Abstract
Objective
To test the fit and stability of 3 alternative models of the metabolic syndrome’s factor structure across 3 developmental stages.
Study design
With data from the Fels Longitudinal Study, confirmatory factor analyses tested 3 alternative models of the factor structure underlying relationships among 8 metabolic syndrome-associated risks. Models tested were a 1-factor model (A), a 4-factor model (B), and a second-order latent factor model (C). Developmental stages assessed were prepuberty (ages 8–10), puberty (ages 11–15), and postpuberty (ages 16–20).
Results
Convergence was achieved for all developmental stages for model A, but the fit was poor throughout (root mean square error of approximation > 0.1). Standardized factor loadings for waist circumference and body mass index were much stronger than those for fasting insulin at all 3 time points. Although prepuberty and postpuberty models converged for models B and C, each model had problems with Heywood cases. The puberty model did not converge for either model B or C.
Conclusions
The hypothetical structures commonly used to support the metabolic syndrome concept do not provide adequate fit in a pediatric sample and may be variable by maturation stage. A components-based approach to cardiovascular risk reduction, with emphasis on obesity prevention and control, may be a more appropriate clinical strategy for children and youth than a syndromic approach.
Despite the large amount of recent research on metabolic syndrome (MetS), its definition and existence remain controversial, especially in pediatrics. 1–5 Not only is the categorical definition of MetS unclear, debate continues about the structure underlying MetS. 6,7 Most work on the structure of MetS has relied on exploratory factor analysis (EFA), an analytic approach, particularly principal components analysis with orthogonal rotation. These EFA studies have consistently found between 2 to 4 factors (obesity, dyslipidemia, impaired glucose tolerance, and hypertension) depending on the number and types of risks included the analyses.8 The heterogeneity of the factors may indicate that they represent separate metabolic processes, rather than a single unifying pathogenic phenomenon. 9,10 Whether insulin resistance or obesity is a primary linking entity also remains controversial.11–13
EFA is an empirical procedure that identifies the number of latent constructs and the underlying factor structure of a set of measured variables. The goal in this exploratory approach is to let the structure of the groupings emerge from the data. There is no preconceived idea or theory being tested. The atheoretical nature of exploratory factor analysis has caused some to question its ability to provide useful insights to biologic phenomena, particularly a syndrome such as MetS.14 In contrast, confirmatory factor analysis (CFA) is a method for confirming a priori hypotheses about the latent constructs underlying relationships between observed variables. CFA is a type of structural equation modeling and has a number of advantages over exploratory factor analysis. First, CFA is a theory-driven approach. Second, CFA is more flexible than exploratory factor analysis. For example, exploratory factor analysis requires all or more commonly none of the factors to correlate, and CFA allows individual factors to be correlated with each other as specified by the investigator. Correlations between some factors and not others are more likely to represent physiological responses. Thus CFA is a more appropriate approach to studying phenomena like the clustering of cardiovascular risks than EFA.
Despite its advantages over EFA, few studies have used a CFA approach to test hypotheses about the factor structure underlying the MetS. The small number of published CFA studies have tested various hypothetical models, including 1-factor, 2-factor, 4-factor and second-order latent factor models,6,7,15–18 but there continues to be debate regarding which model best represents the factor structure underlying cardiovascular risk factor clustering. In addition, all the CFA studies have been cross-sectional, and most have studied adults. No CFA study has assessed the relationships among these factors in prepubertal children, and none has assessed the factor structure of MetS within a cohort over time or across development stages.
The purpose of this study was to fill this gap in the literature. Using data from the Fels Longitudinal Study, we assess the fit and stability of 3 alternative models of the hypothetical structure underlying MetS across 3 developmental stages— prepuberty, puberty, and postpuberty. The 3 models we chose were based on the work of Shen et al,15 which set the stage for CFA studies of the MetS structure.
Methods
These data were drawn from the Fels Longitudinal Study. The Fels Longitudinal Study has been active since 1929, studying individuals within families. There are more than 1722 living, active participants. Regular examinations occur at birth, 1, 3, 6, 9, and 12 months and then every 6 months to 18 years. Examinations occur biennially thereafter. All procedures were approved by the Institutional Review Board of Wright State University, and all participants provided written consent to join the Fels Longitudinal Study. Beginning in 1976, body composition, fasting plasma lipids, and lipoproteins were included in the study for participants 8 years and older. Data on fasting glucose and insulin began to be collected in the Fels Study in 1989 on subjects who were 8 years of age and older. Thus this study draws on data collected from 1989 onward. Inclusion criteria for this study were (1) non-Hispanic white, (2) nonpregnant, and (3) at least 2 visits between 8, the age when all 8 MetS related variables began to be assessed, and 20 years of age. We identified a total of 14 152 observations since 1989 from 963 individuals who were between 8 and 20 years of age and who were seen more than once. From these 14 152 observations, we identified 1124 visits from 257 unique individuals in which all 8 measured variables we used to develop the factor structures (see below) were assessed.
We developed our 3 developmental stage samples on the basis of these 1124 visits. Because a direct measure of pubertal stage was not present in Fels, we defined the 3 developmental stages by age in this non-Hispanic white sample. Prepubertal was defined as age between 8 and 10 years. Pubertal was defined as age between 11 and 14.99 years. Postpubertal was defined as between 16 and 20 years of age. A single visit per individual was chosen for each of these stages. For the prepubertal sample, we chose the youngest visit for an individual that occurred between 8 and 10 years of age (n = 86). For the pubertal sample, if subjects had more than 1 visit between 11 and 14.99 years of age, the visit closest to age 13 (a mid-point in this age group) was chosen. There were a total of 180 visits/individuals in the pubertal sample (n = 180). The postpubertal sample was comprised of the last visit subjects had between the ages of 16 and 20 (n = 176). Almost all of those in the prepubertal sample had a visit during puberty (n = 75 [87.2%]). Nearly two thirds of pubertal subjects had a postpubertal visit (n = 115, 63.8%). A total of 46.5% of the prepubertal subjects (n = 40) were seen at all 3 stages. When aggregated together, these 3 developmental stage samples included 251 Fels participants (135 males and 116 females). Descriptive information on the 3 developmental stage samples is shown in Table I.
Table I.
Descriptive characteristics of the 3 developmental stage samples drawn from the Fels Longitudinal Study (Aggregate N across the 3 stages = 251)
| Prepuberty (n = 86) | Puberty (n = 180) | Postpuberty (n = 176) | |
|---|---|---|---|
| Demographic characteristics | |||
| Male | 45 (52.3%) | 98 (54.4%) | 95 (54.0%) |
| Number of developmental stages assessed | |||
| 1 | 10 (11.6%) | 30 (16.7%) | 60 (34.1%) |
| 2 | 36 (41.9%) | 110 (61.1%) | 71 (43.2%) |
| 3 | 40 (46.5% | 40 (22.2%) | 40 (22.7%) |
| Age (years) | 8.9 ± 0.64 | 14.2 ± 0.68 | 18.3 ± 1.0 |
| Measured cardiovascular risks | |||
| BMI | 16.9 ± 2.9 | 21.3 ± 4.4 | 22.8 ± 4.7 |
| Waist circumference (cm) | 62.1 ± 7.8 | 78.1 ± 12.0 | 82.4 ±11.6 |
| Fasting insulin (mIU/mL) | 7.1 ± 3.8 | 14.2 ± 10.4 | 10.5 ± 7.0 |
| Fasting glucose (mg/dL) | 88.8 ± 28.8 | 93.2 ± 21.1 | 89.5 ± 23.4 |
| Triglycerides (mg/dL) | 78.6 ± 31.0 | 111.9 ± 76.3 | 113.4 ± 150.0 |
| HDL (mg/dL) | 54.2 ± 9.8 | 53.5 ± 10.5 | 47.1 ± 10.7 |
| SBP (mm Hg) | 94.9 ± 8.4 | 108.6 ± 8.9 | 108.2 ± 10.2 |
| DBP (mm Hg) | 49.7 ± 11.5 | 65.1 ± 8.7 | 66.0 ± 10.6 |
Data are either N(%) for categorical variables or mean (SD) for continuous variables.
Measured Cardiovascular Risks Used to Determine the Factor Structures
Eight cardiovascular risks (fasting plasma insulin, glucose, triglycerides, and high-density lipoprotein [HDL] cholesterol, body mass index [BMI], waist circumference, systolic blood pressure [SBP], and diastolic blood pressure [DBP]) were used to define the factor structures. Descriptive information on these risks is also found in Table I. Correlations between these eight risks is shown in Table II. Physiological measures had been assayed by use of fasting blood specimens. In addition, at each study visit, weight had been measured to 0.1 kg with a Seca scale (Seca, Hamburg, Germany) and height had been measured to 0.1 cm with a Holtain stadiometer (Holtain Ltd., Crosswell, United Kingdom), and waist circumference at the iliac crest had been measured twice, and the average values were used for analyses. Measured height and weight were used to determine BMI (kg/m2).
Table II.
Pearson correlation coefficients between measured cardiovascular risks from participants in the Fels Longitudinal Study at 3 developmental stages
| BMI | WC | Fasting insulin | Fasting glucose | Triglycerides | HDL | SBP | DBP | |
|---|---|---|---|---|---|---|---|---|
| Prepuberty (n = 86) | ||||||||
| BMI | 1 | |||||||
| Waist Circumference (cm) | .945* | 1 | ||||||
| Fasting insulin (mIU/mL) | .361* | .333* | 1 | |||||
| Fasting glucose (mg/dL) | .034 | −.005 | .515* | 1 | ||||
| Triglycerides (mg/dL) | .356* | .330* | .085 | −.042 | 1 | |||
| HDL (mg/dL) | −.318* | −.324* | −.070 | −.001 | −.598* | 1 | ||
| SBP (mm Hg) | .321* | .297* | .114 | .062 | .058 | −.012 | 1 | |
| DBP (mm Hg) | .187 | .214† | .112 | −.032 | .081 | −.009 | .203 | 1 |
| Puberty (n = 180) | ||||||||
| BMI | 1 | |||||||
| Waist circumference (cm) | .948* | 1 | ||||||
| Fasting insulin (mIU/mL) | .439* | .436* | 1 | |||||
| Fasting glucose (mg/dL) | −.046 | −.057 | .573* | 1 | ||||
| Triglycerides (mg/dL) | .343* | .359* | .341* | −.037 | 1 | |||
| HDL (mg/dL) | −.430* | −.443* | −.237* | .022 | −.340* | 1 | ||
| SBP (mm Hg) | .403* | .410* | .173† | −.071 | .282* | −.252* | 1 | |
| DBP (mm Hg) | .115 | .115 | .078 | .012 | .049 | −.013 | .294* | 1 |
| Postpuberty (n = 176) | ||||||||
| BMI | 1 | |||||||
| Waist Circumference (cm) | .915* | 1 | ||||||
| Fasting insulin (mIU/mL) | .390* | .412* | 1 | |||||
| Fasting glucose (mg/dL) | .010 | .035 | .459* | 1 | ||||
| Triglycerides (mg/dL) | .203* | .214* | .274* | −.038 | 1 | |||
| HDL (mg/dL) | −.221* | −.250* | −.183† | −.013 | −.302* | 1 | ||
| SBP (mm Hg) | .347* | .392* | .180† | .103 | .097 | −.295* | 1 | |
| DBP (mm Hg) | .190† | .219* | .039 | .131 | −.073 | .007 | .497* | 1 |
Correlation is significant at the 0.01 level (2-tailed).
Correlation is significant at the 0.05 level (2-tailed).
Hypothesized Metabolic Syndrome Factor Structures
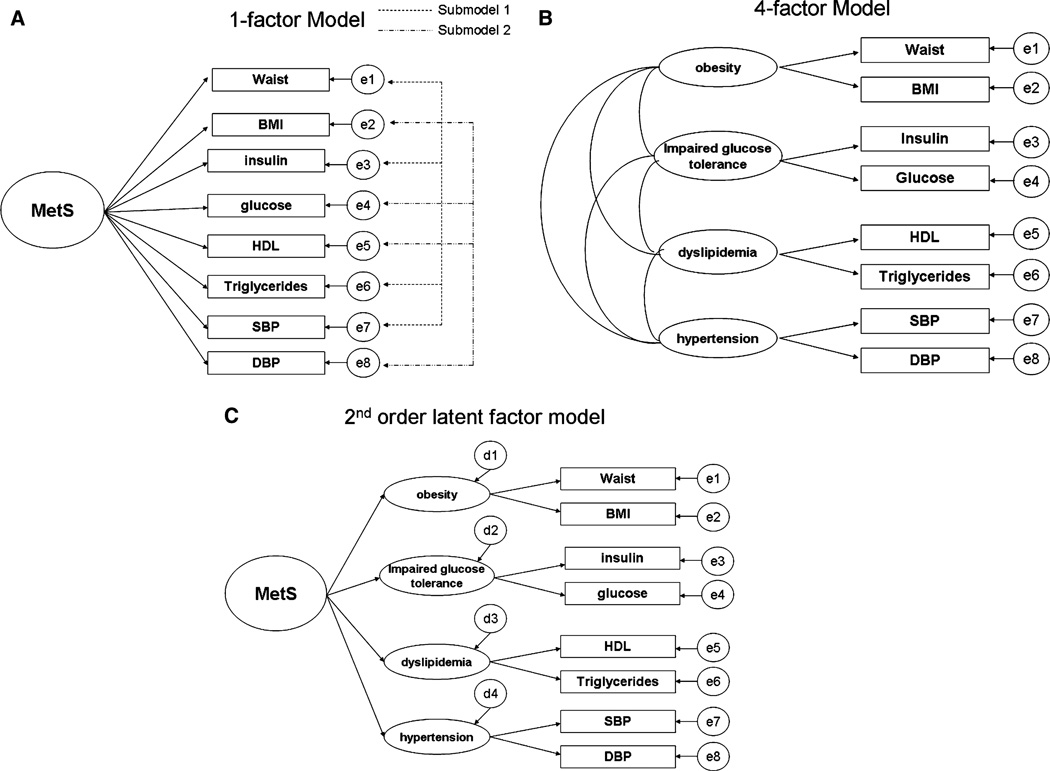
After the first published CFA study of the metabolic syndrome structure by Shen et al,15 we tested 3 hypothetical models for the factor structure underlying MetS. The factor structures were based on the 8 measured cardiovascular risks. The hypothesized structures included a 1-factor model, 4-factor model, and second-order latent factor model (Figure 1). Of note, the 4-factor model does not specify a MetS factor but outlines relationships between 4 metabolic traits, and the 1-factor and second-order latent factor models specify a MetS factor. Each of the 3 models was evaluated in each of the 3 developmental stages. In addition, because some have suggested that a reduced 4-variable 1-factor model may provide the best fit,6,18 we evaluated 2 submodels for the 1-factor structure. Submodel 1, which had been evaluated in a prior study,18 included waist circumference, fasting insulin, triglyceride levels, and SBP. The alternative 4-variable 1-factor model (submodel 2, including BMI, fasting glucose, HDL cholesterol, and DBP) was also assessed.
Figure 1.
Hypothesized metabolic syndrome factor structures. A, 1-factor model. B, 4-factor model. C, Second-order latent factor model.
Analyses
Descriptive analyses were performed with SPSS (Version 15; SPSS Inc., Chicago, Illinois), and CFA analyses were performed with Amos (version 7.0) software (Amos Developmental Corp., Chicago, Illinois). Because preliminary analyses revealed no substantial difference between analyses performed with log-transformed or -untransformed variables, no transformation of original variables was undertaken in the final CFAs reported. We present the completely standardized solution in which measured variables and latent factors are standardized so that their variances become unity. In the completely standardized solution, the factor loadings between latent factors and their measured variables can be interpreted as the correlation coefficients. Residual terms and variances are not presented for the sake of clarity. An α = 0.05 was used as the significance level for 2-tailed statistical tests.
We present 2 measures used to evaluate model fit. First, to assess the fit between the model and the empirical data, we report the χ2 test. As the null hypothesis for CFA is that the proposed model fits the data perfectly, a model with adequate fit would fail to be rejected by the χ2 test. Conversely, when a model shows a large χ2 value with a small P value, this indicates an inadequate fit of the model. The χ2 test is, however, sensitive to sample size, so we also use root mean square error of approximation (RMSEA) to assess the model fit. The RMSEA, which corrects for the number of degree of freedom in the model, ranges from 0 to 1 with <0.05 indicating close model fit.19
Results
Model A: One-Factor Model
Results for model A and its submodels are presented in Figure 2. The χ2 values are statistically significant, and RMSEA was >0.1 for all 3 models, indicating that the 1-factor model did not fit the data. Waist circumference and BMI dominated the model at all 3 developmental stages. Factor loadings for waist circumference and BMI, which represent the correlation between the MetS latent factor and those measured risks were above 0.9 and were more than 2-fold greater than the factor loading for fasting insulin for all 3 developmental stages. Fasting glucose had the weakest correlation with MetS.
Figure 2.
MODEL A = 1-factor model including submodels.
Submodels 1 and 2
In the prepuberty stage, the adiposity measure in each of the submodels was a Heywood case, because the error variance was negative for waist circumference in submodel 1 and for BMI in submodel 2. The negative error variances caused the factor loadings to be greater than 1, which is not plausible because these factor loadings represent correlation coefficients. Heywood cases invalidate the factor solution. The puberty and postpuberty stages did not have this problem, but the model fit was generally poor. Submodel 2 during puberty was the only model that demonstrated adequate fit, but the factor loading for glucose was very small (−0.06), indicating that glucose was not closely associated with the MetS latent factor. As in the 8-variable 1-factor model, measures of adiposity tended to be most closely correlated with the MetS latent factor.
Four-Factor Model
Results for model B are presented in Figure 3. Prepuberty and postpuberty models achieved convergence, but the puberty model did not. However, there were problems with the Heywood case for both models. The error variance for fasting insulin was negative in both models, as was the error variance for systolic blood pressure in the postpuberty. Removal of 2 outliers for fasting insulin did alleviate the Heywood case problem for insulin. However, the Heywood case for SBP remained, and waist circumference developed negative error variance. Therefore none of the 4-factor model has a meaningful interpretation.
Figure 3.
MODEL B = 4-factor model.
Second-Order Latent Factor Model
Results for model C are presented in Figure 4. These results were similar to those of 4-factor models shown in Figure 3. The puberty model did not achieve convergence, and the Heywood cases persisted. In addition, the error variance for obesity latent factor was also negative, adding a second Hey-wood case to the prepuberty model. As with the 4-factor model, removal of the insulin outliers did not resolve the Heywood case problems. Therefore none of the second-order latent factor models have a meaningful interpretation.
Figure 4.
MODEL C = Second-order latent factor model.
Discussion
This study assessed alternative hypothetical models for the factor structure of the MetS across 3 developmental stages in the Fels Longitudinal Study. Among the models, measures of adiposity were most closely associated with a latent MetS factor. However, none of the hypothesized models functioned well across development, and the fit was generally poor. The only model that demonstrated adequate fit was a previously untested 4-variable 1-factor model (submodel 2) during puberty. However, this same model did not demonstrate adequate fit during postpuberty and had an invalid factor solution during prepuberty. These inconsistencies mirror findings of marked instability in the clinical diagnosis of MetS in both children and adolescents.4,5
It is possible that we could have improved the fit of these models and increased consistency across development by allowing some of the errors to correlate. Shah et al16 allowed for correlated errors in both the 2-factor structure and 4-factor structure they assessed with data from the Insulin Resistance Atherosclerosis Study and determined that the 4-factor structure fit the data well. In our preliminary work, allowing for correlated errors did increase the model fit for models A and C. However, the presence of correlated error terms suggests that the latent factor represented by the measured variables does not extract all the covariance between the items with correlated errors, which, in turn, suggests additional latent factors should be present in the model. This is particularly problematic for factors with only 2 measured variables, such as the 4-factor and second-order latent factor models we assessed here. It is difficult to justify the residual correlation in such cases. Correlated errors are fallback options, which, while nearly always increasing model fit, detract from the theoretical sophistication of confirmatory factor analysis and decrease interpretability.20 Therefore we decided against allowing for correlated errors in our final models.
The decision whether to allow for correlated errors is emblematic of a serious concern with the use of factor analysis to justify physiological phenomena. Many of the decisions required in factor analysis, be it exploratory or confirmatory, are arbitrary and may be influenced by investigators’ preconceived ideas of what MetS should be. These preconceived ideas lead to errors in logic and reasoning which undermine the usefulness of MetS for science and practitioners. 3,14 For example, Pladevall et al6 developed a simplified 4-variable, 1-factor model to define MetS because they believed “the single most likely reason for the failure to show a single unifying factor is that previous EFAs used 2 or more measures for the same trait, ensuring that these highly correlated measures will cluster together under a separate factor instead of loading onto a common factor.”6 The essence of this argument is that MetS exists but the hypothesized factor structure is wrong and by improving the structure through reductionism, we will arrive at the truth. This is an example of the kind of circular logic that has plagued the metabolic syndrome field.3 Ultimately, as each complex latent factor is reduced to a single measured variable, a single latent factor structure will be the only possible structure to be assessed. Such a simplified solution may be stable across development in comparison with a more “complex” structure like the ones tested here, but it is not clear what such a simplified structure represents physiologically or pathologically.
Regardless of how well specified the model is or its goodness of fit, factor analysis does not and cannot “prove” trait clustering or its physiological mechanism. Even “confirmatory” analyses are a function of a hypothetical model. In theory, there are an infinite number of alternative models that could fit data equally well or better and thereby produce the same covariance matrix. This is known as the equivalent models problem in structural equation modeling.21 Equivalent models are of particular concern for metabolic syndrome research and theory because such equivalent models may produce conflicting interpretations. In this study, submodels 1 and 2 are examples of such conflicting models. A prior CFA study had tested submodel 1 and found it to be valid and invariant across race and sex groups of adolescents.18 In our study, this model did not fit the data well in puberty, but submodel 2, an alternative 4-variable 1-factor model that has not been assessed in prior studies, did.
The clinical implications of this are significant. Submodel 1 requires measurement of waist circumference and insulin. Measurement protocols for these are not standard in pediatric practice. On the other hand, submodel 2 calls for measurement of glucose and BMI, both of which are already assessed in pediatrics. In addition, whether either model actually reflects a syndrome which should be clinically diagnosed remains open to question.3
There are some noteworthy limitations to this study. Despite the fact that we used data from one of the most well established longitudinal studies in existence, our sample size, especially for the prepuberty stage, was relatively small. There is no large, long-term longitudinal study of children that measured the 8 cardiovascular risks we needed to assess these factor structures. Studies like the Bogalusa Heart Study22 and the National Growth and Health Study23 measured subsets of these variables, but no other study had measures of all 8 over the span of ages we assessed. Without such longitudinal cohorts, questions of the stability and developmental trajectory of metabolic syndrome and its constituent traits will remain unanswered. The relatively small size of our samples is important because confirmatory factor analysis is a type of structural equation modeling and structural equation modeling is based on large sample theory. The small sample size may be the reason some of our models had Heywood cases, which were most common in the prepuberty stage. Because of the small number of subjects seen at all 3 developmental stages, we could not assess the hypothesized structures in the same individuals across development. However, most of the 251 subjects from whom these data were drawn were seen in more than one developmental stage, lending credence to the idea that these structures are unstable across development within individuals. These data were drawn from non-Hispanic white subjects, because this racial/ethnic group comprises most of the Fels subjects, and therefore the findings may not apply to other racial/ethnic groups. Obesity, cardiovascular risks, and metabolic syndrome are more prevalent in other racial/ethnic groups.24–26 We did not have a direct assessment of puberty and so had to base our stages on relationships between age and pubertal development noted in other studies; for non-Hispanic white girls. Biro et al27 have shown that mean onset of puberty is 10.2 years, mean age of menarche, a pubertal event is 12.6 years, and achievement of adult height is 17.1 years. Such data support our developmental stage age groups. Finally, we did not assess the possibility of threshold effects, which can be done with complex CFA modeling. The small factor loading of glucose in many of the models we tested may reflect such a threshold effect, in which glucose does not contribute to the pathologic clustering until it reaches a certain threshold.
In conclusion, this study indicates that the factor structure underlying the clustering of cardiovascular risk in childhood and adolescence is variable in non-Hispanic white children and that obesity, whether measured by BMI or waist circumference, is most closely tied to the clustering phenomenon. The lack of fit and instability of the 3 hypothesized structures across development indicate that there is little evidence supporting the hypothesized factor structures of the MetS in pediatric population. Therefore a components-based approach to cardiovascular risk reduction, with emphasis on obesity prevention and control, may be a more appropriate clinical strategy for children and youth than a syndromic approach.
Acknowledgments
We thank Dr. Roger M. Siervogel, PhD, at Lifespan Health Research Center, Department of Community Health, Wright State University School of Medicine, Dayton, Ohio, for his many contributions to the Fels Longitudinal Study. We thank the children and families who participated in the Fels Longitudinal Study.
Glossary
- BMI
Body mass index
- CFA
Confirmatory factor analysis
- DBP
Diastolic blood pressure
- EFA
Exploratory factor analysis
- HDL
High-density lipoprotein
- MetS
Metabolic Syndrome
- RMSEA
Root mean square error of approximation
- SBP
Systolic blood pressure
Footnotes
The contents of this paper do not necessarily represent the views of policies of the National Institutes of Health or the official position of the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry.
Author Disclosures
The following authors have no financial arrangement or affiliation with a corporate organization or a manufacturer of a product discussed in this supplement: Elizabeth Goodman, MD, Chaoyang Li, MD, PhD, Yu-Kang Tu, PhD, Earl Ford, MD, MPH, Shumei Sun, PhD, Terry T.-K. Huang, PhD, MPH.
References
- 1.Reaven GM. The metabolic syndrome: requiescat in pace. Clin Chem. 2005;51:931–938. doi: 10.1373/clinchem.2005.048611. [DOI] [PubMed] [Google Scholar]
- 2.Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28:2289–2304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 3.Goodman E. Metabolic syndrome and the mismeasure of risk. J Adolesc Health. 2008;42:538–540. doi: 10.1016/j.jadohealth.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Goodman E, Daniels SR, Meigs JB, Dolan LM. Instability in the diagnosis of metabolic syndrome in adolescents. Circulation. 2007;115:2316–2322. doi: 10.1161/CIRCULATIONAHA.106.669994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gustafson J, Easter B, Keil M, Brady S, Han J, Roberts M, et al. Instability of the diagnosis of metabolic syndrome in children. Obesity. 2007;15(Suppl):A172. [Google Scholar]
- 6.Pladevall M, Singal B, Williams LK, Brotons C, Guyer H, Sadurni J, et al. A single factor underlies the metabolic syndrome: a confirmatory factor analysis. Diabetes Care. 2006;29:113–122. doi: 10.2337/diacare.29.1.113. [DOI] [PubMed] [Google Scholar]
- 7.Novak S, Stapleton LM, Litaker JR, Lawson KA. A confirmatory factor analysis evaluation of the coronary heart disease risk factors of metabolic syndrome with emphasis on the insulin resistance factor. Diabetes Obes Metab. 2003;5:388–396. doi: 10.1046/j.1463-1326.2003.00289.x. [DOI] [PubMed] [Google Scholar]
- 8.Meigs JB. Invited commentary: insulin resistance syndrome? Syndrome X? Multiple metabolic syndrome? A syndrome at all? Factor analysis reveals patterns in the fabric of correlated metabolic risk factors. Am J Epidemiol. 2000;152:908–911. doi: 10.1093/aje/152.10.908. [DOI] [PubMed] [Google Scholar]
- 9.Lindblad U, Langer RD, Wingard DL, Thomas RG, Barrett-Connor EL. Metabolic syndrome and ischemic heart disease in elderly men and women. Am J Epidemiol. 2001;153:481–489. doi: 10.1093/aje/153.5.481. [DOI] [PubMed] [Google Scholar]
- 10.Hanson RL, Imperatore G, Bennett PH, Knowler WC. Components of the “metabolic syndrome” and incidence of type 2 diabetes. Diabetes. 2002;51:3120–3127. doi: 10.2337/diabetes.51.10.3120. [DOI] [PubMed] [Google Scholar]
- 11.Anderson PJ, Critchley JA, Chan JC, Cockram CS, Lee ZS, Thomas GN, et al. Factor analysis of the metabolic syndrome: obesity vs insulin resistance as the central abnormality. Int J Obes Relat Metab Disord. 2001;25:1782–1788. doi: 10.1038/sj.ijo.0801837. [DOI] [PubMed] [Google Scholar]
- 12.Chen W, Srinivasan SR, Elkasabany A, Berenson GS. Cardiovascular risk factors clustering features of insulin resistance syndrome (Syndrome X) in a biracial (black-white) population of children, adolescents, and young adults: the Bogalusa Heart Study. Am J Epidemiol. 1999;150:667–674. doi: 10.1093/oxfordjournals.aje.a010069. [DOI] [PubMed] [Google Scholar]
- 13.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. for the Conference Participants Definition of Metabolic Syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on Scientific Issues Related to Definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 14.Lawlor DA, Ebrahim S, May M, DaveySmith G. (Mis)use of factor analysis in the study of insulin resistance syndrome. Am J Epidemiol. 2004;159:1013–1018. doi: 10.1093/aje/kwh150. [DOI] [PubMed] [Google Scholar]
- 15.Shen BJ, Todaro JF, Niaura R, McCaffery JM, Zhang J, Spiro A3rd, et al. Are metabolic risk factors one unified syndrome? Modeling the structure of the metabolic syndrome X. Am J Epidemiol. 2003;157:701–711. doi: 10.1093/aje/kwg045. [DOI] [PubMed] [Google Scholar]
- 16.Shah S, Novak S, Stapleton LM. Evaluation and comparison of models of metabolic syndrome using confirmatory factor analysis. Eur J Epidemiol. 2006;21:343–349. doi: 10.1007/s10654-006-9004-2. [DOI] [PubMed] [Google Scholar]
- 17.Shen BJ, Goldberg RB, Llabre MM, Schneiderman N. Is the factor structure of the metabolic syndrome comparable between men and women and across three ethnic groups: the Miami Community Health Study. Ann Epidemiol. 2006;16:131–137. doi: 10.1016/j.annepidem.2005.06.049. [DOI] [PubMed] [Google Scholar]
- 18.Li C, Ford ES. Is there a single underlying factor for the metabolic syndrome in adolescents? A confirmatory factor analysis. Diabetes Care. 2007;30:1556–1561. doi: 10.2337/dc06-2481. [DOI] [PubMed] [Google Scholar]
- 19.Brown T. Confirmatory factor analysis for applied research. New York: Guilford Press; 2006. [Google Scholar]
- 20.Gerbing D, Anderson J. On the meaning of within-factor correlated measurement errors. J Consumer Res. 1984;11:572–580. [Google Scholar]
- 21.Raykov T, Marcoulides G. Can there be infinitely many models equivalent to a given covariance structure model? Structural Equation Modeling. 2001;8:142–149. [Google Scholar]
- 22.Berenson GS, Srinivasan SR, Bao W, Newman WP, 3rd, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children young adults The Bogalusa Heart Study. N Engl J Med. 1998;338:1650–1656. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 23.Kimm SYS, Barton BA, Obarzanek E, McMahon RP, Kronsberg SS, Waclawiw MA, et al. Obesity development during adolescence in a biracial cohort: The NHLBI Growth and Health Study. Pediatrics. 2002;110:e54. doi: 10.1542/peds.110.5.e54. [DOI] [PubMed] [Google Scholar]
- 24.Goodman E, Daniels SR, Morrison JA, Huang B, Dolan LM. Contrasting prevalence of and demographic disparities in the World Health Organization and National Cholesterol Education Program Adult Treatment Panel III definitions of metabolic syndrome among adolescents. J Pediatr. 2004;145:445–451. doi: 10.1016/j.jpeds.2004.04.059. [DOI] [PubMed] [Google Scholar]
- 25.Morrison JA, Friedman LA, Harlan WR, Harlan LC, Barton BA, Schreiber GB, et al. Development of the metabolic syndrome in black and white adolescent girls: a longitudinal assessment. Pediatrics. 2005;116:1178–1182. doi: 10.1542/peds.2004-2358. [DOI] [PubMed] [Google Scholar]
- 26.Halley Castillo E, Borges G, Talavera JO, Orozco R, Vargas-Alema´n C, Huitro´n-Bravo G, et al. Body mass index and the prevalence of metabolic syndrome among children and adolescents in two Mexican populations. J Adolesc Health. 2007;40:521–526. doi: 10.1016/j.jadohealth.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 27.Biro FM, Huang B, Crawford PB, Lucky AW, Striegel-Moore R, Barton BA, et al. Pubertal correlates in black and white girls. J Pediatr. 2006;148:234–240. doi: 10.1016/j.jpeds.2005.10.020. [DOI] [PubMed] [Google Scholar]