Abstract
Microvolt level T-wave alternans (MTWA), a phenomenon of beat-to-beat variability in the repolarization phase of the ventricles, has been closely associated with an increased risk of ventricular tachyarrhythmic events (VTE) and sudden cardiac death (SCD) during medium- and long-term follow-up. Recent observations also suggest that heightened MTWA magnitude may be closely associated with short-term risk of impending VTE. At the sub-cellular and cellular level, perturbations in calcium transport processes likely play a primary role in the genesis of alternans, which then secondarily lead to alternans of action potential morphology and duration (APD). As such, MTWA may play a role not only in risk stratification but also more fundamentally in the pathogenesis of VTE. In this paper, we outline recent advances in understanding the pathogenesis of MTWA and also the utility of T-wave alternans testing for clinical risk stratification. We also highlight emerging clinical applications for MTWA.
Keywords: repolarization alternans, arrhythmia risk stratification, arrhythmia prevention, sudden cardiac death, T-wave alternans
Introduction
Electrocardiographic alternans, a phenomenon of beat-to-beat oscillation in electrocardiographic waveforms, was first described by Hering in 1908 1. Much of the interest in the alternans phenomenon has focused on alternans during the repolarization phase of the cardiac action potential (AP), also known as repolarization alternans (RA). Over the last several decades, physiologic studies have demonstrated that alternans is an important marker of cardiac electrical instability and ventricular tachyarrhythmic events (VTE) 2, 3.
Specifically, the presence of microvolt level beat-to-beat alternation in T-wave morphology during low level exercise has been identified as a marker of ventricular arrhythmia susceptibility in patients with structural heart disease and lead to the advent of microvolt T-wave alternans (MTWA) testing for clinical risk stratification. Since the seminal publication by Rosenbaum et al. demonstrating the efficacy of MTWA testing in risk-stratifying patients for VTE 4, an extensive body of literature has evolved on the clinical utility and the underlying pathophysiologic mechanisms of MTWA. In this paper, we aim to provide a contemporary perspective on clinical MTWA testing for arrhythmia risk stratification and also provide a review of emerging applications for alternans.
Emerging insights on the pathophysiology of MTWA
T-wave alternans likely occurs as a result of beat-to-beat alternation in AP duration (APD) at the cellular level. Two prevailing hypotheses have emerged to explain alternation of APD: the APD restitution hypothesis and the calcium restitution hypothesis (Figure 1).
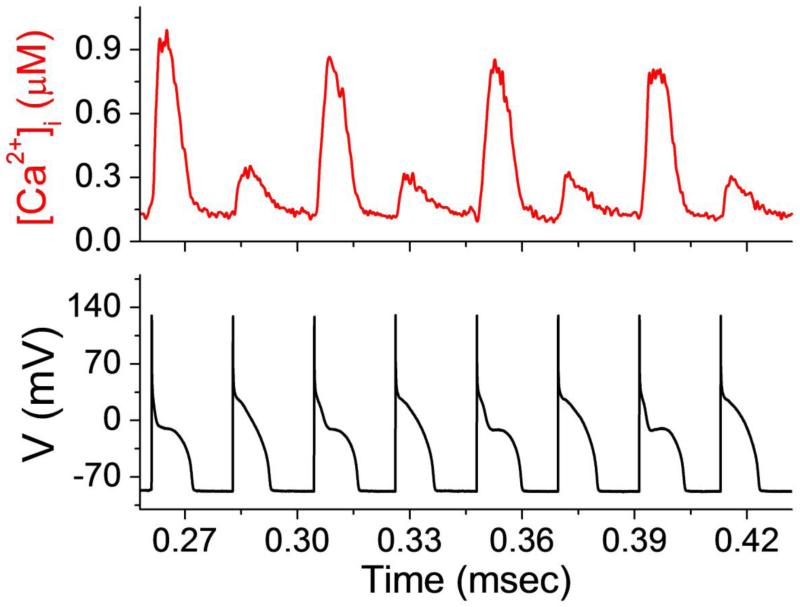
Figure 1.
Illustration of [Ca2+]i and AP alternans. Representative example of intracellular calcium concentration ([Ca2+]i) and action potential (AP) voltage alternans in a left ventricular canine myocyte.
The APD restitution hypothesis suggests that alternation in sarcolemmal currents, membrane voltage and AP morphology lead to beat-to-beat fluctuations in intracellular calcium concentration ([Ca2+]i). The calcium restitution hypothesis posits that alternation of [Ca2+]i is the primary event which then secondarily leads to alternans of membrane voltage and AP morphology 5-12. According to the second hypothesis, [Ca2+]i alternans can result from stress-induced 5, 13 perturbations in any number of Ca2+ transport processes including Ca2+ entry into the cytoplasm 14, recovery of ryanodine receptors (RyRs) from inactivation, triggering of sarcoplasmic reticulum (SR) Ca2+ release 6, 10, SR Ca2+ uptake 15, intra-SR Ca2+ redistribution 16, 17 and linking of intracellular Ca2+ handling to surface membrane voltage 8.
Early work demonstrated significant variation across species in the ability to induce alternans and also demonstrated that APD alternans is more easily induced at lower temperatures 18, which tend to prolong APD, therefore suggesting a primary role for membrane voltage dynamics in alternans at the tissue level. Further support of this hypothesis comes from data showing that modulation of sarcolemmal Ca2+ 19 and K+ 14, 20 currents based on changes in AP morphology 8 has a significant effect on the stability of Ca2+ handling processes and the transition to stable alternans 21,22. These results are in agreement with the findings of Weiss et al 23 in computer simulations which have shown that at the cellular level, both steep APD restitution (the relationship between APD and the previous diastolic interval) slope and [Ca2+]i cycling dynamics cause APD and [Ca2+]i to alternate.
However, despite the demonstration that sustained APD alternans occurs when the APD restitution slope is >1 at a given cycle length, additional experimental evidence indicates that the onset of APD alternans is primarily attributable to an instability in [Ca2+]i cycling dynamics rather than steep APD restitution 23, 24. Voltage clamp experiments in isolated myocytes 7 have demonstrated that [Ca2+]i exhibits alternans despite a constant beat-to-beat AP (voltage) waveform, suggesting that APD alternans is typically driven by [Ca2+]i dynamics and not by voltage dynamics (i.e. steep APD restitution slope). Furthermore, in both isolated ventricular myocytes 5 and intact tissue 25, the onset of APD alternans has been demonstrated at a constant cycle length at which APD restitution slope is still considerably <1 and interventions that suppress [Ca2+]SR cycling have eliminated AP alternans irrespective of the APD restitution 5. Overall, a preponderance of recent data has emerged in support of the calcium restitution hypothesis, suggesting the primacy of perturbations in Ca2+ handling processes as the fundamental event in the genesis of cellular alternans (for a detailed review of the calcium cycling hypothesis, see Merchant and Armoundas 26).
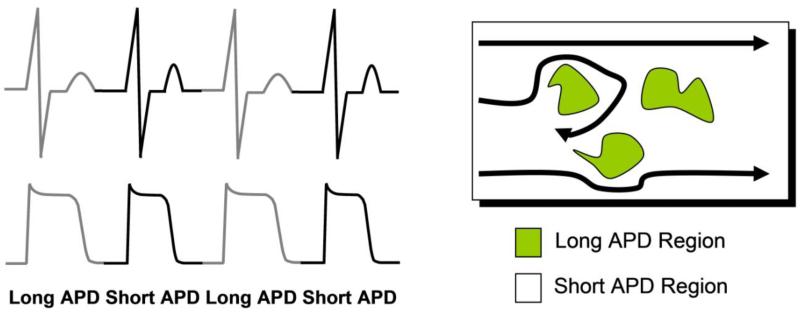
Optical mapping experiments in isolated perfused hearts 11, 13, 27-32 and single cell electrophysiological studies 5-12, 14, 19, 20, 33, have shown that cardiac alternans has its origin at the single cell level. Weiss et al 23 have demonstrated that at the tissue level, additional factors such as conduction velocity restitution and ectopic beats promote spatially discordant alternans, a condition where adjacent regions of myocardium demonstrate long and short APD sequences which oscillate out-of-phase with each other. Overall, it is believed that localized regions of AP alternans (spatial alternans), exhibiting delayed recovery on an every other beat basis, are intrinsically related to increased repolarization gradients sufficient to produce unidirectional block, wave break, reentry and arrhythmia on-set (Figure 2). This mechanism linking alternans and arrhythmogenesis has been explored by Kuo et al 34 who have shown that increased dispersion of repolarization (DR) is an important condition for the development of reentrant arrhythmias. The mechanistic link between the onset of alternans and the substrate for reentrant arrhythmias also lends support to the notion that beyond medium- and long-term risk stratification, MTWA may also play a role in predicting short-term arrhythmia susceptibility. In aggregate, evidence supports the idea that at a minimum, the mechanisms that generate alternans (functional spatial dispersion of refractoriness 11, 13, 27-32, 35, 36) are likely to also lead to ventricular tachycardia/ventricular fibrillation (VT/VF), such that the heart either passes through a state of heightened alternans on the way to VT/VF or heightened alternans occurs in close conjunction with developing VT/VF.
Figure 2.
Functional relationship of alternans and re-entry. Localized action potential alternans is manifested as repolarization alternans on the electrocardiogram. Localized regions of tissue exhibiting action potential alternans are associated with delayed recovery on an every other beat basis. These tissue areas of delayed recovery may lead to wavebreak and the development of reentry.
The preponderance of data in support of the calcium restitution hypothesis may also have important clinical implications. Impairments in intracellular calcium cycling machinery have been identified as a major cause of contractile dysfunction/heart failure and may provide a common mechanism linking contractile dysfunction with electrophysiologic risk. In support of this idea, it has recently been demonstrated in animal models that impairment of sarcoplasmic reticulum Ca2+ ATPase 2a (SERCA 2a) function is associated with enhanced susceptibility to alternans 37 and SERCA 2a gene transfer improves both contractile function and reduces arrhythmia susceptibility 38, 39. Extension of these findings holds the exciting promise of developing therapeutic strategies that not only improve myocardial contractile function but also reduce the risk of VTE. Improved understanding of the pathophysiology of T-wave alternans and the specific cellular and sub-cellular defects which lead to alternans may also shed light on which patients are at particularly high, or low, arrhythmic risk.
Another area of emerging interest in the pathogenesis of malignant arrhythmias is the role of the autonomic nervous system. The effect of sympathetic nervous system stimulation on the heart is complex and is governed by the state of the myocardium. In the normal ventricle, sympathetic stimulation shortens the APD and reduces the dispersion of repolarization, both associated with a decrease in arrhythmogenic tendency 40. However, in pathological states associated with reductions in repolarizing capacity of the ventricular myocardium, such as congestive heart failure, sympathetic stimulation is a potent stimulus for the generation of arrhythmias, perhaps by enhancing the dispersion of repolarization, which may be why β-adrenergic blockade reduces sudden death mortality in patients with heart failure 41, 42. Consistent with these observations, pre-clinical studies have demonstrated that sympathetic stimulation tends to reduce the threshold for RA and increase arrhythmia susceptibility whereas parasympathetic stimulation has the opposite effect 43, 44. In this context, interventions that reduce cardiac sympathetic activity have been shown to protect against arrhythmias 45-47, whereas those that enhance sympathetic activity provoke malignant arrhythmias 45, 46, 48, 49.
Clinical MTWA testing
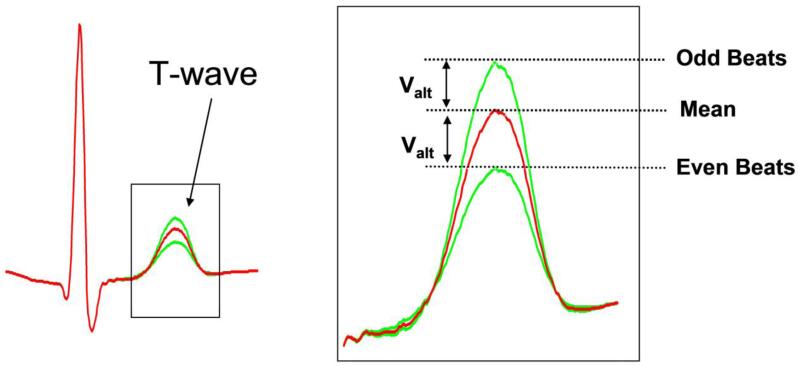
The presence of microvolt level beat-to-beat alternation in T-wave morphology (Figure 3) during low-level exercise has been identified as a marker of ventricular arrhythmia susceptibility and has lead to the advent of MTWA testing for clinical risk stratification. Over the last two decades, numerous studies have assessed the ability of MTWA testing to predict risk of VTE in both ischemic and non-ischemic heart failure and other forms of structural heart disease 50, 51. In general, patients with a positive MTWA test have consistently been found to a have a significantly increased risk of ventricular arrhythmias during follow-up whereas those with negative MTWA tests have a very low arrhythmic risk. In the era of primary prevention ICD therapy, there has been widespread recognition that new tools, beyond LVEF, are necessary to better risk stratify patients and determine which patients are most, or least, likely to benefit from ICDs. Specifically, among patients who are currently candidates for primary prevention ICD therapy (i.e. LVEF ≤ 35%), only a small percentage of patients (~ 2-5% per year) will suffer a ventricular arrhythmia resulting in sudden cardiac death (SCD) 52. Conversely, the majority of SCD events occur in patients with only mildly impaired or even preserved LV systolic function 53, 54.
Figure 3.
Beat-to-beat alternation in T-wave morphology. Illustration of microvolt level (Valt) alternation in T-wave amplitude which serves as the premise of clinical microvolt T-wave alternans (MTWA) testing.
Two studies have specifically assessed the utility of MTWA testing to predict arrhythmic risk in patients implanted with ICDs. The Microvolt T-wave Alternans Testing for Risk Stratification of Post-Myocardial Infarction Patient trial (MASTER) 55 studied 575 patients meeting Multicenter Automatic Defibrillator Implantation Trial II (MADIT II) criteria 56, all of whom underwent MTWA testing followed by ICD implantation. During an average follow-up of just over 2 years, MTWA status (negative vs. non-negative) was not found to be a significant predictor of VTE (defined as SCD or appropriate ICD therapy). However, a non-negative MTWA test result was associated with a significantly heightened risk of all-cause mortality. The MTWA sub-study of the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) reported on 490 patients enrolled in a sub-study of the larger trial 57. Again, MTWA status was not found to be a significant predictor of the primary composite endpoint of SCD, sustained VTE or appropriate ICD therapy. In large, part due to these results, there has been a dampening of enthusiasm for clinical MTWA testing as a tool to guide ICD implantation.
What might account for the discrepancy between the results of MASTER and the SCD-HeFT MTWA sub-study and the majority of earlier studies documenting a robust role for MTWA testing in predicting arrhythmic risk? One plausible explanation may lie in the confounding effect of including “appropriate” ICD therapy as a clinical endpoint. It is well recognized that many “appropriate” ICD therapies treat arrhythmias that would have self-terminated or that ICDs may induce arrhythmias that they subsequently treat 58-60. In fact, the number of “appropriate” ICD therapies may overestimate the true incidence of SCD in the placebo arm of primary prevention ICD trials by a factor of 2 or greater 59. In light of this, a recent meta-analysis has suggested that MTWA testing maintains robust predictive capacity in studies where relatively few patients are implanted with ICDs (i.e. ≤ 15%) and “appropriate” ICD therapies account for a small percentage of the overall outcome events, whereas the predictive capacity of MTWA is significantly obscured when a larger percentage of patients are implanted with ICDs 58. These data suggest that MTWA testing is not as good a predictor of “appropriate” ICD therapy, as it is a predictor of VTE/SCD in patients without ICDs. It is likely that MTWA testing is best utilized for patients who do not already have ICDs in order to determine whether they are at risk and should be considered for ICD therapy.
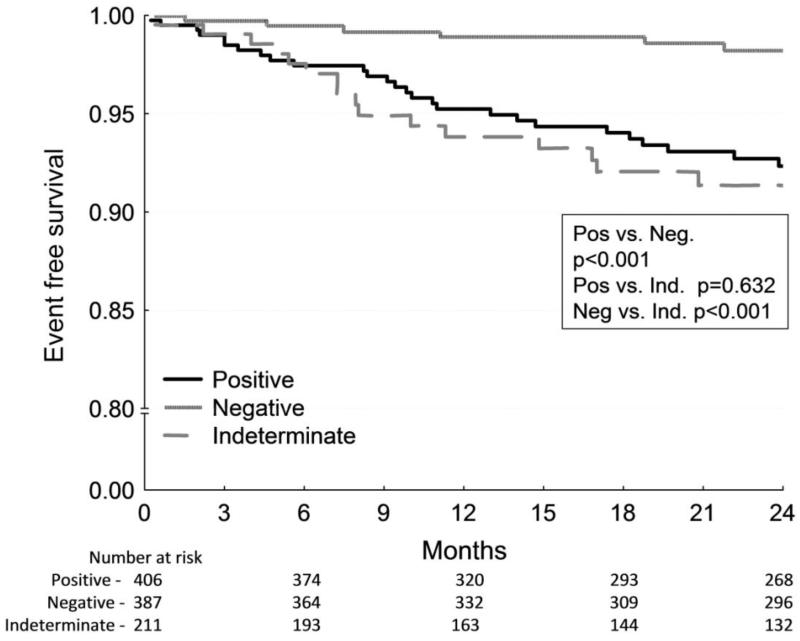
To overcome the potential cofounding influence of ICD therapy on clinical endpoints, we have recently shown that in a pooled cohort of 2883 patients without ICDs, a negative MTWA test in patients with LVEF ≤ 35% predicts a very low risk for SCD (0.9% per year), whereas the risk of SCD was significantly increased among patients with positive MTWA tests (approximately 4% per year) 61 (Figure 4A). In this study, MTWA testing was also a significant predictor of SCD risk among patients with LVEF > 35% with annual SCD rates of 3.0% in the positive MTWA cohort versus 0.3% in the negative MTWA group (Figure 4B). Further data in support of this idea come from a prospective study of patients with LVEF ≤ 35% which showed that ICD therapy reduced annual mortality by approximately 50% among patients with a non-negative MTWA test and provided no benefit among those with a negative test 62.
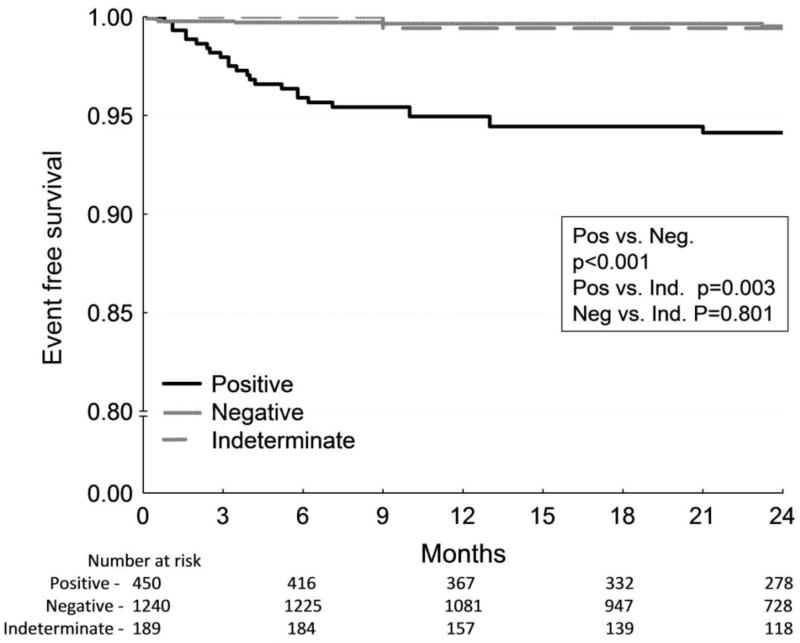
Figure 4.
Kaplan-Meier event-free survival curves for arrhythmic mortality/sudden cardiac death stratified by MTWA test result. A, Among patients with left ventricle ejection fraction (LVEF) ≤ 35%, patients with a positive or indeterminate MTWA test result demonstrate significantly higher mortality than those with negative MTWA results. B, In contrast, among patients with LVEF > 35%, only a positive MTWA test predicts increased mortality. All p values are generated by the log-rank test. (From: Merchant FM, Ikeda T, Pedretti RF, Salerno-Uriarte JA, Chow T, Chan PS, Bartone C, Hohnloser SH, Cohen RJ, Armoundas AA. Clinical utility of microvolt T-wave alternans testing in identifying patients at high or low risk of sudden cardiac death. Heart Rhythm. 2012;9(8):1256-1264 e1252) [61••].
A generally consistent finding in the MTWA literature is that patients with a negative MTWA test are at very low risk of arrhythmic death during follow-up 51, 55, 58, 63, 64. In the Alternans Before Cardioverter Defibrillator (ABCD) Trial, among patients with LVEF 40%, coronary artery disease and non-sustained ventricular tachycardia, a negative MTWA test demonstrated a negative predictive value of approximately 95% and 90% at 1 and 2 years, respectively, for the primary endpoint of SCD or appropriate ICD discharge 64. The negative predictive value of a negative MTWA test in the ABCD trial was comparable to invasive electrophysiology testing. Recent studies of patients meeting MADIT II 56 or SCD-HeFT 65 criteria for ICD therapy have demonstrated that approximately one-quarter to one-third of patients with impaired LVEF will have a negative MTWA test result 55, 57, 66 and based on the pooled analysis described above 61, it can be estimated that the risk of SCD in these patients is approximately 0.9% per year. No study has ever demonstrated a benefit to primary prevention ICD therapy in patients with a SCD risk as low as <1% per year, suggesting that the excellent negative predictive value of a negative MTWA test may still have an important clinical role in identifying patients who are unlikely to benefit from ICD therapy. The ability to safely withhold device therapy from these patients represents a major opportunity to reduce unnecessary exposure to an invasive treatment with well-established short- and long-term complications 67, while at the same time improving resource allocation and reducing the cost burden to the healthcare system.
The above described clinical data reference primarily the Fast Fourier Transform (FFT)-based spectral method for estimating MTWA during a graded increase in heart rate via low-level exercise, chronotropic stimulation or atrial pacing. However, several other methods have been applied for the detection and quantification of MTWA including autocorrelation 68, complex demodulation 69, autoregression 70 and the modified moving average (MMA) 71, 72. To date, the FFT based method remains the most studied and best validated method for measuring RA due in large part to its ability to differentiate between true alternans and non-specific noise in the ECG. The FFT method serves as the basis for the commercially available Cambridge Heart system, while the MMA method, which can be employed during both exercise and during ambulatory (Holter) monitoring, is manufactured by GE Healthcare®. Although both methods are designed to detect the same phenomenon, head-to-head comparative data on the clinical performance of the two methods are lacking 73.
Emerging clinical applications of RA
Although MTWA testing has traditionally been used for predicting medium- and long-term risk of ventricular arrhythmias, the concept of MTWA may have several novel clinical applications. The pathophysiology of alternans suggests that alternation in APD may play a critical role in generating the necessary electrophysiologic substrate for arrhythmia onset. Numerous experimental 11, 12, 74, 75 and computational 76-78 studies have demonstrated that discordant APD alternans, where the APDs of adjacent regions of myocardium oscillate out-of-phase (Figure 2), is associated with increased DR and VT/VF (DR is greater at sites of discordant vs. concordant alternans) 11. In this paradigm, the presence of APD alternans may also serve as an important marker of short-term arrhythmia susceptibility. Several lines of clinical evidence support this idea. Analysis of ambulatory body-surface electrograms (Holter monitors) from patients with various forms of heart disease has demonstrated a sharp upsurge in repolarization alternans (measured by time-domain techniques) within the minutes prior to spontaneous VTE 79. Similarly, analysis of body-surface electrocardiograms from patient hospitalized with decompensated heart failure has demonstrated a significant upsurge in MTWA during the 15-30 mins prior to the onset of spontaneous VTE 80.
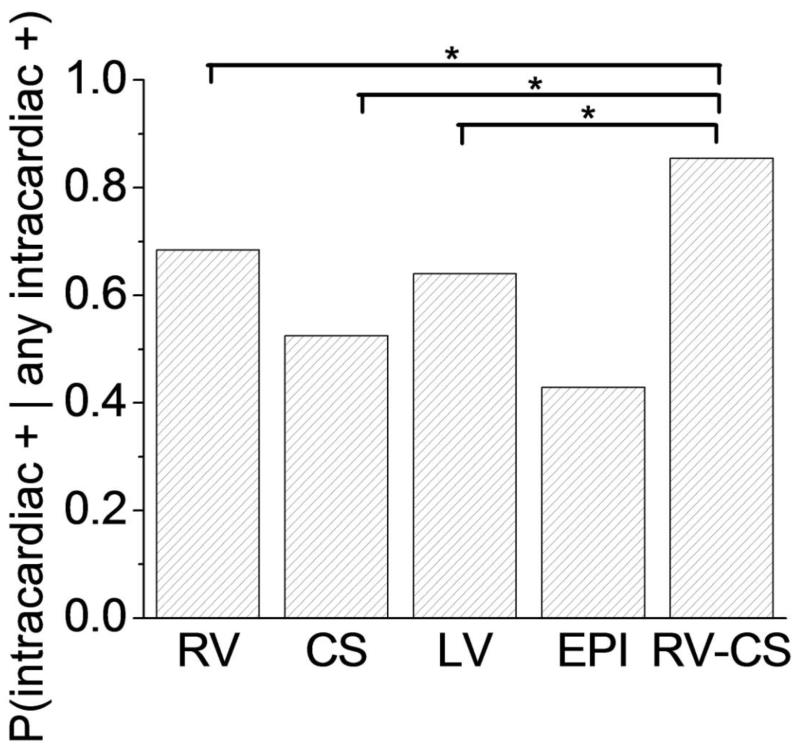
Analysis of intra-cardiac electrograms from ICDs has also demonstrated a sharp increase in MTWA magnitude immediately prior to spontaneous ventricular arrhythmias 81-84. However, a similar upsurge in MTWA has not been observed prior to induced ventricular arrhythmias or preceding inappropriate ICD shocks 81, suggesting that the presence of increased MTWA magnitude is not just a by-product of a ventricular arrhythmia or a consequence of an ICD shock. Simultaneous measurement of MTWA from body-surface and intracardiac electrograms by our group 63 and others 85 has shown a high degree of correlation suggesting that these measurements are detecting the same electrical phenomenon. Additionally, we have recently developed a novel intra-cardiac lead configuration consisting of leads in the right ventricle and the coronary sinus that provides an “optimal” sensitivity in detecting intra-cardiac MTWA 63 (Figure 5).
Figure 5.
Sensitivity of alternans detection in intra-cardiac leads. Probability that a far-field bipolar intra-cardiac lead is positive for repolarization alternans (RA), given that at least 1 intra-cardiac far-field lead is positive for RA, for each of the right ventricle (RV), coronary sinus (CS), left ventricle (LV), epicardial (EPI), and triangular right ventricle-coronary sinus (RV-CS) far-field intra-cardiac lead configurations. The RV-CS positive percentage was significantly larger than for the RV configuration (P=0.040), the CS configuration (P=0.004), and the LV configuration (P=0.035) but not for the EPI configuration (P=0.270). Statistically significant comparisons are marked by an asterisk. (From: Weiss EH, Merchant FM, d’Avila A, Foley L, Reddy VY, Singh JP, Mela T, Ruskin JN, Armoundas AA. A novel lead configuration for optimal spatio-temporal detection of intracardiac repolarization alternans. Circ Arrhythm Electrophysiol. Jun 1, 2011: 407-417) [63•].
The ability to detect acute surges in RA/TWA from intra-cardiac or body-surface electrograms, respectively, immediately prior to the onset of spontaneous VTE holds significant promise for developing tools to warn patients of impending arrhythmias and possibly to deliver upstream therapy and preempt arrhythmia onset. For instance, there has been significant interest in the use of dynamic pacing protocols to control APD and suppress discordant RA. Experimental studies have demonstrated that stimulation during the absolute refractory period is capable of controlling APD, in part through modulation of cellular calcium transients 86,87. Based on this premise, our group has recently developed a method for in situ dynamic control of MTWA in a large animal model 88. In this model, the delivery of sub-threshold pacing stimuli during the absolute refractory period is capable of suppressing MTWA in a swine heart failure model, even when the pacing site (right ventricle) was located at a distance from the site of alternans genesis (left ventricle).
Extension of these findings raises the possibility of incorporating adaptive pacing protocols into implantable devices such that if the device detects an unstable myocardial substrate (as evidenced by heightened MTWA magnitude), the adaptive pacing protocol would be activated to deliver electrical therapy that is specific to the underlying electrical instability to re-stabilize the electrical substrate and prevent the onset of a spontaneous VTE. The adaptive pacing protocol could be terminated when the MTWA magnitude falls below a predetermined threshold. Beyond adaptive pacing protocols, detection of MTWA by implantable devices may also be coupled to other forms of suppressive therapy. For instance, there is significant interest in coupling micro-electromechanical systems (MEMS) to implantable devices to facilitate localized delivery of pharmacologic agents for treating various aspects of chronic heart failure (i.e. neurohormonal antagonists, diuretics, anti-arrhythmic agents) 89. It’s conceivable that timely and potentially localized delivery of such agents may be capable of suppressing MTWA and re-stabilizing the electrical substrate. In an analogous manner, detection of heightened MTWA in patients hospitalized with decompensated heart failure may prompt down-titration of inotropic/chronotropic agents or temporary initiation of anti-arrhythmic therapy to quell the arrhythmogenic substrate and preempt VTE onset.
Although ventricular RA/TWA has traditionally been implicated in the pathogenesis and risk stratification of ventricular arrhythmias, another emerging application in the clinical arena involves atrial arrhythmogenesis. Alternation of atrial APD has been implicated in the transition from atrial flutter to atrial fibrillation (AF) 90 and atrial APD restitution dynamics have been implicated as a mechanism for why premature atrial depolarizations from pulmonary vein foci may trigger AF in susceptible substrates 24. Additionally, alternation of atrial APD has been documented to occur at lower heart rates in patients with persistent AF versus those with paroxysmal AF or controls, thus revealing important differences in the underlying substrate among these groups 91. Furthermore, the presence of atrial APD alternans preceded the transition to AF in all instances. These findings suggest that atrial alternans may play a crucial role in the genesis of AF and mechanistic insights into the pathophysiology of atrial alternans may lead to novel risk stratification tools and therapeutic options.
Conclusions
Numerous experimental and computational studies have demonstrated that APD alternans gives rise to T-wave alternans and can provide the substrate for reentrant arrhythmias. Although MTWA testing has traditionally been utilized as a clinical tool for SCD risk stratification, as highlighted in this paper, the role of MTWA testing in the context of primary prevention ICD therapy continues to evolve. However, as the pathogenesis of repolarization alternans is better elucidated, it is likely that MTWA will play a broader clinical role in both short-term arrhythmia prediction and management of congestive heart failure.
Acknowledgments
The work was supported by NIA grant 1R21AG035128, by a Fellowship and a Science Award from the Center for Integration of Medicine and Innovative Technology (CIMIT), a Post-doctoral Fellowship (#12POST9310001) from the American Heart Association and the Deane Institute for Integrative Research in Atrial Fibrillation and Stroke and the Cardiovascular Research Society.
Footnotes
Conflict of Interest
Faisal M. Merchant, Omid Sayadi, Kasra Moazzami, Dheeraj Puppala, andAntonis A. Armoundas declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Hering HE. Das Wesen des Herzalternans. Munchen. Med. Wchenshr. 1908;4:1417–1421. [Google Scholar]
- 2.Ritzenberg AL, Adam DR, Cohen RJ. Period multupling-evidence for nonlinear behaviour of the canine heart. Nature. 1984;307(5947):159–161. doi: 10.1038/307159a0. [DOI] [PubMed] [Google Scholar]
- 3.Smith JM, Clancy EA, Valeri CR, Ruskin JN, Cohen RJ. Electrical alternans and cardiac electrical instability. Circulation. 1988;77(1):110–121. doi: 10.1161/01.cir.77.1.110. [DOI] [PubMed] [Google Scholar]
- 4.Rosenbaum DS, Jackson LE, Smith JM, Garan H, Ruskin JN, Cohen RJ. Electrical alternans and vulnerability to ventricular arrhythmias. N Engl J Med. 1994;330(4):235–241. doi: 10.1056/NEJM199401273300402. [DOI] [PubMed] [Google Scholar]
- 5.Goldhaber JI, Xie LH, Duong T, Motter C, Khuu K, Weiss JN. Action potential duration restitution and alternans in rabbit ventricular myocytes: the key role of intracellular calcium cycling. Circ Res. 2005;96(4):459–466. doi: 10.1161/01.RES.0000156891.66893.83. [DOI] [PubMed] [Google Scholar]
- 6.Diaz ME, Eisner DA, O’Neill SC. Depressed ryanodine receptor activity increases variability and duration of the systolic Ca2+ transient in rat ventricular myocytes. Circ Res. 2002;91(7):585–593. doi: 10.1161/01.res.0000035527.53514.c2. [DOI] [PubMed] [Google Scholar]
- 7.Chudin E, Goldhaber J, Garfinkel A, Weiss J, Kogan B. Intracellular Ca(2+) dynamics and the stability of ventricular tachycardia. Biophys J. 1999;77(6):2930–2941. doi: 10.1016/S0006-3495(99)77126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jordan PN, Christini DJ. Action potential morphology influences intracellular calcium handling stability and the occurrence of alternans. Biophys J. 2006;90(2):672–680. doi: 10.1529/biophysj.105.071340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kockskamper J, Zima AV, Blatter LA. Modulation of sarcoplasmic reticulum Ca2+ release by glycolysis in cat atrial myocytes. J Physiol. 2005;564(Pt 3):697–714. doi: 10.1113/jphysiol.2004.078782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hüser J, Wang YG, Sheehan KA, Cifuentes F, Lipsius SL, Blatter LA. Functional coupling between glycolysis and excitation-contraction coupling underlies alternans in cat heart cells. J Physiol (Lond) 2000;524(Pt 3):795–806. doi: 10.1111/j.1469-7793.2000.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pastore JM, Girouard SD, Laurita KR, Akar FG, Rosenbaum DS. Mechanism linking T-wave alternans to the genesis of cardiac fibrillation. Circulation. 1999;99(10):1385–1394. doi: 10.1161/01.cir.99.10.1385. [DOI] [PubMed] [Google Scholar]
- 12.Pastore JM, Rosenbaum DS. Role of structural barriers in the mechanism of alternans-induced reentry. Circ Res. 2000;87(12):1157–1163. doi: 10.1161/01.res.87.12.1157. [DOI] [PubMed] [Google Scholar]
- 13.Laurita KR, Pastore JM, Rosenbaum DS. How restitution, repolarization, and alternans form arrhythmogenic substrates: insights from high-resolution optical mapping. In: Zipes DP, Jalife J, editors. Cardiac Electrophysiology: From Cell to Bedside. 2nd ed. W.B.Saunders; Philadelphia, PA: 1999. pp. 239–248. [Google Scholar]
- 14.Fox JJ, McHarg JL, Gilmour RF., Jr. Ionic mechanism of electrical alternans. Am J Physiol Heart Circ Physiol. 2002;282(2):H516–530. doi: 10.1152/ajpheart.00612.2001. [DOI] [PubMed] [Google Scholar]
- 15.Kameyama M, Hirayama Y, Saitoh H, Maruyama M, Atarashi H, Takano T. Possible contribution of the sarcoplasmic reticulum Ca(2+) pump function to electrical and mechanical alternans. J Electrocardiol. 2003;36(2):125–135. doi: 10.1054/jelc.2003.50021. [DOI] [PubMed] [Google Scholar]
- 16.Kihara Y, Morgan JP. Abnormal Cai2+ handling is the primary cause of mechanical alternans: study in ferret ventricular muscles. Am J Physiol. 1991;261(6 Pt 2):H1746–1755. doi: 10.1152/ajpheart.1991.261.6.H1746. [DOI] [PubMed] [Google Scholar]
- 17.Lab MJ, Lee JA. Changes in intracellular calcium during mechanical alternans in isolated ferret ventricular muscle. Circ Res. 1990;66(3):585–595. doi: 10.1161/01.res.66.3.585. [DOI] [PubMed] [Google Scholar]
- 18.Spear JF, Moore EN. A comparison of alternation in myocardial action potentials and contractility. Am J Physiol. 1971;220(6):1708–1716. doi: 10.1152/ajplegacy.1971.220.6.1708. [DOI] [PubMed] [Google Scholar]
- 19.Mahajan A, Sato D, Shiferaw Y, Baher A, Xie LH, Peralta R, Olcese R, Garfinkel A, Qu Z, Weiss JN. Modifying L-type calcium current kinetics: consequences for cardiac excitation and arrhythmia dynamics. Biophys J. 2008;94(2):411–423. doi: 10.1529/biophysj.106.98590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hua F, Johns DC, Gilmour RF., Jr. Suppression of electrical alternans by overexpression of HERG in canine ventricular myocytes. Am J Physiol Heart Circ Physiol. 2004;286(6):H2342–2351. doi: 10.1152/ajpheart.00793.2003. [DOI] [PubMed] [Google Scholar]
- 21.Allen DG, Orchard CH. Myocardial contractile function during ischemia and hypoxia. Circ Res. 1987;60(2):153–168. doi: 10.1161/01.res.60.2.153. [DOI] [PubMed] [Google Scholar]
- 22.Armoundas AA. Mechanism of abnormal sarcoplasmic reticulum calcium release in canine left-ventricular myocytes results in cellular alternans. IEEE Trans Biomed Eng. 2009;56(2):220–228. doi: 10.1109/TBME.2008.2003283. [DOI] [PubMed] [Google Scholar]
- 23.Weiss JN, Karma A, Shiferaw Y, Chen PS, Garfinkel A, Qu Z. From pulsus to pulseless: the saga of cardiac alternans. Circ Res. 2006;98(10):1244–1253. doi: 10.1161/01.RES.0000224540.97431.f0. [DOI] [PubMed] [Google Scholar]
- 24.Narayan SM, Bayer JD, Lalani G, Trayanova NA. Action potential dynamics explain arrhythmic vulnerability in human heart failure: a clinical and modeling study implicating abnormal calcium handling. J Am Coll Cardiol. 2008;52(22):1782–1792. doi: 10.1016/j.jacc.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pruvot EJ, Katra RP, Rosenbaum DS, Laurita KR. Role of calcium cycling versus restitution in the mechanism of repolarization alternans. Circ Res. 2004;94(8):1083–1090. doi: 10.1161/01.RES.0000125629.72053.95. [DOI] [PubMed] [Google Scholar]
- 26.Merchant FM, Armoundas AA. Role of substrate and triggers in the genesis of cardiac alternans, from the myocyte to the whole heart: implications for therapy. Circulation. 2012;125(3):539–549. doi: 10.1161/CIRCULATIONAHA.111.033563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi BR, Salama G. Simultaneous maps of optical action potentials and calcium transients in guinea-pig hearts: mechanisms underlying concordant alternans. J Physiol. 2000;529(Pt 1):171–188. doi: 10.1111/j.1469-7793.2000.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laurita KR, Girouard SD, Akar FG, Rosenbaum DS. Modulated dispersion explains changes in arrhythmia vulnerability during premature stimulation of the heart. Circulation. 1998;98(24):2774–2780. doi: 10.1161/01.cir.98.24.2774. [DOI] [PubMed] [Google Scholar]
- 29.Laurita KR, Rosenbaum DS. Implications of ion channel diversity to ventricular repolarization and arrhythmogenesis: insights from high resolution optical mapping. Can J Cardiol. 1997;13(11):1069–1076. [PubMed] [Google Scholar]
- 30.Lee HC, Mohabir R, Smith N, Franz MR, Clusin WT. Effect of ischemia on calcium-dependent fluorescence transients in rabbit hearts containing indo 1. Correlation with monophasic action potentials and contraction. Circulation. 1988;78(4):1047–1059. doi: 10.1161/01.cir.78.4.1047. [DOI] [PubMed] [Google Scholar]
- 31.Qian YW, Clusin WT, Lin SF, Han J, Sung RJ. Spatial heterogeneity of calcium transient alternans during the early phase of myocardial ischemia in the blood-perfused rabbit heart. Circulation. 2001;104(17):2082–2087. doi: 10.1161/hc4201.097136. [DOI] [PubMed] [Google Scholar]
- 32.Wu Y, Clusin WT. Calcium transient alternans in blood-perfused ischemic hearts: observations with fluorescent indicator fura red. Am J Physiol. 1997;273(5 Pt 2):H2161–2169. doi: 10.1152/ajpheart.1997.273.5.H2161. [DOI] [PubMed] [Google Scholar]
- 33.Armoundas AA. Mechanism of Abnormal Sarcoplasmic Reticulum Calcium Release in Canine Left Ventricular Myocytes Results in Cellular Alternans. IEEE Trans Biomed Eng. 2009 doi: 10.1109/TBME.2008.2003283. In press. [DOI] [PubMed] [Google Scholar]
- 34.Kuo CS, Amlie JP, Munakata K, Reddy CP, Surawicz B. Dispersion of monophasic action potential durations and activation times during atrial pacing, ventricular pacing, and ventricular premature stimulation in canine ventricles. Cardiovasc Res. 1983;17(3):152–161. doi: 10.1093/cvr/17.3.152. [DOI] [PubMed] [Google Scholar]
- 35.Chinushi M, Restivo M, Caref EB, El-Sherif N. Electrophysiological basis of arrhythmogenicity of QT/T alternans in the long-QT syndrome: tridimensional analysis of the kinetics of cardiac repolarization. Circ Res. 1998;83(6):614–628. doi: 10.1161/01.res.83.6.614. [DOI] [PubMed] [Google Scholar]
- 36.Chinushi M, Kozhevnikov D, Caref EB, Restivo M, El-Sherif N. Mechanism of discordant T wave alternans in the in vivo heart. J Cardiovasc Electrophysiol. 2003;14(6):632–638. doi: 10.1046/j.1540-8167.2003.03028.x. [DOI] [PubMed] [Google Scholar]
- 37.Wilson LD, Jeyaraj D, Wan X, Hoeker GS, Said TH, Gittinger M, Laurita KR, Rosenbaum DS. Heart failure enhances susceptibility to arrhythmogenic cardiac alternans. Heart Rhythm. 2009;6(2):251–259. doi: 10.1016/j.hrthm.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.del Monte F, Lebeche D, Guerrero JL, Tsuji T, Doye AA, Gwathmey JK, Hajjar RJ. Abrogation of ventricular arrhythmias in a model of ischemia and reperfusion by targeting myocardial calcium cycling. Proc Natl Acad Sci U S A. 2004;101(15):5622–5627. doi: 10.1073/pnas.0305778101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39••.Cutler MJ, Wan X, Plummer BN, Liu H, Deschenes I, Laurita KR, Hajjar RJ, Rosenbaum DS. Targeted sarcoplasmic reticulum Ca2+ATPase 2a gene delivery to restore electrical stability in the failing heart. Circulation. 2012;126(17):2095–2104. doi: 10.1161/CIRCULATIONAHA.111.071480. This paper highlights the close relationship between contractile dysfunction and arrhythmogenesis in pre-clinical models.
- 40.Takei M, Sasaki Y, Yonezawa T, Lakhe M, Aruga M, Kiyosawa K. The autonomic control of the transmural dispersion of ventricular repolarization in anesthetized dogs. J Cardiovasc Electrophysiol. 1999;10(7):981–989. doi: 10.1111/j.1540-8167.1999.tb01269.x. [DOI] [PubMed] [Google Scholar]
- 41.MERIT-HF Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet. 1999;353(9169):2001–2007. [PubMed] [Google Scholar]
- 42.Poole-Wilson PA, Swedberg K, Cleland JG, Di Lenarda A, Hanrath P, Komajda M, Lubsen J, Lutiger B, Metra M, Remme WJ, Torp-Pedersen C, Scherhag A, Skene A. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362(9377):7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- 43.Ng GA, Brack KE, Patel VH, Coote JH. Autonomic modulation of electrical restitution, alternans and ventricular fibrillation initiation in the isolated heart. Cardiovasc Res. 2007;73(4):750–760. doi: 10.1016/j.cardiores.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 44.Euler DE, Guo H, Olshansky B. Sympathetic influences on electrical and mechanical alternans in the canine heart. Cardiovasc Res. 1996;32(5):854–860. [PubMed] [Google Scholar]
- 45.Corr PB, Yamada KA, Witkowski FX. Mechanisms controlling cardiac autonomic function and their relationships to arrhythmogenesis. In: Fozzard HA, Haber E, Jennings RB, et al., editors. The Heart and Cardiovascular System. Raven Press; New York: 1986. pp. 1343–1404. [Google Scholar]
- 46.Rubart M, Zipes DP. Mechanisms of sudden cardiac death. J Clin Invest. 2005;115(9):2305–2315. doi: 10.1172/JCI26381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwartz PJ, Zipes DP. Autonomic modulation of cardiac arrhythmias. In: Zipes DP, Jalife J, editors. Cardiac Electophysiology: From Cell to Bedside. 3rd ed. Saunders W.B.; 2000. pp. 300–314. [Google Scholar]
- 48.Janse MJ, Schwartz PJ, Wilms-Schopman F, Peters RJ, Durrer D. Effects of unilateral stellate ganglion stimulation and ablation on electrophysiologic changes induced by acute myocardial ischemia in dogs. Circulation. 1985;72(3):585–595. doi: 10.1161/01.cir.72.3.585. [DOI] [PubMed] [Google Scholar]
- 49.Billman GE. A comprehensive review and analysis of 25 years of data from an in vivo canine model of sudden cardiac death: implications for future anti-arrhythmic drug development. Pharmacol Ther. 2006;111(3):808–835. doi: 10.1016/j.pharmthera.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 50.Armoundas AA, Hohnloser SH, Ikeda T, Cohen RJ. Can microvolt T-wave alternans testing reduce unnecessary defibrillator implantation? Nat Clin Pract Cardiovasc Med. 2005;2(10):522–528. doi: 10.1038/ncpcardio0323. [DOI] [PubMed] [Google Scholar]
- 51.Gehi AK, Stein RH, Metz LD, Gomes JA. Microvolt T-wave alternans for the risk stratification of ventricular tachyarrhythmic events: a meta-analysis. J Am Coll Cardiol. 2005;46(1):75–82. doi: 10.1016/j.jacc.2005.03.059. [DOI] [PubMed] [Google Scholar]
- 52.Fishman GI, Chugh SS, Dimarco JP, Albert CM, Anderson ME, Bonow RO, Buxton AE, Chen PS, Estes M, Jouven X, Kwong R, Lathrop DA, Mascette AM, Nerbonne JM, O’Rourke B, Page RL, Roden DM, Rosenbaum DS, Sotoodehnia N, Trayanova NA, Zheng ZJ. Sudden cardiac death prediction and prevention: report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation. 2010;122(22):2335–2348. doi: 10.1161/CIRCULATIONAHA.110.976092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stecker EC, Vickers C, Waltz J, Socoteanu C, John BT, Mariani R, McAnulty JH, Gunson K, Jui J, Chugh SS. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol. 2006;47(6):1161–1166. doi: 10.1016/j.jacc.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 54.Rautaharju PM, Surawicz B, Gettes LS, Bailey JJ, Childers R, Deal BJ, Gorgels A, Hancock EW, Josephson M, Kligfield P, Kors JA, Macfarlane P, Mason JW, Mirvis DM, Okin P, Pahlm O, van Herpen G, Wagner GS, Wellens H. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009;53(11):982–991. doi: 10.1016/j.jacc.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 55.Chow T, Kereiakes DJ, Onufer J, Woelfel A, Gursoy S, Peterson BJ, Brown ML, Pu W, Benditt DG. Does microvolt T-wave alternans testing predict ventricular tachyarrhythmias in patients with ischemic cardiomyopathy and prophylactic defibrillators? The MASTER (Microvolt T Wave Alternans Testing for Risk Stratification of Post-Myocardial Infarction Patients) trial. J Am Coll Cardiol. 2008;52(20):1607–1615. doi: 10.1016/j.jacc.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 56.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346(12):877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 57.Gold MR, Ip JH, Costantini O, Poole JE, McNulty S, Mark DB, Lee KL, Bardy GH. Role of microvolt T-wave alternans in assessment of arrhythmia vulnerability among patients with heart failure and systolic dysfunction: primary results from the T-wave alternans sudden cardiac death in heart failure trial substudy. Circulation. 2008;118(20):2022–2028. doi: 10.1161/CIRCULATIONAHA.107.748962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58•.Hohnloser SH, Ikeda T, Cohen RJ. Evidence regarding clinical use of microvolt T-wave alternans. Heart Rhythm. 2009;6(3 Suppl):S36–44. doi: 10.1016/j.hrthm.2008.10.011. This paper elucidates the confounding role that including ICD therapies as an endpoint in risk stratification trials may have on defining the utility of T-wave alternans testing.
- 59.Ellenbogen KA, Levine JH, Berger RD, Daubert JP, Winters SL, Greenstein E, Shalaby A, Schaechter A, Subacius H, Kadish A. Are implantable cardioverter defibrillator shocks a surrogate for sudden cardiac death in patients with nonischemic cardiomyopathy? Circulation. 2006;113(6):776–782. doi: 10.1161/CIRCULATIONAHA.105.561571. [DOI] [PubMed] [Google Scholar]
- 60.Germano JJ, Reynolds M, Essebag V, Josephson ME. Frequency and causes of implantable cardioverter-defibrillator therapies: is device therapy proarrhythmic? Am J Cardiol. 2006;97(8):1255–1261. doi: 10.1016/j.amjcard.2005.11.048. [DOI] [PubMed] [Google Scholar]
- 61••.Merchant FM, Ikeda T, Pedretti RF, Salerno-Uriarte JA, Chow T, Chan PS, Bartone C, Hohnloser SH, Cohen RJ, Armoundas AA. Clinical utility of microvolt T-wave alternans testing in identifying patients at high or low risk of sudden cardiac death. Heart Rhythm. 2012;9(8):1256–1264. e1252. doi: 10.1016/j.hrthm.2012.03.014. This paper highlights the robust role of T-wave alternans testing in clinical risk stratification among patients without ICDs.
- 62.Chow T, Kereiakes DJ, Bartone C, Booth T, Schloss EJ, Waller T, Chung E, Menon S, Nallamothu BK, Chan PS. Microvolt T-wave alternans identifies patients with ischemic cardiomyopathy who benefit from implantable cardioverter-defibrillator therapy. J Am Coll Cardiol. 2007;49(1):50–58. doi: 10.1016/j.jacc.2006.06.079. [DOI] [PubMed] [Google Scholar]
- 63•.Weiss EH, Merchant FM, d’Avila A, Foley L, Reddy VY, Singh JP, Mela T, Ruskin JN, Armoundas AA. A novel lead configuration for optimal spatio-temporal detection of intracardiac repolarization alternans. Circ Arrhythm Electrophysiol. 2011 Jun 1;:407–417. doi: 10.1161/CIRCEP.109.934208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Costantini O, Hohnloser SH, Kirk MM, Lerman BB, Baker JH, 2nd, Sethuraman B, Dettmer MM, Rosenbaum DS. The ABCD (Alternans Before Cardioverter Defibrillator) Trial: strategies using T-wave alternans to improve efficiency of sudden cardiac death prevention. J Am Coll Cardiol. 2009;53(6):471–479. doi: 10.1016/j.jacc.2008.08.077. [DOI] [PubMed] [Google Scholar]
- 65.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352(3):225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 66.Bloomfield DM, Steinman RC, Namerow PB, Parides M, Davidenko J, Kaufman ES, Shinn T, Curtis A, Fontaine J, Holmes D, Russo A, Tang C, Bigger JT., Jr. Microvolt T-wave alternans distinguishes between patients likely and patients not likely to benefit from implanted cardiac defibrillator therapy: a solution to the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II conundrum. Circulation. 2004;110(14):1885–1889. doi: 10.1161/01.CIR.0000143160.14610.53. [DOI] [PubMed] [Google Scholar]
- 67.Reynolds MR, Cohen DJ, Kugelmass AD, Brown PP, Becker ER, Culler SD, Simon AW. The frequency and incremental cost of major complications among medicare beneficiaries receiving implantable cardioverter-defibrillators. J Am Coll Cardiol. 2006;47(12):2493–2497. doi: 10.1016/j.jacc.2006.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zareba W, Moss AJ, le Cessie S, Hall WJ. T wave alternans in idiopathic long QT syndrome. J Am Coll Cardiol. 1994;23(7):1541–1546. doi: 10.1016/0735-1097(94)90653-x. [DOI] [PubMed] [Google Scholar]
- 69.Nearing BD, Huang AH, Verrier RL. Dynamic tracking of cardiac vulnerability by complex demodulation of the T wave. Science. 1991;252(5004):437–440. doi: 10.1126/science.2017682. [DOI] [PubMed] [Google Scholar]
- 70.Zareba W, Moss AJ, le Cessie S, Locati EH, Robinson JL, Hall WJ, Andrews ML. Risk of cardiac events in family members of patients with long QT syndrome. J Am Coll Cardiol. 1995;26(7):1685–1691. doi: 10.1016/0735-1097(95)60383-2. [DOI] [PubMed] [Google Scholar]
- 71.Verrier RL, Nearing BD, Ghanem RN, Olson RE, Garberich RF, Katsiyiannis WT, Gornick CC, Tang CY, Henry TD. Elevated T-Wave Alternans Predicts Nonsustained Ventricular Tachycardia in Association with Percutaneous Coronary Intervention in ST-Segment Elevation Myocardial Infarction (STEMI) Patients. J Cardiovasc Electrophysiol. 2012 doi: 10.1111/jce.12102. [DOI] [PubMed] [Google Scholar]
- 72.Takasugi N, Kubota T, Nishigaki K, Verrier RL, Kawasaki M, Takasugi M, Ushikoshi H, Hattori A, Ojio S, Aoyama T, Takemura G, Minatoguchi S. Continuous T-wave alternans monitoring to predict impending life-threatening cardiac arrhythmias during emergent coronary reperfusion therapy in patients with acute coronary syndrome. Europace. 2012;13(5):708–715. doi: 10.1093/europace/euq512. [DOI] [PubMed] [Google Scholar]
- 73.Nieminen T, Nanbu DY, Datti IP, Vaz GR, Tavares CA, Pegler JR, Nearing BD, Belardinelli L, Verrier RL. Antifibrillatory effect of ranolazine during severe coronary stenosis in the intact porcine model. Heart Rhythm. 2011;8(4):608–614. doi: 10.1016/j.hrthm.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 74.Tachibana H, Kubota I, Yamaki M, Watanabe T, Tomoike H. Discordant S-T alternans contributes to formation of reentry: a possible mechanism of reperfusion arrhythmia. Am J Physiol. 1998;275(1 Pt 2):H116–121. doi: 10.1152/ajpheart.1998.275.1.H116. [DOI] [PubMed] [Google Scholar]
- 75.Shimizu W, Antzelevitch C. Cellular and ionic basis for T-wave alternans under long-QT conditions. Circulation. 1999;99(11):1499–1507. doi: 10.1161/01.cir.99.11.1499. [DOI] [PubMed] [Google Scholar]
- 76.Fox JJ, Riccio ML, Hua F, Bodenschatz E, Gilmour RF., Jr. Spatiotemporal transition to conduction block in canine ventricle. Circ Res. 2002;90(3):289–296. doi: 10.1161/hh0302.104723. [DOI] [PubMed] [Google Scholar]
- 77.Qu Z, Garfinkel A, Chen PS, Weiss JN. Mechanisms of discordant alternans and induction of reentry in simulated cardiac tissue. Circulation. 2000;102(14):1664–1670. doi: 10.1161/01.cir.102.14.1664. [DOI] [PubMed] [Google Scholar]
- 78.Watanabe MA, Fenton FH, Evans SJ, Hastings HM, Karma A. Mechanisms for discordant alternans. J Cardiovasc Electrophysiol. 2001;12(2):196–206. doi: 10.1046/j.1540-8167.2001.00196.x. [DOI] [PubMed] [Google Scholar]
- 79.Shusterman V, Goldberg A, London B. Upsurge in T-wave alternans and nonalternating repolarization instability precedes spontaneous initiation of ventricular tachyarrhythmias in humans. Circulation. 2006;113(25):2880–2887. doi: 10.1161/CIRCULATIONAHA.105.607895. [DOI] [PubMed] [Google Scholar]
- 80.Nearing BD, Wellenius GA, Mittleman MA, Josephson ME, Burger AJ, Verrier RL. Crescendo in depolarization and repolarization heterogeneity heralds development of ventricular tachycardia in hospitalized patients with decompensated heart failure. Circ Arrhythm Electrophysiol. 2012;5(1):84–90. doi: 10.1161/CIRCEP.111.965434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim JW, Pak HN, Park JH, Nam GB, Kim SK, Lee HS, Jang JK, Choi JI, Kim YH. Defibrillator electrogram T wave alternans as a predictor of spontaneous ventricular tachyarrhythmias in defibrillator recipients. Circ J. 2009;73(1):55–62. doi: 10.1253/circj.cj-08-0311. [DOI] [PubMed] [Google Scholar]
- 82.Armoundas AA, Albert CM, Cohen RJ, Mela T. Utility of implantable cardioverter defibrillator electrograms to estimate repolarization alternans preceding a tachyarrhythmic event. J Cardiovasc Electrophysiol. 2004;15(5):594–597. doi: 10.1046/j.1540-8167.2004.03411.x. [DOI] [PubMed] [Google Scholar]
- 83.Swerdlow CD, Zhou X, Voroshilovsky O, Abeyratne A, Gillberg J. High amplitude T-wave alternans precedes spontaneous ventricular tachycardia or fibrillation in ICD electrograms. Heart Rhythm. 2008;5(5):670–676. doi: 10.1016/j.hrthm.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 84••.Swerdlow C, Chow T, Das M, Gillis AM, Zhou X, Abeyratne A, Ghanem RN. Intracardiac electrogram T-wave alternans/variability increases before spontaneous ventricular tachyarrhythmias in implantable cardioverter-defibrillator patients: a prospective, multicenter study. Circulation. 2011;123(10):1052–1060. doi: 10.1161/CIRCULATIONAHA.110.986364. This paper demonstrates the feasibility of intra-cardiac detection of repolarization alternans in predicting ICD therapy and opens the door to delivery of upstream, abortive anti-arrhythmic therpaies.
- 85.Paz O, Zhou X, Gillberg J, Tseng HJ, Gang E, Swerdlow C. Detection of T-wave alternans using an implantable cardioverter-defibrillator. Heart Rhythm. 2006;3(7):791–797. doi: 10.1016/j.hrthm.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 86.Brunckhorst CB, Shemer I, Mika Y, Ben-Haim SA, Burkhoff D. Cardiac contractility modulation by non-excitatory currents: studies in isolated cardiac muscle. Eur J Heart Fail. 2006;8(1):7–15. doi: 10.1016/j.ejheart.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 87.Winter J, Brack KE, Ng GA. The acute inotropic effects of cardiac contractility modulation (CCM) are associated with action potential duration shortening and mediated by beta1-adrenoceptor signalling. J Mol Cell Cardiol. 2011;51(2):252–262. doi: 10.1016/j.yjmcc.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Armoundas AA, Weiss EH, Sayadi O, Laferriere S, Sajja N, Mela T, Singh JP, Barrett CD, Kevin Heist E, Merchant FM. A novel pacing method to suppress repolarization alternans in vivo: Implications for arrhythmia prevention. Heart Rhythm. 2012 doi: 10.1016/j.hrthm.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 89.Merchant FM, Dec GW, Singh JP. Implantable sensors for heart failure. Circ Arrhythm Electrophysiol. 2010;3(6):657–667. doi: 10.1161/CIRCEP.110.959502. [DOI] [PubMed] [Google Scholar]
- 90.Narayan SM, Bode F, Karasik PL, Franz MR. Alternans of atrial action potentials during atrial flutter as a precursor to atrial fibrillation. Circulation. 2002;106(15):1968–1973. doi: 10.1161/01.cir.0000037062.35762.b4. [DOI] [PubMed] [Google Scholar]
- 91.Narayan SM, Franz MR, Clopton P, Pruvot EJ, Krummen DE. Repolarization alternans reveals vulnerability to human atrial fibrillation. Circulation. 2011;123(25):2922–2930. doi: 10.1161/CIRCULATIONAHA.110.977827. [DOI] [PMC free article] [PubMed] [Google Scholar]