Abstract
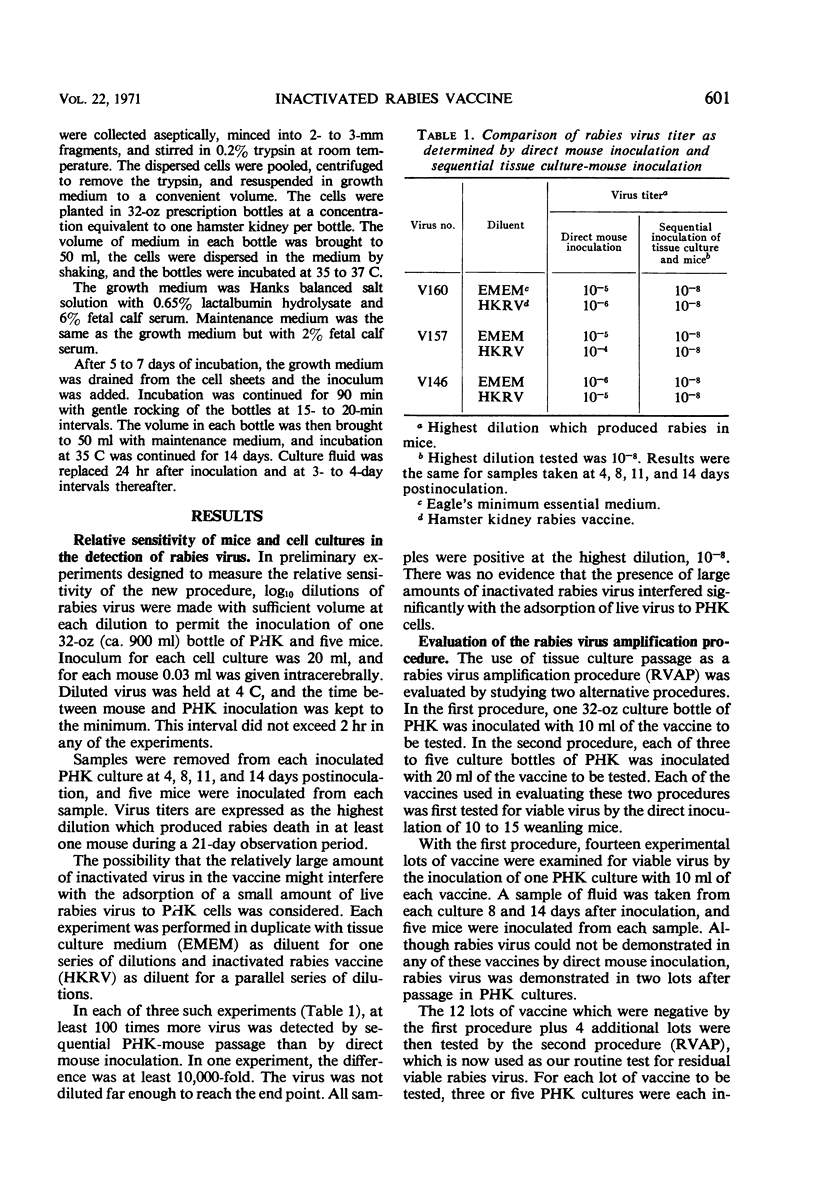
A procedure for testing inactivated rabies vaccines of tissue culture origin for residual viable virus is reported in which the vaccine to be tested is passed in primary hamster kidney cell culture (PHK) before mouse inoculation. In preliminary experiments, titrations of rabies virus in which each dilution was passed in PHK before inoculating mice yielded titers 100 to 10,000 times higher than the titers obtained for the same virus by direct mouse inoculation. This rabies virus amplification procedure was evaluated by testing 18 lots of inactivated rabies vaccine of tissue culture origin. No viable virus was found in these vaccine lots when tested by direct intracerebral inoculation of mice. Eight of these 18 lots were found to contain viable virus, however, when tested by passage in PHK cell culture. The significance of low levels of viable virus in rabies vaccines is discussed. It is recommended that the amplification procedure described in this report be used in the safety testing of rabies vaccines of tissue culture origin and that it be evaluated for use in testing other rabies vaccines of low tissue content.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- KISSLING R. E. Growth of rabies virus in non-nervous tissue culture. Proc Soc Exp Biol Med. 1958 Jun;98(2):223–225. doi: 10.3181/00379727-98-23997. [DOI] [PubMed] [Google Scholar]


