRAP1 led to loosened chromatin structure, particularly at subtelomeric VSG loci, in PF cells. The amount of FAIRE-extracted DNA after depletion of TbRAP1 for 2.5 days was quantified by PCR using primers specific to indicated gene loci and divided by that obtained before depletion of TbRAP1. The average was calculated from at least three independent experiments. Standard deviations are shown as error bars. Top, results from PRi-pool cells; Bottom, results from control cells. The y-axes of both diagrams are in the same scale. Within the PRi-pool cells, BES-linked VSG FAIRE values were compared with the group of chromosome internal genes, and significant P-values are indicated with asterisks. Between PRi-pool and the control cells, the values for each gene were compared and significant differences are marked with asterisks between the two corresponding columns from the two histograms. Control genes are listed using the last 4 digits of their gene ID. Tb2430: Tb927.2.2430; Tb2440: Tb927.2.2440; Tb1510: Tb09.211.1510. *, 0.01 < P ≤ 0.05; **, 0.001 < P ≤ 0.01, ***, P ≤ 0.001.
To rule out the possibility that the chromatin structure change resulted from elevated transcription rather than directly from loss of TbRAP1, we examined the VSG671 locus, which is located at the subtelomere of a minichromosome (Supplementary Figure S3) and not derepressed on TbRAP1 depletion. Depletion of TbRAP1 led to an ∼5-fold increase in FAIRE extracted VSG671 DNA, which was significantly different from that in control cells (Figure 4), indicating that the VSG671 chromatin structure was affected by TbRAP1, and that this effect was not due to elevated transcription of VSG671.
We subsequently performed the same FAIRE analyses in BF cells in multiple independent TbRAP1 RNAi cell lines (Ri-CT, Ri-BRCT, Ri-2 and Ri-9) at 24 and 36 h after TbRAP1 depletion. A mild increase in the amount of FAIRE-extracted BES-linked, metacyclic and minichromosome VSG DNA was visible in all TbRAP1 RNAi and control cells at both time points (Supplementary Figure S7). A similar change was also observed for the chromosome internal genes and the BES promoter loci (1–3-fold) (Supplementary Figure S7). Unpaired t-test showed that all VSG loci behaved similarly to chromosome internal loci, and all TbRAP1 RNAi cells behaved similarly to the control cells (P > 0.05). Therefore, we did not detect significant changes in chromatin structure on depletion of TbRAP1 at the infectious stage.
To rule out the possibility that inefficient depletion of TbRAP1 was the reason for the lack of significant chromatin structure change in BF TbRAP1 RNAi cells, we examined TbRAP1 protein levels by western analysis. At 24 h after TbRAP1 RNAi induction, the TbRAP1 protein level typically reduced to 6 or 26% of the WT level in Ri-CT and Ri-BRCT cells, respectively (Supplementary Figure S6C). By 36 h, Ri-2 and Ri-9 cells had ∼10% or less TbRAP1 left (Supplementary Figure S6A and B). Although in PRi-pool cells, TbRAP1 was typically depleted to ∼16% of the WT level after 2.5 days of induction (Supplementary Figure S6C). Therefore, the different phenotypes we observed in BF and PF cells did not result from unequal levels of TbRAP1 depletion.
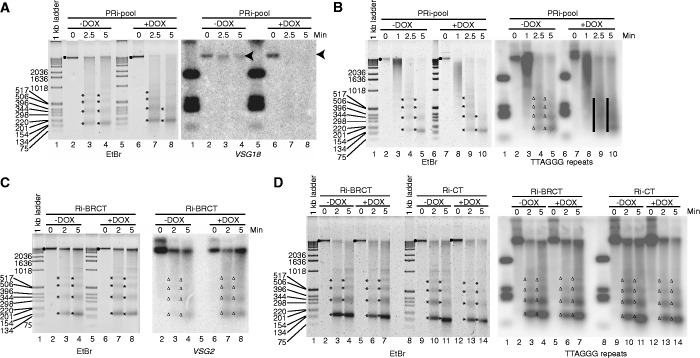
To further validate our conclusion, we examined the accessibility of chromatin by MNase before and after depletion of TbRAP1. PRi-pool cells were induced for 2.5 days and treated with a fixed amount of MNase for an increasing length of time. Subsequently, the genomic DNA was isolated and separated on agarose gel. Without MNase treatment, nearly all of the genomic DNA was in the limiting mobility size range (Figure 5, EtBr, dots). After 2.5–5 min of digestion with MNase, a portion of the chromatin was digested into mono-, di-, tri-nucleosomes and so forth, and the isolated DNA gave a laddering pattern with the smallest bands of ∼150, 350 and 500 bp (Figure 5, EtBr, asterisks). After depletion of TbRAP1, the chromatin was more accessible to MNase digestion, and less material was left undigested while more DNA was in the mono-nucleosome fraction (Figure 5A, lanes 7 and 8; Figure 5B, lanes 9 and 10). The samples from TbRAP1-depleted cells also appeared more smeared, whereas samples from un-induced cells gave sharper bands (Figure 5A, compare lanes 4 and 8; Figure 5B, compare lanes 4 and 9), indicating that in TbRAP1-depleted cells, DNA was less protected by nucleosomes, resulting in more DNA degradation. Depletion of TbRAP1 altered the MNase digestion pattern mildly in ethidium bromide-stained gels, suggesting that TbRAP1 had an effect on chromatin structure at multiple loci in the genome. On the contrary, adding doxycycline to the control cells did not change the MNase digestion pattern (Supplementary Figure S8E and F).
Figure 5.
Depletion of TbRAP1 led to chromatin structure changes in PF cells. Trypanosoma brucei cells were treated with MNase for an increasing length of time (min) before genomic DNA was isolated, separated on agarose gel, blotted onto nylon membranes and hybridized with specific probes. The PRi-pool cells were treated and hybridized with the VSG18 (A) or the TTAGGG repeat probes (B). The Ri-CT and Ri-BRCT cells were treated and hybridized with the VSG2 (C) or the TTAGGG repeat probes (D). Black dots and the arrow head represent limiting mobility in ethidium bromide (EtBr)-stained gels and hybridized blots, respectively. Asterisks and triangles represent mono-, di-, tri- and tetra- nucleosomes on EtBr-stained gels and hybridized blots, respectively. Bars represent smeared DNA without distinctive nucleosome laddering pattern.
To examine chromatin structure at specific loci, we carried out Southern blotting using a VSG18 probe, a VSG2 probe, an mVSG397 probe and a TTAGGG repeat probe. In un-induced cells, most of the VSG18 DNA was not digested by a 5-min treatment of MNase, whereas in TbRAP1-depleted cells, a 2.5-min digestion had already degraded most of the VSG18 DNA (Figure 5A, VSG18 hybridized blot, arrow heads). Depletion of TbRAP1 also had similar effects on the VSG2 (Supplementary Figure S8A), mVSG397 (Supplementary Figure S8C), and the telomere chromatin (Figure 5B, black bars). Furthermore, we observed the same phenotype in PRi-C2 cells but not the control cells (Supplementary Figure S8B, E and F). The chromatin structure at the ES promotor region appears to be only mildly affected by TbRAP1 depletion (Supplementary Figure S8D), similar to what we observed in FAIRE analysis (Figure 4).
We also performed the MNase digestion in BF cells. A normal laddering pattern was observed in ethidium bromide-stained gels after TbRAP1 was depleted, which was similar to that obtained before TbRAP1 depletion (Figure 5C and D; Supplementary Figure S9A and B). No significant difference was visible between un-induced and induced cells when the chromatin structure at the VSG18 (Supplementary Figure S9A), VSG2 (Figure 5C and Supplementary Figure S9B) and the telomere (Figure 5D) loci were examined. Control cells were not affected by doxycycline, either (Supplementary Figure S9C–E). Therefore, no significant change in chromatin structure was detected in BF cells on depletion of TbRAP1.
DISCUSSION
TbRAP1 as a key regulator of VSG silencing at different developmental stages
Trypanosoma brucei has evolved sophisticated mechanisms to regulate VSG expression at different life cycle stages. At the insect stage, silencing both BF VSGs and mVSGs avoid their degradation by proteases in the midgut of tsetse (9). In BF cells, silencing of all mVSGs and all but one BES-linked VSGs is critical for successful antigenic variation and a long-term infection. Recent studies have unveiled several factors that affect BES-linked VSG silencing: Depletion of TbRAP1 in BF cells led to derepression of all BES-linked VSGs for typically several 10-fold (23); deletion of a histone methyltransferase, DOT1b, led to 10-fold higher of BES-linked VSG mRNAs in BF cells (20); depletion of TbORC1, a component of ORC that is involved in DNA replication also led to several fold increase of BES-linked VSG mRNA in BF and PF cells (38). In addition, a number of factors including Imitation Switch (ISWI) (17,39), Nucleoplasmin-Like Protein (NLP) (40), Spt16 (18) and DAC3 (19) are necessary for proper regulation of BES promoter activities without affecting VSG silencing. It is clear that regulation of BES-linked VSG expression involves multiple mechanisms (14,15,17–20,23,36). On the contrary, regulation of mVSG expression appears to be entirely at the transcription initiation level (41,42), which is unusual in T. brucei, where posttranscriptional regulation is predominant (43). However, mVSG silencing is poorly understood except that TbORC1, when depleted, led to mVSG derepression in PF cells (44).
In this study, we found that depletion of TbRAP1 in PF cells has a similar effect as in BF cells—BES-linked VSG mRNA levels were increased several 10- to several 100-fold. In addition, TbRAP1 is also required for mVSG silencing at both BF and PF stages. Therefore, TbRAP1 is required for silencing both BES-linked and mVSGs, the two types of VSG that can be expressed under normal physiological conditions, and TbRAP1 apparently has a much stronger effect on VSG silencing than other known VSG regulation factors.
Regulation of VSG expression/silencing at BF and PF stages
VSGs are expressed in a strictly monoallelic manner in BF cells and are silenced at the PF stage. In this study, using qRT-PCR analysis, we showed that the steady state mRNA levels of several BES-linked VSGs are much lower in PF cells than that in BF cells. A previous study indicated that the 3′ untranslated region of the VSG gene renders its mRNA less stable in PF cells (36), which most likely is the reason for a lower VSG mRNA level in PF cells than in BF cells.
Regardless of the steady–state levels of VSG mRNA in WT BF and PF cells, the changes in VSG mRNA levels induced by depletion of TbRAP1 reflect the silencing effect of TbRAP1. The strength of TbRAP1-mediated silencing at the BES-linked VSG loci is significantly stronger in PF cells than in BF cells. Although we do not have all the proper cell lines to carry out a comprehensive comparison for all BES-linked VSGs, comparison between our current and previous results suggest that most BES-linked VSGs are derepressed at a higher level in PF cells (several 10- to several 100-fold) than in BF cells (several to several 10-fold) (23). However, we noticed that different BES-linked VSGs were derepressed to different levels on depletion of TbRAP1. It is therefore possible that for some VSGs, the derepression levels are at more comparable levels between BF and PF than other VSGs. Importantly, depletion of TbRAP1 affects chromatin structure significantly in PF cells, but not in BF cells, suggesting that modulating chromatin structure in PF cells is a mechanism of TbRAP1-mediated VSG silencing.
TbRAP1 modulates chromatin structure in PF cells
A number of recent studies have shown that modulation of chromatin structure is important for regulation of BES expression in BF cells. First, chromatin structures of the active and silent BESs are dramatically different (21,22). Second, several proteins with known functions in chromatin remodeling, including ISWI (17,39), DAC3 (19), Spt16 (18) and DOT1b (20) have been shown to play important roles in regulation of BES promoter activity. In this study, we showed that depletion of TbRAP1 led to a more loosely packed chromatin structure at all genomic loci tested and more significantly at subtelomeric VSG regions in PF cells, suggesting that TbRAP1-mediated VSG silencing involves modulation of chromatin structure.
Depletion of TbRAP1 in PF cells led to a striking change in the chromatin structure at subtelomeric VSG loci, including the minichromosome VSG671 locus, whereas the VSG671 mRNA level was not affected, indicating that the change in chromatin structure is not a consequence of elevated gene transcription, and that modulation of chromatin structure is a primary effect of TbRAP1. VSG671 was not derepressed, presumably because it lacks a functional upstream promoter. However, for BES-linked VSGs and mVSGs, a loosened chromatin structure most likely is the cause or one of the reasons of their derepression. We noticed that changes in FAIRE values at some BES-linked VSG loci (including VSGs 2, 18, 15, 9 and 11) are slightly higher than that at the VSG671 locus (P < 0.05). As all BES-linked VSGs were strongly derepressed on depletion of TbRAP1, it is possible that the elevated transcription of BES-linked VSGs may induce a further opening of the chromatin structure in addition to the initial effect resulting from the depletion of TbRAP1.
At the insect stage, FAIRE and MNase digestion results indicated that TbRAP1 depletion affects chromatin structure mildly at many loci throughout the genome, which is much broader than we previously anticipated. In both yeast and mammalian cells, RAP1 homologs have been found to locate at non-telomeric regions and act as transcription regulators (45–49), and yeast RAP1 has been found to help determine genome-wide chromatin structure (50). We previously observed that some TbRAP1 appears to localize at loci other than telomeres (23). Therefore, TbRAP1 may have a conserved function as its yeast and mammalian homologs in determination of genome-wide chromatin structure.
To our surprise, we did not detect significant chromatin structure changes in BF cells on TbRAP1 depletion. Our observations suggest that the significantly more severe chromatin structure change and the significantly stronger gene derepression at BESs in PF cells than those in BF cells are linked. Currently, we favor several possible explanations (not necessarily mutually exclusive) for the different phenotypes observed in BF and PF cells. First, the BES chromatin may have different structures in BF and PF cells, and TbRAP1 may be the responsible factor for this difference. An earlier work by Navarro et al. (51) found that T7 polymerase-mediated transcription from a chromosomally integrated T7 promoter is repressed along the entire length of the BES in PF cells, but not in BF cells. Therefore, the chromatin structure at the silent BES appears to be more tightly packed in PF cells. It is possible that TbRAP1 is the factor that maintains a more closed BES structure in PF cells. Second, as BF cells undergo antigenic variation and silent BESs need to be ready to switch to the active state, their silent state is likely metastable, similar to what is often observed for telomeric silencing in yeast (24). Therefore, the BES may switch between the open and closed states more frequently in BF cells than in PF cells. In this case, TbRAP1 may still be involved in determination of chromatin structures in BF cells, but our techniques are not sensitive enough to detect a significant change owing to the fast switching status of the BES. Another possibility is that more factors are involved in modulating BES chromatin structure and BES silencing independent of TbRAP1 at the BF stage. Therefore, depletion of TbRAP1 in BF cells may still affect the chromatin structure, but a lesser degree of change or no quantifiable differences can be detected. Glover and Horn showed that an rRNA promoter-driven reporter gene targeted 5 kb from the telomere in a silent BES was expressed in PF cells, but not in BF cells (52), suggesting that additional factors are involved in silencing BESs in BF cells. A recent study suggested that depletion of histone H1 led to a chromatin opening at multiple genomic loci only in BF cells, but not in PF cells (53), which is also consistent with this hypothesis.
In this study, we further demonstrated that TbRAP1 is a key regulator of VSG expression in T. brucei cells, and that VSG silencing appears to involve chromatin structure. In PF cells, TbRAP1 helps to determine the chromatin structure, particularly at the subtelomeric BES loci, which presumably contributes to TbRAP1-mediated silencing. The effect of TbRAP1 on chromatin structure also appears to be more global than we anticipated.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online: Supplementary Tables 1 and 2, Supplementary Figures 1–9 and Supplementary Methods.
FUNDING
National Institutes of Heath (NIH) [AI066095 to B.L.]. Funding for open access charge: [NIH R01].
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Dr George A. M. Cross for VSG antibodies and Dr Keith Gull for the TAT-1 tubulin antibody. Members of the Li laboratory are acknowledged for their comments on the manuscript and technical support. They give special thanks to Mr. Jie Liu for technical support on the Opticon II computer.
REFERENCES
- 1.Cross GAM. Identification, purification and properties of clone-specific glycoprotein antigens constituting the surface coat of Trypanosoma brucei. Parasitology. 1975;71:393–417. doi: 10.1017/s003118200004717x. [DOI] [PubMed] [Google Scholar]
- 2.Reinitz DM, Mansfield JM. T-cell-independent and T-cell-dependent B-cell responses to exposed variant surface glycoprotein epitopes in trypanosome-infected mice. Infect. Immun. 1990;58:2337–2342. doi: 10.1128/iai.58.7.2337-2342.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barry JD, McCulloch R. Antigenic variation in trypanosomes: enhanced phenotypic variation in a eukaryotic parasite. Adv. Parasitol. 2001;49:1–70. doi: 10.1016/s0065-308x(01)49037-3. [DOI] [PubMed] [Google Scholar]
- 4.Berriman M, Ghedin E, Hertz-Fowler C, Blandin G, Renauld H, Bartholomeu DC, Lennard NJ, Caler E, Hamlin NE, Haas B, et al. The genome of the African trypanosome Trypanosoma brucei. Science. 2005;309:416–422. doi: 10.1126/science.1112642. [DOI] [PubMed] [Google Scholar]
- 5.Gunzl A, Bruderer T, Laufer G, Schimanski B, Tu LC, Chung HM, Lee PT, Lee MG. RNA polymerase I transcribes procyclin genes and variant surface glycoprotein gene expression sites in Trypanosoma brucei. Eukaryot. Cell. 2003;2:542–551. doi: 10.1128/EC.2.3.542-551.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Lange T, Borst P. Genomic environment of the expression-linked extra copies of genes for surface antigens of Trypanosoma brucei resembles the end of a chromosome. Nature. 1982;299:451–453. doi: 10.1038/299451a0. [DOI] [PubMed] [Google Scholar]
- 7.Navarro M, Cross GAM. DNA rearrangements associated with multiple consecutive directed antigenic switches in Trypanosoma brucei. Mol. Cell. Biol. 1996;16:3615–3625. doi: 10.1128/mcb.16.7.3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hertz-Fowler C, Figueiredo LM, Quail MA, Becker M, Jackson A, Bason N, Brooks K, Churcher C, Fahkro S, Goodhead I, et al. Telomeric expression sites are highly conserved in Trypanosoma brucei. PLoS One. 2008;3:e3527. doi: 10.1371/journal.pone.0003527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gruszynski AE, van Deursen FJ, Albareda MC, Best A, Chaudhary K, Cliffe LJ, del Rio L, Dunn JD, Ellis L, Evans KJ, et al. Regulation of surface coat exchange by differentiating African trypanosomes. Mol. Biochem. Parasitol. 2006;147:211–223. doi: 10.1016/j.molbiopara.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Tetley L, Turner CMR, Barry JD, Crowe JS, Vickerman K. Onset of expression of the variant surface glycoproteins of Trypanosoma brucei in the tsetse fly studied using immunoelectron microscopy. J. Cell Sci. 1987;87:363–372. doi: 10.1242/jcs.87.2.363. [DOI] [PubMed] [Google Scholar]
- 11.Barry JD, Graham SV, Fotheringham M, Graham VS, Kobryn K, Wymer B. VSG gene control and infectivity strategy of metacyclic stage Trypanosoma brucei. Mol. Biochem. Parasitol. 1998;91:93–105. doi: 10.1016/s0166-6851(97)00193-x. [DOI] [PubMed] [Google Scholar]
- 12.Ginger ML, Blundell PA, Lewis AM, Browitt A, Gunzl A, Barry JD. Ex vivo and in vitro identification of a consensus promoter for VSG genes expressed by metacyclic–stage trypanosomes in the tsetse fly. Eukaryot. Cell. 2002;1:1000–1009. doi: 10.1128/EC.1.6.1000-1009.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alarcon CM, Son HJ, Hall T, Donelson JE. A monocistronic transcript for a trypanosome variant surface glycoprotein. Mol. Cell. Biol. 1994;14:5579–5591. doi: 10.1128/mcb.14.8.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Navarro M, Gull K. A pol I transcriptional body associated with VSG mono–allelic expression in Trypanosoma brucei. Nature. 2001;414:759–763. doi: 10.1038/414759a. [DOI] [PubMed] [Google Scholar]
- 15.Vanhamme L, Poelvoorde P, Pays A, Tebabi P, Van Xong H, Pays E. Differential RNA elongation controls the variant surface glycoprotein gene expression sites of Trypanosoma brucei. Mol. Microbiol. 2000;36:328–340. doi: 10.1046/j.1365-2958.2000.01844.x. [DOI] [PubMed] [Google Scholar]
- 16.Ansorge I, Steverding D, Melville S, Hartmann C, Clayton C. Transcription of ‘inactive' expression sites in African trypanosomes leads to expression of multiple transferrin receptor RNAs in bloodstream forms. Mol. Biochem. Parasitol. 1999;101:81–94. doi: 10.1016/s0166-6851(99)00060-2. [DOI] [PubMed] [Google Scholar]
- 17.Hughes K, Wand M, Foulston L, Young R, Harley K, Terry S, Ersfeld K, Rudenko G. A novel ISWI is involved in VSG expression site downregulation in African trypanosomes. EMBO J. 2007;26:2400–2410. doi: 10.1038/sj.emboj.7601678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denninger V, Fullbrook A, Bessat M, Ersfeld K, Rudenko G. The FACT subunit TbSpt16 is involved in cell cycle specific control of VSG expression sites in Trypanosoma brucei. Mol. Microbiol. 2010;78:459–474. doi: 10.1111/j.1365-2958.2010.07350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang QP, Kawahara T, Horn D. Histone deacetylases play distinct roles in telomeric VSG expression site silencing in African trypanosomes. Mol. Microbiol. 2010;77:1237–1245. doi: 10.1111/j.1365-2958.2010.07284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Figueiredo LM, Janzen CJ, Cross GAM. A histone methyltransferase modulates antigenic variation in African trypanosomes. PLoS Biol. 2008;6:e161. doi: 10.1371/journal.pbio.0060161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Figueiredo LM, Cross GAM. Nucleosomes are depleted at the VSG expression site transcribed by RNA polymerase I in African trypanosomes. Eukaryot. Cell. 2010;9:148–154. doi: 10.1128/EC.00282-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stanne TM, Rudenko G. Active VSG expression sites in Trypanosoma brucei are depleted of nucleosomes. Eukaryot. Cell. 2010;9:136–147. doi: 10.1128/EC.00281-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang X, Figueiredo LM, Espinal A, Okubo E, Li B. RAP1 is essential for silencing telomeric variant surface glycoprotein genes in Trypanosoma brucei. Cell. 2009;137:99–109. doi: 10.1016/j.cell.2009.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gottschling DE, Aparicio OM, Billington BL, Zakian VA. Position effect at S. cerevisiae telomeres: reversible repression of pol II transcription. Cell. 1990;63:751–762. doi: 10.1016/0092-8674(90)90141-z. [DOI] [PubMed] [Google Scholar]
- 25.Baur JA, Zou Y, Shay JW, Wright WE. Telomere position effect in human cells. Science. 2001;292:2075–2077. doi: 10.1126/science.1062329. [DOI] [PubMed] [Google Scholar]
- 26.Pedram M, Sprung CN, Gao Q, Lo AW, Reynolds GE, Murnane JP. Telomere position effect and silencing of transgenes near telomeres in the mouse. Mol. Cell. Biol. 2006;26:1865–1878. doi: 10.1128/MCB.26.5.1865-1878.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horn D, Cross GAM. A developmentally regulated position effect at a telomeric locus in Trypanosoma brucei. Cell. 1995;83:555–561. doi: 10.1016/0092-8674(95)90095-0. [DOI] [PubMed] [Google Scholar]
- 28.Freitas-Junior LH, Hernandez-Rivas R, Ralph SA, Montiel-Condado D, Ruvalcaba-Salazar OK, Rojas-Meza AP, Mancio-Silva L, Leal-Silvestre RJ, Gontijo AM, Shorte S, et al. Telomeric heterochromatin propagation and histone acetylation control mutually exclusive expression of antigenic variation genes in malaria parasites. Cell. 2005;121:25–36. doi: 10.1016/j.cell.2005.01.037. [DOI] [PubMed] [Google Scholar]
- 29.Duraisingh MT, Voss TS, Marty AJ, Duffy MF, Good RT, Thompson JK, Freitas-Junior LH, Scherf A, Crabb BS, Cowman AF. Heterochromatin silencing and locus repositioning linked to regulation of virulence genes in Plasmodium falciparum. Cell. 2005;121:13–24. doi: 10.1016/j.cell.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 30.Munoz-Jordan JL, Cross GAM, de Lange T, Griffith JD. t-loops at trypanosome telomeres. EMBO J. 2001;20:579–588. doi: 10.1093/emboj/20.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li B, Espinal A, Cross GAM. Trypanosome telomeres are protected by a homologue of mammalian TRF2. Mol. Cell. Biol. 2005;25:5011–5021. doi: 10.1128/MCB.25.12.5011-5021.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giresi PG, Lieb JD. Isolation of active regulatory elements from eukaryotic chromatin using FAIRE (Formaldehyde Assisted Isolation of Regulatory Elements) Methods. 2009;48:233–239. doi: 10.1016/j.ymeth.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wirtz E, Leal S, Ochatt C, Cross GAM. A tightly regulated inducible expression system for dominant negative approaches in Trypanosoma brucei. Mol. Biochem. Parasitol. 1999;99:89–101. doi: 10.1016/s0166-6851(99)00002-x. [DOI] [PubMed] [Google Scholar]
- 34.Kolev NG, Ramey-Butler K, Cross GAM, Ullu E, Tschudi C. Developmental progression to infectivity in Trypanosoma brucei triggered by an RNA-binding protein. Science. 2012;338:1352–1353. doi: 10.1126/science.1229641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Z, Morris JC, Drew ME, Englund PT. Inhibition of Trypanosoma brucei gene expression by RNA interference using an integratable vector with opposing T7 promoters. J. Biol. Chem. 2000;275:40174–40179. doi: 10.1074/jbc.M008405200. [DOI] [PubMed] [Google Scholar]
- 36.Berberof M, Vanhamme L, Tebabi P, Pays A, Jefferies D, Welburn S, Pays E. The 3'-terminal region of the mRNAs for VSG and procyclin can confer stage specificity to gene expression in Trypanosoma brucei. EMBO J. 1995;14:2925–2934. doi: 10.1002/j.1460-2075.1995.tb07292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kyrion G, Liu K, Liu C, Lustig AJ. RAP1 and telomere structure regulate telomere position effects in Saccharomyces cerevisiae. Genes Dev. 1993;7:1146–1159. doi: 10.1101/gad.7.7a.1146. [DOI] [PubMed] [Google Scholar]
- 38.Benmerzouga I, Concepcion-Acevedo J, Kim HS, Vandoros AV, Cross GAM, Klingbeil MM, Li B. Trypanosoma brucei Orc1 is essential for nuclear DNA replication and affects both VSG silencing and VSG switching. Mol. Microbiol. 2013;87:196–210. doi: 10.1111/mmi.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stanne TM, Kushwaha M, Wand M, Taylor JE, Rudenko G. TbISWI regulates multiple polymerase I (Pol I)-transcribed loci and is present at Pol II transcription boundaries in Trypanosoma brucei. Eukaryot. Cell. 2011;10:964–976. doi: 10.1128/EC.05048-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Narayanan MS, Kushwaha M, Ersfeld K, Fullbrook A, Stanne TM, Rudenko G. NLP is a novel transcription regulator involved in VSG expression site control in Trypanosoma brucei. Nucleic Acids Res. 2011;39:2018–2031. doi: 10.1093/nar/gkq950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Graham SV, Barry JD. Transcriptional regulation of metacyclic variant surface glycoprotein gene expression during the life cycle of Trypanosoma brucei. Mol. Cell. Biol. 1995;15:5945–5956. doi: 10.1128/mcb.15.11.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pedram M, Donelson JE. The anatomy and transcription of a monocistronic expression site for a metacyclic variant surface glycoprotein gene in Trypanosoma brucei. J. Biol. Chem. 1999;274:16876–16883. doi: 10.1074/jbc.274.24.16876. [DOI] [PubMed] [Google Scholar]
- 43.Clayton C, Shapira M. Post-transcriptional regulation of gene expression in trypanosomes and leishmanias. Mol. Biochem. Parasitol. 2007;156:93–101. doi: 10.1016/j.molbiopara.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 44.Tiengwe C, Marcello L, Farr H, Dickens N, Kelly S, Swiderski M, Vaughan D, Gull K, Barry JD, Bell SD, et al. Genome-wide analysis reveals extensive functional interaction between DNA replication initiation and transcription in the genome of Trypanosoma brucei. Cell Rep. 2012;2:185–197. doi: 10.1016/j.celrep.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shore D. RAP1: a protean regulator in yeast. Trends Genet. 1994;10:408–412. doi: 10.1016/0168-9525(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 46.Yarragudi A, Parfrey LW, Morse RH. Genome-wide analysis of transcriptional dependence and probable target sites for Abf1 and Rap1 in Saccharomyces cerevisiae. Nucleic Acids Res. 2007;35:193–202. doi: 10.1093/nar/gkl1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang D, Xiong Y, Kim H, He Q, Li Y, Chen R, Songyang Z. Human telomeric proteins occupy selective interstitial sites. Cell Res. 2011;21:1013–1027. doi: 10.1038/cr.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teo H, Ghosh S, Luesch H, Ghosh A, Wong ET, Malik N, Orth A, de Jesus P, Perry AS, Oliver JD, et al. Telomere-independent Rap1 is an IKK adaptor and regulates NF-kappaB-dependent gene expression. Nat. Cell. Biol. 2010;12:758–767. doi: 10.1038/ncb2080. [DOI] [PubMed] [Google Scholar]
- 49.Martinez P, Thanasoula M, Carlos AR, Gomez-Lopez G, Tejera AM, Schoeftner S, Dominguez O, Pisano DG, Tarsounas M, Blasco MA. Mammalian Rap1 controls telomere function and gene expression through binding to telomeric and extratelomeric sites. Nat. Cell. Biol. 2010;12:768–780. doi: 10.1038/ncb2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ganapathi M, Palumbo MJ, Ansari SA, He Q, Tsui K, Nislow C, Morse RH. Extensive role of the general regulatory factors, Abf1 and Rap1, in determining genome-wide chromatin structure in budding yeast. Nucleic Acids Res. 2011;39:2032–2044. doi: 10.1093/nar/gkq1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Navarro M, Cross GAM, Wirtz E. Trypanosoma brucei variant surface glycoprotein regulation involves coupled activation/inactivation and chromatin remodeling of expression sites. EMBO J. 1999;18:2265–2272. doi: 10.1093/emboj/18.8.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glover L, Horn D. Repression of polymerase I-mediated gene expression at Trypanosoma brucei telomeres. EMBO Rep. 2006;7:93–99. doi: 10.1038/sj.embor.7400575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Povelones ML, Gluenz E, Dembek M, Gull K, Rudenko G. Histone H1 Plays a Role in Heterochromatin Formation and VSG Expression Site Silencing in Trypanosoma brucei. PLoS Pathog. 2012;8:e1003010. doi: 10.1371/journal.ppat.1003010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.