Abstract
Generating long-term humoral immunity is a crucial component of successful vaccines and requires interactions between T cells and B cells in germinal centers (GC). In GCs, a specialized subset of CD4+ helper T cells, called T follicular helper cells (Tfh), provide help to B cells; this help directs the magnitude and quality of the antibody response. Tfh cell help influences B cell survival, proliferation, somatic hypermutation, class switch recombination, and differentiation. Sustained contact between Tfh cells and B cells is necessary for the provision of help to B cells. SAP (Signaling lymphocytic activation molecule (SLAM)-associated protein, encoded by Sh2d1a) regulates the duration of T:B cell interactions and is required for long-term humoral immunity in animal models and in humans. SAP binds to SLAM family receptors and mediates signaling that affects cell adhesion, cytokine secretion, and TCR signaling strength. Therefore, the modulation of SAP and SLAM family receptor expression represents a major axis by which the quality and duration of an antibody response is controlled after vaccination.
Introduction
Today we face urgent challenges to human health by viruses and other infectious diseases. Vaccination has saved countless lives at an affordable monetary cost. The polio vaccine alone prevents approximately 1,000,000 cases of paralysis a year, making it so successful that it is virtually invisible. Advancing our understanding of how to generate protective immune responses via vaccination will aid in confronting worldwide epidemics such as HIV, malaria, and influenza. The efficacy of most vaccines relies on antibody responses to block pathogen infection. T follicular helper cells are a subset of CD4+ helper T cells specialized for —helping B cells to generate these protective antibodies responses [1]. Tfh cells, which express the transcriptional regulator Bcl6 [2–4], interact with B cells in the germinal center. The germinal center is a complex cellular niche within secondary lymphoid tissue optimized for the production of pathogen-specific antibodies. Antigen-specific B cells acquire pathogen proteins via the B cell receptor (BCR). B cells then present pathogen-derived peptides, in the context of MHC class II, to Tfh cells in order to receive the —help signals required to survive. These multifaceted help signals from Tfh cells are also required for germinal center B cells to undergo antibody class switch recombination and progress through successive rounds of proliferation and selection resulting in increased affinity for the foreign antigen [1,5,6]. Tfh cells then direct the differentiation of germinal center B cells into either long-lived memory B cells or plasma cells leading to lasting antibody-mediated immunity. Tfh cells orchestrate the germinal center response by providing survival, proliferation, differentiation, or apoptotic signals to B cells via cell surface molecules and secreted cytokines. For Tfh cells to faithfully transmit —help signals preferentially to the best individual B cells, stable T:B interactions must occur. The SAP/SLAM family of adhesion molecules is essential for these long-duration contacts. A more thorough understanding of the —help signals and the necessity for sustained interactions between Tfh cells and B cells that lead to germinal center B cell decision making would provide an opportunity to manipulate this interaction in order to make more effective vaccines.
Tfh cells control the magnitude of the antibody response
In mice, experimentally increasing the number of Tfh cells in the germinal center increased the number of germinal center B cells and resulted in higher plasma cell numbers and antibody production [2]. The observation that Tfh help to B cells is limiting in the germinal center held in both acute viral infections and protein immunizations [2,7,8]. Blocking Tfh development during influenza infection of mice caused impaired anti-influenza germinal center B cell, plasma cell, and antibody responses [9]. In SIV (Simian Immunodeficiency Virus) infected macaques, Tfh cell numbers correlate with germinal center B cells numbers, and SIV Env-specific IgG production [10]. Developing vaccine regimens that favor the development of Tfh cells would likely lead to increased antibody responses. However, the quality of the Tfh response is equally important to the magnitude of the response [11]. During HIV infection, lymph node Tfh cells are expanded in number and are correlated with germinal center B cell numbers [12], but provide less efficient help to B cells in vitro (Cubas et al., Nat Med, in press). Lack of quality Tfh cell help to B cells may account for the reduced antibody responses to standard vaccinations in HIV+ individuals [13–17].
Tfh cells control germinal center B cell survival and differentiation
To assess the quality of —help that Tfh cells provide to B cells in the germinal center, we can examine the molecules important for the major functions of Tfh cells. Helper CD4+ T cells are separated into different subsets classically defined by the production of cytokines. Tfh cell function is dependent on cytokines and cell surface molecules. CD40L and SAP/SLAM-family receptors are important as well as cytokines IL-21 and IL-4, and the chemokine CXCL13. Within the germinal center, Tfh cells express high levels of CD40L, which is partially regulated by Bcl6 expression [18]. CD40L signaling to CD40-expressing germinal center B cells is vital. Germinal center B cells are highly apoptotic, in part due to high Fas expression, a pro-apoptotic molecule. Tfh cells must interact with germinal center B cells to provide a CD40L signal for survival [19–21]. Humans or mice lacking CD40L or CD40 expression are unable to generate germinal centers in response to T cell dependent antigens, such as viruses and most vaccines, making CD40L-CD40 signals a requirement for antibody mediated immunity.
Tfh cells induce germinal center B cells to proliferate and differentiate by providing the cytokines IL-21 and IL-4. In combination with CD40L, IL-21 is a powerful proliferative signal to B cells that can also drive the differentiation of naïve B cells into plasma cells in vitro [22,23]. Humans harboring mutations in STAT3, a key signaling molecule downstream of IL-21, have lower levels of antigen specific IgG in response to vaccination. Stimulated with IL-21 in vitro, these STAT3-deficient B cells produce less immunoglobulin, have lower expression of plasma cell differentiation genes, and cannot differentiate into antibody secreting cells [24]. Immunization of mice with genetic deletions of IL-21 or IL-21R results in reduced plasma cells numbers, reduced germinal center persistence, and limited antibody affinity maturation [25,26]. These defects were found to be intrinsic to B cells. Bcl6, a critical transcription factor for the germinal center B cell program, was reduced without IL-21 signals. Bcl6 expression in germinal center B cells is driven by STAT3 and JunD/AP-1 transcription factors [27]. Together, the data support the view that Tfh cells provided IL-21 signals through STAT3 to upregulate Bcl6 expression in germinal center B cells for survival, maturation of antibody affinity, and generation of plasma cells.
Secretion of IL-4 by Tfh cells enhances B cell survival and proliferation through increases in B cell glucose uptake and metabolism [1]. IL-4-producing CD4+ T cells in the germinal center are Tfh cells, not Th2 cells [28,29]. Although IL-4 is classically associated with Th2 cells and expression of the transcription factor GATA3, Tfh cells do not express high levels of GATA3 [29,30]. Moreover, transcription of IL-4 in Th2 and Tfh cells is regulated by different enhancers in the Il4 gene locus [31–33], implying different modes of IL-4 production. This supports a previously surprising finding that IL-4 production by Tfh cells is largely dependent on SAP/SLAM family signaling [29] as will be discussed in more detail below. The transcription factor Maf (a.k.a., c-maf) is necessary for IL-4 production [34] and can facilitate IL-21 expression in CD4+ T cells [18,35–37]. In summary, CD40L, IL-21, and IL-4 are critical signals by which Tfh cells direct germinal center B cell survival, proliferation, and differentiation into memory B cells and plasma cells capable of mounting protective antibody responses.
Tfh cells control somatic hypermutation and isotype switching
Activation-induced cytidine deaminase (AID) expression is required for both class switch recombination and affinity maturation of antibodies through somatic hypermutation in germinal center B cells [5]. Tfh cells produce cytokines to influence class switch recombination. Tfh cells can induce and regulate B cell expression of BCL6, which can positively regulate AID expression via repression of microRNA inhibition [38]. Further work must elucidate additional mechanisms by which Tfh cell help influences class switch recombination and somatic hypermutation.
Affinity maturation of antibody responses is an important part of generating highly protective antibodies against pathogens by vaccination. Interestingly, broadly neutralizing antibodies generated against HIV have undergone dramatic levels of affinity maturation, evidenced by major divergence from germline sequences [39]. Germinal center B cell maintenance and high mutational frequency are dependent on Tfh cells and will likely need to be maximized for the generation of broadly HIV-neutralizing antibodies by vaccination [11,40].
SAP-dependent regulation of germinal center responses
Regulation of Tfh cell function is important for vaccine development due to the ability of Tfh cells to impact the quantity and quality of protective antibodies. However, the generation of Tfh cells in itself is insufficient to support GC responses unless Tfh cells and B cells can form contacts and exchange signals. SAP is an important regulator of the GC response and impacts Tfh:B cell contacts and the exchange of signals.
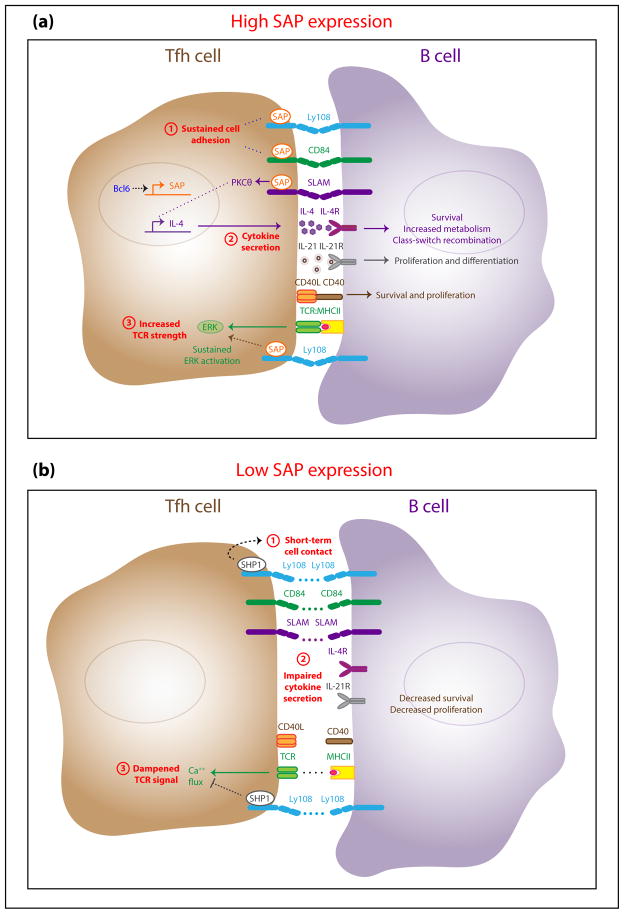
SAP is an intracellular adaptor protein that regulates immune responses. Intrinsic SAP expression in CD4+ T cells, but not in B cells, is important for the formation of germinal centers, long-lived plasma cells, and memory B cells [41,42]. In both mice and humans, SAP is expressed most highly in Tfh cells—specifically, Tfh cells located within the GC [18,29,43]. Bcl6, which is also expressed more highly in Tfh cells found in the GC, promotes SAP expression [18]. SAP binds to members of the SLAM family receptors, four of which are expressed by CD4+ T cells: SLAM (Slamf1), Ly9 (Slamf3), CD84 (Slamf5), and Ly108 (Slamf6; Ly108 in mice, NTB-A in humans). These receptors are upregulated upon activation and are more highly expressed on Tfh cells in comparison to other T cell subsets [42,44,45]. Additionally, B cells upregulate SLAM, CD84, and Ly108 upon activation [44] and constitutively express Ly9 [46,47]. Because these SLAM family receptors bind in a homophilic fashion, expression on both Tfh cells and B cells is necessary for SLAM family receptor-mediated interactions. Although it was shown that SAP is essential for germinal center responses [41], the role of SAP in this process was unclear for many years. Recent data demonstrated that SAP regulates GC responses by modulating Tfh:B cell interactions, cytokine secretion, and TCR signal strength (Figure 1). The roles of SAP in these processes will be discussed below.
Figure 1. SAP sustains Tfh:B cell contacts and allows for adequate Tfh cell help to B cells in germinal centers (GC).
(a) The presence of high SAP expression allows SAP to bind SLAM family receptors and mediate positive signaling. Positive signaling leads to sustained Tfh:B cell adhesion, cytokine secretion, and increased TCR signaling. As a result, a GC B cell receives sufficient Tfh cell help via IL-21, IL-4, and CD40L:CD40 receptor ligation that induces GC B cell survival and proliferation. (b) In the presence of low SAP expression, SHP-1 binds to Ly108 and mediates a potent negative signal that shortens Tfh:B cell contacts and curtails Tfh cell help to the GC B cell.
SAP-dependent regulation of T:B cell adhesion
In GCs, antigen-specific Tfh cells provide help to the highest affinity B cells that are best at capturing and presenting antigen [1,5,7]. Tfh cell help to the highest affinity B cells is necessary for the evolution of high affinity antibodies in the GC. The study of SAP deficiency provides insight into the requirements for Tfh cell help to B cells. To explain the humoral defects in SAP-deficient mice, early studies identified molecules that were differentially expressed in CD4+ T cells that lacked SAP [48–50]. It was later found that SAP sustained adhesion between T cells and B cells, and that sustained adhesion was necessary for the delivery of adequate Tfh cell help to B cells [51]. Even though CXCR5 and Bcl6 could be induced in SAP-deficient CD4 T cells [52,53], short-term Tfh:B cell contacts mediated through TCR ligation and integrin-mediated adhesion were insufficient to promote B cell proliferation or Tfh cell retention within the GC [51]. This discovery marked a substantial step forward in our understanding of how SAP regulates GC responses.
More recent work has further defined the molecular mechanism underlying SAP-mediated regulation of T:B cell adhesion [45,54,55]. SAP binds cytoplasmic tails of SLAM family receptors and mediates positive signaling by recruiting the Src family kinase Fyn [56] or other molecules [55,57]. Given the role of SAP in positive signaling, it was thought that the defect in long-term humoral immunity in SAP-deficient mice and humans was caused by the lack of positive signaling through one or more SLAM family receptors. Because deletions of single SLAM family receptors in mice did not lead to a phenotype as severe as that seen in SAP-deficient mice [29,45,57–59], it was then hypothesized that the absence of positive signaling through two or more redundant SLAM family receptors was responsible for the antibody response defect of SAP-deficient mice. Quite unexpectedly, it was recently shown that abrogation of signaling through Ly108 rescued GC responses in mice that lacked SAP (Kageyama-Immunity-2012). This led to the realization that defects in humoral responses in SAP-deficient mice were due to a combination of two things: 1) the lack of positive signaling through SLAM family receptors and 2) potent negative signaling through Ly108 that shortened Tfh:B cell contacts. Thus, SAP is not only important for mediating positive signals, but it is also important for competing with mediators of negative signaling for binding to Ly108. The balance between the expression of SAP and the expression of negative mediators may dictate the length of Tfh:B cell contacts. In support of this, it was found that the absence of SAP favored binding of the tyrosine phosphatase SHP-1 to the cytoplasmic tail of Ly108 in Tfh cells [45]. The phosphatase activity of SHP-1 reduces the duration of T:B adhesion, and therefore truncates signals between Tfh cells and B cells, leading to an impairment of GC responses [45]. Knowledge of the underlying molecular mechanism of SAP-mediated regulation of GC responses may be used to improve GC responses to vaccines. For example, increasing SAP expression may favor positive signaling and dampen negative signaling through Ly108, allowing for sustained T:B contacts and more robust GC responses. On the other hand, repressing negative signaling through Ly108 by blocking SHP-1 may sustain T:B contacts and increase the amount of Tfh cell help given to GC B cells.
It should be noted that Tfh:B cell interactions do not only occur in the GC. Tfh:B cell interactions start well before GC formation. Bcl6+CXCR5+ Tfh cells develop early during immune responses [53,60,61] and these Bcl6-dependent T:B interactions are important for early B cell proliferation [62]. Therefore, SAP may also be important for regulating T:B contacts before the formation of GCs. During the early humoral response, prolonged T:B interactions are seen at the border between the T cell zone and follicle (the T-B border) [63]. At this time, most B cells have low affinity for the antigen because they have not undergone affinity maturation in the GC. Given that SAP enhances adhesion between T cells and low avidity cells [54], SAP may play an important role in prolonging Tfh cell interactions with low-avidity B cells, thus providing adequate help for a broader range of B cells with diverse BCRs. This may increase the epitope breadth of antibodies generated after vaccination, which is likely to be important for protection from highly mutating pathogens [64,65]. Therefore, regulation of SAP at early as well as later stages of the humoral response may be important for the generation of a diverse and high affinity antibodies after vaccination.
SAP-dependent regulation of cytokine secretion
In addition to prolonging adhesion, SLAM family receptors regulate the secretion of cytokines. Understanding how SLAM family receptors increase Tfh cell cytokine secretion is important for understanding how to shape the germinal center response during vaccination. It has been known for more than a decade that SAP deficiency alters the cytokine profile of T cells after activation [66–68]. More recently, it was discovered that SAP specifically regulates IL-4 secretion by GC Tfh cells [29]. Production of IL-4 by Tfh cells is dependent on SLAM [29] and on positive signaling through SAP and PKC-θ [57]. GC Tfh cells have increased potential to initiate positive signaling through SLAM due to their increased expression of SAP. Moreover, GC B cells can elicit Tfh cell help through increased SLAM expression. GC B cells are located in two anatomically defined regions of the GC called the light zone (LZ) and the dark zone (DZ), while Tfh cells are localized mainly in the LZ [5]. GC B cells in the LZ upregulate SLAM expression [69,70], thereby having an increased capacity to elicit IL-4 production from Tfh cells. Therefore, upregulation of SAP in GC Tfh cells and upregulation of SLAM in LZ B cells may allow for maximal coordinated induction of IL-4 synthesis by GC Tfh cells. GC B cell modulation of other SLAM family receptors may also elicit additional forms of help from GC Tfh cells. Further research into the modulation of SLAM family receptors on B cells will provide insight into how B cells elicit particular signals from Tfh cells in the GC.
SAP-dependent amplification of TCR signals
SAP-dependent regulation of positive and negative signaling through SLAM family receptors can amplify or dampen TCR signals, which results in modified functions in response to TCR signaling [54,57]. This is especially relevant in GCs, where Tfh cells are constantly being exposed to antigen presented by GC B cells. The strength of TCR signaling allows Tfh cells to determine which B cells have the highest affinity B cell receptor (BCR) [5,7,71]. However, strong agonistic signaling through TCRs causes downregulation of TCR expression [72,73], which may decrease the strength of TCR signaling in Tfh cells over time. In the context of constant TCR stimulation in the GC, SLAM family receptors may be important for sustaining TCR signals that allow Tfh cells to selectively provide help to B cells. For example, positive signaling through Ly108 and SAP can amplify TCR signaling by sustaining ERK activation [54], which in turn prolongs TCR signaling by inhibiting dephosphorylation of the TCR [74]. This amplification of TCR signals could result in higher quality interactions between Tfh cells and B cells within the GC. In contrast, dampening of the TCR signal would be expected to inhibit Tfh cell help to B cells. In the absence of SAP, negative signaling occurs via SHP-1 binding to Ly108 at the center of the T:B synapse [45,54]. Positioning of SHP-1 to the TCR-rich center of the synapse may increase the access of SHP-1 to proteins involved in TCR signaling. Indeed, SAP-deficient T cells displayed decreased phosphorylation of Src family kinases consistent with increased SHP-1 localization near TCRs [54]. Additional signaling events can be inhibited by negative signaling through SLAM family receptors, including Vav-1 phosphorylation and calcium flux [54,55]. By amplifying or inhibiting TCR signaling, SLAM family receptors can fine-tune Tfh cell help to B cells. Enhancing positive signaling through SLAM family receptors may therefore be a potential way to sustain Tfh cell help to GC B cells to improve vaccine responses.
Future directions
Tfh cell help is essential for long-term humoral immunity, which is a key component of successful vaccines. SAP is essential for the delivery of Tfh cell help to B cells because it regulates the balance of positive and negative signaling through SLAM family receptors. This receptor family regulates cell adhesion, cytokine secretion, and TCR signaling. Importantly, B cells can elicit Tfh cell help by modulating their SLAM family receptor expression. The modulation of Tfh cell help to B cells affects GC B cell survival, proliferation, and differentiation, all of which influence the resulting protective antibody response. Regulating SLAM family receptor signaling is a promising approach for shaping the humoral response during vaccination. In support of this, a recent strategy was used to enhance SLAM receptor-mediated cytokine secretion by dendritic cells (DCs) and macrophages during vaccination [75]. Inducing expression of EAT-2, a SAP-related adaptor that binds to SLAM family receptors in DCs and macrophages, led to enhanced cytokine secretion and increased activation of innate and adaptive immune cells during vaccination. Similarly, by increasing positive signaling and inhibiting negative signaling through SLAM family receptors on Tfh cells, it may be possible to enhance T:B cell adhesion and increase Tfh signals to B cells during the GC response. Further investigation into the regulation of SLAM family receptor expression, signaling, and function in Tfh cells will be beneficial for improving rational vaccination strategies.
Highlights.
Tfh cells regulate the quantity and quality of the germinal center response.
SAP regulates the magnitude of Tfh cell help to B cells.
SAP impacts T:B adhesion, cytokine secretion, and TCR signaling strength.
Modulation of SAP and SLAM family receptors can improve responses to vaccination.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 2.Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, Wang Y-H, Dong C. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, Srivastava M, Linterman M, Zheng L, Simpson N, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31:457–468. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 6.Vinuesa CG, Cyster JG. How T cells earn the follicular rite of passage. Immunity. 2011;35:671–680. doi: 10.1016/j.immuni.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Victora GD, Schwickert TA, Fooksman DR, Kamphorst AO, Meyer-Hermann M, Dustin ML, Nussenzweig MC. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143:592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rolf J, Fairfax K, Turner M. Signaling pathways in T follicular helper cells. J Immunol. 2010;184:6563–6568. doi: 10.4049/jimmunol.1000202. [DOI] [PubMed] [Google Scholar]
- 9.Ballesteros-Tato AE, Leon B, Graf BA, Moquin A, Adams PS, Lund FE, Randall TD. Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity. 2012;36:847–856. doi: 10.1016/j.immuni.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrovas C, Yamamoto T, Gerner MY, Boswell KL, Wloka K, Smith EC, Ambrozak DR, Sandler NG, Timmer KJ, Sun X, et al. CD4 T follicular helper cell dynamics during SIV infection. J Clin Invest. 2012;122:3281–3294. doi: 10.1172/JCI63039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Streeck H, D’Souza MP, Littman DR, Crotty S. Harnessing CD4(+) T cell responses in HIV vaccine development. Nat Med. 2013;19:143–149. doi: 10.1038/nm.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindqvist M, van Lunzen J, Soghoian DZ, Kuhl BD, Ranasinghe S, Kranias G, Flanders MD, Cutler S, Yudanin N, Muller MI, et al. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J Clin Invest. 2012;122:3271–3280. doi: 10.1172/JCI64314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bekker V, Scherpbier H, Pajkrt D, Jurriaans S, Zaaijer H, Kuijpers TW. Persistent humoral immune defect in highly active antiretroviral therapy-treated children with HIV-1 infection: loss of specific antibodies against attenuated vaccine strains and natural viral infection. Pediatrics. 2006;118:e315–22. doi: 10.1542/peds.2005-2616. [DOI] [PubMed] [Google Scholar]
- 14.Mehta N, Cunningham CK, Flynn P, Pepe J, Obaro S, Kapogiannis BG, Bethel J, Luzuriaga K. Adolescent Trials Network for HIV/AIDS Interventions: Impaired generation of hepatitis B virus-specific memory B cells in HIV infected individuals following vaccination. Vaccine. 2010;28:3672–3678. doi: 10.1016/j.vaccine.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Titanji K, De Milito A, Cagigi A, Thorstensson R, Grutzmeier S, Atlas A, Hejdeman B, Kroon FP, Lopalco L, Nilsson A, et al. Loss of memory B cells impairs maintenance of long-term serologic memory during HIV-1 infection. Blood. 2006;108:1580–1587. doi: 10.1182/blood-2005-11-013383. [DOI] [PubMed] [Google Scholar]
- 16.Pallikkuth S, Parmigiani A, Silva SY, George VK, Fischl M, Pahwa R, Pahwa S. Impaired peripheral blood T-follicular helper cell function in HIV-infected nonresponders to the 2009 H1N1/09 vaccine. Blood. 2012;120:985–993. doi: 10.1182/blood-2011-12-396648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pallikkuth S, Pilakka Kanthikeel S, Silva SY, Fischl M, Pahwa R, Pahwa S. Upregulation of IL-21 receptor on B cells and IL-21 secretion distinguishes novel 2009 H1N1 vaccine responders from nonresponders among HIV-infected persons on combination antiretroviral therapy. The Journal of Immunology. 2011;186:6173–6181. doi: 10.4049/jimmunol.1100264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kroenke MA, Eto D, Locci M, Cho M, Davidson T, Haddad EK, Crotty S. Bcl6 and Maf cooperate to instruct human follicular helper CD4 T cell differentiation. The Journal of Immunology. 2012;188:3734–3744. doi: 10.4049/jimmunol.1103246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armitage RJ, Fanslow WC, Strockbine L, Sato TA, Clifford KN, Macduff BM, Anderson DM, Gimpel SD, Davis-Smith T, Maliszewski CR. Molecular and biological characterization of a murine ligand for CD40. Nature. 1992;357:80–82. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- 20.Lederman S, Yellin MJ, Inghirami G, Lee JJ, Knowles DM, Chess L. Molecular interactions mediating T-B lymphocyte collaboration in human lymphoid follicles. Roles of T cell-B-cell-activating molecule (5c8 antigen) and CD40 in contact-dependent help. J Immunol. 1992;149:3817–3826. [PubMed] [Google Scholar]
- 21.Noelle RJ, Roy M, Shepherd DM, Stamenkovic I, Ledbetter JA, Aruffo A. A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. Proc Natl Acad Sci USA. 1992;89:6550–6554. doi: 10.1073/pnas.89.14.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ettinger R, Sims GP, Fairhurst A-M, Robbins R, da Silva YS, Spolski R, Leonard WJ, Lipsky PE. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol. 2005;175:7867–7879. doi: 10.4049/jimmunol.175.12.7867. [DOI] [PubMed] [Google Scholar]
- 23.Bryant VL, Ma CS, Avery DT, Li Y, Good KL, Corcoran LM, de Waal Malefyt R, Tangye SG. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol. 2007;179:8180–8190. doi: 10.4049/jimmunol.179.12.8180. [DOI] [PubMed] [Google Scholar]
- 24.Avery DT, Deenick EK, Ma CS, Suryani S, Simpson N, Chew GY, Chan TD, Palendira U, Bustamante J, Boisson-Dupuis S, et al. B cell-intrinsic signaling through IL-21 receptor and STAT3 is required for establishing long-lived antibody responses in humans. J Exp Med. 2010;207:155–71. S1–5. doi: 10.1084/jem.20091706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zotos D, Coquet JM, Zhang Y, Light A, D’Costa K, Kallies A, Corcoran LM, Godfrey DI, Toellner K-M, Smyth MJ, et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med. 2010;207:365–378. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, Verma NK, Smyth MJ, Rigby RJ, Vinuesa CG. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arguni E. JunD/AP-1 and STAT3 are the major enhancer molecules for high Bcl6 expression in germinal center B cells. Int Immunol. 2006;18:1079–1089. doi: 10.1093/intimm/dxl041. [DOI] [PubMed] [Google Scholar]
- 28.Reinhardt RL, Liang H-E, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol. 2009;10:385–393. doi: 10.1038/ni.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yusuf I, Kageyama R, Monticelli L, Johnston RJ, DiToro D, Hansen K, Barnett B, Crotty S. Germinal center T follicular helper cell IL-4 production is dependent on signaling lymphocytic activation molecule receptor (CD150) The Journal of Immunology. 2010;185:190–202. doi: 10.4049/jimmunol.0903505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang H-E, Reinhardt RL, Bando JK, Sullivan BM, Ho I-C, Locksley RM. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat Immunol. 2011 doi: 10.1038/ni.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vijayanand P, Seumois GEG, Simpson LJ, Abdul-Wajid S, Baumjohann D, Panduro M, Huang X, Interlandi J, Djuretic IM, Brown DR, et al. Interleukin-4 production by follicular helper T cells requires the conserved Il4 enhancer hypersensitivity site V. Immunity. 2012;36:175–187. doi: 10.1016/j.immuni.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harada Y, Tanaka S, Motomura Y, Harada Y, Ohno S-I, Ohno S, Yanagi Y, Inoue H, Kubo M. The 3’ enhancer CNS2 is a critical regulator of interleukin-4-mediated humoral immunity in follicular helper T cells. Immunity. 2012;36:188–200. doi: 10.1016/j.immuni.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka S, Motomura Y, Suzuki Y, Yagi R, Inoue H, Miyatake S, Kubo M. The enhancer HS2 critically regulates GATA-3-mediated Il4 transcription in T(H)2 cells. Nat Immunol. 2011;12:77–85. doi: 10.1038/ni.1966. [DOI] [PubMed] [Google Scholar]
- 34.Kim JI, Ho IC, Grusby MJ, Glimcher LH. The transcription factor c-Maf controls the production of interleukin-4 but not other Th2 cytokines. Immunity. 1999;10:745–751. doi: 10.1016/s1074-7613(00)80073-4. [DOI] [PubMed] [Google Scholar]
- 35.Hiramatsu Y, Suto A, Kashiwakuma D, Kanari H, Kagami S-I, Ikeda K, Hirose K, Watanabe N, Grusby MJ, Iwamoto I, et al. c-Maf activates the promoter and enhancer of the IL-21 gene, and TGF-beta inhibits c-Maf-induced IL-21 production in CD4+ T cells. J Leukoc Biol. 2010;87:703–712. doi: 10.1189/jlb.0909639. [DOI] [PubMed] [Google Scholar]
- 36.Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho I-C, Sharpe AH, Kuchroo VK. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol. 2009;10:167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rani A, Afzali B, Kelly A, Tewolde-Berhan L, Hackett M, Kanhere AS, Pedroza-Pacheco I, Bowen H, Jurcevic S, Jenner RG, et al. IL-2 regulates expression of C-MAF in human CD4 T cells. The Journal of Immunology. 2011;187:3721–3729. doi: 10.4049/jimmunol.1002354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Basso K, Schneider C, Shen Q, Holmes AB, Setty M, Leslie C, Dalla-Favera R. BCL6 positively regulates AID and germinal center gene expression via repression of miR-155. J Exp Med. 2012;209:2455–2465. doi: 10.1084/jem.20121387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien J-P, Wang S-K, Ramos A, Chan-Hui P-Y, Moyle M, et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burton DR, Ahmed R, Barouch DH, Butera ST, Crotty S, Godzik A, Kaufmann DE, McElrath MJ, Nussenzweig MC, Pulendran B, et al. A Blueprint for HIV Vaccine Discovery. Cell Host and Microbe. 2012;12:396–407. doi: 10.1016/j.chom.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171:4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 42.Cannons JL, Tangye SG, Schwartzberg PL. SLAM family receptors and SAP adaptors in immunity. Annu Rev Immunol. 2011;29:665–705. doi: 10.1146/annurev-immunol-030409-101302. [DOI] [PubMed] [Google Scholar]
- 43.Ma CS, Suryani S, Avery DT, Chan A, Nanan R, Santner-Nanan B, Deenick EK, Tangye SG. Early commitment of naïve human CD4(+) T cells to the T follicular helper (T(FH)) cell lineage is induced by IL-12. Immunol Cell Biol. 2009;87:590–600. doi: 10.1038/icb.2009.64. [DOI] [PubMed] [Google Scholar]
- 44.Cannons JL, Qi H, Lu KT, Dutta M, Gomez-Rodriguez J, Cheng J, Wakeland EK, Germain RN, Schwartzberg PL. Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity. 2010;32:253–265. doi: 10.1016/j.immuni.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kageyama R, Cannons JL, Zhao F, Yusuf I, Lao C, Locci M, Schwartzberg PL, Crotty S. The Receptor Ly108 Functions as a SAP Adaptor-Dependent On-Off Switch for T Cell Help to B Cells and NKT Cell Development. Immunity. 2012 doi: 10.1016/j.immuni.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.la Fuente de MA, Tovar V, Villamor N, Zapater N, Pizcueta P, Campo E, Bosch J, Engel P. Molecular characterization and expression of a novel human leukocyte cell-surface marker homologous to mouse Ly-9. Blood. 2001;97:3513–3520. doi: 10.1182/blood.v97.11.3513. [DOI] [PubMed] [Google Scholar]
- 47.Sintes J, Vidal-Laliena M, Romero X, Tovar V, Engel P. Characterization of mouse CD229 (Ly9), a leukocyte cell surface molecule of the CD150 (SLAM) family. Tissue Antigens. 2007;70:355–362. doi: 10.1111/j.1399-0039.2007.00909.x. [DOI] [PubMed] [Google Scholar]
- 48.Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. 2005;5:853–865. doi: 10.1038/nri1714. [DOI] [PubMed] [Google Scholar]
- 49.Ma CS, Hare NJ, Nichols KE, Dupré L, Andolfi G, Roncarolo M-G, Adelstein S, Hodgkin PD, Tangye SG. Impaired humoral immunity in X-linked lymphoproliferative disease is associated with defective IL-10 production by CD4+ T cells. J Clin Invest. 2005;115:1049–1059. doi: 10.1172/JCI23139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cannons JL, Yu LJ, Hill B, Mijares LA, Dombroski D. ScienceDirect.com - Immunity - SAP Regulates TH2 Differentiation and PKC-θ-Mediated Activation of NF-κB1. Immunity. 2004 doi: 10.1016/j.immuni.2004.09.012. [no volume] [DOI] [PubMed] [Google Scholar]
- 51.Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008;455:764–769. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008;455:764–769. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, Lao C, Crotty S. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34:932–946. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao F, Cannons JL, Dutta M, Griffiths GM, Schwartzberg PL. Positive and Negative Signaling through SLAM Receptors Regulate Synapse Organization and Thresholds of Cytolysis. Immunity. 2012;36:1003–1016. doi: 10.1016/j.immuni.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dong Z, Davidson D, Perez-Quintero LA, Kurosaki T, Swat W, Veillette AE. The adaptor SAP controls NK cell activation by regulating the enzymes Vav-1 and SHIP-1 and by enhancing conjugates with target cells. Immunity. 2012;36:974–985. doi: 10.1016/j.immuni.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 56.Latour S, Roncagalli R, Chen R, Bakinowski M, Shi X, Schwartzberg PL, Davidson D, Veillette AE. Binding of SAP SH2 domain to FynT SH3 domain reveals a novel mechanism of receptor signalling in immune regulation. Nature Publishing Group. 2003;5:149–154. doi: 10.1038/ncb919. [DOI] [PubMed] [Google Scholar]
- 57.Cannons JL, Wu JZ, Gomez-Rodriguez J, Zhang J, Dong B, Liu Y, Shaw S, Siminovitch KA, Schwartzberg PL. Biochemical and Genetic Evidence for a SAP-PKC- Interaction Contributing to IL-4 Regulation. The Journal of Immunology. 2010;185:2819–2827. doi: 10.4049/jimmunol.0902182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Graham DB, Bell MP, McCausland MM, Huntoon CJ, van Deursen J, Faubion WA, Crotty S, McKean DJ. Ly9 (CD229)-deficient mice exhibit T cell defects yet do not share several phenotypic characteristics associated with SLAM- and SAP-deficient mice. J Immunol. 2006;176:291–300. doi: 10.4049/jimmunol.176.1.291. [DOI] [PubMed] [Google Scholar]
- 59.McCausland MM, Yusuf I, Tran H, Ono N, Yanagi Y, Crotty S. SAP Regulation of Follicular Helper CD4 T Cell Development and Humoral Immunity Is Independent of SLAM and Fyn Kinase. The Journal of. 2007 doi: 10.4049/jimmunol.178.2.817. [no volume] [DOI] [PubMed] [Google Scholar]
- 60.Kitano M, Moriyama S, Ando Y, Hikida M, Mori Y, Kurosaki T, Okada T. Bcl6 protein expression shapes pre-germinal center B cell dynamics and follicular helper T cell heterogeneity. Immunity. 2011;34:961–972. doi: 10.1016/j.immuni.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 61.Kerfoot SM, Yaari G, Patel JR, Johnson KL, Gonzalez DG, Kleinstein SH, Haberman AM. Germinal center B cell and T follicular helper cell development initiates in the interfollicular zone. Immunity. 2011;34:947–960. doi: 10.1016/j.immuni.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee SK, Rigby RJ, Zotos D, Tsai LM, Kawamoto S, Marshall JL, Ramiscal RR, Chan TD, Gatto D, Brink R, et al. B cell priming for extrafollicular antibody responses requires Bcl-6 expression by T cells. J Exp Med. 2011;208:1377–1388. doi: 10.1084/jem.20102065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Okada T, Miller MJ, Parker I, Krummel MF, Neighbors M, Hartley SB, O’Garra A, Cahalan MD, Cyster JG. Antigen-Engaged B Cells Undergo Chemotaxis toward the T Zone and Form Motile Conjugates with Helper T Cells. PLoS Biol. 2005;3:e150. doi: 10.1371/journal.pbio.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pollard AJ, Hill AVS. Antibody repertoire: embracing diversity. Science Translational Medicine. 2011;3:93ps32. doi: 10.1126/scitranslmed.3002694. [DOI] [PubMed] [Google Scholar]
- 65.Purtha WE, Tedder TF, Johnson S, Bhattacharya D, Diamond MS. Memory B cells, but not long-lived plasma cells, possess antigen specificities for viral escape mutants. J Exp Med. 2011;208:2599–2606. doi: 10.1084/jem.20110740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Czar MJ, Kersh EN, Mijares LA, Lanier G, Lewis J, Yap G, Chen A, Sher A, Duckett CS, Ahmed R, et al. Altered lymphocyte responses and cytokine production in mice deficient in the X-linked lymphoproliferative disease gene SH2D1A/DSHP/SAP. Proc Natl Acad Sci USA. 2001;98:7449–7454. doi: 10.1073/pnas.131193098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Latour S, Gish G, Helgason CD, Humphries RK, Pawson T, Veillette A. Regulation of SLAM-mediated signal transduction by SAP, the X-linked lymphoproliferative gene product. Nat Immunol. 2001;2:681–690. doi: 10.1038/90615. [DOI] [PubMed] [Google Scholar]
- 68.Wu C, Nguyen KB, Pien GC, Wang N, Gullo C, Howie D, Sosa MR, Edwards MJ, Borrow P, Satoskar AR, et al. SAP controls T cell responses to virus and terminal differentiation of TH2 cells. Nat Immunol. 2001;2:410–414. doi: 10.1038/87713. [DOI] [PubMed] [Google Scholar]
- 69.Victora GD, Schwickert TA, Fooksman DR, Kamphorst AO, Meyer-Hermann M, Dustin ML, Nussenzweig MC. Germinal Center Dynamics Revealed by Multiphoton Microscopy with a Photoactivatable Fluorescent Reporter. Cell. 2010;143:592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Victora GD, Dominguez-Sola D, Holmes AB, Deroubaix S, Dalla-Favera R, Nussenzweig MC. Identification of human germinal center light and dark zone cells and their relationship to human B-cell lymphomas. 2012 doi: 10.1182/blood-2012-03-415380. [no volume] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Allen CDC, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007;27:190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martin S, Bevan MJ. Transient alteration of T cell fine specificity by a strong primary stimulus correlates with T cell receptor down-regulation. Eur J Immunol. 1998;28:2991–3002. doi: 10.1002/(SICI)1521-4141(199810)28:10<2991::AID-IMMU2991>3.0.CO;2-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.San José E, Borroto A, Niedergang F, Alcover A, Alarcón B. Triggering the TCR complex causes the downregulation of nonengaged receptors by a signal transduction-dependent mechanism. Immunity. 2000;12:161–170. doi: 10.1016/s1074-7613(00)80169-7. [DOI] [PubMed] [Google Scholar]
- 74.Stefanová I, Hemmer B, Vergelli M, Martin R, Biddison WE, Germain RN. TCR ligand discrimination is enforced by competing ERK positive and SHP-1 negative feedback pathways. Nat Immunol. 2003;4:248–254. doi: 10.1038/ni895. [DOI] [PubMed] [Google Scholar]
- 75.Aldhamen YA, Appledorn DM, Seregin SS, Liu C-JJ, Schuldt NJ, Godbehere S, Amalfitano A. Expression of the SLAM family of receptors adapter EAT-2 as a novel strategy for enhancing beneficial immune responses to vaccine antigens. The Journal of Immunology. 2011;186:722–732. doi: 10.4049/jimmunol.1002105. [DOI] [PMC free article] [PubMed] [Google Scholar]