Abstract
Diabetes mellitus is a disease with considerable morbidity and mortality worldwide. Breakdown of the blood–retinal barrier and leakage from the retinal vasculature leads to diabetic macular edema, an important cause of vision loss in patients with diabetes. Although epidemiologic studies and randomized clinical trials suggest that glycemic control plays a major role in the development of vascular complications of diabetes, insulin therapies for control of glucose metabolism cannot prevent long-term retinal complications. The phenomenon of temporary paradoxical worsening of diabetic macular edema after insulin treatment has been observed in a number of studies. In prospective studies on non–insulin-dependent (type 2) diabetes mellitus patients, a change in treatment from oral drugs to insulin was often associated with a significant increased risk of retinopathy progression and visual impairment. Although insulin therapies are critical for regulation of the metabolic disease, their role in the retina is controversial. In this study with diabetic mice, insulin treatment resulted in increased vascular leakage apparently mediated by betacellulin and signaling via the epidermal growth factor (EGF) receptor. In addition, treatment with EGF receptor inhibitors reduced retinal vascular leakage in diabetic mice on insulin. These findings provide unique insight into the role of insulin signaling in mediating retinal effects in diabetes and open new avenues for therapeutics to treat the retinal complications of diabetes mellitus.
Diabetic maculopathy, an important cause of vision loss in patients with type 2 diabetes, is characterized by hyperpermeability of retinal blood vessels and subsequent formation of macular edema and hard exudates. Although the increase in retinal vascular permeability occurs both diffusely and in focal regions, the basic physiological defect that causes retinal vascular leakage is unknown. The blood–retinal barrier (BRB) isolates the retina from the bloodstream, establishing a favorable environmental milieu with the regulation of ionic balance, nutrient availability, and blockage of potentially toxic molecules that allows for optimal retinal function. The BRB consists of an inner BRB, formed by endothelial cells lining the retinal blood vessels and the outer BRB formed by the retinal pigment epithelium (RPE), a layer of epithelial cells between the retina and the non-neuronal choroid.1,2 Disruption of the BRB is an important feature of diabetic retinopathy.
Based on data from the Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR), a prospective population-based cohort study of patients with type 1 and 2 diabetes mellitus, the prevalence of clinically significant macular edema is 5.9% for type 1 and 7.5% for type 2 diabetes.3 Although epidemiologic studies and randomized clinical trials suggest that glycemic control plays a major role in the development of vascular complications of diabetes,4 insulin therapies for control of glucose metabolism may not prevent long-term complications.5,6 Even though both laser photocoagulation and anti-VEGF therapies have shown significant promise in the treatment of proliferating vessels in proliferative diabetic retinopathy, diabetic macular edema (DME) appears to be more resistant to these treatment approaches, suggesting that other factors might contribute to this complication. We have recently reported the potential role of betacellulin (Btc) in inducing retinal vascular permeability in diabetes.7 Clinical trials and other studies have determined that initiation of acute intensive insulin therapy in patients with long-standing poor glycemic control results in a transient worsening of diabetic retinopathy.8–13 A change in treatment from oral drugs to insulin in patients with non–insulin-dependent (type 2) diabetes mellitus was associated with a significantly increased risk of retinopathy progression and visual impairment.14–16 In addition, it has been reported that patients who undergo total pancreatectomy for cancer develop severe diabetes because of the complete absence of insulin but rarely if ever develop proliferative diabetic retinopathy,17 even when they survive for more than one or two decades. These reports led us to model and evaluate the pathophysiological effects of insulin on the retinal vasculature and the potential crosstalk between insulin and Btc in the regulation of retinal vascular permeability.
Materials and Methods
Materials
Recombinant human vascular endothelial growth factor (VEGF) was a kind gift from Genentech (South San Francisco, CA). Recombinant mouse Btc was from R&D Systems (Minneapolis. MN). Goat polyclonal anti-Btc antibody (sc-5802), goat polyclonal anti-actin antibody (sc-1615), rabbit polyclonal anti–zonula occludens-1 (anti–ZO-1) antibody (sc-10804), horseradish peroxidase–conjugated anti-goat IgG (sc-2353), normal rabbit IgG (sc-2027), and normal goat IgG (sc-2028) were from Santa Cruz Biotechnology (Santa Cruz, CA). Horseradish peroxidase–conjugated anti-rabbit IgG antibody (NA934V) was from GE Healthcare (Chalfont St Giles, UK). Rabbit polyclonal anti–hypoxia-inducible factor-1α (HIF-1α) antibody (NB100-449) was from Novus Biologicals (Littleton, CO). Calbiochem rabbit polyclonal anti-ADAM10 antibody (pc528) was from EMD Millipore (Billerica, MA). Alexa Fluor 488 anti-goat IgG and Alexa Fluor 594 anti-rabbit IgG were from Life Technologies–Invitrogen (Carlsbad, CA). Two EGFR inhibitors were used: Calbiochem AG1478 from EMD Millipore and cetuximab (Erbitux) from Bristol-Myers Squibb (Princeton, NJ).
Animals and Experimental Diabetes or Insulin-Treated Model
All animal studies were approved by the Institutional Animal Care and Use Committee of the Cleveland Clinic and conformed to current NIH guidelines (Guide for the Care and Use of Laboratory Animals in Research, 8th edition, 2011). The animals were cared for in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Visual Research (http://www.arvo.org/about_arvo/policies/statement_for_the_use_of_animals_in_ophthalmic_and_visual_research, last accessed November 2010). Female C57BL/6 mice, 6 to 8 weeks old, were purchased from the Jackson Laboratory (Bar Harbor, ME). For induction of diabetes, mice received three consecutive daily injections of 55 mg/kg streptozotocin (STZ; Sigma-Aldrich, St. Louis, MO) freshly dissolved in 10 mmol/L citrate buffer, pH 4.5. Control nondiabetic mice received three consecutive injections of citrate buffer alone. One week after the first STZ injection, blood glucose levels were evaluated with a glucometer (One Touch Ultra Test strips and One Touch UltraMini glucometer; LifeScan, Milpitas, CA). Mice with blood glucose levels greater than 250 mg/dL were deemed diabetic. The animals were then assigned to the various treatment groups. Insulin pellets were implanted 3 weeks after the first STZ injection. Cetuximab (1 mg per mouse) was administered i.p. once a week for two consecutive weeks, with the first dose being given 3 weeks after the first STZ injection. Animals were euthanized and examined 2 weeks after the first cetuximab dose.
Implantation of Insulin Pellets
Implantation of insulin pellets was performed as described previously.18 In brief, mice were anesthetized with isoflurane, and one controlled-release insulin implant (LinBit, Sustained Release Insulin Implant; LinShin Canada Inc., Toronto, ON, Canada) was placed in the subcutaneous space of their backs.
Determination of Protein Expression by Immunoblot Analysis
Cells and mouse retina were lysed on ice for 20 minutes in lysis buffer consisting of 1% NP-40, 50 mmol/L Tris/HCl (pH 7.5), 150 mmol/L NaCl, and protease inhibitor cocktail (cOmplete, Mini; Roche Applied Bioscience, Mannheim, Germany). Lysates were clarified by centrifugation at 18,363 × g rpm for 10 minutes at 4°C and subjected to SDS-PAGE and Western blotting onto a nitrocellulose or polyvinylidene difluoride membrane (filter paper sandwich; Life Technologies–Invitrogen). Immunoreactive bands were visualized after sequential incubation with a primary antibody, either horseradish peroxidase–conjugated anti-goat or anti-rabbit antibody as a secondary antibody, and enhanced chemiluminescence Western blotting detection reagents (ECL Plus Western blotting detection system; GE Healthcare) according to standard procedures. The blots were stripped with Pierce ReStore Western blot stripping buffer (Thermo Fisher Scientific, Rockford, IL) and then were reprobed according to the manufacturer’s instructions. Protein expression was quantified by densitometry (Quantity One software, version 4.2.3; Bio-Rad Laboratories, Hercules, CA).
Cell Culture and Immunofluorescence
Human retinal pigment epithelium cells (ARPE-19; ATCC, Manassas, VA) were grown in Dulbecco’s modified Eagle’s medium/Ham’s F-12 medium supplemented with 10% fetal bovine serum (HyClone; Thermo Scientific, Logan, UT), 50 units/mL penicillin, and 50 μg/mL streptomycin in a 5% CO2–enriched atmosphere with constant humidity. Human retinal endothelium cells (HRECs; ACBR1 181) were grown in CSC complete medium (CSC complete medium kit; Applied Cell Biology Research Institute, Kirkland, WA) with 10% fetal bovine serum, 50 units/mL penicillin and 50 μg/mL streptomycin in a 5% CO2–enriched atmosphere with constant humidity. Human Müller cells (MIO-M1) were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 50 U/mL penicillin, and 50 μg/mL streptomycin in a 5% CO2–enriched atmosphere with constant humidity. Before each experiment, cells were placed in serum-free medium for 24 hours.
On the day of the experiments, reagents were added at the appropriate concentrations and times. For immunofluorescence micrography, cells grown at a density of 2 × 104 cells/mL on 12-mm coverslips were fixed in 10% tricarboxylic acid for 10 minutes at 4°C, then treated with 0.5% Triton X-100 for 15 minutes. To detect Btc, goat polyclonal anti-Btc antibody was used as the primary antibody, and Alexa Fluor 488 anti-goat IgG was used as the secondary antibody. To visualize ZO-1, cells were reacted with rabbit polyclonal anti–ZO-1 antibody as a primary antibody. Alexa Fluor 594 anti-rabbit IgG was used as a secondary antibody. For staining of paraffin sections, slides were deparaffinized in xylene and rehydrated through an alcohol series. Endogenous peroxidase was blocked by immersion in 0.3% hydrogen peroxidase for 30 minutes. Slides were incubated with goat polyclonal anti-Btc antibody followed by Alexa Fluor 488 anti-goat IgG. Samples were imaged using a fluorescence microscope (BX61; Olympus, Tokyo, Japan) equipped with a charge-coupled device monochrome camera (Hamamatsu Photonics, Bridgewater, NJ) or using a Leica TCS SP2 confocal microscope (Leica Microsystems, Wetzlar Germany). Z-stacks were taken during confocal image acquisition.
Statistical Analysis
All experiments were repeated at least three times. Data were analyzed by one-way or two-way nonrepeated analysis of variance followed by post hoc Bonferroni tests for comparison among means. Statistical significance was set at P < 0.05. Data are expressed as means ± SEM.
Results
Insulin Treatment Is a Predictor of Macular Edema in Diabetes
Long-term data on the 10-year incidences of DME from the WESDR in patients with type II diabetes on insulin was 25%, compared with 14% in those with type 2 diabetes not using insulin.19 Although the decrease in the incidence of DME in patients not taking insulin was attributed to the likelihood of those patients having had a shorter duration of diabetes or less severe hyperglycemia, results from the same study evaluating the incidence of DME against disease duration found an increase in DME in patients taking insulin irrespective of disease duration. To address whether insulin is a significant predictor of macular edema, we analyzed the WESDR type 2 diabetes cohort and selected subjects with 15 or more years of diabetes and defined two groups: subjects who had not taken insulin within 6 months of diagnosis and were not currently taking insulin and subjects who had been currently taking insulin for 10 or more years.19 Using logistic regression analysis with prevalence of macular edema as the dependent variable, and adjusting for independent variables including duration of diabetes, glycosylated hemoglobin, systolic blood pressure and patient group, we determined that people with type 2 diabetes using insulin were significantly more likely to have macular edema (P = 0.03; odds ratio = 2.4; 95% confidence interval 1.1, 5.2).
Insulin Treatment in Diabetic Mice Leads to Increased Retinal Vascular Permeability
To determine whether we could model the effect of insulin on the BRB in vivo, we used a mouse model of STZ-induced diabetes and an Evans Blue assay as described previously.7,18 We generated four groups of mice: i) control mice (normoglycemic); ii) control insulin-treated mice (diabetic normoglycemic); iii) STZ-induced diabetic mice not treated with insulin (hyperglycemic; blood glucose >250 mg/dL); and iv) STZ-induced diabetic mice treated with controlled-release insulin implants administered 3 weeks after STZ induction (normoglycemic; blood glucose <200 mg/dL); and (Figure 1A).
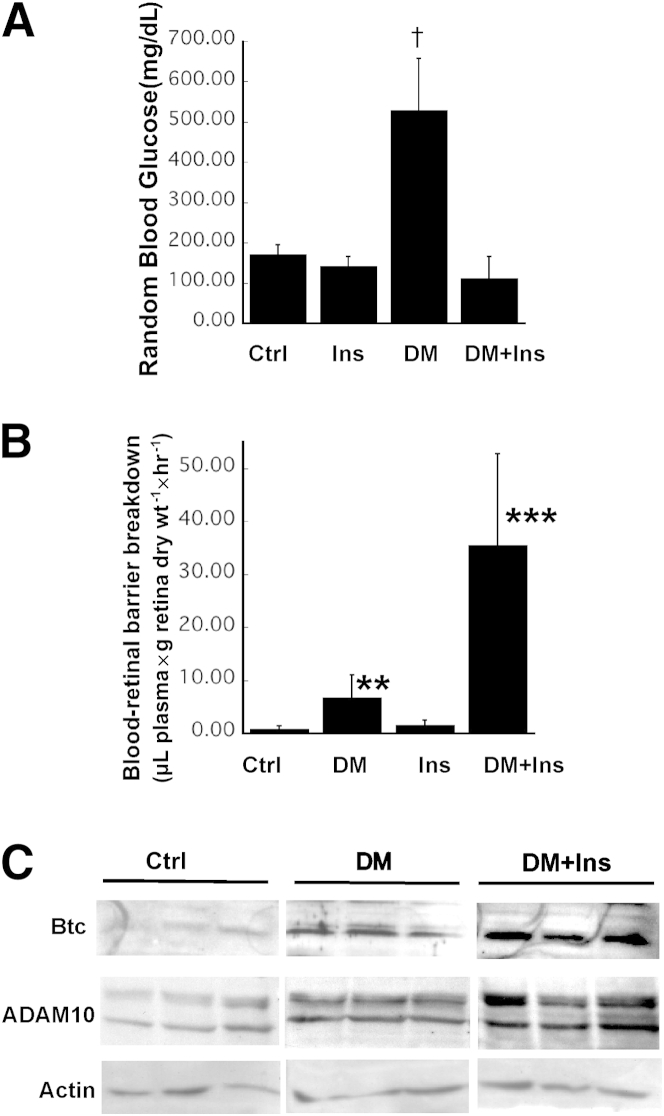
Figure 1.
Insulin treatment exacerbates retinal vascular permeability and induces increased expression of ADAM10 and cleaved Btc in the retina. A: Blood glucose levels in normal (Ctrl), insulin-treated (Ins), STZ-induced diabetic (DM), and STZ-induced diabetic, insulin-treated mice (DM+Ins). B: Leakage of Evans Blue dye from mouse retinal vessels in Ctrl, DM, Ins, and DM+Ins mice was quantitated in whole-mount retinas 1 hour after perfusion with the dye. C: Representative Western blots of soluble Btc, ADAM10, and actin in retinas from Ctrl, DM, and DM+Ins mice. Data are expressed as means ± SEM. n ≥ 5 mice per group. ∗∗∗P = 0.001, †P < 0.0005 analysis of variance.
Retinal vascular permeability was evaluated at 4 weeks after induction of diabetes and was found to be increased from 0.73 μL plasma × g retinal dry wt− 1 × hour−1 in nondiabetic mice (n = 10) to 6.873 μL plasma × g retinal dry wt− 1 × hour−1 in STZ-induced diabetic mice (n = 7) (P < 0.0005). Diabetic mice treated with insulin implants (n = 5) showed a dramatic increase in retinal vascular permeability, from 6.87 to 35.58 μL plasma × g retinal dry wt−1 × hour−1 (P = 0.001) (Figure 1B). These results suggest that increased retinal vascular permeability may not be directly related to hyperglycemia and that intensive insulin therapy in the presence of hyperglycemia may result in significant increase in BRB breakdown, which suggests that this may be a good model for understanding the early worsening of diabetic retinopathy observed after acute intensive insulin therapy in type 1 diabetes patients.20–22
Insulin-Induced Retinal Vascular Permeability Is Mediated via ADAM10 and Btc
We have previously reported that the increased retinal vascular permeability in diabetic mice is regulated by Btc and disintegrin and metalloproteinase domain-containing protein 10 (ADAM10).7 To determine whether insulin treatment in diabetic mice induces increased activation of ADAM10 and Btc, we analyzed retinas from diabetic mice that were treated or untreated with insulin, using Western blot analysis. Both ADAM10 and soluble Btc were increased in retinal tissue from diabetic mice on insulin, compared with diabetic mice not treated with insulin (Figure 1C).
Btc Can Disrupt Tight Junctions in Endothelial Cells and RPE Cells
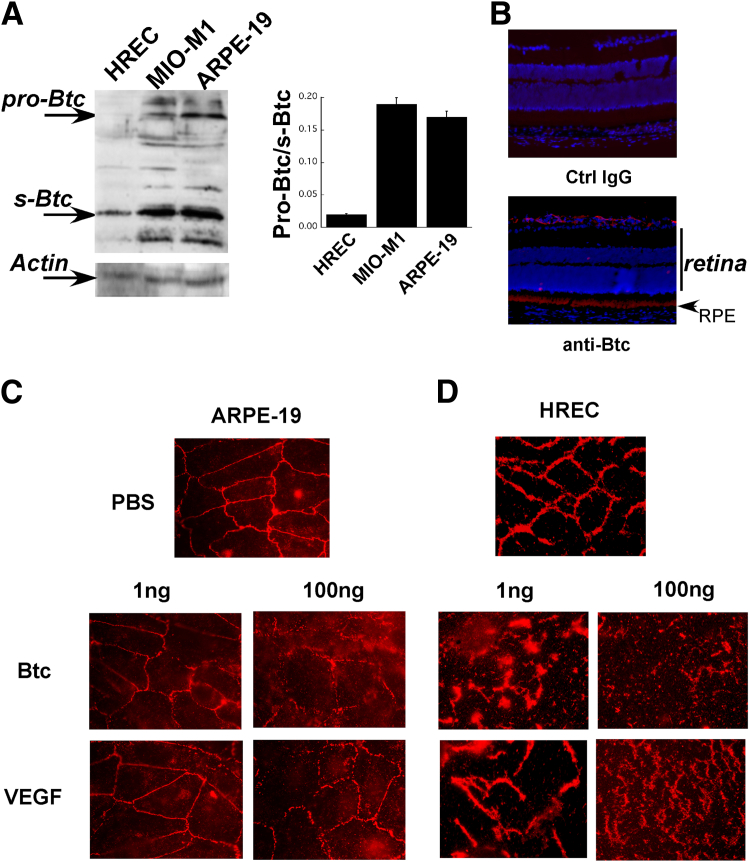
To evaluate whether Btc can disrupt tight junctions in retinal endothelial and pigment epithelial cells, we first determined which cells in the retina express Btc, using Western blot analysis with Btc antibodies on lysates from HRECs, MIO-M1 Müller cells, and ARPE-19 human RPE cells. Müller cells and RPE cells expressed pro-Btc and soluble cleaved Btc, whereas HRECs expressed predominantly soluble Btc (Figure 2A). Immunohistochemistry with polyclonal Btc antibodies using normal mouse retina identified strong expression of Btc in the RPE and astrocytes (Figure 2B). Staining with control nonspecific IgG showed no background staining.
Figure 2.
Btc can disrupt tight junctions in retinal endothelial and pigment epithelial cells. A: Representative immunoblots of pro-Btc and soluble Btc (s-Btc) in HRECs, MIO-M1 human Müller cells, and ARPE-19 human RPE cells, with quantitation of the pro-Btc/s-Btc ratio. B: Localization of Btc in normal mouse retina by immunohistochemistry. C and D: Immunofluorescent ZO-1 expression in ARPE-19 cells (C) and HRECs (D) after exposure to PBS and either Btc (1 or 100 ng) or VEGF (1 or 100 ng) for 24 hours. Data are expressed as means ± SEM. n ≥ 5 mice per group. RPE, retinal pigment epithelium.
We have previously demonstrated that Btc induces retinal vascular leakage in vivo in mice.7 In the present study, we evaluated the effects of Btc on the tight junctions of RPE (Figure 2C) and endothelial cells (Figure 2D). Soluble active Btc was added to confluent ARPE-19 cells (Figure 2C) or HRECs (Figure 2D) at doses of 1 ng or 100 ng and the effects on tight junction (ZO-1) expression were compared with those of similar doses of VEGF. Comparisons were made with ZO-1 expression in control RPE cells (Figure 2C) and HRECs (Figure 2D) exposed to PBS. Btc disrupted tight junctions in HRECs (and to a lesser extent in RPE cells), similar to the disruption induced by VEGF. Although it does appear that HRECs are more sensitive to Btc than are RPE cells in vitro, this remains to be verified in vivo.
Insulin-Mediated Disruption of Tight Junctions via ADAM10 and Btc
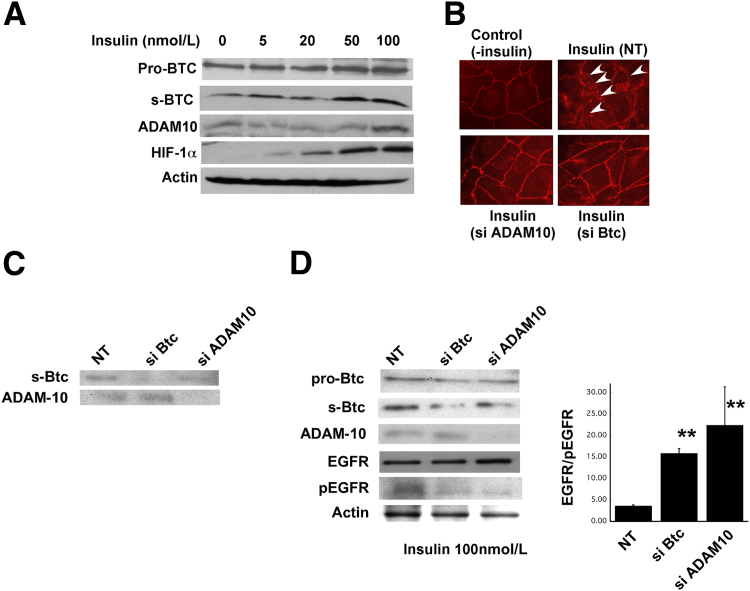
To determine whether insulin can disrupt tight cell junctions in RPE cells, we examined the expression of ZO-1 in confluent ARPE-19 cells after treatment with insulin. The insulin treatment resulted in a disruption of ZO-1 in 24 hours, compared with control untreated cells (Figure 3B). Western blot analysis of soluble Btc and ADAM10 in insulin-treated cells showed an increase in both cleaved soluble Btc and ADAM10 (Figure 3A). Interestingly, HIF-1α was also increased with increasing doses of insulin (Figure 3A), suggesting that insulin might activate ADAM10, which in turn can cleave Btc to its soluble form.
Figure 3.
Inhibition of Btc can prevent insulin-mediated disruption of tight junctions in retinal pigment epithelial cells. A: Representative immunoblots of soluble Btc, ADAM10, HIF-1α, and actin in ARPE-19 human RPE cells exposed to increasing concentrations of insulin. B: Immunofluorescent ZO-1 expression in ARPE-19 cells exposed to insulin in the presence or absence of ADAM10 siRNA or Btc siRNA. Arrowheads indicate the disruption of ZO-1. C: Representative immunoblots of soluble Btc and ADAM10 in control ARPE-19 cells treated with Btc siRNA or ADAM10 siRNA. D: Representative immunoblots of soluble Btc, ADAM10, EGFR, phosphorylated EGFR (pEGFR), and actin in ARPE-19 human RPE cells exposed to insulin (100 nmol/L) in the presence of Btc siRNA or ADAM10 siRNA, with quantitation of the EGFR/pEGFR ratio after no treatment or treatment with Btc siRNA or ADAM10 siRNA. ∗P < 0.05. Data are expressed as means ± SEM. n ≥ 5 mice per group. NT, non-targeting control RNA.
To evaluate whether the increase in Btc is causative for the breakdown of tight junctions we tested whether treatment of RPE cells with Btc siRNA can rescue the insulin-mediated disruption of tight junctions. We exposed cells treated with insulin to Btc siRNA and then examined ZO-1 expression. No disruption of ZO-1 occurred in cells treated with Btc siRNA (Figure 3B). In addition, Western blot analysis of lysates from control cells (Figure 3C) and insulin-treated cells (Figure 3D) showed a decrease in soluble Btc protein in cells treated with Btc siRNA but no effect on ADAM10 protein, which suggests that ADAM10 is upstream of Btc activation.
To ascertain whether inhibiting ADAM10 has the same effect in rescuing insulin-mediated disruption of tight junctions, we added ADAM10 siRNA to cells that had been treated with insulin and evaluated ZO-1 disruption. The inhibition of ADAM10 resulted in an intact tight-junction complex on the surface of the RPE cells (Figure 3B).
EGFR Inhibitor Can Prevent Insulin-Mediated Increase in Retinal Vascular Permeability
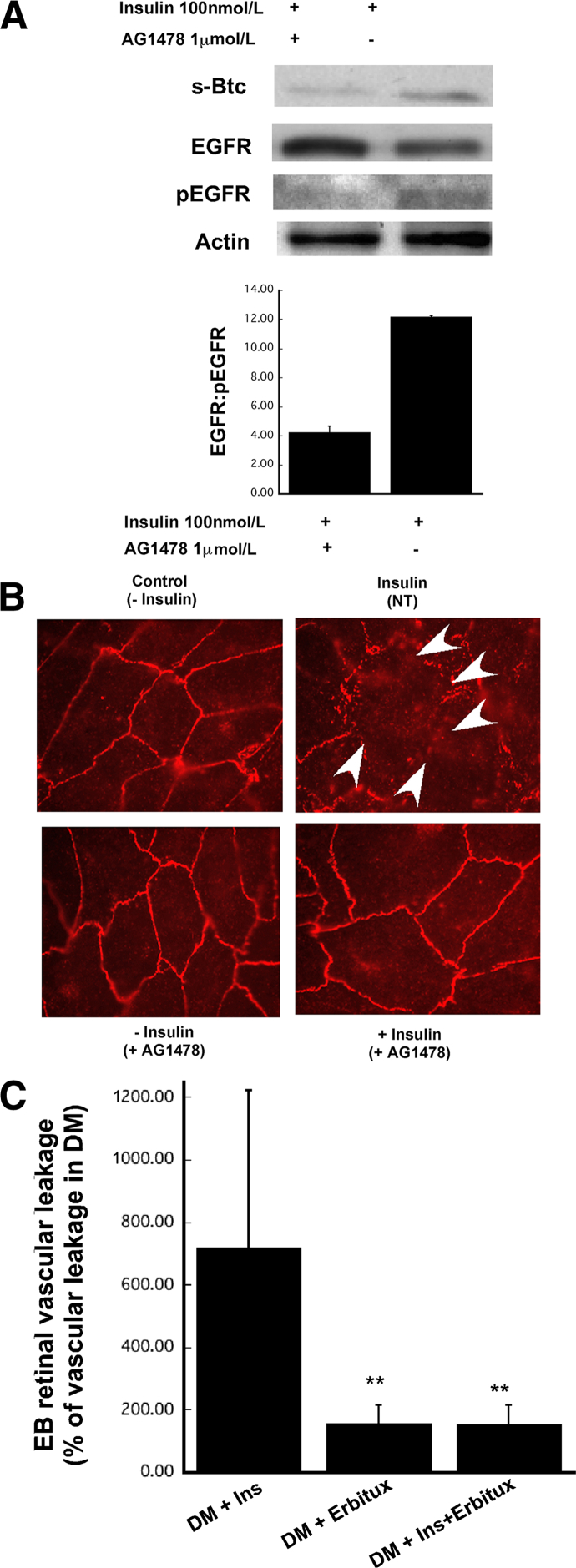
EGF receptors belong to the ErbB family of tyrosine kinase receptors and include EGFR (alias ErbB-1, HER1), ErbB-2 (alias Neu, HER2), ErbB-3 (alias HER3), and ErbB-4 (alias HER4).23 After binding of a ligand, ErbB receptors form homo- or heterodimers, followed by autophosphorylation of tyrosine residues and secondary messenger recruitment. Btc has been shown to bind and signal via all four EGF receptors.24 Although the role of Btc25–34 and EGF receptor signaling35 in pancreatic differentiation has been well documented, its function in the retina has not been explored in detail. We observed that insulin activated RPE cells treated with Btc siRNA or ADAM10 siRNA showed a decrease in EGFR phosphorylation (Figure 3D), which suggests that treatment with an EGFR inhibitor might inhibit insulin-mediated tight-junction disruption and retinal vascular leakage. We therefore treated cells exposed to insulin with AG1478, a tyrosine kinase inhibitor that is specific for EGFR. Western blot analysis after treatment with insulin and AG1478 showed a decrease in EGFR phosphorylation, compared with cells not exposed to AG1478 (Figure 4A). We observed that AG1478 prevented the tight-junction breakdown induced by insulin, compared with cells exposed to insulin but not treated with AG1478 (Figure 4B). We evaluated the efficacy of systemic intraperitoneal administration of cetuximab (a potent EGFR inhibitor) to inhibit retinal vascular leakage in diabetic mice on insulin.
Figure 4.
EGFR inhibitors can prevent insulin-mediated disruption of tight junctions in retinal pigment epithelial cells (in vitro) and retinal vascular leakage in vivo. A: Representative immunoblots of EGFR, pEGFR, and actin in human RPE cell line (ARPE-19) exposed to 100 nmol/L insulin in the presence of EGFR inhibitor AG1478. B: Immunofluorescent ZO-1 expression in ARPE-19 cells exposed to insulin in the presence or absence of 1 μmol/L EGFR inhibitor AG1478. C: Leakage of Evans Blue dye (EB) from retinal vessels of DM+cetuximab, DM+Ins, and DM+Ins+cetuximab mice. The cetuximab dose was 1 mg per mouse. Evans Blue dye leakage was quantitated in whole-mount retinas at 1 hour after perfusion with the dye. Data are expressed as means ± SEM. n ≥ 5 mice per group. ∗∗P = 0.01 versus DM+Ins.
To determine whether EGFR inhibitors can prevent the insulin-induced retinal vascular leakage in mice, we generated four groups of mice: i) diabetic mice without insulin and without cetuximab treatment; ii) diabetic mice with insulin but without cetuximab treatment; iii) diabetic mice without insulin but with cetuximab treatment, and iv) diabetic mice with both insulin and cetuximab treatment. Mice were treated once a week with cetuximab, and retinal vascular leakage was evaluated at week 4 after induction of diabetes. Retinal vascular leakage was calculated as a percentage of vascular leakage observed in untreated diabetic mice. The increase in retinal vascular leakage observed in diabetic mice on insulin was dramatically reduced when diabetic mice were treated with cetuximab (Figure 4C).
Discussion
Macular edema, an important cause of vision impairment in patients with diabetes, is a consequence of increased retinal vascular permeability and breakdown of the BRB. Based on the results of early randomized trials such as the UK Prospective Diabetic Study36 and the Diabetes Control and Complications Trial,37 which compared intensive versus conventional therapies for hyperglycemia and showed the effectiveness of intensive glycemic control in minimizing the risk of developing microvascular complications, the American Diabetes Association recommended a target of <7% for glycosylated hemoglobin (HbA1C) for nonpregnant adults in general.38 Investigators in the ACCORD Trial found a statistically significant increase in overall mortality in the intensive glycemic control group, leading to early termination of this arm of the study.39 At the same time, the ACCORD eye study group reported that the intensive glycemic control therapy significantly reduced the risk of progression of diabetic retinopathy but did not reduce the risk of moderate vision loss.40 Moreover, additional WESDR data indicated that higher amounts of exogenous insulin are related to an increased incidence of macular edema.41 The Epidemiology of Diabetes Interventions and Complications study determined a higher risk for development of complications of DR in patients on conventional insulin therapy even 4 and 10 years after switching to intensive insulin therapy, despite normal HbA1C levels.42,43 Our findings from the present study analyzing the WESDR study samples and normalizing for HbA1C levels also suggest that insulin may be correlated with the incidence of diabetic macular edema independent of blood sugar control. Although our data are preliminary, the findings certainly bring into question the role of blood sugar control in the regulation of retinal vascular leakage.
Adding to this conundrum is the observation that patients with varying degrees of retinopathy often experience a paradoxical worsening of their retinopathy symptoms concomitant with the rapid lowering of high levels of glycemia associated with the onset of insulin therapy to control their hyperglycemia.8–13,44 Such worsening of diabetic retinopathy has been reported to be related to upregulation of insulin-like growth factor (IGF).14,45–47 Nonetheless, the molecular mechanisms that contribute to the early-worsening paradox have remained elusive. Elucidation of these mechanisms should allow the design of therapies to prevent symptoms of early worsening of retinopathy during insulin therapy. An early report in 2002 suggested that acute intensive insulin therapy exacerbates diabetic BRB breakdown via hypoxia-inducible factor-1α and VEGF18; the authors used an STZ mouse model similar to that in the present study.
The present results suggest that the association of macular edema with insulin use may be a consequence of the effect of Btc and EGF receptor signaling in the RPE that regulates the outer BRB. It is conceivable that a subset of patients may be more susceptible to these effects that result in DME.
Crosstalk between the insulin receptor and EGF receptor signaling systems has been reported to play a role in growth and differentiation in Drosophila melanogaster.48 The authors found that phospholipase C-γ activation by the insulin pathway positively regulates EGF pathway during cell growth and negatively regulates the same pathway during differentiation. This implies that there may be other factors contributing to the link between insulin and EGF signaling pathways that may be dependent on tissue type and or physiological homeostatic state. Insulin has also been shown to increase the proteolytic activities of ADAM10 and stimulate the cleavage and release of the extracellular domain of Klotho.49 EGF and Btc are biologically important substrates of ADAM10.50
With the present study, we have identified an additional mechanism involving ADAM10, Btc, and signaling via the EGF receptor for the transient BRB disruption that is a consequence of intensive insulin therapy. In addition, we have demonstrated that EGFR inhibitor can attenuate the increased retinal vascular leakage after insulin therapy. However, given the absence of specific inhibitors to the ADAM10/Btc pathway that could be used in vivo, the role of other pathways of EGF receptor activation that lead to this phenomenon cannot be excluded.
Mice lacking insulin receptors or insulin-like growth factor receptors are resistant to developing retinal neovascularization.51 Insulin edema (the term was coined by Leifer52 in 1928) refers to describe the development of severe acute edema in insulin-treated diabetic patients.53 A report that insulin increases the permeability of subdermal vessels54 suggests that insulin treatment could have global effects on vascular permeability. With the present study, we have demonstrated that insulin can disrupt tight junctions in RPE cells and up-regulate ADAM10 and the subsequent soluble active Btc. That the soluble form of Btc plays a role in the disruption of tight junctions was demonstrated by the ability of Btc siRNA to prevent insulin-induced disruption of tight junctions.
In diabetic mice, insulin signaling in retinal endothelial cells is differentially regulated, relative to insulin signaling in classic target tissues such as liver.55 In STZ-induced diabetic mice and ob/ob mouse retinas, insulin receptor (IR) and insulin receptor substrate-2 (IRS-2) protein and tyrosine phosphorylation were increased by insulin.55 These findings suggest that, in the diabetic state, there may be an increase in the tonic activity of insulin receptor in the retina, which results in activation of ADAM10, cleavage of Btc to its active form, disruption of RPE and/or endothelial tight junctions, retinal vascular leakage, and DME and diabetic retinopathy. This retinal response may be further exacerbated with insulin therapy leading to worsening of symptoms. Binding of insulin to the IR receptor activates the intrinsic tyrosine kinase activity in the β subunit, induces autophosphorylation on tyrosine residues within its tyrosine kinase domain, and initiates a cascade of phosphorylation events on downstream substrates that include insulin receptor substrates (IRS-1, -2, -3, and -4), Grb-2 associated binder (Gab-1), Shc, and Cbl; these in turn recruit other adapter and enzymatic proteins (specifically, phosphatidylinositol 3′-OH kinase and the PI3K and Akt pathway) to propagate signal transduction and induce a biological effect.
Although insulin signaling has been extensively characterized in liver, adipose tissue, and skeletal muscle, its ability to modulate biological activity in the retina has begun to be recognized only in the past decade.56 Retinal insulin receptor kinase activity is equivalent to that of the brain; it does not fluctuate with the feeding/fasting cycle and is maintained in a tonic state of activity. Although immunohistochemistry studies demonstrate a signal for IR in all layers of the retina, specific binding has been identified in retinal endothelial cells, pericytes, and the RPE. However, the controversy in the literature as to whether insulin signaling is accentuated55 or attenuated57 in STZ-induced diabetes remains unresolved. In addition, it is likely that insulin might signal uniquely in different tissues, based on metabolic requirements. If insulin-mediated Btc signaling is specific to retinal tissue, then targeting the retina with ADAM10 blockers or EGFR inhibitors offers a potentially useful therapeutic approach to the insulin-induced retinal vascular leakage seen in some patients with diabetes.
Conclusions
Insulin treatment of diabetes results in increased retinal vascular permeability mediated via the Btc/ADAM10 pathway. Inhibition of EGF signaling and/or ADAM10 may be a useful therapeutic approach in combination with insulin to prevent retinal vascular leakage.
Footnotes
Supported in part by NIH grants EY016490, CA106415, EY015638; Howard Hughes Medical Institute Medical Student Fellowship no. 57007464 (B.S.); a Research to Prevent Blindness challenge grant; a Foundation Fighting Blindness center grant; and a Research to Prevent Blindness Lew R. Wasserman award (B.A.).
References
- 1.Erickson K.K., Sundstrom J.M., Antonetti D.A. Vascular permeability in ocular disease and the role of tight junctions. Angiogenesis. 2007;10:103–117. doi: 10.1007/s10456-007-9067-z. [DOI] [PubMed] [Google Scholar]
- 2.Neuwelt E., Abbott N.J., Abrey L., Banks W.A., Blakley B., Davis T., Engelhardt B., Grammas P., Nedergaard M., Nutt J., Pardridge W., Rosenberg G.A., Smith Q., Drewes L.R. Strategies to advance translational research into brain barriers. Lancet Neurol. 2008;7:84–96. doi: 10.1016/S1474-4422(07)70326-5. [DOI] [PubMed] [Google Scholar]
- 3.Hirai F.E., Knudtson M.D., Klein B.E.K., Klein R. Clinically significant macular edema and survival in type 1 and type 2 diabetes. Am J Ophthalmol. 2008;145:700–706. doi: 10.1016/j.ajo.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Diabetes Control and Complications Trial Research Group Implications of the Diabetes Control and Complications Trial. Diabetes. 1993;42:1555–1558. doi: 10.2337/diab.42.11.1555. [DOI] [PubMed] [Google Scholar]
- 5.Keenan H.A., Costacou T., Sun J.K., Doria A., Cavellerano J., Coney J., Orchard T.J., Aiello L.P., King G.L. Clinical factors associated with resistance to microvascular complications in diabetic patients of extreme disease duration: the 50-year medalist study. Diabetes Care. 2007;30:1995–1997. doi: 10.2337/dc06-2222. [DOI] [PubMed] [Google Scholar]
- 6.Wong T.Y., Liew G., Tapp R.J., Schmidt M.I., Wang J.J., Mitchell P., Klein R., Klein B.E.K., Zimmet P., Shaw J. Relation between fasting glucose and retinopathy for diagnosis of diabetes: three population-based cross-sectional studies. Lancet. 2008;371:736–743. doi: 10.1016/S0140-6736(08)60343-8. [Erratum appeared in Lancet 2008, 371:1838] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anand-Apte B., Ebrahem Q., Cutler A., Farage E., Sugimoto M., Hollyfield J., Folkman J. Betacellulin induces increased retinal vascular permeability in mice. PLoS One. 2010;5:e13444. doi: 10.1371/journal.pone.0013444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Kroc Collaborative Study Group Blood glucose control and the evolution of diabetic retinopathy and albuminuria–a preliminary multicenter trial. N Engl J Med. 1984;311:365–372. doi: 10.1056/NEJM198408093110604. [DOI] [PubMed] [Google Scholar]
- 9.The Kroc Collaborative Study Group Diabetic retinopathy after two years of intensified insulin treatment. Follow-up of the Kroc Collaborative Study. JAMA. 1988;260:37–41. doi: 10.1001/jama.1988.03410010045032. [DOI] [PubMed] [Google Scholar]
- 10.Keen H. Normoglycaemic re-entry and diabetic complications. Diabet Med. 1984;1:85–87. doi: 10.1111/j.1464-5491.1984.tb01933.x. [DOI] [PubMed] [Google Scholar]
- 11.Lauritzen T., Frost-Larsen K., Larsen H.W., Deckert T. Effect of 1 year of near-normal blood glucose levels on retinopathy in insulin-dependent diabetics. Lancet. 1983;1:200–204. doi: 10.1016/s0140-6736(83)92585-0. [DOI] [PubMed] [Google Scholar]
- 12.Lawson P.M., Champion M.C., Canny C., Kingsley R., White M.C., Dupré J., Kohner E.M. Continuous subcutaneous insulin infusion (CSII) does not prevent progression of proliferative and preproliferative retinopathy. Br J Ophthalmol. 1982;66:762–766. doi: 10.1136/bjo.66.12.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chantelau E., Kohner E.M. Why some cases of retinopathy worsen when diabetic control improves. BMJ. 1997;315:1105–1106. doi: 10.1136/bmj.315.7116.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henricsson M., Berntorp K., Berntorp E., Fernlund P., Sundkvist G. Progression of retinopathy after improved metabolic control in type 2 diabetic patients. Relation to IGF-1 and hemostatic variables. Diabetes Care. 1999;22:1944–1949. doi: 10.2337/diacare.22.12.1944. [DOI] [PubMed] [Google Scholar]
- 15.Henricsson M., Janzon L., Groop L. Progression of retinopathy after change of treatment from oral antihyperglycemic agents to insulin in patients with NIDDM. Diabetes Care. 1995;18:1571–1576. doi: 10.2337/diacare.18.12.1571. [DOI] [PubMed] [Google Scholar]
- 16.Henricsson M., Nilsson A., Janzon L., Groop L. The effect of glycaemic control and the introduction of insulin therapy on retinopathy in non-insulin-dependent diabetes mellitus. Diabet Med. 1997;14:123–131. doi: 10.1002/(SICI)1096-9136(199702)14:2<123::AID-DIA306>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 17.Tiengo A., Segato T., Briani G., Setti A., Del Prato S., Devide A., Padovan D., Virgili F., Crepaldi G. The presence of retinopathy in patients with secondary diabetes following pancreatectomy or chronic pancreatitis. Diabetes Care. 1983;6:570–574. doi: 10.2337/diacare.6.6.570. [DOI] [PubMed] [Google Scholar]
- 18.Poulaki V., Qin W., Joussen A.M., Hurlbut P., Wiegand S.J., Rudge J., Yancopoulos G.D., Adamis A.P. Acute intensive insulin therapy exacerbates diabetic blood-retinal barrier breakdown via hypoxia-inducible factor-1alpha and VEGF. J Clin Invest. 2002;109:805–815. doi: 10.1172/JCI13776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein R., Moss S.E., Klein B.E.K., Davis M.D., DeMets D.L. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. XI. The incidence of macular edema. Ophthalmology. 1989;96:1501–1510. doi: 10.1016/s0161-6420(89)32699-6. [DOI] [PubMed] [Google Scholar]
- 20.The Diabetes Control and Complications Trial Research Group The effect of intensive diabetes treatment on the progression of diabetic retinopathy in insulin-dependent diabetes mellitus. Arch Ophthalmol. 1995;113:36–51. doi: 10.1001/archopht.1995.01100010038019. [DOI] [PubMed] [Google Scholar]
- 21.Agardh C.D., Eckert B., Agardh E. Irreversible progression of severe retinopathy in young type I insulin-dependent diabetes mellitus patients after improved metabolic control. J Diabetes Complications. 1992;6:96–100. doi: 10.1016/1056-8727(92)90018-g. [DOI] [PubMed] [Google Scholar]
- 22.Brinchmann-Hansen O., Dahl-Jørgensen K., Sandvik L., Hanssen K.F. Blood glucose concentrations and progression of diabetic retinopathy: the seven year results of the Oslo study. BMJ. 1992;304:19–22. doi: 10.1136/bmj.304.6818.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Citri A., Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 24.Shin H.S., Lee H.J., Nishida M., Lee M.S., Tamura R., Yamashita S., Matsuzawa Y., Lee I.K., Koh G.Y. Betacellulin and amphiregulin induce upregulation of cyclin D1 and DNA synthesis activity through differential signaling pathways in vascular smooth muscle cells. Circ Res. 2003;93:302–310. doi: 10.1161/01.RES.0000086803.64109.9E. [DOI] [PubMed] [Google Scholar]
- 25.Brun T., Duhamel D.L., Hu He K.H., Wollheim C.B., Gauthier B.R. The transcription factor PAX4 acts as a survival gene in INS-1E insulinoma cells. Oncogene. 2007;26:4261–4271. doi: 10.1038/sj.onc.1210205. [DOI] [PubMed] [Google Scholar]
- 26.Buteau J., Foisy S., Joly E., Prentki M. Glucagon-like peptide 1 induces pancreatic beta-cell proliferation via transactivation of the epidermal growth factor receptor. Diabetes. 2003;52:124–132. doi: 10.2337/diabetes.52.1.124. [DOI] [PubMed] [Google Scholar]
- 27.Demeterco C., Beattie G.M., Dib S.A., Lopez A.D., Hayek A. A role for activin A and betacellulin in human fetal pancreatic cell differentiation and growth. J Clin Endocrinol Metab. 2000;85:3892–3897. doi: 10.1210/jcem.85.10.6848. [DOI] [PubMed] [Google Scholar]
- 28.Huotari M.A., Miettinen P.J., Palgi J., Koivisto T., Ustinov J., Harari D., Yarden Y., Otonkoski T. ErbB signaling regulates lineage determination of developing pancreatic islet cells in embryonic organ culture. Endocrinology. 2002;143:4437–4446. doi: 10.1210/en.2002-220382. [DOI] [PubMed] [Google Scholar]
- 29.Kawaguchi M., Hosotani R., Kogire M., Ida J., Doi R., Koshiba T., Miyamoto Y., Tsuji S., Nakajima S., Kobayashi H., Masui T., Imamura M. Auto-induction and growth stimulatory effect of betacellulin in human pancreatic cancer cells. Int J Oncol. 2000;16:37–41. [PubMed] [Google Scholar]
- 30.Kojima H., Fujimiya M., Matsumura K., Younan P., Imaeda H., Maeda M., Chan L. NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nat Med. 2003;9:596–603. doi: 10.1038/nm867. [DOI] [PubMed] [Google Scholar]
- 31.Li L., Yi Z., Seno M., Kojima I. Activin A and betacellulin: effect on regeneration of pancreatic beta-cells in neonatal streptozotocin-treated rats. Diabetes. 2004;53:608–615. doi: 10.2337/diabetes.53.3.608. [DOI] [PubMed] [Google Scholar]
- 32.Mashima H., Ohnishi H., Wakabayashi K., Mine T., Miyagawa J., Hanafusa T., Seno M., Yamada H., Kojima I. Betacellulin and activin A coordinately convert amylase-secreting pancreatic AR42J cells into insulin-secreting cells. J Clin Invest. 1996;97:1647–1654. doi: 10.1172/JCI118591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogata T., Dunbar A.J., Yamamoto Y., Tanaka Y., Seno M., Kojima I. Betacellulin-delta4, a novel differentiation factor for pancreatic beta-cells, ameliorates glucose intolerance in streptozotocin-treated rats. Endocrinology. 2005;146:4673–4681. doi: 10.1210/en.2005-0456. [DOI] [PubMed] [Google Scholar]
- 34.Watada H., Kajimoto Y., Miyagawa J., Hanafusa T., Hamaguchi K., Matsuoka T., Yamamoto K., Matsuzawa Y., Kawamori R., Yamasaki Y. PDX-1 induces insulin and glucokinase gene expressions in alphaTC1 clone 6 cells in the presence of betacellulin. Diabetes. 1996;45:1826–1831. doi: 10.2337/diab.45.12.1826. [DOI] [PubMed] [Google Scholar]
- 35.Miettinen P., Ormio P., Hakonen E., Banerjee M., Otonkoski T. EGF receptor in pancreatic beta-cell mass regulation. Biochem Soc Trans. 2008;36:280–285. doi: 10.1042/BST0360280. [DOI] [PubMed] [Google Scholar]
- 36.The U.K. Prospective Diabetes Study (UKPDS) Research Group: Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [Erratum appeared in Lancet 1999, 354:602] [PubMed] [Google Scholar]
- 37.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 38.American Diabetes Association Summary of revisions for the 2009 clinical practice recommendations. Diabetes Care. 2009;32(Suppl 1):S3–S5. doi: 10.2337/dc09-S003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ismail-Beigi F., Craven T., Banerji M.A., Basile J., Calles J., Cohen R.M., Cuddihy R., Cushman W.C., Genuth S., Grimm R.H., Jr., Hamilton B.P., Hoogwerf B., Karl D., Katz L., Krikorian A., O'Connor P., Pop-Busui R., Schubart U., Simmons D., Taylor H., Thomas A., Weiss D., Hramiak I. ACCORD Trial Group: Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419–430. doi: 10.1016/S0140-6736(10)60576-4. [Erratum appeared in Lancet 2010, 376:1466] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chew E.Y., Ambrosius W.T., Davis M.D., Danis R.P., Gangaputra S., Greven C.M., Hubbard L., Esser B.A., Lovato J.F., Perdue L.H., Goff D.C., Jr., Cushman W.C., Ginsberg H.N., Elam M.B., Genuth S., Gerstein H.C., Schubart U., Fine L.J. ACCORD Study Group; ACCORD Eye Study Group: Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363:233–244. doi: 10.1056/NEJMoa1001288. [Erratum appeared in N Engl J Med 2011, 364:190 and in N Engl J Med 2012, 367:2458] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klein R., Klein B.E.K., Moss S.E. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. XVI. The relationship of C-peptide to the incidence and progression of diabetic retinopathy. Diabetes. 1995;44:796–801. doi: 10.2337/diab.44.7.796. [DOI] [PubMed] [Google Scholar]
- 42.The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000;342:381–389. doi: 10.1056/NEJM200002103420603. [Erratum appeared in N Engl J Med 2000, 342:1376] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.White N.H., Sun W., Cleary P.A., Danis R.P., Davis M.D., Hainsworth D.P., Hubbard L.D., Lachin J.M., Nathan D.M. Prolonged effect of intensive therapy on the risk of retinopathy complications in patients with type 1 diabetes mellitus: 10 years after the Diabetes Control and Complications Trial. Arch Ophthalmol. 2008;126:1707–1715. doi: 10.1001/archopht.126.12.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dahl-Jørgensen K., Brinchmann-Hansen O., Hanssen K.F., Sandvik L., Aagenaes O. Rapid tightening of blood glucose control leads to transient deterioration of retinopathy in insulin dependent diabetes mellitus: the Oslo study. Br Med J (Clin Res Ed) 1985;290:811–815. doi: 10.1136/bmj.290.6471.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chantelau E. Evidence that upregulation of serum IGF-1 concentration can trigger acceleration of diabetic retinopathy. Br J Ophthalmol. 1998;82:725–730. doi: 10.1136/bjo.82.7.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chantelau E., Meyer-Schwickerath R., Klabe K. Downregulation of serum IGF-1 for treatment of early worsening of diabetic retinopathy: a long-term follow-up of two cases. Ophthalmologica. 2010;224:243–246. doi: 10.1159/000260231. [DOI] [PubMed] [Google Scholar]
- 47.Henricsson M., Berntorp K., Fernlund P., Sundkvist G. Progression of retinopathy in insulin-treated type 2 diabetic patients. Diabetes Care. 2002;25:381–385. doi: 10.2337/diacare.25.2.381. [DOI] [PubMed] [Google Scholar]
- 48.Murillo-Maldonado J.M., Zeineddine F.B., Stock R., Thackeray J., Riesgo-Escovar J.R. Insulin receptor-mediated signaling via phospholipase C-gamma regulates growth and differentiation in Drosophila. PLoS One. 2011;6:e28067. doi: 10.1371/journal.pone.0028067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen C.D., Podvin S., Gillespie E., Leeman S.E., Abraham C.R. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci USA. 2007;104:19796–19801. doi: 10.1073/pnas.0709805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sahin U., Weskamp G., Kelly K., Zhou H.M., Higashiyama S., Peschon J., Hartmann D., Saftig P., Blobel C.P. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J Cell Biol. 2004;164:769–779. doi: 10.1083/jcb.200307137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kondo T., Vicent D., Suzuma K., Yanagisawa M., King G.L., Holzenberger M., Kahn C.R. Knockout of insulin and IGF-1 receptors on vascular endothelial cells protects against retinal neovascularization. J Clin Invest. 2003;111:1835–1842. doi: 10.1172/JCI17455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leifer A. A case of insulin edema. J Am Med Assoc. 1928;90:610–611. [Google Scholar]
- 53.Evans D.J., Pritchard-Jones K., Trotman-Dickenson B. Insulin oedema. Postgrad Med J. 1986;62:665–668. doi: 10.1136/pgmj.62.729.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garcia-Leme J., Bohm G.M., Migliorini R.H., de Souza M.Z. Possible participation of insulin in the control of vascular permeability. Eur J Pharmacol. 1974;29:298–306. doi: 10.1016/0014-2999(74)90030-2. [DOI] [PubMed] [Google Scholar]
- 55.Kondo T., Kahn C.R. Altered insulin signaling in retinal tissue in diabetic states. J Biol Chem. 2004;279:37997–38006. doi: 10.1074/jbc.M401339200. [DOI] [PubMed] [Google Scholar]
- 56.Reiter C.E., Gardner T.W. Functions of insulin and insulin receptor signaling in retina: possible implications for diabetic retinopathy. Prog Retin Eye Res. 2003;22:545–562. doi: 10.1016/s1350-9462(03)00035-1. [DOI] [PubMed] [Google Scholar]
- 57.Reiter C.E., Wu X., Sandirasegarane L., Nakamura M., Gilbert K.A., Singh R.S., Fort P.E., Antonetti D.A., Gardner T.W. Diabetes reduces basal retinal insulin receptor signaling: reversal with systemic and local insulin. Diabetes. 2006;55:1148–1156. doi: 10.2337/diabetes.55.04.06.db05-0744. [DOI] [PubMed] [Google Scholar]