Abstract
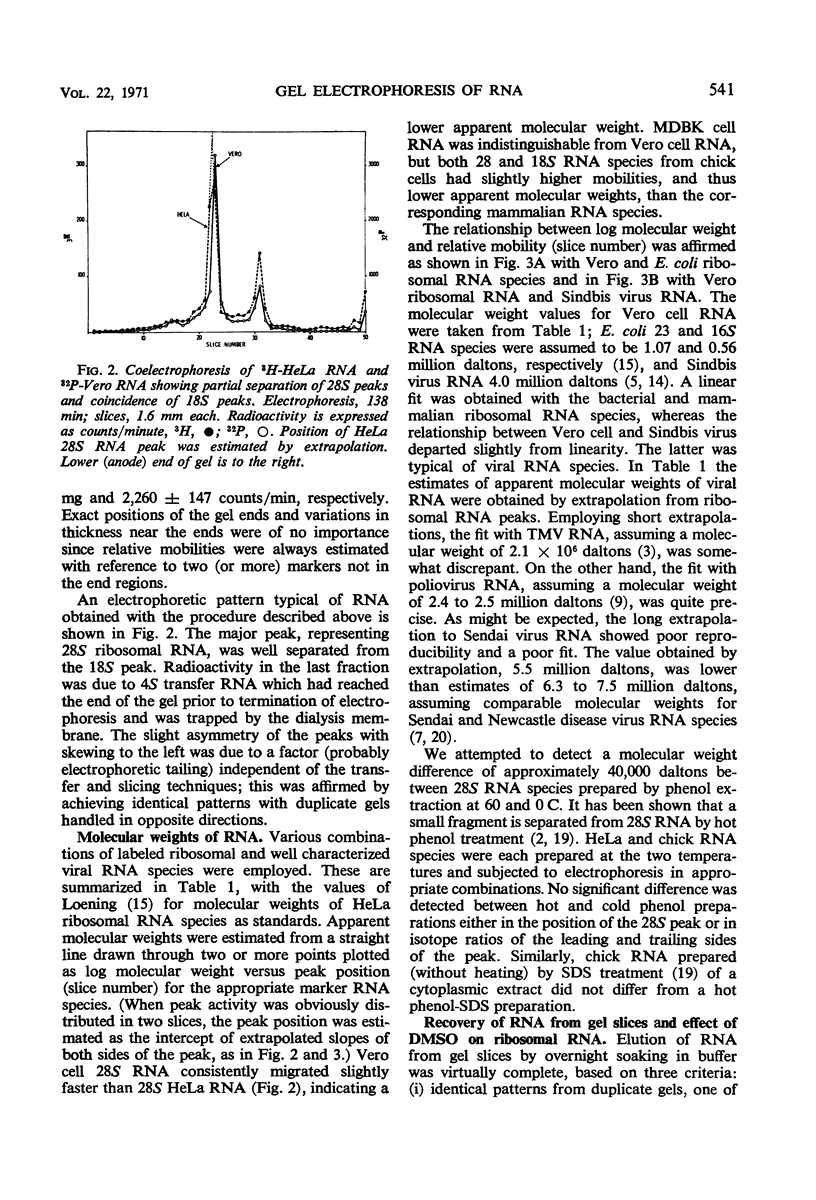
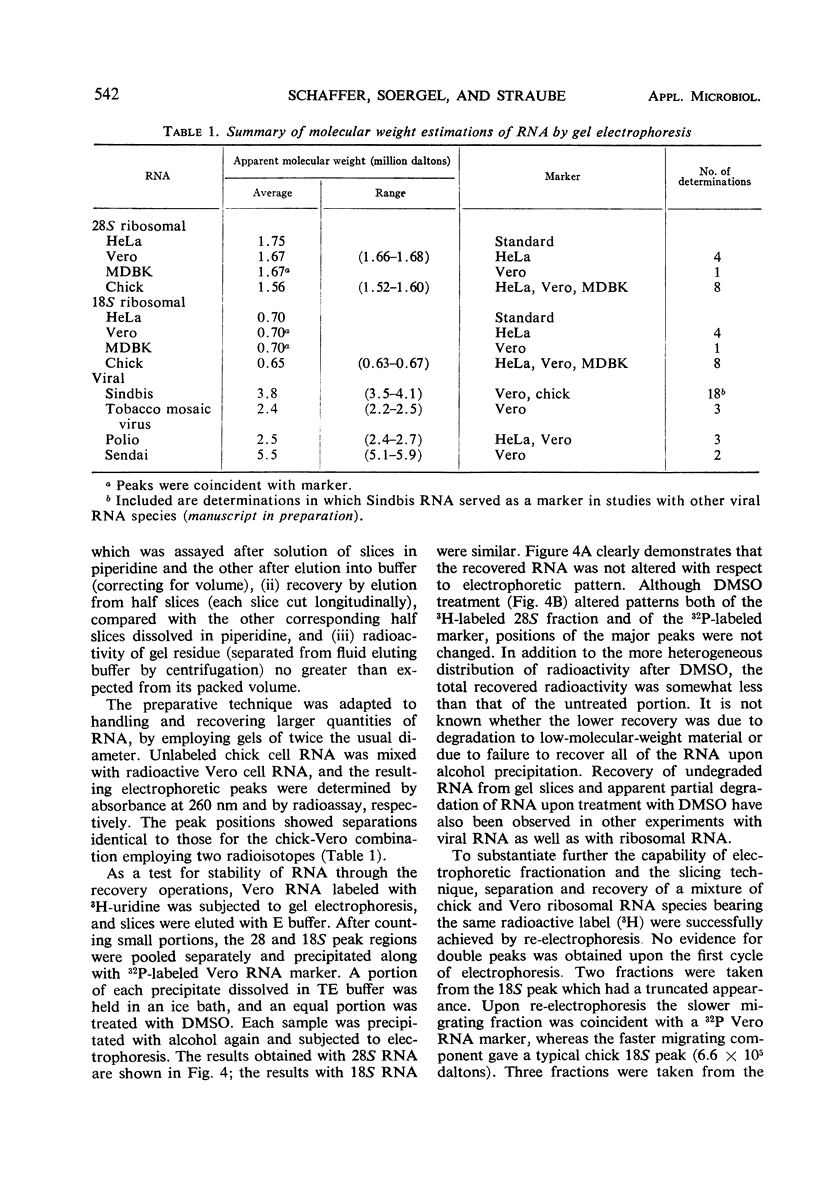
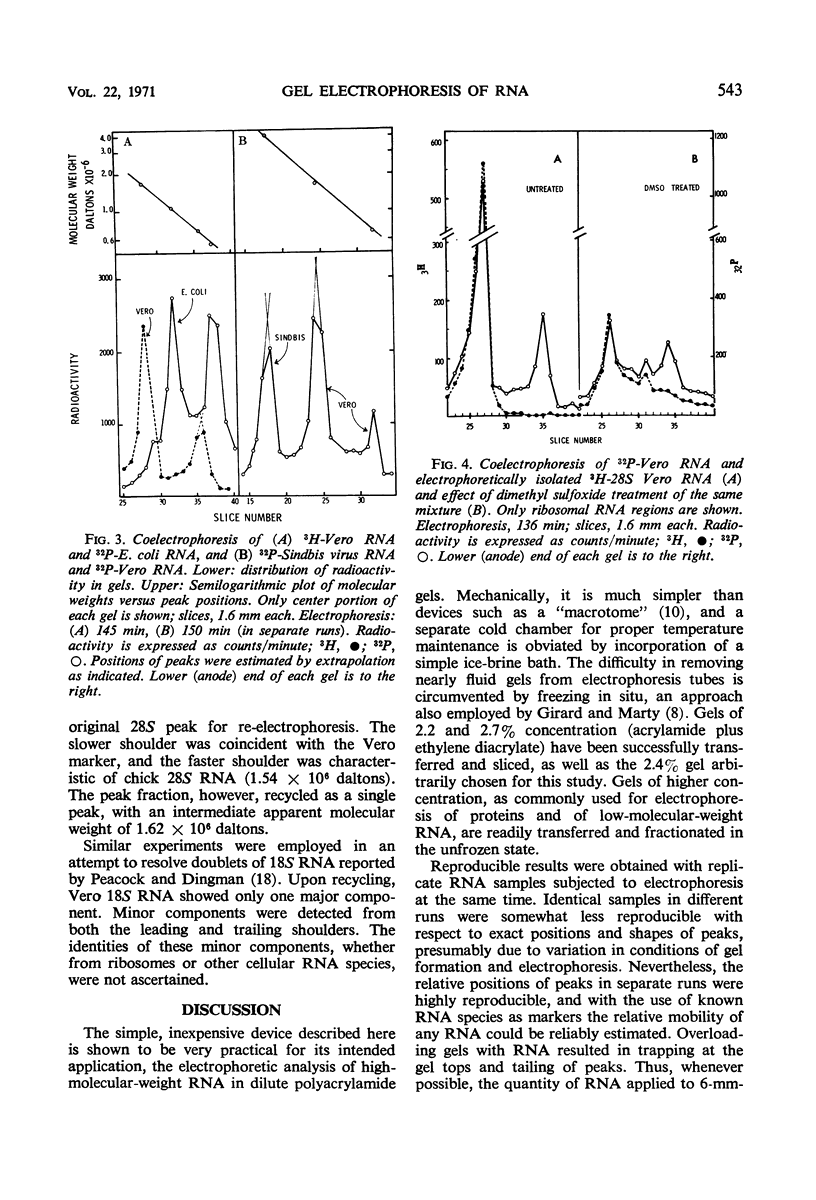
The electrophoretic mobilities of ribosomal ribonucleic acids (RNA) from cultured mammalian (HeLa, Vero, MDBK), avian (chick embryo), and bacterial (Escherichia coli) cells, and RNA species extracted from selected viruses (Sindbis, polio, tobacco mosaic, Sendai) were compared, employing a simple, inexpensive technique for slicing low-concentration polyacrylamide gels. The procedure provides for rapid fractionation of gels used for characterization of RNA, incorporating extrusion and serial sectioning of frozen gels. Among 28S ribosomal RNA species, Vero and MDBK were indistinguishable, whereas HeLA RNA had a slightly lower mobility (higher apparent molecular weight) and chick RNA had a higher mobility (lower apparent molecular weight). The 18S ribosomal RNA species of the three mammalian sources were indistinguishable, but chick 18S RNA had a slightly lower apparent molecular weight. The inverse relation between mobility and log-molecular weight among the ribosomal and viral RNA species, though not highly precise, demonstrates the applicability of the technique to the study of molecular weights of viral RNA species.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop D. H., Claybrook J. R., Spiegelman S. Electrophoretic separation of viral nucleic acids on polyacrylamide gels. J Mol Biol. 1967 Jun 28;26(3):373–387. doi: 10.1016/0022-2836(67)90310-5. [DOI] [PubMed] [Google Scholar]
- Bishop J. M., Levinson W. E., Quintrell N., Sullivan D., Fanshier L., Jackson J. The low molecular weight RNAs of Rous sarcoma virus. I. The 4 S RNA. Virology. 1970 Sep;42(1):182–195. doi: 10.1016/0042-6822(70)90251-5. [DOI] [PubMed] [Google Scholar]
- Boedtker H. Dependence of the sedimentation coefficient on molecular weight of RNA after reaction with formaldehyde. J Mol Biol. 1968 Jul 14;35(1):61–70. doi: 10.1016/s0022-2836(68)80036-1. [DOI] [PubMed] [Google Scholar]
- Clausen T. Measurement of 32P activity in a liquid scintillation counter without the use of scintillator. Anal Biochem. 1968 Jan;22(1):70–73. doi: 10.1016/0003-2697(68)90260-1. [DOI] [PubMed] [Google Scholar]
- Dobos P., Faulkner P. Molecular weight of Sindbis virus ribonucleic acid as measured by polyacrylamide gel electrophoresis. J Virol. 1970 Jul;6(1):145–147. doi: 10.1128/jvi.6.1.145-147.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H. Physical properties of Rous Sarcoma Virus RNA. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1511–1518. doi: 10.1073/pnas.60.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H. The RNA of influenza virus. Proc Natl Acad Sci U S A. 1968 Mar;59(3):930–937. doi: 10.1073/pnas.59.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard M., Marty L. Séparation électrophorétique en gels de polyacrylamide des formes mono, bi, et pluricaténaires de l'acide ribonucléique du poliovirus. Bull Soc Chim Biol (Paris) 1969 Jun 4;51(1):31–45. [PubMed] [Google Scholar]
- Granboulan N., Girard M. Molecular weight of poliovirus ribonucleic acid. J Virol. 1969 Oct;4(4):475–479. doi: 10.1128/jvi.4.4.475-479.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gressel J., Wolowelsky J. A "Macrotome" for polyacrylamide gel and tissue slicing. Anal Biochem. 1968 Feb;22(2):352–354. doi: 10.1016/0003-2697(68)90328-x. [DOI] [PubMed] [Google Scholar]
- HACKETT A. J. A POSSIBLE MORPHOLOGIC BASIS FOR THE AUTOINTERFERENCE PHENOMENON IN VESICULAR STOMATITIS VIRUS. Virology. 1964 Sep;24:51–59. doi: 10.1016/0042-6822(64)90147-3. [DOI] [PubMed] [Google Scholar]
- Hackett A. J., Schaffer F. L., Madin S. H. The separation of infectious and autointerfering particles in vesicular stomatitis virus preparations. Virology. 1967 Jan;31(1):114–119. doi: 10.1016/0042-6822(67)90014-1. [DOI] [PubMed] [Google Scholar]
- Iglewski W. J., Franklin R. M. Denaturation and renaturation of viral ribonucleic acd. II. Characterization of the products resulting from annealing R17 ribonucleic acid with denatured replicative form or with denatured replicative intermediate. J Virol. 1967 Aug;1(4):804–809. doi: 10.1128/jvi.1.4.804-809.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J. G., Friedman R. M. Analysis of arbovirus ribonucleic acid forms by polyacrylamide gel electrophoresis. J Virol. 1971 Apr;7(4):504–514. doi: 10.1128/jvi.7.4.504-514.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening U. E. Molecular weights of ribosomal RNA in relation to evolution. J Mol Biol. 1968 Dec;38(3):355–365. doi: 10.1016/0022-2836(68)90391-4. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The determination of the molecular weight of ribonucleic acid by polyacrylamide-gel electrophresis. The effects of changes in conformation. Biochem J. 1969 Jun;113(1):131–138. doi: 10.1042/bj1130131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Pene J. J., Knight E., Jr, Darnell J. E., Jr Characterization of a new low molecular weight RNA in HeLa cell ribosomes. J Mol Biol. 1968 May 14;33(3):609–623. doi: 10.1016/0022-2836(68)90309-4. [DOI] [PubMed] [Google Scholar]
- SCHERRER K., DARNELL J. E. Sedimentation characteristics of rapidly labelled RNA from HeLa cells. Biochem Biophys Res Commun. 1962 Jun 4;7:486–490. doi: 10.1016/0006-291x(62)90341-8. [DOI] [PubMed] [Google Scholar]
- Schaffer F. L., Gordon M. Differential inhibitory effects of actinomycin D among strains of poliovirus. J Bacteriol. 1966 Jun;91(6):2309–2316. doi: 10.1128/jb.91.6.2309-2316.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer F. L., Hackett A. J., Soergel M. E. Vesicular stomatitis virus RNA: complementarity between infected cell RNA and RNA's from infectious and autointerfering viral fractions. Biochem Biophys Res Commun. 1968 Jun 10;31(5):685–692. doi: 10.1016/0006-291x(68)90616-5. [DOI] [PubMed] [Google Scholar]




