Abstract
Medulloblastoma is a malignant pediatric brain tumor. Current treatment following patient stratification into standard and high-risk groups using clinical features, has improved survival. However, a subset of patients with standard-risk features have unanticipated aggressive disease, underscoring the need for a better understanding of tumor biology and development of novel treatments. Poor differentiation, a hallmark of medulloblastomas is associated with elevated expression levels of the repressor of neuronal differentiation REST. Here, we assessed if elevated REST expression levels had prognostic significance and if its pharmacological manipulation would promote neurogenesis and block tumor cell growth. REST levels in patient tumors were measured by immunohistochemistry (IHC) and stratified into low/moderate- (+/++/+++) and high-REST (+++++) groups. Kaplan-Meier curves revealed that patients with high-REST tumors had worse overall and event-free survival compared to patients with REST-negative or REST-low tumors. Since histone deacetylases (HDACs), are required for REST-dependent repression of neurogenesis, we evaluated a panel of HDAC inhibitors (HDACIs) for their effects on growth and differentiation of established and primary REST-positive cell lines. MS-275, trichostatin-A (TSA), valproic acid (VPA) and suberoylanilide hydroxamic acid (SAHA) upregulated expression of the REST-target neuronal differentiation gene, Syn1, suggesting a potential effect of these HDACIs on REST function. Interestingly, VPA and TSA substantially increased histone acetylation at the REST promoter and activated its transcription, whereas SAHA unexpectedly promoted its proteasomal degradation. A REST-dependent decrease in cell growth was also observed following SAHA treatment. Thus, our studies suggest that HDACIs may have therapeutic potential for patients with REST-positive tumors. This warrants further investigation.
Keywords: REST, medulloblastoma, HDAC inhibitor, prognosis, differentiation
Introduction
Brain tumors are the most common solid malignancy and the leading cause of cancer-related morbidity and mortality in pediatrics (1). Medulloblastoma, a malignant tumor of the cerebellum with a high propensity for leptomeningeal dissemination, represents approximately 20% of all childhood brain tumors (2). Current prognostication and treatment strategies are primarily based on patient stratification into standard and high-risk groups using clinical factors such as patient’s age, tumor resection, metastases and tumor histology (3-6). The 5-year overall survival (OS) is approximately 75-85% and 50-70% for standard-risk and high-risk patients respectively (3, 7, 8). Unfortunately, approximately 30% of patients develop recurrent disease, and a proportion of patients with “standard” risk features exhibit unanticipated clinically aggressive disease (9, 10). A number of patients with recurrent and progressive disease who fail first-line therapies eventually succumb to their illness (9, 10). There is a great movement towards using disease-specific molecular features to help delineate the different risk groups, so that treatment strategies can be tailored for each patient to maximize survival and minimize toxicities (11-15). However, despite an improved understanding of tumor etiology, only a few of the molecules uncovered as key regulators of medulloblastoma biology have been studied as therapeutic targets or as prognostic factors in up-front protocol strategies for newly diagnosed patients (4, 16-18).
Medulloblastomas are poorly differentiated cerebellar tumors. The Repressor Element-1 Silencing Transcription Factor (REST) is a transcriptional repressor of a number of genes involved in terminal neuronal differentiation (19-22). Importantly, our previous studies demonstrated abnormally elevated expression of REST in human medulloblastoma tumors (23, 24). REST knockdown in human medulloblastoma cells abrogated their tumorigenic potential in mouse orthotopic models whereas its constitutive expression in Myc-immortalized neural progenitors promoted tumor formation in vivo (23, 24). These findings highlight the importance of REST in medulloblastoma genesis.
In the current study, we evaluated the prognostic significance of increased REST levels in human medulloblastoma samples. We show that increased REST levels are correlated with poor overall and event free survival in patients with the disease. Given its poor prognostic significance, we investigated if REST activity could be pharmacologically manipulated for further development as a therapeutic target. Transcriptional repression of terminal neuronal differentiation genes such as Syn1, TUBIII and SCG10 requires the activity of histone deacetylases-1 and 2 (HDAC1/2) that are complexed with the amino- and carboxy-terminal repression domains of REST (19, 21, 25). We therefore studied the ability of a panel of HDAC inhibitors (HDACIs) to attenuate REST-mediated blockade of neuronal differentiation and promotion of cell growth. We observed that MS-275, valproic acid (VPA), trichostatin A (TSA) and suberoyl anilide hydroxamic acid (SAHA/vorinostat) induced the expression of the REST-target gene Syn1, which is required for synaptic function (26), and blocked cell growth of medulloblastoma cell lines. Unexpectedly, REST transcription was upregulated by VPA and TSA, whereas SAHA promoted its proteasomal degradation. MS-275 caused only a small change in REST transcription or protein levels, suggesting that it likely induced differentiation by inhibiting REST activity. These data suggest that MS-275 and SAHA may warrant further pre-clinical investigation as potential therapeutic agents for patients with high REST expressing medulloblastoma.
Materials and Methods
Analyses of patient samples
Institutional Review Board (IRB) approval was obtained for immunohistochemical (IHC) staining of medulloblastoma samples for REST levels and for retrospective chart analyses of patient correlates. Baseline information on each patient, including, age, sex, histology, metastases, surgical resection (documented by post-operative MRI), treatments, recurrences and date of last follow-up or death was collected. Sections of paraffin-embedded tissue were studied by hematoxylin eosin (H&E) staining and IHC to measure REST protein levels using anti-REST antibody (Sigma-Aldrich, Inc., St. Louis, MO, 1:150 dilution) and DAB staining (Thermo Scientific, Rockford, IL). Hematoxylin (Richard-Allen Scientific, Kalamazoo, MI) was used as a counterstain. Slides were semi-quantitatively evaluated for REST levels by 2 observers (JF and VR) utilizing a 5-point grading scale as described in Fig.1B.
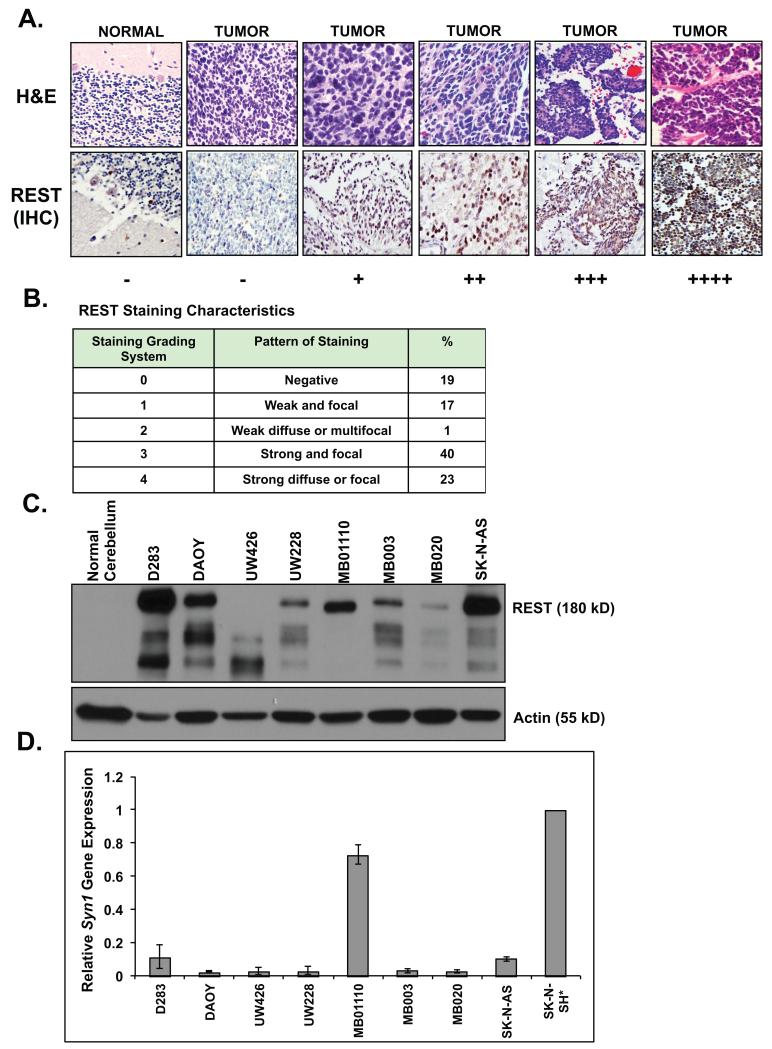
Figure 1. REST protein is elevated in human medulloblastoma cells.
(A) REST protein levels in human medulloblastomas and normal fetal cerebellum was determined by IHC using anti-REST antibody. Staining was evaluated and scored by our neuropathologist as −/+/++/+++/++++ (B) Description of REST staining characteristics in tumor tissue (C) Western blot analysis to measure REST levels in a panel of human medulloblastoma cultures was performed using anti-REST antibody. SK-N-AS neuroblastoma cell line and normal cerebellum were included as positive and negative controls respectively. Actin served as a loading control. (D) REST-target gene Syn1 expression in medulloblastoma cell lines was measured by Q-RT-PCR analysis. Retinoic acid-treated SK-N-SH neuroblastoma cells (*) served as a positive control. Experiments were performed in triplicate and values were normalized to 18sRNA and then to SK-N-SH* (set to 1) and reported as mean +/− standard error.
Plasmids
Human REST (hREST) transgene was cloned into a modified pcDNA3.1-V5/His plasmid wherein the cytomegalovirus (CMV) promoter was replaced by a 1kb region of the NeuroD2 (ND2) promoter (27). A 6× His/3× HA epitope tag was added to the amino-terminus of hREST to generate the pcDNA3.1/ND2/REST plasmid.
Cell culture
DAOY and D283 (ATCC, Manassas, VA) were cultivated in the recommended media. Cell identity was verified using SNP analysis. Conditions for the cultivation of primary medulloblastoma cultures (UW426, UW228, MB01110, MB003 and MB020) are described in supplemental material. NSC-M and NSC-MR were maintained as previously described (24).
Drug treatment
Tumor cells were treated with 2.5 μM MS-275 (Alexis, San Diego, CA), 33 nM TSA (Millipore, Billercia, CA), 1.5 mM VPA (Sigma, St Louis, MO), 5 μM SAHA (Caymen Chemicals, Ann Arbor, MI), 100 μg/ml CHX (Sigma, St Louis, MO) or 20 μM MG132 (Calbiochem, La Jolla, CA) for various time periods and processed as outlined below. The structures of these HDACIs were obtained through the NCI PubChem Compound Database using their unique chemical structure identifiers CID: 4216, 444732, 3121, 5311 (28) (Fig. 3A). The specificities of these HDACIs are also shown in Figure 3A (6, 29-32).
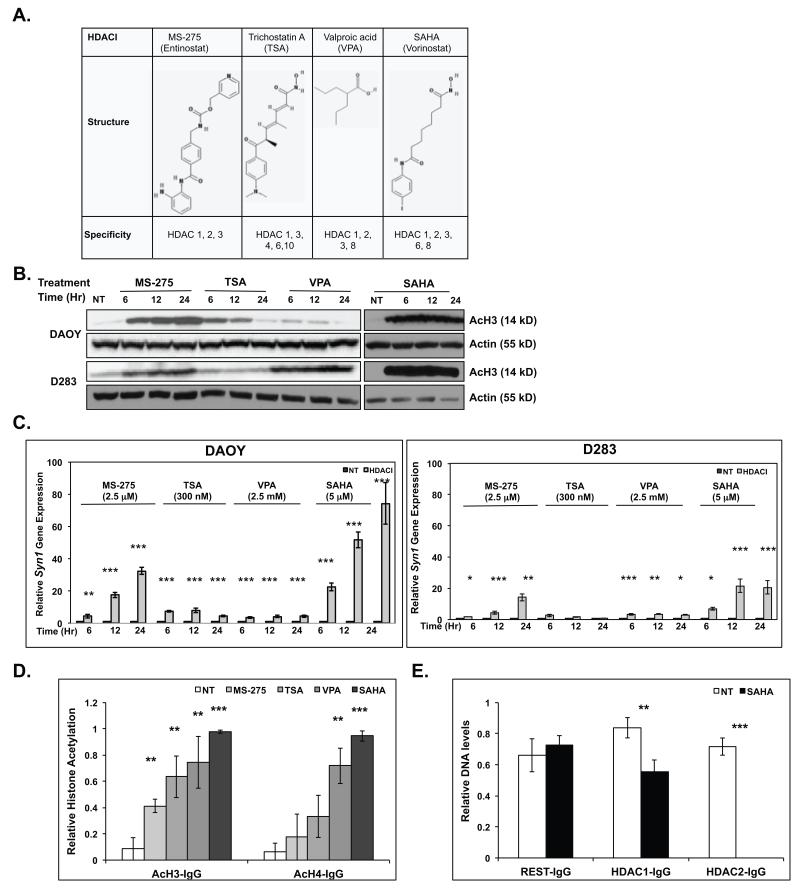
Figure 3. HDACIs upregulate Syn1 gene expression.
(A) Structure and specificities of HDACIs used in this study (30) (B) Western blot analysis and (C) Q-RT-PCR were performed to measure acetylation of histone H3 (AcH3) and changes in Syn1 gene expression respectively in DAOY and D283 in response to MS-275 (2.5 μM), TSA (300 nM), VPA (2.5 mM), or SAHA (5 μM) treatment for the indicated time-periods. Actin was used as a loading control for Western blots. Syn1 expression is reported relative to 18sRNA levels and compared with untreated controls (NT) set to 1. ChIP analyses were performed to compare changes in (D) acetylation of histones H3 and H4 or (E) REST, HDAC1 and HDAC2 binding to the Syn1 RE1 element in DAOY cells following HDACI treatment. Samples were normalized to input DNA and compared with DNA pulled down by non-immune sera (IgG). Assays were performed in triplicate and results reported as mean +/− standard error. Statistical significance is shown as *(0.1 >p>0.05), **(0.05≥p>0.01) or ***(p≤0.01).
Western blot
Equal amount of protein extracts prepared from untreated or drug-treated cells were subjected to polyacrylamide gel electrophoresis and Western blotting using the following antibodies: anti-REST (1:1000, Millipore, Billercia, CA); anti-AcH3 (1:1000, Millipore, Billercia, CA), anti-Actin HRP (1:50,000, Cell Signaling, Beverly, MA), anti-GAPDH HRP (1:2,000, Abcam, Cambridge, MA).
Q-RT-PCR and Q-PCR
Q-PCR and Q-RT-PCR analyses and normalization and statistics for assays performed in triplicate were performed as described previously (33, 34). The following primers were used for our analyses:
Q-RT-PCR
Human Syn1
Forward primer 5′ CAACGGAGACTACCGCAGTTTGGTC 3′,
Reverse primer 5′ GGGGTAGAAGGTCTGATCAATTAGAGGG 3′
Mouse Syn1
Forward primer 5′ CTCATTCCTCAGTATGTCCCTTGAGAAAC 3′
Reverse primer 5′ GAAATCACCCTTTAGATGTACCAGAAGTAGAGG 3′
Human REST
Forward primer 5′ GAAACACCTGGAGCGGAGGACAAAG 3′
Reverse primer 5′ CTTCTGCAGTGGAAGAGCCAGATTCC 3′
Human 18s RNA
Forward primer 5′ GTGGTGTTGAGGAAAGCAGAC 3′,
Reverse primer 5′ CATCCTTCTGTCTGTTCAAGA 3′
Mouse 18s RNA
Forward primer 5′ GAACTCACGGAGGATGAGGTG 3′,
Reverse primer 5′ GTTGGCCAGAACCTGGCTGTA 3′
Q-PCR
Human REST
(−2.0 kb)
Forward primer 5′ CAAGTTCATAGCAACAGCTTCCCT C 3′
Reverse primer 5′ GAGGCCCTTGTTCAAGGGATG 3′
(−1.5 kb)
Forward primer 5′ CCCTTCCTTCCTGTCTCTTTGGTTC 5′,
Reverse primer 5′ GGACCTCTGTTTCCCTCTATCTGG 3′;
(−1.0 kb)
Forward primer 5′ CCATCTTCCTACTGGCAAACCCC 3′,
Reverse primer 5′ CGTAAGTCACACCTGTCCTCAGAAGC 3′,
(−0.5 kb)
Forward primer 5′ CACGCTTTCTGAGTCCATAACCTCCTTC 3′,
Reverse primer 5′ CTCTTCAAGTCTCCACCCATAGCTGTC 3′.
Human Syn1-RE1
Forward primer 5′ CAACACTACAAACCGAGTATCTGC 3′,
Reverse primer 5′ GCCTCATCCTGGTCCTAAAA 3′.
Chromatin immunoprecipitation (ChIP)
ChIP was done as previously described using anti-AcH3 (Millipore, Billercia, CA), anti-AcH4 (Millipore, Billercia, CA), anti-REST (Millipore, Billercia, CA), anti-HDAC1 (Millipore, Billercia, CA), anti-HDAC2 (Millipore, Billercia, CA) or non-immune sera (rabbit or mouse IgG) (35). Primers used for Q-PCR are listed above. The signal obtained with IgG was subtracted from that with specific antibodies and reported.
MTT
DAOY, DAOY-REST, NSC-M and NSC-MR cells were treated with various concentrations of SAHA for different time-periods. Growth of drug-treated cells relative to untreated cells was measured by MTT assays (35). Each sample was run in quadruplicate and repeated at least four times.
Statistical methods
Estimated 3- and 5-year EFS and OS rates were calculated using the Kaplan-Meier method, and p-values determined by log-rank tests. Recurrences were considered as events while calculating EFS, whereas death was used to calculate EFS and OS. Statistical analyses for patient analysis was performed using SAS (v9.2) and a p-value < 0.05 was considered significant. Statistical analysis for all other experiments was done using Statistica (v6.0., Statsoft, Tulsa, OK). Significance is indicated as 0.1>p>0.05(*), 0.05≥p>0.01(**) or p≤0.01(***).
RESULTS
REST is elevated in human medulloblastoma samples and cell lines
Tumors from 58 patients with a new diagnosis (2000-2010) were used for our analyses. Of these, 60% were male and the median and mean age at diagnosis was 7.23 and 7.28 years, respectively (Supplemental Table S1A). Histologically, 62.1%, 25.9% and 12% of patients had tumors with classic, anaplastic, and desmoplastic histology, respectively (Supplemental Table S1A). Approximately 64% of patients underwent a gross total resection (GTR). The remainder had less than a GTR (Supplemental Table S1A). 70.7% of patient had no metastases at diagnosis whereas 29.3% did have metastases, either documented by MRI or lumbar spinal fluid cytology (Supplemental Table S1A). The mean follow-up for patients was 43.2 months (0.7-121.5).
The 5-year EFS and OS for the entire cohort were 81.6% and 81.8%, respectively. Univariate analysis (Supplemental Tables S1B and 1C) revealed that patients less than 3-years-of age at diagnosis had 5-year EFS and OS of 46.9% and 54.6%, respectively, whereas those diagnosed after age 3-years-old who had a 5-year EFS and OS of 90.8% and 88.6%, respectively (EFS, p=0.0003 and OS, p=0.004). Patients with less than a GTR had a 5-year EFS and OS of 64.3% and 68.4%, respectively in contrast to a 5-year EFS and OS of 91.5% and 90.0% respectively in children with GTR (EFS, p=0.01 and OS, p=0.03). Also, patients with tumor metastases at diagnosis had a 5-year EFS and OS of 44.1% and 47.5%, respectively, in contrast to a 5-year EFS and OS of 94.9% and 94.5% (EFS, p<0.0001 and OS, p<0.0001) respectively for patients without metastases.
Once we established that the prognostic variables of our dataset were similar to that described in the literature, tumor samples were stained for REST protein and graded from no expression (−) to elevated expression (+/++/+++/++++) (Fig. 1A, bottom panel). The corresponding hematoxylin eosin (H&E) staining is shown in the top panel (Fig. 1A). Infant cerebellum was used as a negative control (Fig. 1A). REST staining appeared to be solely nuclear in some cases and was both the nuclear and cytoplasmic in other cases (Fig. 1A). Approximately 81% of the samples exhibited higher levels of REST staining compared to normal cerebellum (Fig. 1A and 1B). Of the REST-positive samples, quantitation was as follows: 17% (+), 1% (++), 40% (+++) and 23% (++++) (Fig. 1A and 1B). REST protein was also detected by Western blotting in a panel of established (DAOY and D283) and primary cultures (UW228, UW426, MB01110, MB003 and MB020) of human medulloblastoma (Fig 1C). Interestingly, a faster migrating possibly a degradation or alternatively spliced form of REST (~125 kD) was detected in UW426 cells whereas the expected 180kD form of the protein was seen in other cell lines. REST levels were variable amongst these cells although it was elevated relative to normal cerebella, where REST was absent (Fig. 1C). SK-N-AS neuroblastoma cells and actin served as positive and loading controls respectively.
Since REST is a repressor of neuronal differentiation genes, we studied the relationship between REST levels and tumor differentiation in medulloblastoma cell lines by measuring the expression of its target gene, Synapsin1 (Syn1) by Q-RT-PCR analyses. Syn1 expression in SK-N-SH neuroblastoma cells treated with retinoic acid served as a positive control (34). As seen in Fig. 1D, most tumor cells with the exception of MB01110 exhibited poor Syn1 gene expression compared to normal cerebella, suggesting a blockade of neuronal differentiation in most REST-positive cells.
High REST levels in tumors correlates with poor prognostic significance
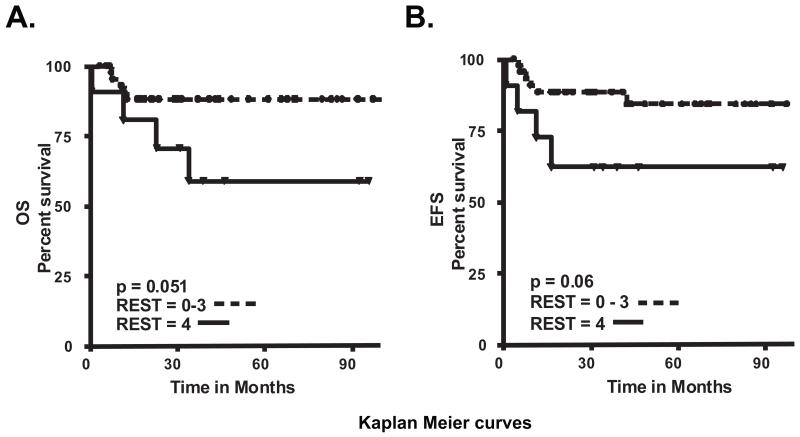
To determine if high REST levels in tumors correlated with patient survival, we grouped patients into 5 categories (−/+/++/+++/++++) based on the level of REST staining in tumors. OS and EFS were determined and Kaplan-Meier curves plotted (Figs. 2A and 2B). Patients with tumors graded as 4+ had the worst prognosis with a 5-year OS and EFS of 58.9% and 62.3% respectively. Patients with REST grading of 0-3+ had a 5-year OS and EFS of 88.2% and 84.2% respectively (OS and EFS, p=0.06 and p=0.05 respectively) (Figs. 2A and 2B).
Figure 2. Elevated REST levels in human medulloblastoma is associated with poor outcome.
Kaplan-Meier curves were plotted to compare (A) Overall survival (OS) and (B) Event free survival (EFS) in patients with high-REST (++++) and REST-negative (−) or low-moderate REST (+/++/+++) staining. P-values were established by log-rank test.
HDACI treatment induces neuronal differentiation
Since high REST protein levels were associated with poor survival in patient samples, we asked if REST activity could be targeted through pharmacological approaches. Consistent with our previous work, and as shown in Figures 1C and 1D, a number of REST-positive medulloblastoma cell lines were poorly differentiated (23, 24). Work from other groups has shown the involvement of HDAC (HDACs1/2) associated with the amino and carboxy-terminal-repression domains of REST for its repression of neuronal genes (19, 21, 25, 36, 37). We therefore asked if inhibition of HDAC activity would attenuate REST-mediated blockade of neuronal differentiation. We tested a panel of HDAC inhibitors (HDACIs) including benzamides (MS-275), hydroxamic acids (TSA and SAHA/vorinostat) and aliphatics (VPA) with activity against HDACs (Fig 3A), for their ability to upregulate the expression of the REST-target gene, Syn1 in DAOY and D283 cells. IC50 doses of these agents were established in our previously published work and that from other groups (35, 38). Drug activity was first confirmed by Western blotting measurement of changes in the acetylation of histone H3 (AcH3) after various times of treatment of DAOY and D283 cells with MS-275, TSA, VPA, and SAHA (Fig. 3B). Syn1 gene expression was then assessed by Q-RT-PCR analyses and found to be elevated in these cells following HDACI treatment, although the extent of elevation differed between the two cell lines (5-80-fold and 4-20 fold respectively) (Fig. 3C). Levels of 18s RNA were used for normalization. SAHA caused the largest increase in Syn1 expression (20-80 fold and 5-20-fold in DAOY, and D283 cells respectively). To further examine the mechanism by which SAHA modulated Syn1 expression, we measured changes in histone acetylation at the REST-binding RE1 element within the Syn1 promoter by ChIP analysis (Fig. 3D). A significant increase in histone H3 acetylation at the RE1 element was observed for all four HDACIs (Fig. 3D), whereas a statistically significant increase in histone H4 acetylation in this region was seen for VPA and SAHA only. The binding of REST, HDAC1 and HDAC2 at the Syn1 RE1 element in the presence and absence of SAHA treatment was also evaluated by ChIP assays. Since SAHA was found to degrade REST (Fig. 4C), ChIP assays were done after 6 hours of SAHA treatment, a time at which REST levels were not significantly reduced. While REST binding was unaffected by SAHA treatment at 6 hours, HDACs1 and 2 showed a partial to complete dissociation from the RE1 element to which REST was also bound (Fig. 3E). These observations suggest that SAHA may facilitate dissolution of the REST/HDAC/DNA complex at this time point.
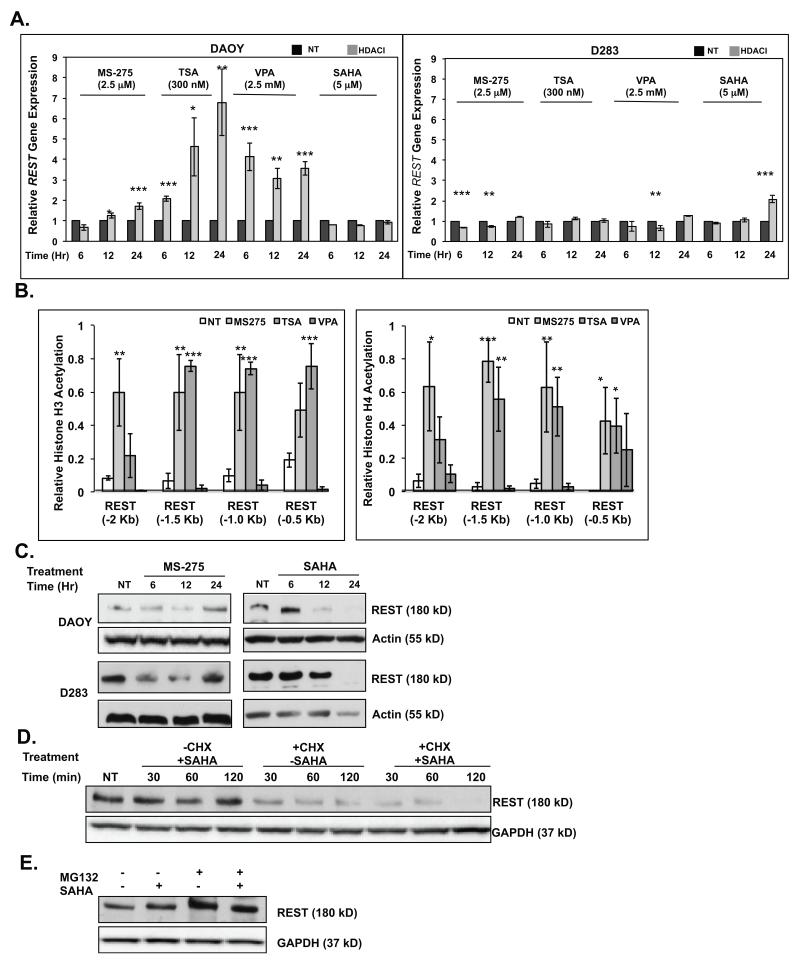
Figure 4. HDACIs modulate REST gene expression and protein stability.
(A) Q-RT-PCR analysis were performed to measure REST gene expression changes in DAOY and D283 cells in response to HDACI treatment. Normalization and statistical calculations were carried out as described in Figure 3. (B) ChIP analyses were done in HDACI-treated DAOY cells to assess changes in acetylation of histones H3 and H4 within a 2 kb region of the REST promoter. Normalization and statistical calculations were carried out as described in Figure 3. Western blot analysis were done to measure REST levels in DAOY cells treated with (C) various HDACIs (D) with SAHA (5 μM) in the presence or absence of cycloheximide (CHX) (10 mg/ml) for 0-120 minutes (mins) (E) with SAHA (5 μM) in the presence or absence of MG132 (20 μM) for 4 hours (hrs). Actin or GAPDH were used as loading controls.
HDACIs modulate REST transcription and protein levels
The effect of HDACIs on REST gene expression and protein levels were also studied to ensure that the observed changes in Syn1 expression stemmed from HDAC targeting and not from changes in REST gene or protein levels. Interestingly, Q-RT-PCR analyses revealed that MS-275 caused a small (1.5 fold) increase in REST transcription, whereas TSA and VPA promoted a larger increase in REST gene expression (1.5-8-fold) in DAOY cells (Fig. 4A, left panel). A similar change was not seen in D283 cells at most of the time-points tested (Fig. 4A, right panel). However, SAHA caused a small (2-fold) increase in REST transcript in D283 cells at 24 hours post-treatment (Fig. 4A, right panel). Further confirmation of these findings was obtained by ChIP assays using anti-histone H3/H4 antibodies and control IgG and primers specific to various regions of the REST promoter (−0.5 kb to −2.0 kb). As seen in Figure 4B (left panel), a significant increase in acetylation of histone H3 was observed, around the −0.5 kb, −1.0 kb and −1.5 kb of the REST promoter 24 hours after treatment with MS-275 and TSA, but not VPA. However, VPA promoted a change in acetylation of histone H3 at these regions at 6- and 12-hours post-treatment with HDACIs (data not shown). Acetylation of histone H3 at the −2.0 kb region was seen only in response to MS-275. ChIP assays also revealed a significant increase in acetylation of histone H4 at the REST promoter after treatment with MS-275 and TSA, but not with VPA (Fig. 4B, right panel). Together these results indicate that the increase in REST transcription upon treatment with MS-275 and TSA was likely caused by chromatin remodeling at the cognate promoter by these agents.
Since MS-275 and SAHA caused the least transcriptional upregulation of REST, further studies were carried out with these agents. Western blotting showed that REST protein levels in MS-275 treated DAOY and D283 cells paralleled the small fluctuations in its transcript levels (Fig. 4C). Importantly, because Syn1 expression was upregulated even at time-points when REST levels were elevated in MS-275 (24hr)- and SAHA (6hr)-treated cells, it suggested that inhibition HDAC activity (presumably REST-associated) contributed to Syn1 upregulation (Figs. 4A and 4C). However, SAHA also caused an unexpected decrease in REST protein levels in both cell lines (after 6hrs), suggesting that the loss of REST itself may contribute to the increase in Syn1 expression at longer time points of SAHA exposure (Fig. 4C).
Since SAHA did not affect REST transcription significantly, we asked if the SAHA-dependent decline in REST protein levels occurred through post-transcriptional mechanisms. We blocked translation of newly synthesized mRNA using cycloheximide (CHX) for 0-120 minutes and examined the effect of SAHA on pre-existing REST protein levels by Western blotting. As expected, in the absence of CHX, SAHA did not cause a substantial change in REST protein levels within 120 minutes (Fig. 4D). However in the presence of CHX, SAHA caused a rapid decline in REST protein levels (Fig 4D). These results confirmed that SAHA-dependent decline in REST protein levels occurred through post-transcriptional mechanisms. REST protein levels are known to be modulated by its proteasomal degradation (39, 40). We therefore examined the contribution of the proteasomal machinery to the SAHA-dependent decrease in REST protein levels by blocking proteasomal activity using MG132. As seen in Figure 4E, MG132 treatment for 4 hours caused an accumulation of REST, which was accentuated by co-incubation with SAHA. As expected, treatment with SAHA alone caused only a very modest decrease in REST protein levels within the 4-hour window of the assay.
Ectopic REST expression causes resistance to SAHA-dependent decrease in cell growth
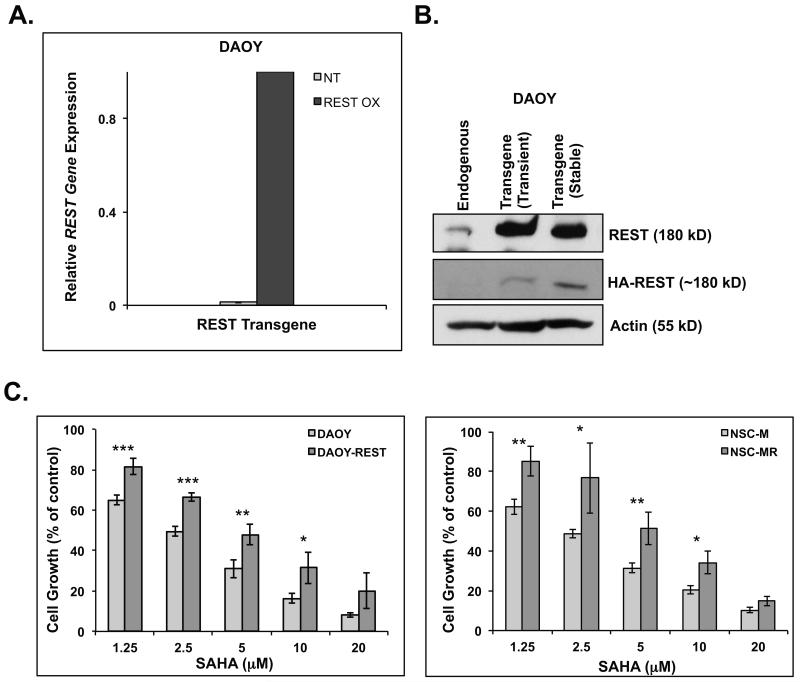
We and others previously showed that REST loss blocked the growth of medulloblastoma cell lines in vitro and in vivo (23, 24). The effect of SAHA-dependent decline in REST levels on cell growth was monitored by MTT assays. To determine if this decrease in cell growth was through an effect on REST, we generated stable cells (DAOY-REST) that constitutively expressed HA-tagged REST transgene (27). Transgene expression was confirmed by Q-RT-PCR and Western blotting (Figs. 5A and 5B). DAOY-REST cells were treated with the doses of SAHA utilized for DAOY cells, and cell-growth relative to untreated controls was measured. As shown in Figure 5C (left panel), constitutive REST transgene expression in DAOY-REST cells caused a significant blockade to the SAHA-mediated decline in cell growth seen with DAOY cells at all doses tested (except 20μM). To further validate this observation, we compared the response of immortalized mouse neural progenitor cells (NSC-M) with that of isogenic cells NSC-MR cells constitutively expressing hREST transgene. We had previously shown that constitutive REST expression facilitated tumor formation by NSC-MR in the murine cerebellum, whereas NSC-M cells were non-tumorigenic (24). In this study, we observed that elevated hREST expression also caused a significant impediment to SAHA-dependent decline in NSC-MR cell growth compared to that in NSC-M cells (Fig. 5C, right panel). This correlated with a lack of Syn1 upregulation in NSC-MR cells after SAHA treatment. In contrast, the decline in cell growth in NSC-M cells following SAHA exposure was accompanied by a significant increase in Syn1 expression (Supplemental Fig. S1). Collectively, these results suggest that the decline in cell growth and the induction of differentiation in response to SAHA occurs at least in part through an effect on REST protein.
Figure 5. Ectopic REST expression counters SAHA-dependent decline in cell growth.
DAOY cells stably expressing HA-tagged human REST transgene under the control of ND2 promoter (DAOY-REST) were generated by G418 selection. Transgene expression was confirmed by (A) by Q-RT-PCR analyses using transgene-specific primers specific and (B) Western blot analysis using antibodies against REST or the epitope-tag. Actin was used as a loading control. (C) MTT assays were performed to compare cell growth in DAOY and DAOY-REST cells (left panel) or NSC-M and NSC-MR cells (right panel) following treatment with various doses of SAHA for 48 hrs. Assays were done at least four times and plotted relative to untreated controls. Normalization and statistical calculations were done as previously stated (35).
Discussion
Medulloblastomas are poorly differentiated cerebellar tumors in which expression of the repressor of neuronal differentiation, REST is often elevated (23, 24, 41). REST elevation in tumor samples and cell lines was recapitulated in this study and correlated with a block in neuronal differentiation in tumor cell lines (23, 24, 41). However, the prognostic significance of REST levels in medulloblastomas or its pharmacological manipulation for potential therapeutic intervention had not been investigated prior to this study.
The initial analysis of our data set with respect to previously known parameters such as age, resection, metastasis were consistent with what has been previously documented in the literature (2). Clinically, histology it also an important prognostic factor, whereas patients with anaplastic histology have an inferior outcome compared to patients with desmoplastic and classic histology (42, 43). Interestingly, while our analyses revealed REST levels to be increased in all histological sub types of medulloblastoma, there was an interesting and unexpected trend wherein 3 of the 7 patients with desmoplastic tumors that exhibited high REST protein (++++) staining also had the worst outcome. While the reason for this finding is not clear, a potential explanation could be that the recognition and diagnosis of desmoplastic histology has evolved and improved over the last few years and may have varied during the 10 year period over which the patient data were collected. A larger data set and re-evaluation of tumors based on their gene expression profiling may provide a more accurate statistical understanding of the relationship between survival and tumor type (11-15). As we have shown for the first time in this study, high REST levels conferred poor OS as well as EFS. The basis for this inferior survival outcome although not evident in our analysis may be because of the tendency for metastatic disease in patients with high REST levels in their tumors (44). Interestingly, REST expression in our tumor samples was often focal and required staining of tumor sections larger than that used in tissue microarrays (TMAs). Although REST is a transcription factor and its nuclear localization is well accepted, we found cytoplasmic REST staining in several of our tumor samples. The significance of this unexpected staining pattern remains to be determined.
The overall poor prognosis associated with elevated REST levels led us to investigate if REST activity could be modulated in in vitro experiments for future preclinical and clinical applications in patients with REST-expressing tumors. Previous work from other groups has demonstrated the involvement of HDACs1/2 in REST-dependent repression of neuronal differentiation genes, suggesting that HDACIs may have therapeutic applications for REST-positive tumors (19, 21, 25, 36, 37). Clinically, HDACIs are not only associated with a more tolerable side-effect profile than traditional cytotoxic chemotherapy, but they have also shown potential as effective treatment strategies in pediatric brain tumors (45). A number of clinically relevant inhibitors of HDACs such as MS-275, VPA and SAHA are available and have been studied in the context of medulloblastoma cell lines, but not specifically for REST-positive tumors (35, 38, 46, 47).
The HDACIs we studied upregulated the expression of the REST-target gene Syn1 presumably through inhibition of REST activity. Interestingly, MB1110 had high levels of base-line Syn1 expression although REST was expressed in these cells. In this case, other components of the REST-repression complex may have aberrant expression or activity, a possibility that remains to be investigated. Some of the HDACIs we tested also caused an unexpected increase in REST gene expression. This effect was particularly significant with TSA and VPA, whereas a more modest increase was seen with MS-275. The regions within the REST promoter where histone acetylation and chromatin remodeling was detected upon HDACI treatment also house Retinoic Acid Receptor Element (RARE) elements (−0.5 −1.0, −1.2, −1.5, and −2.0 kb from transcription start). Previous studies have implicated these elements in the regulation of REST transcription during differentiation of normal neural stem/progenitor cells (48). The induction of Syn1 expression despite REST upregulation by TSA, VPA and MS-275 suggests attenuation of REST activity by these agents.
In contrast, SAHA predominantly influenced REST stability. The reason for these variable effects of HDACIs on REST biology may be because of their different specificities for HDACIs or alternatively, effects on non-histone proteins that are yet to be discovered (32). The abrogation of SAHA-dependent REST degradation in response to proteasomal inhibition led us to investigate the contribution of known regulators of REST protein stability to its decline in SAHA-treated cells. The E-3 ligase β-TRCP, and the deubiquitylase USP7/HAUSP are play opposing and balancing roles in controlling REST stability in neural progenitors and tumors (34, 39, 40, 49). Aberrations in β-TRCP biology levels, or its ability to interact with REST contributes to chemo-resistance in the neural tumor, neuroblastoma (34, 40). In our studies, the levels of β-TRCP were transiently upregulated in response to SAHA treatment whereas that of HAUSP remained unaffected in DAOY cells (data not shown). Whether these transient changes are sufficient to perturb the balance between REST ubiquitination and its degradation or its deubiquitination and stabilization remains to be evaluated. HDACIs are also known to modulate expression of the proteasomal beta sub-units (50) which could also be a potential mechanistic explanation for SAHA-induced REST degradation. High-throughput genomic, epigenomic and proteomic screens may also provide an alternative approach to uncovering molecular mechanisms underlying the differential effects of HDACIs on REST.
In summary, the current study has identified increased REST levels to be a poor prognostic indicator for patients with medulloblastoma. We have also identified MS-275 and SAHA as HDACIs with potential therapeutic relevance for medulloblastomas. This remains to be determined in more detailed studies in mouse orthotopic models. SAHA has been evaluated in phase I clinical trials for pediatric patients and is currently being utilized as part of the up-front strategy treating infants with medulloblastoma in a Pediatric Brain Tumor Consortium (PBTC) study-026. Similar studies with MS-275 have not been initiated in pediatric patients with cancer. Our results may provide the impetus for stratifying patients based on their REST levels in clinical trials involving SAHA or MS-275 and may help better prognosticate patient outcome based on a specific biologic abnormality.
Supplementary Material
Acknowledgements
We are grateful to Dr. Joya Chandra for insightful comments and to Ms. Donna Kersey for her assistance with the IHC analyses of medulloblastoma samples. We thank Drs. James Olson and James Silber for providing primary medulloblastoma cell cultures.
Grant Support: American Cancer Society Research Scholar Grant 118165-RSG-09-273-01-DDC (VG), Matthew Larson Pediatric Brain Tumor Foundation (VG and JF), National Institutes of Health/National Institute of Neurological Disorders,Stroke 1R03NS077021-01 (VG) and The Rolf-Dierichs-Foundation (MH and LR).
Footnotes
Conflict of Interest: None
References
- 1.Fangusaro J, Chi S. Introduction to a special issue on pediatric neuro-oncology. J Child Neurol. 2009;24:1341–2. doi: 10.1177/0883073809338959. [DOI] [PubMed] [Google Scholar]
- 2.Dhall G. Medulloblastoma. J Child Neurol. 2009;24:1418–30. doi: 10.1177/0883073809341668. [DOI] [PubMed] [Google Scholar]
- 3.Castellino RC, De Bortoli M, Lu X, Moon SH, Nguyen TA, Shepard MA, et al. Medulloblastomas overexpress the p53-inactivating oncogene WIP1/PPM1D. J Neurooncol. 2008;86:245–56. doi: 10.1007/s11060-007-9470-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crawford JR, MacDonald TJ, Packer RJ. Medulloblastoma in childhood: new biological advances. Lancet Neurol. 2007;6:1073–85. doi: 10.1016/S1474-4422(07)70289-2. [DOI] [PubMed] [Google Scholar]
- 5.Eberhart CG, Kratz J, Wang Y, Summers K, Stearns D, Cohen K, et al. Histopathological and molecular prognostic markers in medulloblastoma: c-myc, N-myc, TrkC, and anaplasia. J Neuropathol Exp Neurol. 2004;63:441–9. doi: 10.1093/jnen/63.5.441. [DOI] [PubMed] [Google Scholar]
- 6.Suliman BA, Xu D, Williams BR. HDACi: molecular mechanisms and therapeutic implications in the innate immune system. Immunol Cell Biol. 2012;90:23–32. doi: 10.1038/icb.2011.92. [DOI] [PubMed] [Google Scholar]
- 7.Jakacki RI. Treatment strategies for high-risk medulloblastoma and supratentorial primitive neuroectodermal tumors. Review of the literature. J Neurosurg. 2005;102:44–52. doi: 10.3171/ped.2005.102.1.0044. [DOI] [PubMed] [Google Scholar]
- 8.Packer RJ, Gajjar A, Vezina G, Rorke-Adams L, Burger PC, Robertson PL, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24:4202–8. doi: 10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]
- 9.Bakst RL, Dunkel IJ, Gilheeney S, Khakoo Y, Becher O, Souweidane MM, et al. Reirradiation for recurrent medulloblastoma. Cancer. 2011;117:4977–82. doi: 10.1002/cncr.26148. [DOI] [PubMed] [Google Scholar]
- 10.Gajjar A, Pizer B. Role of high-dose chemotherapy for recurrent medulloblastoma and other CNS primitive neuroectodermal tumors. Pediatr Blood Cancer. 2010;54:649–51. doi: 10.1002/pbc.22378. [DOI] [PubMed] [Google Scholar]
- 11.Gilbertson RJ. Finding the perfect partner for medulloblastoma prognostication. J Clin Oncol. 2011;29:3841–2. doi: 10.1200/JCO.2011.37.5709. [DOI] [PubMed] [Google Scholar]
- 12.Kool M, Koster J, Bunt J, Hasselt NE, Lakeman A, van Sluis P, et al. Integrated genomics identifies five medulloblastoma subtypes with distinct genetic profiles, pathway signatures and clinicopathological features. PLoS One. 2008;3:e3088. doi: 10.1371/journal.pone.0003088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Northcott PA, Korshunov A, Witt H, Hielscher T, Eberhart CG, Mack S, et al. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 2011;29:1408–14. doi: 10.1200/JCO.2009.27.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfister S, Remke M, Benner A, Mendrzyk F, Toedt G, Felsberg J, et al. Outcome prediction in pediatric medulloblastoma based on DNA copy-number aberrations of chromosomes 6q and 17q and the MYC and MYCN loci. J Clin Oncol. 2009;27:1627–36. doi: 10.1200/JCO.2008.17.9432. [DOI] [PubMed] [Google Scholar]
- 15.Schwalbe EC, Lindsey JC, Straughton D, Hogg TL, Cole M, Megahed H, et al. Rapid diagnosis of medulloblastoma molecular subgroups. Clin Cancer Res. 2011;17:1883–94. doi: 10.1158/1078-0432.CCR-10-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlotti CG, Jr., Smith C, Rutka JT. The molecular genetics of medulloblastoma: an assessment of new therapeutic targets. Neurosurg Rev. 2008;31:359–68. doi: 10.1007/s10143-008-0146-4. discussion 68-9. [DOI] [PubMed] [Google Scholar]
- 17.de Bont JM, Packer RJ, Michiels EM, den Boer ML, Pieters R. Biological background of pediatric medulloblastoma and ependymoma: a review from a translational research perspective. Neuro Oncol. 2008;10:1040–60. doi: 10.1215/15228517-2008-059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guessous F, Li Y, Abounader R. Signaling pathways in medulloblastoma. J Cell Physiol. 2008;217:577–83. doi: 10.1002/jcp.21542. [DOI] [PubMed] [Google Scholar]
- 19.Chong JA, Tapia-Ramirez J, Kim S, Toledo-Aral JJ, Zheng Y, Boutros MC, et al. REST: a mammalian silencer protein that restricts sodium channel gene expression to neurons. Cell. 1995;80:949–57. doi: 10.1016/0092-8674(95)90298-8. [DOI] [PubMed] [Google Scholar]
- 20.Lonnerberg P, Schoenherr CJ, Anderson DJ, Ibanez CF. Cell type-specific regulation of choline acetyltransferase gene expression. Role of the neuron-restrictive silencer element and cholinergic-specific enhancer sequences. J Biol Chem. 1996;271:33358–65. doi: 10.1074/jbc.271.52.33358. [DOI] [PubMed] [Google Scholar]
- 21.Schoenherr CJ, Anderson DJ. The neuron-restrictive silencer factor (NRSF): a coordinate repressor of multiple neuron-specific genes. Science. 1995;267:1360–3. doi: 10.1126/science.7871435. [DOI] [PubMed] [Google Scholar]
- 22.Schoenherr CJ, Paquette AJ, Anderson DJ. Identification of potential target genes for the neuron-restrictive silencer factor. Proc Natl Acad Sci U S A. 1996;93:9881–6. doi: 10.1073/pnas.93.18.9881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawinger P, Venugopal R, Guo ZS, Immaneni A, Sengupta D, Lu W, et al. The neuronal repressor REST/NRSF is an essential regulator in medulloblastoma cells. Nat Med. 2000;6:826–31. doi: 10.1038/77565. [DOI] [PubMed] [Google Scholar]
- 24.Su X, Gopalakrishnan V, Stearns D, Aldape K, Lang FF, Fuller G, et al. Abnormal expression of REST/NRSF and Myc in neural stem/progenitor cells causes cerebellar tumors by blocking neuronal differentiation. Mol Cell Biol. 2006;26:1666–78. doi: 10.1128/MCB.26.5.1666-1678.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Battaglioli E, Andres ME, Rose DW, Chenoweth JG, Rosenfeld MG, Anderson ME, et al. REST repression of neuronal genes requires components of the hSWI.SNF complex. J Biol Chem. 2002;277:41038–45. doi: 10.1074/jbc.M205691200. [DOI] [PubMed] [Google Scholar]
- 26.Ferreira A, Rapoport M. The synapsins: beyond the regulation of neurotransmitter release. Cell Mol Life Sci. 2002;59:589–95. doi: 10.1007/s00018-002-8451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hallahan AR, Pritchard JI, Hansen S, Benson M, Stoeck J, Hatton BA, et al. The SmoA1 mouse model reveals that notch signaling is critical for the growth and survival of sonic hedgehog-induced medulloblastomas. Cancer Res. 2004;64:7794–800. doi: 10.1158/0008-5472.CAN-04-1813. [DOI] [PubMed] [Google Scholar]
- 28.National Center for Biotechnology Information [accessed March 5, 2012]; PubChem Compound Database;CID=4261, http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgicid4261,CID=444732, http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgicid444732,CID=3121, http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgicid3221,CID=5311, http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgicid5311.
- 29.Bradner JE, West N, Grachan ML, Greenberg EF, Haggarty SJ, Warnow T, et al. Chemical phylogenetics of histone deacetylases. Nat Chem Biol. 2010;6:238–43. doi: 10.1038/nchembio.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marks PA, Breslow R. Dimethyl sulfoxide to vorinostat: Development of this histone deacetylase inhibitor as an anticancer drug. Nat Biotechnol. 2007;25:84–90. doi: 10.1038/nbt1272. [DOI] [PubMed] [Google Scholar]
- 31.Rikiishi H. Autophagic and Apoptotic Effects of HDAC Inhibitors on Cancer Cells. Journal of Biomedicine and Biotechnology. 2011 doi: 10.1155/2011/830260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu WS, Parmigiani RB, Marks PA. Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene. 2007;26:5541–52. doi: 10.1038/sj.onc.1210620. [DOI] [PubMed] [Google Scholar]
- 33.Das CM, Zage PE, Taylor P, Aguilera D, Wolff JE, Lee D, et al. Chromatin remodelling at the topoisomerase II-beta promoter is associated with enhanced sensitivity to etoposide in human neuroblastoma cell lines. Eur J Cancer. 2010 doi: 10.1016/j.ejca.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh A, Rokes C, Gireud M, Fletcher S, Baumgartner J, Fuller G, et al. Retinoic acid induces REST degradation and neuronal differentiation by modulating the expression of SCF(beta-TRCP) in neuroblastoma cells. Cancer. 2011 doi: 10.1002/cncr.26145. [DOI] [PubMed] [Google Scholar]
- 35.Aguilera DG, Das CM, Sinnappah-Kang ND, Joyce C, Taylor PH, Wen S, et al. Reactivation of death receptor 4 (DR4) expression sensitizes medulloblastoma cell lines to TRAIL. J Neurooncol. 2009;93:303–18. doi: 10.1007/s11060-008-9788-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gopalakrishnan V. REST and the RESTless: in stem cells and beyond. Future Neurol. 2009;4:317–29. doi: 10.2217/fnl.09.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ooi L, Wood IC. Chromatin crosstalk in development and disease: lessons from REST. Nat Rev Genet. 2007;8:544–54. doi: 10.1038/nrg2100. [DOI] [PubMed] [Google Scholar]
- 38.Furchert SE, Lanvers-Kaminsky C, Juurgens H, Jung M, Loidl A, Fruhwald MC. Inhibitors of histone deacetylases as potential therapeutic tools for high-risk embryonal tumors of the nervous system of childhood. Int J Cancer. 2007;120:1787–94. doi: 10.1002/ijc.22401. [DOI] [PubMed] [Google Scholar]
- 39.Huang Z, Wu Q, Guryanova OA, Cheng L, Shou W, Rich JN, et al. Deubiquitylase HAUSP stabilizes REST and promotes maintenance of neural progenitor cells. Nat Cell Biol. 2011;13:142–52. doi: 10.1038/ncb2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westbrook TF, Hu G, Ang XL, Mulligan P, Pavlova NN, Liang A, et al. SCFbeta-TRCP controls oncogenic transformation and neural differentiation through REST degradation. Nature. 2008;452:370–4. doi: 10.1038/nature06780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weis DC, Visco DP, Jr., Faulon JL. Data mining PubChem using a support vector machine with the Signature molecular descriptor: classification of factor XIa inhibitors. J Mol Graph Model. 2008;27:466–75. doi: 10.1016/j.jmgm.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 42.Holthouse DJ, Dallas PB, Ford J, Fabian V, Murch AR, Watson M, et al. Classic and desmoplastic medulloblastoma: complete case reports and characterizations of two new cell lines. Neuropathology. 2009;29:398–409. doi: 10.1111/j.1440-1789.2008.00989.x. [DOI] [PubMed] [Google Scholar]
- 43.Leary SE, Zhou T, Holmes E, Geyer JR, Miller DC. Histology predicts a favorable outcome in young children with desmoplastic medulloblastoma: a report from the children’s oncology group. Cancer. 2011;117:3262–7. doi: 10.1002/cncr.25856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu X, Northcott PA, Dubuc A, Dupuy AJ, Shih DJ, Witt H, et al. Clonal selection drives genetic divergence of metastatic medulloblastoma. Nature. 2012;482:529–33. doi: 10.1038/nature10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spiller SE, Ravanpay AC, Hahn AW, Olson JM. Suberoylanilide hydroxamic acid is effective in preclinical studies of medulloblastoma. J Neurooncol. 2006;79:259–70. doi: 10.1007/s11060-006-9142-0. [DOI] [PubMed] [Google Scholar]
- 46.Hacker S, Dittrich A, Mohr A, Schweitzer T, Rutkowski S, Krauss J, et al. Histone deacetylase inhibitors cooperate with IFN-gamma to restore caspase-8 expression and overcome TRAIL resistance in cancers with silencing of caspase-8. Oncogene. 2009;28:3097–110. doi: 10.1038/onc.2009.161. [DOI] [PubMed] [Google Scholar]
- 47.Sonnemann J, Kumar KS, Heesch S, Muller C, Hartwig C, Maass M, et al. Histone deacetylase inhibitors induce cell death and enhance the susceptibility to ionizing radiation, etoposide, and TRAIL in medulloblastoma cells. Int J Oncol. 2006;28:755–66. [PubMed] [Google Scholar]
- 48.Ballas N, Grunseich C, Lu DD, Speh JC, Mandel G. REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell. 2005;121:645–57. doi: 10.1016/j.cell.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 49.Frescas D, Pagano M. Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the scales of cancer. Nat Rev Cancer. 2008;8:438–49. doi: 10.1038/nrc2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller CP, Singh MM, Rivera-Del Valle N, Manton CA, Chandra J. Therapeutic strategies to enhance the anticancer efficacy of histone deacetylase inhibitors. J Biomed Biotechnol. 2011;2011:514261. doi: 10.1155/2011/514261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.