Abstract
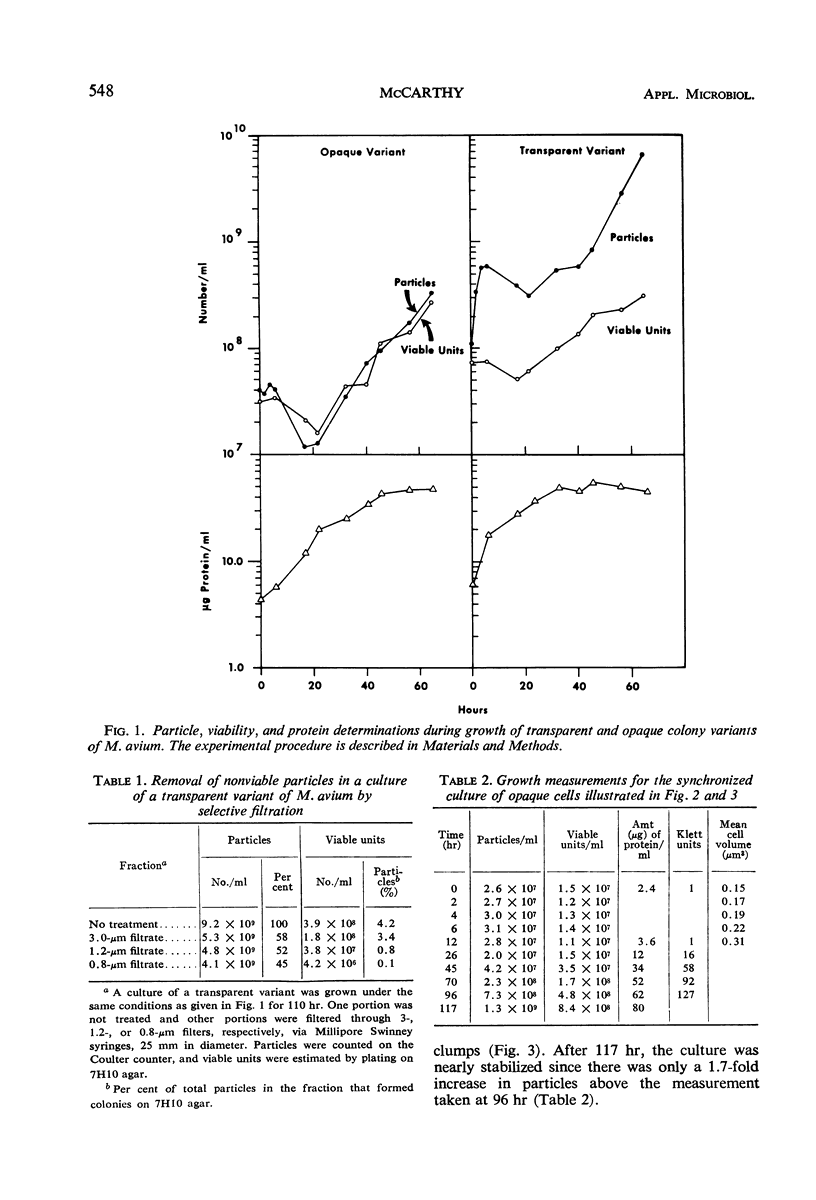
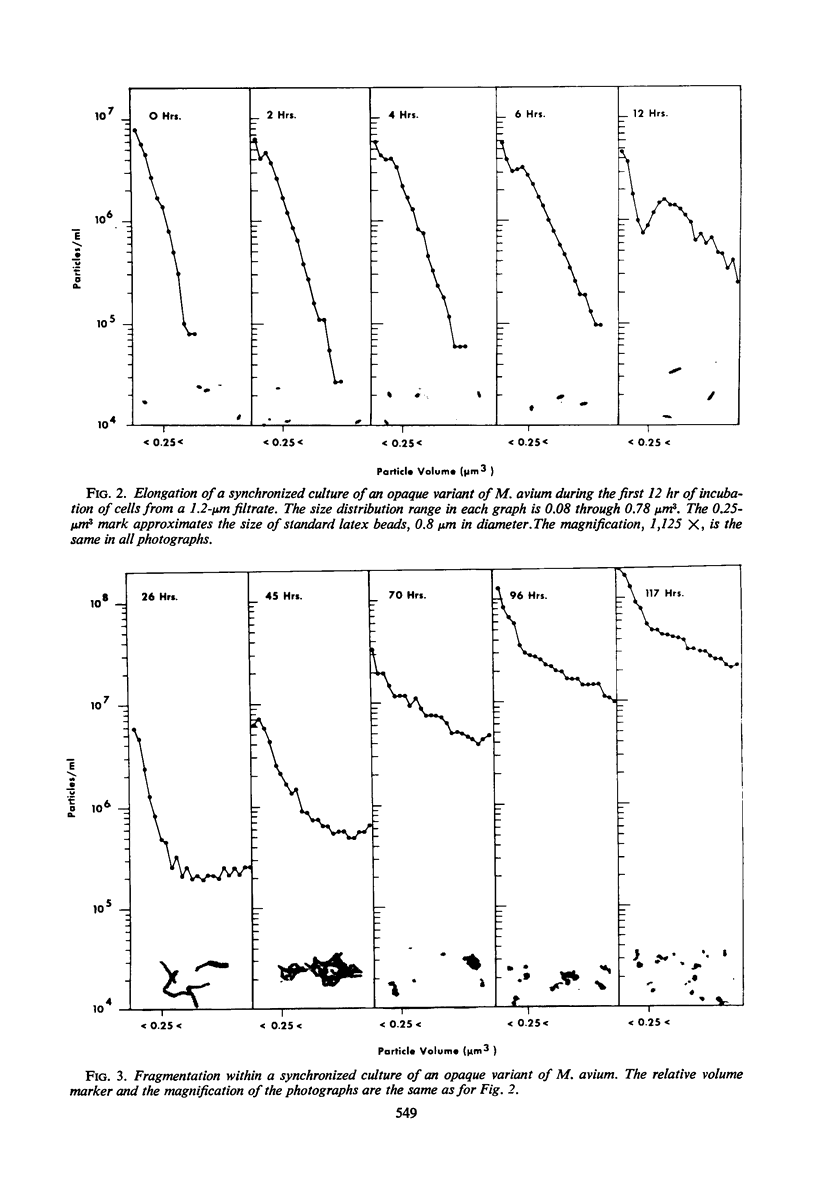
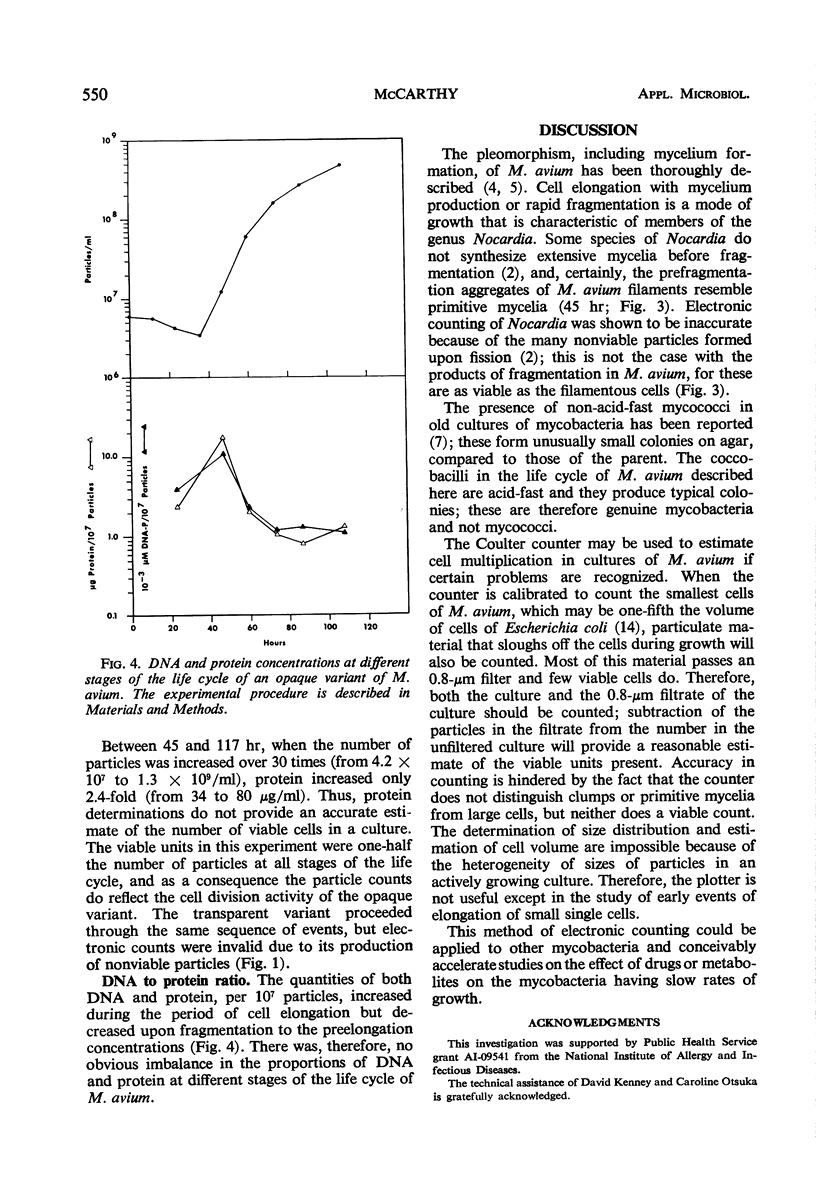
Metabolically uniform cells of Mycobacterium avium were obtained by selective filtration. The life cycle of these cells was followed by photomicrographs, electronic enumeration, and sizing and by viability and protein determinations. The cells elongate to form filaments several times their initial length; the increase in mass is reflected by a five- to sixfold increase of total protein in the culture. The filaments then fragment causing the production of viable coccobacilli. The techniques employed to obtain this form of growth are described and comparisons with nocardial growth are noted.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS J. N., McCLUNG N. M. Comparison of the developmental cycles of some members of the genus Nocardia. J Bacteriol. 1962 Aug;84:206–216. doi: 10.1128/jb.84.2.206-216.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AUBERT E. Cold stain for acid-fast bacteria. Can J Public Health. 1950 Jan;41(1):31–31. [PubMed] [Google Scholar]
- Adams J. N. Studies on the fragmentation of Nocardia erythropolis in liquid medium. Can J Microbiol. 1966 Jun;12(3):433–441. doi: 10.1139/m66-064. [DOI] [PubMed] [Google Scholar]
- BRIEGER E. M., GLAUERT A. M. A phase-contrast study of reproduction in mycelial strains of avian tubercle bacilli. J Gen Microbiol. 1952 Nov;7(3-4):287–294. doi: 10.1099/00221287-7-3-4-287. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csillag A. A simple method to obtain the mycococcus form of Mycobacterium phlei. J Gen Microbiol. 1970 Aug;62(2):251–259. doi: 10.1099/00221287-62-2-251. [DOI] [PubMed] [Google Scholar]
- Engbaek H. C., Vergmann B., Baess I. Non-photochromogenic mycobacteria serotype Davis. The inhomogeneity within the serological group and the relationship to Mycobacterium avium. Acta Pathol Microbiol Scand B Microbiol Immunol. 1970;78(5):619–631. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MIDDLEBROOK G., COHN M. L. Bacteriology of tuberculosis: laboratory methods. Am J Public Health Nations Health. 1958 Jul;48(7):844–853. doi: 10.2105/ajph.48.7.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy C. Spontaneous and Induced Mutation in Mycobacterium avium. Infect Immun. 1970 Sep;2(3):223–228. doi: 10.1128/iai.2.3.223-228.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olitzki A. L., Davis C. L., Schaefer W. B., Cohn M. L. Colony variants of avian-Battey group Mycobacteria intracerebrally injected into mice. Pathol Microbiol (Basel) 1969;34(5):316–323. doi: 10.1159/000162176. [DOI] [PubMed] [Google Scholar]
- Schaefer W. B., Davis C. L., Cohn M. L. Pathogenicity of transparent, opaque, and rough variants of Mycobacterium avium in chickens and mice. Am Rev Respir Dis. 1970 Oct;102(4):499–506. doi: 10.1164/arrd.1970.102.4.499. [DOI] [PubMed] [Google Scholar]
- Shehata T. E., Marr A. G. Synchronous growth of enteric bacteria. J Bacteriol. 1970 Sep;103(3):789–792. doi: 10.1128/jb.103.3.789-792.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestal A. L., Kubica G. P. Differential colonial characteristics of mycobacteria on Middlebrook and Cohn 7H10 agar-base medium. Am Rev Respir Dis. 1966 Aug;94(2):247–252. doi: 10.1164/arrd.1966.94.2.247. [DOI] [PubMed] [Google Scholar]





