Abstract
Whether some hypothalamic neurons have direct access to circulating metabolic cues represents a crucial question that has been intensely debated. New findings reveal that fasting promotes “leakiness” of some hypothalamic blood vessels, increasing the access of circulating factors to certain hypothalamic neurons that control feeding (Langlet et al., 2013).
The brain coordinates many aspects of energy balance and metabolism (Morton et al., 2006; Myers and Olson, 2012; Williams and Elmquist, 2012). Many of the neurons that mediate these effects lie in the brainstem and hypothalamus—especially in the hypothalamic arcuate nucleus (ARC), which lies at the base of the third cerebral ventricle, immediately above the median eminence (ME) (see Figure 1). Many ARC neurons sense circulating signals relevant to energy balance and metabolic state. Most neurons lie behind the blood-brain barrier (BBB; formed by tight junctions between the endothelial cells lining cerebral microvessels) and are not directly exposed to circulating factors, but rather sense these substances following their active transport into the brain (Banks et al., 1996). Importantly, while active transport across the BBB provides access to these circulating factors for most neurons, the proximity of the ME (which contains “leaky” fenestrated capillaries) to neurons in the medial basal ARC (Norsted et al., 2008) has prompted a significant amount of interest and debate into the issue of whether some of these ARC neurons might be able to directly (and sensitively) sense circulating factors independent of transport across the BBB. While structural studies suggest that most ARC neurons lie behind the BBB (Norsted et al., 2008), some functional studies suggest the direct sensing of circulating factors by subsets of ARC cells (Faouzi et al., 2007). This question of how peripheral signals gain access to crucial ARC neurons represents an issue central to our understanding of the mechanisms controlling energy balance and metabolism.
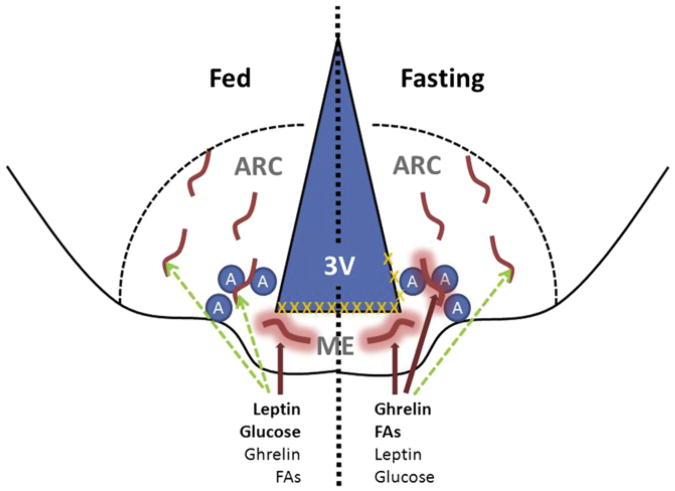
Figure 1. Model for the Fasting-Induced Control of Vascular Permeability at the ARC/ME Junction.
The arcuate nucleus of the hypothalamus (ARC) lies lateral to the third cerebral ventricle (3V) and immediately dorsolateral to the median eminence (ME). Orexigenic AgRP/NPY-expressing neurons (A) lie near the ME/ARC junction. Under normal fed conditions (left half of figure), the ME contains fenestrated blood vessels that permit the local diffusion of macromolecules from the circulation (thick red lines with halo), while the vessels in the ARC proper exhibit BBB properties that do not permit diffusion (no halo). Hence, peptides and metabolites that are high in the fed state (e.g., leptin and glucose) require BBB transport (dashed green lines) to access ARC cells. Tight junctions (orange Xs) lie above the ME under these conditions, preventing the diffusion of circulating factors into the 3V and CSF. During energy restriction (right half of figure), peptides such as ghrelin rise, along with substrates derived from lipolysis (e.g., fatty acids [FAs]), while leptin and glucose fall. Concomitantly, some of the vessels that lie along the ME/ARC junction become fenestrated, while barrier formation along the 3V extends dorsally. This change allows freer diffusion of circulating signals that indicate energy restriction to the ARC neurons (presumably AgRP cells) that lie at the ME/ARC junction, while preventing free access of these substances further into the brain and CSF. This focal plasticity in the BBB enhances the orexigenic/anabolic response to energy deficits.
A report in this issue of Cell Metabolism (Langlet et al., 2013) provides additional evidence not only that fenestrated capillaries permit enhanced access of circulating factors to the region of the ARC that adjoins the ME, but also that the extent of this process is regulated by nutritional status. While capillary fenestrations are restricted to the ME in fed animals, fasting promotes the fenestration of capillaries that reach into the medial basal ARC and increases access of circulating macromolecules into this area. Tanycytes, which are specialized glial cells that line the cerebral ventricles and surround microvessels of the ME, increase their production of the vascular permeability factor VEGF-A during fasting. Furthermore, exogenous VEGF mimics the capillary changes that are induced by fasting, while treatment with the VEGF receptor inhibitor Axitinib or tanycyte-specific ablation of Vegfa blocks capillary fenestration in fasted animals. Simultaneously, tanycytes reorganize their tight-junction complexes (the ultrastructural barrier to diffusion) in a manner that suggests that they serve to prevent the passage of blood-derived substances beyond the ME-adjacent ARC, into other brain areas and the cerebrospinal fluid (CSF; see Figure 1).
Increased capillary permeability should enhance the penetration and action of circulating metabolic signals on ARC neurons. Indeed, the authors monitored the cellular response to leptin, an adipocytederived circulating signal of replete energy stores that acts on ARC neurons, demonstrating enhanced leptin-responsiveness in fasting. This enhanced response is blocked by Axitinib, while VEGF mimics the effects of fasting in fed animals. While the investigators used leptin in this experiment because of the availability of robust assays for its cellular actions, it should be noted that physiological fasting decreases circulating leptin concentrations. One would then rather predict that the increased permeability/penetration of circulating substances into the ME-adjacent ARC during fasting would serve to accentuate the action of substances that are increased in fasting, thereby promoting feeding and other anabolic processes opposed by leptin action. Indeed, the authors found that Axitinib blunts the increase in body weight and food intake during the refeeding of fasted animals. Thus, these data suggest that the fasting-induced increase in permeability of ME-adjacent ARC vessels increases sensitivity to fasting-induced circulating factors, potentially including ghrelin and fatty acids.
The process identified by these investigators is replicated by glucoprivation and blunted by glucose treatment, revealing an important role for decreased glucose in these rearrangements. It is not clear whether glucose sensing represents the unique signal underlying this process; other metabolites (fatty acids, amino acids, etc.) or hormonal signals, including leptin, insulin, or ghrelin, could potentially contribute as well. Similarly, it will be important to clarify the extent to which this fasting-induced vascular/tight-junction reorganization contributes to the action of specific fasting signals, such as fatty acids or ghrelin.
The ME-adjacent region of the ARC, within which fasting increases vascular permeability, contains AgRP- and NPY-expressing neurons that are activated by signals of nutrient depletion and play a crucial role in the orexigenic and anabolic response to fasting (Morton et al., 2006; Myers and Olson, 2012; Williams and Elmquist, 2012). It is thus tempting to speculate that the rearrangements in vascular permeability and barrier junctions during fasting serve specifically to enhance the response of AgRP/NPY cells, promoting a stronger anabolic response. Determining whether these specific cells are more strongly affected by fasting-induced circulating factors and/or whether their exposure to the circulation is increased by this capillary/tight-junction remodeling process will represent an important line of future investigation. Relatedly, the hind-brain region surrounding the area postrema (AP; a ME-like region with its own fenestrated capillaries [Maolood and Meister, 2009]) lies close to other neurons that sense and integrate energy balance and metabolism, and it will be interesting to know whether the process identified by Langlet et al. also occurs in the AP to mediate increased access of circulating factors to these important neurons.
In addition to understanding the physiological role for this vascular/tight-junction reorganization, it will also be important to determine the contribution of the process to potential pathophysiologic processes and/or how the reorganization itself might be altered in such states. For instance, is this process normal, decreased, or accentuated in dietary or genetic obesity? Does the production of VEGF-A by tanycytes during fasting or its suppression in the fed state contribute to the apparent hypothalamic vascular proliferation and endothelial dysfunction that accompany diet-induced obesity (Yi et al., 2012)? Clearly, even as the exciting findings of Langlet et al. have helped us to understand the processes underlying direct sensing of the circulation by a subset of the hypothalamic neurons that control energy balance and metabolism, they also raise many new and important questions for the field.
References
- Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Peptides. 1996;17:305–311. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- Faouzi M, Leshan R, Björnholm M, Hennessey T, Jones J, Münzberg H. Endocrinology. 2007;148:5414–5423. doi: 10.1210/en.2007-0655. [DOI] [PubMed] [Google Scholar]
- Langlet F, Levin BE, Luquet S, Mazzone M, Messina A, Dunn-Meynell AA, Balland E, Lacombe A, Mazur D, Carmeliet P, et al. Cell Metab. 2013;17(this issue):607–617. doi: 10.1016/j.cmet.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maolood N, Meister B. J Chem Neuroanat. 2009;37:182–195. doi: 10.1016/j.jchemneu.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- Myers MG, Jr, Olson DP. Nature. 2012;491:357–363. doi: 10.1038/nature11705. [DOI] [PubMed] [Google Scholar]
- Norsted E, Gömüç B, Meister B. J Chem Neuroanat. 2008;36:107–121. doi: 10.1016/j.jchemneu.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Williams KW, Elmquist JK. Nat Neurosci. 2012;15:1350–1355. doi: 10.1038/nn.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi CX, Gericke M, Krüger M, Alkemade A, Kabra DG, Hanske S, Filosa J, Pfluger P, Bingham N, Woods SC, et al. Molecular Metabolism. 2012;1:95–100. doi: 10.1016/j.molmet.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]