Abstract
The Tinnitus Retraining Therapy Trial (TRTT) is a National Institutes of Health-sponsored, multi-centered, placebo-controlled, randomized trial evaluating the efficacy of tinnitus retraining therapy (TRT) and its component parts, directive counseling and sound therapy, as treatments for subjective debilitating tinnitus in the military. The TRTT will enroll 228 individuals at an allocation ratio of 1:1:1 to: (1) directive counseling and sound therapy using conventional sound generators; (2) directive counseling and placebo sound generators; or (3) standard of care as administered in the military. Study centers include a Study Chair’s Office, a Data Coordinating Center, and six Military Clinical Centers with treatment and data collection standardized across all clinics. The primary outcome is change in Tinnitus Questionnaire (TQ) score assessed longitudinally at 3, 6, 12, and 18-month follow-up visits. Secondary outcomes include: Change in TQ sub-scales, Tinnitus Handicap Inventory, Tinnitus Functional Index, and TRT interview visual analog scale; audiometric and psychoacoustic measures; and change in quality of life. The TRTT will evaluate TRT efficacy by comparing TRT (directive counseling and conventional sound generators) with standard of care; directive counseling by comparing directive counseling plus placebo sound generators versus standard of care; and sound therapy by comparing conventional versus placebo sound generators. We hypothesize that full TRT will be more efficacious than standard of care, directive counseling and placebo sound generators more efficacious than standard of care, and conventional more efficacious than placebo sound generators in habituating the tinnitus awareness, annoyance, and impact on the study participant’s life.
Keywords: Randomized controlled trials, tinnitus, tinnitus retraining therapy
Introduction
Tinnitus is the perception of sound in the absence of a corresponding external sound.[1] The tinnitus percept varies widely from person to person, ranging from a pure tone to a roaring noise. Tinnitus may be heard continuously or intermittently within one or both ears or within the head. It may be acute or chronic, occurring either with or without measurable audiometric hearing loss and/or associated sound tolerance complaints. Tinnitus may or may not be associated with a known causal event or suspected pathology.
Estimates broadly place the prevalence of tinnitus between 10% and 20%, corresponding to ~30–60 million affected persons in the US population. Roughly 5% of the affected population seek healthcare for their tinnitus (~15 million persons).[2] The tinnitus problem is severe and distressing for about 1–2% of the general population,[3] representing ~3–6 million Americans. Symptoms of tinnitus-related distress vary across individuals, but may include insomnia, anxiety, depressive symptoms, reclusive behavior, and even suicide.
Currently, there is no reliable means of eliminating (i.e., curing) tinnitus at its source.[4,5] Moreover, the current literature,[3–6] as well as evidence from formalized reviews of tinnitus treatment trials [Table 1],[7–14] indicates that no medical or non-medical treatment is more effective than a placebo in eliminating tinnitus (although there is some indication that cognitive behavioral therapy may help alleviate some symptoms of distress).[10]
Table 1.
Published Cochrane reviews of tinnitus treatment trials
| Reviewed treatment | No. trials | No. participants | Authors |
|---|---|---|---|
| Antidepressants | 6 | 610 | Baldo, Doree, Lazzarini, Molin, and McFarran[7] |
| Ginkgo biloba | 3 | 1143 | Hilton and Stuart[8] |
| Hyperbaric oxygen | 7 | 392 | Bennett, Kertesz, Perleth, and Yeung[9] |
| Cognitive behavioral therapy* | 8 | 468 | Martinez-Devesa, Perera, Theodoulou, and Waddell[10] |
| Anticonvulsants | 7 | 453 | Hoekstra, Rynja, van Zanten, and Rovers[11] |
| Sound therapy (masking) | 6 | 553 | Hobson, Chisholm, El Refaie[12] |
| Tinnitus retraining therapy | 1 | 123 | Phillips and McFerran[13] |
| Repetitive transcranial magnetic stimulation | 5 | 233 | Meng, Liu, Zheng, Phillips[14] |
No intervention showed efficacy on any outcome when compared to a placebo except for CBT. Evidence of improved quality of life, as revealed by a decrease in global tinnitus severity, was reported in five studies of CBT relative to no treatment or another intervention
Tinnitus retraining therapy
At this time, one of the most popular and influential, but controversial, treatments for distressing tinnitus is an habituation-based non-medical intervention, Tinnitus Retraining Therapy (TRT).[15] TRT represents an amalgam of a sound therapy protocol, originally described by Hazell and Sheldrake,[16] with an expanded directive counseling protocol based on Jastreboff’s neurophysiological model of tinnitus.[17,18] The neurophysiological model, which is described below, provides a theoretical basis and clinical structure for conducting TRT.[15,19,20]
The neurophysiological model of tinnitus
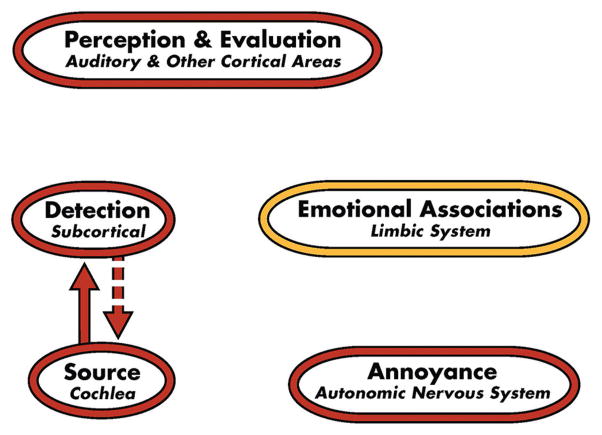
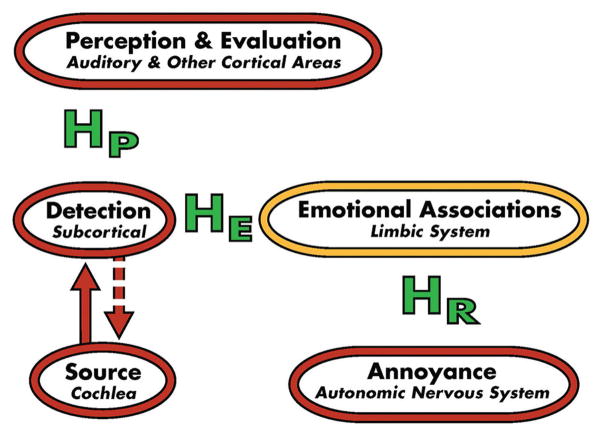
TRT theory and the TRT-induced habituation process are based on the neurophysiological model of tinnitus,[17,21,22] which is depicted in Figures 1–3. For the vast majority of tinnitus patients, the tinnitus signal is believed to arise in the cochlea or auditory periphery (source), and is filtered out at a subcortical level as shown in Figure 1. Although tinnitus may be detectable, it is not bothersome in this conceptual representation of the model. Accordingly, the majority of Americans who experience tinnitus at one time or another are neither routinely aware nor annoyed by their tinnitus. However, in severely debilitated patients, estimated at 1–2% of the US population, conscious and subconscious neuronal circuitry is activated by the distress accompanying the tinnitus.[22] Consequently, the full model is engaged as shown in Figure 2. This engagement includes activation of the subcortical, associated cortical areas, and auditory cortex, as well as the limbic and autonomic nervous system structures depicted in the model. If at the conclusion of treatment TRT is ultimately successful in habituating the negative reactions (HR), emotional associations (HE), and tinnitus perception (HP), as shown in Figure 3, then the model for the successfully treated, previously distressed tinnitus patient will be the same as that shown in Figure 1. He/she will then fall back in the pack with the vast majority of tinnitus patients who are not inordinately bothered by their tinnitus. Thus, at the conclusion of TRT, tinnitus may still be detectable, but the patient will not be annoyed routinely nor constantly aware of it even though the psychoacoustic properties of the tinnitus (i.e., pitch and loudness properties) may still be similar to those experienced at the start of the TRT intervention.[23] As a consequence, the tinnitus will no longer impact the patient’s well being nor his/her daily activities.
Figure 1.
Neurophysiological model of tinnitus depicting detection of the neutral tinnitus signal and activation of subcortical structures that filter out the tinnitus signal generated at a peripheral source (cochlea). Other components of the model are not activated and, therefore, there is neither awareness nor annoyance in response to the tinnitus. This depiction of the model is representative of the vast majority of persons with tinnitus, who are neither bothered nor distressed by their tinnitus (After Jastreboff[21])
Figure 3.
Neurophysiological model of tinnitus depicting complete tinnitus retraining therapy-induced habituation of the emotional (HE) and negative (HR) reactions to the tinnitus and it perception (HP). The resulting representation of the model, subsequent to the habituation, is the same as that shown in Figure 1 for individuals who are neither bothered nor distressed by their tinnitus (After Jastreboff[21])
Figure 2.
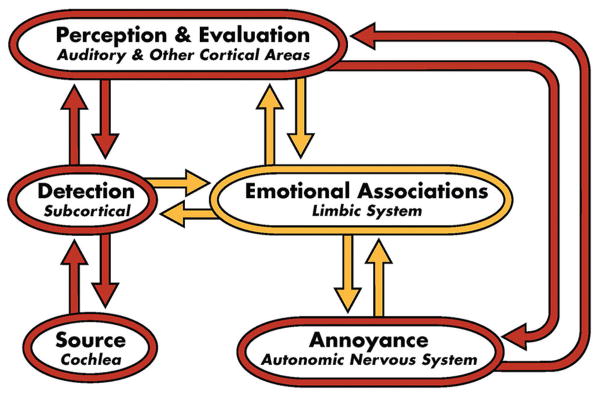
Neurophysiological model of tinnitus depicting full activation, including activation of non-auditory limbic and autonomic nervous system structures that are responsible for the emotional and negative reactions to the tinnitus. This engagement of both conscious and subconscious neuronal circuitry gives rise to the awareness and annoyance associated with distressing tinnitus (After Jastreboff[21])
TRT clinical protocol
Directive counseling, as implemented in the TRT clinical protocol, initiates the habituation process by neutralizing the negative and emotional reactions (i.e., annoyance) to the tinnitus.[23] This neutralization and demystification phase of the habituation protocol in the neurophysiological model of tinnitus operates at the levels of the non-auditory limbic and autonomic nervous systems by gradually extinguishing the associated negative learned response to the tinnitus signal.[15,21,22] In time, the tinnitus ceases to have relevance in the patient’s life and is ignored. Sound therapy reinforces the habituation process and facilitates the habituation of the perception (awareness) of the tinnitus through enrichment of the tinnitus patient’s sound environment.[24,25] The aim of the sound therapy is to decrease the perceived contrast between the patient’s background and tinnitus-related neuronal activity. Exposure to low-level background noise from sound therapy makes tinnitus more difficult to resolve, while also resetting central auditory gain processes that are thought to play a critical role in tinnitus.[26] The patient’s focus and fixation on the tinnitus are diminished concomitantly with its diminished detection in the sound therapy-enhanced background noise. Habituation of the tinnitus perception, which follows after habituation of the negative reaction, ostensibly occurs automatically.[15] This incremental process of passive extinction takes place over a period of at least 6 months as the conditioned reflex between subcortical auditory structures and limbic/autonomic neural structures is extinguished. Sound therapy may be achieved by any reasonable means that enriches the patient’s sound environment, including the use of personalized bilateral ear-worn sound generators and hearing aids.[15,24]
The evolution of TRT as an international treatment for tinnitus
TRT currently is provided in over 100 clinical settings around the world.[27] The international rise of TRT over the past 20 years is attributable to a number of factors.
First and foremost, are the following significant characteristics of TRT:
TRT is a comprehensive protocol including diagnostic (medical/audiological), educational, and treatment components to achieve habituation of tinnitus annoyance and awareness.[23]
In most clinics where TRT has been applied, about 80% of the TRT-managed patients have demonstrated reduced distress related to tinnitus.[23,28]
TRT is a non-invasive treatment that does not interfere with hearing or communication.[23]
TRT has broad applicability independent of the tinnitus etiology; hence, after intervention is ruled out for a medically manageable disease or other contributing medical problems, almost all patients qualify for treatment.[29]
Second, in the absence of widespread training in treatment approaches for severe debilitating tinnitus in the 1990s, TRT filled a void in the clinical armamentarium of the audiology community. The structured intervention approach offered clinicians a systematic method to isolate and manage issues related to an individual patient’s tinnitus as well as confounding effects from sound tolerance problems (i.e., hyperacusis, misophonia, and phonophobia) and hearing loss, both of which are common conditions that may be associated with and exacerbate the perceived negative effects of tinnitus.[15]
Third, a growing body of scientific evidence from independent sources, most notably from neuro-imaging studies, has provided objective support for Jastreboff’s neurophysiological model of tinnitus.[15,17,21] Specifically, imaging studies of tinnitus patients have revealed high activation of the non-auditory limbic structures and cortical areas.[30–34] The former, in particular, are engaged in Jastreboff’s model and contribute to the debilitating negative emotional reactions to the tinnitus. This neuroimaging evidence, in turn, has provided support for the concept of directive counseling and its importance in the TRT protocol in initiating habituation of the inordinate emotional and negative responses to the tinnitus.[15] Also, evidence from other independent sources has supported the objectives of sound therapy in the TRT protocol. For example, one of the ideas motivating sound therapy is that exposure to an enriched sound environment is critical in regulating a compensatory process, operating within the central auditory pathways, to amplify otherwise undetectable tinnitus activity.[26] This adaptive central auditory gain control process was hypothesized early on as an important mechanism in the production of tinnitus and hyperacusis.[16,17,35] Basic science[36–39] and clinical evidence[40–44] now lend credence to the idea of a central gain process and, in turn, support the use of sound therapy in TRT to reset the gain of the tinnitus patient.[45]
Finally, Jastreboff and Hazell have been very effective in advocating and promoting TRT relentlessly to the clinical community, offering structured training courses internationally for clinicians to learn how to conduct TRT. Participation in and completion of a TRT training course has effectively served the tinnitus clinical and patient community as qualification to conduct TRT. These ongoing training efforts, together with numerous presentations at professional and scientific meetings by Jastreboff, Hazell, and independent clinicians using TRT, propelled TRT to the forefront of treatment for debilitating tinnitus at the turn of this century.[28]
Indeed, by 2000, a substantial body of uncontrolled clinical evidence had accrued in support of the efficacy of TRT. In the foreword for a special issue of the Journal of the American Academy of Audiology devoted to tinnitus, Jacobson stated that “TRT has become the non-medical management of this decade” for treating severe tinnitus.[46] This same perspective was echoed 2 years later by Dobie, who proclaimed TRT to be “the most widely and enthusiastically advocated treatment for tinnitus” (p. 4).[6] Consistent with this adulation for TRT, various reports have touted the successes of the intervention (or its components) in a series of uncontrolled studies. Many of these reports appeared at the Sixth and Seventh International Tinnitus Seminars in 1999 and 2002, respectively. A review of some of these early reports and other relevant studies [Table 2] revealed clinical success rates approaching or exceeding 80% (as judged by a minimum of 20% change on two or more tinnitus-specific, health-related, quality-of-life scales) across various study populations.[47–54] Other successes with TRT have subsequently been reported by multiple centers internationally using TRT or TRT-like protocols.[55–58]
Table 2.
Tinnitus Retraining Therapy efficacy in uncontrolled treatment studies
Jastreboff and Jastreboff promoted these and other positive results as providing “evidence by consensus” for the validity and efficacy of TRT.[51] They contended that an appropriate test of TRT would be a “meta-analysis” of studies from multiple sites using the protocol. They also argued that “a randomized trial with some agreeable controls” is unnecessary because “the validity of this study, time frame, and sample of patients will be questionable and will face many ethical issues” (p. 91).[51]
Controversy surrounding TRT in the absence of randomized controlled trials
Notwithstanding the abundance of uncontrolled clinical evidence of benefit widely attributed to TRT, nor the Jastreboffs’ contentions that a systematic review is more appropriate than a randomized controlled trial for evaluating the efficacy of TRT, the TRT protocol has been and continues to be a lightning rod for controversy. The most valid objection to TRT continues to be the lack of scientific evidence from rigorously controlled randomized trials to support the efficacy of TRT, its component parts (directive counseling and sound therapy), or the underlying mechanisms. A Wessex Institute report pointed out multiple methodological shortcomings in the early observational studies of TRT.[59] The report highlighted problems in the selection of validated outcome measures and stressed the lack of prospective studies, double-blind designs, randomized treatment assignments, and placebo controls. The Wessex report concluded that the available literature simply could not support the validity of TRT in light of such methodological weaknesses, and ended with the statement “There is clearly a need for properly controlled research trials into the effectiveness of retraining therapy.” Wilson et al.[60] reinforced the Wessex Institute’s criticisms of methodological limitations in the early TRT research, calling for “randomized, controlled studies that include no-treatment and placebo conditions.” They also stated, “studies are required in which the efficacy of the counseling and white noise components can be clearly isolated” (p. 273).[60] This call for randomized controlled trials of TRT was echoed early on, and has since been repeated by concerned clinicians and investigators.[61–63]
In 2004, the Washington State Department of Labor and Industries conducted a review of the literature on TRT efficacy.[64] The conclusions were that (1) in the absence of prospective trials with comparison groups, the efficacy of TRT remains to be established and (2) TRT therefore should continue to be viewed as “investigational and controversial.” A recent Cochrane review of evidence from clinical trials of TRT was published in 2010.[13] The reviewers considered four trials,[55–58] but ultimately found that only one trial met the review criteria; the remaining three trials used a TRT-like intervention rather than TRT as developed by Jasteboff.[29] The included trial, conducted by Henry et al.,[58] found that both TRT and a conventional masking protocol were efficacious in treating tinnitus over a treatment period of 18 months. TRT, however, yielded a superior outcome for patients with greater tinnitus severity at the start of treatment. The Cochrane reviewers concluded that “a simple, low-quality randomized controlled trial[58] suggests that TRT is more effective as a treatment for patients with tinnitus than tinnitus masking.”[13] The reviewers criticized Henry et al.’s study[58] for flawed allocation bias, unblinded treatments, and concerns about the generalizability of the results based on the Veterans Administration population under study.[13]
Subsequent to the publication of this latest Cochrane review of TRT in 2010, three new clinical trials have been completed, two of which have now been published. Bauer and Brozoski[65] reported that both TRT and general counseling, without additional sound therapy, are efficacious in reducing the annoyance and impact of tinnitus. The effect size was greater for TRT (1.13) than for general counseling (0.78). The TRT-related improvements accrued over an 18-month period and were reported to be robust and clinically significant. Westin et al.[1] compared the treatment effects of acceptance and commitment therapy (ACT) with TRT for reducing the impact of tinnitus. They reported that ACT yielded significantly greater reductions in tinnitus impact than did TRT at 10 weeks, 6 months, and 18 months after onset of the assigned treatment. The results from a third trial, led by Richard Tyler at the University of Iowa, are yet to be published.[13]
It would suffice to say that the current clinical trials’ literature for TRT, although now beginning to provide some mixed support for the protocol, continues to be criticized because of the lack of one or more of the following trial features: Prospective and double-blind designs, randomized treatment assignments, placebo controls, baseline measurements, appropriate comparison groups, predetermined outcome measurements, patient homogeneity, and adherence to the TRT protocol. The latter trial feature, adherence to protocol, has been especially problematic. Specifically, it was noted in the Cochrane review of TRT to be a primary factor, eliminating many TRT-related studies from review consideration.[13] The problem is that the directive counseling component of TRT has often been modified by individual practitioners and clinics. In some cases, the counseling has been expanded to include aspects or features of cognitive behavioral therapy, which may have influenced or confounded previous reports of TRT efficacy. Moreover, no published randomized study to date has controlled for the treatment effects of directive counseling or sound therapy apart from their combined effects in TRT, nor have investigators examined the relative contributions of these components to TRT success in a rigorous trial. Furthermore, no definitive randomized controlled trials of TRT have been reported previously at the Phase III level (i.e., comparing the efficacy of TRT with that of the current standard of care). Thus, in the persistent absence of a rigorous and definitive randomized controlled trial, we proposed and were funded by the National Institute of Deafness and Other Communication Disorders (NIDCD), National Institutes of Health, to conduct the first large-scale, randomized, placebo-controlled, multi-site clinical trial of TRT, the Tinnitus Retraining Therapy Trial (TRTT), which is described in the remainder of this report.
Implementing the Tinnitus Retraining Therapy Trial
Design of the clinical trial
The TRTT is a multi-center, placebo-controlled, randomized clinical trial testing the efficacy of TRT versus the standard-of-care treatment in individuals who have self-perceived intolerable tinnitus. The primary objective of the TRTT is to assess the efficacy of TRT as a treatment for severe debilitating tinnitus. The TRTT includes persons with functionally adequate hearing sensitivity who do not need to use hearing aids.
Aims
The specific aims of the TRTT are to:
Evaluate the efficacy of TRT by comparing full TRT (directive counseling and sound therapy achieved with conventional ear-level sound generators) with the standard of care in habituating the perceived tinnitus sensation, awareness, annoyance, and overall impact on life;
Evaluate the efficacy of directive counseling by comparing partial TRT (directive counseling combined with sound therapy achieved with placebo sound generators) with the standard of care;
Evaluate the efficacy of sound therapy by comparing sound therapy achieved with conventional ear-level sound generators with sound therapy provided by placebo sound generators in participants assigned to directive counseling; and
Develop statistical models and methods to establish and predict the success of TRT based on important study participant baseline characteristics.
Individuals meeting the eligibility criteria have an equal probability of being randomized to one of the three treatment groups in the TRTT. The goal is to enroll 76 participants per treatment group for a total of 228 study participants.
Treatment groups include:
Directive counseling and sound therapy implemented with conventional sound generators;
Directive counseling and sound therapy implemented with placebo sound generators; and
Standard of care as administered in the military based on American Speech, Language, and Hearing Association guidelines for tinnitus management and treatment[66] and surveys of clinicians at participating clinical centers.
The standard-of-care treatment is similar to that typically provided to patients with severe tinnitus at the participating military medical centers. The tinnitus management is based on the patient’s complaints, history, audiologic evaluation, and self-assessment. The goal of the treatment is to reduce negative cognitive, affective, physical, and behavioral reactions to tinnitus and to improve the patient’s well being and quality of life. Specific treatment recommendations are individualized to reflect the participant’s concerns and coping abilities as well as his or her engagement in the decision-making process regarding treatment options.
The double-blind sound therapy, implemented with a placebo-controlled sound-generator device, is an innovative feature of the TRTT. Neither the study participant nor the treating audiologist will know the type of sound generator that the study participant receives. Because it is not possible to blind study participants, nor the treating audiologist, to directive counseling versus the standard of care, all audiological outcomes will be measured by a second audiologist who is blinded to the treatment assignment. There is an inherent risk of information bias in the TRTT in that the tinnitus health-related quality-of-life outcomes are being completed by the study participant.
The TRTT is being conducted at US military medical centers with active-duty and retired military personnel and their dependents, all of whom qualify because of their severely distressing tinnitus. Although the incidence and prevalence of tinnitus among US military personnel is unknown, it is probably much higher than that among the overall population because of increased exposure to loud noise.[67,68] Indeed, tinnitus is now the primary service-related disability among veterans returning from the Middle East conflicts.[69] The likely higher incidence of noise-induced tinnitus in the military compared with that of the general population and the great diversity of this study population make the US Armed Forces an ideal study group for a clinical trial of TRT. The six military clinical centers now participating in the TRTT include three Navy and two Air Force sites and the newly integrated-service site at the Walter Reed National Military Medical Center. The participating Navy sites are the Naval Medical Center Portsmouth, the Naval Hospital Camp Pendleton, and the Naval Medical Center San Diego. The participating Air Force sites are the David Grant Medical Center at Travis Air Force Base and the Wilford Hall Ambulatory Surgical Center at Lackland Air Force Base. US Army clinical centers are also expected to join the TRTT as new treatment sites over the next year to enhance and expedite study recruitment. The main feature of the TRTT study population is that patients have severely debilitating subjective tinnitus with onset of symptoms at least 1 year prior to treatment. Specific eligibility criteria are listed in Table 3.
Table 3.
Tinnitus Retraining Therapy Trial eligibility criteria
| Inclusion criteria |
| Primary complaint of continuous, chronic (≥12 months) subjective tinnitus |
| Tinnitus questionnaire[70] score ≥ 40 |
| Functionally normal hearing sensitivity by audiometric thresholds ≤ 30 dB Hearing Level (HL) at and below 2000 Hz and ≤40 dB HL at 4000 Hz |
| Ability to understand counseling and comprehend and complete english language questionnaires |
| Willing and able to give informed consent |
| Eligibility for health care at a participating military medical clinical center |
| Age 18 or older |
| Exclusion criteria |
| Predisposing disease with tinnitus symptoms amenable to medical or surgical intervention |
| Clinical treatment for tinnitus within previous year |
| Evidence of malingering or exaggeration of tinnitus or hearing symptoms; emotional, psychological, or psychiatric condition precluding full participation or follow-up |
| Active involvement in tinnitus-related litigation |
| Diagnosis of pulsatile tinnitus, somatosounds, or objective tinnitus (i.e., spontaneous otoacoustic emissions) that would account for the tinnitus problem |
| Brain or head trauma requiring treatment 24 months before screening or enrollment |
| Inability to complete audiological testing or clinical trial protocol |
The primary outcome to be measured in the TRTT is the difference in scores on the Tinnitus Questionnaire (TQ) between baseline and follow-up, assessed longitudinally after 3, 6, 12, and 18 months of treatment. The TQ is a broad-spectrum 52-item assessment tool that features a 3-point response scale.[70,71] This comprehensive self-report instrument has been widely used for clinical and research purposes, and is respected for its robust psychometric characteristics.[72,73] The five sub-scales of the TQ include intrusiveness, emotional distress, sleep disturbance, auditory perceptual difficulties, and somatic complaints.[70,71] Our primary objective is to test the hypothesis that TRT will significantly reduce the severity of debilitating tinnitus over 18 months of follow-up compared with the standard of care, as currently offered to tinnitus patients in the military clinical centers. This longitudinal analysis assumes a linear treatment effect over time; therefore, we will also compare the difference in TQ score at 18 months (end of treatment) for individuals assigned to TRT with those assigned to the standard of care as a secondary analysis.
Secondary outcome data include change in the sub-scales of the TQ[70,71] and change in scores for additional tinnitus-specific, health-related, and quality-of-life questionnaires, including:
Tinnitus Handicap Inventory (THI)[74]
Tinnitus Functional Index (TFI)[75]
Visual analog scale of the TRT interview.[76]
Additional secondary outcomes include change in the Digit Symbol Substitution Task[77] and the EuroQol.[78]
We are also measuring change in pure-tone and speech audiometry, loudness discomfort levels, and psychoacoustic measures of tinnitus pitch and loudness. Additionally, we are evaluating the impact of change in tinnitus severity on outcomes related to the participant’s health by assessing change from baseline in the Hearing Handicap Inventory,[79] Beck Depression Inventory Fast Screen,[80] Positive And Negative Affect Schedule,[81] and state sub-scale of the State-Trait Anxiety Inventory.[82]
Eligibility for the TRTT is being determined at a baseline eligibility visit, which consists of a medical and tinnitus history, physical examination, and baseline audiological/ tinnitus/hyperacusis evaluation. Treatment assignment is performed at a randomization visit. Immediately before treatment assignment, each study participant completes the tinnitus health-related quality-of-life instruments and other psychological profile tests. The initial treatment visit takes place following the randomization visit, and is followed by a second treatment visit 1 month later. Follow-up visits at clinical centers take place at 3, 6, 12, and 18 months, and include completion of tinnitus outcome questionnaires at all visits. Psychoacoustic testing and the audiological/tinnitus/ hyperacusis evaluation are also performed at 6, 12, and 18 month visits.
Recruitment to the TRTT began in August 2011, and the trial is expected to be completed within 5 years. In the interim, a more detailed description of the TRTT study design and its development will be forthcoming in a separate report.
Acknowledgments
Source of Support: NIH,
The TRTT project and the preparation of this manuscript are supported by NIDCD awards U01DC007411 to C. Formby, TRTT Study Chair, and U01DC007422 to R. Scherer, Data Coordinating Center Director. The authors thank the US Navy and Air Force for their continued support of this project, acknowledge the significant contributions of General Hearing Instruments, Inc. (GHI) to develop and implement the innovative sound therapy treatments for the TRTT, and recognize the significant contributions of Connie Formby in the preparation of the illustrations and Gordon Hughes for his guidance and support as the NIDCD project officer for the TRTT. This manuscript is based on a presentation at the 5th International Tinnitus Research Initiative Conference held in Grand Island, NY in August 2011. This research has been approved by the Institutional Review Boards at all participating military clinical sites, the University of Alabama, and Johns Hopkins University. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Departments of the Navy and Air Force, Department of Defense, or the United States Government.This work was prepared as part of official duties performed by the military service members and civilian employees participating in the TRTT project. Title 17 U.S.C. 105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. 101 defines a United States Government work as a work prepared by a military service member or employee of the United States Government as part of that person’s official duties.
Footnotes
Conflict of Interest: None declared.
References
- 1.Westin VZ, Schulin M, Hesser H, Karlsson M, Noe RZ, Olofsson U, et al. Acceptance and commitment therapy versus tinnitus retraining therapy in the treatment of tinnitus: A randomised controlled trial. Behav Res Ther. 2011;49:737–47. doi: 10.1016/j.brat.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 2.McFadden D. Tinnitus: Facts, Theories, and Treatments. Washington DC: National Academy Press; 1982. Introduction; p. 8. [PubMed] [Google Scholar]
- 3.Dobie RA. A review of randomized clinical trials in tinnitus. Laryngoscope. 1999;109:1202–11. doi: 10.1097/00005537-199908000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Parnes SM. Current concepts in the clinical management of patients with tinnitus. Eur Arch Otorhinolaryngol. 1997;254:406–9. doi: 10.1007/BF02439968. [DOI] [PubMed] [Google Scholar]
- 5.Dobie RA. Clinical trials and drug therapy for tinnitus. In: Snow JB, editor. Tinnitus: Theory and Management. Hamilton, ONT: BC Decker; 2004. pp. 266–77. [Google Scholar]
- 6.Dobie RA. Randomized clinical trials for tinnitus: Not the last word? In: Patuzzi R, editor. Proceedings of the 7th International Tinnitus Seminar. Perth, W.A: University of Western Australia; 2002. pp. 3–6. [Google Scholar]
- 7.Baldo P, Doree C, Lazzarini R, Molin P, McFerran DJ. Antidepressants for patients with tinnitus. Cochrane Database Syst Rev. 2006;4:Art No.:CD003853. doi: 10.1002/14651858.CD003853.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Hilton M, Stuart E. Ginkgo biloba for tinnitus. Cochrane Database Syst Rev. 2004;2:CD003852. doi: 10.1002/14651858.CD003852.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Bennett MH, Kertesz T, Perleth M, Yeung P, Lehm JP. Hyperbaric oxygen for idiopathic sudden sensorineural hearing loss and tinnitus. Cochrane Database Syst Rev. 2012;2(10):CD004739. doi: 10.1002/14651858.CD004739.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez-Devesa P, Perera R, Theodoulou M, Waddell A. Cognitive behavioural therapy for tinnitus. Cochrane Database Syst Rev. 2010;1:CD005233. doi: 10.1002/14651858.CD005233.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoekstra CE, Rynja SP, van Zanten GA, Rovers MM. Anticonvulsants for tinnitus. Cochrane Database Syst Rev. 2011;7:CD007960. doi: 10.1002/14651858.CD007960.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hobson J, Chisholm E, El Refaie A. Sound therapy (masking) in the management of tinnitus in adults. Cochrane Database Syst Rev. 2010;12:CD006371. doi: 10.1002/14651858.CD006371.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Phillips JS, McFerran D. Tinnitus Retraining Therapy (TRT) for tinnitus. Cochrane Database Syst Rev. 2010;3:CD007330. doi: 10.1002/14651858.CD007330.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meng Z, Liu S, Zheng Y, Phillips JS. Repetitive transcranial magnetic stimulation for tinnitus. Cochrane Database Syst Rev. 2011;10:CD007946. doi: 10.1002/14651858.CD007946.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Jastreboff PJ, Hazell JW. Tinnitus Retraining Therapy: Implementing the Neurophysiological Model. Cambridge, UK: Cambridge University Press; 2004. The neurophysiological model of tinnitus and decreased sound tolerance; pp. 16–62. [Google Scholar]
- 16.Hazell JW, Sheldrake JB. Hyperacusis and tinnitus. In: Aran JM, Dauman R, editors. Proceedings of the 4th International Tinnitus Seminar. New York: Kugler; 1992. pp. 245–8. [Google Scholar]
- 17.Jastreboff PJ. Phantom auditory perception (tinnitus): Mechanisms of generation and perception. Neurosci Res. 1990;8:221–54. doi: 10.1016/0168-0102(90)90031-9. [DOI] [PubMed] [Google Scholar]
- 18.Jastreboff PJ. Clinical implications of the neurophysiological model of tinnitus. In: Vernon JA, Reich G, editors. Proceedings of the 5th International Tinnitus Seminar. Portland, Oregon: American Tinnitus Association; 1996. pp. 500–7. Formby and Scherer: Tinnitus Retraining Therapy Trial 141 Noise & Health, March-April 2013, Volume 15. [Google Scholar]
- 19.Jastreboff PJ, Hazell JW. A neurophysiological approach to tinnitus: Clinical implications. Br J Audiol. 1993;27:7–17. doi: 10.3109/03005369309077884. [DOI] [PubMed] [Google Scholar]
- 20.Jastreboff PJ, Hazell JW. Treatment of tinnitus based on a neurophysiological model. In: Vernon J, editor. Tinnitus and Relief. Boston: Allyn and Bacon; 1998. pp. 201–17. [Google Scholar]
- 21.Jastreboff PJ. Tinnitus as a phantom perception: Theories and clinical implications. In: Vernon J, Moller AR, editors. Mechanisms of Tinnitus. Boston: Allyn and Bacon; 1995. pp. 73–94. [Google Scholar]
- 22.Jastreboff PJ. The neurophysiological model of tinnitus. In: Snow JB, editor. Tinnitus: Theory and Management. Hamilton, ONT: Decker BC; 2004. pp. 96–106. [Google Scholar]
- 23.Gold SL, Formby C, Gray WC. Celebrating a decade of evaluation and treatment: The University of Maryland Tinnitus and Hyperacusis Center. Am J Audiol. 2000;9:69–74. doi: 10.1044/1059-0889(2000/014). [DOI] [PubMed] [Google Scholar]
- 24.Gold SL, Gray WC, Hu S, Jastreboff PJ. Proceedings of the 5th International Tinnitus Seminar. Portland, Oregon: American Tinnitus Association; 1995. Selection and fitting of noise generators and hearing aids for tinnitus patients; pp. 312–4. [Google Scholar]
- 25.Jastreboff PJ, Gray WC, Gold SL. Neurophysiological approach to tinnitus patients. Am J Otol. 1996;17:236–40. [PubMed] [Google Scholar]
- 26.Jastreboff PJ, Jastreboff MM. Tinnitus Retraining Therapy (TRT) as a method for treatment of tinnitus and hyperacusis patients. J Am Acad Audiol. 2000;11:162–77. [PubMed] [Google Scholar]
- 27.Henry JA, Zaugg TL, Myers PJ, Schechter MA. Using therapeutic sound with progressive audiologic tinnitus management. Trends Amplif. 2008;12:188–209. doi: 10.1177/1084713808321184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hazell JW, editor. Proceedings of the 6th International Tinnitus Seminar. London, UK: The Tinnitus and Hyperacusis Centre; 1999. [Google Scholar]
- 29.Jastreboff PJ. Tinnitus Habituation Therapy (THT) and Tinnitus Retraining Therapy (TRT) In: Tyler RS, editor. Tinnitus Handbook. San Diego: Singular; 2000. pp. 357–76. [Google Scholar]
- 30.Lockwood AH, Salvi RJ, Coad ML, Towsley ML, Wack DS, Murphy BW. The functional neuroanatomy of tinnitus: Evidence for limbic system links and neural plasticity. Neurology. 1998;50:114–20. doi: 10.1212/wnl.50.1.114. [DOI] [PubMed] [Google Scholar]
- 31.Lockwood AH, Salvi RJ, Burkard RF. Tinnitus. N Engl J Med. 2002;347:904–10. doi: 10.1056/NEJMra013395. [DOI] [PubMed] [Google Scholar]
- 32.Mirz F, Pedersen B, Ishizu K, Johannsen P, Ovesen T, Stødkilde-Jørgensen H, et al. Positron emission tomography of cortical centers of tinnitus. Hear Res. 1999;134:133–44. doi: 10.1016/s0378-5955(99)00075-1. [DOI] [PubMed] [Google Scholar]
- 33.Mirz F, Gjedde A, Ishizu K, Pedersen CB. Cortical networks subserving the perception of tinnitus – A PET study. Acta Otolaryngol Suppl. 2000;543:241–3. doi: 10.1080/000164800454503. [DOI] [PubMed] [Google Scholar]
- 34.Cacace AT. The limbic system and tinnitus. In: Snow JB, editor. Tinnitus: Theory and Management. Hamilton, ONT: Decker BC; 2004. pp. 162–70. [Google Scholar]
- 35.Hazell JW. Tinnitus masking therapy. In: Hazell JW, editor. Tinnitus. London: Churchill Livingston; 1987. pp. 96–117. [Google Scholar]
- 36.Formby C, Sherlock LP, Gold SL. Adaptive plasticity of loudness induced by chronic attenuation and enhancement of the acoustic background. J Acoust Soc Am. 2003;114:55–8. doi: 10.1121/1.1582860. [DOI] [PubMed] [Google Scholar]
- 37.Formby C, Sherlock LP, Gold SL, Hawley ML. Adaptive recalibration of chronic auditory gain. Semin Hear. 2007;28:295–302. [Google Scholar]
- 38.Munro KJ, Blount J. Adaptive plasticity in brainstem of adult listeners following earplug-induced deprivation. J Acoust Soc Am. 2009;126:568–71. doi: 10.1121/1.3161829. [DOI] [PubMed] [Google Scholar]
- 39.Noreña AJ. An integrative model of tinnitus based on a central gain controlling neural sensitivity. Neurosci Biobehav Rev. 2011;35:1089–109. doi: 10.1016/j.neubiorev.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Formby C. Hyperacusis and related sound tolerance complaints: Differential diagnosis, treatment effects, and models. Semin Hear. 2007;28:227–59. [Google Scholar]
- 41.Gold SL, Frederick EA, Formby C. Shifts in dynamic range for hyperacusis patients receiving Tinnitus Retraining Therapy (TRT) In: Hazell JW, editor. Proceeding of the 6th International Tinnitus Seminar. London, UK: The Tinnitus and Hyperacusis Centre; 1999. pp. 297–301. [Google Scholar]
- 42.Gold SL, Formby C, Frederick EA, Suter C. Incremental shifts in loudness discomfort level among tinnitus patients with and without hyperacusis. In: Patuzzi R, editor. 7th International Tinnitus Seminar. Tinnitus Treatment, Devices and Research; 2002. pp. 170–2. [Google Scholar]
- 43.McKinney CJ, Hazell JW, Graham RL. Changes in loudness discomfort level and sensitivity to environmental sound with habituation based therapy. Proceedings of the 6th International Tinnitus Seminar; London, UK: The Tinnitus and Hyperacusis Centre; 1999b. pp. 499–501. [Google Scholar]
- 44.Noreña AJ, Chery-Croze S. Enriched acoustic environment rescales auditory sensitivity. Neuroreport. 2007;18:1251–5. doi: 10.1097/WNR.0b013e3282202c35. [DOI] [PubMed] [Google Scholar]
- 45.Formby C, Gold SL. Modification of loudness discomfort level: Evidence for adaptive chronic auditory gain and its clinical relevance. Semin Hear. 2002;23:21–34. [Google Scholar]
- 46.Jacobson GP. Editorial: Special issue on tinnitus. J Am Acad Audiol. 2000;11 preface. [Google Scholar]
- 47.Jastreboff PJ. Tinnitus. In: Gates GA, editor. Current Therapy in Otolaryngoloav-Head and Neck Surgery. 6. St. Louis: Mosby-Year Book Inc; 1998. pp. 90–5. [Google Scholar]
- 48.Bartnick G, Fabijanska A, Rogowski M. Our experience in treatment of patients with tinnitus and/or hyperacusis using the habituation method. Proceedings of the 6th International Tinnitus Seminar; London, UK: The Tinnitus and Hyperacusis Centre; 1999. pp. 415–7. [Google Scholar]
- 49.Heitzmann T, Rubio L, Cardenas MR, Zofio E. The importance of continuity in TRT patients: Results at 18 months. Proceedings of the 6th International Tinnitus Seminar; London, UK: The Tinnitus and Hyperacusis Centre; 1999. pp. 509–11. [Google Scholar]
- 50.Herraiz C, Hernandez FJ, Machado A, De Lucas P, Tapia MC. Tinnitus retraining therapy: Our experience. Proceedings of the 6th International Tinnitus Seminar; London, UK: The Tinnitus and Hyperacusis Centre; 1999. pp. 483–4. [Google Scholar]
- 51.Jastreboff PJ, Jastreboff MM. How TRT derives from the neurophysiological model. Proceedings of the 6th International Tinnitus Seminar; London, UK: The Tinnitus and Hyperacusis Centre; 1999. pp. 87–91. [Google Scholar]
- 52.McKinney CJ, Hazell JW, Graham RL. An evaluation of the TRT method. Proceedings of the 6th International Tinnitus Seminar; London, UK: The Tinnitus and Hyperacusis Centre; 1999a. pp. 99–105. [Google Scholar]
- 53.Sheldrake JB, Hazell JW, Graham RL. Results of tinnitus retraining therapy. Proceedings of the 5th International Tinnitus Seminar; London, UK: The Tinnitus and Hyperacusis Centre; 1999. pp. 292–6. [Google Scholar]
- 54.Herraiz C, Hernandez FJ, Plaza G, de los Santos G. Long-term clinical trial of tinnitus retraining therapy. Otolaryngol Head Neck Surg. 2005;133:774–9. doi: 10.1016/j.otohns.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 55.Goebel G, Rubler D, Hiller W, Heuser J, Fichter MM. Evaluation of tinnitus retraining therapy in comparison to cognitive therapy and broad-band noise generator therapy. Laryngo-Rhino-Otologie. 2000;79(Suppl 1):S88. [Based on Goebel G, Rubler D, Seppurat F, Hiller W, Heuser J, Fichter MM. Controlled prospective study of tinnitus retraining therapy compared to tinnitus coping therapy and broad-band noise generator therapy. In: Hazell JW, editor. Proceedings of the International Tinnitus Seminar. London, UK: The Tinnitus and Hyperacusis Centre; 1999. p. 302–6.] [Google Scholar]
- 56.Schmitt C, Kroner-Herwig B. In: Patuzzi R, editor. Comparison of tinnitus coping training and TRT: Are they superior to education?; Proceedings of the 7th International Tinnitus Seminar; Freemantle, Australia. 5–7 March; University of Western Australia; 2002. [Google Scholar]
- 57.Caffier PP, Haupt H, Scherer H, Mazurek B. Outcomes of long-term outpatient tinnitus-coping therapy: Psychometric changes and value of tinnitus-control instruments. Ear Hear. 2006;27:619–27. doi: 10.1097/01.aud.0000240504.77861.1a. [DOI] [PubMed] [Google Scholar]
- 58.Henry JA, Schechter MA, Zaugg TL, Griest S, Jastreboff PJ, Vernon JA, et al. Outcomes of clinical trial: Tinnitus masking versus tinnitus retraining therapy. J Am Acad Audiol. 2006;17:104–32. doi: 10.3766/jaaa.17.2.4. [DOI] [PubMed] [Google Scholar]
- 59.Leal P, Milne R. Development and Evaluation Committee report no 83. The Wessex Institute for Health Research and Development; 1998. [Last accessed on 2012 June 27]. Tinnitus retraining therapy. Available from: http://www.epi.bris.ac.uk/rd. [Google Scholar]
- 60.Wilson PH, Henry JL, Andersson G, Hallam RS, Lindberg P. A critical analysis of directive counselling as a component of tinnitus retraining therapy. Br J Audiol. 1998;32:273–86. doi: 10.3109/03005364000000078. [DOI] [PubMed] [Google Scholar]
- 61.Coles RA. Placebo effects and placebo treatment. Proceedings of the 5th International Tinnitus Seminar; Portland, Oregon: American Tinnitus Association; 1996. pp. 6–8. [Google Scholar]
- 62.Baguley DM. Chairman’s introduction to Plenary 4; Tinnitus retraining therapy-Tinnitus and the nature of evidence. The 6th International Tinnitus Seminar; London, UK: The Tinnitus and Hyperacusis Centre; 1999. p. 107. [Google Scholar]
- 63.Kroener-Herwig B, Biesinger E, Gerhards F, Goebel G, Verena Greimel K, Hiller W. Retraining therapy for chronic tinnitus. A critical analysis Formby and Scherer: Tinnitus Retraining Therapy Trial Noise & Health, March-April 2013, Volume 15, 142 of its status. Scand Audiol. 2000;29:67–78. doi: 10.1080/010503900424471. [DOI] [PubMed] [Google Scholar]
- 64.Wang G. Health Technology Assessment Report. Olympia, WA: Washington State Department of Labor and Industries (WSDLI); 2004. Tinnitus retraining therapy; pp. 1–17. [Google Scholar]
- 65.Bauer CA, Brozoski TJ. Effect of tinnitus retraining therapy on the loudness and annoyance of tinnitus: A controlled trial. Ear Hear. 2011;32:145–55. doi: 10.1097/AUD.0b013e3181f5374f. [DOI] [PubMed] [Google Scholar]
- 66.American Speech-Language-Hearing Association. Preferred practice patterns in audiology. [Last accessed on 2012 June 27];ASHA Practice Policy. 2007 Available from: http://www.asha.org/docs/html/PP2006-00274.html.
- 67.U.S. Army Environmental Hygiene Agency. Hearing Conservation Data Profile No 51-34-PU83-93.1 Army-Wide Database. Aberdeen Proving Ground; MD: May, 1993. [Google Scholar]
- 68.Humes LE, Joellenbeck LM, Durch JS. Noise and Military Service: Implications for Hearing Loss and Tinnitus. Washington, DC: The National Academics Press; 2005. Tinnitus; pp. 116–45. [Google Scholar]
- 69.American Tinnitus Association. [Last accessed on 2012 June 27];How tinnitus affects our military personnel. Available from: http://www.ata.org/form-patients/at-risk.
- 70.Hallam RS. Manual of the Tinnitus Questionnaire (TQ) London: Psychological Corporation; 1996. [Google Scholar]
- 71.Hallam RS, Jakes SC, Hinchcliffe R. Cognitive variables in tinnitus annoyance. Br J Clin Psychol. 1988;27:213–22. doi: 10.1111/j.2044-8260.1988.tb00778.x. [DOI] [PubMed] [Google Scholar]
- 72.Goebel G, Hiller W. Quality management in the therapy of chronic tinnitus. Proceedings of the 6th International Tinnitus Seminar; London, UK. 1999. pp. 357–63. [Google Scholar]
- 73.Newman CW, Sandridge SA. Tinnitus’ questionnaires. In: Snow JB, editor. Tinnitus Theory and Management. ONT: Decker BC; 2004. pp. 237–54. [Google Scholar]
- 74.Newman CW, Jacobson GP, Spitzer JB. Development of the Tinnitus Handicap Inventory. Arch Otolaryngol Head Neck Surg. 1996;122:143–8. doi: 10.1001/archotol.1996.01890140029007. [DOI] [PubMed] [Google Scholar]
- 75.Meikle MB, Henry JA, Griest SE, Stewart BJ, Abrams HB, McArdle R, et al. The tinnitus functional index: Development of a new clinical measure for chronic, intrusive tinnitus. Ear Hear. 2012;33:153–76. doi: 10.1097/AUD.0b013e31822f67c0. [DOI] [PubMed] [Google Scholar]
- 76.Henry JA, Jastreboff MM, Jastreboff PJ, Schechter MA, Fausti SA. Guide to conducting tinnitus retraining therapy initial and follow-up interviews. J Rehabil Res Dev. 2003;40:157–78. [PubMed] [Google Scholar]
- 77.Wechsler D. Manual of the Wechsler Adult Intelligence Scale-Revised. New York: The Psychological Corporation: Harcourt Brace Jovanovich; 1981. [Google Scholar]
- 78.EuroQol – A new facility for the measurement of health-related quality of life. The EuroQol Group. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 79.Newman CW, Weinstein BE, Jacobson GP, Hug GA. The Hearing Handicap Inventory for Adults: Psychometric adequacy and audiometric correlates. Ear Hear. 1990;11:430–3. doi: 10.1097/00003446-199012000-00004. [DOI] [PubMed] [Google Scholar]
- 80.Beck A, Steer R, Brown G. Manual for the BDI FastScreen for Medical Patients Manual. San Antonio, TX: The Psychological Corporation: Harcourt Assessment; 2003. [Google Scholar]
- 81.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 82.Speilberger CD, Gorshuch RL, Lushene RE, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory (Form Y) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]