Abstract
The cerebellum plays an important role in motor learning and cognition, and structural cerebellar abnormalities have been associated with cognitive impairment. In tuberous sclerosis complex, neurological outcome is highly variable, and no consistent imaging or pathological determinant of cognition has been firmly established. The cerebellum calls for specific attention as mouse models of tuberous sclerosis complex have demonstrated a loss of cerebellar Purkinje cells and cases of human histological data have demonstrated a similar loss in patients. We hypothesized that there might be a common cerebellar finding in tuberous sclerosis complex that could be measured as morphometric changes with magnetic resonance imaging. Using a robust, automated image analysis procedure, we studied 36 patients with tuberous sclerosis complex and age-matched controls and observed significant volume loss among patients in the cerebellar cortices and vermis. Furthermore, this effect was strongest in a subgroup of 19 patients with a known, pathogenic mutation of the tuberous sclerosis 2 gene and impacted all cerebellar structures. We conclude that patients with tuberous sclerosis complex exhibit volume loss in the cerebellum, and this loss is larger and more widespread in patients with a tuberous sclerosis 2 mutation.
Introduction
Tuberous sclerosis complex is a rare, genetic, neurocutaneous disorder affecting 1 in 6000 live births with approximately 40,000 cases in the U.S. and over one million worldwide [1, 2]. While some children with the disease remain cognitively intact, over 40% have intelligence quotients (IQs) below 70[3] and many are eventually diagnosed with an autism spectrum disorder [4, 5]. The vast majority of children with tuberous sclerosis complex will also suffer from epilepsy during their lifetime [6].
Tuberous sclerosis complex is an autosomal dominant disorder caused by germ line mutations in either of two genes: TSC1 and TSC2. Pathogenic mutations in either gene lead to dysregulation of mammalian target of rapamycin signaling and, in turn, excessive translation of proteins associated with cell growth and with the physical manifestations of the disease. Within the brain, these physical signs include tubers, subependymal nodules, and subependymal giant cell astrocytomas. The relationship between these gross morphological brain abnormalities and cognitive phenotype remains difficult to establish.
While previous morphological studies using magnetic resonance imaging (MRI) have focused largely on cortical tubers, subependymal giant cell astrocytomas, and subependymal nodules, recent evidence indicates that there may be other structural abnormalities more strongly related to cognitive phenotype [7]. Recent findings of Purkinje cell loss in a mouse model of tuberous sclerosis complex [8] and in several cases of human histology [8, 9], combined with evidence that the cerebellum plays a key role in learning and cognition [10, 11], motivated us to test whether there are morphological differences in the cerebellum in tuberous sclerosis complex that are observable in vivo using magnetic resonance imaging.
Methods
Subjects
A cohort of 36 patients with tuberous sclerosis complex (ages 1-27 years) was recruited from the Multi-Disciplinary Tuberous Sclerosis Center at Children’s Hospital Boston and 36 age-matched controls (ages 1-25 years) were recruited from the community. Control subjects underwent imaging as part of their routine care or as part of this research study. Each MRI was reviewed by a pediatric neuroradiologist (S.P.P.), and all controls had normal MRI results and normal neurologic examinations. Subject demographics are presented in Table 1. Patients fulfilled the clinical criteria for definite tuberous sclerosis complex, as defined by the Tuberous Sclerosis Complex Consensus Conference [12], and underwent genetic testing that included TSC1 and TSC2 gene sequencing and micro-deletion analysis. Genetic testing was performed by two clinical laboratories: Athena Diagnostics (Worcester, MA) and Boston University School of Medicine Center for Human Genetics (Boston, MA). Twenty-six patients had their diagnoses confirmed by genetic testing (19 TSC2, 7 TSC1). In other patients, either testing was not performed or failed to find a known, pathogenic mutation.
Table 1.
Study Demographics
| Characteristic | TSC | Controls | |
|---|---|---|---|
| sample (TSC1/TSC2/unknown) | 36 (7/19/6) | 36 | |
| gender (M:F) | 23:13 | 17:19 | |
| age (mean±sd) | 9.7±6.6 | 9.7±6.5 | paired t-test t=0.41 p=0.68 |
| phenytoin exposure (TSC1/TSC2/unknown) | 7 (1/4/1) | ||
| ACTH exposure (TSC1/TSC2/unknown) | 3 (0/2/1) | ||
| cerebellar tubers (TSC1/TSC2/unknown) | 3 (0/1/2) |
M = Male; F = Female. TSC1/TSC2 = Patients with known, likely pathogenic TSC1/TSC2 mutation. ACTH = adrenocorticotropic hormone.
Standard Protocol Approvals, Registrations, and Patient Consents
All recruitment and imaging was performed in accordance with an institutional review board (IRB) approved protocol. Written, informed consent was obtained for each participant.
Data Acquisition
To enable morphometric examination of the cerebellum in vivo, magnetic resonance imaging (MRI) was employed. Imaging was performed on a 3 Tesla Siemens Tim Trio (Siemens, Erlangen, Germany) magnetic resonance imager using a 32-channel head coil. Computational morphometry studies of the cerebellum utilized images of each patient from two pulse sequences: 1) T1-weighted images acquired using MPRAGE (TE 2.27ms/TI 800ms/TR 1410ms), FOV 240, matrix 256, slice thickness 1.0mm, spacing 0 and 2) T2-weighted images acquired using Turbo Spin Echo (TE 85ms/TR 16000ms), field-of-view 200, matrix 192, echo train length 11, slice thickness 1.2mm, spacing 0. Images for this study were acquired without contrast media.
Data Analysis
The primary measure in this study was regional tissue volume. While automatic delineation of the cerebellum can be challenging due to unexpected size and shape variation in pathology, it is necessary in order to avoid the bias and variability associated with hand-drawn measurements and to enable observer-independent analysis of large patient data sets. In order to reliably label the cerebellum in these patients, we employed a two-step procedure in which image voxels were first labeled based on image appearance as gray matter, white matter, or non-brain tissue. Voxels labeled as gray or white matter were then further subdivided, based on anatomical position, into left cerebellar hemisphere, right cerebellar hemisphere, vermis, and non-cerebellar tissue. This two-step procedure, utilizing both appearance and location features, allowed us to avoid errors likely if location cues alone were used. Details of this procedure are as follows.
The initial segmentation of gray matter and white matter using image appearance features was performed using a previously validated automatic segmentation procedure [13, 14] based on the joint distribution of image intensities in each subject’s T1 and T2-weighted images. This technique uses highly flexible, non-linear registration (Advanced Normalization Tools)[15] to warp example segmentations to the target image under study. These example segmentations are then used to train a supervised classifier to generate the probability of each tissue class at each voxel. Unlike label fusion approaches, our algorithm learns the pattern of intensities specific to a particular structure, in a particular individual’s image, and is therefore robust to changes in anatomy.
The separation of the cerebellum from the rest of the brain based on location cues, as well as the separation of the hemispheres and vermis from one another, was performed using a label fusion procedure [16]. Each of fifteen labeled example images was warped to the target subject and a consensus was generated for the left and right hemispheres and vermis using the MAP STAPLE algorithm [17, 18]. Figure 1 shows three orthogonal views of a single subject with a color-coded overlay of the cerebellar segmentation showing left and right cerebellar cortices, central cerebellar white matter, and vermis.
Figure 1. The cerebellum was segmented automatically without examiner interaction.
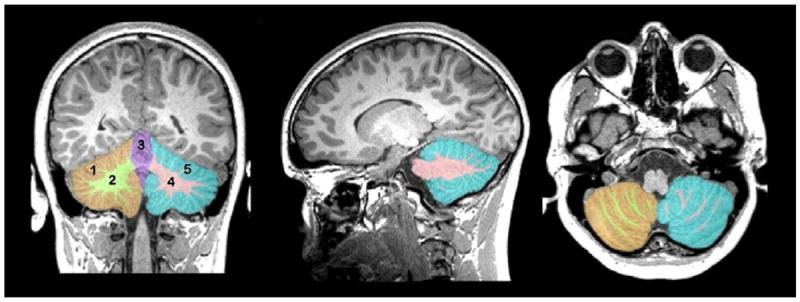
Displayed from left to right are coronal, sagittal, and axial views of a single subject’s T1-weighted MRI (see text) with the five identified cerebellar regions overlaid in color. Shown are the (1,5) cerebellar cortex, (2,4) central cerebellar white matter, and the vermis (3).
Results
Tissue volumes were generated for the cerebellar vermis, and for gray matter and white matter in each of the left and right cerebellar hemispheres, for a total of five measures per subject. Comparisons were performed using linear regression to explain regional tissue volume based on group membership (e.g. tuberous sclerosis complex versus control). Given the wide range of ages studied (1-27), it was necessary to correct for differences in overall brain size due to development. Total intracranial volume (brain parenchyma and cerebrospinal fluid) is suitable for this purpose [19] and was used as a confound to correct for global size. Because long-term exposure to phenytoin has been associated with cerebellar atrophy[20], it was necessary to test for an effect related to the drug. Given the limited degrees of freedom afforded by the sample size, separate regression models were fit for each structure and false discovery rate control [21] was used to account for multiple comparisons. Each regression model was of the form:
where volume is the measured tissue volume (response), TSC is a group indicator for tuberous sclerosis complex, TIV is the total intracranial volume to control for head size, and PHT is an indicator for any exposure to phenytoin during the patient’s lifetime.
Regression analyses are shown in Table 2. The significance of each coefficient was assessed using a two-tailed t-test with the null hypothesis that the coefficient is zero and therefore does not contribute to the model. Group membership (TSC) contributed significantly to the volume of the cerebellar cortices (left and right) and vermis, with tuberous sclerosis complex patients exhibiting significant reductions in volume in these structures. There was no significant effect for phenytoin (PHT) exposure.
Table 2.
Patients with tuberous sclerosis complex have widespread cerebellar volume loss.
| TSC v. Controls n=36/36 | TSC2 v. Controls n=19/19 | ||||||
|---|---|---|---|---|---|---|---|
| Structure | Variable | Coeff. | t-value (df=68) | p-value (*sig) | Coeff. | t-value (df=34) | p-value (*sig) |
| Cortex L | TSC | -3.65e+3 | -3.39 | 0.00118* | -6.22e+3 | -4.40 | 0.0001* |
| PHT | -8.63e+2 | -0.475 | 0.636 | 1.72e+3 | 0.747 | 0.460 | |
| Cortex R | TSC | -3.82e+3 | -3.77 | 0.000343* | -6.18e+3 | -5.11 | .0000123* |
| PHT | -4.22e+2 | -0.248 | 0.805 | 1.162e+2 | 0.59 | 0.559 | |
| White Matter L | TSC | -4.34e+2 | -1.40 | 0.166 | -1.03e+3 | -2.40 | 0.0219* |
| PHT | -5.62e+2 | -1.08 | 0.286 | -4.12e+2 | -0.588 | 0.5603 | |
| White Matter R | TSC | -3.98e+2 | -1.28 | 0.206 | -9.77e+2 | -2.18 | 0.0366* |
| PHT | -7.99e+2 | -1.523 | 0.132 | -9.02e+2 | -1.23 | 0.227 | |
| Vermis | TSC | -6.57e+2 | -2.90 | 0.00501* | -8.83e+2 | -2.89 | 0.00675* |
| PHT | 4.51e+2 | 1.18 | 0.242 | 1.80e+3 | 2.171 | 0.03704 | |
Regression analysis: TSC (n=36) and TSC2 (n=19) versus age-matched controls. Tuberous sclerosis complex patients as a whole (TSC) exhibit volume loss in the cerebellar cortices and vermis, while patients with a confirmed, pathogenic TSC2 mutation (TSC2) exhibit a stronger, more widespread effect. No significant effect of phenytoin (PHT) was observed. Significant values after false discovery rate control with (q*=0.05, alpha=0.05) are marked with an asterisk.
The data were further subdivided based on genetic analysis. Nineteen patients had known, pathogenic mutations of TSC2 whereas only seven patients had known mutations of TSC1. Patients with mutations of unknown significance were not considered. Due to the small sample size of TSC1 patients, only TSC2 patients were compared statistically with controls in this subgroup analysis. The TSC2 regression analysis is shown in Table 2 (right column) and demonstrates significant volume loss for TSC2 patients in all cerebellar regions, with an increased effect compared to that seen in the overall patient group. A potential effect for phenytoin exposure was seen only in the cerebellar vermis, and this did not reach statistical significance after false discovery rate control. The cerebellar volumes, as a fraction of total intracranial volume, are illustrated in Figure 2 and a regression analysis is shown in Figure 3.
Figure 2. Cerebellar volume is decreased in patients with TSC2 mutations.
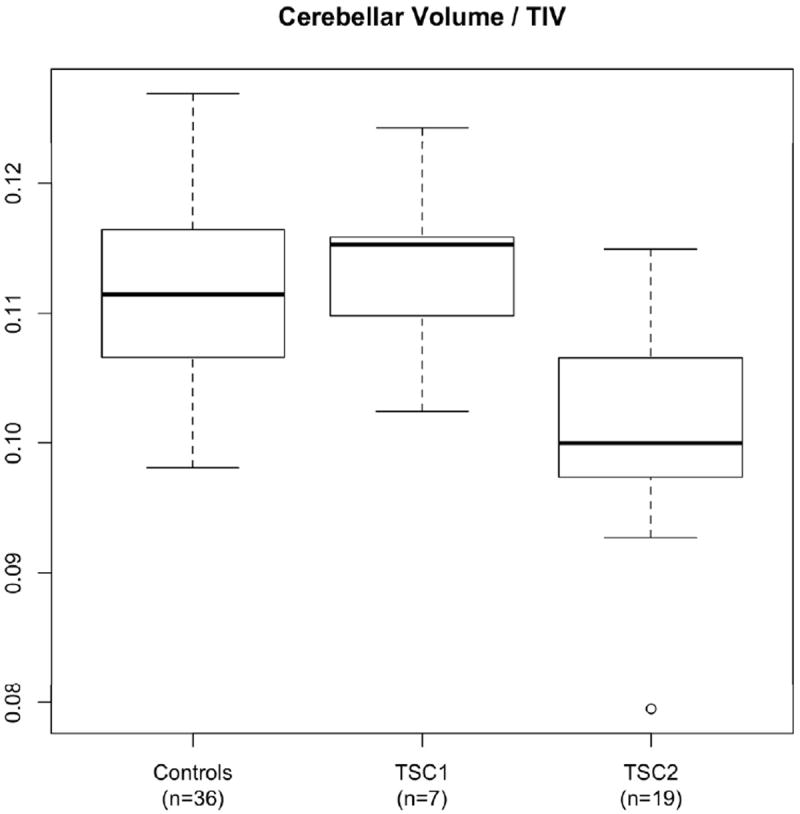
Ratio of cerebellar volume to total intracranial volume (TIV) is shown for Controls, TSC1, and TSC2. Only patients with known pathogenic mutations are included. Boxes show the first to third quartile with the median indicated by the bold line. The “whiskers” indicate the most extreme data points within 1.5 times the size of the box. Outliers beyond this range are plotted as circles.
Figure 3. Cerebellar volume is compared to total intracranial volume (TIV) to control for maturational growth.
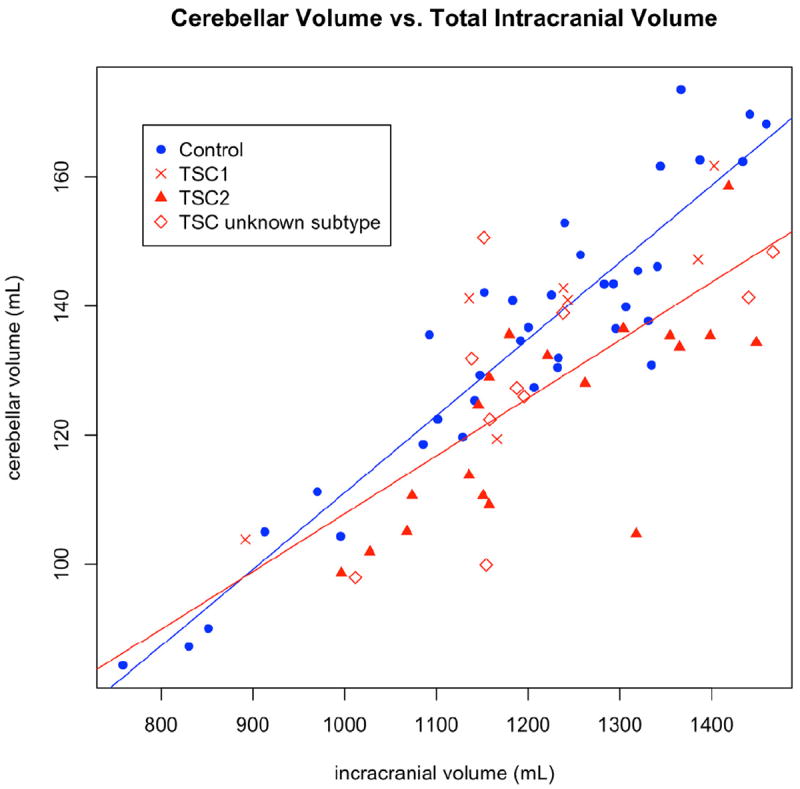
Regression lines are displayed comparing all controls (blue line) to all patients (red line). Patients are coded by symbol based on whether a known pathogenic mutation was found and, if so, whether it was TSC1 or TSC2. The TSC2 patients (triangles) appear to have smaller cerebella than the TSC1 patients (crosses). Patients whose genetic status is unknown appear evenly distributed (diamonds).
Discussion
Most imaging studies in tuberous sclerosis complex to date have focused on the study of lesions associated with the disease. Several authors have investigated the relationship between lesion number, volume, location, and functional outcomes such as epilepsy, autism, and cognitive disorders [9, 22-26]. None of these measures is sufficient to explain the range of disease phenotypes observed, with some patients exhibiting widespread tubers but only mild cognitive effects, and other patients with only mild lesion burden, but profound disability. Several groups are investigating alternate indicators of disease, such as white matter microstructure. Our group was the first to report on the relationship between microstructural abnormalities in normal appearing white matter, as measured using diffusion imaging, and neurodevelopmental outcome [7].
A few studies have examined regional tissue volumes in tuberous sclerosis complex. Ridler and colleagues undertook a general morphometric study of 10 patients and 8 controls and demonstrated widespread volumetric differences with the disease, with an increase in white matter in affected patients in a region that appears to include the cerebellar peduncles and a gray matter loss in the cerebellar cortex [27]. A larger study of 25 adult patients and controls [28] reported a gray matter loss with tuberous sclerosis complex in the cerebellum, as well as deficits in the basal ganglia and brainstem, but did not investigate white matter changes. The present study reinforces these gray matter observations but finds contradicting evidence with respect to the cerebellar white matter, with our results suggesting a reduction in cerebellar white matter volume in tuberous sclerosis complex patients.
Cerebellar involvement in tuberous sclerosis complex has been observed in mouse models and in human histology. Boer and colleagues [9] presented immunohistochemical findings from the autopsy of a 32-year old patient with a TSC2 mutation. Supratentorial findings in this patient included cortical tubers and subependymal nodules. Examination of the cerebellum revealed no apparent tubers, but found cerebellar folia with “prominent” Purkinje cell loss. Based on their observation of reactive changes including gliosis and calcifications, they hypothesized that these changes resulted from an ongoing degenerative process, rather than a developmental abnormality.
Recently, Reith and colleagues [8] generated a mouse mutant where Tsc2 was specifically deleted in cerebellar Purkinje cells. These mutants displayed Purkinje cell apoptosis beginning at 1 month of age with increasing cell loss demonstrated as mutants aged. The authors subsequently examined four cerebellum samples from human tuberous sclerosis complex patients and age-matched controls and reported finding a reduction in Purkinje cell densities in two of the four patients. The authors did not specify whether these patients had TSC1 or TSC2 mutations. They did however indicate that the samples that exhibited Purkinje cell loss came from patients with a history of seizures and exposure to phenytoin.
In a mouse model, Di Nardo and colleagues found that absence of the TSC1/2 gene product complex leads to increased vulnerability to endoplasmic reticular stress and therefore TSC1/2 serves a neuroprotective role [29]. Reith confirmed this, and found that the Purkinje cell loss they observed was due to oxidative and endoplasmic reticular stress[8]. It is unclear if the loss of this neuroprotective effect alone leads to progressive cellular injury in tuberous sclerosis complex patients, or whether seizures, medication or other stressors may contribute as well.
Possibly as many as ninety percent of tuberous sclerosis complex patients suffer seizures at one time or another [6], and several therapeutic agents may impact the cerebellum. Phenytoin is known to have potential cerebellar effects when used long-term [20], however such use has become rare due to its challenging pharmacokinetics, narrow therapeutic window, prominent drug-drug interactions, and long-term side-effects. Nonetheless we tested for, and failed to find, an effect due to the drug. Another potential effect is due to glucocorticoid treatment of infantile spasms. Both acute and chronic exposure during early development may lead to cerebellar volume loss, possibly through apoptosis of the external granule layer [30, 31]. While vigabatrin is the first-line treatment for infantile spasms in tuberous sclerosis complex, three patients were exposed to adrenocorticotropic hormone (ACTH), and two of these patients were in the TSC2 group. For this small number of patients, a potential role of the steroids in their cerebellar atrophy cannot be excluded.
Cerebellar tubers have been reported in the literature as common in tuberous sclerosis complex [32, 33], but their relationship to genotype and cerebellar volume is not currently known. Images in this study were reviewed for cerebellar tubers by a pediatric neuroradiologist (S.P.P.). Only three patients were affected, one with a TSC2 mutation and two for whom a known mutation could not be identified. Due to the small numbers encountered in our cohort, we were unable to assess the significance of these lesions.
With respect to the genetics of tuberous sclerosis complex, functional mutations in either TSC1 or TSC2 have been associated with the disease, and TSC1 and TSC2 are thought to have complementary roles [34]. TSC1 (hamartin) and TSC2 (tuberin) heterodimerize to form a GTPase activating protein complex that inhibits Rheb, itself a key activator of mammalian target of rapamycin, a critical regulator of protein synthesis. While TSC2 possesses the functional domain, TSC1 is required for stability of TSC2 by preventing its degradation; thus, both proteins are required for proper function of the complex with mutations in either sufficient to result in clinical disease.
Our data may indicate differences in cerebellar volumes based on genotype of tuberous sclerosis complex mutation. Although our TSC1 mutation possessing cohort is too small to allow for a firm comparison between genotypes, overall reduced cerebellar volumes appear to be associated with TSC2 mutation. Despite their complementary nature, the TSC1 and TSC2 protein products may serve different roles in nervous system tissue. Gutmann and colleagues [35] noted a different subcellular distribution of TSC1 and TSC2 within the mouse cerebellum, hinting at potential independent functions. Indeed, while both are required for either protein’s stability, enzymatic activity lies on TSC2. Thus, TSC2 may retain some function in the absence of TSC1, while TSC1, lacking a catalytic domain, would be unable to inhibit mammalian target of rapamycin signaling without TSC2 [36]. Furthermore, there are compelling data that patients and mouse models with TSC2 mutations exhibit more severe neurological phenotypes [37-43]. The observed difference between TSC1 and TSC2 patients is therefore plausible and warrants further investigation in a larger cohort.
The cerebellum plays an important role in learning, motor control and memory [10, 11]. Structural abnormalities including congenital lesions and aberrant morphology are strongly associated with cognitive impairment [28, 33, 44], and there is a growing body of literature reporting on the potential role of the cerebellum in autism [44-47]. In tuberous sclerosis complex specifically, the presence of tubers in the cerebellum has been linked to autism [26, 33].
We found that TSC patients have smaller cerebella than control subjects, with statistically significant reductions in the left and right cerebellar cortex and vermis, and no statistically significant reduction in left and right cerebellar white matter. When analyzing a subgroup of 19 patients with TSC2 mutations, we identified a larger and more widespread pattern of cerebellar volume loss. In this subgroup, we identified statistically significant reductions in left and right cerebellar cortex, in left and right cerebellar white matter, and in the vermis.
Future work should be aimed at uncovering the precise mechanism behind the cerebellar volume loss in tuberous sclerosis complex and its possible relationship to TSC2 mutations. Histological and animal model findings suggest a potential degenerative process, but the precise mechanism, and any developmental role, remains unclear. The relationship between this volume change and cognitive outcome in these patients, including autism, will be a topic of future investigation by our group.
Acknowledgments
The authors are indebted to the children and families who participated in this study. We also wish to thank the staff of the Multi-Disciplinary Tuberous Sclerosis Clinic, as well as other staff at Boston Children’s Hospital who were critical to this work. This investigation was supported in part by NIH grants R01 RR021885, R01 EB008015, R03 EB008680 and R01 LM010033 (NIW, SKW) and Boston Children’s Hospital Translational Research Program to (MS, SKW). PTT was supported by the American Academy of Neurology and the Nancy Lurie Marks Foundation. MS was supported by the John Merck Scholars Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hyman MH, Whittemore VH. National Institutes of Health consensus conference: tuberous sclerosis complex. Arch Neurol. 2000;57:662–5. doi: 10.1001/archneur.57.5.662. [DOI] [PubMed] [Google Scholar]
- 2.Curatolo P, Bombardieri R, Jozwiak S. Tuberous sclerosis. Lancet. 2008;372:657–68. doi: 10.1016/S0140-6736(08)61279-9. [DOI] [PubMed] [Google Scholar]
- 3.Winterkorn EB, Pulsifer MB, Thiele EA. Cognitive prognosis of patients with tuberous sclerosis complex. Neurology. 2007;68:62–4. doi: 10.1212/01.wnl.0000250330.44291.54. [DOI] [PubMed] [Google Scholar]
- 4.Jeste SS, Sahin M, Bolton P, Ploubidis GB, Humphrey A. Characterization of autism in young children with tuberous sclerosis complex. J Child Neurol. 2008;23:520–5. doi: 10.1177/0883073807309788. [DOI] [PubMed] [Google Scholar]
- 5.Curatolo P, Porfirio MC, Manzi B, Seri S. Autism in tuberous sclerosis. Eur J Paediatr Neurol. 2004;8:327–32. doi: 10.1016/j.ejpn.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Yates JR, Maclean C, Higgins JN, Humphrey A, le Marechal K, Clifford M, Carcani-Rathwell I, Sampson JR, Bolton PF. The Tuberous Sclerosis 2000 Study: presentation, initial assessments and implications for diagnosis and management. Arch Dis Child. 2011;96:1020–5. doi: 10.1136/adc.2011.211995. [DOI] [PubMed] [Google Scholar]
- 7.Peters JM, Sahin M, Vogel-Farley VK, Jeste SS, Nelson CA, 3rd, Gregas MC, Prabhu SP, Scherrer B, Warfield SK. Loss of white matter microstructural integrity is associated with adverse neurological outcome in tuberous sclerosis complex. Acad Radiol. 2012;19:17–25. doi: 10.1016/j.acra.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reith RM, Way S, McKenna J, 3rd, Haines K, Gambello MJ. Loss of the tuberous sclerosis complex protein tuberin causes Purkinje cell degeneration. Neurobiol Dis. 2011;43:113–22. doi: 10.1016/j.nbd.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boer K, Troost D, Jansen F, Nellist M, van den Ouweland AM, Geurts JJ, Spliet WG, Crino P, Aronica E. Clinicopathological and immunohistochemical findings in an autopsy case of tuberous sclerosis complex. Neuropathology. 2008;28:577–90. doi: 10.1111/j.1440-1789.2008.00920.x. [DOI] [PubMed] [Google Scholar]
- 10.O’Halloran CJ, Kinsella GJ, Storey E. The cerebellum and neuropsychological functioning: a critical review. J Clin Exp Neuropsychol. 2012;34:35–56. doi: 10.1080/13803395.2011.614599. [DOI] [PubMed] [Google Scholar]
- 11.Gordon N. The cerebellum and cognition. Eur J Paediatr Neurol. 2007;11:232–4. doi: 10.1016/j.ejpn.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Roach ES, Gomez MR, Northrup H. Tuberous sclerosis complex consensus conference: revised clinical diagnostic criteria. J Child Neurol. 1998;13:624–8. doi: 10.1177/088307389801301206. [DOI] [PubMed] [Google Scholar]
- 13.Weisenfeld NI, Warfield SK. Automatic segmentation of newborn brain MRI. Neuroimage. 2009;47:564–72. doi: 10.1016/j.neuroimage.2009.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weisenfeld NI, Warfield SK. Learning likelihoods for labeling (L3): a general multi-classifier segmentation algorithm. Med Image Comput Comput Assist Interv. 2011;14:322–9. doi: 10.1007/978-3-642-23626-6_40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54:2033–44. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rohlfing T, Russakoff DB, Maurer CR., Jr Performance-based classifier combination in atlas-based image segmentation using expectation-maximization parameter estimation. IEEE Trans Med Imaging. 2004;23:983–94. doi: 10.1109/TMI.2004.830803. [DOI] [PubMed] [Google Scholar]
- 17.Commowick O, Warfield SK. Incorporating priors on expert performance parameters for segmentation validation and label fusion: a maximum a posteriori STAPLE. Med Image Comput Comput Assist Interv. 2010;13:25–32. doi: 10.1007/978-3-642-15711-0_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warfield SK, Zou KH, Wells WM. Simultaneous truth and performance level estimation (STAPLE): an algorithm for the validation of image segmentation. IEEE Trans Med Imaging. 2004;23:903–21. doi: 10.1109/TMI.2004.828354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitwell JL, Crum WR, Watt HC, Fox NC. Normalization of cerebral volumes by use of intracranial volume: implications for longitudinal quantitative MR imaging. AJNR Am J Neuroradiol. 2001;22:1483–9. [PMC free article] [PubMed] [Google Scholar]
- 20.Crooks R, Mitchell T, Thom M. Patterns of cerebellar atrophy in patients with chronic epilepsy: a quantitative neuropathological study. Epilepsy Res. 2000;41:63–73. doi: 10.1016/s0920-1211(00)00133-9. [DOI] [PubMed] [Google Scholar]
- 21.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Statist Soc Ser B. 1995;57:289–300. [Google Scholar]
- 22.DiMario FJ., Jr Brain abnormalities in tuberous sclerosis complex. J Child Neurol. 2004;19:650–7. doi: 10.1177/08830738040190090401. [DOI] [PubMed] [Google Scholar]
- 23.Ridler K, Suckling J, Higgins N, Bolton P, Bullmore E. Standardized whole brain mapping of tubers and subependymal nodules in tuberous sclerosis complex. J Child Neurol. 2004;19:658–65. doi: 10.1177/08830738040190090501. [DOI] [PubMed] [Google Scholar]
- 24.Numis AL, Major P, Montenegro MA, Muzykewicz DA, Pulsifer MB, Thiele EA. Identification of risk factors for autism spectrum disorders in tuberous sclerosis complex. Neurology. 2011;76:981–7. doi: 10.1212/WNL.0b013e3182104347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bolton PF, Park RJ, Higgins JN, Griffiths PD, Pickles A. Neuro-epileptic determinants of autism spectrum disorders in tuberous sclerosis complex. Brain. 2002;125:1247–55. doi: 10.1093/brain/awf124. [DOI] [PubMed] [Google Scholar]
- 26.Weber AM, Egelhoff JC, McKellop JM, Franz DN. Autism and the cerebellum: evidence from tuberous sclerosis. J Autism Dev Disord. 2000;30:511–7. doi: 10.1023/a:1005679108529. [DOI] [PubMed] [Google Scholar]
- 27.Ridler K, Bullmore ET, De Vries PJ, Suckling J, Barker GJ, Meara SJ, Williams SC, Bolton PF. Widespread anatomical abnormalities of grey and white matter structure in tuberous sclerosis. Psychol Med. 2001;31:1437–46. doi: 10.1017/s0033291701004561. [DOI] [PubMed] [Google Scholar]
- 28.Ridler K, Suckling J, Higgins NJ, de Vries PJ, Stephenson CM, Bolton PF, Bullmore ET. Neuroanatomical correlates of memory deficits in tuberous sclerosis complex. Cereb Cortex. 2007;17:261–71. doi: 10.1093/cercor/bhj144. [DOI] [PubMed] [Google Scholar]
- 29.Di Nardo A, Kramvis I, Cho N, Sadowski A, Meikle L, Kwiatkowski DJ, Sahin M. Tuberous sclerosis complex activity is required to control neuronal stress responses in an mTOR-dependent manner. J Neurosci. 2009;29:5926–37. doi: 10.1523/JNEUROSCI.0778-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noguchi KK, Walls KC, Wozniak DF, Olney JW, Roth KA, Farber NB. Acute neonatal glucocorticoid exposure produces selective and rapid cerebellar neural progenitor cell apoptotic death. Cell Death Differ. 2008;15:1582–92. doi: 10.1038/cdd.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noguchi KK, Lau K, Smith DJ, Swiney BS, Farber NB. Glucocorticoid receptor stimulation and the regulation of neonatal cerebellar neural progenitor cell apoptosis. Neurobiol Dis. 2011;43:356–63. doi: 10.1016/j.nbd.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ertan G, Arulrajah S, Tekes A, Jordan L, Huisman TA. Cerebellar abnormality in children and young adults with tuberous sclerosis complex: MR and diffusion weighted imaging findings. J Neuroradiol. 2010;37:231–8. doi: 10.1016/j.neurad.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Eluvathingal TJ, Behen ME, Chugani HT, Janisse J, Bernardi B, Chakraborty P, Juhasz C, Muzik O, Chugani DC. Cerebellar lesions in tuberous sclerosis complex: neurobehavioral and neuroimaging correlates. J Child Neurol. 2006;21:846–51. doi: 10.1177/08830738060210100301. [DOI] [PubMed] [Google Scholar]
- 34.Tsai P, Sahin M. Mechanisms of neurocognitive dysfunction and therapeutic considerations in tuberous sclerosis complex. Curr Opin Neurol. 2011;24:106–13. doi: 10.1097/WCO.0b013e32834451c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gutmann DH, Zhang Y, Hasbani MJ, Goldberg MP, Plank TL, Petri Henske E. Expression of the tuberous sclerosis complex gene products, hamartin and tuberin, in central nervous system tissues. Acta Neuropathol. 2000;99:223–30. doi: 10.1007/pl00007431. [DOI] [PubMed] [Google Scholar]
- 36.Benvenuto G, Li S, Brown SJ, Braverman R, Vass WC, Cheadle JP, Halley DJ, Sampson JR, Wienecke R, DeClue JE. The tuberous sclerosis-1 (TSC1) gene product hamartin suppresses cell growth and augments the expression of the TSC2 product tuberin by inhibiting its ubiquitination. Oncogene. 2000;19:6306–16. doi: 10.1038/sj.onc.1204009. [DOI] [PubMed] [Google Scholar]
- 37.Dabora SL, Jozwiak S, Franz DN, Roberts PS, Nieto A, Chung J, Choy YS, Reeve MP, Thiele E, Egelhoff JC, Kasprzyk-Obara J, Domanska-Pakiela D, Kwiatkowski DJ. Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. Am J Hum Genet. 2001;68:64–80. doi: 10.1086/316951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeng LH, Rensing NR, Zhang B, Gutmann DH, Gambello MJ, Wong M. Tsc2 gene inactivation causes a more severe epilepsy phenotype than Tsc1 inactivation in a mouse model of tuberous sclerosis complex. Hum Mol Genet. 2011;20:445–54. doi: 10.1093/hmg/ddq491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Langkau N, Martin N, Brandt R, Zugge K, Quast S, Wiegele G, Jauch A, Rehm M, Kuhl A, Mack-Vetter M, Zimmerhackl LB, Janssen B. TSC1 and TSC2 mutations in tuberous sclerosis, the associated phenotypes and a model to explain observed TSC1/ TSC2 frequency ratios. Eur J Pediatr. 2002;161:393–402. doi: 10.1007/s00431-001-0903-7. [DOI] [PubMed] [Google Scholar]
- 40.Lewis JC, Thomas HV, Murphy KC, Sampson JR. Genotype and psychological phenotype in tuberous sclerosis. J Med Genet. 2004;41:203–7. doi: 10.1136/jmg.2003.012757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Au KS, Williams AT, Roach ES, Batchelor L, Sparagana SP, Delgado MR, Wheless JW, Baumgartner JE, Roa BB, Wilson CM, Smith-Knuppel TK, Cheung MY, Whittemore VH, King TM, Northrup H. Genotype/phenotype correlation in 325 individuals referred for a diagnosis of tuberous sclerosis complex in the United States. Genet Med. 2007;9:88–100. doi: 10.1097/gim.0b013e31803068c7. [DOI] [PubMed] [Google Scholar]
- 42.Sancak O, Nellist M, Goedbloed M, Elfferich P, Wouters C, Maat-Kievit A, Zonnenberg B, Verhoef S, Halley D, van den Ouweland A. Mutational analysis of the TSC1 and TSC2 genes in a diagnostic setting: genotype--phenotype correlations and comparison of diagnostic DNA techniques in Tuberous Sclerosis Complex. Eur J Hum Genet. 2005;13:731–41. doi: 10.1038/sj.ejhg.5201402. [DOI] [PubMed] [Google Scholar]
- 43.Jones AC, Shyamsundar MM, Thomas MW, Maynard J, Idziaszczyk S, Tomkins S, Sampson JR, Cheadle JP. Comprehensive mutation analysis of TSC1 and TSC2-and phenotypic correlations in 150 families with tuberous sclerosis. Am J Hum Genet. 1999;64:1305–15. doi: 10.1086/302381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bolduc ME, du Plessis AJ, Sullivan N, Guizard N, Zhang X, Robertson RL, Limperopoulos C. Regional Cerebellar Volumes Predict Functional Outcome in Children with Cerebellar Malformations. Cerebellum. 2011 doi: 10.1007/s12311-011-0312-z. [DOI] [PubMed] [Google Scholar]
- 45.Courchesne E, Saitoh O, Townsend JP, Yeung-Courchesne R, Press GA, Lincoln AJ, Haas RH, Schriebman L. Cerebellar hypoplasia and hyperplasia in infantile autism. Lancet. 1994;343:63–4. doi: 10.1016/s0140-6736(94)90923-7. [DOI] [PubMed] [Google Scholar]
- 46.Hodge SM, Makris N, Kennedy DN, Caviness VS, Jr, Howard J, McGrath L, Steele S, Frazier JA, Tager-Flusberg H, Harris GJ. Cerebellum, language, and cognition in autism and specific language impairment. J Autism Dev Disord. 2010;40:300–16. doi: 10.1007/s10803-009-0872-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsai PT, Hull C, Chu Y, Greene-Colozzi E, Sadowski AR, Leech JM, Steinberg J, Crawley JN, Regehr WG, Sahin M. Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature. 2012;488:647–51. doi: 10.1038/nature11310. [DOI] [PMC free article] [PubMed] [Google Scholar]


