Abstract
Vascular endothelial growth factor (VEGF) A is implicated in aberrant angiogenesis and intravitreous neovascularization (IVNV) in retinopathy of prematurity (ROP). However, VEGFA also regulates retinal vascular development and functions as a retinal neural survival factor. By using a relevant ROP model, the 50/10 oxygen-induced retinopathy (OIR) model, we previously found that broad inhibition of VEGFA bioactivity using a neutralizing antibody to rat VEGF significantly reduced IVNV area compared with control IgG but also significantly reduced body weight gain in the pups, suggesting an adverse effect. Therefore, we propose that knockdown of up-regulated VEGFA in cells that overexpress it under pathological conditions would reduce IVNV without affecting physiological retinal vascular development or overall pup growth. Herein, we determined first that the VEGFA mRNA signal was located within the inner nuclear layer corresponding to CRALBP-labeled Müller cells of pups in the 50/10 OIR model. We then developed a lentiviral-delivered miR-30–embedded shRNA against VEGFA that targeted Müller cells. Reduction of VEGFA by lentivector VEGFA-shRNA–targeting Müller cells efficiently reduced 50/10 OIR up-regulated VEGFA and IVNV in the model, without adversely affecting physiological retinal vascular development or pup weight gain. Knockdown of VEGFA in rat Müller cells by lentivector VEGFA-shRNA significantly reduced VEGFR2 phosphorylation in retinal vascular endothelial cells. Our results suggest that targeted knockdown of overexpressed VEGFA in Müller cells safely reduces IVNV in a relevant ROP model.
Retinopathy of prematurity (ROP) remains a leading cause of childhood blindness and is increasing in frequency in developing countries. The hypothetical proposed pathophysiological characteristics of ROP have been recently refined to be that stresses in prematurity cause delayed physiological retinal vascular development and potentially some high oxygen-induced capillary constriction that results in avascular retina.1–4 Once supplemental oxygen is removed from the preterm infant, the retina becomes hypoxic, and hypoxia stimulates the release of angiogenic factors with growth of new blood vessels into the vitreous as intravitreous neovascularization (IVNV). Many angiogenic factors can result in pathological IVNV in animal models, such as insulin-like growth factor-1,5,6 hepatocyte growth factor,7 erythropoietin,8–10 platelet-derived growth factor,11 and angiopoietins,12,13 but vascular endothelial cell growth factor A (VEGFA) has become one of the most studied factors leading to IVNV. VEGFA mRNA was found in the retina of a preterm infant eye with severe ROP,14 and VEGFA protein was increased in vitreous from preterm infants who underwent surgery for stage 4 ROP compared with controls.15 VEGFA inhibitors reduce pathological angiogenesis in adult retinal diseases, including diabetic retinopathy16,17 and age-related macular degeneration.18–20 Therefore, there is reason to consider VEGFA in the pathological characteristics of human ROP. However, in the preterm infant retina, VEGFA is also important in the development of retinal blood vessels21–23 and other organs.24,25 After a recent clinical trial testing intravitreal delivery of a broad anti-VEGFA antibody in infants with severe ROP, there have been reports of persistent avascular retina and reactivation of IVNV with subsequent total retinal detachment, even 1 year after treatment.26 In addition, by using a relevant ROP model, we found that inhibition of VEGFA bioactivity using a neutralizing antibody to rat VEGF significantly reduced IVNV area without adversely affecting physiological retinal vascular development 6 days after antibody injection, but significantly reduced body weight gain in the pups, suggesting an adverse effect.27 Therefore, safer ways to inhibit pathological IVNV while preserving physiological retinal vascularization are needed.
One way to target pathological IVNV is to determine the cells within the retina that overproduce VEGFA during pathological stress. In preterm infant eyes, it is not possible to safely localize where VEGFA is produced. Therefore, we used a relevant model of ROP today, the rat 50/10 oxygen-induced retinopathy (OIR) model, to localize the VEGFA signal within the retina and determine its role in pathological IVNV in ROP. This model causes features of severe ROP and produces extrauterine growth restriction, a risk for ROP in human preterm infants.28 The oxygen exposure recreates arterial oxygen fluctuations similar to those experienced by infants with severe ROP.29 Previously, we found that VEGFA and VEGFR2 were both increased as early as at postnatal day 8 (p8) in whole retinas from eyes of pups in the 50/10 OIR model compared with room air–raised counterparts.30
In the retina, several cells have been shown to produce VEGFA to support retinal development and physiological functioning. These include ganglion cells,31 astrocytes,32 Müller cells, and retinal pigment epithelium.33 In pathological IVNV, the VEGFA signal has been localized to many of the same cells: Müller cells,34,35 astrocytes,36 and, possibly, ganglion cells. However, there has been disagreement as to the cell type that overproduced VEGFA to cause IVNV,34,36 and these articles also used the mouse model of OIR that exposes pups to constant and higher oxygen levels than currently used in the management of ROP.37
In the current study, we generated shRNAs to VEGFA and used a lentiviral miRNA-based system to target Müller cells, where the VEGFA message was localized in the 50/10 OIR model. We tested the hypothesis that knocking down VEGFA to physiological levels in Müller cells would inhibit IVNV without adversely affecting physiological retinal vascular development.
Materials and Methods
Rat Model of the 50/10 OIR Model
All animal studies were performed in compliance with the University of Utah (Salt Lake City) Guide for the Care and Use of Laboratory Animals and the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. The rat 50/10 OIR model has been previously described.38 Entire litters of newborn Sprague-Dawley rat pups (Charles River, Wilmington, MA) and dams were placed into an oxygen environment (Oxycycler; Biospherix, Lacona, NY) that cycled the oxygen concentration between 50% and 10% every 24 hours for 14 days. At postnatal day 14 (p14), litters were placed into room air for an additional 4 days. Pup number was maintained at 12 to 14 pups per litter. At least two litters were used for each condition. Protein, in situ hybridization, or immunohistochemistry (IHC) was performed in one eye, and flat mount analysis was performed in the fellow similarly treated eye.
In Situ Hybridization of VEGFA Splice Variants
Serial sections (10 μm thick) from fresh frozen p14 50/10 OIR uninjected eyes were processed for in situ hybridization to detect the message of VEGF120, VEGF164, and VEGF188 splice variants using the fluorescence in situ hybridization (FISH) kit (Invitrogen, Carlsbad, CA), per manufacturer’s instructions. In comparison, serial retinal sections from a room air–raised pup at developmental day p14 were processed at the same time. Labeling of nuclei was performed using Hoechst 33342 (Invitrogen, Carlsbad, CA).
Construction of VEGFA shRNA Lentivectors
Studies have shown that double-stranded RNAs can bind the surface of cells and activate toll-like receptor 3.39 We chose shRNAs packaged within a virus, rather than as siRNA, so the RNA will not have access to the surface toll-like receptor 3. We embedded shRNAs into the endogenous precursor miR-30 context, so that the miRNA-based shRNA will be involved in the endogenous gene-silencing machinery that is transcribed predominantly by polymerase II promoters.
Target sequences for rat VEGFA (NM_031836) were selected from five online prediction algorithms based on regions predicted to work in more than two of the algorithms. Sequences were blasted to ensure no off-target effects. For VEGFA, the target sequences were selected in the region of bases between 100 and 419 so that no alternative splice sites were targeted and to ensure that all splice variants would be silenced. A sequence-targeting nonmammalian gene, luciferase (M15077), was developed as a control. The lentiviral transfer vector with a CD44 promoter and the red fluorescence protein (RFP) or green fluorescence protein (GFP) (pFmCD44.1GW), along with viral packaging constructs (VSV-G, pMDLg/pRRE, and pRSV-REV), were shown to target Müller cells in rats in vivo.40 shRNAs were designed against rat VEGFA (VEGFA-shRNA) or luciferase (luciferase-shRNA) and cloned into lentiviral transfer vectors (pFmCD44.1GW). Viral titers were determined using real-time quantitative PCR for viral particles, with final viral working stocks of 1 × 109 viral particles/mL. The sequences of VEGFA-shRNA and luciferase-shRNA were 5′-TGCTGTTGACAGTGAGCGCCCAAAGAAAGATAGAACAAAGTAGTGAAGCCACAGATGTACTTTGTTCTATCTTTCTTTGGTTGCCTACTGCCTCGGA-3′ and 5′-TGCTGTTGACAGTGAGCGCGCTGAGTACTTCGAAATGTCTAGTGAAGCCACAGATGTAGACATTTCGAAGTACTCAGCGTGCCTACTGCCTCGGA-3′, respectively.
Generation of VEGF120 and VEGF164 HEK Reporter Cell Lines
Two HEK reporter cell lines were generated by cloning rat VEGF120 and VEGF164 cDNA sequences into a pTK642 lentiviral transfer vector to test the efficacy of shRNAs to inhibit all splice variants of VEGFA. pTK642 contains a cytomegalovirus promoter, followed by an interferon response element and blasticidin/GFP. The GFP reporter was used to assess transfection efficiency, whereas the blasticidin permitted selection of cells that expressed rat VEGF120 and rat VEGF164. Both HEK VEGFA reporter cell lines were transfected with plasmid DNA pFmCD44.1GW containing VEGFA-shRNA expressed with RFP or an empty vector without shRNA as control. The knockdown efficiency of VEGFA-shRNA was determined by reading GFP fluorescence 48 hours after transfection of HEK VEGF reporter cell lines with flow cytometry. Red cells were gated, and the mean fluorescence intensity of GFP was measured. Silencing was calculated as the difference in GFP mean fluorescence intensity between cells transfected with the vector containing the VEGFA-shRNA and cells containing the control vector. Silencing was expressed as a percentage of control vector transfected cells.
Cell Culture and Transduction with pFmCD44-shRNA Lentivectors
rMC-1 cells, a rat Müller cell line (kindly provided by Dr. Vijay Sarthy, Northwestern University, Evanston, IL), were maintained in Dulbecco’s modified Eagle’s medium/high glucose (Gibco/Life Technologies, Grand Island, NY), supplemented with 10% fetal bovine serum/1% penicillin-streptomycin and grown to 80% confluence in 6-well plates (Corning, Inc., Corning, NY). Human primary retinal microvascular endothelial cells at passage 3 (hRMVECs; Cell Systems, Kirkland, WA) were maintained in basal endothelial growth medium (Lonza, Hopkinton, MA), supplemented with 5% fetal bovine serum/1% penicillin-streptomycin and grown to 80% confluence in 6-well plates (Corning, Inc., Tewksbury, MA). Cells were infected in triplicate with VEGFA-shRNA or luciferase-shRNA lentivector-containing media (5.0 × 106 viral particles/mL) or left uninfected. After 24 hours, media were replaced, and cells were then incubated for another 18 hours at 37°C at 1% O2 (Biospherix). GFP expression was imaged using an inverted fluorescence microscope (Olympus IX81; Olympus, Tokyo, Japan) at ×20 magnification with a fluorescein isothiocyanate filter. rMC-1 cells were collected for RNA extraction and analysis with real-time PCR. Media were harvested for VEGFA enzyme-linked immunosorbent assay (ELISA). All samples were frozen at −80°C until analysis.
Co-Culture of rMC-1s and hRMVECs
hRMVECs were grown on inserts (Transwell; Corning, Inc.) with 1-μm-diameter pores that were too small to allow cell migration but still allowed hRMVEC and rMC-1 cells grown on the underside of the inserts to make contact. In some experiments, rMC-1 cells were infected with lentivector VEGFA-shRNA or luciferase-shRNA, as previously described. At 48 hours after contact and virus infection, hRMVECs were collected from the tops of the inserts and processed for VEGFR2 phosphorylation.
Subretinal Injections
At the beginning of the 50% oxygen cycle of the 50/10 OIR model on p8, pups were anesthetized by i.p. injection (IP) of 20 mg/kg ketamine and 6 mg/kg xylazine. By using a 33-gauge needle attached to a Hamilton syringe, 1 μL (1 × 109 viral particles/mL) of lentivectors containing VEGFA-shRNA or luciferase-shRNA was delivered into the subretinal space. Sterile PBS (1 μL) was used as an additional control. Successful injections produced a retinal detachment, which was transient, and retinas reattached within 24 hours without lens injury or injury to the choroid. Both eyes of each pup were injected with the same lentivector preparation or with PBS. Each litter typically had an equal distribution of lentivector- and PBS-injected pups. After the injection, 0.5% erythromycin was topically applied to each eye, and pups were allowed to recover on a warming pad before being returned to the oxygen environment (Oxycycler). Litters were typically out of the oxygen cycler for 3 hours. At postnatal day 18 (p18), a time point that we have found maximum IVNV in this model,41 and to provide sufficient time for lentivirus transduction and VEGFA knockdown, pups were euthanized by IP of ketamine (60 mg/kg) and xylazine (18 mg/kg), followed by IP of pentobarbital (80 mg/kg). One eye was processed for retinal flat mounts, and the fellow eye was processed with the same treatment for protein or RNA analysis.
In Vivo Retinal Imaging
Pups were anesthetized for in vivo imaging of the retinas with Micron III retinal imaging microscope (Phoenix Research Laboratories, Inc., Pleasanton, CA) using a GFP filter during the 50% oxygen cycle and returned to the oxygen chamber for recovery within 3 hours. For euthanasia, pups received IP of pentobarbital (80 mg/kg) after deep anesthesia.
Retinal Flat Mount Preparation, Imaging, and Analysis
Lectin-stained retinal flat mounts were prepared using 5 μg/mL Alexa Fluor 568–conjugated Griffonia simplicifolia (Bandeiraea) isolectin B4 (Molecular Probes, Eugene, OR), as previously described,10 imaged using an inverted fluorescence microscope (Olympus). Flat mounts were generated using the scan-slide stitching function of Metamorph imaging software version 7.0 (Molecular Devices, Inc., Sunnyvale, CA). Measurements were made by two masked reviewers (Y.J. and M.M.) using ImageJ software version 1.46 (NIH, Bethesda, MD). The avascular retinal area (AVA) and IVNV area were calculated as a percentage of total retinal area for each flat mount.
Retinal Section Preparation and Staining and Retinal Thickness Measurement
Eyes were fixed in 4% paraformaldehyde containing 10 mmol/L sodium orthovanadate for 10 minutes, and retinas were removed and placed into 4% paraformaldehyde for another 15 minutes, followed by incubation in 30% sucrose/PBS overnight. Each retina was immersed in optimal cutting temperature compound (Tissue Tek; EMS, Hatfield, PA). Eyes cut into cryosections (10 μm thick) were processed, as described previously,10 and incubated overnight at 4°C with primary antibody, rabbit anti-phosphorylated VEGFR2 (p-VEGFR2 at Y951; Santa Cruz Biotechnology, Santa Cruz, CA), and mouse isolectin B4; then, they were washed and incubated for 1 hour with a 1:500 dilution of Alexa 594–conjugated goat anti-mouse secondary antibody (Invitrogen) for isolectin B4 or Alexa 488–conjugated goat anti-rabbit secondary antibody (Invitrogen) for p-VEGFR2. Some sections stained with only secondary antibody were controls. Cryosections stained with DAPI were used to measure retinal thickness. Images were captured using confocal microscopy (Olympus IX81). Exposure times for images were the same. Integrated density per image area of p-VEGFR2 and thickness of overall retina and different retinal layers were measured using ImageJ software.
VEGFA ELISA
Protein samples (50 μg) were used in the Quantikine Rat VEGFA ELISA kit (RRV00; R&D Systems, Minneapolis, MN) to measure total retinal VEGFA concentration between treatment groups, per manufacturer’s instructions. All samples were run in duplicate.
Protein Extraction and Western Blot Analysis
The hRMVECs were lysed in modified radioimmunoprecipitation assay buffer (20 mmol/L Tris base, 120 mmol/L NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, and 10% glycerol) with 1:100 protease inhibitors (Roche Diagnostics, Indianapolis, IN) and 2 mmol/L orthovanadate, and were homogenized and centrifuged at 16,000 × g for 10 minutes at 4°C. Total protein concentration (g/mL) in the supernatant fluid was quantified by BCA protein assay (Pierce, Rockford, IL). Total protein (30 μg) for each sample was used for Western blot analyses, as described previously.10 Membranes were incubated overnight at 4°C with primary antibodies: p-VEGFR2, VEGFR2 (1:500; Santa Cruz Biotechnology), cleaved caspase 3, and total caspase 3 (1:1000; Cell Signaling Technology Inc., Danvers, MA). Blots were visualized, and the relative densities of bands were calculated as previously described.10 The relative activities of STAT3 and VEGFR2 were calculated as phosphorylated/total protein and expressed as fold difference. Caspase 3 activity was analyzed by Western blot analysis and quantified as the density of the bands for the cleaved form/density of total caspase bands.
RNA Isolation and Real-Time PCR Analysis
Samples were removed from RNAlater, and total RNA was extracted with the RNeasy Mini kit (Qiagen, Valencia, CA). RNA was quantified using the NanoDrop spectrophotometer (Thermo Scientific, Wilmington, DE). cDNA was generated using a High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). Real-time PCR was performed on a Mastercycler ep Realplex (Eppendorf, Westbury, NY) with the use of TaqMan probes (Applied Biosystems). Expression levels for VEGFA were normalized to the mean value of internal control, glyceraldehyde-3-phosphate dehydrogenase.
Statistical Analysis
Significant differences between treatment groups were determined by one-way analysis of variance with the Bonferroni multiple-comparison post hoc test. A minimum value of P < 0.05 was considered statistically significant.
Results
VEGFA Splice Variants Localize to Layers Corresponding to CRALBP-Labeled Müller Cells in the 50/10 OIR Model
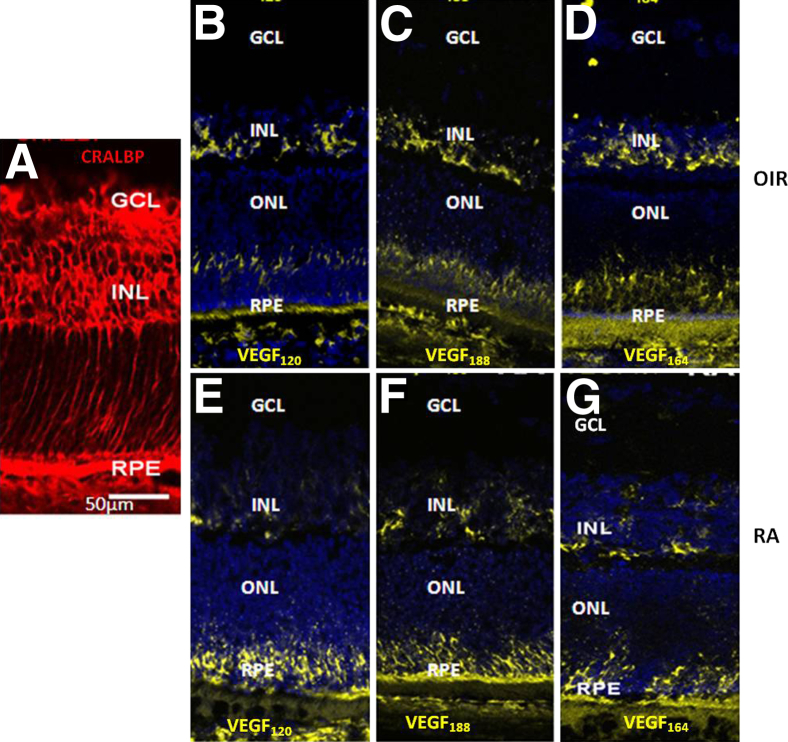
We previously reported that VEGFA splice variant expression increased in the retinas of rat pups exposed to the 50/10 OIR model at time points including p14 and p18, compared with retinas from age-matched pups raised in room air. 30,41 We, therefore, localized VEGFA mRNA splice variants, VEGF120, VEGF164, and VEGF188, within the retinas of rat pups raised in the 50/10 OIR model and in room air at p14. The mRNA signals in both room air–raised pups and the 50/10 OIR model, determined by FISH, were mainly in the inner nuclear layer, corresponding to CRALBP-labeled Müller cells, and retinal pigment epithelium in both the 50/10 OIR model and room air (Figure 1). These results support the hypothesis that Müller cell–produced VEGFA may be involved in VEGFA-mediated pathological angiogenesis in the 50/10 OIR model.
Figure 1.
mRNA of VEGFA splice variants is localized in the inner nuclear layer (INL) corresponding to Müller cells in the rat 50/10 OIR model. FISH of VEGFA splice variants (VEGF120, VEGF188, and VEGF164) in retinal cryosections at p14 shows the VEGFA splice variant message in regions where CRALBP-labeled Müller cells were present (A) in the rat 50/10 OIR model (B–D) and in a room air–raised pup at p14 (RA; E–G). The expression of VEGFA is also present in the external limiting layer, retinal pigment epithelium (RPE), and photoreceptor regions. GCL, ganglion cell layer; ONL, outer nuclear layer.
Generation of Lentivectors for VEGFA-shRNA Delivery to Müller Cells
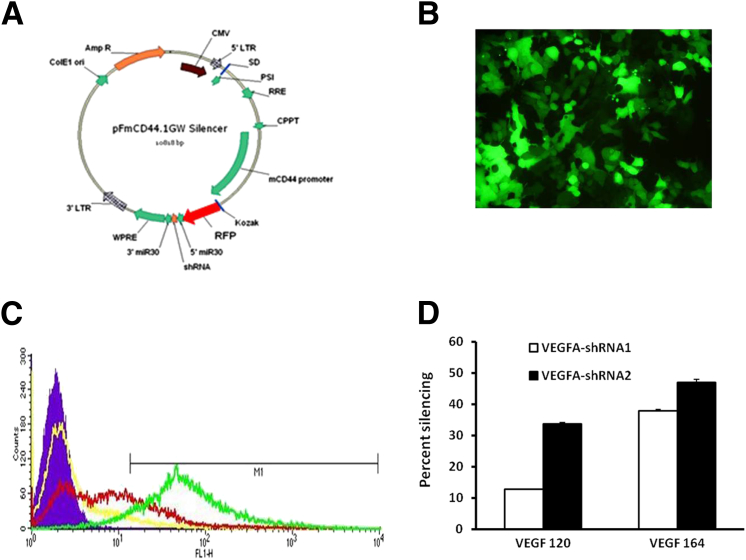
To investigate whether Müller cell–derived VEGFA contributed to IVNV, we generated lentivectors from pFmCD44.1 GW, which contains a CD44 promoter that targets Müller cells,40 and used this to drive an miR-30–based shRNA cassette and RFP (Figure 2A) or GFP (Figure 3A) to deliver VEGFA shRNAs to Müller cells in vitro or in vivo. To ensure knockdown efficiency, two shRNAs targeting rat VEGFA coding sequences were designed, and an empty lentivector was used as a control. To test if designed shRNAs reduced VEGFA expression, HEK reporter cell lines were generated that expressed GFP-tagged rat VEGF120 or VEGF164 (Figure 2B). Lentivector plasmid DNA containing VEGFA-shRNA with RFP expression was transfected into HEK reporter cell lines. Fluorescence-activated cell sorter analysis of GFP fluorescence in RFP-positive cells was used as a readout for the expression level of VEGF120 and VEGF164, two splice variants of VEGFA (Figure 2C). As shown in Figure 2D, VEGFA-shRNA2 showed better knockdown efficiency than VEGFA-shRNA1, with 35% reduction in VEGF120 expression and approximately 50% reduction in VEGF164 expression. Therefore, VEGFA-shRNA2 was used for in vivo experiments.
Figure 2.
Generation of lentivector-delivered shRNA for specific knockdown of VEGFA in Müller cells. A: Diagram of the pFmCD44.1GW lentivector containing the glia-specific CD44 promoter driving an miR-30–based shRNA cassette and an RFP marker. B: HEK293 reporter cell line expressed GFP-tagged rat VEGF120 or VEGF164. C: HEK reporter cell lines to VEGF120 and VEGF164 were transfected with RFP-expressed lentivector VEGFA-shRNA plasmids, and GFP fluorescence (infectivity) was measured by flow cytometry. D: Quantification of percentage silencing of VEGF120 and VEGF164 by VEGFA-shRNAs from fluorescence-activated cell sorter analysis (n = 3). Results are means ± SEM.
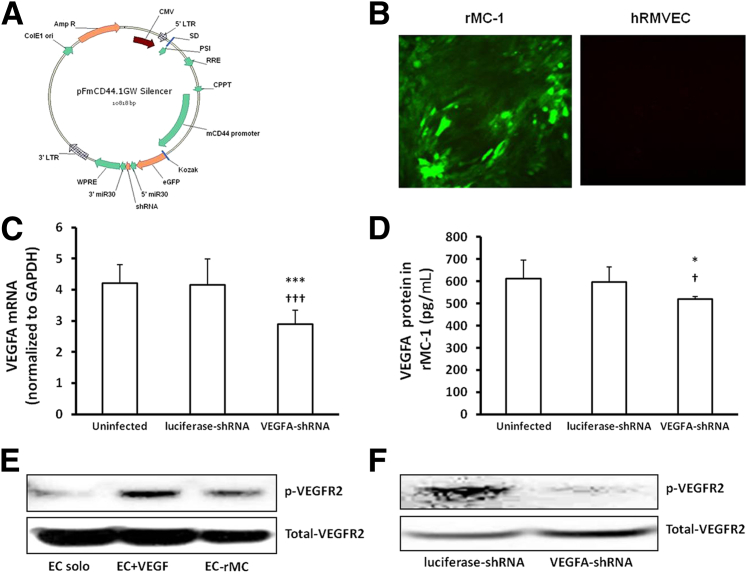
Figure 3.
In vitro analysis of lentivector-delivered shRNA transduction and VEGFA knockdown in rMC-1 cells, and VEGFA signaling in a co-culture model of rMC-1s and hRMVECs. A: Diagram of the pFmCD44.1GW lentivector plasmid containing the glia-specific CD44 promoter driving an miR-30–based shRNA cassette and a GFP marker. B: GFP expression in rMC-1s and hRMVECs. Real-time PCR of VEGFA mRNA in rMC-1s (C) and ELISA of VEGFA protein in culture media of rMC-1s transduced with lentivirus (D). E and F: Knockdown of VEGFA in rMC-1s by lentivector-VEGFA-shRNA reduces co-culture–induced p-VEGFR2 in hRMVECs. Representative gels of p-VEGFR2 in hRMVECs grown in contact with lentivector VEGFA-shRNA or luciferase-shRNA–transduced rMC-1s (E) (solo cultured, treated with VEGFA, and grown in contact with rMC-1s) and in hRMVECs grown in contact with lentivector VEGFA-shRNA– or luciferase-shRNA–transduced rMC-1s (F). *P < 0.05, ∗∗∗P < 0.001 versus uninfected; †P < 0.05, †††P < 0.001 versus luciferase-shRNA. Data shown in C and D are representative of six independent samples. Results are means ± SEM. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
To determine whether the lentivector specifically targeted Müller cells, a rat Müller cell line, rMC-1s, and, as a comparison, hRMVECs, were infected with GFP-expressed lentivirus carrying VEGFA-shRNA2 or luciferase-shRNA control virus (Figure 3A). Specificity to Müller cells was determined by GFP expression, as shown in Figure 3B, where rMC-1s were GFP positive, and no hRMVECs were GFP positive. At 48 hours after viral transduction, VEGFA mRNA was significantly reduced in VEGFA-shRNA–transduced rMC-1s (Figure 3C), and VEGFA protein was significantly decreased in the culture media of VEGFA-shRNA–transduced rMC-1s compared with uninfected cells or cells transduced with lentivector with luciferase-shRNA (Figure 3D). These results suggest that lentivectors transporting shRNAs to VEGFA yielded a satisfactory reduction of VEGFA expression at both mRNA and protein levels.
To further test if knockdown of VEGFA in rMC-1s is able to reduce VEGFA signaling in retinal endothelial cells, a co-culture model of rMC-1s and hRMVECs was used. As shown in Figure 3E, compared with solo cultured hRMVECs, p-VEGFR2 was increased in hRMVECs either grown in contact with rMC-1 for 24 hours or treated with human VEGFA for 30 minutes, suggesting the co-culture model was feasible to test our hypotheses. To further examine the effect of Müller cell–derived VEGFA on the activation of hRMVEC VEGFA signaling, activation of VEGFR2 was determined in hRMVECs grown in contact with rMC-1 cells transduced with lentivector VEGFA-shRNA or control luciferase-shRNA. Similar to the results shown in Figure 3C, VEGFA mRNA was significantly reduced in rMC-1s transduced with lentivector VEGFA-shRNA, and p-VEGFR2 was also decreased in hRMVECs when cells were in contact with lentivector VEGFA-shRNA–transduced rMC-1s, compared with control luciferase-shRNA (Figure 3F). These findings support the hypothesis that modulating VEGFA expression in rMC-1s changed VEGF-mediated angiogenic signaling in hRMVECs.
Knockdown of VEGFA in Müller Cells Reduces IVNV without Affecting Retinal Physiological Vascularization
In the 50/10 OIR model, we found VEGFA was increased as early as p8 and was up-regulated at several time points through p18,30 when IVNV is at a maximal level.37 To investigate whether knockdown of VEGFA in Müller cells reduced IVNV in the 50/10 OIR model, lentivectors carrying VEGFA-shRNA or control luciferase-shRNA were delivered to rat pups via subretinal injections at p8, a time point that allowed sufficient time for viral transduction. We (data not shown) and Greenberg et al40 previously found that intravitreal injections did not yield Müller cell transduction. Only pups weighing within ±2 g of the average weight of the litters received injections and were included in the following experiments; thus, body weights at p8 were similar between the virus injection and control groups. In addition, pup number was maintained between 12 and 14 in all litters to ensure consistency in the 50/10 OIR model.28
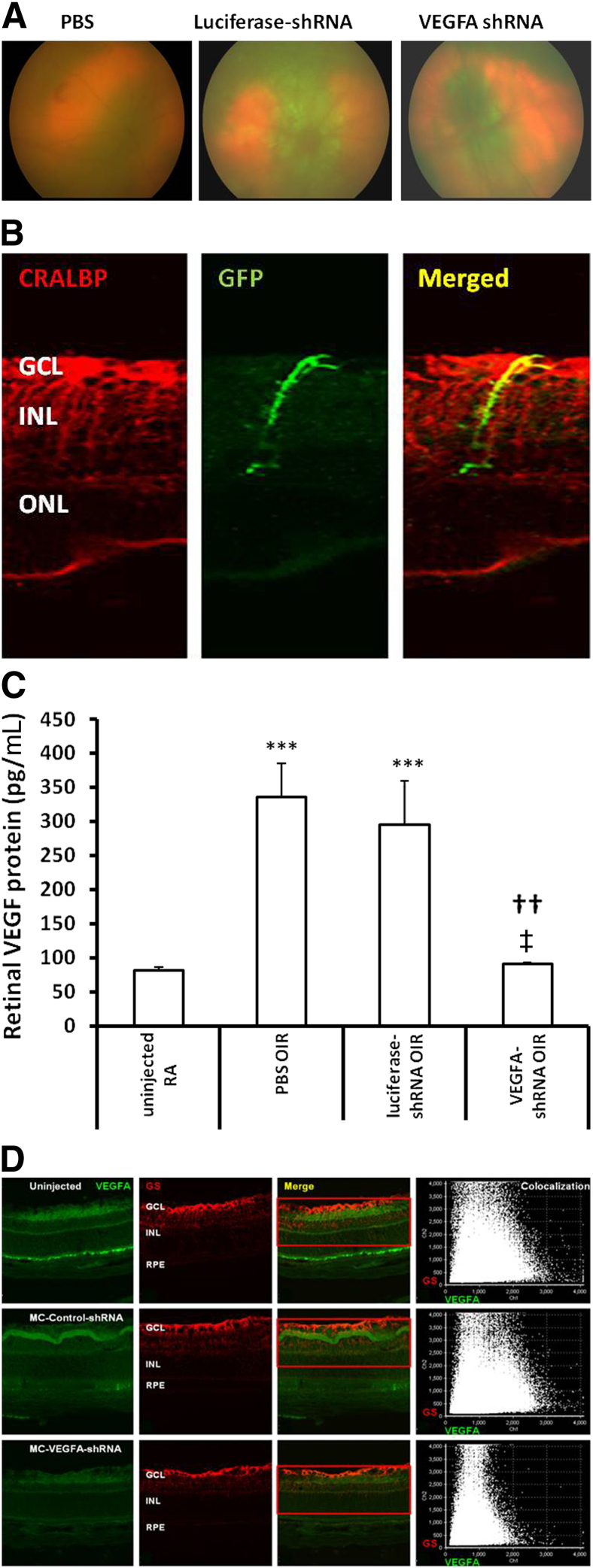
To determine the extent of viral transduction in the retina, retinal images were captured in vivo using the Micron III retinal imaging microscope. As shown in Figure 4A, the retina had GFP fluorescence by p18. Rat pups were sacrificed at p18, and one eye was analyzed for IVNV and avascular retina, and the other for protein or IHC. By IHC, we found GFP colocalized with CRALBP-labeled Müller cells, as shown in an experiment using control lentivirus with the CD44 promoter to drive GFP (Figure 4B), providing additional support that the lentivirus was transduced in Müller cells. To determine the silencing effect of the lentivector-delivered shRNA in vivo, total retinal VEGFA protein was measured by ELISA. Consistent with our previous findings, VEGFA protein was increased in retinas from pups raised in the 50/10 OIR model at p18 compared with room air–raised pups (Figure 4C). Compared with pups receiving subretinal injections of PBS or luciferase-shRNA controls, retinal VEGFA was significantly reduced in pups that received VEGFA-shRNA virus injections (Figure 4C). However, compared with room air–raised pups of the same postnatal day ages, there was no significant difference in VEGFA levels in retinas from p18 pups in the 50/10 OIR model and treated with VEGFA-shRNA virus, suggesting that lentivector VEGFA-shRNA reduced VEGFA to physiological levels required for normal retinal vascular development. To determine the VEGFA knockdown, we colabeled retinal cryosections with VEGFA and glutamine synthetase from eyes injected with lentivector VEGFA-shRNA or luciferase-shRNA. Immunoreactivity of VEGFA was reduced in sections from VEGFA-shRNA injection compared with luciferase-shRNA or uninjected eyes from the 50/10 OIR model at p18 (Figure 4D).
Figure 4.
In vivo analysis of lentivector-delivered shRNA transduction in retina of pups raised in the rat 50/10 OIR model at p18 after subretinal injection at p8. A: Images by Micron III retinal imaging microscope showed GFP expression in retina of pups after lentivirus injection compared with the PBS-injected pup. B: GFP expression is localized with CRALBP-labeled Müller cells in retinal cryosections at p18 taken from pup eye injected at p8 with lentivirus containing CD44 promoter driving GFP expression. C: ELISA of retinal VEGFA protein at p18. ∗∗∗P < 0.001 versus uninjected room air raised (RA), ††P < 0.01 versus PBS, and ‡P < 0.05 versus luciferase-shRNA. Results are means ± SEM (n = 6 to 8). D: IHC of VEGFA protein in glutamine-synthetase–labeled Müller cells at p18, showing reduced immunoreactivity of VEGFA in VEGFA-shRNA–treated eyes compared with uninjected or control injected eyes from the 50/10 OIR model at p18. GCL, ganglion cell layer; INL, inner nuclear layer; IPL, inner plexiform layer; ONL, outer nuclear layer; OPL, outer plexiform layer; RPE, retinal pigment epithelium.
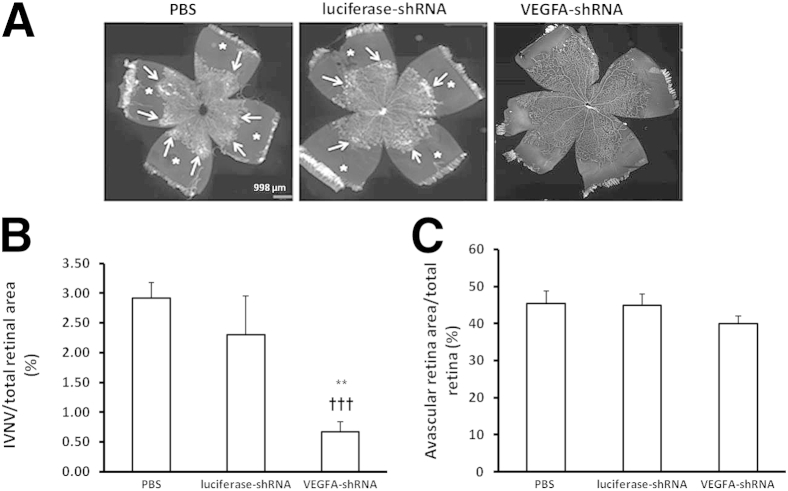
We then determined the effect of lentivector VEGFA-shRNA subretinal injections on IVNV and percentage AVA. For AVA, there was no difference in control or PBS-treated eyes with VEGFA-shRNA–treated eyes (Figure 5, A and C). However, there was a significant reduction in percentage IVNV in lentivector VEGFA-shRNA–treated eyes compared with the other groups (Figure 5, A and B). These findings provide evidence that Müller cell–derived VEGFA contributed to IVNV, and suggest that knockdown of up-regulated VEGFA in Müller cells reduced pathological IVNV without interfering with physiological retinal vascular development or affecting retinal apoptosis in the 50/10 OIR model at p18.
Figure 5.
Lentivector-VEGFA-shRNA reduces IVNV without interfering with physiological retinal vascular development in the rat 50/10 OIR model. A: Images of retinal flat mounts at p18 after subretinal injections in each group (PBS, control luciferase-shRNA, and VEGA-shRNA). B and C: Quantification of IVNV (∗∗P < 0.01 versus PBS; †††P < 0.001 versus luciferase-shRNA; B) and AVA (P = 0.85, luciferase-shRNA versus PBS; P = 0.15, VEGFA-shRNA versus PBS; C) in each group. Data shown in B and C are representative of ≥12 independent samples. Results are means ± SEM. In panel A, asterisks indicate AVA; arrows, area with IVNV.
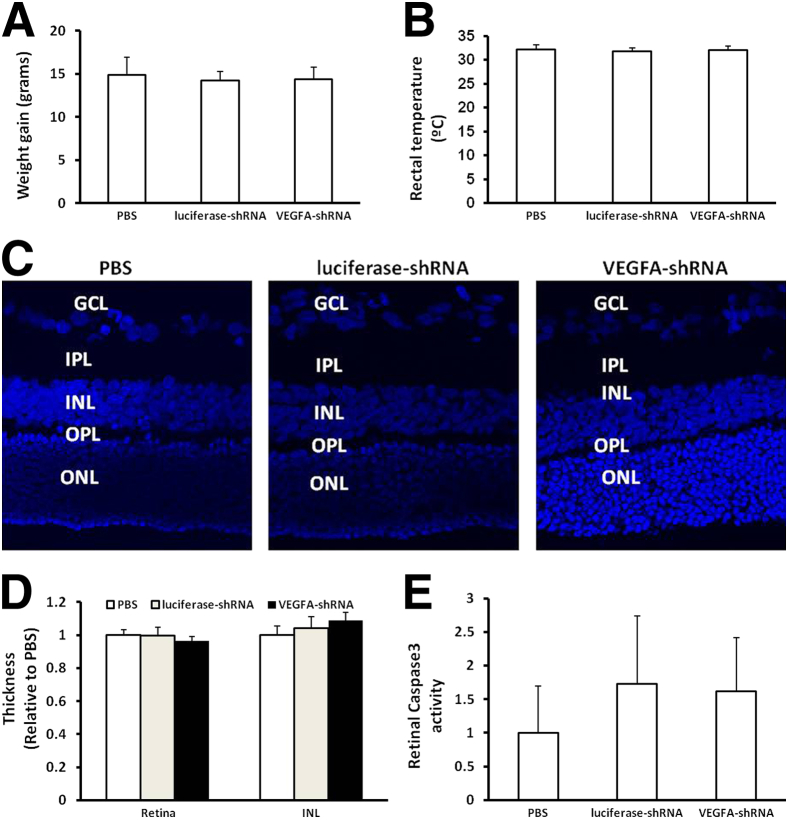
Reduced pup growth rate, or extrauterine growth restriction, is an associated risk factor for severe ROP in human infants.42 We found no significant differences in mean pup weights measured at p18 in all groups, as shown in Figure 6A. Rectal temperature measured at p18 as an indicator of viral-induced systemic inflammation was also not different between the PBS, control, or VEGFA-shRNA lentivirus–injected groups (Figure 6B). In addition, lentivirus injection did not cause changes in retinal morphological characteristics, retinal thickness, or thickness of the inner nuclear (Figure 6, C and D), outer nuclear, inner plexiform, or outer plexiform layers (quantification is not shown). To determine whether lentivirus injection caused increased apoptosis, we measured retinal caspase 3 activity and found that there was no significant difference in retinas between PBS-treated eyes and either VEGFA-shRNA– or luciferase-shRNA–treated eyes (Figure 6E).
Figure 6.
Injection of lentivirus does not cause systemic effects or retinal morphological changes in the rat 50/10 OIR model. A: Weight gain of pups from p8 to p18. B: Rectal temperature at p18. C and D: Retinal cross sections stained with DAPI at p18 (C) show no difference in thickness of overall retina and inner nuclear layer (D). E: Retinal-activated caspase 3 at p18. P > 0.05 for VEGFA-shRNA versus either PBS or luciferase-shRNA. Results are means ± SEM (n = 5 to 15). GCL, ganglion cell layer; INL, inner nuclear layer; IPL, inner plexiform layer; ONL, outer nuclear layer; OPL, outer plexiform layer.
Knockdown of VEGFA in Müller Cells Reduces VEGFA Signaling in Retinal Vascular Endothelial Cells
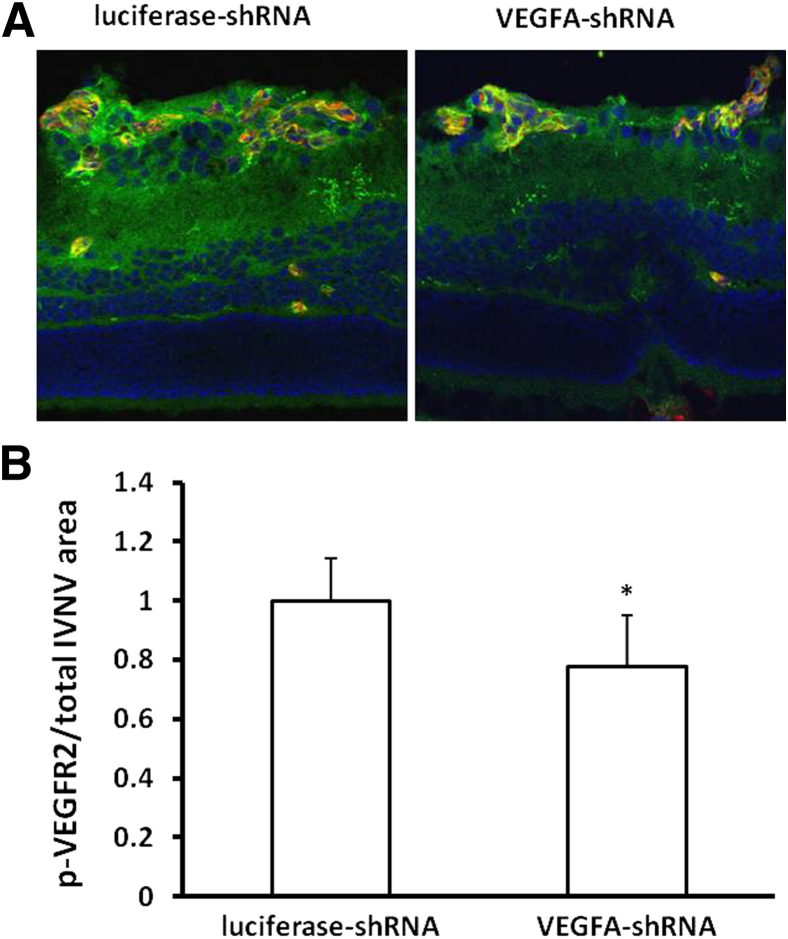
Based on the previously described results, we hypothesized that down-regulation of VEGFA in Müller cells reduced VEGF receptor signaling in retinal vascular endothelial cells. To test this hypothesis, we measured p-VEGFR2 in retinal cryosections by IHC (Figure 7). We found that, compared with luciferase-shRNA, the density of p-VEGFR2 (Figure 7, A and B) labeling normalized to the IVNV area, determined by lectin-positive staining, was qualitatively decreased in sections from pups that received lentivector VEGFA-shRNA in the 50/10 OIR model.
Figure 7.
Knockdown of VEGFA in Müller cells by lentivector-VEGFA-shRNA reduces VEGFA signaling in vivo. A: p-VEGFR2 labeling (green) in a cross section through isolectin-stained (red) IVNV or DAPI (blue). B: Quantification of p-VEGFR2 density in IVNV. ∗P < 0.05 for VEGFA-shRNA versus luciferase-shRNA (n = 3). Results are means ± SEM.
Discussion
Elevated VEGFA has been found in the vitreous of infants with ROP.15 Evidence from animal studies indicates that hypoxia induces VEGFA expression and causes the pathological phases seen in ROP.30,37,43 Several types of retinal cells produce VEGFA either in pathological or physiological conditions, making broad and total inhibition of VEGFA signaling in the retina a concern, particularly in preterm infants in whom vasculature, neurons, and the neurovascular connections are developing.37,43 Therefore, targeting specific cells that overproduce VEGFA to knock down excessive expression without inhibiting physiological expression may reduce pathological features without interfering with development and maturation. By using the mouse model of OIR, investigators concluded that Müller cell34 or astrocyte-derived VEGFA36 was important in pathological angiogenesis. To address current-day ROP in countries that have resources to avoid high oxygen at birth, we used a rat model of variable OIR, the 50/10 OIR model, to assess overexpressed VEGFA that developed after birth from stresses relevant to those experienced by current-day human preterm infants.30,43
We previously reported that repeated fluctuations in oxygen up-regulated VEGFA expression in the retina at several time points in the rat 50/10 OIR model, compared with room air–raised pups, that corresponded with persistent avascular retina and IVNV, which are features similar to those in human severe ROP.30,44 By using the same model, we also found that inhibiting rat VEGFA with a neutralizing antibody significantly reduced IVNV area by approximately 3.5-fold, but caused adverse effects, including reduced pup growth between the time of intravitreal injection at postnatal day 12 and the time of sacrifice at p18.27 Therefore, to better target cells that overexpress VEGFA, we localized VEGFA mRNA in retinal sections using fluorescence in situ hybridization (FISH). Fourteen days after pups were exposed to repeated fluctuations in oxygen, mRNA signals of retinal VEGFA splice variants were detected in the inner nuclear layer and colocalized with CRALBP. Therefore, we postulated that Müller cell–derived VEGFA might mediate pathological angiogenesis in the form of IVNV. To knock down Müller cell–derived VEGFA, shRNAs to VEGFA were designed and inserted into a lentiviral miR-30–based system driven by a CD44 promoter (as shown in Figures 2A and 3A), which was shown to exclusively target Müller cells in vivo in the rat.40 Usually, shRNA expression is under the control of polymerase III promoters, such as the U6 promoter, which drives shRNA expression in all cells. To express shRNA only in Müller cells driven by the CD44 promoter that is regulated by polymerase II, we embedded the shRNAs within an miR-30 context. The expression of the miR-30–based shRNAs is transcribed by the polymerase II–regulated CD44 promoter. The use of a polymerase II promoter to drive miR-30–based shRNAs has been shown to yield more efficient knockdown of the target gene than standard shRNA constructs.45,46
We found that the lentivector-driven miR-30–VEGFA–shRNA was specifically expressed in Müller cells. By using several cell models and molecular approaches, we determined an optimal lentivector pFmCD44-driven VEGFA-shRNA that efficiently and specifically reduced Müller cell–derived VEGFA in vitro, even though the knockdown efficiency was not high. That the CD44 promoter may have lower activity in vitro than in vivo40,46 may explain the relatively low silencing of VEGFA by lentivector-VEGFA-shRNA. However, the VSV-G-CD44 promoter configuration was shown to yield high transduction efficiency and specificity for Müller cells in vivo.40
We then determined if the lentivectors used would specifically target Müller cells in vivo and knock down VEGFA expression. We found p18 to be the time for development of maximal IVNV in the rat 50/10 OIR model.41 For the lentivirus to transduce Müller cells, approximately a week is needed. Injections administered too early might adversely affect developing Müller cells. Based on these concerns and previous experiments, the time point, p8, was chosen to administer subretinal injections. Our previous study showed that retinal VEGF expression increased in the 50/10 OIR model as early as postnatal day 8 and became maximal at p14.41 In this study, pups received lentivirus at p8, which we reasoned would provide 1 week for effective and safe shRNA transduction and knockdown of up-regulated VEGFA. Ten days after subretinal injections at p18, live imaging using the Micron III retinal imaging microscope showed GFP expression in retinas from pups injected with either lentivector luciferase–shRNA or VEGFA-shRNA, and this GFP expression was colocalized with CRALBP-labeled Müller cells in retinal cryosections. ELISA analysis showed that pups injected with VEGFA-shRNA had decreased VEGFA protein in retina compared with uninjected pups and pups injected with luciferase-shRNA–delivered virus, but there was no difference compared with pups raised in room air, suggesting lentivector-VEGFA-shRNA reduced 50/10 OIR-induced VEGFA from pathological levels to the levels required to maintain physiological retinal development. We then showed that transduction of Müller cells with VEGFA-shRNA in retinas of pups in the 50/10 OIR model caused almost complete inhibition of IVNV and some, albeit insignificant, inhibition of AVA compared with control (P = 0.15). In addition, the vascular morphological characteristics of VEGFA-shRNA–treated retinas appeared more normal than those of control or PBS-injected eyes, suggesting that reducing VEGF to physiological levels permits physiological-appearing retinal vascularization. In previous studies, comparing flat mounts from pups administered intravitreal anti-VEGF antibody injections with nonimmune IgG control injections,27 IVNV was reduced, but vascular morphological features still lacked physiological morphological features compared with eyes that had been treated with lentivector-VEGFA-shRNA (Supplemental Figure S1). Although there can be associated damage with subretinal injections and subsequent retinal detachment, we and others47 found that the bleb, or limited retinal detachment, associated with a successful subretinal injection was temporary and resolved within 24 hours, as determined using Micron III retinal imaging microscope. In addition, there was no increase in retinal apoptosis or changes in retinal morphological features with lentivector injections. Ocular injections of lentivirus also did not cause adverse effects on pup weight gain or systemic inflammation, as determined by rectal temperature.
Therefore, these results support the hypothesis that targeted partial silencing in cells that overexpress VEGFA (ie, Müller cells) can effectively inhibit pathological IVNV without interfering with physiological retinal vascular development or reducing weight gain during development using a model of OIR that is relevant to current-day ROP. Müller cells provide nutrition for retinal neurons, and ablation of Müller cells causes retinal vascular pathological features in a transgenic mouse model,48 suggesting interactions may exist between Müller cells and retinal vascular endothelial cells. To identify whether knockdown of Müller cell–derived VEGFA, using these lentivector-delivered shRNAs, caused decreased VEGFA signaling in hRMVECs, we measured p-VEGFR2 in IVNV labeled with isolectin in retinal cryosections and found significantly reduced p-VEGFR2 staining per area of IVNV in pups injected with lentivirus-delivered VEGFA-shRNA. To further test our hypothesis, a co-culture of rMC-1s and hRMVECs was performed. We found no significant difference in the activation of VEGFR2 in hRMVECs after growth with rMC-1s compared with that with human VEGFA stimulation, supporting the use of the rat/human co-culture to address this question. We then determined that knockdown of VEGFA in rMC-1s by lentiviral-delivered VEGFA-shRNA reduced VEGFR2 signaling in hRMVECs in co-culture. The results together indicated that knockdown of Müller cell–derived VEGFA effectively inhibited VEGFA-regulated angiogenesis in retinal vascular endothelial cells.
In summary, we localized VEGFA overexpression in Müller cells and generated a lentiviral-delivered miR-30–embedded shRNA that targeted Müller cells and efficiently reduced VEGFA from pathological levels to levels required for physiological retinal vascular development using a model relevant to ROP today, the 50/10 OIR model. Although we do not propose either subretinal injections or gene therapy to treat human infants with ROP, this study provides a novel concept to examine mechanisms involved in pathological IVNV and may be useful to determine pharmacological targets to safely reduce pathological features of ROP without interfering with the beneficial effect of major growth factors. In future studies, we will analyze the effects of targeting Müller cell–derived VEGF on the regression of IVNV and physiological retinal vascularization at later time points in the model.
Footnotes
Supported by NIH grants R01 R01EY015130 and R01EY017011 (M.E.H.) and Research to Prevent Blindness, Inc. (New York, NY), unrestricted grant (Department of Ophthalmology and Visual Sciences, University of Utah).
H.W. and G.W.S. contributed equally to this work.
Supplemental Data
Knockdown of Müller cell–up-regulated VEGFA in the 50/10 OIR model permits physiological-appearing retinal vascularization. Representative retinal flat mounts at p18 after intravitreal injection of anti-VEGF antibody given at postnatal day 12 (p12; left panel) or subretinal injection of lentivirus-delivered VEGF-shRNA given at p8 (right panel).
Supplemental Data
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2013.05.011.
References
- 1.Ashton N. Editorial: retrolental fibroplasia now retinopathy of prematurity. Br J Ophthalmol. 1984;68:689. [Google Scholar]
- 2.Patz A. Current therapy of retrolental fibroplasia: retinopathy of prematurity. Ophthalmology. 1983;90:425–427. doi: 10.1016/s0161-6420(83)34536-x. [DOI] [PubMed] [Google Scholar]
- 3.Michaelson I.C. The mode of development of the vascular system of the retina, with some observations on its significance for certain retinal diseases. Trans Ophthalmol Soc U K. 1948;68:137–180. [Google Scholar]
- 4.Shah P., Narendran V., Kalpana N., Gilbert C. Severe retinopathy of prematurity in big babies in India: history repeating itself? Indian J Pediatr. 2009;76:801–804. doi: 10.1007/s12098-009-0175-1. [DOI] [PubMed] [Google Scholar]
- 5.Hellstrom A., Peruzzu C., Ju M., Engstrom E., Hard A.-L., Liu J.-L., Albertsson-Wickland K., Niklasson A., Sjodell L., LeRoith D., Senger D., Smith L.E.H. Low IGF-1 suppresses VEGF-survival signalling in retinal endothelial cells: direct correlation with clinical retinopathy of prematurity. Proc Natl Acad Sci U S A. 2001;98:5804–5808. doi: 10.1073/pnas.101113998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kondo T., Vicent D., Suzuma K., Yanagisawa M., King G.L., Holzenberger M., Kahn C.R. Knockout of insulin and IGF-1 receptors on vascular endothelial cells protects against retinal neovascularization. J Clin Invest. 2003;111:1835. doi: 10.1172/JCI17455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding S., Merkulova-Rainon T., Han Z.C., Tobelem G. HGF receptor up-regulation contributes to the angiogenic phenotype of human endothelial cells and promotes angiogenesis in vitro. Blood. 2003;101:4816. doi: 10.1182/blood-2002-06-1731. [DOI] [PubMed] [Google Scholar]
- 8.Chen J., Connor K.M., Aderman C.M., Smith L.E. Erythropoietin deficiency decreases vascular stability in mice. J Clin Invest. 2008;118:526–533. doi: 10.1172/JCI33813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J., Connor K.M., Aderman C.M., Willett K.L., Aspegren O.P., Smith L.E.H. Suppression of retinal neovascularization by erythropoietin siRNA in a mouse model of proliferative retinopathy. Invest Ophthalmol Vis Sci. 2009;50:1329–1335. doi: 10.1167/iovs.08-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H., Byfield G., Jiang Y., Smith G.W., McCloskey M., Hartnett M.E. VEGF-mediated STAT3 activation inhibits retinal vascularization by down-regulating local erythropoietin expression. Am J Pathol. 2012;180:1243–1253. doi: 10.1016/j.ajpath.2011.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilkinson-Berka J.L., Babic S., de Gooyer T., Stitt A.W., Jaworski K., Ong L.G.T., Kelly D.J., Gilbert R.E. Inhibition of platelet-derived growth factor promotes pericyte loss and angiogenesis in ischemic retinopathy. Am J Pathol. 2004;164:1263–1273. doi: 10.1016/s0002-9440(10)63214-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdulmalek K., Ashur F., Ezer N., Ye F., Magder S., Hussain S.N. Differential expression of Tie-2 receptors and angiopoietins in response to in vivo hypoxia in rats. Am J Physiol Lung Cell Mol Physiol. 2001;281:L582–L590. doi: 10.1152/ajplung.2001.281.3.L582. [DOI] [PubMed] [Google Scholar]
- 13.Sarlos S., Rizkalla B., Moravski C.J., Cao Z., Cooper M.E., Wilkinson-Berka J.L. Retinal angiogenesis is mediated by an interaction between the angiotensin type 2 receptor, VEGF, and angiopoietin. Am J Pathol. 2003;163:879–887. doi: 10.1016/S0002-9440(10)63448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young T.L., Anthony D.C., Pierce E., Foley E., Smith L.E. Histopathology and vascular endothelial growth factor in untreated and diode laser-treated retinopathy of prematurity. J AAPOS. 1997;1:105–110. doi: 10.1016/s1091-8531(97)90008-2. [DOI] [PubMed] [Google Scholar]
- 15.Sonmez K., Drenser K.A., Capone A., Jr., Trese M.T. Vitreous levels of stromal cell-derived factor 1 and vascular endothelial growth factor in patients with retinopathy of prematurity. Ophthalmology. 2008;115:1065–1070. doi: 10.1016/j.ophtha.2007.08.050. [DOI] [PubMed] [Google Scholar]
- 16.Adamis A.P., Miller J.W., Bernal M.T., D’Amico D.J., Folkman J., Yeo T.K., Yeo K.T. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994;118:445–450. doi: 10.1016/s0002-9394(14)75794-0. [DOI] [PubMed] [Google Scholar]
- 17.Clermont A.C., Aiello L.P., Mori F., Aiello L.M., Bursell S.E. Vascular endothelial growth factor and severity of nonproliferative diabetic retinopathy mediate retinal hemodynamics in vivo: a potential role for vascular endothelial growth factor in the progression of nonproliferative diabetic retinopathy. Am J Ophthalmol. 1997;124:433–446. doi: 10.1016/s0002-9394(14)70860-8. [DOI] [PubMed] [Google Scholar]
- 18.Bhutto I.A., McLeod D.S., Hasegawa T., Kim S.Y., Merges C., Tong P., Lutty G.A. Pigment epithelium-derived factor (PEDF) and vascular endothelial growth factor (VEGF) in aged human choroid and eyes with age-related macular degeneration. Exp Eye Res. 2006;82:99–110. doi: 10.1016/j.exer.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Churchill A.J., Carter J.G., Lovell H.C., Ramsden C., Turner S.J., Yeung A., Escardo J., Atan D. VEGF polymorphisms are associated with neovascular age-related macular degeneration. Hum Mol Genet. 2006;15:2955–2961. doi: 10.1093/hmg/ddl238. [DOI] [PubMed] [Google Scholar]
- 20.Rosenfeld P.J., Brown D.M., Heier J.S., Boyer D.S., Kaiser P.K., Chung C.Y., Kim R.Y., MARINA Study Group Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 21.Chan-Ling T., Halasz P., Stone J. Development of retinal vasculature in the cat: processes and mechanisms. Curr Eye Res. 1990;9:459–478. doi: 10.3109/02713689008999612. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y., Porat R.M., Alon T., Keshet E., Stone J. Tissue oxygen levels control astrocyte movement and differentiation in developing retina. Brain Res Dev Brain Res. 1999;118:135–145. doi: 10.1016/s0165-3806(99)00140-6. [DOI] [PubMed] [Google Scholar]
- 23.Baba T., McLeod D.S., Edwards M.M., Merges C., Sen T., Sinha D., Lutty G.A. VEGF 165b in the developing vasculatures of the fetal human eye. Dev Dyn. 2012;241:595–607. doi: 10.1002/dvdy.23743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y., Berendsen A.D., Jia S., Lotinun S., Baron R., Ferrara N., Olsen B.R. Intracellular VEGF regulates the balance between osteoblast and adipocyte differentiation. J Clin Invest. 2012;122:3101–3113. doi: 10.1172/JCI61209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saint-Geniez M., Maldonado A.E., D’Amore P.A. VEGF expression and receptor activation in the choroid during development and in the adult. Invest Ophthalmol Vis Sci. 2006;47:3135–3142. doi: 10.1167/iovs.05-1229. [DOI] [PubMed] [Google Scholar]
- 26.Hu J., Blair M.P., Shapiro M.J., Lichtenstein S.J., Galasso J.M., Kapur R. Reactivation of retinopathy of prematurity after bevacizumab injection. Arch Ophthalmol. 2012;130:1000–1006. doi: 10.1001/archophthalmol.2012.592. [DOI] [PubMed] [Google Scholar]
- 27.McCloskey M., Wang H., Jiang Y., Smith G.W., Strange J., Hartnett M.E. Anti-VEGF antibody leads to later atypical intravitreous neovascularization and activation of angiogenic pathways in a rat model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2013;54:2020–2026. doi: 10.1167/iovs.13-11625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holmes J.M., Duffner L.A. The effect of postnatal growth retardation on abnormal neovascularization in the oxygen exposed neonatal rat. Curr Eye Res. 1996;15:403–409. doi: 10.3109/02713689608995831. [DOI] [PubMed] [Google Scholar]
- 29.Cunningham S., Fleck B.W., Elton R.A., Mclntosh N. Transcutaneous oxygen levels in retinopathy of prematurity. Lancet. 1995;346:1464–1465. doi: 10.1016/s0140-6736(95)92475-2. [DOI] [PubMed] [Google Scholar]
- 30.Budd S.J., Thompson H., Hartnett M.E. Association of retinal vascular endothelial growth factor with avascular retina in a rat model of retinopathy of prematurity. Arch Ophthalmol. 2010;128:1014–1021. doi: 10.1001/archophthalmol.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandercoe T.M., Geller S.F., Hendrickson A.E., Stone J., Provis J.M. VEGF expression by ganglion cells in central retina before formation of the foveal depression in monkey retina: evidence of developmental hypoxia. J Comp Neurol. 2003;462:42–54. doi: 10.1002/cne.10705. [DOI] [PubMed] [Google Scholar]
- 32.Behzadian M.A., Wang X.L., Shabrawey M., Caldwell R.B. Effects of hypoxia on glial cell expression of angiogenesis-regulating factors VEGF and TGF-beta. Glia. 1998;24:216–225. [PubMed] [Google Scholar]
- 33.Saint-Geniez M., Kurihara T., Sekiyama E., Maldonado A.E., D’Amore P.A. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc Natl Acad Sci U S A. 2009;106:18751–18756. doi: 10.1073/pnas.0905010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bai Y., Ma J.X., Guo J., Wang J., Zhu M., Chen Y., Le Y.Z. Müller cell-derived VEGF is a significant contributor to retinal neovascularization. J Pathol. 2009;219:446–454. doi: 10.1002/path.2611. [DOI] [PubMed] [Google Scholar]
- 35.Pierce E.A., Avery R.L., Foley E.D., Aiello L.P., Smith L.E.H. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci U S A. 1995;92:905–909. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weidemann A., Krohne T.U., Aguilar E., Kurihara T., Takeda N., Dorrell M.I., Simon M.C., Haase V.H., Friedlander M., Johnson R.S. Astrocyte hypoxic response is essential for pathological but not developmental angiogenesis of the retina. Glia. 2010;58:1177–1185. doi: 10.1002/glia.20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hartnett M.E., Penn J.S. Mechanisms and management of retinopathy of prematurity. N Engl J Med. 2012;367:2515–2526. doi: 10.1056/NEJMra1208129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Penn J.S., Henry M.M., Tolman B.L. Exposure to alternating hypoxia and hyperoxia causes severe proliferative retinopathy in the newborn rat. Pediatr Res. 1994;36:724–731. doi: 10.1203/00006450-199412000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Kleinman M.E., Yamada K., Takeda A., Chandrasekaran V., Nozaki M., Baffi J.Z., Albuquerque R.J.C., Yamasaki S., Itaya M., Pan Y., Appukuttan B., Gibbs D., Yang Z., Kariko K., Ambati B.K., Wilgus T., DiPietro L.A., Sakurai E., Zhang K., Smith J.R., Taylor E.W., Ambati J. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature. 2008;452:591–597. doi: 10.1038/nature06765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greenberg K.P., Geller S.F., Schaffer D.V., Flannery J.G. Targeted transgene expression in Muller glia of normal and diseased retinas using lentiviral vectors. Invest Ophthalmol Vis Sci. 2007;48:1844–1852. doi: 10.1167/iovs.05-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geisen P., Peterson L.J., Martiniuk D., Uppal A., Saito Y., Hartnett M.E. Neutralizing antibody to VEGF reduces intravitreous neovascularization and may not interfere with ongoing intraretinal vascularization in a rat model of retinopathy of prematurity. Mol Vis. 2008;14:345–357. [PMC free article] [PubMed] [Google Scholar]
- 42.Allegaert K., Vanhole C., Casteels I., Naulaers G., Debeer A., Cossey V., Devlieger H. Perinatal growth characteristics and associated risk of developing threshold retinopathy of prematurity. J AAPOS. 2003;7:34–37. doi: 10.1067/mpa.2003.S1091853102420150. [DOI] [PubMed] [Google Scholar]
- 43.Hartnett M.E. Studies on the pathogenesis of avascular retina and neovascularization into the vitreous in peripheral severe retinopathy of prematurity (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2010;108:96–119. [PMC free article] [PubMed] [Google Scholar]
- 44.McColm J.R., Geisen P., Hartnett M.E. VEGF isoforms and their expression after a single episode of hypoxia or repeated fluctuations between hyperoxia and hypoxia: relevance to clinical ROP. Mol Vis. 2004;10:512–520. [PMC free article] [PubMed] [Google Scholar]
- 45.Stegmeier F., Hu G., Rickles R.J., Hannon G.J., Elledge S.J. A lentiviral microRNA-based system for single-copy polymerase II-regulated RNA interference in mammalian cells. Proc Natl Acad Sci U S A. 2005;102:13212–13217. doi: 10.1073/pnas.0506306102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giering J.C., Grimm D., Storm T.A., Kay M.A. Expression of shRNA from a tissue-specific pol II promoter is an effective and safe RNAi therapeutic. Mol Ther. 2008;16:1630–1636. doi: 10.1038/mt.2008.144. [DOI] [PubMed] [Google Scholar]
- 47.Tian L., Yang P., Lei B., Shao J., Wang C., Xiang Q., Wei L., Peng Z., Kijlstra A. AAV2-mediated subretinal gene transfer of hIFN-alpha attenuates experimental autoimmune uveoretinitis in mice. PLoS One. 2011;6:e19542. doi: 10.1371/journal.pone.0019542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen W., Fruttiger M., Zhu L., Chung S.H., Barnett N.L., Kirk J.K., Lee S., Coorey N.J., Killingsworth M., Sherman L.S., Gillies M.C. Conditional Müller cell ablation causes independent neuronal and vascular pathologies in a novel transgenic model. J Neurosci. 2012;32:15715–15727. doi: 10.1523/JNEUROSCI.2841-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Knockdown of Müller cell–up-regulated VEGFA in the 50/10 OIR model permits physiological-appearing retinal vascularization. Representative retinal flat mounts at p18 after intravitreal injection of anti-VEGF antibody given at postnatal day 12 (p12; left panel) or subretinal injection of lentivirus-delivered VEGF-shRNA given at p8 (right panel).