Summary
Tea is the most popular beverage in the world, second only to water. Tea contains an infusion of the leaves from the Camellia sinensis plant rich in polyphenolic compounds known as catechins, the most abundant of which is (−)-EGCG. Although tea has been consumed for centuries, it has only recently been studied extensively as a health-promoting beverage that may act to prevent a number of chronic diseases and cancers. The results of several investigations indicate that green tea consumption may be of modest benefit in reducing the plasma concentration of cholesterol and preventing atherosclerosis. Additionally, the cancer-preventive effects of green tea are widely supported by results from epidemiological, cell culture, animal and clinical studies. In vitro cell culture studies show that tea polyphenols potently induce apoptotic cell death and cell cycle arrest in tumor cells but not in their normal cell counterparts. Green tea polyphenols were shown to affect several biological pathways, including growth factor-mediated pathway, the mitogen-activated protein (MAP) kinase-dependent pathway, and ubiquitin/proteasome degradation pathways. Various animal studies have revealed that treatment with green tea inhibits tumor incidence and multiplicity in different organ sites such as skin, lung, liver, stomach, mammary gland and colon. Recently, phase I and II clinical trials have been conducted to explore the anticancer effects of green tea in humans. A major challenge of cancer prevention is to integrate new molecular findings into clinical practice. Therefore, identification of more molecular targets and biomarkers for tea polyphenols is essential for improving the design of green tea trials and will greatly assist in a better understanding of the mechanisms underlying its anti-cancer activity.
Keywords: Tea polyphenols, Molecular targets, Cancer prevention, Cancer treatment
Introduction
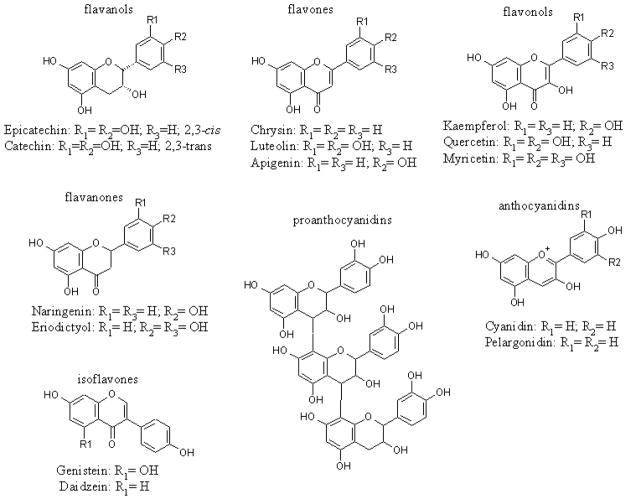
Polyphenols are a group of chemical substances widely distributed in the plant kingdom and present in human diets (Cheynier, 2005; Scalbert et al., 2005). They are characterized by the presence of more than one phenol group per molecule and are responsible for the coloring of some plants. The main polyphenol dietary sources are fruits, vegetables, and beverages (fruit juice, wine, tea, coffee and chocolate). The total intake of polyphenols is approximately 1 gram per day, depending on lifestyle and dietary preferences (Scalbert and Williamson, 2000). Polyphenols can be divided into four subgroups: flavonoids, anthocyanins, proanthocyanidins, and xanthones. The flavonoids constitute a large family of compounds including flavanols (e.g., catechins and epicatechins found in cocoa and dark chocolate), flavones (e.g., apigenin and luteolin found in parsley, celery and broccoli), flavonols (e.g., quercetin and kaempferol found in citrus fruits), flavanones (e.g., naringenin found in orange and grapefruits), anthocyanidins, proanthocyanidins and isoflavones (e.g., genistein found in soy beans) (Fig. 1) (Mukhtar and Ahmad, 1999b).
Fig. 1.
Chemical structures of flavonoids.
The history of tea began over 5,000 years ago in ancient China. Currently, tea is the most popular beverage consumed by 2/3 of the world’s population. Green tea, black tea, and oolong tea are all derived from the Camellia sinensis plant and contain an assortment of compounds, the most significant components of which are polyphenols. The differences between green, black, and oolong tea lie in the fermentation process. While green tea does not undergo fermentation, black tea is completely fermented, and oolong tea contains a mixture of both fermented and non-fermented leaves. Among all teas consumed in the world, green tea is the best studied for health benefits, including chemopreventive efficacy (Mukhtar and Ahmad, 1999a), and tea polyphenols are considered to contribute to the prevention of various degenerative diseases, including cardiovascular diseases and cancers.
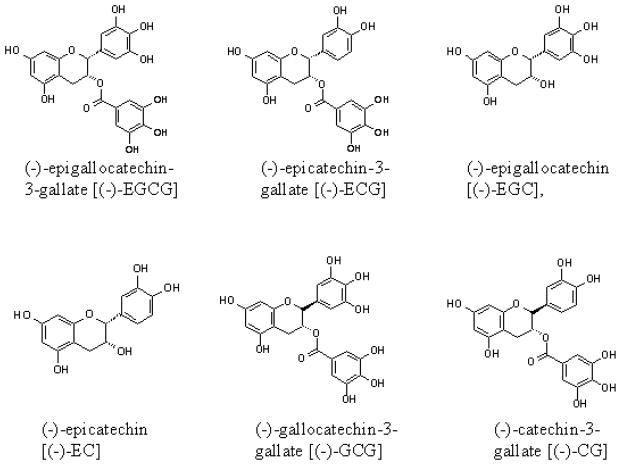
The predominant source of tea polyphenols are catechins, which constitute approximately 30% of the dry leaf weight from the Camellia sinensis plant (Graham, 1992). Catechins contain a benzopyran skeleton with a phenyl group substituted at the 2-position and a hydroxyl (or ester) function at the 3-position. Variations to the catechin structure include the stereochemistry of the 2,3-substituents and the number of hydroxyl groups in the B- and D-ring. Belonging to the flavan-3-ol class of flavonoids, the most abundant catechins found in tea leaves are (−)-epigallocatechin-3-gallate [(−)-EGCG], (−)-epigallocatechin [(−)-EGC], (−)-epicatechin-3-gallate [(−)-ECG], and (−)-epicatechin [(−)-EC] (Fig. 2). In this review we will focus on tea polyphenols, their biological effects and potential molecular targets.
Fig. 2.
Chemical structures of tea polyphenols.
Tea polyphenols and cardiovascular diseases
A major risk factor for the development of heart disease is an elevated level of plasma cholesterol, and epidemiological studies in Japan showed that green tea could lower that level (Imai and Nakachi, 1995). Results from other studies suggested that the lowering of plasma cholesterol by green tea is most likely the result of an increased expression of the low-density lipoprotein receptor (LDLR), which is required for cholesterol removal from the circulation (Brown and Goldstein, 1998). It has also been reported that tea polyphenols could lower plasma cholesterol and inhibit the process of atherosclerosis (Kuhn et al., 2004; Vinson et al., 2004). Moreover, we found that (−)-EGCG potently inhibited the proteasomal activity in hepatocellular and cervical carcinoma cell lines, HepG2 and HeLa, respectively, associated with up-regulation of the LDLR and increased level of the cleaved, activated form of the sterol regulatory element-binding protein 2 (SREBP-2), an essential factor for LDLR transcription (Kuhn et al. 2004). The results from an animal study using a hamster model of atherosclerosis demonstrated that green and black teas were equally effective in inhibiting atherosclerosis when used in a low (0.0625% solution) and high dose (1.25% solution), decreasing atherosclerosis incidence by 26–46% and 48–63%, respectively (Vinson et al., 2004). This study suggested that tea inhibited atherosclerosis by three mechanisms: removing reactive oxygen species, inducing hypolipemia, and decreasing antifibrinolysis (Vinson et al., 2004). Taken together, tea polyphenols appear to play an important role in the prevention of cardiovascular diseases, but the involved mechanisms and molecular targets should be further investigated.
Tea polyphenols and cancer
The process of cancer development, carcinogenesis, may be divided into at least three stages: initiation, promotion, and progression (Pitot, 1993). The first stage of carcinogenesis, initiation, results from changes in gene constructions such as an irreversible genetic alteration. Most likely, genetic alterations occur via simple mutations, transversions, transitions, and/or small deletions in DNA. Additionally, genetic alterations may result from changes in gene function, without alteration of the DNA sequence, including histone modification, transcriptional activity and DNA methylation (Baylin and Ohm, 2006). The second stage of carcinogenesis is promotion, a reversible process that does not involve changes in the structure of DNA but rather in the expression of the genome mediated through promoter-receptor interactions. The final stage of carcinogenesis, progression, can occur spontaneously and is characterized by karyotypic instability and malignant growth. Progression is enhanced by formation and propagation of genetic errors that occur due to increased cellular proliferation (Pitot, 1993; Pitot and Dragan, 1991).
Since advanced metastasized cancers are mostly incurable, an effort to prolong or block the process of carcinogenesis through chemoprevention has become an important, feasible strategy for cancer control and management. The concept of chemoprevention is to reduce the occurrence of cancer by slowing, blocking, or reversing the development of the disease by the administration of natural or synthetic compounds (Khan et al., 2006), and numerous studies have demonstrated that green tea and tea polyphenols possess a chemopreventative potential (Imai et al., 1997; Fujiki, 1999; Mukhtar and Ahmad, 1999b). Central to the biology of cancer is a disrupted intracellular signaling network, which transmits aberrant signals resulting in abnormal cellular function. Targeting deregulated intracellular signaling cascades is considered to be a rational approach in achieving chemoprevention. Recent studies have elucidated that dietary cancer chemopreventive agents exert their effects by modulating multiple cell signaling pathways in a manner that interrupts the carcinogenic process (Surh, 2003). Furthermore, various studies indicate that diet-derived compounds are capable of prolonging one or more stages of the carcinogenic process (Khan et al., 2006; Middleton et al., 2000; Mukhtar and Ahmad, 1999a). It is generally agreed that much of cancer chemopreventive effects of green tea are mediated by its most abundant polyphenol, (−)-EGCG. Based on the current literature, we will summarize the biological role of green tea and (−)-EGCG in cancer prevention.
Green tea and (−)-EGCG can inhibit progression of carcinogenesis
The cancer-preventive effects of green tea and its main constituent (−)-EGCG are widely supported by results from epidemiological, cell culture, animal and clinical studies in the past decade. An epidemiological study conducted among 384 cancer patients showed that cancer onset was delayed by 8.7 and 3.0 years in women and men, respectively, who increased the consumption of green tea from less than three to over ten cups per day (Imai et al., 1997). It was also reported that in 472 stage I and II cancer patients, a lower recurrence rate (16.7%) and a longer disease-free period (3.6 years) were observed in those patients consuming more than five cups per day, compared to those consuming fewer than four cups per day (Nakachi et al., 1998). Therefore, it was called two stages of cancer prevention with tea, cancer prevention before cancer onset and cancer prevention following cancer treatment (Fujiki, 1999).
The inhibition of carcinogenesis by tea has been demonstrated in many different animal models such as lung, skin, esophagus and liver cancer (Dreosti et al., 1997; Conney et al., 1999; Yang et al., 1999). It was reported that green tea infusion (e.g., 1.25 g of tea leaves brewed in 100-ml boiling water) as the sole source of drinking fluid to A/J mice significantly decreased N-nitrosodiethylamine (NDEA)-induced lung tumor incidence (by 36 to 44%) and tumor multiplicity (by 44 to 60%) (Wang et al., 1992).
The inhibition of skin carcinogenesis by tea was investigated by Mantena et al. (2005). In this study, SKH-1 hairless mice were exposed to UVB (180 mJ/cm2) 3 times per week for 24 weeks. Oral administration of green tea polyphenols (GTPs) was found to reduce UVB-induced tumor incidence (35%), tumor multiplicity (63%), and tumor growth (55%). Furthermore, the inhibitory effect of GTPs was associated with reduced expression of the matrix metalloproteinases (MMP-2 and MMP-9), which have crucial roles in tumor growth and metastasis, and with reduced expressions of CD31 and vascular endothelial growth factor, which are essential for angiogenesis (Mantena et al., 2005). Furthermore, SEN-CAR mice were initiated with 7,12- dimethylbenz[a]anthracene (DMBA) and promoted with 12-O-tetradecano-ylphorbol-13- acetate (TPA) or mezerein (MEZ) (Katiyar et al., 1997). Topically application of GTPs (6 mg/animal) to the skin of DMBA-initiated mice, 30 minutes prior to promotion by TPA or MEZ, resulted in significant protection against skin tumor formation in terms of tumor incidence (32–60%), multiplicity (49–63%) and tumor volume/mouse (73–90%) (Katiyar et al., 1997).
In a study conducted to test the inhibitory effect of green tea on hepatocarcinogenesis, mice were given pentachlorophenol (PCP) as a carcinogen following treatment with the initiator diethylnitrosamine (DEN). In mice that were pretreated with a green tea infusion and continuously exposed to the same infusion during the process of initiation and promotion, incidence of hepatocellular tumors was decreased by 40% (Umemura et al., 2003).
In an esophageal tumor model, Sprague-Dawley rats were given 0.6% decaffeinated green tea or decaffeinated black tea extracts as the drinking fluid during an N-nitrosomethylbenzylamine (NMBzA) treatment period. Esophageal tumor incidence and multiplicity were reduced by approximately 70% in both groups (Wang et al., 1995).
Lastly, we have shown that human breast MDA-MB-231 tumors induced in nude mice, followed by treatment with (−)-EGCG for 31 days resulted in a significant inhibition of tumor growth (23%). Interestingly, a prodrug, more biologically stable form of EGCG, Pro-EGCG (1), was shown to accumulate (−)-EGCG in cultured MDA-MB-231 cells and inhibited tumor growth by 56% (Landis-Piwowar et al., 2007).
The results from above experiments indicate that tea has broad inhibitory activity against carcinogenesis and is effective when administered during the initiation, promotion or progression of carcinogenesis. However, the molecular mechanisms for these inhibitory actions are not fully understood. They are most likely related to the biochemical activity of the tea polyphenols, including their antioxidative effect, protection of DNA from damage and/or methylation, inhibition of proteasome activity in tumor cells, induction of apoptosis in tumor or transformed cells, cell cycle regulation and inhibition of cell proliferation and tumor promotion-related events, as discussed below.
Green tea and (−)-EGCG protect DNA from methylation and damage
In the process of carcinogenesis, a carcinogen may cause changes in gene functions and/or in gene constructions. Epigenetics is the study of reversible, heritable changes in gene function that occur without a change in the sequence of nuclear DNA (Baylin and Ohm, 2006). Epigenetic mechanisms control eukaryotic development beyond DNA-stored information, but can also contribute to carcinogenesis by altering chromatin structure, histones, transcriptional activity and DNA methylation. Epigenetic silencing by hypermethylation of tumor suppressor or DNA repair-related genes occurs most frequently during the early stages of the neoplastic process (Feinberg and Tycko, 2004). Moreover, the epigenetic change may result in additional changes in gene constructions. For example, silencing of the O6-methylguanine-DNA methyltransferase gene (MGMT) results in cells with the ability to acquire a specific type of genetic mutation in p53 and subsequently an inability to repair DNA guanosine adducts (Esteller et al., 2001).
Fang et al. (2003) reported that (−)-EGCG could inhibit the activity of DNA methyltransferase (DNMT), resulting in CpG demethylation and reactivation of methylation-silenced genes in human esophageal cancer KYSE 510 cells. In this study, EGCG treatment of KYSE 510 cells caused a concentration- and time-dependent reversal of hypermethylated p16INK4α, retinoic acid receptor β (RARβ), MGMT, and human mutL homologue 1 (hMLH1) genes. Also, an epidemiological study conducted among 73 patients with gastric carcinoma showed that increased intake of green tea was significantly associated with the Cdx2 methylation frequency (P=0.02) (Yuasa et al., 2005). Tumor suppressor gene that encodes caudal-related homeobox transcription factor (Cdx2) is frequently inactivated by promoter hypermethylation in gastric carcinoma and colorectal cancer cells. Green tea decreased the Cdx2 methylation frequency in a dose-dependent manner. It was observed that 10/25 (40%), 7/18 (39%), 2/8 (25%), and 0/6 (0%) of Cdx2 methylation frequency in tested group of people who consumed three or less, four to six, seven to nine and ten cups or more a day respectively (Yuasa et al., 2005).
Ultraviolet radiation (UVR) can induce DNA damage that results in carcinogenesis. It was reported that topical treatment with (−)-EGCG or GTPs resulted in significant prevention of UVB (290–320 nm)-induced depletion of antioxidant enzymes, such as glutathione peroxidase (78–100%) and catalase (51–92%). Furthermore, an animal study showed that GTPs in drinking water also significantly prevented single or multiple UVB irradiation-induced depletion of antioxidant enzymes (44–61%), oxidative stress (33–71%) and phosphorylation of ERK1/2, JNK and p38 proteins (Vayalil et al., 2003). In a more mechanistic study, Zykova et al. reported that (−)-EGCG could block UVB-induced STAT1 (Ser727) phosphorylation through the inhibition of ERKs, JNKs, PDK1 and p90RSK in mouse epidermal JB6 Cl41 cells (Zykova et al., 2005).
Pre-incubation with (−)-EGCG was also shown to significantly decrease DNA damage induced by UVR in three cell lines of human skin fibroblasts, lung fibroblasts and epidermal keratinocytes (Morley et al., 2005). Furthermore, an in vivo experiment demonstrated a photoprotective effect by green tea. After human volunteers consumed 180 ml of green tea, peripheral blood samples were taken and exposed to UVR for 12 min. It was observed that the peripheral blood cells showed lower levels of DNA damage from individuals who consumed green tea compared to the controls (Morley et al., 2005).
A phase II trial, reported in 2003 (Hakim et al., 2003), was a randomized controlled tea intervention trial designed to study the effect of high decaffeinated green or black tea consumption (4 cups/day) on oxidative DNA damage, as measured by urinary 8-hydroxy-deoxyguanosine (8-OHdG) among smokers over a 4 month-period. The level of urinary 8-OHdG was measured monthly in 143 heavy smokers, aged 18–79 years old, who were randomly grouped to drink green tea, black tea, or water. Assessment of urinary 8-OHdG, after adjustment for baseline measurements and other potential confounders, revealed a significant decrease in urinary 8-OHdG (31%) after 4 months of drinking decaffeinated green tea (P = 0.002) (Hakim et al., 2003). These data suggest that regular green tea drinking might protect smokers from oxidative damage and could reduce risk for cancer or other diseases caused by free radicals associated with smoking.
Green tea and (−)-EGCG inhibit proteasome activity in tumor cells
The ubiquitin/proteasome system controls the turnover of regulatory proteins involved in critical cellular processes such as cell cycle and apoptosis (Ciechanover, 1994; Hochstrasser, 1995). Under normal conditions the lysosomal pathway degrades extracellular proteins imported into the cell by endocytosis or pinocytosis, whereas the proteasome controls degradation of intracellular proteins (Goldberg, 1995; Hochstrasser, 1995). The eukaryotic proteasome contains at least three known catalytic activites: chymotrypsin-like, trypsin-like, and caspase-like or peptidyl-glutamyl peptide-hydrolyzing (PGPH)-like activities (Seemuller et al., 1995). It has been suggested that proteasomal activity is essential for tumor cell proliferation and drug resistance development (Hideshima et al., 2001). Therefore, the proteasome-mediated degradation pathway has been considered to be an important target for cancer therapy and prevention. We and others have reported that inhibition of the proteasomal chymotrypsin-like activity is associated with induction of apoptosis in tumor cells (Lopes et al., 1997; An et al., 1998). The proteasome inhibitor Bortezomib (Velcade, PS-341) has been used in clinical trials and its antitumor activity has been reported in a variety of tumor models (Adams, 2002; Dou and Goldfarb, 2002; Kane et al., 2006).
The study in our laboratory showed that (−)-EGCG potently and specifically inhibited the chymotrypsin-like activity of the proteasome in vitro (IC50=86–194 nM), and that (−)-EGCG could induce tumor cell growth arrest in G1 phase of the cell cycle (Nam et al., 2001). We reported, for the first time, that an ester bond within (−)-EGCG played a critical role in its inhibitory activity of the proteasome (Nam et al., 2001). We also found that synthetic (−)-EGCG amides and (−)-EGCG analogs with modifications in the A-ring, C-ring or ester bond inhibited the chymotrypsin-like activity of purified 20S proteasome with altered potencies, induced growth arrest in the G1 phase of the cell cycle in leukemia Jurkat T cells and suppressed colony formation of human prostate cancer LNCaP cells (Kazi et al., 2004).
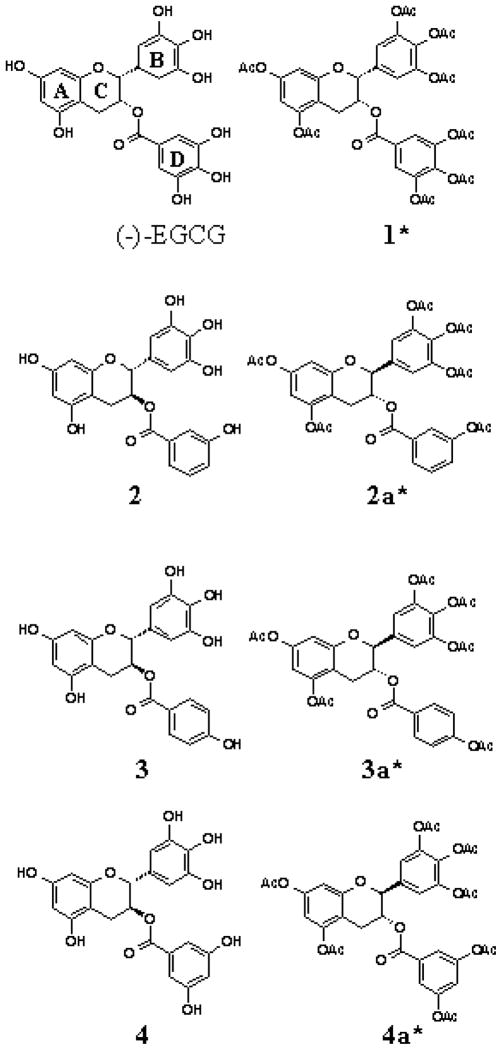
While (−)-EGCG remains to be the most potent polyphenol in green tea, it is unstable in neutral or alkaline conditions (i.e. physiologic pH). In an effort to discover more stable polyphenol proteasome inhibitors, we synthesized several novel (−)-EGCG analogs with -OH groups eliminated from the B- and/or D-rings. In addition, we also synthesized their putative prodrugs with -OH groups protected by acetate (Fig. 3) that can be removed by cellular cytosolic esterases. We first examined the structure-activity relationship of these unprotected and protected compounds with respect to their proteasome inhibitory potentials. We found that decreasing the number of -OH groups from either the B-or D-ring leads to diminished proteasome inhibitory activity in vitro. However, in cultured tumor cells, the protected analogs were capable of potently inhibiting the proteasomal chymotrypsin-like activity by as much as 97% (Landis-Piwowar et al., 2005). Furthermore, we found that, compared to (−)-EGCG, protected analogs exhibited greater potency to inhibit proliferation and induce apoptosis in human leukemic, prostate, breast, and simian virus 40-transformed cells (Kuhn et al., 2005). The protected analogs were non-toxic to human normal and non-transformed cells (Kuhn et al., 2005).
Fig. 3.
Chemical structure of (−)-EGCG and synthetic tea polyphenols with or without protected hydroxyl groups (racemic compounds). *: Peracetate-protected synthetic green tea polyphenols.
Green tea and (−)-EGCG regulate cell cycle progression and induce apoptosis in tumor cells
Human and animals use two main mechanisms for the elimination of cells: necrosis and apoptosis. Necrosis consists of the rupture of the plasma membrane and the formation of an inflammatory process that damages the cells and their surrounding tissues. Apoptosis, instead, involves a ‘cleaner’ type of death, where the chromatin is condensed, the DNA becomes fragmented and vesicles, known as ‘apoptotic bodies’, are formed. These are rapidly phagocytized by the macrophages and the cell disappears without any inflammatory phenomena (Kerr et al., 1972). Recent knowledge on apoptosis has provided the basis for novel anti-cancer therapies that target proteins such as p53 and the proteasome pathway, ultimately affecting the extrinsic/intrinsic pathways of apoptosis (Russo et al., 2006).
Hastak et al. (2003) reported that (−)-EGCG-induced apoptosis in human prostate carcinoma LNCaP cells is mediated via modulation of p53 stabilization. This stabilization results in p53-activated downstream targets, p21/WAF1 and Bax, and negative regulation of NF-kappa B and Bcl-2. Thus, (−)-EGCG has a concurrent effect on two important transcription factors, p53 and NF-kappa B, causing a change in the ratio of Bax/Bcl-2 in a manner that favors apoptosis. This altered expression of Bcl-2 family members triggers the activation of initiator capsases, -9 and -8, followed by activation of the effector caspase, caspase-3. Activation of these caspases is followed by poly (ADP-ribose) polymerase cleavage and induction of apoptosis (Hastak et al., 2003). (−)-EGCG is also known to directly initiate apoptosis as demonstrated by the loss of mitochondrial membrane potential, cytochrome C release, DNA laddering, and dramatic increase in caspase-3 activity (Chen et al., 2003).
Antioxidative effect of green tea and (−)-EGCG
In the natural process of oxidation, human bodies produce free radicals. These molecules can cause damage to proteins, lipids and DNA, but are generally cleaned up by substances called antioxidants and systems of antioxidant enzymes before they can insult cells. Several human diseases have a strong association with the oxidative damage in tissues, such as cancer, heart disease, diabetes, and Alzheimer’s disease, as well as aging (Cutler, 2005; Cutler et al., 2005). Organic polyphenols found in plants are known to have antioxidant activity. For this reason, a diet rich in plant material is required for the health of most mammals. More than 1,000 research articles may be found in the current literature with an emphasis on green tea as an antioxidant. In fact, many of the antiproliferative effects of (−)-EGCG are attributable to its antioxidant properties (Mukhtar and Ahmad, 1999b). Rah et al. (2005) investigated the potential protective role of GTPs against the injurious effects of reactive oxygen species in human microvascular endothelial cells (HUMVECs). They found that alterations induced by hydrogen peroxide (H2O2) or xanthine oxidase (XO) were completely prevented by pre-incubation of the endothelial cells with 10 μg/ml GTPs for 1 h. These results demonstrate that GTPs can act as biological antioxidants in a cell culture experimental model and can prevent oxidative stress-induced cytotoxicity in the endothelial cells.
Coimbra et al. (2006) investigated the effect of green tea in protecting the human body from oxidative stress. In 34 human subjects, they evaluated the total antioxidant status (TAS) by measuring two markers of lipid peroxidation products, malonyldialdehyde (MDA) and malonyldialdehyde+4-hydroxy-2(E)-nonenal (MDA+4-HNE), and the two markers of oxidative changes in erythrocyte membrane, called membrane bound haemoglobin (MBH) and band 3 (an erythrocyte transmembrane protein) profile. After green tea consumption (1 liter green tea daily for 4 weeks), significantly reduced serum levels of MDA (by 30%) and MDA+4-HNE (by 39 %), significantly lower value of MBH (25%) and improved band 3 profile were observed (Coimbra et al., 2006). In another in vivo study, the total antioxidant capacity of plasma was measured in 10 healthy people who consumed 150 ml of green tea. The results showed an increase of 1.1% and 2.1% in total antioxidant capacity, 60 min and 120 min after green tea ingestion respectively, which was not statistically significant. However, total plasma antioxidant capacity after consuming 300 ml of green tea showed a significant increase of 7.0% after 60 min and 6.2% after 120 min (P<0.0001). An higher increase was found after consuming 450 ml of green tea, 12.0% after 60 min and 12.7% after 120 min over baseline value (P<0.0001) (Sung et al., 2000).
Other molecular targets for the cancer preventive activity of green tea and (−)-EGCG
Tumor suppressor p53
The tumor suppressor protein p53 plays a critical role in cell cycle regulation. A mutated form of p53 protein is found in more than 50% of all human cancers (Vogelstein et al., 2000). Several studies found that green tea, and (−)-EGCG in particular, can stabilize p53, inducing G1 phase cell cycle arrest and apoptosis (Hastak et al., 2003; Kuo and Lin, 2003). Similar results were found in bovine aortic smooth muscle cells after the treatment with 80 mg/ml of (−)-EGCG for 8 hours. Nuclear p53 protein levels were found to be increased around 9-fold, resulting in cell apoptosis (Hofmann and Sonenshein, 2003). In a human liver cancer HepG2 cell line, treatment with (−)-EGCG could inhibit the cell proliferation, induce apoptosis and block cell cycle progression in the G1 phase by significantly increasing the expression of p53 protein (Kuo and Lin, 2003).
Bcl-XL and Bcl-2
In cancer cells, Bcl-XL is one of many important anti-apoptotic proteins (Green and Reed, 1998). We reported that human prostate cancer LNCaP and PC-3 cells expressed high levels of a hyperphosphorylated Bcl-XL in mitochondria. Treatment with 50 mM of (−)-EGCG blocked expression of the hyper-, but not hypophosphorylated Bcl-XL in mitochondria, accompanied by cytochrome c release, caspase activation, and apoptosis (Kazi et al., 2002). Another research group investigated the interaction of tea polyphenols with Bcl-XL and Bcl-2 proteins using a combination of nuclear magnetic resonance binding assays, fluorescence polarization assay, and computational docking studies. The results indicated that certain green tea catechins such as (−)-EGCG and (−)-ECG were very potent inhibitors (Ki in the nanomolar range) of the antiapoptotic Bcl-2-family proteins, Bcl-XL and Bcl-2 (Leone et al., 2003).
Vascular endothelial growth factor (VEGF)
Angiogenesis is a neo-vascularization process required for the growth of tumors beyond a few millimeters in diameter, and also for tumor invasion and metastasis (Eatock et al., 2000; Fox et al., 2001). One of the important factors for angiogenesis is a vascular endothelial growth factor (VEGF). GTPs and (−)-EGCG were shown to decrease VEGF expression by reducing VEGF promoter activity and transcription in human breast cancer and umbilical vein endothelial cells (Sartippour et al., 2002). Chung et al. treated an H-ras transformed mouse epidermal cell line (30.7b Ras 12) with 20 mM (−)-EGCG and results showed a significant decrease in levels of phospho-Erk 1/2 and phospho-Mek 1/2, downstream VEGF targets (Chung et al., 2001). In another in vivo study, mice bearing human HT29 colon cancer xenografts were i. p. injected with 1.5 mg/mouse/day of (−)-EGCG or (−)-EC as a control for 22 days, followed by analysis of tumor masses and characteristics (Jung et al., 2001). The results showed that (−)-EGCG inhibited tumor volume by 61% compared with (−)-EC treatment, as well as reduction in tumor vessel counts and increase in apoptosis after treatment with (−)-EGCG (Jung et al., 2001). The authors further examined the effect of (−)-EGCG on VEGF expression in HT29 cells and found that (−)-EGCG dramatically inhibited VEGF expression in a dose-dependent manner, but EC did not affect VEGF expression (Jung et al., 2001).
Heterogenous nuclear ribonucleoprotein B1 (hnRNP B1)
hnRNP B1 is an RNA-binding protein required for maturation of mRNA precursors (Sueoka et al., 1999). It was reported that hnRNP was over-expressed in the early clinical stage of lung cancer (Tockman et al., 1988). Immunohistochemical staining with the hnRNP B1 antibody revealed that hnRNP B1 protein was specifically stained in the nuclei of human cancer cells but not in the nuclei of normal adjacent lung epithelial cells (Sueoka et al., 1999). Therefore, this protein may serve as a biomarker for early detection of lung cancer. Fujimoto et al reported that treatment with 31, 62 or 125 mM of (−)-EGCG and (−)-ECG inhibited expression of hnRNP B1 mRNA examined by real-time RT-PCR and hnRNP B1 protein measured by Western blot in human lung cancer A549 cell lines in dose-dependent manner (Fujimoto et al., 2002).
Telomerase
Telomeres are the ends of the DNA strand, and the integrity of telomeres is maintained by the enzyme telomerase. The activity of this enzyme is typically absent in somatic cells but is found in tumors, where it promotes immortalization (Akiyama et al., 2002; Fu et al., 1999). Several studies are suggesting that telomerase has DNA repair capabilities with possible anti-apoptotic capacity (Akiyama et al., 2002; Fu et al., 1999). (−)-EGCG was found to exhibit significant inhibition on telomerase with an IC50 of ~1 mM in U937 monoblastoid leukemia cells in vitro (Naasani et al., 1998). In an in vivo study, Naasani et al. used nude mice models bearing human colon carcinoma cells, both telomerase-dependent (HCT-L2) and -independent (HCT-S2R) xenograft. Only the telomerase-dependent HCT-L2 tumors responded to oral administration of (−)-EGCG (1.2 mg/mouse/day), shown by decreased tumor size and decreased telomeric signals in tumor sections (Naasani et al., 2003).
Conclusions
Although tea has been consumed for centuries, it has only recently been studied extensively as a health-promoting beverage that may act to prevent a number of chronic diseases and cancers. Numerous studies have indicated that green tea consumption may be of modest benefit in reducing the plasma concentration of cholesterol and preventing atherosclerosis. Although one mechanism for cholesterol reduction may be increased expression of the LDLR, additional mechanisms and molecular targets should be explored to elucidate the prevention of cardiovascular diseases. The cancer-preventive effects of green tea are widely supported by results from epidemiological, cell culture, animal and clinical studies. Studies showed that tea polyphenols potently induce apoptotic cell death and cell cycle arrest in tumor cells but not in their normal cell counterparts and that green tea polyphenols affect several biological pathways. Various animal studies have revealed that treatment with green tea inhibits tumor incidence and multiplicity in different organ sites such as skin, lung, liver, stomach, mammary gland and colon and recently, phase I and II clinical trials have been conducted to explore the anticancer effects of green tea in humans. Studies focusing on the purified tea polyphenol compound (−)-EGCG should continue to provide researchers an improved understanding of tea polyphenol absorption, distribution, role in anti-cancer reactions, metabolism and anti-cancer mechanisms. On the other hand, work should continue on synthesizing and evaluating more analogs of green tea polyphenols to find more potent, stable and specific polyphenol proteasome inhibitors as novel anti-cancer agents. A major challenge of cancer prevention is to integrate new molecular findings into clinical practice. Identification of more molecular targets or biomarkers for tea polyphenols is paramount to cancer prevention and treatment by green tea and will greatly assist in a better understanding of its anti-cancer mechanisms.
Acknowledgments
This work is supported in part by research grants from the National Cancer Institute-National Institutes of Health (to Q. P. D.; 1R01CA120009; 5R03CA112625), the Areas of Excellence Scheme established under the University Grants Committee of the Hong Kong Special Administrative Region, China (to T. H. C.; Project No. AoE/P-10/01), and VAMC Merit Review (to A. W.).
References
- Adams J. Development of the proteasome inhibitor PS-341. Oncologist. 2002;7:9–16. doi: 10.1634/theoncologist.7-1-9. [DOI] [PubMed] [Google Scholar]
- Akiyama M, Yamada O, Kanda N, Akita S, Kawano T, Ohno T, Mizoguchi H, Eto Y, Anderson KC, Yamada H. Telomerase overexpression in K562 leukemia cells protects against apoptosis by serum deprivation and double-stranded DNA break inducing agents, but not against DNA synthesis inhibitors. Cancer Lett. 2002;178:187–197. doi: 10.1016/s0304-3835(01)00838-2. [DOI] [PubMed] [Google Scholar]
- An B, Goldfarb RH, Siman R, Dou QP. Novel dipeptidyl proteasome inhibitors overcome Bcl-2 protective function and selectively accumulate the cyclin-dependent kinase inhibitor p27 and induce apoptosis in transformed, but not normal, human fibroblasts. Cell Death Differ. 1998;5:1062–1075. doi: 10.1038/sj.cdd.4400436. [DOI] [PubMed] [Google Scholar]
- Baylin SB, Ohm JE. Epigenetic gene silencing in cancer -a mechanism for early oncogenic pathway addiction? Nat Rev Cancer. 2006;6:107–116. doi: 10.1038/nrc1799. [DOI] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. Sterol regulatory element binding proteins (SREBPs): controllers of lipid synthesis and cellular uptake. Nutr Rev. 1998;56:S1–3. doi: 10.1111/j.1753-4887.1998.tb01680.x. discussion S54–75. [DOI] [PubMed] [Google Scholar]
- Chen C, Shen G, Hebbar V, Hu R, Owuor ED, Kong AN. Epigallocatechin-3-gallate-induced stress signals in HT-29 human colon adenocarcinoma cells. Carcinogenesis. 2003;24:1369–1378. doi: 10.1093/carcin/bgg091. [DOI] [PubMed] [Google Scholar]
- Cheynier V. Polyphenols in foods are more complex than often thought. Am J Clin Nutr. 2005;81:223S–229S. doi: 10.1093/ajcn/81.1.223S. [DOI] [PubMed] [Google Scholar]
- Chung JY, Park JO, Phyu H, Dong Z, Yang CS. Mechanisms of inhibition of the Ras-MAP kinase signaling pathway in 30.7b Ras 12 cells by tea polyphenols (−)-epigallocatechin-3-gallate and theaflavin-3,3′-digallate. FASEB J. 2001;15:2022–2024. doi: 10.1096/fj.01-0031fje. [DOI] [PubMed] [Google Scholar]
- Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell. 1994;79:13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- Coimbra S, Castro E, Rocha-Pereira P, Rebelo I, Rocha S, Santos-Silva A. The effect of green tea in oxidative stress. Clin Nutr. 2006 doi: 10.1016/j.clnu.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Conney AH, Lu Y, Lou Y, Xie J, Huang M. Inhibitory effect of green and black tea on tumor growth. Proc Soc Exp Biol Med. 1999;220:229–233. doi: 10.1046/j.1525-1373.1999.d01-39.x. [DOI] [PubMed] [Google Scholar]
- Cutler RG. Oxidative stress profiling: part I. Its potential importance in the optimization of human health. Ann NY Acad Sci. 2005;1055:93–135. doi: 10.1196/annals.1323.027. [DOI] [PubMed] [Google Scholar]
- Cutler RG, Plummer J, Chowdhury K, Heward C. Oxidative stress profiling: part II. Theory, technology, and practice. Ann NY Acad Sci. 2005;1055:136–158. doi: 10.1196/annals.1323.031. [DOI] [PubMed] [Google Scholar]
- Dou QP, Goldfarb RH. Bortezomib (millennium pharmaceuticals) IDrugs. 2002;5:828–834. [PubMed] [Google Scholar]
- Dreosti IE, Wargovich MJ, Yang CS. Inhibition of carcinogenesis by tea: the evidence from experimental studies. Crit Rev Food Sci Nutr. 1997;37:761–770. doi: 10.1080/10408399709527801. [DOI] [PubMed] [Google Scholar]
- Eatock MM, Schatzlein A, Kaye SB. Tumour vasculature as a target for anticancer therapy. Cancer Treat Rev. 2000;26:191–204. doi: 10.1053/ctrv.1999.0158. [DOI] [PubMed] [Google Scholar]
- Esteller M, Risques RA, Toyota M, Capella G, Moreno V, Peinado MA, Baylin SB, Herman JG. Promoter hypermethylation of the DNA repair gene O(6)-methylguanine-DNA methyltransferase is associated with the presence of G:C to A:T transition mutations in p53 in human colorectal tumorigenesis. Cancer Res. 2001;61:4689–4692. [PubMed] [Google Scholar]
- Fang MZ, Wang Y, Ai N, Hou Z, Sun Y, Lu H, Welsh W, Yang CS. Tea polyphenol (−)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003;63:7563–7570. [PubMed] [Google Scholar]
- Feinberg AP, Tycko B. The history of cancer epigenetics. Nat Rev Cancer. 2004;4:143–153. doi: 10.1038/nrc1279. [DOI] [PubMed] [Google Scholar]
- Fox SB, Gasparini G, Harris AL. Angiogenesis: pathological, prognostic, and growth-factor pathways and their link to trial design and anticancer drugs. Lancet Oncol. 2001;2:278–289. doi: 10.1016/S1470-2045(00)00323-5. [DOI] [PubMed] [Google Scholar]
- Fu W, Begley JG, Killen MW, Mattson MP. Anti-apoptotic role of telomerase in pheochromocytoma cells. J Biol Chem. 1999;274:7264–7271. doi: 10.1074/jbc.274.11.7264. [DOI] [PubMed] [Google Scholar]
- Fujiki H. Two stages of cancer prevention with green tea. J Cancer Res Clin Oncol. 1999;125:589–597. doi: 10.1007/s004320050321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto N, Sueoka N, Sueoka E, Okabe S, Suganuma M, Harada M, Fujiki H. Lung cancer prevention with (−)-epigallocatechin gallate using monitoring by heterogeneous nuclear ribonucleoprotein B1. Int J Oncol. 2002;20:1233–1239. [PubMed] [Google Scholar]
- Goldberg AL. Functions of the proteasome: the lysis at the end of the tunnel. Science. 1995;268:522–523. doi: 10.1126/science.7725095. [DOI] [PubMed] [Google Scholar]
- Graham HN. Green tea composition, consumption, and polyphenol chemistry. Prev Med. 1992;21:334–350. doi: 10.1016/0091-7435(92)90041-f. [DOI] [PubMed] [Google Scholar]
- Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Hakim IA, Harris RB, Brown S, Chow HH, Wiseman S, Agarwal S, Talbot W. Effect of increased tea consumption on oxidative DNA damage among smokers: a randomized controlled study. J Nutr. 2003;133:3303S–3309S. doi: 10.1093/jn/133.10.3303S. [DOI] [PubMed] [Google Scholar]
- Hastak K, Gupta S, Ahmad N, Agarwal MK, Agarwal ML, Mukhtar H. Role of p53 and NF-kappaB in epigallocatechin-3-gallate-induced apoptosis of LNCaP cells. Oncogene. 2003;22:4851–4859. doi: 10.1038/sj.onc.1206708. [DOI] [PubMed] [Google Scholar]
- Hideshima T, Richardson P, Chauhan D, Palombella VJ, Elliott PJ, Adams J, Anderson KC. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61:3071–3076. [PubMed] [Google Scholar]
- Hochstrasser M. Ubiquitin, proteasomes, and the regulation of intracellular protein degradation. Curr Opin Cell Biol. 1995;7:215–223. doi: 10.1016/0955-0674(95)80031-x. [DOI] [PubMed] [Google Scholar]
- Hofmann CS, Sonenshein GE. Green tea polyphenol epigallocatechin-3 gallate induces apoptosis of proliferating vascular smooth muscle cells via activation of p53. FASEB J. 2003;17:702–704. doi: 10.1096/fj.02-0665fje. [DOI] [PubMed] [Google Scholar]
- Imai K, Nakachi K. Cross sectional study of effects of drinking green tea on cardiovascular and liver diseases. Br Med J. 1995;310:693–696. doi: 10.1136/bmj.310.6981.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K, Suga K, Nakachi K. Cancer-preventive effects of drinking green tea among a Japanese population. Prev Med. 1997;26:769–775. doi: 10.1006/pmed.1997.0242. [DOI] [PubMed] [Google Scholar]
- Jung YD, Kim MS, Shin BA, Chay KO, Ahn BW, Liu W, Bucana CD, Gallick GE, Ellis LM. EGCG, a major component of green tea, inhibits tumour growth by inhibiting VEGF induction in human colon carcinoma cells. Br J Cancer. 2001;84:844–850. doi: 10.1054/bjoc.2000.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane RC, Farrell AT, Sridhara R, Pazdur R. United States food and drug administration approval summary: bortezomib for the treatment of progressive multiple myeloma after one prior therapy. Clin Cancer Res. 2006;12:2955–2960. doi: 10.1158/1078-0432.CCR-06-0170. [DOI] [PubMed] [Google Scholar]
- Katiyar SK, Mohan RR, Agarwal R, Mukhtar H. Protection against induction of mouse skin papillomas with low and high risk of conversion to malignancy by green tea polyphenols. Carcinogenesis. 1997;18:497–502. doi: 10.1093/carcin/18.3.497. [DOI] [PubMed] [Google Scholar]
- Kazi A, Smith DM, Zhong Q, Dou QP. Inhibition of bcl-x(l) phosphorylation by tea polyphenols or epigallocatechin-3-gallate is associated with prostate cancer cell apoptosis. Mol Pharmacol. 2002;62:765–771. doi: 10.1124/mol.62.4.765. [DOI] [PubMed] [Google Scholar]
- Kazi A, Wang Z, Kumar N, Falsetti SC, Chan TH, Dou QP. Structure-activity relationships of synthetic analogs of (−)-epigallocatechin-3-gallate as proteasome inhibitors. Anticancer Res. 2004;24:943–954. [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N, Afaq F, Saleem M, Ahmad N, Mukhtar H. Targeting multiple signaling pathways by green tea polyphenol (−)-epigallocatechin-3-gallate. Cancer Res. 2006;66:2500–2505. doi: 10.1158/0008-5472.CAN-05-3636. [DOI] [PubMed] [Google Scholar]
- Kuhn DJ, Burns AC, Kazi A, Dou QP. Direct inhibition of the ubiquitin-proteasome pathway by ester bond-containing green tea polyphenols is associated with increased expression of sterol regulatory element-binding protein 2 and LDL receptor. Biochim Biophys Acta. 2004;1682:1–10. doi: 10.1016/j.bbalip.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Kuhn DJ, Lam WH, Kazi A, Daniel KG, Song S, Chow LM, Chan TH, Dou QP. Synthetic peracetate tea polyphenols as potent proteasome inhibitors and apoptosis inducers in human cancer cells. Front Biosci. 2005;10:1010–1023. doi: 10.2741/1595. [DOI] [PubMed] [Google Scholar]
- Kuo PL, Lin CC. Green tea constituent (−)-epigallocatechin-3-gallate inhibits Hep G2 cell proliferation and induces apoptosis through p53-dependent and Fas-mediated pathways. J Biomed Sci. 2003;10:219–227. doi: 10.1007/BF02256057. [DOI] [PubMed] [Google Scholar]
- Landis-Piwowar KR, Kuhn DJ, Wan SB, Chen D, Chan TH, Dou QP. Evaluation of proteasome-inhibitory and apoptosis-inducing potencies of novel (−)-EGCG analogs and their prodrugs. Int J Mol Med. 2005;15:735–742. [PubMed] [Google Scholar]
- Landis-Piwowar KR, Hou C, Chen D, Milacic V, Shi G, Chan TH, Dou Q. A Novel Pro-drug of the Green Tea Polyphenol (−)-Epigallocatechin-3-Gallate as a Potential Anti-Cancer Agent. Cancer Res. 2007;67 doi: 10.1158/0008-5472.CAN-06-4699. [DOI] [PubMed] [Google Scholar]
- Leone M, Zhai D, Sareth S, Kitada S, Reed JC, Pellecchia M. Cancer prevention by tea polyphenols is linked to their direct inhibition of antiapoptotic Bcl-2-family proteins. Cancer Res. 2003;63:8118–8121. [PubMed] [Google Scholar]
- Lopes UG, Erhardt P, Yao R, Cooper GM. p53-dependent induction of apoptosis by proteasome inhibitors. J Biol Chem. 1997;272:12893–12896. doi: 10.1074/jbc.272.20.12893. [DOI] [PubMed] [Google Scholar]
- Mantena SK, Meeran SM, Elmets CA, Katiyar SK. Orally administered green tea polyphenols prevent ultraviolet radiation-induced skin cancer in mice through activation of cytotoxic T cells and inhibition of angiogenesis in tumors. J Nutr. 2005;135:2871–2877. doi: 10.1093/jn/135.12.2871. [DOI] [PubMed] [Google Scholar]
- Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- Morley N, Clifford T, Salter L, Campbell S, Gould D, Curnow A. The green tea polyphenol (−)-epigallocatechin gallate and green tea can protect human cellular DNA from ultraviolet and visible radiation-induced damage. Photodermatol Photoimmunol Photomed. 2005;21:15–22. doi: 10.1111/j.1600-0781.2005.00119.x. [DOI] [PubMed] [Google Scholar]
- Mukhtar H, Ahmad N. Cancer chemoprevention: future holds in multiple agents. Toxicol Appl Pharmacol. 1999a;158:207–210. doi: 10.1006/taap.1999.8721. [DOI] [PubMed] [Google Scholar]
- Mukhtar H, Ahmad N. Mechanism of cancer chemopreventive activity of green Tea. Proc Soc Exp Biol Med. 1999b;220:234–238. doi: 10.1046/j.1525-1373.1999.d01-40.x. [DOI] [PubMed] [Google Scholar]
- Naasani I, Seimiya H, Tsuruo T. Telomerase inhibition, telomere shortening, and senescence of cancer cells by tea catechins. Biochem Biophys Res Commun. 1998;249:391–396. doi: 10.1006/bbrc.1998.9075. [DOI] [PubMed] [Google Scholar]
- Naasani I, Oh-Hashi F, Oh-Hara T, Feng WY, Johnston J, Chan K, Tsuruo T. Blocking telomerase by dietary polyphenols is a major mechanism for limiting the growth of human cancer cells in vitro and in vivo. Cancer Res. 2003;63:824–830. [PubMed] [Google Scholar]
- Nakachi K, Suemasu K, Suga K, Takeo T, Imai K, Higashi Y. Influence of drinking green tea on breast cancer malignancy among Japanese patients. Jpn J Cancer Res. 1998;89:254–261. doi: 10.1111/j.1349-7006.1998.tb00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam S, Smith DM, Dou QP. Ester bond-containing tea polyphenols potently inhibit proteasome activity in vitro and in vivo. J Biol Chem. 2001;276:13322–13330. doi: 10.1074/jbc.M004209200. [DOI] [PubMed] [Google Scholar]
- Pitot HC. The molecular biology of carcinogenesis. Cancer. 1993;72:962–970. doi: 10.1002/1097-0142(19930801)72:3+<962::aid-cncr2820721303>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Pitot HC, Dragan YP. Facts and theories concerning the mechanisms of carcinogenesis. FASEB J. 1991;5:2280–2286. [PubMed] [Google Scholar]
- Rah DK, Han DW, Baek HS, Hyon SH, Park JC. Prevention of reactive oxygen species-induced oxidative stress in human microvascular endothelial cells by green tea polyphenol. Toxicol Lett. 2005;155:269–275. doi: 10.1016/j.toxlet.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Russo A, Terrasi M, Agnese V, Santini D, Bazan V. Apoptosis: a relevant tool for anticancer therapy. Ann Oncol. 2006;17(Suppl 7):vii115–vii123. doi: 10.1093/annonc/mdl963. [DOI] [PubMed] [Google Scholar]
- Sartippour MR, Shao ZM, Heber D, Beatty P, Zhang L, Liu C, Ellis L, Liu W, Go VL, Brooks MN. Green tea inhibits vascular endothelial growth factor (VEGF) induction in human breast cancer cells. J Nutr. 2002;132:2307–2311. doi: 10.1093/jn/132.8.2307. [DOI] [PubMed] [Google Scholar]
- Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr. 2000;130:2073S–2085S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- Scalbert A, Manach C, Morand C, Remesy C, Jimenez L. Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr. 2005;45:287–306. doi: 10.1080/1040869059096. [DOI] [PubMed] [Google Scholar]
- Seemuller E, Lupas A, Stock D, Lowe J, Huber R, Baumeister W. Proteasome from Thermoplasma acidophilum: a threonine protease. Science. 1995;268:579–582. doi: 10.1126/science.7725107. [DOI] [PubMed] [Google Scholar]
- Sueoka E, Goto Y, Sueoka N, Kai Y, Kozu T, Fujiki H. Heterogeneous nuclear ribonucleoprotein B1 as a new marker of early detection for human lung cancers. Cancer Res. 1999;59:1404–1407. [PubMed] [Google Scholar]
- Sung H, Nah J, Chun S, Park H, Yang SE, Min WK. In vivo antioxidant effect of green tea. Eur J Clin Nutr. 2000;54:527–529. doi: 10.1038/sj.ejcn.1600994. [DOI] [PubMed] [Google Scholar]
- Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- Tockman MS, Gupta PK, Myers JD, Frost JK, Baylin SB, Gold EB, Chase AM, Wilkinson PH, Mulshine JL. Sensitive and specific monoclonal antibody recognition of human lung cancer antigen on preserved sputum cells: a new approach to early lung cancer detection. J Clin Oncol. 1988;6:1685–1693. doi: 10.1200/JCO.1988.6.11.1685. [DOI] [PubMed] [Google Scholar]
- Umemura T, Kai S, Hasegawa R, Kanki K, Kitamura Y, Nishikawa A, Hirose M. Prevention of dual promoting effects of pentachlorophenol, an environmental pollutant, on diethyl-nitrosamine-induced hepato- and cholangiocarcinogenesis in mice by green tea infusion. Carcinogenesis. 2003;24:1105–1109. doi: 10.1093/carcin/bgg053. [DOI] [PubMed] [Google Scholar]
- Vayalil PK, Elmets CA, Katiyar SK. Treatment of green tea polyphenols in hydrophilic cream prevents UVB-induced oxidation of lipids and proteins, depletion of antioxidant enzymes and phosphorylation of MAPK proteins in SKH-1 hairless mouse skin. Carcinogenesis. 2003;24:927–936. doi: 10.1093/carcin/bgg025. [DOI] [PubMed] [Google Scholar]
- Vinson J, Teufel K, Wu N. Green and black teas inhibit atherosclerosis by lipid, antioxidant, and fibrinolytic mechanisms. J Agric Food Chem. 2004;52:3661–3665. doi: 10.1021/jf035255l. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Hong JY, Huang MT, Reuhl KR, Conney AH, Yang CS. Inhibition of N-nitrosodiethylamine- and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced tumorigenesis in A/J mice by green tea and black tea. Cancer Res. 1992;52:1943–1947. [PubMed] [Google Scholar]
- Wang ZY, Wang LD, Lee MJ, Ho CT, Huang MT, Conney AH, Yang CS. Inhibition of N-nitrosomethylbenzylamine-induced esophageal tumorigenesis in rats by green and black tea. Carcinogenesis. 1995;16:2143–2148. doi: 10.1093/carcin/16.9.2143. [DOI] [PubMed] [Google Scholar]
- Yang CS, Kim S, Yang GY, Lee MJ, Liao J, Chung JY, Ho CT. Inhibition of carcinogenesis by tea: bioavailability of tea polyphenols and mechanisms of actions. Proc Soc Exp Biol Med. 1999;220:213–217. doi: 10.1046/j.1525-1373.1999.d01-36.x. [DOI] [PubMed] [Google Scholar]
- Yuasa Y, Nagasaki H, Akiyama Y, Sakai H, Nakajima T, Ohkura Y, Takizawa T, Koike M, Tani M, Iwai T, Sugihara K, Imai K, Nakachi K. Relationship between CDX2 gene methylation and dietary factors in gastric cancer patients. Carcinogenesis. 2005;26:193–200. doi: 10.1093/carcin/bgh304. [DOI] [PubMed] [Google Scholar]
- Zykova TA, Zhang Y, Zhu F, Bode AM, Dong Z. The signal transduction networks required for phosphorylation of STAT1 at Ser727 in mouse epidermal JB6 cells in the UVB response and inhibitory mechanisms of tea polyphenols. Carcinogenesis. 2005;26:331–342. doi: 10.1093/carcin/bgh334. [DOI] [PubMed] [Google Scholar]