Abstract
In addition to its effects on bone metabolism, osteoprotegerin (OPG), a soluble member of the tumor necrosis factor family of receptors, promotes smooth muscle cell proliferation and migration and may act as a survival factor for tumor cells. We hypothesized that these cellular mechanisms of OPG may be involved in the growth and proliferation of lymphangioleiomyomatosis (LAM) cells, abnormal smooth muscle-like cells with mutations in one of the tuberous sclerosis complex tumor-suppressor genes (TSC1/TSC2) that cause LAM, a multisystem disease characterized by cystic lung destruction, lymphatic infiltration, and abdominal tumors. Herein, we show that OPG stimulated proliferation of cells cultured from explanted LAM lungs, and selectively induced migration of LAM cells identified by the loss of heterozygosity for TSC2. Consistent with these observations, cells with TSC2 loss of heterozygosity expressed the OPG receptors, receptor activator of NF-κB ligand, syndecan-1, and syndecan-2. LAM lung nodules showed reactivities to antibodies to tumor necrosis factor–related apoptosis-inducing ligand, receptor activator of NF-κB ligand, syndecan-1, and syndecan-2. LAM lung nodules also produced OPG, as shown by expression of OPG mRNA and colocalization of reactivities to anti-OPG and anti-gp100 (HMB45) antibodies in LAM lung nodules. Serum OPG was significantly higher in LAM patients than in normal volunteers. Based on these data, it appears that OPG may have tumor-promoting roles in the pathogenesis of lymphangioleiomyomatosis, perhaps acting as both autocrine and paracrine factors.
Osteoprotegerin (OPG; TNFRSF11B), a soluble member of the tumor necrosis factor (TNF) receptor family, is best known as a regulator of bone metabolism that promotes bone formation by inhibiting osteoclast development, thus protecting against osteoporosis.1,2 OPG, acting as a decoy receptor, binds to receptor activator of NF-κB ligand (RANKL), preventing the interaction of RANKL with its receptor RANK, resulting in the inhibition of osteoclast activation and bone resorption. Polymorphisms in the OPG gene have been linked to development of osteoporosis.3–6 Patients with juvenile Paget disease, a rare inherited disease affecting children, show increased bone turnover, leading to skeletal deformity. Mutations in the OPG gene determine the severity of the juvenile Paget disease phenotype,7 with the loss of the entire gene or mutations leading to the loss of OPG structure resulting in a severe phenotype.
More recently, the role of OPG in vascular cell biological characteristics has been studied. OPG knockout mice have both severe osteoporosis and significant arterial calcification,8 suggesting that OPG plays a protective role against arterial calcification in mice. OPG serum levels are associated with the severity of cardiovascular disease in humans.9–11 OPG levels may be higher either directly, through a proatherosclerotic effect, or indirectly, because of an incomplete compensatory mechanism in which increases in serum OPG levels are seen as a response to RANKL activity.9–11 This compensatory effect may also be invoked to explain high serum levels of OPG, sometimes seen in subjects with osteoporosis.12
Vascular smooth muscle cells express OPG, and aortic smooth muscle cells proliferate in response to OPG.13 OPG induced both the proliferation and migration of pulmonary artery smooth muscle cells14 and human microvascular endothelial cells.15 The effects of OPG on human microvascular endothelial cells were mediated through integrins αVβ3 and αVβ5 and the extracellular signal–regulated kinase 1/2. OPG can also stimulate monocyte migration; this effect was shown to involve syndecans and phosphatidylinositol-3-OH kinase/Akt, protein kinase C, and tyrosine kinases.16
OPG also has roles in tumor development and metastasis.17,18 OPG can bind TNF-related apoptosis-inducing ligand (TRAIL), blocking TRAIL’s apoptotic effects on cancer cells.19–23 Serum OPG levels may be higher in cancer patients compared with healthy controls, and levels may correlate with cancer stage.24–27 Tumor growth and metastasis are also supported by OPG’s promotion of endothelial cell survival and angiogenesis.28,29 Interestingly, some malignant breast cancer tumors show endothelial OPG expression, whereas neighboring normal endothelium does not express high levels of the protein.29
Lymphangioleiomyomatosis (LAM) cells are abnormal neoplastic smooth muscle-like cells, with mutations in one of two tuberous sclerosis complex tumor-suppressor genes (TSC1 or TSC2). TSC1 (encoding hamartin) and TSC2 (tuberin) form a complex that regulates the serine/threonine kinase, mammalian target of rapamycin.30 Mutations in TSC1/TSC2 lead to uncontrolled mammalian target of rapamycin activity, resulting in increased cell proliferation and size.30 These LAM cells form nodules covered with type II pneumocytes, with surrounding areas of cystic destruction in the lungs of patients with LAM. In addition to the cystic destruction of lung parenchyma, LAM, a rare multisystem disease affecting women,31 is characterized by lymphatic abnormalities and abdominal tumors (eg, angiomyolipomas). LAM cells can metastasize, as LAM cells from lung lesions and angiomyolipomas in the same patient have the same TSC2 mutation.32 Consistent with their migratory behavior, LAM cells have been isolated from blood and other body fluids of patients with LAM.33,34 LAM cells have characteristics of both smooth muscle cells, such as reactivity with antibodies to smooth muscle actin and desmin, and of melanocytes, with reactivity with HMB45,35 an antibody recognizing gp100, a melanosomal protein.36–38
In this study, we investigated the effect of OPG on the neoplastic smooth muscle cell-like LAM cells. OPG promoted proliferation of cells grown from explanted LAM lungs and specifically induced LAM cell migration. Three OPG receptors, RANKL, syndecan-1, and syndecan-2, were detected on LAM cells and LAM lung nodules. Furthermore, LAM cells produced OPG, and OPG levels were elevated in serum from patients with LAM compared with healthy volunteers, suggesting both autocrine and paracrine effects of OPG in LAM.
Materials and Methods
Study Population
Research was approved by the Institutional Review Board of the National Heart, Lung, and Blood Institute, Bethesda, MD (protocols 95-H-0186 and 96-H-0100). All participants gave written informed consent. Patients with LAM, for serum protein measurements, had a mean age of 50.5 ± 1.1 years and included 71 whites, four Asians, four African Americans, and one Hispanic. Healthy volunteers were seen at the NIH and were screened for pulmonary health by medical history and physical examination. The exclusion criteria for participants in the healthy volunteer group were as follows: aged <18 or >80 years, serum test result positive for HIV or hepatitis virus, and inability to perform reliable pulmonary function tests. The principal investigator (J.M.) reviewed applicants with minor health problems on a case-by-case basis. Healthy volunteers for the serum protein measurements had a mean age of 41.4 ± 2.0 years and included 20 whites, five Asians, four African Americans, one Hispanic, and one of unknown ethnicity. Concentrations of OPG, RANKL, and TRAIL in serum were quantified using kits from Alpco Diagnostics (Windham, NH) and Pierce Biotechnology (Rockford, IL).
Tissue Samples and Cell Culture
Lung tissue was collected from patients with LAM undergoing transplantation, and cells were grown, as previously described,39 in mesenchymal stem cell medium (Lonza, Walkersville, MD). Briefly, lung sections obtained at transplant were placed on plastic dishes with mesenchymal stem cell medium containing 0.05 U/mL penicillin, 0.05 μg/mL streptomycin, 4 mmol/L glutamine, and 10% fetal bovine serum. When colonies of cells formed, the tissue sections were removed and cells were allowed to grow for 3 to 7 days before trypsinization and transfer to new dishes. This procedure resulted in a heterogeneous mixture of cells that have functional TSC2 and those with TSC2 loss of heterozygosity (LOH). These cultures have been previously characterized.39–41
LOH Analysis
Kg8 or D16S3395 microsatellite markers from chromosome 16 were amplified with appropriate primer pairs [Kg8 forward (5′-CTCCCAGGGTGGAGGAAGGTG-3′) and Kg8 reverse (5′-Fam-GCAGGCACAGCCAGCTCCGAG-3′) or D16S3395 forward (5′-CTAACCCTCAGCAGAGTTCTG-3′) and D16S3395 reverse (5′-Fam-CCTGGCAGTAAGTCCTGAAA-3′)], and products were analyzed on a 3100 Genetic Analyzer (Applied Biosystems, Carlsbad, CA). LOH was quantified as previously described.33 Briefly, the heights of the two alleles in the sample were determined (L1 and L2), as were the heights of the alleles of the control (N1 and N2). QLOH is defined as (L1/L2)/(N1/N2), where L1 is the diminished allele. If the sample had a QLOH score <0.6, then it had LOH.
Definition of a LAM Cell
In this study, we are defining a LAM cell as a cell with a mutation or LOH of TSC2. Although many protein markers have been suggested to be expressed by LAM cells, none has been shown to be exclusive to LAM cells.36 Therefore, we chose to use the genetic definition of TSC2 LOH to specifically distinguish LAM cells. No one has reported a homogeneous LAM cell culture from lung; all in vitro studies using LAM lung cell cultures were performed on heterogeneous mixtures that have both LAM cells (TSC2 LOH) and non-LAM cells (TSC2 functional). The heterogeneous mixtures of cells grown from LAM lung contain 20% to 40% TSC2 LOH LAM cells by fluorescent in situ hybridization.39 In general, these LAM cells are undetectable by PCR analysis of LOH because the altered allele of the LAM cell is obscured by the alleles of the non-LAM cells, unless fractionation or selection is performed to remove some of the non-LAM cells. Separation of populations of cells by fluorescence-activated cell sorting (FACS) using the marker CD44v639 has been shown to enrich for and allow detection of LAM cells having TSC2 LOH.
Proliferation
A total of 2000 cells per well of four heterogeneous mixtures of cells grown from four different LAM lungs, pulmonary artery smooth muscle (PASM) cells, or normal human fibroblasts were plated in 96-well plates and incubated overnight at 37°C with 10, 50, or 100 ng/mL of OPG, RANKL, or TRAIL. Cell growth was measured using a colorimetric assay (Cell Counting Kit-8; Dojindo Molecular Technologies, Inc., Gaithersburg, MD). Data were reported as a percentage of the appropriate vehicle-containing control, and the LAM data were the average of the results from four mixtures of cells.
Chemotaxis Assays
Chemotaxis was assayed in 24-well plates with 8-μm pores (Chemicon, San Diego, CA), using 1 × 106 cells per well. Heterogeneous mixtures of cells were added to the upper chamber; 10% fetal bovine serum, 100 ng/mL OPG, or chemokine ligand (CCL) 2 was added to serum-free medium in the bottom chamber, and cells were permitted to migrate from the upper chamber overnight at 37°C. DNA was prepared from migrated cells (QIAamp DNA Mini Kit; Qiagen, Valencia, CA), and LOH analysis was performed.
FACS Analysis
Approximately 1 × 106 cells were incubated with indicated antibodies: fluorescein isothiocyanate (FITC) mouse anti-human CD44v6 (clone VFF-7; Invitrogen, Carlsbad, CA), phycoerythrin mouse anti-human TRAIL (clone 75402; R&D Systems, Minneapolis, MN), Alexa Fluor 647 rat anti-mouse RANKL (clone IK22-5; BD Biosciences, San Jose, CA), phycoerythrin rat anti-human syndecan-1 (clone 359103; R&D Systems), and allophycocyanin rat anti-human syndecan-2 (clone 305515; R&D Systems). Cell sorting was performed on a MoFlo Flow Cytometer (Beckman Coulter, Hialeah, FL). DNA was prepared (QIAamp DNA Mini Kit; Qiagen) from the subpopulations, and LOH analysis was performed.
OPG in Cell Supernatants
Serum-starved PASM cells and three heterogeneous mixtures of cells from LAM lungs (each from a different patient) (100,000 cells per well of a 6-well plate) were incubated overnight with serum-free medium, with or without 100 ng/mL CCL2 or 100 ng/mL TNF-α, or complete medium, and culture supernatants were collected. OPG concentrations in the supernatants were measured by enzyme-linked immunosorbent assay (ELISA; Alpco Diagnostics) and normalized with the final number of cells in each well. PASM cells were tested six times, as were each of the mixtures of cells. Data are reported as a percentage of the serum-free medium control. A LAM-specific average was calculated.
RT-PCR
Laser-capture microdissection was performed on frozen LAM lung tissue samples. LAM cells (5000 to 7000 spots) were collected with the VERITAS microdissection system (Arcturus Engineering, Mountain View, CA). RNA was prepared (Arcturus Picopure RNA Isolation Kit; Applied Biosystems). First-strand cDNA was primed with oligo dT and random primers (Superscript First-Strand Synthesis System for RT-PCR; Invitrogen) and PCR amplified with primers OPGCS (5′-Hex-CTGGATTTGGAGTGGTGCAAGC-3′) and OPGCAS (5′-TGTTTCCGGAACATATGTTGTCGTG-3′). Cells from two heterogeneous mixtures were spun down and lysed in cell lysis buffer (Signosis, Inc., Sunnyvale, CA). OPG gene fragments were produced using the One Step RT-PCR Kit (Qiagen) and primers OPGCS and OPGCAS. Products were analyzed on a 3100 Genetic Analyzer. The plots show PCR product peaks. The 186-bp peak represents OPG RNA. Amplification of genomic DNA would result in a peak >4000 bp because primer pair OPGCS binds to a sequence in exon 2, whereas OPGCAS binds to a sequence in exon 3. Amplification of actin was performed as a positive control.
IHC Assays
Immunohistochemical (IHC) assays were performed on formalin-fixed, paraffin-embedded sections. Slides were incubated with rabbit polyclonal antibody against OPG (H-249; Santa Cruz Biotechnologies, Inc., Santa Cruz, CA) overnight, then washed, incubated with biotinylated goat anti-rabbit antibody (HRP/DAB Detection System; Spring Bioscience, Fremont, CA), and washed again before development with 3,3′-diaminobenzidine tetrahydrochloride, and counterstained with hematoxylin. A negative control was treated with normal rabbit IgG instead of primary antibody. Slides were inspected with a Nikon DXM-1200 charge-coupled device microscope (Nikon, Inc., Melville, NY), and results were analyzed with Nikon ACT-1 software version 2.
Fluorescent IHC Assays
Slides for fluorescent IHC assays were prepared from formalin-fixed, paraffin-embedded or frozen LAM lung tissue sections (10 or 20 μm thick). Normal human lung slides were obtained from Imgenex (San Diego, CA). Dual labeling for immunofluorescence evaluated colocalization of a monoclonal antibody reaction with OPG (labeled with Texas Red; R&D Systems) paired with HMB45 (FITC; Dako, Carpinteria, CA) or antibodies against either CD31 (FITC; Abcam, Cambridge, MA) or podoplanin (FITC; R&D Systems). The characterization of OPG receptors in LAM lung tissue was accomplished by pairing a monoclonal antibody to α-smooth muscle actin (α-SMA) [FITC; Epitomics (Burlingame, CA) or Sigma (St. Louis, MO)] with anti-RANKL, anti–syndecan-2 (monoclonal antibodies), anti-TRAIL, or anti–syndecan-1 (polyclonal antibodies) (all Texas Red and purchased from R&D Systems). Slides were inspected with a Zeiss LSM 510 META laser-scanning confocal microscope equipped with 405 diode, argon, and helium-neon laser sources, using a Plan Neofluar 40× oil objective (numerical aperture, 1.3) (Carl Zeiss Microscopy, LLC, Thornwood, NY). Results were analyzed using LSM Image Examiner software version 4.2 SP1 (Zeiss). Controls consisted of replacing the primary antibody with PBS or normal mouse, goat, or rabbit IgG and were consistently negative.
Statistical Analysis
Two-tailed t-tests were performed using Excel (Microsoft, Redmond, WA). Tests were considered significant at P = 0.05.
Results
OPG Stimulates Proliferation of Cells from a Heterogeneous Mixture from LAM Lung
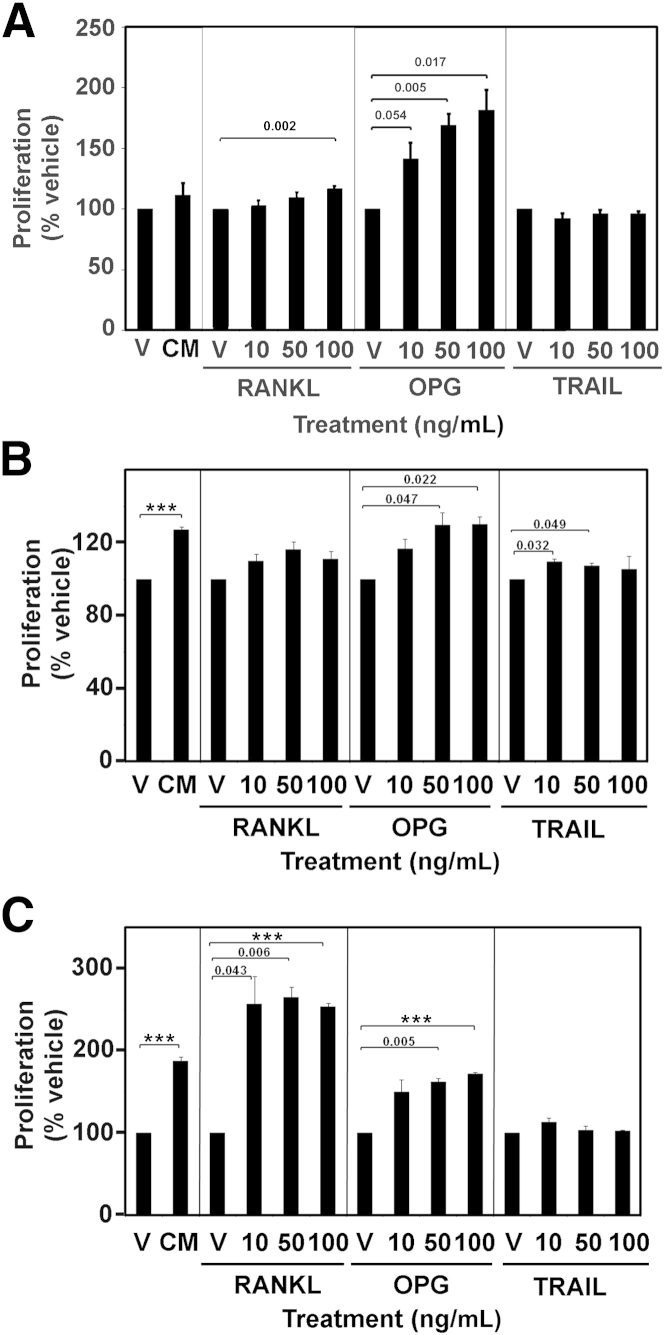
To examine the possible effects of OPG on LAM lung cells, we started by testing whether OPG could induce cell proliferation. We also tested the effects of OPG’s binding partners, RANKL and TRAIL, on the proliferation of LAM cell mixtures. Four heterogeneous cell cultures from four different LAM patients, PASM cells, and normal human fibroblasts were plated in 96-well plates and incubated overnight with 10, 50, or 100 ng/mL of OPG, RANKL, or TRAIL, and cell growth was measured. PASM cells were used as a control for the proliferation of the abnormal smooth muscle-like LAM cells, whereas the normal human fibroblasts represented a control for the non-LAM cells of the heterogeneous cultures. Data are reported as a percentage of the appropriate vehicle-containing control, and the LAM data are the average of the four cell mixtures. Proliferation of cells in the LAM mixtures was not significantly different in medium without or with 10% fetal bovine serum (112% ± 10%) (Figure 1A), whereas complete medium significantly stimulated proliferation of both PASM cells (127% ± 2%, P < 0.001) (Figure 1B) and normal human fibroblasts (188% ± 5%, P < 0.001) (Figure 1C). OPG induced proliferation of the heterogeneous LAM cultures in a dose-dependent manner, with the lowest concentration (10 ng/mL) inducing growth to 142% ± 13% of the vehicle control (P = 0.054), 50 ng/mL stimulating 169% ± 10% growth (P = 0.005), and 100 ng/mL stimulating 182% ± 17% growth (P = 0.017). OPG also stimulated proliferation of PASM cells and normal human fibroblasts, with the highest two concentrations stimulating proliferation significantly over the vehicle control. The highest concentration of RANKL (100 ng/mL) significantly induced proliferation of cells in the LAM mixtures by 118% ± 2% of its vehicle control (P = 0.002), whereas the lower concentrations did not have significant effects. RANKL did not stimulate proliferation of PASM cells at any concentration, but significantly stimulated proliferation of normal human fibroblasts at all three concentrations. TRAIL did not stimulate proliferation of the cells of the LAM mixtures or normal human fibroblasts at any concentration, but significantly stimulated proliferation of PASM cells at the lower two concentrations. Because more cells in the heterogeneous cultures are non-LAM than LAM cells, the proliferative response to OPG may be due primarily to the non-LAM cells. Because the proliferation assay did not allow us to identify the specific cell type responding to OPG, we next examined which cells in the mixtures expressed receptors that could bind OPG and mediate its effects.
Figure 1.
Cell proliferation induced by OPG in mixtures of LAM cells (A), pulmonary artery smooth muscle cells (B), and normal human fibroblasts (C). A total of 2000 cells per well were plated in 96-well plates and incubated overnight in serum-free medium with 10, 50, or 100 ng/mL of OPG, RANKL, TRAIL, or complete medium (CM), and cell growth was measured using a colorimetric assay that produced a compound that was directly proportional to the number of living cells. Experiments were performed three times. Data are reported as a percentage of the appropriate vehicle-containing control (V), and the LAM data are the average of results from four cell lines. P values for treatment versus control are above bars. Data are means ± SEM. ***P < 0.001.
OPG Receptors
Cells in our studies were heterogeneous mixtures, with both abnormal smooth muscle-like LAM cells and non-LAM cells, and we identified LAM cells genetically by confirming TSC2 LOH. These mixtures have 20% to 40% LAM cells, as determined by fluorescent in situ hybridization,39 and approximately 20% of the cells reacted with antibodies to smooth muscle actin. The effects of OPG on LAM cells may be mediated by OPG receptors, such as type II membrane forms of RANKL42,43 and TRAIL,44 and heparin sulfate–containing proteoglycans, such as syndecans.16
Receptors for OPG Are Expressed by Cells from Heterogeneous Mixtures from LAM Lungs
Cells from three heterogeneous mixtures with both LAM and non-LAM cells from explanted lungs (each from a different LAM patient) were reacted with antibodies against RANKL, TRAIL, syndecan-1, and syndecan-2, in addition to antibodies against CD44v6, a marker of metastasis used for LAM cell separation,39 and subjected to FACS cell sorting followed by TSC2 LOH analysis. All three heterogeneous cultures reacted with antibodies to OPG receptors, with an average of 40% ± 14% of cells reacting with anti–syndecan-2, 22% ± 9% reacting with anti-RANKL, 13% ± 2% reacting with anti–syndecan-1, 12% ± 3% reacting with anti-TRAIL, and 7% ± 2% reacting with anti-CD44v6. After incubation with anti-CD44v6 plus antibodies against one of the receptors, four cell populations were detected with anti–syndecan-1 and anti-TRAIL antibodies, but only three with anti-RANKL or anti–syndecan-2 antibodies. No cells were CD44v6+RANKL− or CD44v6+syndecan-2− (Table 1). Cells that expressed CD44v6 had a higher level of expression of OPG receptor, as determined by mean fluorescence intensity, than cells expressing the OPG receptor alone (Table 1). Thus, cells within the mixtures contained OPG receptors.
Table 1.
FACS Analysis of Heterogeneous Cells Grown from LAM Lung, with Percentage of Cells in Population, Mean Fluorescence Intensity of the Marker, and TSC2 LOH Analysis of Cell Populations
| Variable | Cells in population (%)∗ | Mean fluorescence intensity |
TSC2 analysis (%)† |
||
|---|---|---|---|---|---|
| CD44v6 | Receptor | ROH | LOH | ||
| CD44v6−RANKL− | 52 ± 2 | 4.8 ± 0.4 | 5.1 ± 0.3 | 80 | 20 |
| CD44v6−RANKL+ | 7 ± 3 | 9.1 ± 0.7 | 113.6 ± 49.3 | 70 | 30 |
| CD44v6+RANKL− | 0 ± 0 | NA | NA | NA | NA |
| CD44v6+RANKL+ | 5 ± 1 | 141.4 ± 34.5 | 350.2 ± 17.8 | 0 | 100 |
| CD44v6−syndecan-1− | 60 ± 3 | 5.8 ± 0.1 | 3.2 ± 0.1 | 89 | 11 |
| CD44v6−syndecan-1+ | 5 ± 1 | 5.1 ± 0.5 | 39.1 ± 4.2 | 56 | 44 |
| CD44v6+syndecan-1− | 3 ± 2 | 80.4 ± 6.9 | 3.7 ± 0.1 | 33 | 67 |
| CD44v6+syndecan-1+ | 3 ± 1 | 209.4 ± 97.3 | 193.9 ± 115.3 | 29 | 71 |
| CD44v6−syndecan-2− | 37 ± 6 | 4.8 ± 0.8 | 3.9 ± 0.3 | 100 | 0 |
| CD44v6−syndecan-2+ | 18 ± 6 | 7.8 ± 0.8 | 48.1 ± 4.8 | 67 | 33 |
| CD44v6+syndecan-2− | 0 ± 0 | NA | NA | NA | NA |
| CD44v6+syndecan-2+ | 5 ± 1 | 91.6 ± 7.5 | 92.1 ± 13.4 | 13 | 88 |
| CD44v6−TRAIL− | 68 ± 4 | 5.8 ± 0.1 | 2.9 ± 0.2 | 100 | 0 |
| CD44v6−TRAIL+ | 2 ± 1 | 7.2 ± 0.2 | 32.1 ± 0.3 | 44 | 56 |
| CD44v6+TRAIL− | 2 ± 0 | 87.2 ± 20.0 | 3.4 ± 0.1 | 43 | 57 |
| CD44v6+TRAIL+ | 4 ± 1 | 108.9 ± 13.4 | 81.5 ± 24.5 | 43 | 57 |
NA, not applicable; ROH, retention of heterozygosity.
Data are means ± SEM of values from three heterogeneous cell mixtures grown from LAM lungs from three different patients, each assayed at least three times. The four cell populations do not total 100% because specific gates were drawn encompassing each population relative to negative controls.
Data are percentage of times DNA isolated from the sorted population showed ROH or LOH. Experiments were performed on three heterogeneous cell mixtures grown from LAM lungs from three patients, at least three times each.
Receptors for OPG Are Expressed by TSC2 LOH Cells
DNA prepared from the sorted subpopulations of cells was tested for TSC2 LOH (Table 1). In three different heterogeneous cultures, DNA from the populations of CD44v6+RANKL+, CD44v6+syndecan-1+, and CD44v6+syndecan-2+ showed TSC2 LOH in several experiments (100%, 71%, and 88% of the time, respectively). FACS using anti-TRAIL antibody did not separate a population that consistently showed TSC2 LOH (CD44v6+TRAIL+ cells showed LOH only 57% of the time). Therefore, LAM cells with TSC2 LOH have the surface markers CD44v6, RANKL, syndecan-1, and syndecan-2.
Receptors for OPG Are Expressed in LAM Lung Tissue
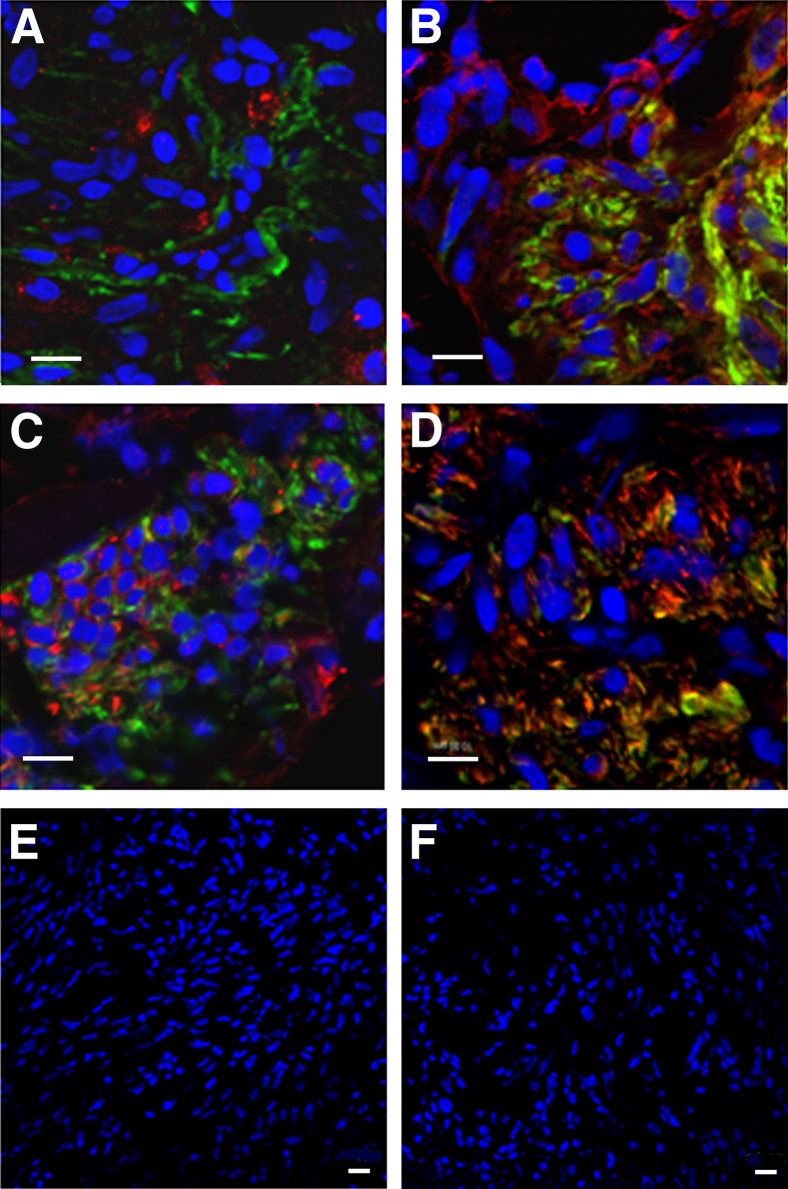
LAM lung tissue sections from three different patients were reacted with antibodies to RANKL, TRAIL, syndecan-1, or syndecan-2. Confocal imaging showed reactivity for RANKL (Figure 2A) and TRAIL (Figure 2B) in LAM nodules, but the reactivity was present in fewer cells than the reactivity for syndecan-1 and syndecan-2 in the proliferative areas of LAM lung tissue (Figure 2, C and D). Some LAM lung tissue cells reactive to syndecan-1, syndecan-2, or TRAIL were also reactive to anti–α-SMA antibody, a marker of LAM nodules. Therefore, receptors for OPG are expressed in LAM lung nodules in vivo.
Figure 2.
Expression of OPG receptors in LAM lung nodules demonstrated by confocal microscopy. Tissue sections were immunoreacted with anti–α-SMA antibody (FITC, green) and anti-RANKL, anti-TRAIL, anti–syndecan-1, or anti–syndecan-2 antibodies (Texas Red, red). The four receptors are expressed in areas of cellular proliferation (A–D). A: Cells positive for RANKL are seen adjacent to anti–α-SMA antibody–reactive cells. Some cells within the nodule express reactivity to both anti–α-SMA antibody and anti-TRAIL (B), anti–syndecan-1 (C), or anti–syndecan-2 (D) antibodies. E and F: The nuclei of cells were counterstained with DAPI. Control sections (deletion of primary antibody) were negative. Scale bar = 10 μm (A–F). Frozen lung sections from three patients with LAM showed comparable results.
OPG Specifically Induces Migration of TSC2 LOH LAM Cells from Heterogeneous Mixtures of Cells from LAM lung
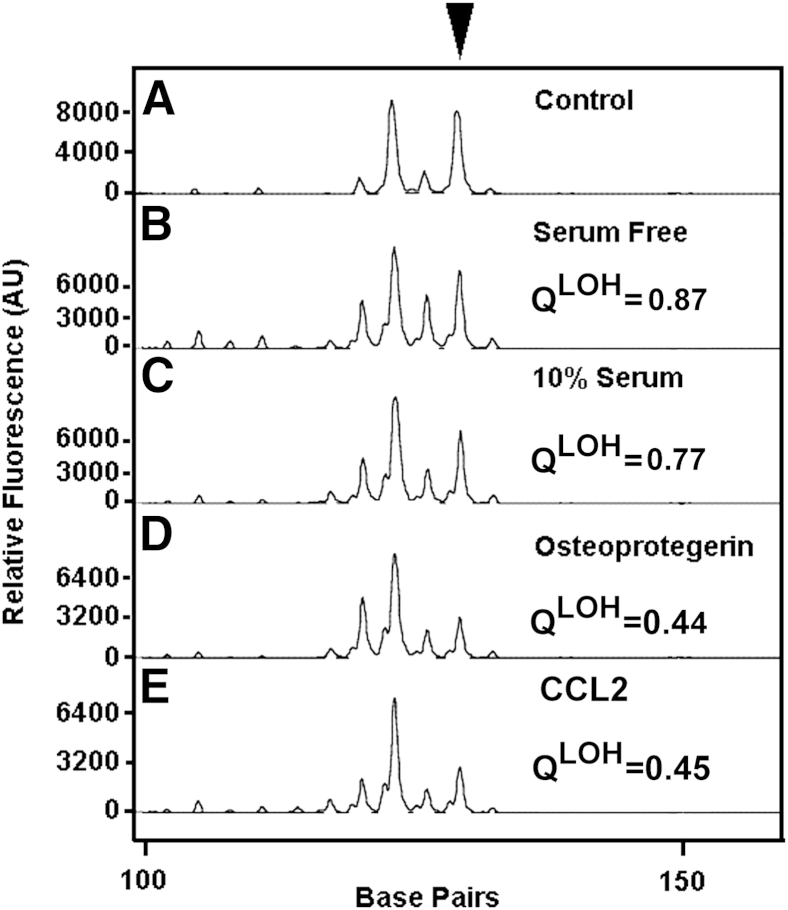
To determine whether TSC2 LOH LAM cells have functional OPG receptors, we tested the effect of OPG on the migration of LAM cells. Because TSC2 LOH LAM cells migrate in response to the chemokine, CCL2,41 and OPG induces migration of monocytes through syndecan-1,16 the effect of OPG on LAM cell chemotaxis was examined. A heterogeneous mixture of cells from LAM lung was placed in the upper chamber, 100 ng/mL OPG or CCL2 or 10% fetal bovine serum was added to serum-free medium in the bottom chamber, and cells were allowed to migrate from the upper chamber overnight. Those cells that migrated were collected, and DNA was prepared. As shown by the presence of TSC2 LOH in the DNA of migrated cells (Figure 3), OPG or CCL2 stimulated migration of TSC2 LOH LAM cells from the heterogeneous mixture. Serum-free medium and 10% fetal bovine serum did not selectively stimulate chemotaxis of cells with TSC2 LOH. Thus, OPG can selectively induce migration of TSC2 LOH LAM cells.
Figure 3.
TSC2 LOH in cells that migrated in response to OPG. Cells were stimulated overnight with medium without serum (B) or medium with 10% serum (C) or 100 ng/mL of OPG (D) or CCL2 (E) in the lower chamber of a Boyden chamber. DNA was prepared from cells that migrated, and PCR was performed to quantify chromosome 16 marker D16S3395. QLOH values were calculated relative to peak heights in samples amplified from the blood (A, control) of the patient who was the source of the heterogeneous mixtures of cells grown from LAM lung. A sample with a QLOH score of <0.6 was categorized as having LOH. Experiments were performed with two different heterogeneous mixtures of cells grown from LAM lungs (from two different patients) at least two times each. OPG and CCL2 each selectively stimulated migration of cells with TSC2 LOH. AU, arbitrary unit.
Sources of OPG
Endothelial and vascular smooth muscle cells are major sources of circulating OPG.18 Because LAM cells and smooth muscle cells express some genes in common, we investigated OPG expression by LAM cells in three ways: expression in heterogeneous cultures grown from LAM lung, expression of OPG mRNA in samples isolated from LAM lung nodules, and expression of OPG protein in LAM tissue by IHC.
OPG Is Expressed by Heterogeneous Cultures Containing Both LAM and Non-LAM Cells
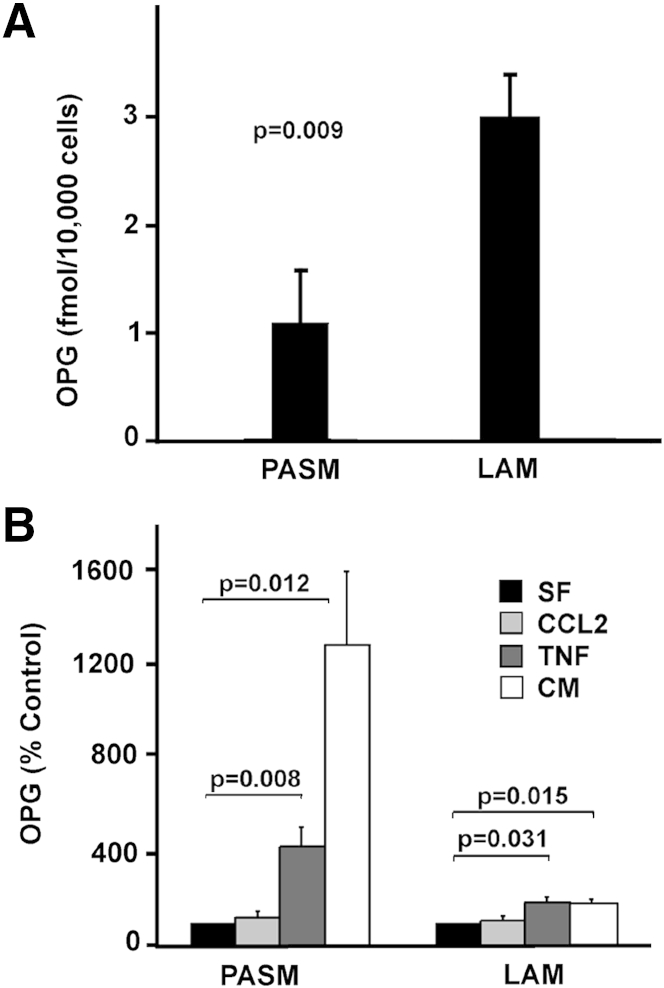
Three mixtures of cells grown from explanted LAM lungs and PASM cells were serum starved overnight, then incubated overnight in medium, serum free or with TNF-α or CCL2, as indicated, and supernatant was collected. OPG in medium was quantified by ELISA, and OPG concentrations were normalized to the final number of cells per well. Data for the amount of OPG produced in complete medium or with TNF-α or CCL2 are expressed as a percentage of serum-free control. Heterogeneous LAM cell cultures incubated with serum-free medium produced 2.7 times more OPG than did PASM cells treated with serum-free medium alone (P = 0.009); thus, basal OPG expression was higher in the LAM cell cultures than in PASM cells (Figure 4A). OPG levels from the heterogeneous LAM cell cultures and PASM cell cultures grown in both complete medium and serum-free medium containing TNF-α were significantly higher than those from cells grown in serum-free medium (Figure 4B). PASM cells incubated with complete medium produced 1300% ± 310% more OPG than the serum-free control (P = 0.012), whereas the heterogeneous mixtures from LAM lung incubated with complete medium produced 190% ± 10% more OPG than the serum-free control (P = 0.015). Incubation with TNF-α produced 430% ± 80% (P = 0.008) and 190% ± 20% (P = 0.031) more OPG than the serum-free control in PASM cells and the LAM cell mixtures, respectively. Incubation with CCL2 did not stimulate OPG production in either LAM cell mixtures or PASM cells. Thus, LAM cell mixtures contained cells capable of expressing OPG; however, these data did not demonstrate whether OPG was produced by TSC2 LOH LAM cells.
Figure 4.
OPG production by PASM cells and heterogeneous mixtures of cells grown from LAM lung. Serum-starved PASM cells and three different heterogeneous mixtures of cells grown from LAM lung from three different patients (100,000 cells per well of a 6-well plate) were incubated for 24 hours in serum-free medium (SF), with or without 100 ng/mL CCL2 or 100 ng/mL TNF-α, or complete medium (CM), and culture supernatants were collected for quantification of OPG concentrations by ELISA. PASM cells were tested six times, as was each of the heterogeneous mixtures of cells grown from LAM lung. LAM data are reported as a LAM cell average. Data for proliferation stimulated by complete medium or with TNF-α or CCL2 are percentage of serum-free medium control and were normalized to the final number of cells per well. P values for treatment versus control are above bars. A: Amount of OPG per cell expressed by PASM and LAM cells incubated in SF medium. B: OPG production in PASM and LAM cells incubated with CCL2- or TNF-α–containing medium or complete medium expressed as a percentage of the SF control.
OPG mRNA Is Detected in Microdissected LAM Nodules
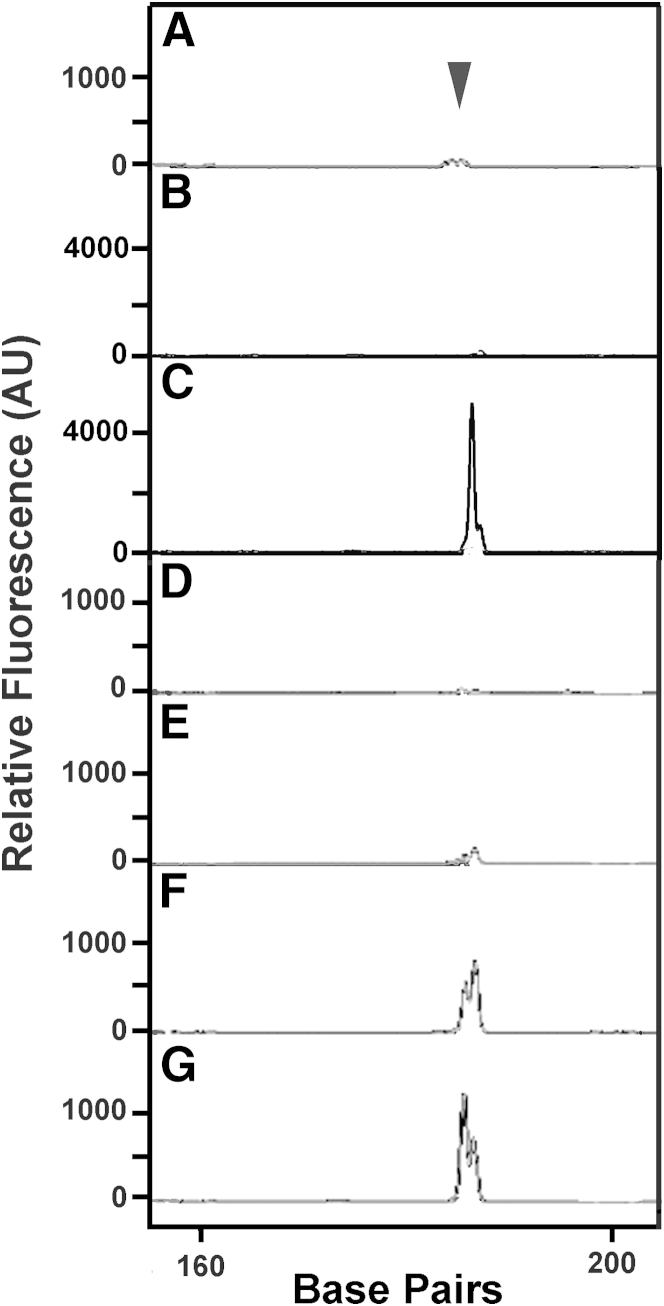
To identify the cells that produced OPG, LAM nodules were microdissected from LAM lung tissue, and RNA from the nodules and from heterogeneous LAM cell mixtures was prepared for RT-PCR. Products were analyzed on a 3100 Genetic Analyzer. OPG RNA was amplified from a LAM cell mixture (Figure 5C). Although the positive control, actin, could be amplified from four of the microdissected samples (data not shown), two of four samples expressed OPG RNA (Figure 5, D–G), suggesting that OPG was expressed by some LAM nodules.
Figure 5.
OPG mRNA in laser-capture microdissected LAM nodules. OPG RNA was amplified by RT-PCR and analyzed on a 3100 Genetic Analyzer. The 186-bp peak (arrowhead) represents OPG RNA. Amplification of genomic DNA would result in a peak >4000 bp because primer OPGCS is from exon 2 and OPGCAS is from exon 3. Representative amplification results from no DNA control (A), no reverse transcriptase control (using the RNA from the LAM cell mixture used in C) (B), RNA from a LAM cell mixture (C), and RNA from microdissected LAM nodules (D–G). RNA isolated from a second LAM cell mixture showed similar results to B and C (data not shown). Actin was a positive control for the amplification reactions in D–G (data not shown). AU, arbitrary unit.
OPG and gp100 Are Co-Expressed by Cells in LAM Nodules
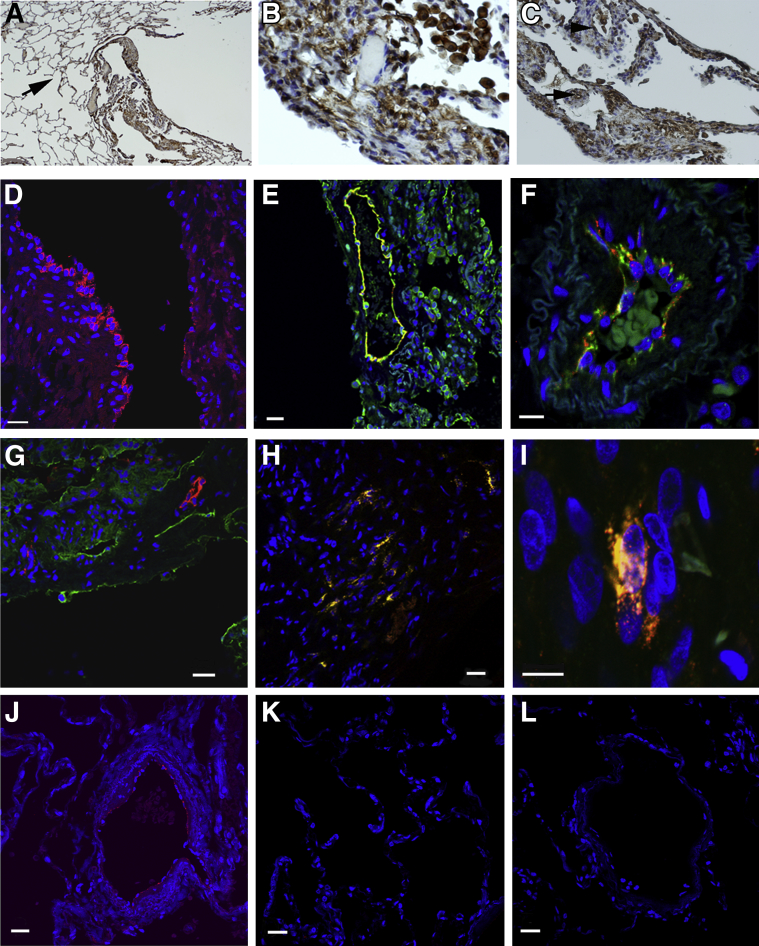
IHC assays were used to investigate further OPG production by LAM cells. An IHC reaction with anti-OPG antibodies was seen in LAM nodules of explanted LAM lungs (Figure 6, A–C). We found both cytoplasmic and membranous reaction for OPG in LAM cells (Figure 6, A and B) and vessel walls (Figure 6C). Immunofluorescent microscopy of explanted LAM lung tissue from six patients showed OPG in endothelial cells lining vascular structures and in alveolar cells. OPG is more concentrated in the vascular structures, resulting in a strong signal, compared with the parenchyma, where the reactivity of OPG appears to be more diffuse (Figure 6D). In normal human lung, OPG was detected in cells lining vascular structures, but was not detected in the alveolar structures (Figure 6, J and K). Reaction with anti-OPG and anti-CD31 (a marker for vascular endothelial cells) antibodies was detected in the cytoplasm of the same vascular endothelial cells (Figure 6, E and F). It is not clear whether the parenchymal cells react with both the anti-OPG and anti-CD31 antibodies, because the reactivity for anti-CD31 was much stronger in these areas than the weaker reactivity for OPG and may have overwhelmed the OPG signal. OPG was not detected in the lymphatic structures that traverse LAM nodules, using podoplanin as a lymphatic vessel marker. Reactivity with both anti-podoplanin and anti-OPG antibodies was seen, but in distinct cells (Figure 6G). LAM cells that reacted with the monoclonal antibody, HMB45, which recognizes gp100, a melanosomal LAM cell marker,35 reacted with anti-OPG antibodies (Figure 6, H and I). Although a small subset of HMB45-positive cells did not react with anti-OPG antibodies, incubation with antibodies against both proteins revealed their profuse colocalization in the cytoplasm of cells in LAM nodules. These data suggest that production of OPG in LAM may be both paracrine and autocrine, with OPG released by vascular structures within LAM lung tissue and by the LAM cells themselves.
Figure 6.
OPG in LAM lung lesions. A and B: Reaction with antibodies against OPG in LAM lung nodules in sections of explanted LAM lungs. C: Reactions in cells lining vessels. Original magnifications: ×40 (A); ×400 (B); ×200 (C). The arrow indicates anti-OPG reactivity in alveolar structures (A). Anti-OPG reactivity in LAM cells and alveolar macrophages (B). The arrowhead indicates a vascular structure, whereas the arrow indicates a LAM nodule (C). D: Confocal fluorescence imaging of lung sections. Strong positive reactivity to anti-OPG antibody (Texas Red, red) is localized to the lining of vascular structures, with weaker reactivity in cells throughout the parenchyma. E: CD31 (FITC, green)– and OPG (Texas Red, red)–positive cells are seen in a vascular structure of a LAM lung section. F: Higher magnification of another LAM tissue section revealed cells with reaction to antibodies against both OPG and CD31 within the same vascular endothelial cells (merge, yellow) lining a vessel. G: Reaction with antibodies against OPG and podoplanin (FITC, green) is evident in a LAM lung section, without, however, colocalization of immunoreactivities within the same cell. H: HMB45 (FITC, green)– and OPG (Texas Red, red)–positive cells in a proliferative region of a LAM lung. I: Higher magnification of a tissue section demonstrated positive reactivity to HMB45 and an antibody to OPG within the same cell (merge, yellow), suggesting that LAM cells express OPG. J: Cells immunopositive for OPG (Texas Red, red) line vascular structures in normal lung tissue. K: No reaction to OPG is observed in normal lung alveolar cells, in contrast to the diffuse expression of OPG in LAM lung tissue. L: Control staining of normal human lung using IgG mouse control. The nuclei of cells were counterstained with DAPI (blue). Results were similar in at least three different samples for each pair of antibodies. LAM lung sections are from different patients (D, E, G, and H). Scale bar = 20 μm (D–L).
Concentrations of Serum OPG Are Significantly Higher in LAM Patients than Healthy Volunteers
OPG concentrations in serum from LAM patients and healthy volunteers were quantified by ELISA. OPG concentrations in serum from LAM patients were significantly higher than those from healthy volunteers [means ± SEM, 6.2 ± 0.3 pmol/L (80 patients) versus 3.8 ± 0.3 pmol/L (34 healthy volunteers); P < 0.001].
As had been previously reported in healthy individuals,45 the concentration of OPG in serum increased significantly with age in LAM patients (P = 0.004) and in healthy volunteers (P = 0.028). Even after adjustment for age, OPG concentrations in serum were significantly higher in LAM patients than in healthy volunteers (P = 0.003).
Because RANKL and TRAIL interact with OPG,18 we measured RANKL and TRAIL concentrations in serum by ELISA. TRAIL [557 ± 27 pg/mL (78 patients) versus 507 ± 26 pg/mL (35 healthy volunteers)] and RANKL [0.16 ± 0.01 pmol/L (80 LAM patients) versus 0.16 ± 0.05 pmol/L (36 healthy volunteers)] concentrations showed no significant difference in serum from LAM patients versus healthy volunteers. Thus, only the serum levels of OPG were found to be different between LAM patients and healthy volunteers of the three interacting proteins, OPG, RANKL, and TRAIL.
Serum Concentrations of OPG and CCL2 Are Correlated in LAM Patients
CCL2 is a chemokine that may play a role in LAM.41 Polymorphisms in the CCL2 gene were associated with susceptibility to LAM, and CCL2 specifically induced migration of LAM cells with TSC2 LOH.41 In a study of healthy normal volunteers,46 CCL2 plasma levels correlated with RANKL concentrations in men and women, whereas in LAM patients, no association was seen between CCL2 and RANKL or TRAIL (data not shown). CCL2 plasma levels correlated positively with OPG in healthy women, but not in men.46 In LAM patients, CCL2 and OPG were positively correlated (n = 58; P < 0.001, adjusted for age) (Figure 7), suggesting that CCL2 and OPG might both participate in LAM pathogenesis, perhaps through effects on migration.
Figure 7.
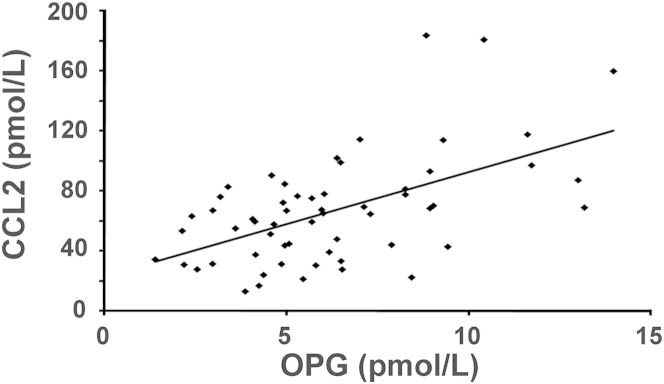
Correlation of OPG and CCL2 concentrations in serum from LAM patients (n = 58). P < 0.001.
Discussion
LAM is a metastatic disease. LAM cells from lung lesions and angiomyolipomas in the same patient have the same TSC2 mutation,32 and recurrent LAM cells of recipient origin have been found in the donor lung of a transplanted patient.47,48 In support of metastatic dissemination, we were able to isolate LAM cells from body fluids, including blood, chyle, and urine.33,34 Herein, we show that LAM cell mixtures proliferate and TSC2 LOH LAM cells migrate selectively in response to OPG, probably via one of the OPG receptors, membranous RANKL, syndecan-1, or syndecan-2. LAM cells expressed OPG, and serum levels of OPG in LAM patients were found to be significantly higher than those in healthy volunteers, perhaps inducing LAM cells to enter the bloodstream. A study comparing serum OPG levels between healthy volunteers and patients with solid tumors found no significant difference between the groups, but did find a trend of higher serum levels of OPG in patients with metastatic disease than in those with localized tumors.24 OPG has also been shown to be involved in the metastasis of prostate and breast cancer cells to bone.49
OPG is expressed by both endothelial cells and by LAM cells within the LAM cell nodules (Figure 6). Reactivity of anti-OPG antibodies resulted in a much stronger signal in the endothelial cells than that seen in the nodules, where most OPG-expressing cells showed a more diffuse cytosolic reactivity. There were, however, some cells of the LAM nodules that showed stronger reactivity to anti-OPG antibodies. Both the endothelial cells and the LAM nodules showed reactivity with an antibody to CD31, a marker that is mainly used to identify endothelial cells. CD31 is expressed on hematopoietic stem cells and endothelial cells,50 but it is also found on circulating monocytes51 and on intratumoral macrophages.52 Although it is clear that some of the anti-CD31 reactivity is on vascular structures, it is not known what type of cell reacts with the anti-CD31 antibodies in the LAM lung parenchyma. LAM cell nodules are heterogeneous, with LAM cells found along alveolar walls, blood vessels, lymphatics, and bronchioles.53 Both blood and lymphatic vessels infiltrate the nodules. LAM cells themselves can be either proliferative spindle-shaped cells or more differentiated epithelioid cells. It has been shown that renal angiomyolipomas from LAM patients contain smooth muscle cells, adipose tissue, and five types of vessels. Interestingly, four of the five types of vessels reacted with HMB45 and showed TSC2 LOH, as did the smooth muscle cells and adipose tissue.54 In LAM lung, we may be seeing a LAM cell type that expresses CD31 antigen or the infiltration of another cell type expressing CD31.
It has been proposed that the growth of LAM cells within the nodule is regulated by soluble factors, metalloproteases, and chemokines. LAM cells have been shown to express receptors for angiotensin,55 erythropoietin,40 insulin-like growth factor,56 epidermal growth factor,57 chemokines,41 and OPG (Table 1); however, the mechanisms of action of some of these receptors have not been clearly delineated. We show that membrane-bound syndecan-1 and syndecan-2 are present in LAM cells (Table 1) and in LAM lung tissue (Figure 2). Syndecans are heparin sulfate proteoglycans that are able to bind extracellular matrix proteins, such as integrins (eg, α5β1, αVβ3, and αVβ5), regulating cell adhesion. Syndecans are also recruiters of growth factors and metalloproteases.58 Thus, it is tempting to speculate that the syndecans present in LAM cells could play a critical role in cell proliferation, migration, and anchoring during metastasis, by modulating the function of growth factors and increasing the on-site concentration of soluble factors.
RANKL and TRAIL were also present in LAM lung tissue (Figure 2), but in fewer cells than syndecan-1 and syndecan-2. Cells positive for RANKL also tended to be adjacent to those reactive to anti–α-SMA antibody instead of having colocalized reactivities of RANKL and α-SMA. Analysis of heterogeneous mixtures of cells cultured from LAM lung tissue indicated, however, that CD44v6+RANKL+ cells showed TSC2 LOH in 100% of our experiments, whereas CD44v6+TRAIL+ cells had TSC2 LOH in only 57% of experiments (Table 1). Because the cells in the LAM nodule have not been well characterized, it is possible that LAM cells have variable amounts of smooth muscle actin, which would account for the presence of TSC2 LOH in vitro in RANKL-positive cells, whereas the RANKL-positive cells in vivo may express low levels of or undetectable smooth muscle actin. There may also be differences in the expression of RANKL and TRAIL in the LAM lung tissue versus cultured cells, which could account for the in vivo versus in vitro discrepancies.
OPG is found in the Weibel-Palade bodies of endothelial cells and is released after stimulation by proinflammatory cytokines, such as TNF-α and IL-1β.59 Although not shown to specifically induce OPG release or expression, a study has shown that the serum levels of CCL2, an inflammatory cytokine, correlate with the serum levels of OPG.46 We also see this association in LAM patients (Figure 7). It is possible that CCL2 stimulates OPG release in LAM patients. We have previously shown that CCL2 stimulated the migration of TSC2 LOH LAM cells,41 as did OPG. These effects on chemotaxis may play a role in LAM cell metastasis. LAM is a sex-specific disease in which estrogen appears to play a role in its progression and metastasis.60 Interestingly, it has been shown that 17β-estradiol increases both OPG and CCL2 production in human dendritic cells.61
Patients with LAM may have secondary clinical manifestations, such as osteoporosis and elevated exercise-induced pulmonary artery pressures.62,63 Of LAM patients, 70% had abnormal bone mineral densities, and the bone mineral density correlated with severity of lung disease and age.62 We did not find a correlation between serum levels of OPG and bone mineral density in LAM patients (data not shown), similar to the findings in a healthy adult population.64 Another study,12 however, found both significantly higher levels of OPG in the serum of postmenopausal women with osteoporosis than in matched normal controls and a correlation of OPG concentrations and bone mineral density in the postmenopausal women with osteoporosis. High levels of serum OPG in women with osteoporosis may be a compensative response to bone loss, as opposed to being a cause of osteoporosis.12 It is also possible that OPG in the serum comes from many different sources, and may not truly reflect the microenvironment of the bone.64
LAM patients also experience higher than normal exercise-induced pulmonary artery pressures.63 More than half of the patients tested showed an increase in pulmonary artery pressures with moderate physical activity, suggesting that the activities of daily living may lead to pulmonary hypertension.63 Pulmonary vascular lesions of patients with idiopathic pulmonary artery hypertension were reactive with anti-OPG antibodies in the concentric and plexiform lesions of the remodeled pulmonary arteries.14 Serum OPG levels from patients with idiopathic pulmonary artery hypertension were higher than those of controls, and OPG induced pulmonary artery smooth muscle cell proliferation and migration.14 It is possible that high serum levels of OPG play a role in pulmonary artery hypertension in LAM by affecting non-LAM cells, such as pulmonary artery smooth muscle cells.
Although sources of OPG include osteoblasts, immune-competent cells (T cells, B cells, and monocytic cells), fibroblasts, smooth muscle cells, and endothelial cells,65 endothelial cells and vascular smooth muscle cells may be the major contributors of OPG in serum.18 Both endothelial cells and HMB45-positive LAM cells in lung tissue expressed OPG. In one study,38 approximately 40% of LAM cells in LAM lung tissue reacted with HMB45, a mouse monoclonal antibody reactive to gp100.35 LAM cells that contained gp100 were primarily the larger, more differentiated, epithelioid cells, not the small, proliferative, spindle-shaped cells. It was reported that the extent of cellular differentiation influenced the level of OPG expression in smooth muscle cells,13 with highly differentiated cells producing the most OPG. These findings are consistent with HMB45-expressing cells producing OPG, because these cells are more highly differentiated than the non–HMB45-expressing spindle-shaped cells.
Non-LAM cells may also be influenced by LAM cells to express OPG. Direct contact with breast cancer cells stimulated endothelial cells to produce OPG,66 with potential effects on tumor angiogenesis. We show that both endothelial cells and LAM cells produce OPG in LAM lung; it is possible that LAM cells in lung, such as breast cancer cells, increase OPG expression by endothelial cells. Because the LAM nodule appears to produce OPG locally, the microenvironment of the nodule may play a role in LAM cell proliferation and dissemination. Similarly, a high local concentration of OPG produced by non-LAM cells may stimulate migration of LAM cells to this region. Therefore, the microenvironment could be involved in a positive feedback mechanism.41
In conclusion, LAM cells express both OPG and receptors that bind OPG, including RANKL, syndecan-1, and syndecan-2. OPG stimulates proliferation of LAM cell cultures and specifically induces migration of TSC2 LOH LAM cells. Patients with LAM have significantly higher serum levels of OPG than healthy volunteers. These data suggest that OPG plays a role in LAM pathogenesis and progression, acting as both a paracrine and an autocrine factor.
Acknowledgments
We thank Dr. Martha Vaughan for helpful discussions and critical review of the manuscript, Dr. J. Philip McCoy, Jr, and Leigh Samsel (Flow Cytometry Core Facility) for help with the FACS analysis, Drs. Christian Combs and Daniela Malide (Light Microscopy Core Facility) and Dr. Zu-Xi Yu (Pathology Core Facility) for help with immunostaining, and the LAM Foundation and the Tuberous Sclerosis Alliance for their assistance in recruiting patients for our studies.
Footnotes
Supported by the Intramural Research Program, the NIH, the National Heart, Lung, and Blood Institute, and an Oak Ridge Institute for Science and Education senior fellowship (Y.I.).
The author J.-P.L. is deceased.
Current address of Y.I., Department of Pathology, National Cerebral and Cardiovascular Center, Osaka, Japan.
References
- 1.Schoppet M., Preissner K.T., Hofbauer L.C. RANK ligand and osteoprotegerin paracrine regulators of bone metabolism and vascular function. Arterioscler Thromb Vasc Biol. 2002;22:549–553. doi: 10.1161/01.atv.0000012303.37971.da. [DOI] [PubMed] [Google Scholar]
- 2.Trouvin A.P., Göeb V. Receptor activator of nuclear factor-κB ligand and osteoprotegerin: maintaining the balance to prevent bone loss. Clin Interv Aging. 2010;5:345–354. doi: 10.2147/CIA.S10153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jurado S., Nogues X., Agueda L., Garcia-Giralt N., Urreizti R., Yoskovitz G., Perez-Edo L., Salo G., Carreras R., Mellibovsky L., Balcells S., Grinberg D., Diez-Perez A. Polymorphisms and haplotypes across the osteoprotegerin gene associated with bone mineral density and osteoporotic fractures. Osteoporos Int. 2010;21:287–296. doi: 10.1007/s00198-009-0956-4. [DOI] [PubMed] [Google Scholar]
- 4.Richards J.B., Rivadeneira F., Inouye M., Pastinen T.M., Soranzo N., Wilson S.G., Andrew T., Falchi M., Gwilliam R., Ahmadi K.R., Valdes A.M., Arp P., Whittaker P., Verlaan D.J., Jhamai M., Kumanduri V., Moorhouse M., van Meurs J.B., Hofman A., Pols H.A.P., Hart D., Zhai G., Kato B.S., Mullin B.H., Zhang F., Deloukas P., Uitterlinden A.G., Spector T.D. Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study. Lancet. 2008;371:1505–1512. doi: 10.1016/S0140-6736(08)60599-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vidal C., Formosa R., Xuereb-Anastasi A. Functional polymorphisms within the TNFRSF11B (osteoprotegerin) gene increase the risk for low bone mineral density. J Mol Endocrinol. 2011;47:327–333. doi: 10.1530/JME-11-0067. [DOI] [PubMed] [Google Scholar]
- 6.Styrkarsdottir U., Halldorsson B.V., Gretarsdottir S., Gudbjartsson D.F., Walters G.B., Ingvarsson T., Jonsdottir T., Saemundsdottir J., Center J.R., Nguyen T.V., Bagger Y., Gulcher J.R., Eisman J.A., Christiansen C., Sigurdsson G., Kong A., Thorsteinsdottir U., Stefansson K. Multiple genetic loci for bone mineral density and fractures. N Engl J Med. 2008;358:2355–2365. doi: 10.1056/NEJMoa0801197. [DOI] [PubMed] [Google Scholar]
- 7.Brunetti G., Marzano F., Colucci S., Ventura A., Cavallo L., Grano M., Faienza M.F. Genotype-phenotype correlation in Juvenile Paget disease: role of molecular alterations of the TNFRSF11B gene. Endocrine. 2012;42:266–271. doi: 10.1007/s12020-012-9705-0. [DOI] [PubMed] [Google Scholar]
- 8.Bucay N., Sarosi I., Dunstan C.R., Morony S., Tarpley J., Capparelli C., Scully S., Tan H.L., Xu W., Lacey D.L., Boyle W.J., Simonet W.S. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jono S., Ikari Y., Shioi A., Mori K., Miki T., Hara K., Nishizawa Y. Serum osteoprotegerin levels are associated with the presence and severity of coronary artery disease. Circulation. 2002;106:1192–1194. doi: 10.1161/01.cir.0000031524.49139.29. [DOI] [PubMed] [Google Scholar]
- 10.Tousoulis D., Siasos G., Maniatis K., Oikonomou R., Kioufis S., Zaromitidou M., Paraskevopoulos T., Michalea S., Kollia C., Miliou A., Kokkou E., Papavassiliou A.G., Stefanadis C. Serum osteoprotegerin and osteopontin levels are associated with arterial stiffness and the presence and severity of coronary artery disease. Int J Cardiol. 2012 doi: 10.1016/j.ijcard.2012.05.001. http://dx.doi.org/10.1016/j.ijcard.2012.05.001 [DOI] [PubMed] [Google Scholar]
- 11.Venuraju S.M., Yerramasu A., Corder R., Lahiri A. Osteoprotegerin as a predictor of coronary artery disease and cardiovascular mortality and morbidity. J Am Coll Cardiol. 2010;55:2049–2061. doi: 10.1016/j.jacc.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Yano K., Tsuda E., Washida N., Kobayashi F., Goto M., Harada A., Ikeda K., Higashio K., Yamada Y. Immunological characterization of circulating osteoprotegerin/osteoclastogenesis inhibitory factor: increase serum concentrations in postmenopausal women with osteoporosis. J Bone Miner Res. 1999;14:518–527. doi: 10.1359/jbmr.1999.14.4.518. [DOI] [PubMed] [Google Scholar]
- 13.Corallini F., Gonelli A., D’Aurizio F., di Iasio M.G., Vaccarezza M. Mesenchymal stem cells-derived vascular smooth muscle cells release abundant levels of osteoprotegerin. Eur J Histochem. 2009;53:19–24. doi: 10.4081/ejh.2009.19. [DOI] [PubMed] [Google Scholar]
- 14.Lawrie A., Waterman E., Southwood M., Evans D., Suntharalingam J., Francis S., Crossman D., Croucher P., Morrell N., Newman C. Evidence of a role for osteoprotegerin in the pathogenesis of pulmonary artery hypertension. Am J Pathol. 2008;172:256–264. doi: 10.2353/ajpath.2008.070395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi-Sakamoto M., Isogai E., Hirose K., Chiba I. Role of αV integrin in osteoprotegerin-induced endothelial cell migration and proliferation. Microvasc Res. 2008;76:139–144. doi: 10.1016/j.mvr.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Mosheimer B.A., Kaneider N.C., Feistritzer C., Djanani A.M., Sturn D.H., Patsch J.R., Wiedermann C.J. Syndecan-1 is involved in osteoprotegerin-induced chemotaxis in human peripheral blood monocytes. J Clin Endocrinol Metab. 2005;90:2964–2971. doi: 10.1210/jc.2004-1895. [DOI] [PubMed] [Google Scholar]
- 17.Holen I., Shipman C.M. Role of osteoprotegerin (OPG) in cancer. Clin Sci. 2006;110:279–291. doi: 10.1042/CS20050175. [DOI] [PubMed] [Google Scholar]
- 18.Zauli G., Melloni E., Capitani S., Secchiero P. Role of full-length osteoprotegerin in tumor cell biology. Cell Mol Life Sci. 2009;66:841–851. doi: 10.1007/s00018-008-8536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neville-Webbe H.L., Cross N.A., Eaton C.L., Nyambo R., Evans C.A., Coleman R.E. Osteoprotegerin (OPG) produced by bone marrow stromal cells protects breast cancer cells from TRAIL-induced apoptosis. Breast Cancer Res Treat. 2004;86:269–279. doi: 10.1023/b:brea.0000036900.48763.b3. [DOI] [PubMed] [Google Scholar]
- 20.Nyambo R., Cross N.A., Lippitt J.M., Holen I., Bryden G., Hamdy F.C., Eaton C.L. Human bone marrow stromal cells protect prostate cancer cells from TRAIL-induced apoptosis. J Bone Miner Res. 2004;10:1712–1721. doi: 10.1359/JBMR.040703. [DOI] [PubMed] [Google Scholar]
- 21.Shipman C.M., Croucher P.I. Osteoprotegerin is a soluble decoy receptor for tumor necrosis factor-related apoptosis-inducing ligand/Apo2 ligand and can function as a paracrine survival factor for human myeloma cells. Cancer Res. 2003;63:912–916. [PubMed] [Google Scholar]
- 22.De Toni E.N., Thieme S.E., Herbst A., Behrens A., Stieber P., Jung A., Blum H., Goke B., Kolligs F.T. OPG is regulated by beta-catenin and mediates resistance to TRAIL-induced apoptosis in colon cancer. Clin Cancer Res. 2008;14:4713–4718. doi: 10.1158/1078-0432.CCR-07-5019. [DOI] [PubMed] [Google Scholar]
- 23.Holen I., Croucher P.I., Hamdy F.C., Eaton C.L. Osteoprotegerin (OPG) is a survival factor for human prostate cancer cells. Cancer Res. 2002;62:1619–1623. [PubMed] [Google Scholar]
- 24.Lipton A., Ali S.M., Leitzel K., Chinchilli V., Witters L., Engle L., Holloway D., Bekker P., Dunstan C.R. Serum osteoprotegerin levels in healthy controls and cancer patients. Clin Cancer Res. 2002;8:2306–2310. [PubMed] [Google Scholar]
- 25.Brown J.M., Vessella R.L., Kostenuik P.J., Dunstan C.R., Lange P.H., Corey E. Serum osteoprotegerin levels are increased in patients with advanced prostate cancer. Clin Cancer Res. 2001;10:2977–2983. [PubMed] [Google Scholar]
- 26.Mizutani Y., Matsubara H., Yamamoto K., Nan Li Y., Mikami K., Okihara K., Kawauchi A., Bonavida B., Miki T. Prognostic significance of serum osteoprotegerin levels in patients with bladder carcinoma. Cancer. 2004;101:1794–1802. doi: 10.1002/cncr.20550. [DOI] [PubMed] [Google Scholar]
- 27.Ito R., Nakayama H., Yoshida K., Kuraoka K., Motoshita J., Oda N., Oue N., Yasui W. Expression of osteoprotegerin correlates with aggressiveness and poor prognosis of gastric carcinoma. Virchows Arch. 2003;443:146–151. doi: 10.1007/s00428-003-0845-8. [DOI] [PubMed] [Google Scholar]
- 28.Malyankar U., Scatena M., Suchland K., Yun T., Clark E., Giachelli C. Osteoprotegerin is an αvβ3-induced, NF-κΒ-dependent survival factor for endothelial cells. J Biol Chem. 2000;275:20959–20962. doi: 10.1074/jbc.C000290200. [DOI] [PubMed] [Google Scholar]
- 29.Cross S.S., Yang Z., Brown N.J., Balasubramanian S.P., Evans C.A., Woodward J.K., Neville-Webbe H.L., Lippitt J.M., Reed M.W., Coleman R.E., Holen I. Osteoprotegerin (OPG): a potential new role in the regulation of endothelial cell phenotype and tumour angiogenesis. Int J Cancer. 2006;118:1901–1908. doi: 10.1002/ijc.21606. [DOI] [PubMed] [Google Scholar]
- 30.Orlova K.A., Crino P.B. The tuberous sclerosis complex. Ann N Y Acad Sci. 2010;1184:87–105. doi: 10.1111/j.1749-6632.2009.05117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steagall W.K., Taveira-DaSilva A.M., Moss J. Clinical and molecular insights into lymphangioleiomyomatosis. Sarcoidosis Vasc Diffuse Lung Dis. 2005;22(Suppl):S49–S66. [PubMed] [Google Scholar]
- 32.Carsillo T., Astrinidis A., Henske E.P. Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci U S A. 2000;97:6085–6090. doi: 10.1073/pnas.97.11.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crooks D.M., Pacheco-Rodriguez G., DeCastro R.M., McCoy J.P., Jr., Wang J.A., Kumaki F., Darling T., Moss J. Molecular and genetic analysis of disseminated neoplastic cells in lymphangioleiomyomatosis. Proc Natl Acad Sci U S A. 2004;101:17462–17467. doi: 10.1073/pnas.0407971101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai X., Pacheco-Rodriguez G., Fan Q.-Y., Haughey M., Samsel L., El-Chemaly S., Wu H.-P., McCoy J.P., Steagall W.K., Lin J.-P., Darling T.N., Moss J. Phenotypic characterization of disseminated cells with TSC2 loss of heterozygosity in patients with lymphangioleiomyomatosis. Am J Respir Crit Care Med. 2010;182:1410–1418. doi: 10.1164/rccm.201003-0489OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adema G.J., de Boer A.J., van’t Hullenaar R., Denijn M., Ruiter D.J., Vogel A.M., Figdor C.G. Melanocyte lineage-specific antigens recognized by monoclonal antibodies NKI-beteb, HMB-50, and HMB-45 are encoded by a single cDNA. Am J Pathol. 1993;143:1579–1585. [PMC free article] [PubMed] [Google Scholar]
- 36.Darling T.N., Pacheco-Rodriguez G., Gorio A., Lesma E., Walker C., Moss J. Lymphangioleiomyomatosis and TSC2-/- cells. Lymphat Res Biol. 2010;8:59–69. doi: 10.1089/lrb.2009.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferrans V.J., Yu Z.-X., Nelson W.K., Valencia J.C., Tatsuguchi A., Avila N.A., Riemenschn W., Matsui K., Travis W.D., Moss J. Lymphangioleiomyomatosis (LAM): a review of clinical and morphological features. J Nippon Med Sch. 2000;67:311–329. doi: 10.1272/jnms.67.311. [DOI] [PubMed] [Google Scholar]
- 38.Matsumoto Y., Horiba K., Usuki J., Chu S.C., Ferrans V.J., Moss J. Markers of cell proliferation and expression of melanosomal antigen in lymphangioleiomyomatosis. Am J Respir Cell Mol Biol. 1999;21:327–336. doi: 10.1165/ajrcmb.21.3.3693. [DOI] [PubMed] [Google Scholar]
- 39.Pacheco-Rodriquez G., Steagall W.K., Crooks D.M., Stevens L.A., Hashimoto H., Li S., Wang J.A., Darling T.N., Moss J. TSC2 loss in lymphangioleiomyomatosis cells correlated with expression of CD44v6, a molecular determinant of metastasis. Cancer Res. 2007;67:10573–10581. doi: 10.1158/0008-5472.CAN-07-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ikeda Y., Taveira-DaSilva A.M., Pacheco-Rodriguez G., Steagall W.K., El-Chemaly S., Gochuico B.R., May R.M., Hathaway O.M., Li S., Wang J.A., Darling T.N., Stylianou M., Moss J. Erythropoietin-driven proliferation of cells with mutations in the tumor suppressor gene TSC2. Am J Physiol Lung Cell Mol Physiol. 2011;300:L64–L72. doi: 10.1152/ajplung.00095.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pacheco-Rodriguez G., Kumaki F., Steagall W.K., Zhang Y., Ikeda Y., Lin J.-P., Billings E.M., Moss J. Chemokine-enhanced chemotaxis of lymphangioleiomyomatosis cells with mutations in the tumor suppressor TSC2 gene. J Immunol. 2009;182:1270–1277. doi: 10.4049/jimmunol.182.3.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hikita A., Tanaka S. Ectodomain shedding of receptor activator of NF-kappaB ligand. Adv Exp Med Biol. 2007;602:15–21. doi: 10.1007/978-0-387-72009-8_2. [DOI] [PubMed] [Google Scholar]
- 43.Nakashima T., Kobayashi Y., Yamasaki S., Kawakami A., Eguchi K., Sasaki H., Sakai H. Protein expression and functional difference of membrane-bound and soluble receptor activator of NF-kappaB ligand: modulation of the expression by osteotropic factors and cytokines. Biochem Biophys Res Commun. 2000;275:768–775. doi: 10.1006/bbrc.2000.3379. [DOI] [PubMed] [Google Scholar]
- 44.Wiley S.R., Schooley K., Smolak P.J., Din W.S., Huang C.-P., Nicholl J.K., Sutherland G.R., Smith T.D., Rauch C., Smith C.A., Goodwin R.G. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 45.Khosla S., Arrighi H.M., Melton L.J., 3rd, Atkinson E.J., O’Fallon W.M., Dunstan C., Riggs B.L. Correlates of osteoprotegerin levels in women and men. Osteoporos Int. 2002;13:394–399. doi: 10.1007/s001980200045. [DOI] [PubMed] [Google Scholar]
- 46.Pantsulaia I., Trofimov S., Kobyliansky E., Livshits G. Contribution of the familial and genetic factors on monocyte chemoattractant protein-1 variation in healthy human pedigrees. Cytokine. 2005;32:117–123. doi: 10.1016/j.cyto.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 47.Bittmann I., Rolf B., Amann G., Lohrs U. Recurrence of lymphangioleiomyomatosis after single lung transplantation: new insights into pathogenesis. Hum Pathol. 2003;34:95–98. doi: 10.1053/hupa.2003.50. [DOI] [PubMed] [Google Scholar]
- 48.Karbowniczek M., Astrinidis A., Balsara B.R., Testa J.R., Lium J.H., Colby T.V., McCormack F.X., Henske E.P. Recurrent lymphangioleiomyomatosis after transplantation: genetic analyses reveal a metastatic mechanism. Am J Respir Crit Care Med. 2003;167:976–982. doi: 10.1164/rccm.200208-969OC. [DOI] [PubMed] [Google Scholar]
- 49.Fili S., Karalaki M., Schaller B. Mechanism of bone metastasis: the role of osteoprotegerin and of host-tissue microenvironment-related survival factors. Cancer Lett. 2009;283:10–19. doi: 10.1016/j.canlet.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 50.Ross E.A., Freeman S., Zhao Y., Dhanjal T.S., Ross E.J., Lax S., Ahmed Z., Hou T.Z., Kalia N., Egginton S., Nash G., Watson S.P., Frampton J., Buckley C.D. A novel role for PECAM-1 (CD31) in regulating haematopoietic progenitor cell compartmentalization between the peripheral blood and bone marrow. PLoS One. 2008;3:e2338. doi: 10.1371/journal.pone.0002338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim S.-J., Kim J.-S., Papadopoulos J., Kim S.W., Maya M., Zhang F., He J., Fan D., Langley R., Fidler I.J. Circulating monocytes expressing CD31 implications for acute and chronic angiogenesis. Am J Pathol. 2009;174:1972–1980. doi: 10.2353/ajpath.2009.080819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McKenney J.K., Weiss S.W., Folpe A.L. CD31 expression in intratumoral macrophages a potential diagnostic pitfall. Am J Surg Pathol. 2001;25:1167–1173. doi: 10.1097/00000478-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 53.Zhang X., Travis W.D. Pulmonary lymphangioleiomyomatosis. Arch Pathol Lab Med. 2010;134:1823–1828. doi: 10.5858/2009-0576-RS.1. [DOI] [PubMed] [Google Scholar]
- 54.Karbowniczek M., Yu J., Henske E.P. Renal angiomyolipomas from patients with sporadic lymphangioleiomyomatosis contain both neoplastic and non-neoplastic vascular structures. Am J Pathol. 2003;162:491–500. doi: 10.1016/S0002-9440(10)63843-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Valencia J.C., Pacheco-Rodriguez G., Carmona A.K., Xavier J., Bruneval P., Riemenschneider W.K., Ikeda Y., Yu Z.-X., Ferrans V.J., Moss J. Tissue-specific renin-angiotensin system in pulmonary lymphangioleiomyomatosis. Am J Respir Cell Mol Biol. 2006;35:40–47. doi: 10.1165/rcmb.2005-0387OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Valencia J.C., Matsui K., Bondy C., Zhou J., Rasmussen A., Cullen K., Yu Z.X., Moss J., Ferrans V.J. Distribution and mRNA expression of insulin-like growth factor system in pulmonary lymphangioleiomyomatosis. J Investig Med. 2001;49:421–433. doi: 10.2310/6650.2001.33787. [DOI] [PubMed] [Google Scholar]
- 57.Lesma E., Grande V., Carelli S., Brancaccio D., Canevini M.P., Alfano R.M., Coggi G., DiGiulio A.M., Gorio A. Isolation and growth of smooth muscle-like cells derived from tuberous sclerosis complex-2 human renal angiomyolipoma. Am J Pathol. 2005;167:1093–1103. doi: 10.1016/S0002-9440(10)61198-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kwon M.-J., Jang B., Yi J.Y., Han I.-O., Oh E.S. Syndecans play dual roles as cell adhesion receptors and docking receptors. FEBS Lett. 2012;586:2207–2211. doi: 10.1016/j.febslet.2012.05.037. [DOI] [PubMed] [Google Scholar]
- 59.Zannettino A.C.W., Holding C.A., Diamond P., Atkins G.J., Kostakis P., Farrugia A., Gamble J., To L.B., Findlay D.M., Haynes D.R. Osteoprotegerin (OPG) is localized to the Weibel-Palade bodies of human vascular endothelial cells and is physically associated with von Willebrand factor. J Cell Physiol. 2005;204:714–723. doi: 10.1002/jcp.20354. [DOI] [PubMed] [Google Scholar]
- 60.Yu J., Henske E.P. mTOR activation, lymphangiogenesis, and estrogen-mediated cell survival: the “perfect storm” of pro-metastatic factors in LAM pathogenesis. Lymphat Res Biol. 2010;8:43–49. doi: 10.1089/lrb.2009.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bengtsson A.K., Ryan E.J., Giordano D., Magaletti D.M., Clark E.A. 17β-Estradiol (E2) modulates cytokine and chemokine expression in human monocyte-derived cells. Blood. 2004;104:1404–1410. doi: 10.1182/blood-2003-10-3380. [DOI] [PubMed] [Google Scholar]
- 62.Taveira-DaSilva A.M., Stylianou M.P., Hedin C.J., Hathaway O., Moss J. Bone mineral density in lymphangioleiomyomatosis. Am J Respir Crit Care Med. 2005;171:61–67. doi: 10.1164/rccm.200406-701OC. [DOI] [PubMed] [Google Scholar]
- 63.Taveira-DaSilva A., Hathaway O.M., Sachdev V., Shizukuda Y., Birdsall C.W., Moss J. Pulmonary artery pressure in lymphangioleiomyomatosis. Chest. 2007;132:1573–1578. doi: 10.1378/chest.07-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kudlacek S., Schneider B., Woloszczuk W., Pietschmann P., Willvonseder R. Serum levels of osteoprotegerin increase with age in a healthy adult population. Bone. 2003;32:681–686. doi: 10.1016/s8756-3282(03)00090-5. [DOI] [PubMed] [Google Scholar]
- 65.Wagner D., Fahrleitner-Pammer A. Levels of osteoprotegerin (OPG) and receptor activator for nuclear factor kappa B ligand (RANKL) in serum: are they of any help? Wien Med Wochenschr. 2010;160:452–457. doi: 10.1007/s10354-010-0818-x. [DOI] [PubMed] [Google Scholar]
- 66.Reid P.E., Brown J., Holen I. Breast cancer cells stimulate osteoprotegerin (OPG) production by endothelial cells through direct cell contact. Mol Cancer. 2009;8:49–58. doi: 10.1186/1476-4598-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]