Abstract
Object
The authors conducted a phase I study of late infantile neuronal ceroid lipofuscinosis using an adeno-associated virus serotype 2 (AAV2) vector containing the deficient CLN2 gene (AAV2CUhCLN2). The operative technique, radiographic changes, and surgical complications are presented.
Methods
Ten patients with late infantile neuronal ceroid lipofuscinosis disease each underwent infusion of AAV2CUhCLN2 (3 × 1012 particle units) into 12 distinct cerebral locations (2 depths/bur hole, 75 minutes/infusion, and 2 μl/minute). Innovative surgical techniques were developed to overcome several obstacles for which little or no established techniques were available. Successful infusion relied on preoperative stereotactic planning to optimize a parenchymal target and diffuse administration. Six entry sites, each having 2 depths of injections, were used to reduce operative time and enhance distribution. A low-profile rigid fixation system with 6 integrated holding arms was utilized to perform simultaneous infusions within a practical time frame. Dural sealant with generous irrigation was used to avoid CSF egress with possible subdural hemorrhage or altered stereotactic registration.
Results
Radiographically demonstrated changes were seen in 39 (65%) of 60 injection sites, confirming localization and infusion. There were no radiographically or clinically defined complications.
Conclusions
The neurosurgical considerations and results of this study are presented to offer guidance and a basis for the design of future gene therapy or other clinical trials in children that utilize direct therapeutic delivery.
Keywords: storage disease, lipofuscinosis, gene therapy, local delivery
Gene therapy has long held the great potential to provide novel treatment options for neurological diseases. While the initial promise of this general field was tempered by slow progress, in recent years there has been a resurgence in the enthusiasm for gene therapy. This revival has been led by several new human clinical trials for a variety of neurodegenerative disorders including Parkinson, Alzheimer, and Canavan disease.2,5,9,14,15,28 One of the unique limitations of CNS gene therapy is the blood-brain barrier, which prevents current-generation genetic vectors from entering the brain from the bloodstream. Therefore, a feature common to all CNS gene therapy clinical trials to date is the need to neurosurgically infuse viral vectors harboring the potential therapeutic gene directly into 1 or more planned brain targets. Direct parenchymal injections in some childhood brain tumors are also being explored and simulate some of the technical considerations in gene therapy.12 Because no commercial system is currently optimized for direct infusions into brain parenchyma, each trial has incorporated different infusion equipment and methods developed by the individual investigators. Although some of the neurosurgical methodology has been briefly described in past reports, detailed discussions of the specific neurosurgical problems addressed in any study have been lacking. Since these issues can be limiting to the success of even very promising new gene therapies, a detailed analysis could significantly affect the future development and widespread use of this technology in the human brain.
We recently conducted a phase I study for the treatment of LINCL using an AAV2 vector containing the deficient CLN2 gene (AAV2CUhCLN2).24,29 Ten children underwent an innovative and hitherto undefined neurosurgical procedure aimed at administering AAV2CUh-CLN2 in a safe and potentially effective manner. In the design of a neurosurgical approach for these children, we used our experience with human CNS gene therapy but also accounted for several unique features of this specific disorder, including the demand for a large-scale target, the presence of global cerebral atrophy, the need for simultaneous infusions at multiple sites, and the potential for large-volume CSF egress. We describe our approach and the implementation of the neurosurgical techniques in this trial. Our results highlight the importance of early involvement and close collaboration of neurosurgeons with other physicians and scientists not only in the execution but also in the design of new therapeutic approaches and their translation into human clinical trials.
Methods
Ten children were selected to participate in an openlabel phase I study of gene therapy for the treatment of LINCL. The study background, selection criteria, AAV vector production methods, and results have been reported in detail.1,3,4,17,22,24,29 The study was completed at the New York Presbyterian Hospital, Weill Cornell Medical College, Cornell University. The research protocol was reviewed and approved by the Weill Cornell Institutional Review Board, Institutional Biosafety Committee, NIH/General Clinical Research Center Pediatric Scientific Advisory Committee, and Data Safety Monitoring Board. The protocol was also reviewed by the NIH/Recombinant DNA Advisory Committee and an Investigational New Drug application was approved by the Center for Biologics Evaluation and Research, US FDA (BBIND 11481). The parents of all participating children provided informed consent.
Preoperative Imaging and Target Planning
General anesthesia was induced in each child, and preoperative MR imaging of the brain was performed for stereotactic planning. Regardless of age, all patients required anesthesia for imaging given their developmental regression. Entry site and trajectory planning was emphasized given the exaggerated degree of cerebral atrophy in this patient population. From the preoperative MR imaging studies, entry sites were selected on a BrainLAB workstation (BrainLAB USA) with 2 principal goals. The first major objective was to achieve a diffuse distribution of the injected virus over the supratentorial compartment. In an effort to realize such spatial orientation, 3 entry sites over each cerebral hemisphere were selected. Bur holes were spaced with the goal of 1 bur hole each at the frontal pole, the midfrontal region, and the parietal-occipital region (Fig. 1). The second major goal in selecting entry sites was to avoid routes into or through a sulcus, thus minimizing vascular injury and subarachnoid instillation of vector (Fig. 2). Given the degree of atrophy in these patients, this second goal was generally limiting in determining bur hole sites and catheter trajectories, particularly when the desire to avoid traversing eloquent cortex was also considered.
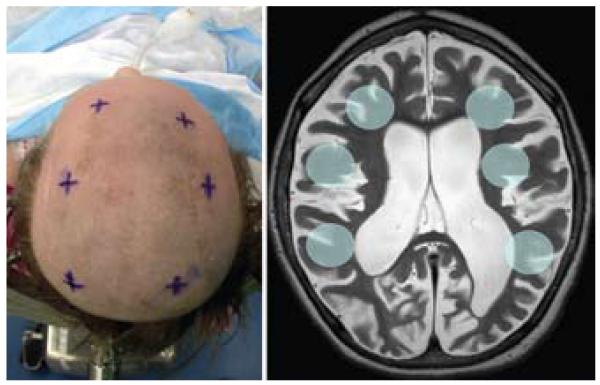
Fig. 1.
Left: Intraoperative image of entry sites selected with preoperative stereotactic planning. Right: Axial T2-weighted MR image demonstrating the typical brain of a patient with Batten disease. Highlighted circles represent the 6 proposed target areas for CLN2 AAV2 vector infusion.
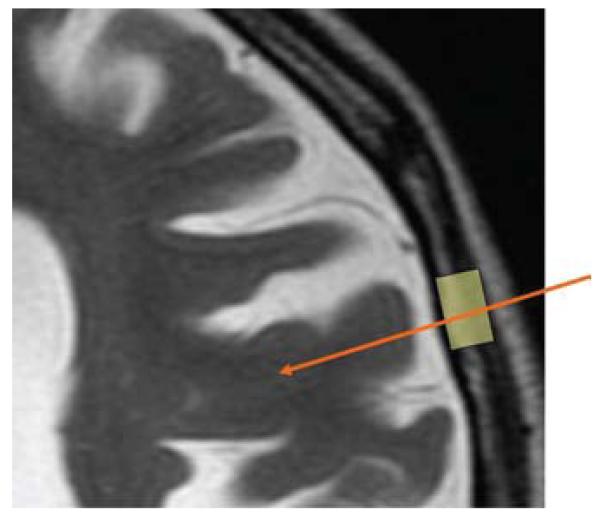
Fig. 2.
Simulated planning for bur hole position (yellow highlight) and cannula trajectory (orange arrow). Planning is conducted with the intent to avoid sulci, whose dimensions are exaggerated in patients with LINCL.
Frameless Navigation and Entry Site Localization
From the MR imaging suite, the child was transported to the operating room, and rigid fixation was applied using a Sugita head frame (Mitzuho Medical, Inc.)—a head frame that provides a secure mount, maintains a low profile over the cranial convexities, and provides access to most of the cranial vault. In anticipating the sites of the bur holes, fixation of the frame was low, based on the inferior temporal-occipital regions, and was angled to be particularly low posteriorly to facilitate placement of the parietal-occipital bur hole (Fig. 3). A single dose of a prophylactic antibiotic was administered: cefazolin 25 mg/kg, maximum of 1 g. Steroids, hyperosmolar agents, and hypocarbia were avoided in attempt to minimize cerebral relaxation, which would likely exacerbate CSF loss in the setting of severe atrophy.
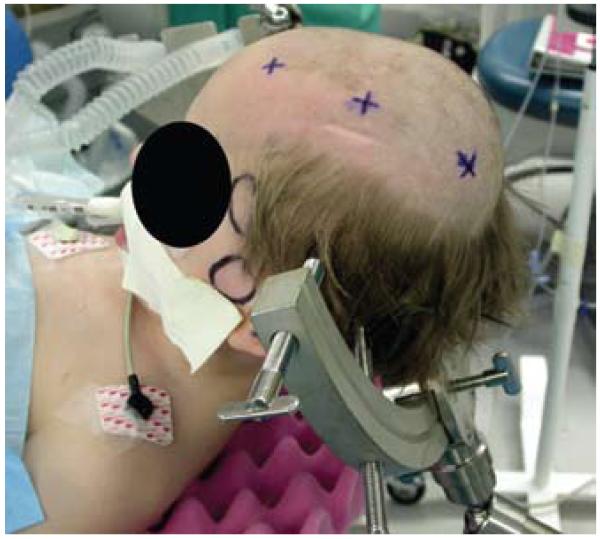
Fig. 3.
Photograph featuring the position of the head frame, secured with 4 points of fixation. The frame is positioned inferiorly on the temporal and occipital bones to allow access to the cranial vault overlying the sites of planned injection.
The patient was registered to the preoperative MR image using a minimum of 5 fiducial markers. Preplanned entry sites were marked on the scalp, and surgical preparations were made. A local anesthetic (0.25% bupivacaine with 1/100,000 parts epinephrine) was administered at each planned entry site. After skin incisions for each bur hole, Alm self-retaining retractors (Miltex, Inc.) were used to maximize access while minimizing obstruction to the workspace. Bur holes were created using an 11-mm perforator (Codman). A bur hole was used instead of a twist drill given the preference for visual verification of a gyrus without a major intervening vascular structure during cannula placement. Dural opening was performed only at the time of cannula placement and was sequenced so that the posterior locations were accessed last in an effort to decrease gravity-dependent CSF loss. A cruciate dural incision and subsequent bipolar coagulation were used for intradural access. The operating microscope was used for greater precision during dural opening to minimize the likelihood of premature violation of the arachnoid and attendant CSF loss.
Catheter Insertion and AAV Infusion
For each site, a 20-gauge spinal needle was used as a rigid guide for insertion of a flexible, fused silica polyimide-coated catheter (inner diameter approximately 150 μm and outer diameter 362 μm, Polymicro Technologies; Fig. 4). Each catheter was connected to a separate syringe, thus maintaining an independent system for each infusion. Before insertion of the spinal needle, the catheter was inserted until its tip was flush with the needle tip. The catheter was then marked with a marking pen at the top of the needle as a measure of the point of interface between the catheter and the brain prior to deeper insertion. Using the operating microscope, the pial surface overlying a gyrus was coagulated. The spinal needle was affixed to a flexible retractor arm of the head frame and oriented to match the predetermined stereotactic trajectory based on the preoperative plan. The guide needle itself was not tracked given the small size and number of infusion sites; rather, the trajectories in nearly all cases were chosen to be orthogonal to the cortical surface. Where an orthogonal tract could not be generated—that is, one that would not traverse a subarachnoid space and would conform to the predetermined safety and efficacy criteria—an angled trajectory was used, and the angle of guide needle insertion was visually determined to match the presurgical plan. Retractor arms and needles were all initially set to the planned orientation prior to skin opening but after fiducial registration to ensure that the orientation of the head frame relative to the planned entry sites would permit proper localization and locking of the arm without excessive torque or tension. The needle was then inserted just below the pial surface, and the retractor arm was secured with rigid fixation. The infusion system was run to flush out the dead space and confirm flow of the vector solution, until a drop of vector solution was seen at the tip of the glass catheter. The droplet was removed with sterile gauze to ensure that gene therapy vector did not flow onto the cortical surface. The guide needle stylet was removed, and a borosilicate catheter was advanced to the initial target depth, which was chosen to be just deep to adjacent sulci in the white matter to facilitate spread to distant structures. Generally, this distance was 2 cm from the cortical surface, with a range of 1.7–2.5 cm. This target depth was based on the zero reference being defined by the visualized cortical surface rather than the preoperative MR image given some expectation of brain shift with dural opening. Each catheter for each site was marked with a folded Steri-strip at the distance from the initial zero mark that would place the tip at the desired depth from the cortical surface. The Steri-strip also served as a stop to hold the glass catheter in place during the infusion.
Fig. 4.

Photograph of a 20-gauge spinal needle with the fused silica catheter prior to stereotactic insertion. The spinal needle serves as a linear guide and a protective sheath for rigid fixation.
Following catheter placement, the entry site was sealed with a thrombin-soaked absorbable gelatin sponge (Pfizer, Inc.) and fibrin sealant (Baxter) to minimize CSF egress (Fig. 5). Five minutes after cannulation, 150 μl of 2 × 109 packaged AAV2 units/μl was injected by a microperfusion pump (Hamilton) at 2 μl/minute to each of the 6 sites in parallel (Fig. 6). Five minutes after completion of the infusion, to minimize backflow along the tract, catheters were retracted 1 cm, a new Steri-strip was used to hold the catheter in place, and the injections were repeated. The goal of the second set of infusions was to maximize infusion within the gyrus just deep to the bur hole. These 2 sets of infusions resulted in 2 infusions through each of the 6 bur holes, for a total administration of 1800 μl (3.6 × 1012 particle units). After an additional 5 minutes to again prevent backflow, catheters were removed, the subdural space was generously irrigated, fibrin sealant was applied to the dural opening, and the wounds were closed in a multilayered fashion.
Fig. 5.
Photograph of an example of an entry site, highlighting the low-profile retractor system, fixation of the spinal needle, and fibrin sealant.
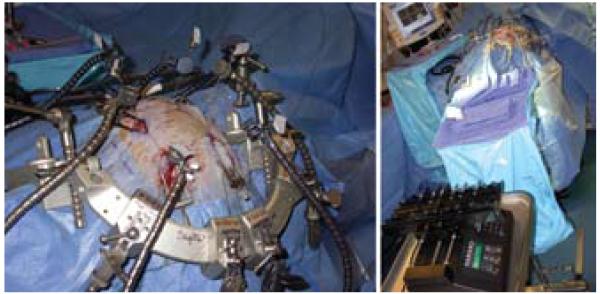
Fig. 6.
Intraoperative images depicting simultaneous infusion through 6 cannulas fixed in place with modified retractor arms of an integrated head holder (left) and the relative positions of the multisyringe microinfusion pump and sterile operative field (right).
Postoperative Imaging
Postoperative MR imaging with FLAIR and gradient relaxed echo sequences was performed within the first 24 hours after the surgical procedure (Fig. 7). Each child was monitored in a pediatric intensive care unit for a minimum of 24 hours postoperatively and then transferred to the Children’s Clinical Research Center unit of the Weill Cornell Medical College. The children were discharged from the hospital at the discretion of the clinical team, typically within 7 days after vector administration.
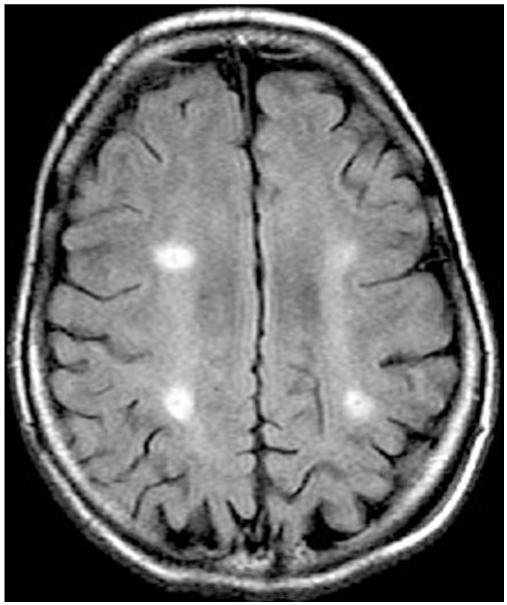
Fig. 7.
Representative postoperative axial FLAIR MR image demonstrating areas of infusion as hyperintense signal change.
Results
Ten patients underwent the vector infusion procedure without perioperative complication. In total, there were 60 cannula insertions and 120 injections. All planned bur hole sites in all patients were localized with adequate precision using BrainLAB frameless navigation. In 1 case, initial orientation of the Sugita head frame had to be adjusted after initial testing given the potential for steric hindrance of the posterior retractor arms, which created a concerning level of torque and/or prevented locking of the arm. After the frame was adjusted and the fiducial markers were reregistered in this case, the issue was resolved prior to skin opening, the planned entry sites were localized, and the retractor arms were able to lock comfortably. All catheters remained at the desired depth with no inadvertent migrations throughout each infusion.
Precise targeting and evidence of hemorrhage were evaluated on postoperative MR imaging. Target site infusion was measurably achieved in 65% of sites (39 of 60 sites), based on increased FLAIR signal in the region of the planned target. Each of these “positive” sites demonstrated a FLAIR change of at least 0.5 cm, which likely represented a concentrated fluid signal from the infusion rather than a tissue reaction to a 150-μm, biologically inert glass catheter. Particular to the anatomical site, the lowest rate of evident injection was 50.0%, whereas the highest rate was 90.0% (Table 1). There were no postoperative infections or clinically relevant hemorrhages. There was no perioperative morbidity or death related to the surgery.
TABLE 1. Postoperative radiographic evidence of successful infusion.
| Infusion Site | Postop Radiographic Success Rate |
|---|---|
| It anterior frontal | 6/10 |
| rt anterior frontal | 5/10 |
| It posterior frontal | 9/10 |
| rt posterior frontal | 8/10 |
| It parietal | 5/10 |
| rt parietal | 6/10 |
| overall (%) | 39/60 (65) |
Discussion
Gene therapy for diseases of the CNS has long represented a theoretical tool of the future, primarily existing at the laboratory bench. Recent advances in understanding gene expression and function in neurological diseases, isolating and controlling viral vectors for gene delivery, and developing neurosurgical techniques in navigational guidance have provided a foundation for translating gene therapy from the bench to the operating room. This conversion has been led by several new human clinical trials for a variety of neurodegenerative disorders including Parkinson, Alzheimer, and Canavan disease.2,5,9,14,15,28 For Parkinson disease, phase I clinical studies have demonstrated the safety of the direct injection of AAV vectors carrying glutamic acid decarboxylase into the subthalamic nucleus and aromatic l-amino acid decarboxylase into the putamen; further efficacy studies are underway. For Canavan disease, phase I trial data showed the safety of intraparenchymally injecting AAV carrying human ASPA cDNA. These clinical experiences with AAV utilization provide a foundation for its application in other trials for genetic diseases.
Given the increasing translation to human studies of gene delivery for a variety of diseases of the nervous system, it is vital that neurosurgeons understand current techniques and nuances to implement current research and future innovation.2,5,9,14,15,28 Further, the evolving role of the neurosurgeon in providing safe therapeutic delivery for oncological purposes in tumors such as the diffuse pontine glioma necessitates similar technical considerations for translation into the clinical arena.11,16,21,26,27 Late infantile neuronal ceroid lipofuscinosis, a form of Batten disease, represents a genetic disorder arising from autosomal recessive mutations in the CLN2 gene. These mutations result in lysosomal protease tripeptidyl peptidase I deficiency and manifest in patients 2–4 years of age with seizures, developmental delay and regression, myoclonus, speech impairment, and ataxia. After extensive animal studies, an AAV2 vector carrying human CLN2 cDNA was developed, thus establishing the framework for therapeutic replacement of the deficient gene product.3,6,10,17,22-25 In addition to the LINCL trial reported on herein, phase I trials for Canavan disease, an autosomal recessive mutation of ASPA causing fatal leukodystrophy, mental retardation, hypotonia, macrocephaly, and seizures are underway to determine the safety and efficacy of the subcortical delivery of AAV carrying ASPA.7,10 As such, targeted delivery of gene therapy represents an important developing application of functional neurosurgery in children.8 The therapeutic replacement of genes within the CNS requires important technical considerations specific for each disease. With regard to LINCL, several features of the disease warranted a unique technical approach toward gene replacement. Primary distinctive features include the global distribution of diseased neurons and considerable cerebral atrophy. A similar set of practical issues has also been addressed with respect to Canavan disease, a childhood leukodystrophy that shares these anatomical features with LINCL.10
Our use of frameless navigation to target areas of the cortex and avoid large subarachnoid spaces combined our experiences with gene therapy to address an issue unique to global pediatric neurogenetic disorders. For adult neurodegenerative diseases that have particular pathophysiological neural pathway deficiencies along with the involvement of particular deep brain nuclei, such as Parkinson disease, stereotaxis is crucial to optimize infusion to a specific target and minimize the risk of off-target adverse effects. Batten disease, Canavan disease, and similar disorders result in widespread pathological changes throughout the cerebral cortex. As such, no single target for CLN2 gene transfer would adequately overcome the pathophysiological progression. A tactic of using multiple sites of administration was relied on to achieve better spatial distribution balanced by the practical considerations of surgery. For this reason, 6 pairs of injection sites were selected (3 in either hemisphere), which mimicked a similar approach used in a prior Canavan disease gene therapy study.15 However, authors in that study did not use stereotaxis and instead chose 6 bur holes based on a standard location in all patients relative to scalp landmarks, which did not account for the underlying brain anatomy. Since animal studies suggest that AAV vectors do not penetrate deep into the brain from CSF spaces, we believed it was important to maximize delivery directly into brain parenchyma.
Another concern was the potential increased risk of developing an immune reaction to the AAV vector by inadvertent delivery of large amounts of virus into the CSF, which could lead to both reduced gene delivery and possible adverse effects. Recombinant AAV vectors contain no viral genes and thus without foreign gene expression evoke a minimal inflammatory response, making it an ideal viral delivery option. Examples in the literature of induced anti-AAV capsid immune responses are likely due to very high doses of the vector delivering relevant quantities of capsid protein or the presence of contaminant viral genes. While there are no published data on targeting success in the Canavan disease study,15 it is interesting to note that the published immunological findings from that trial demonstrated a substantially greater number of patients developing anti-AAV neutralizing antibodies compared with patients in the present study even though the same strain of AAV (AAV2) was used in both studies.29 Although this immunological response could be influenced by a variety of factors, including differences in vector preparation techniques and disease-specific and gene-specific influences, the avoidance of CSF infusion would be expected to reduce the likelihood of developing an anti-AAV immune response. This assertion is further supported by an observation in our gene therapy study of Parkinson disease; even with different patients, different AAV production methods from a distinct facility, a different gene, and the same strain of AAV, no neutralizing antibody response developed in any of those patients following deep infusion into a single parenchymal target.9
The chosen stereotactic approach itself was also the basis for other problematic issues surrounding the operative technique. In our gene therapy study of Parkinson disease, a Leksell frame was used to target catheter insertion for infusion into a single site.9 When multiple targets are selected for vector infusion, frame-based targeting can only be performed serially. An infused volume of 300 μl delivered at 2 μl/minute results in an infusion time of 150 minutes/site (300 minutes total)—this would result in a medically unacceptable operating room time, which would substantially increase the risk of excessive CSF loss and infection. Thus, simultaneous infusion represents not only a technical nuance but also an operative necessity. Successful and accurate concurrent infusions at multiple targets require a protocol devised to cannulate multiple targets with precision and a reliable multisyringe infusion system specifically designed to handle parallel infusions (Fig. 6); therefore, frameless navigation was chosen. To achieve simultaneous infusions at 6 different sites, however, low-profile instruments are vital to ensure an open working space. Based on our experience with frame-based stereotaxis, however, we believed that it was important not simply to insert the borosilicate catheter unguided into the brain, but to use some form of larger guide tube that could be rigidly fixed to increase the precision of catheter placement and to better stabilize the catheter during a long infusion. However, with 6 bur holes and associated fixed cannulas, the 3D working space, which includes the head frame, retractors, adjustable retractor arms, and cannulas, is compromised. Maintaining such an elaborate scheme while retaining stereotactic precision requires an innovative and sequential method of creating a functional operating system. With an organization featured in Fig. 6 right, the step-wise procedure thus followed an order from anterior to posterior and from left to right, resulting in placement of the left frontal catheter first and placement of the right occipital catheter last. Given the variability in skull anatomy and in surgeon placement of the head frame, testing the accuracy and stability of all clamps before incision was critical to avoid brain injury or the recognition of a problem after some of the catheters were inserted and readjustment was no longer possible. With such operative planning, infusion time was reduced to achieve the desired vector distribution in a practical time frame with precise targeting and no injury to the patient.
Lastly, given the degree of cerebral atrophy and the potential for CSF egress, inherent risks, including subdural hemorrhage and inaccurate stereotactic targeting, are considerable. This risk of CSF egress with brain shift was addressed in the surgical plan with a sequential order for dural opening (gravity dependent; posterior entry sites opened last) and diligence in isolating the subarachnoid space from air (saline irrigation and fibrin sealant). These measures appear to have been successful since no patient experienced a perioperative subdural hemorrhage, and radiographic evidence of the infusion did not seem to be dependent on a specific bur hole location (anterior vs posterior). It is important to point out that the current method of MR imaging to assess evidence for infusion is potentially misleading. The reported 65% “success” rate as a measure of infusions/targeted site is an estimate. Using postoperative MR imaging does not guarantee that gene delivery occurred but instead documents areas of increased fluid after infusion. But the latter assumes a concentration of fluid that would be detectable, and the technique may not be sensitive enough to detect small infusion volumes, may not be done in an adequate time frame, and is dependent on the acquisition technique. It is likely that some fluid delivery occurred beyond the observed areas but had sufficiently diluted so that it was below the threshold for detection and that the absence of signal at some sites may not reflect failed delivery but could indicate more widespread delivery with a concomitant reduction in the focal concentration necessary for a change in imaging signal. At present, there is no mechanism to distinguish between these very different possibilities in patients. Neither is there an in vivo methodology for monitoring the distribution of therapeutic compounds following direct delivery. This issue has been on the forefront of clinical applications in which convection enhanced delivery is being used. Surrogate tracers with MR imaging contrast agents or radioisotopes have been successfully used but have as a major drawback the potential to misrepresent the actual distribution of the therapeutic compound.11,16,18-20 Alterations in MR signal, most notably the FLAIR, T2-weighted, and diffusion weighted sequences, have also been used but are problematic when the underlying disease invokes a baseline alteration of that signal prior to delivery.13,20 Furthermore, the use of reporter genes or surrogate tracers may increase the chances of immunological recognition of the therapeutic compound, possibly leading to inflammation and vector clearance.
While neurosurgical gene therapy is in its clinical infancy, it is important for neurosurgeons to embrace this field and to develop appropriate operative techniques. From the bench to bedside, the involvement of neurosurgeons is vital to ensure that this field develops in a surgically safe and efficient manner. Neurosurgical participation and design of delivery schemes will become increasingly important to avoid failure in an otherwise promising biological therapy because of technical limitations not always appreciated by nonneurosurgical investigators.
Conclusions
Gene therapy for global cerebral diseases presents a number of anatomical and technical challenges regarding therapeutic delivery. In the absence of a specifically defined anatomical target, wide distribution can be enhanced using multiple sites of delivery, an approach that does have spatial and temporal constraints. The described neurosurgical technique integrates a number of features into an innovative procedure aimed at minimizing morbidity, optimizing targeting, maximizing spatial distribution, and reducing operative time. The procedure used in this limited clinical study was practical and safe. Reliable methods for measuring the distribution of administered viral particles or the expression of gene products are not yet established but will become increasingly important in assessing treatment efficacy.
Acknowledgments
The authors are grateful for the illustrative work of Mr. Thomas Graves, Thom Graves Media, and the technical assistance of Dr. Paola Leone, University of Medicine and Dentistry of New Jersey.
Glossary
Abbreviations used in this paper
- AAV2
adeno-associated virus 2
- ASPA
aspartoacylase
- LINCL
late infantile neuronal ceroid lipofuscinosis.
Footnotes
Portions of this work were presented at the 2006 annual meetings of the American Society of Pediatric Neurosurgeons held in Great Exuma, Bahamas, and the Congress of Neurological Surgeons held in Chicago, Illinois.
Disclosure
Mark M. Souweidane is a paid consultant and serves on the Aesculap Neuroendoscopic Advisory Committee. Michael G. Kaplitt is a founder of and paid consultant for Neurologix, Inc., which was not involved in this study but is developing AAV gene therapy for other disorders. This study was supported in part by the Nathan’s Battle Foundation (Greenwood, Indiana); by NIH Grant Nos. U01 NS047458 CTSC UL1-RR024996; and the Will Rogers Memorial Fund (Los Angeles, California).
Author contributions to the study and manuscript preparation include the following. Conception and design: Souweidane, Crystal. Acquisition of data: Souweidane, Arkin, Sondhi, Hackett, Kosofsky, Worgall, Crystal, Kaplitt. Analysis and interpretation of data: Souweidane, Fraser, Kaminsky, Heier, Kosofsky, Crystal, Kaplitt. Drafting the article: Souweidane, Fraser, Kaplitt. Critically revising the article: Souweidane. Reviewed final version of the manuscript and approved it for submission: all authors. Statistical analysis: Kaminsky. Administrative/technical/material support: Arkin, Sondhi, Hackett, Kaminsky, Worgall. Study supervision: Sondhi, Crystal.
References
- 1.Arkin LM, Sondhi D, Worgall S, Suh LH, Hackett NR, Kaminsky SM, et al. Confronting the issues of therapeutic misconception, enrollment decisions, and personal motives in genetic medicine-based clinical research studies for fatal disorders. Hum Gene Ther. 2005;16:1028–1036. doi: 10.1089/hum.2005.16.1028. [DOI] [PubMed] [Google Scholar]
- 2.Christine CW, Starr PA, Larson PS, Eberling JL, Jagust WJ, Hawkins RA, et al. Safety and tolerability of putaminal AADC gene therapy for Parkinson disease. Neurology. 2009;73:1662–1669. doi: 10.1212/WNL.0b013e3181c29356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crystal RG, Sondhi D, Hackett NR, Kaminsky SM, Worgall S, Stieg P, et al. Clinical protocol. Administration of a replication-deficient adeno-associated virus gene transfer vector expressing the human CLN2 cDNA to the brain of children with late infantile neuronal ceroid lipofuscinosis. Hum Gene Ther. 2004;15:1131–1154. doi: 10.1089/hum.2004.15.1131. [DOI] [PubMed] [Google Scholar]
- 4.Dyke JP, Voss HU, Sondhi D, Hackett NR, Worgall S, Heier LA, et al. Assessing disease severity in late infantile neuronal ceroid lipofuscinosis using quantitative MR diffusion-weighted imaging. AJNR Am J Neuroradiol. 2007;28:1232–1236. doi: 10.3174/ajnr.A0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feigin A, Kaplitt MG, Tang C, Lin T, Mattis P, Dhawan V, et al. Modulation of metabolic brain networks after subthalamic gene therapy for Parkinson’s disease. Proc Natl Acad Sci U S A. 2007;104:19559–19564. doi: 10.1073/pnas.0706006104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hackett NR, Redmond DE, Sondhi D, Giannaris EL, Vassallo E, Stratton J, et al. Safety of direct administration of AAV2(CU)hCLN2, a candidate treatment for the central nervous system manifestations of late infantile neuronal ceroid lipofuscinosis, to the brain of rats and nonhuman primates. Hum Gene Ther. 2005;16:1484–1503. doi: 10.1089/hum.2005.16.1484. [DOI] [PubMed] [Google Scholar]
- 7.Janson C, McPhee S, Bilaniuk L, Haselgrove J, Testaiuti M, Freese A, et al. Clinical protocol. Gene therapy of Canavan disease: AAV-2 vector for neurosurgical delivery of aspartoacylase gene (ASPA) to the human brain. Hum Gene Ther. 2002;13:1391–1412. doi: 10.1089/104303402760128612. [DOI] [PubMed] [Google Scholar]
- 8.Kaplitt MG, Darakchiev B, During MJ. Prospects for gene therapy in pediatric neurosurgery. Pediatr Neurosurg. 1998;28:3–14. doi: 10.1159/000028611. [DOI] [PubMed] [Google Scholar]
- 9.Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. Lancet. 2007;369:2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- 10.Leone P, Janson CG, McPhee SJ, During MJ. Global CNS gene transfer for a childhood neurogenetic enzyme deficiency: Canavan disease. Curr Opin Mol Ther. 1999;1:487–492. [PubMed] [Google Scholar]
- 11.Lonser RR, Warren KE, Butman JA, Quezado Z, Robison RA, Walbridge S, et al. Real-time image-guided direct convective perfusion of intrinsic brainstem lesions. Technical note. J Neurosurg. 2007;107:190–197. doi: 10.3171/JNS-07/07/0190. [DOI] [PubMed] [Google Scholar]
- 12.Luther N, Cheung NK, Dunkel IJ, Fraser JF, Edgar MA, Gutin PH, et al. Intraparenchymal and intratumoral interstitial infusion of anti-glioma monoclonal antibody 8H9. Neurosurgery. 2008;63:1166–1174. doi: 10.1227/01.NEU.0000334052.60634.84. [DOI] [PubMed] [Google Scholar]
- 13.Mardor Y, Roth Y, Lidar Z, Jonas T, Pfeffer R, Maier SE, et al. Monitoring response to convection-enhanced taxol delivery in brain tumor patients using diffusion-weighted magnetic resonance imaging. Cancer Res. 2001;61:4971–4973. [PubMed] [Google Scholar]
- 14.Marks WJ, Jr, Ostrem JL, Verhagen L, Starr PA, Larson PS, Bakay RA, et al. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson’s disease: an openlabel, phase I trial. Lancet Neurol. 2008;7:400–408. doi: 10.1016/S1474-4422(08)70065-6. [DOI] [PubMed] [Google Scholar]
- 15.McPhee SW, Janson CG, Li C, Samulski RJ, Camp AS, Francis J, et al. Immune responses to AAV in a phase I study for Canavan disease. J Gene Med. 2006;8:577–588. doi: 10.1002/jgm.885. [DOI] [PubMed] [Google Scholar]
- 16.Murad GJ, Walbridge S, Morrison PF, Szerlip N, Butman JA, Oldfield EH, et al. Image-guided convection-enhanced delivery of gemcitabine to the brainstem. J Neurosurg. 2007;106:351–356. doi: 10.3171/jns.2007.106.2.351. [DOI] [PubMed] [Google Scholar]
- 17.Passini MA, Dodge JC, Bu J, Yang W, Zhao Q, Sondhi D, et al. Intracranial delivery of CLN2 reduces brain pathology in a mouse model of classical late infantile neuronal ceroid lipofuscinosis. J Neurosci. 2006;26:1334–1342. doi: 10.1523/JNEUROSCI.2676-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sampson JH, Akabani G, Archer GE, Berger MS, Coleman RE, Friedman AH, et al. Intracerebral infusion of an EGFRtargeted toxin in recurrent malignant brain tumors. Neuro Oncol. 2008;10:320–329. doi: 10.1215/15228517-2008-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sampson JH, Brady ML, Petry NA, Croteau D, Friedman AH, Friedman HS, et al. Intracerebral infusate distribution by convection-enhanced delivery in humans with malignant gliomas: descriptive effects of target anatomy and catheter positioning. Neurosurgery. 2007;60(2 Suppl 1):ONS89–ONS99. doi: 10.1227/01.NEU.0000249256.09289.5F. [DOI] [PubMed] [Google Scholar]
- 20.Sampson JH, Raghavan R, Provenzale JM, Croteau D, Reardon DA, Coleman RE, et al. Induction of hyperintense signal on T2-weighted MR images correlates with infusion distribution from intracerebral convection-enhanced delivery of a tumor-targeted cytotoxin. AJR Am J Roentgenol. 2007;188:703–709. doi: 10.2214/AJR.06.0428. [DOI] [PubMed] [Google Scholar]
- 21.Sandberg DI, Edgar MA, Souweidane MM. Convection-enhanced delivery into the rat brainstem. J Neurosurg. 2002;96:885–891. doi: 10.3171/jns.2002.96.5.0885. [DOI] [PubMed] [Google Scholar]
- 22.Sondhi D, Hackett NR, Apblett RL, Kaminsky SM, Pergolizzi RG, Crystal RG. Feasibility of gene therapy for late neuronal ceroid lipofuscinosis. Arch Neurol. 2001;58:1793–1798. doi: 10.1001/archneur.58.11.1793. [DOI] [PubMed] [Google Scholar]
- 23.Sondhi D, Hackett NR, Peterson DA, Stratton J, Baad M, Travis KM, et al. Enhanced survival of the LINCL mouse following CLN2 gene transfer using the rh.10 rhesus macaque-derived adeno-associated virus vector. Mol Ther. 2007;15:481–491. doi: 10.1038/sj.mt.6300049. [DOI] [PubMed] [Google Scholar]
- 24.Sondhi D, Peterson DA, Edelstein AM, del Fierro K, Hackett NR, Crystal RG. Survival advantage of neonatal CNS gene transfer for late infantile neuronal ceroid lipofuscinosis. Exp Neurol. 2008;213:18–27. doi: 10.1016/j.expneurol.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sondhi D, Peterson DA, Giannaris EL, Sanders CT, Mendez BS, De B, et al. AAV2-mediated CLN2 gene transfer to rodent and non-human primate brain results in long-term TPP-I expression compatible with therapy for LINCL. Gene Ther. 2005;12:1618–1632. doi: 10.1038/sj.gt.3302549. [DOI] [PubMed] [Google Scholar]
- 26.Souweidane MM, Occhiogrosso G, Mark EB, Edgar MA. Interstitial infusion of IL13-PE38QQR in the rat brain stem. J Neurooncol. 2004;67:287–293. doi: 10.1023/b:neon.0000024219.47447.91. [DOI] [PubMed] [Google Scholar]
- 27.Souweidane MM, Occhiogrosso G, Mark EB, Edgar MA, Dunkel IJ. Interstitial infusion of carmustine in the rat brain stem with systemic administration of O6-benzylguanine. J Neurooncol. 2004;67:319–326. doi: 10.1023/b:neon.0000024242.59770.7a. [DOI] [PubMed] [Google Scholar]
- 28.Tuszynski MH, Thal L, Pay M, Salmon DP, U HS, Bakay R, et al. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat Med. 2005;11:551–555. doi: 10.1038/nm1239. [DOI] [PubMed] [Google Scholar]
- 29.Worgall S, Sondhi D, Hackett NR, Kosofsky B, Kekatpure MV, Neyzi N, et al. Treatment of late infantile neuronal ceroid lipofuscinosis by CNS administration of a serotype 2 adeno-associated virus expressing CLN2 cDNA. Hum Gene Ther. 2008;19:463–474. doi: 10.1089/hum.2008.022. [DOI] [PubMed] [Google Scholar]