Abstract
Probiotics possess potential therapeutic and preventative effects for various diseases and metabolic disorders. One important limitation for the oral delivery of probiotics is the harsh conditions of the upper gastrointestinal tract (GIT) which challenge bacterial viability and activity. One proposed method to surpass this obstacle is the use of microencapsulation to improve the delivery of bacterial cells to the lower GIT. The aim of this study is to use alginate-poly-L-lysine-alginate (APA) microcapsules to encapsulate Lactobacillus fermentum NCIMB 5221 and characterize its enzymatic activity and viability through a simulated GIT. This specific strain, in previous research, was characterized for its inherent ferulic acid esterase (FAE) activity which could prove beneficial in the development of a therapeutic for the treatment and prevention of cancers and metabolic disorders. Our findings demonstrate that the APA microcapsule does not slow the mass transfer of substrate into and that of the FA product out of the microcapsule, while also not impairing bacterial cell viability. The use of simulated gastrointestinal conditions led to a significant 2.5 log difference in viability between the free (1.10 × 104 ± 1.00 × 103 cfu/mL) and the microencapsulated (5.50 × 106 ± 1.00 × 105 cfu/mL) L. fermentum NCIMB 5221 following exposure. The work presented here suggests that APA microencapsulation can be used as an effective oral delivery method for L. fermentum NCIMB 5221, a FAE-active probiotic strain.
Keywords: Lactobacillus fermentum, artificial cells, ferulic acid esterase, microcapsules
1. Introduction
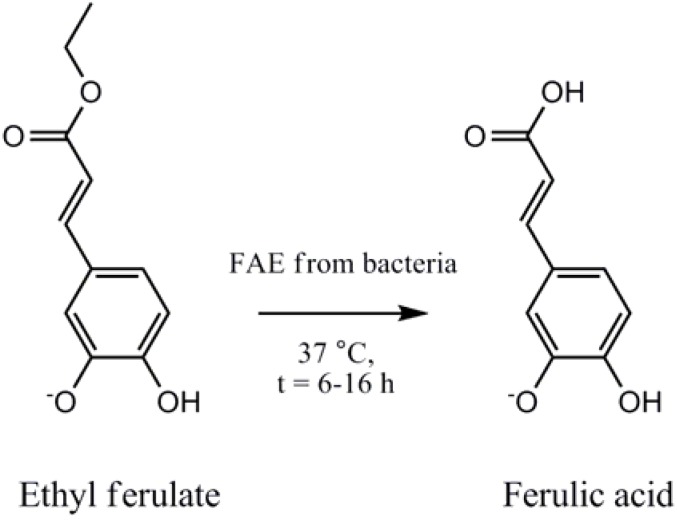
Ferulic acid (FA), a naturally found phenolic acid, is a potent antioxidant able to neutralize free radicals, such as Reactive Oxygen Species (ROS) [1]. ROS have been implicated in DNA damage, cancer and accelerated cell aging [2]. Recent studies suggest that FA has antitumor activity against breast cancer [3,4] liver cancer [5,6] and is effective at preventing cancer induced by the exposure to various carcinogenic compounds such as benzopyrene [7] and 4-nitroquinoline 1-oxide [8]. The oral delivery of free FA is hampered by its quick absorption in the small intestine, specifically in the jejunum, followed by its rapid excretion [9,10]. However, it has been proposed, and shown in previous studies that some GIT bacterial strains produce FAE, an enzyme that has the inherent capability to produce FA from available substrates in the GIT (see Figure 1).
Figure 1.
Ferulic acid esterases (FAE) enable microbes to hydrolyse the ester bond between hydroxyl cinnamic acids and sugars. The hydrolysis of ethyl ferulate by FAE gives rise to ferulic acid a compound with a number of health-promoting benefits.
The oral delivery of conjugated FA, in the form of a dietary source such as wheat bran, is a feasible alternative, with the release of free FA by microbial FAE present in the lower human digestive tract, giving rise to a constant and controlled release of FA [9,10]. The development of probiotic formulations to enhance the FA bioavailability should prove beneficial for the treatment and prevention of inflammatory metabolic disorders [11].Previous research by our group has demonstrated the use of Lactobacillus fermentum NCIMB 5221 as a superior microbial producer of FA [12]. The oral delivery of probiotics, however, is impeded by the harsh conditions of the upper GIT, specifically the presence of bile and a detrimentally acidic pH [13]. Microencapsulation, a method defined as the “entrapment of a compound or a system inside a dispersed material for its immobilization, protection, controlled release, structuration and functionalisation” has been used to overcome the challenge of delivering bacterial cells through the GIT [14]. This article investigates the use of APA microencapsulation for the viable delivery of the FAE active Lactobacillus fermentum NCIMB 5221 to the colon. The results should demonstrate the efficiency of APA microcapsules to increase the viability of this specific probiotic strain in the GIT while preserving enzymatic activity in terms of FA production.
2. Experimental Methods and Materials
2.1. Bacterial Growth Media and Chemicals
Ethyl ferulate (ethyl 4-hydroxy-3-methoxycinnamate, EFA) and ferulic acid (trans-4-hydroxy-3-methoxycinnamate, FA) were purchased from Sigma-Aldrich (Oakville, ON, Canada). De Man, Rogosa, Sharpe (MRS) broth and Methanol of high-performance liquid chromatography (HPLC) grade were obtained from Fisher Scientific (Ottawa, ON, Canada). Water was purified with an EasyPure reverse osmosis system and a NanoPure Diamond Life Science (UV/UF) ultrapure water system from Barnstead (Dubuque, IA, USA). All other chemicals were of analytical or HPLC grade and purchased from commercial sources.
2.2. Bacterial Strains and Culture Conditions
Lactobacillus fermentum NCIMB 5221 was purchased from NCIMB (Aberdeen, Scotland, UK). The bacterial strain was stored at −80 °C in MRS containing 20% (v/v) glycerol. An MRS-agar plate was streaked for isolation from the frozen stock and incubated at 37 °C with 5% CO2 for 24 h to ensure purity. One colony from the MRS-agar plate was inoculated into 5 mL of MRS broth and incubated at 37 °C for 24 h. A 1% (v/v) inoculum was then used for subculturing and incubated at 37 °C for 24 h immediately before use.
2.3. APA Microencapsulation
The microencapsulation of L. fermentum NCIMB 5221 was performed according to the standard protocol, with slight modifications to the flow rate, vibration frequency and voltage [15]. The microcapsules were loaded with 8% (w/v) bacterial pellet. A sodium-alginate solution (1.75% w/v) containing the L. fermentum NCIMB 5221 was extruded into a stirred CaCl2 solution (0.1 M) using a microencapsulator and a 200 μm nozzle (Inotech Corp.). The formed calcium-alginate beads were washed in a physiological solution (PS) followed by their immersion in a poly-L-lysine (PLL) solution (0.1% w/v) for 20 min. Another wash in PS was performed for 5 min followed by immersion in a sodium-alginate solution (0.1% w/v) for 20 min. The resulting microcapsules were stored in minimal storage media at 4 °C for further testing. Viability on microcapsules was performed by exposure to 0.1 M sodium citrate until disruption of the microcapsule was observed. Ten-fold serial dilutions in physiological saline followed by plating on MRS-agar plates were then performed to determine the colony forming units.
2.4. Ferulic Acid Esterase HPLC Assay To Measure FA Production
The bacterial strain was subcultured from MRS broth at 1% (v/v) to MRS-EFA broth at an EFA concentration of 1.33 mM (0.2956 mg/mL). For the APA microcapsules these were added at 10% (w/v) into MRS-EFA broth. Uninoculated MRS-EFA broth was used as a negative control and treated in the exact same way. Each sample was treated in triplicate and incubated at 37 °C during the course of the experiment. An HPLC assay, modified from Mastihuba et al., was used to measure FAE activity [16]. At each time point, 500 μL of sample was added to tubes and centrifuged at 10,000 rpm for 7 min at 4 °C. The resulting supernatant (300 μL) was acidified with 0.35 M H2SO4 (100 μL) and vortexed. 1mM benzoic acid (300 μL) was added, as an internal standard, followed by the addition of 0.7 M NaOH (100 μL) to neutralize the pH. The samples were then stored at −20 °C until all of the samples were collected for the HPLC analysis.
For HPLC analysis, the samples were thawed to room temperature and filtered with a 0.45 μm filter. The analysis was performed on a reverse-phase C-18 column: LiChrosorb RP-18, 25 × 0.46 cm (Grace Davison Discovery Sciences, ON, Canada). The HPLC system consists of a ProStar 335 diode array detector (DAD) set at 280 nm and 320 nm, a ProStar 410 autosampler, and the software Star LC workstation version 6.41. 25 μL was injected for each sample. The mobile phase (solvent A) consists of 37% (v/v) methanol and 0.9% (v/v) acetic acid in water (HPLC grade). Solvent B consisted of 100% (v/v) methanol. The run was initiated with solvent A at 100% for 16 minutes. This was followed by a 1 minute linear gradient to reach 100% of solvent B, attained at the 17th minute. Solvent B was isocratically held at 100% for 12 minutes, until the 29th minute. This was followed by a 1 minute linear gradient to reach 100% of solvent A by the 30th minute. Standard curves of FA and EFA, using peak area quantification, were generated for quantifying the test sample FA and EFA concentrations. The FA standard curve was generated using triplicates and the concentrations 100, 300, 500, 960 and 1,100 μM were plotted against peak area (R2 = 0.9869). The EFA standard curve was generated using triplicates and the concentrations 100, 300, 500, 700, 1,000, 1,400 and 1,800 μM were plotted against peak area (R2 = 0.9785). Standards and quality control samples were prepared and analyzed in the same way as the test samples.
2.5. Simulated Gastrointestinal Conditions to Determine the Stability and Viability of Microencapsulated L. Fermentum NCIMB 5221 Delivered Orally
Simulated gastric (SGF) and intestinal fluids (SIF) were prepared according to the U.S. Pharmacopeia, with some minor modifications [17]. SGF consisted of NaCl (2 g/L), glucose (3.5 g/L) and pepsin (3.2 g/L) in deionized water. The pH of the SGF was adjusted to 1.5 using 2 M HCl. SIF consisted of monobasic potassium phosphate (6.8 g/L), pancreatin (10 g/L), Oxgall (1.5 g/L) and glucose (3.5 g/L) in deionized water. 20 g of APA microcapsules or 2 g of free L. fermentum NCIMB 5221 were added to 200 mL SGF and incubated at 37 °C on a rotary shaker at 75 rpm for 2 h. Following the 2 h, 200 mL of SIF was added into each flask and the pH was increased to 6.8 using 2 M NaOH. At each time point, 1 mL of the solution was sampled into 9 mL of 0.1 M sodium citrate. This was serially diluted in 10-fold dilutions in 0.85% (w/v) NaCl and plated on MRS agar in triplicates. These MRS-agar plates were incubated at 37 °C and 5% CO2, followed by colony counting following 48 h of incubation.
2.6. Statistical Analysis
Values are expressed as means ± Standard Deviation. Statistical analysis was carried out using Minitab (Minitab, Version 14, Minitab Inc., State College, PA, USA). Statistical comparisons between EFA/FA concentrations were carried out by using the general linear model (GLM) and post-hoc analysis. Statistical significance was set at p < 0.05. All interaction terms were treated as fixed terms and p-values less than 0.01 were considered highly significant.
3. Results and Discussion
3.1. Results
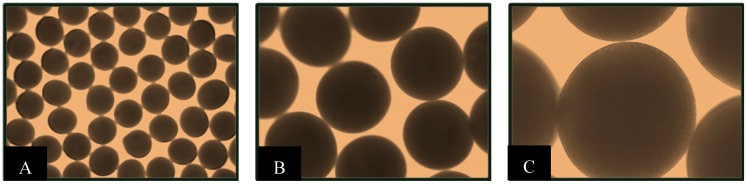
L. fermentum NCIMB 5221 microcapsules were formed to investigate the suitability of the APA microcapsule for the oral delivery of an FAE active probiotic. L. fermentum NCIMB 5221 microencapsulation was optimized—by controlling the flow rate, stirring time, stirring speed, coating time, vibration frequency, and voltage—to obtain monodispersed and spherical microcapsules. The obtained microcapsules were observed under light microscopy at magnifications of 40×, 100× and 200×. The APA microcapsules obtained were monodispersed, as seen in Figure 2A, with an approximate size of 400 ± 25 μm in diameter. Figure 2B demonstrates the spherical shape of the microcapsules and Figure 2C allows for visualisation of the outer coat of the APA microcapsule loaded with L. fermentum NCIMB 5221 at 8% of the total microcapsule weight. The viability of the bacteria prior to microencapsulation, in the alginate solution, was determined to be 1.95 × 109 ± 2.1 × 108 cfu/mL and the microcapsules had a final viability of 1.21 × 109 ± 9.54 × 107 cfu/g, suggesting no significant loss of bacterial viability due to the process of APA microencapsulation.
Figure 2.
Morphology of APA microcapsule containing L. fermentum NCIMB 5221 taken by light microscope (A) 40× (B) 100× (C) 200×. The approximate diameter of the microcapsules was 400 ± 25 μm.
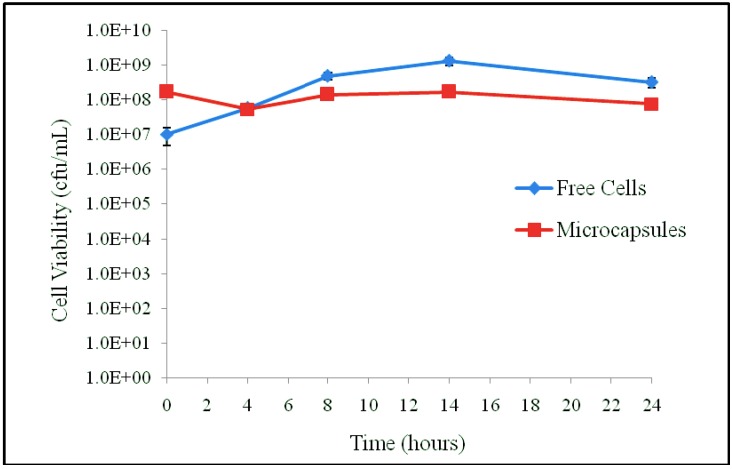
The FAE activity of APA microencapsulated L. fermentum NCIMB 5221 was then determined to ensure that the APA microcapsule does not impede the uptake of the substrate, EFA, and the release of the desired product, FA. For this, the viability of free and microencapsulated L. fermentum NCIMB 5221 during the FAE assay was determined, as can be observed in Figure 3.
Figure 3.
The viability of the free and microencapsulated L. fermentum NCIMB 5221 during the FAE assay (MRS-EFA 0.2956 mg/mL at 37 °C). Each point represents the mean of triplicates and the error bars the standard deviation. During the assay, there was no significant difference in viability between the free and microencapsulated bacteria.
At the start of the assay, the free cells had a viability of 1.53 × 109 ± 9.02 × 107 cfu/mL and the microcapsules a viability of 6.77 × 108 ± 7.77 × 107 cfu/mL. Following 24 h of incubation, the free cells had a viability of 3.27 × 108 ± 1.00 × 103 cfu/mL and the microcapsules a viability of 7.72 × 107 ± 1.00 × 105 cfu/mL.
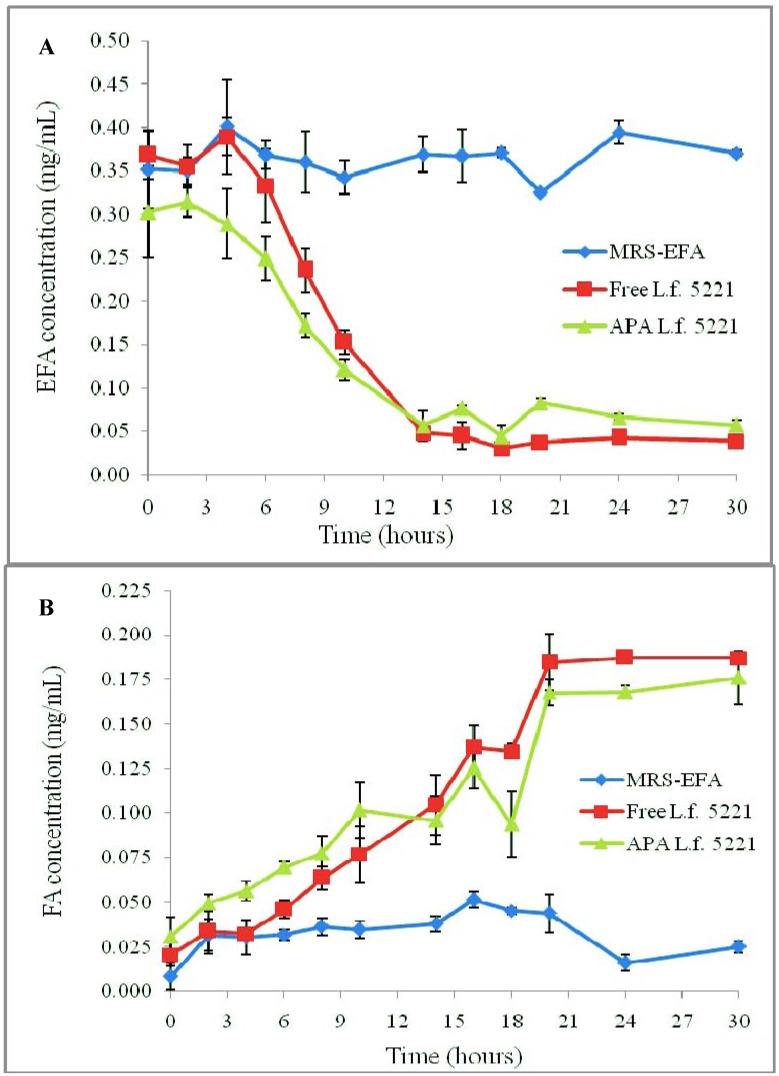
In terms of FAE activity, the initial EFA concentration was 0.2956 mg/mL. Following 30 h of incubation, the free cells had 0.0382 ± 0.0011 mg/mL (12.92 ± 0.37%) and the microcapsules had 0.057 ± 0.0054 mg/mL (19.28 ± 1.83%) EFA remaining in solution, as seen in Figure 4A. At this point, the free cells had a total production of 0.1872 ± 0.0033 mg/mL FA and APA microcapsules had a final production of 0.1760 ± 0.0149 mg/mL FA, as seen in Figure 4B.
Figure 4.
FAE quantitative HPLC assay of free (free L.f. 5221) and microencapsulated L. fermentum NCIMB 5221 (APA L.f. 5221). Uninoculated MRS-EFA was used as a negative control. The presented data represents the amount of unhydrolysed EFA remaining in solution (A) and the amount of FA produced (B), as measured by HPLC peak area data. Each point represents the mean of triplicates and the error bars represent the standard deviations.These results demonstrate no significant difference in FA production between the free and encapsulated L. fermentum NCIMB 5221.
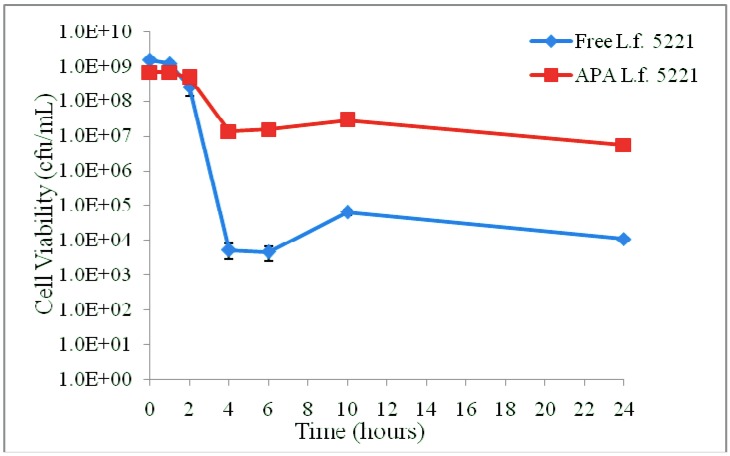
The viability of L. fermentum NCIMB 5221 in free and encapsulated form was determined upon exposure to the simulated gastrointestinal conditions, Figure 5. The initial viability of L. fermentum NCIMB 5221 was 1.53 × 109 ± 9.02 × 107 cfu/mL for the free cells and 6.77 × 108 ± 7.77 × 107 cfu/mL for the microencapsulated cells. Following 2 h of exposure to the simulated conditions of the stomach the viability was 2.60 × 108 ± 1.22 × 108 cfu/mL for the free cells and 4.73 × 108 ± 4.93 × 107 cfu/mL for the encapsulated cells. Following the further exposure to simulated intestinal conditions for 24 h, the free L. fermentum NCIMB 5221 demonstrated a viability of 1.10 × 104 ± 1.00 × 103 cfu/mL and the microencapsulated L. fermentum NCIMB 5221 had a viability of 5.50 × 106 ± 1.00 × 105 cfu/mL. The viability of L. fermentum NCIMB 5221, through the in vitro gastrointestinal passage, and the associated conditions of the gastric and intestinal exposure, are summarized in Table 1. It is also noted that, following the transition to the intestinal conditions, the microcapsules lost some of their integrity due to a change in pH and osmotic conditions.
Figure 5.
The viability of L. fermentum NCIMB 5221 in free and encapsulated form was determined upon exposure to the simulated GI conditions using standard colony counting methods. Each point represents the mean of triplicates and the error bars the standard deviations. A significant difference in viability between free and microencapsulated bacterial cells following simulated GI exposure was observed (p < 0.001; Tukey’s HSD).
Table 1.
The viability of L. fermentum NCIMB 5221, through the in vitro GI passage and the associated conditions of the gastric and intestinal exposure. Free = free L. fermentum NCIMB 5221; APA = microencapsulated L. fermentum NCIMB 5221. The data values represent the mean of triplicates ± SD. These results demonstrate a significant difference in viability between the free and microencapsulated cells (p < 0.001; Tukey’s HSD).
| Time (hours) | GIT section | pH | Solutes | Viability (cfu/mL) | Viability (%) | ||
|---|---|---|---|---|---|---|---|
| Free | APA | Free | APA | ||||
| 0 | Stomach | 1.5 | Sodium chloride Peptic enzymes Glucose | 1.53 × 109 ± 9.02 × 107 | 6.77 × 108 ± 7.77 × 107 | 100.00 ± 0.059 | 100.00 ± 0.115 |
| 1 | 1.18 × 109 ± 2.04 × 108 | 6.83 × 108 ± 4.51 × 107 | 77.51 ± 13.365 | 100.99 ± 6.664 | |||
| 2 | 2.60 × 108 ± 1.22 × 108 | 4.73 × 108 ± 4.93 × 107 | 17.03 ± 7.969 | 69.95 ± 7.290 | |||
| 4 | Small / Large intestines | 6.8 | Potassium phosphate Pancreatic enzymes Bile Glucose | 5.33 × 103 ± 2.52 × 103 | 1.35 × 107 ± 1.12 × 106 | 0.0004 ± 0.0002 | 2.00 ± 0.165 |
| 6 | 4.67 × 103 ± 2.08 × 103 | 1.53 × 107 ± 1.15 × 106 | 0.0003 ± 0.0001 | 2.26 ± 0.170 | |||
| 10 | 6.37 × 104 ± 1.33 × 104 | 2.82 × 107 ± 1.23 × 106 | 0.0042 ± 0.0009 | 4.17 ± 0.182 | |||
| 24 | 1.10 × 104 ± 1.00 × 103 | 5.50 × 106 ± 1.00 × 105 | 0.0007 ± 0.0001 | 0.813 ± 0.015 | |||
3.2. Discussion
Past and recent research has looked at probiotics for use as therapeutics. These formulations are defined as dietary supplements containing bacteria which, when administered in adequate amounts, confer a health benefit on the host [13,18,19]. Probiotics, as natural compounds, are generally considered safe, but can also be tested for set-out safety parameters [20]. A number of studies have investigated bacterial strains for a range of conditions, including infections, allergies and metabolic disorders such as ulcerative colitis and Crohn’s disease [21,22,23,24,25]. Promising research focuses on the microbial secretion and production of beneficial biologically active enzymes and proteins [26,27]. These include the use of ornithine decarboxylase as a powerful antioxidant for the treatment of autoimmune diseases and accelerated cell apoptosis [28], the use of bile salt hydrolase for hypercholesterolemia [29,30,31], and the use of bile transport and tolerance proteins for the efficient delivery of probiotics [32]. In recent studies, the products of, another microbial protein, cinnamoyl esterase, have shown significant levels of antioxidant activity [12,33] and other effects, including stimulation of insulin secretion [34,35], prevention of oxidative stress [33], lipid peroxidation [36], cholesterol-lowering capabilities [11] and inhibition of diabetic nephropathy progression [37]. FA, a well-characterised antioxidant, is one of the desired products of hydrolysis by FAE [12].
Previously we have screened strains for FAE activity and selected L. fermentum NCIMB 5221 as the best FA producer of the investigated Lactobacilli strains [12]. For the development of an efficient probiotic therapeutic formulation there is a requirement for a carrier system. The delivery of bacterial cells through the GIT is impaired by the harsh conditions of the upper GIT. Microencapsulation, specifically APA has been suggested as a method to overcome this obstacle [13]. This method is investigated in the presented research, specifically with relation to L. fermentum NCIMB 5221 and its FA producing capabilities.
This work investigated the FA production of L. fermentum NCIMB 5221 in both the microencapsulated and the free form. The FAE activity of L. fermentum NCIMB 5221 free and encapsulated was determined by HPLC, to ensure that the microcapsule does not hamper the flow of substrate into and the FA product out of the microcapsule. Cell viability was determined during the assay to ensure that the cell count remained equal. Although FA production was non-significantly higher for free cells, this is explained by slightly higher free cell viability during the course of the experiment Figure 3. This is supported by the previous research that directly correlates bacterial cell counts of L. fermentum NCIMB 5221 with its FA production [12]. Keeping this in mind, the higher the cell count delivered to the colon, the higher the FA production. The successful delivery requires a carrier method such as microencapsulation.
Research has previously been presented on the use of microencapsulated cells for FA production, but fails to demonstrate a comparison between free and microencapsulated bacterial cells under the same conditions, as investigated in the presented research [38]. In terms of microencapsulation technology for the storage of microcapsules, Kailasapathy has demonstrated a significant increase in viability of microencapsulated cells in yoghurt cultures stored over 7 weeks, at a pH as low as 4 [39]. However, in terms of oral delivery, a pH of approximately 1.5 is encountered in the stomach. Hence, a microcapsule capable of delivering optimal numbers of bacteria through the GIT needs to sustain viability at such a low pH. In this flask study, the exposure to the simulated gastrointestinal conditions clearly illustrates the requirement for a carrier system when delivering live bacterial cells to the lower GIT Figure 5. A significant 2.5 log difference in viability following exposure to the simulated conditions could be detected between the free 1.10 × 104 ± 1.00 × 103 cfu/mL and the microencapsulated 5.50 × 106 ± 1.00 × 105 L. fermentum NCIMB 5221.
Work is being undertaken to determine the FAE activity of free vs. microencapsulated L. fermentum NCIMB 5221 under GIT conditions. Unfortunately, the simple substrate EFA is labile under the enzymatic and pH conditions used in this study, which quickly resulted in EFA degradation when added to the SGF and SIF solutions. Normally, in the diet, the FA substrate would be present in a more complex form, such as wheat bran, which would permit FA release in the colon due to fermentation processes. This type of food matrix, however, is undefined, rendering it difficult to quantify the low levels of FA that are released. Our research continues to explore the enzymatic process, looking at other in vitro and, potentially, in vivo methods to comprehend the FAE activity in the GIT.
4. Conclusions
This work supports the use of APA microencapsulation for the oral delivery of the investigated probiotic. The presented work successfully demonstrated the advantage of using APA microencapsulation for L. fermentum NCIMB 5221 for use in oral delivery. The FAE enzymatic activity and bacterial viability were maintained post-encapsulation and the viability of encapsulated cells was greater than free cells in simulated gastrointestinal conditions.
Future work should involve further optimisation of the microencapsulation process, since a significant loss of cell count was still evident with the microencapsulated formulation. Additional characterisation of a final formulation, in terms of the mechanisms of action and safety of the probiotic strain, the produced FA, and the use of the microencapsulation with in vitro and in vivo studies should be performed. In terms of the gastrointestinal tract, FAE activity should be characterized in vivo in appropriate animal models to ensure enzyme activity remains stable and efficient under potentially harsh conditions. The fate of the APA microcapsule should also be investigated in vivo. This work, nonetheless, opens up future potentials for the use of a synergistic formulation of microencapsulated L. fermentum NCIMB 5221 with its intrinsic microbial FAE activity for both industrial and therapeutic applications.
Acknowledgments
The authors would like to acknowledge Micropharma Limited grants and a Canadian Institute of Health Research (CIHR) grant (MOP 264308) to Satya Prakash, the support of the Industrial Innovation Scholarship (IIS) BMP Innovation – NSERC, FQRNT and Micropharma Limited to Catherine Tomaro-Duchesneau, a FRSQ Master’s award to Michael Coussa-Charley and a FRSQ Doctoral award to Meenakshi Malhotra.
Conflict of Interest
The authors declare that no financial support or other compensation has been received relating to any aspect of this research or its publication that could be construed as a potential conflict of interest.
References
- 1.Rice-Evans C.A., Miller N.J., Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 2.Lombard D.B., Chua K.F., Mostoslavsky R., Franco S., Gostissa M., Alt F.W. DNA Repair, Genome Stability, and Aging. Cell. 2005;120:497–512. doi: 10.1016/j.cell.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 3.Kampa M., Alexaki V.-I., Notas G., Nifli A.-P., Nistikaki A., Hatzoglou A., Bakogeorgou E., Kouimtzoglou E., Blekas G., Boskou D., et al. Antiproliferative and apoptotic effects of selective phenolic acids on T47D human breast cancer cells: Potential mechanisms of action. Breast Canacer Res. 2003;6:R63–R74. doi: 10.1186/bcr752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang C.J., Chiu J.H., Tseng L.M., Chang C.H., Chien T.M., Wu C.W., Lui W.Y. Modulation of HER2 expression by ferulic acid on human breast cancer MCF7 cells. Eur. J. Clin. Investig. 2006;36:588–596. doi: 10.1111/j.1365-2362.2006.01676.x. [DOI] [PubMed] [Google Scholar]
- 5.Lee Y. Role of NADPH oxidase-mediated generation of reactive oxygen species in the mechanism of apoptosis induced by phenolic acids in HepG2 human hepatoma cells. Arch. Pharm. Res. 2005;28:1183–1189. doi: 10.1007/BF02972984. [DOI] [PubMed] [Google Scholar]
- 6.Taniguchi H., Hosoda A., Tsuno T., Maruta Y., Nomura E. Preparation of ferulic acid and its application for the synthesis of cancer chemopreventive agents. Anticancer Res. 1999;19:3757–3761. [PubMed] [Google Scholar]
- 7.Lesca P. Protective effects of ellagic acid and other plant phenols on benzo[a]pyrene-induced neoplasia in mice. Carcinogenesis. 1983;4:1651–1653. doi: 10.1093/carcin/4.12.1651. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka T., Kojima T., Kawamori T., Wang A., Suzui M., Okamoto K., Mori H. Inhibition of 4-nitroquinoline-1-oxide-induced rat tongue carcinogenesis by the naturally occurring plant phenolics caffeic, ellagic, chlorogenic and ferulic acids. Carcinogenesis. 1993;14:1321–1325. doi: 10.1093/carcin/14.7.1321. [DOI] [PubMed] [Google Scholar]
- 9.Zhao Z., Egashira Y., Sanada H. Digestion and absorption of ferulic acid sugar esters in rat gastrointestinal tract. J. Agric. Food Chem. 2003;51:5534–5539. doi: 10.1021/jf034455u. [DOI] [PubMed] [Google Scholar]
- 10.Spencer J.P.E., Chowrimootoo G., Choudhury R., Debnam E.S., Srai S.K., Rice-Evans C. The small intestine can both absorb and glucuronidate luminal flavonoids. FEBS Lett. 1999;458:224–230. doi: 10.1016/S0014-5793(99)01160-6. [DOI] [PubMed] [Google Scholar]
- 11.Bhathena J., Martoni C., Kumar A., Urbanska A., Malhotra M., Prakash S. Orally delivered microencapsulated live probiotic formulation lowers serum lipids in hypercholesterolemic hamsters. J. Med. Food. 2009;12:310–319. doi: 10.1089/jmf.2008.0166. [DOI] [PubMed] [Google Scholar]
- 12.Tomaro-Duchesneau C., Saha S., Malhotra M., Coussa-Charley M., Al-Salami H., Jones M.L., Labbé A., Prakash S. Lactobacillus fermentum NCIMB 5221 has a greater ferulic acid production compared to other ferulic acid esterase producing Lactobacilli. Int. J. Probiotics Prebiotics. 2012;7 in press. [Google Scholar]
- 13.Prakash S., Tomaro-Duchesneau C., Saha S., Cantor A. The gut microbiota and human health with an emphasis on the use of microencapsulated bacterial cells. J. Biomed. Biotechnol. 2011;2011 doi: 10.1155/2011/981214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poncelet D. Microencapsulation: Fundamentals, methods and applications. In: Blitz J.P., Gun'ko V.M., editors. Surface Chemistry in Biomedical and Environmental Science. Springer; Dordrecht, The Netherlands: 2006. pp. 23–34. [Google Scholar]
- 15.Chang T., Prakash S. Procedures for microencapsulation of enzymes, cells and genetically engineered microorganisms. Mol. Biotechnol. 2001;17:249–260. doi: 10.1385/MB:17:3:249. [DOI] [PubMed] [Google Scholar]
- 16.Mastihuba V., Kremnicky L., Mastihubova M., Willett J.L., Cote G.L. A spectrophotometric assay for feruloyl esterases. Anal. Biochem. 2002;309:96–101. doi: 10.1016/s0003-2697(02)00241-5. [DOI] [PubMed] [Google Scholar]
- 17.U.S. Pharmacopeia. Test Solutions. 2010.
- 18.Prakash S., Rodes L., Coussa-Charley M., Tomaro-Duchesneau C. Gut microbiota: Next frontier in understanding human health and development of biotherapeutics. Biol. Targets Ther. 2011;2011:71–86. doi: 10.2147/BTT.S19099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.FAO and WHO. Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria; 1–4 October 2001.Cordoba, Argentina: Food and Agricultural Organization of the United Nations; [Google Scholar]
- 20.Branton W.B., Jones M.L., Tomaro-Duchesneau C., Martoni C.J., Prakash S. In vitro characterization and safety of the probiotic strain Lactobacillus reuteri Cardioviva NCIMB 30242. Int. J. Probiotics Prebiotics. 2011;6:1–12. [Google Scholar]
- 21.Wolvers D., Antoine J.M., Myllyluoma E., Schrezenmeir J., Szajewska H., Rijkers G.T. Guidance for substantiating the evidence for beneficial effects of probiotics: Prevention and management of infections by probiotics. J. Nutr. 2010;140:698S–712S. doi: 10.3945/jn.109.113753. [DOI] [PubMed] [Google Scholar]
- 22.Jankovic I., Sybesma W., Phothirath P., Ananta E., Mercenier A. Application of probiotics in food products – challenges and new approaches. Curr. Opin. Biotechnol. 2010;21:175–181. doi: 10.1016/j.copbio.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Floch M.H. Probiotic therapy for ulcerative colitis. J. Clin. Gastroenterol. 2010;44:237–238. doi: 10.1097/MCG.0b013e3181cf837f. [DOI] [PubMed] [Google Scholar]
- 24.Al-Salami H., Butt G., Fawcett J., Tucker I., Golocorbin-Kon S., Mikov M. Probiotic treatment reduces blood glucose levels and increases systemic absorption of gliclazide in diabetic rats. Eur. J. Drug Metab. Pharmacokinet. 2008;33:101–106. doi: 10.1007/BF03191026. [DOI] [PubMed] [Google Scholar]
- 25.Luoto R., Laitinen K., Nermes M., Isolauri E. Impact of maternal probiotic-supplemented dietary counselling on pregnancy outcome and prenatal and postnatal growth: A double-blind, placebo-controlled study. Br. J. Nutr. 2010;103:1792–1799. doi: 10.1017/S0007114509993898. [DOI] [PubMed] [Google Scholar]
- 26.Azcarate-Peril M.A., Altermann E., Hoover-Fitzula R.L., Cano R.J., Klaenhammer T.R. Identification and inactivation of genetic loci involved with Lactobacillus acidophilus acid tolerance. Appl. Environ. Microbiol. 2004;70:5315–5322. doi: 10.1128/AEM.70.9.5315-5322.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guglielmetti S., de Noni I., Caracciolo F., Molinari F., Parini C., Mora D. Bacterial cinnamoyl esterase activity screening for the production of a novel functional food product. Appl. Environ. Microbiol. 2008;74:1284–1288. doi: 10.1128/AEM.02093-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mates J.M., Perez-Gomez C., de Castro I.N., Asenjo M., Marquez J. Glutamine and its relationship with intracellular redox status, oxidative stress and cell proliferation/death. Int. J. Biochem. Cell Biol. 2002;34:439–458. doi: 10.1016/S1357-2725(01)00143-1. [DOI] [PubMed] [Google Scholar]
- 29.Martoni C., Bhathena J., Urbanska A.M., Prakash S. Microencapsulated bile salt hydrolase producing Lactobacillus reuteri for oral targeted delivery in the gastrointestinal tract. Appl. Microbiol. Biotechnol. 2008;81:225–233. doi: 10.1007/s00253-008-1642-8. [DOI] [PubMed] [Google Scholar]
- 30.Christopher M., Jasmine B., Mitchell Lawrence J., Aleksandra Malgorzata U., Hongmei C., Satya P. Investigation of microencapsulated BSH active Lactobacillus in the simulated human GI tract. J. Biomed. Biotechnol. 2007 doi: 10.1155/2007/13684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanaka H., Doesburg K., Iwasaki T., Mierau I. Screening of lactic acid bacteria for bile salt hydrolase activity. J. Dairy Sci. 1999;82:2530–2535. doi: 10.3168/jds.S0022-0302(99)75506-2. [DOI] [PubMed] [Google Scholar]
- 32.Pfeiler E.A., Klaenhammer T.R. Role of transporter proteins in bile tolerance of Lactobacillus acidophilus. Appl. Environ. Microbiol. 2009;75:6013–6016. doi: 10.1128/AEM.00495-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Srinivasan M., Sudheer A.R., Menon V.P. Ferulic acid: Therapeutic potential through its antioxidant property. J. Clin. Biochem. Nutr. 2007;40:92–100. doi: 10.3164/jcbn.40.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adisakwattana S., Moonsan P., Yibchok-anun S. Insulin-releasing properties of a series of cinnamic acid derivatives in vitro and in vivo. J. Agric. Food Chem. 2008;56:7838–7844. doi: 10.1021/jf801208t. [DOI] [PubMed] [Google Scholar]
- 35.Sri Balasubashini M., Rukkumani R., Menon V.P. Protective effects of ferulic acid on hyperlipidemic diabetic rats. Acta Diabetol. 2003;40:118–122. doi: 10.1007/s00592-003-0099-6. [DOI] [PubMed] [Google Scholar]
- 36.Balasubashini M.S., Rukkumani R., Viswanathan P., Menon V.P. Ferulic acid alleviates lipid peroxidation in diabetic rats. Phytother. Res. 2004;18:310–314. doi: 10.1002/ptr.1440. [DOI] [PubMed] [Google Scholar]
- 37.Atsuyo F., Hideyuki S., Asako D., Kunihisa O., Shohei M., Hiroto F., Masahiro N., Taisei N., Takuo T., Hisaji T., et al. Ferulic acid prevents pathological and functional abnormalities of the kidney in Otsuka Long-Evans Tokushima Fatty diabetic rats. Diabetes Res. Clin. Pract. 2008;79:11–17. doi: 10.1016/j.diabres.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Bhathena J., Kulamarva A., Martoni C., Urbanska A.M., Prakash S. Preparation and in vitro analysis of microencapsulated live Lactobacillus fermentum 11976 for augmentation of feruloyl esterase in the gastrointestinal tract. Biotechnol. Appl. Biochem. 2008;50:1–9. doi: 10.1042/BA20070007. [DOI] [PubMed] [Google Scholar]
- 39.Kailasapathy K. Survival of free and encapsulated probiotic bacteria and their effect on the sensory properties of yoghurt. LWT - Food Sci. Technol. 2006;39:1221–1227. doi: 10.1016/j.lwt.2005.07.013. [DOI] [Google Scholar]