Abstract
First trimester human decidua is composed of decidual cells, CD56brightCD16− decidual natural killer (dNK) cells, and macrophages. Decidual cells incubated with NK cell–derived IFN-γ and either macrophage-derived TNF-α or IL-1β synergistically enhanced mRNA and protein expression of IP-10 and I-TAC. Both chemokines recruit CXCR3-expressing NK cells. This synergy required IFN-γ receptor 1 and 2 mediation via JAK/STAT and NFκB signaling pathways. However, synergy was not observed on neutrophil, monocyte, and NK cell–recruiting chemokines. Immunostaining of first trimester decidua localized IP-10, I-TAC, IFN-γR1, and -R2 to vimentin-positive decidual cells versus cytokeratin-positive interstitial trophoblasts. Flow cytometry identified high CXCR3 levels on dNK cells and minority peripheral CD56brightCD16− pNK cells and intermediate CXCR3 levels on the majority of CD56dimCD16+ pNK cells. Incubation of pNK cells with either IP-10 or I-TAC elicited concentration-dependent enhanced CXCR3 levels and migration of both pNK cell subsets that peaked at 10 ng/mL, whereas each chemokine at a concentration of 50 ng/mL inhibited CXCR3 expression and pNK cell migration. Deciduae from women with preeclampsia, a leading cause of maternal and fetal morbidity and mortality, displayed significantly lower dNK cell numbers and higher IP-10 and I-TAC levels versus gestational age–matched controls. Significantly elevated IP-10 levels in first trimester sera from women eventually developing preeclampsia compared with controls, identifying IP-10 as a novel, robust early predictor of preeclampsia.
In normal human pregnancy, blastocyst-derived extravillous cytotrophoblasts (EVTs) traverse the underlying decidua and inner third of the myometrium. As they cross the decidua, EVTs detach from anchoring placental villous columns, then breech spiral arteries and arterioles to mediate replacement of the smooth muscle tunica media and endothelium. This invasive process can occur either from the vessel lumen into the tunica media, mediated by endovascular EVTs, or from the surrounding decidualized stroma into the tunica media, mediated by interstitial EVTs. On entering the vessel, the epithelial cell adhesion molecule phenotype of trophoblasts is converted to an endothelial cell–like adhesion molecule phenotype,1 and spiral vessels are transformed into low-resistance, high-capacity conduits that increase uteroplacental blood flow to the developing fetal–placental unit.1,2
Preeclampsia, a major cause of maternal and perinatal morbidity and mortality,3 is frequently associated with shallow trophoblast invasion leading to incomplete uterine vascular remodeling.4 The resulting decreased uteroplacental blood flow can elicit fetal growth restriction and/or elaboration of antiangiogenic and proinflammatory placental factors that mediate the maternal syndrome of hypertension and proteinuria, which usually occurs later in pregnancy and can produce end-organ damage.5
At the human implantation site, the decidua is composed primarily of resident decidual cells (50%) and a diverse immune cell population (40%). The latter is dominated by decidual natural killer (dNK) cells (70%), macrophages (20%), and T lymphocytes (10%) with small percentages of dendritic cells and B lymphocytes.6 Unlike the major antigen-presenting cells, macrophages and dendritic cells, NK cells act as specialized lymphocytes and normally mediate innate immunity by killing tumor and virus-infected cells without prior sensitization before the onset of T- and B-cell–mediated adaptive immunity. In the circulation, NK cells comprise approximately 5% to 15% of the lymphocyte population and consist primarily of two functionally distinct subsets. The majority, CD56dimCD16+ peripheral NK (pNK) cells (90%), exhibit greater cytotoxicity, express high levels of killer cell immunoglobulin-like receptors (KIRs), as well as CD57, and usually do not secrete cytokines. By contrast, the absence of CD16 expression by the minority, less mature, CD56brightCD16− pNK cells (10%), accounts for their inability to mediate antibody-dependent cell toxicity.7 These CD56brightCD16− pNK cells do not display KIRs, but express low levels of perforin and high levels of the CD94/NKG2 receptor and adhesion-mediating L-selectin.8 They also serve as the major pNK cell source of secreted immunoregulatory cytokines. Chief among these is interferon-gamma (IFN-γ). This prototypic NK cell cytokine is expressed by CD56brightCD16− pNK cells in response to IL-12 acting in concert with either other cytokines (ie, IL-1, IL-2, IL-15, or IL-18) or engagement of either the CD16 (FcγRIIIa) or NKG2D pNK cell-activating receptors.9 Recently, the microRNA (miR155) was also shown to function as a positive regulator of IFN-γ expression in pNK cells.10 Other immunoregulatory cytokines expressed by CD56brightCD16− pNK cells include tumor necrosis factor-β (TNF-β), granulocyte-macrophage colony stimulating factor (GM-CSF), and IL-10 and -13.7
Like the minority circulating NK cell population, approximately 80% of dNK cells are also CD56brightCD16−.7,11 Extensive investigation indicates that dNK cells represent a unique immune cell subtype that plays a crucial pregnancy-supporting role by fostering immune tolerance of the semiallogeneic fetal–placental unit while promoting EVT invasion and spiral artery and arteriole remodeling via expression of vascular endothelial and placental growth factors.7,11–13
The current study postulates that at the human implantation site, targeting of the majority resident decidual cells by paracrine effectors, secreted primarily by dNK cells and macrophages, recruits additional CD56brightCD16− pNK cells into the decidua. In support of this hypothesis, flow cytometric analysis singled out high CXCR3 expression by CD56brightCD16− dNK cells and CD56brightCD16− pNK cells, and lower expression by CD56dimCD16+ pNK cells. Complementing these observations, mRNA and protein expression of IFN-γ–induced protein 10 (IP-10/CXCL10) and IFN-inducible T cell–α chemoattractant (I-TAC/CXCL11), chemokines that specifically recruit CXCR3-bearing pNK cells,14 proved to be synergistically enhanced during incubation of human leukocyte-free first trimester decidual cells with the primary NK cell–derived cytokine, IFN-γ,12 and either of the primary macrophage-derived cytokines, TNF-α or IL-1β.15 These observations were augmented by: i) evaluating the potential involvement of both IFN-γ receptors (IFN-γR1 and IFN-γR2),16 as well as signaling pathways in mediating this synergistic up-regulation of IP-10 and I-TAC in decidual cells; ii) functional studies that assessed the individual and combined effects of IP-10 and I-TAC on CXCR3 expression and migration of both subsets of pNK cells; iii) in situ studies that assessed cellular localization of IP-10, I-TAC, and IFN-γR1 and -R2 at the human implantation site and compared IP-10 and I-TAC levels and the abundance of CD56brightCD16− NK cells in preeclamptic versus gestational age–matched control decidua; and iv) measurement of IP-10 and I-TAC levels in first trimester sera to determine whether either chemokine is an early predictor of preeclampsia.
Materials and Methods
Tissues and Blood Samples
Decidual samples obtained from patients undergoing termination of pregnancy (6 to 12 weeks gestation) at the New York University Medical Center and Yale-New Haven Hospital, under New York University Institutional Review Board and Yale Human Investigative Committee approval, were used to isolate decidual cells and for flow cytometric analysis of dNK cells. Decidual samples (6 to 12 weeks and 37 to 40 weeks) were also obtained under institutional review board approval at the Mackay Memorial Hospital in Taiwan and used for immunostaining and tissue enzyme-linked immunosorbent assays (ELISAs). Peripheral blood samples from volunteers were obtained from the Harvard Department of Molecular and Cellular Biology for use in flow cytometric analysis, and from the Yale-New Haven Hospital for use in measuring CXCR3 expression by NK cells, as well as their migration in a Transwell assay, under institutional review board and human investigative committee approval, respectively.
Isolation of Decidual Cells and NK Cells
Decidual tissues derived from first trimester elective terminations were washed in sterile PBS until clear of blood, minced, and then digested with 0.1% collagenase type IV and 0.01% DNase (Sigma-Aldrich, St. Louis, MO) in RPMI 1640 containing 20 μg/mL penicillin/streptomycin, 1 μL/mL fungizone (Invitrogen, Carlsbad, CA) in a 37°C shaking water bath for 30 minutes followed by washing with PBS. The digestate was subjected to consecutive filtration through 100-μm, 70-μm, and 40-μm Millipore filters (Millipore, Billerica, MA). The resulting pellet was resuspended in complete RPMI (Invitrogen), then seeded onto six 10-cm Petri dishes and incubated at 37°C in a humidified 95% O2:5% CO2 incubator for 2 hours to enrich for decidual cells (plastic adherent) and lymphocytes (nonadherent). The leukocyte-enriched supernatants were washed with complete RPMI and cell pellets resuspended in complete RPMI, layered over Ficoll-Hypaque (Pharmacia and Upjohn, Chicago, IL), and centrifuged for 20 minutes at 509 × g. The decidual leukocyte band at the interface was washed twice with PBS plus 2% fetal calf serum, and then subjected to flow cytometric analysis (see below).
Adherent decidual cells were detached with 0.1% trypsin-EDTA (Sigma-Aldrich), resuspended in RPMI, grown to confluence on polystyrene tissue culture dishes, harvested using trypsin-EDTA, and analyzed by flow cytometry with anti-CD45 and anti-CD14 monoclonal antibodies (BD Pharmingen, San Diego, CA) to monitor the presence of leukocytes after each passage. After three to four passages, cell cultures were found to be leukocyte-free (<1%). Confluent decidual cells were vimentin-positive and cytokeratin-negative, and displayed progesterone-induced decidualization-related morphological and biochemical changes, including elevated prolactin and plasminogen activator inhibitor-1 (PAI-1), and reduced interstitial collagenase and stromelysin-1 expression (results not shown). Cell aliquots were frozen in fetal calf serum/dimethyl sulfoxide (9:1 ratio) (Sigma-Aldrich) and stored in liquid nitrogen.
As previously described, peripheral blood from anonymous healthy reproductive-age donors was used to isolate NK cells using Ficoll followed by enrichment for NK cells with RosetteSep according to the manufacturer's instructions (Stem Cell Technologies, Vancouver, BC, Canada).17
Experimental Incubations
Thawed decidual cells were incubated in basal medium (BM), a phenol red–free 1:1 v/v mix of Dulbecco's modified Eagle's medium (Invitrogen) and Ham's F-12 (Flow Labs, Rockville, MD), with 100 U/mL penicillin, 100 μg/mL streptomycin, 0.25 μg/mL fungizone supplemented with 10% charcoal-stripped calf serum (BMS). After two additional passages, confluent cultures were incubated in parallel in BMS containing 10−8 mol/L estradiol (E2) with 10−7 mol/L medroxyprogesterone acetate (MPA) (Sigma-Aldrich), which was used in place of progesterone because of its greater stability in culture.18 After 7 days, the cultures were washed twice with HBSS to remove residual serum elements and then switched to a defined medium consisting of BM plus ITS+ (Collaborative Research, Waltham, MA), 5 μmol/L FeSO4, 0.5 μmol/L ZnSO4, 1 nmol/L CuSO4, 20 nmol/L Na2SeO3, trace elements (Invitrogen), 50 μg/mL ascorbic acid (Sigma-Aldrich), and 50 ng/mL epidermal growth factor (Becton-Dickinson, Bedford, MA) with steroids alone or with 0.01 to 10 ng/mL IFN-γ, IL-1β, TNF-α, or with IFN-γ plus IL-1β or TNF-α (R&D Systems). After the incubation, cells were harvested by scraping into ice-cold PBS, pelleted, and extracted in ice-cold lysis buffer. Conditioned medium supernatants and cell lysates were stored at −70°C. Total RNA was extracted from parallel incubations with Tri Reagent (Sigma-Aldrich).
Signaling Pathway Mediation of IP-10 and I-TAC Expression
Confluent first trimester decidual cells were pretreated in defined medium ± 10 μmol/L NFκB inhibitor BAY-11-7082 (BAY; EMD Chemical, San Diego, CA), or 100 μmol/L Janus kinase/signal transducers and activators of transcription (JAK/STAT) inhibitor 5′-deoxy-5′-(methylthio)adenosine (MTA) (EMD Chemical) or BAY + MTA. The cultures were then incubated with control (vehicle) or TNF-α or IFN-γ or TNF-α + IFN-γ in parallel either for 24 hours and then the conditioned medium was collected for ELISAs, or for 30 minutes and then subjected to nuclear extraction for ELISA-based electromobility shift assay (E-EMSA).
E-EMSA
First trimester decidual cells were treated for 30 minutes as described above, then rinsed in ice-cold PBS containing a protease inhibitor cocktail, and immediately stored at −80°C. A Nuclear Extraction Kit was used according to the manufacturer's instruction (Active Motive, Carlsbad, CA). NFκB transcriptional activity was determined using a TransAM NFκB transcription factor ELISA kit (Active Motive) following the manufacturer's instructions. This kit measures both p65 and p50 NFκB subunits that bind to 96-well plates precoated with NFκB DNA response elements (NFκB-RE). A colorimetric reaction was obtained using a 96-well plate luminometer at 450 nm and an optional reference wavelength of 655 nm.
Flow Cytometry
Flow cytometry was performed as described previously.17 Briefly, cell sorting and fluorescence measurements were carried out on a MoFlo high-performance cell sorter (Cytomation, Fort Collins, CO). For fluorescence measurements, the following mouse anti-human monoclonal antibodies were obtained from BD Pharmingen: CD16–fluorescein isothiocyanate (IgG1), CD56-phycoerythrin (IgG1), and CD3-Cyc (IgG1), and from R&D Systems: Phycoerythrin-conjugated mouse monoclonal anti-human CCR1 (IgG2b), CCR9 (IgG2a), CXCR1 (IgG2a), CXCR3 (IgG1), CXCR4 (IgG2a), and CCR7 (IgG2a). The CCR7 antibody was used together with goat anti-mouse IgG-phycoerythrin as a secondary fluorochrome conjugate (Caltag, Burlingame, CA). All IgG isotype control antibodies were obtained from BD Biosciences (Los Angeles, CA). For flow cytometric analysis, cells were incubated with 10% human serum to block Fc-receptors before incubation with monoclonal antibodies on ice for 30 minutes, followed by washing twice in PBS with 0.5% bovine serum albumin. Surface expression of chemokine receptors on pNK and dNK cells was detected with a standard FACSCalibur flow cytometer (Immunocytometry Systems; Becton Dickinson, San Jose, CA). In these measurements, data from 10,000 single-cell events were collected and analyzed using CellQuest version 2.0 (Becton Dickinson) or FlowJo version 7.6.5 (TreeStar, San Carlos, CA).
ELISA
Total cell protein levels were measured by a modified Lowry assay (Bio-Rad Laboratories, Hercules, CA). Commercial ELISA kits measured immunoreactive levels of IP-10, I-TAC, IL-6, IL-8, IL-11, and monocyte chemotactic protein (MCP)-1 in decidual cell–conditioned medium supernatants according to the manufacturer's instructions (Duoset kits; R&D Systems). The IP-10 and I-TAC ELISAs have sensitivities of 8 and 2 pg/mL, respectively. The intra-assay coefficients of variation (COV) for IP-10 and I-TAC are each <5.0%, and interassay COV are each <10%. The IL-6 ELISA has a sensitivity of 3 pg/mL; intra-assay and interassay COV are 3.1% and 2.7%, respectively. The IL-8 ELISA has a sensitivity of 8 pg/mL, and intra-assay and interassay COV are 4.6% and 6.7%, respectively. The IL-11 ELISA has a sensitivity of 10 pg/mL, and intra-assay and interassay COV are 2.4% and 6.9%, respectively. The MCP-1 ELISA has a sensitivity of 4 pg/mL, and intra-assay and interassay COV are 5.0% and 5.1%, respectively.
Microarray Analysis
Total RNA from decidual cell cultures was extracted as previously described.19 For microarray studies, cells harvested with QIAzol lysis reagent (Qiagen, Valencia, CA) were used to prepare total RNA, which was cleaned and precipitated using the RNeasy Mini Kit (Qiagen). The quality of RNA was confirmed by a Bio-Rad bioanalyzer. Array processing was performed at the W. M. Keck Foundation Biotechnology Resource Laboratory at Yale University using HG_U133 Plus 2.0 chips (Affymetrix, Santa Clara, CA). Raw data without normalization generated from Affymetrix GeneChip Operating Software version 1.2 (GCOS 1.2) (Affymetrix) were analyzed by GeneSpring software version 7.2 (Agilent Technologies/Silicon Genetics, Redwood City, CA). Gene readouts were normalized to the 50th percentile of the distribution of all measurements in each chip. Normalization for each gene across chips was performed using the median value of each gene throughout different chips in the same experimental condition. Normalized data were filtered to eliminate those genes whose mRNA were found to be absent in all experimental conditions and replicates. A parametric test assuming unequal variance was then used to assess statistical significance. Fold-ratios were derived by comparing normalized data between E2 + MPA versus E2 + MPA + IL-1β or TNF-α groups with a cutoff of ≥2-fold. Microarray results were confirmed by quantitative RT-PCR.
Quantitative RT-PCR
Total RNA from decidual cell cultures derived from eight patients was isolated and purified using a column kit with DNase treatment (Ambion, Austin, TX). Generation of cDNA from purified DNA-free RNA used the SuperScript II Reverse Transcriptase kit (Invitrogen). qPCR was performed using TaqMan gene expression assays for IP-10, I-TAC, and the reference gene β-actin (Applied Biosystems, Foster City, CA). Standard curves were created from serial dilutions of a known sample for each gene assay. All samples were run in duplicate and the average used for each sample. The efficiency for each TaqMan gene assay was >94%. The TaqMan Assay ID#s were: Hs00171042_m1 (for IP-10), Hs00171138_m1 (for I-TAC), and Hs99999903_m1 (for β-actin). The relative standard curve method was used (versus the comparative CT method, ΔΔCT). For each standard curve, an independent sample known to contain very high expression of the gene of interest was used. Serial dilutions were made from this independent sample and used in the standard curve to calculate the unknowns according to the manufacturer's instructions.
Western Blotting
Western blot analysis was performed on conditioned medium supernatants, diluted 1:6 in reducing Laemmli 6× sample buffer (Boston Bioproducts, Ashland, MA), then boiled for 5 minutes. Recombinant human IP-10 and I-TAC proteins (R&D Systems) were treated similarly as positive controls. Centrifuged conditioned medium supernatants and positive controls were subjected to SDS-PAGE on 15% Tris-HCl gels (BioRad) with subsequent electroblotting transfer onto 0.20-μm nitrocellulose membranes (BioRad). After transfer, membranes were blocked for 3 hours at room temperature in Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE) and then incubated overnight at 4°C with goat antibodies to IP-10 and I-TAC (R&D Systems). After washing, membranes were incubated in donkey anti-goat IRDye 680 (LI-COR) at room temperature for 1 hour and washed again before visualizing with the Odyssey infrared imaging system (LI-COR).
siRNA Transfection
Transient transfections used a modified protocol.20 Briefly, to prepare siRNA/lipid solutions for a 12-well plate scale transfection, 64 pmol (equivalent of 20 μL of a 3 μmol/L stock) of each siRNA was diluted in 200 μL of OPTI-MEMI (Invitrogen) and incubated at room temperature for 5 minutes. For IFN-γR1 and IFN-γR2 double knockdown, 32 pmol of each siRNA was combined in a final amount of 64 pmol. In a separate tube, 8 μL of Lipofectamine 2000 (Invitrogen) was diluted in 200 μL of OPTI-MEMI and incubated at room temperature for 5 minutes. The contents of both tubes were mixed by gentle pipetting and then incubated at room temperature for 30 to 50 minutes. The resulting 400-μL solution was used to transfect 2 × 105 cells (see below). The siRNAs specific for IFN-γR1 (siR1) (ON-TARGETplus SMARTpool L-011057-00-0005) and IFN-γR2 (siR2) (ON-TARGETplus SMARTpool L-012713-00-0005), and control nontarget siRNA (D-001810-10−05) were from Thermo Fisher Scientific/Dharmacon Products (Lafayette, CO).
To prepare cell pellets for transfection, first trimester leukocyte-free decidual cells grown in BM were dissociated using 0.25% Trypsin/EDTA (Sigma-Aldrich). Cell pellets were collected by centrifugation at 700 × g for 2 minutes. Each cell pellet containing about 2 × 105 cells was gently resuspended in 400 μL of the siRNA/lipid solution prepared as described above, and the cell suspension was incubated at room temperature for 10 minutes (note, incubation for >15 minutes decreases cell viability). At the end of the incubation, 1.6 mL of BM was added and the resulting cell suspension transferred to a well of a 12-well plate, followed by incubation in a CO2 tissue culture incubator overnight. The medium was replaced on the second day of incubation.
Immunohistochemistry
First and third trimester deciduae were snap-frozen in liquid nitrogen for 5-μm cryosections. Morphology was confirmed after H&E staining. Sections were air-dried and fixed in ice-cold acetone for 10 minutes, then rehydrated with PBS for 5 minutes, subjected to protein block (Dako, Atlanta, GA) for 20 minutes, and incubated in goat anti-human IP-10 (dilution 1:10; R&D Systems), rabbit anti-human I-TAC, or mouse anti-human IFN-γR1/R2 (dilution 1:250; Abcam, Cambridge, MA) antibodies for 1 hour at room temperature. After 3 washes in PBS, sections were incubated with fluorescein isothiocyanate–conjugated rabbit anti-goat (dilution 1:20; Dako), donkey anti-mouse (dilution 1:50; Millipore), or donkey anti-rabbit (dilution 1:50; Millipore) antibodies for 1 hour.
Sections were double stained by washing 3× with PBS and incubating in either goat anti-human vimentin antibody (dilution 1:500; Sigma-Aldrich) or mouse anti-human vimentin antibody (dilution 1:400; Millipore), mouse anti-human cytokeratin 7 (CK7) antibody (dilution 1:100; Dako), goat anti-CD16 antibody (dilution 1:100; R&D Systems), or mouse anti-CD56 antibody (dilution 1:100; R&D Systems) for 1 hour at room temperature. After three additional washes in PBS, the sections were incubated with rhodamine-conjugated goat anti-mouse antibody (dilution 1:100; Sigma-Aldrich) or donkey anti-goat antibody (dilution 1:100; Millipore), or goat fluorescein isothiocyanate–conjugated anti-mouse antibody (dilution 1:100; Sigma-Aldrich) for 1 hour. Staining specificity was confirmed by substituting isotype IgG antibody for the primary antibody. Finally, the sections were washed 3× in PBS, stained with DAPI diluted 1:5 × 105 in double-distilled H2O (Sigma-Aldrich), and mounted in a non-fade mounting medium (Life Sciences International, Basingstoke, UK). Immunofluorescence was assessed at ×400 with a Zeiss microscope (Carl Zeiss Vision, München-Hallbergmoos, Germany) equipped with a cooled charge-coupled device camera (AxioCam HR; Carl Zeiss Microimaging, Jena, Germany). No cross-reaction was found among these antibodies. Five randomly selected fields from each section (three sections/tissue) from third trimester deciduae were examined. The number of cells per field (106 pixel2) was counted and calculated as the mean of 15 fields. All of the sections were evaluated by two individuals blinded to the diagnosis.
Functional pNK Cell Studies
Circulating NK cells were isolated from healthy donors using the human NK Cell Isolation Kit (130-092-657; Miltenyi Biotec, Bergisch Gladbach, Germany) and incubated at 5 × 105 cells/mL in BM containing IP-10 or I-TAC or both at a final concentration of 0.1, 1, 10, or 50 ng/mL each as indicated. After 48 hours incubation at 37°C and 5% CO2, cells were collected followed by immunofluorescence staining and flow cytometric analysis to assess purity and CXCR3 expression. For the migration assay, 2 × 105 pNK cells in 100 μL of BM were incubated with either IP-10 or I-TAC or both at a final concentration of 0.1, 1, 10, or 50 ng/mL for 48 hours and then loaded onto Transwell filters (5-μm pore size, 24-well cell clusters; Corning, Corning, NY). The filters were then placed in wells containing 600 μL of medium supplemented with IP-10 or I-TAC or both. Although the pre-incubation was carried out at various concentrations, the concentration of IP-10 or I-TAC or both in the bottom chamber was 100 ng/mL. To determine nonspecific migration, two wells without chemokine(s) were used as controls. After 150 minutes incubation at 37°C and 5% CO2, the upper chambers were removed, and cells in the bottom chamber were collected and counted. The percentage of migrated cells was calculated by subtracting the value of spontaneously migrated cells from the number of migrated cells at a given chemokine concentration, and the result was divided by the total number of cells initially loaded onto the Transwell filter.
Peripheral Blood IP-10 and I-TAC Concentrations in Patients Developing Preeclampsia Versus Patients with Uncomplicated Pregnancies
Serum samples from consenting pregnant women undergoing first trimester aneuploidy screening were collected under institutional review board approval at Mackay Memorial Hospital, Taiwan. Among women who eventually gave birth at Mackay Memorial Hospital from April 2010 to February 2011, 30 developed preeclampsia in the third trimester. Preeclampsia was defined according to standard criteria.21 Gestational age–matched first trimester control (n = 90) blood samples were obtained from women with subsequent uneventful term deliveries. Gestational ages did not differ significantly between preeclamptic patients (12.4 ± 0.4 weeks) and controls (12.5 ± 0.5 weeks) (P = 0.402) (Table 1). Immunoreactive levels of IP-10 and I-TAC in conditioned media were measured by commercial ELISA kits according to the manufacturer's instructions (Duoset kits; R&D Systems).
Table 1.
Characteristics of Pregnant Women with Preeclampsia and of Controls
| Controls (n = 90) | Preeclampsia (n = 30) | P value | |
|---|---|---|---|
| Age, years | 30.8 ± 2.8 | 32.1 ± 3.4 | 0.06 |
| Parity | 1.2 ± 0.4 | 1.3 ± 0.5 | 0.179 |
| Gestational age of delivery, weeks | 38.7 ± 1.1 | 37.7 ± 2.2 | 0.001 |
| Birth weight, g | 3215.8 ± 284.4 | 2755.5 ± 658.5 | <0.001 |
| Gestational age of sample collection, weeks | 12.5 ± 0.5 | 12.4 ± 0.4 | 0.402 |
Tissue ELISA Measurement of IP-10 and I-TAC Concentrations in Preeclamptic Versus Gestational Age-Matched Control Decidua
Frozen deciduae from patients with preeclampsia and gestational age–matched controls were homogenized in radioimmunoprecipitation assay lysis buffer (NP-40 2%, SDS 0.02%, sodium deoxycholate 1%, 1× PBS) with protease inhibitor cocktail (Sigma-Aldrich). Total protein was measured using protein assay dye reagent (PAK500; Strong Biotech, Taipei, Taiwan). Plates coated with 25 μg/mL sample were incubated overnight at 25°C, followed by blocking for 1 hour with PBS containing 2% bovine serum albumin. Subsequently, anti-human IP-10 or I-TAC (1 μg/mL; R&D Systems) antibody was added and incubated for 1 hour. The plates were incubated with horseradish peroxidase–conjugated secondary antibody (dilution 1:5000; Chemicon/Millipore) for 1 hour, then incubated with NeA-Blue (Clinical, Mansfield, MA) for 1 hour. The reaction was stopped by 2N H2SO4. Relative chemokine levels were expressed as absorbance.
Statistical Analysis
For ELISA and RT-qPCR IP-10 and I-TAC data from decidual cell cultures, nonparametric tests were used because data were not distributed normally. Comparisons of control and treatment groups used the Kruskal-Wallis analysis of variance on ranks test followed by the Student-Newman-Keuls post hoc test with a P value <0.05 representing statistical significance. Student's t-tests were used for all pNK cell data, with P values <0.01 representing statistical significance. For both serum and tissue ELISA data and fluorescent immunohistochemistry data from preeclamptic versus control patients, the Student's t-test was used, with a P value <0.05 representing statistical significance.
Results
Flow Cytometric Analysis of NK Cell Chemokine Receptors
Figure 1A displays representative histograms for the expression of six chemokine receptors (CCR1, CCR7, CCR9, CXCR1, CXCR3, and CXCR4) obtained by gating on CD56brightCD16− dNK cells or on either CD56brightCD16− or CD56dimCD16+ pNK cells. Comparison of mean fluorescence intensities in Figure 1B indicates that compared with the CD56dimCD16+ population of pNK cells, CD56brightCD16− dNK cells express relatively higher levels of CXCR3, CCR9, CCR1, and CXCR4. Of these chemokine receptors, CXCR3 is also highly expressed on minority circulating CD56brightCD16− pNK cells. In this subset, CXCR3 is expressed at higher levels than CCR7, and much higher than CXCR4, CCR1, CCR9, and CXCR1. On the majority circulating CD56dimCD16+ pNK cells, only CXCR1, a receptor for IL-8, is highly expressed, whereas CCR7 and CXCR3 are expressed at low-to-intermediate levels, and CCR1 and CXCR4 at low levels (Figure 1B). By indicating that both decidual and peripheral CD56brightCD16− NK cell subsets express significantly elevated levels of CXCR3, Figure 1 suggests a potential role for CXCR3 in mediating preferential trafficking of circulating CD56bright CD16− NK cells to the decidua, thereby explaining the unusual abundance of these cells at this site.
Figure 1.
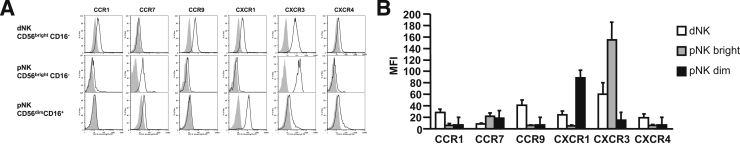
Chemokine receptor expression by decidual and peripheral NK cells. Decidual and peripheral lymphocyte suspensions were stained with fluorescence-conjugated monoclonal antibodies reactive with CD3, CD16, CD56, the indicated chemokine receptors, or with the appropriate isotype control antibodies. Initially, cells were gated by forward scatter/side scatter characteristics. CD56 and CD16 expression were each analyzed after setting a gate on CD3− cells. A: Representative histograms of chemokine receptor expression in NK cells. Isotype: filled gray curve; anti-chemokine receptor antibody: solid black curve. CD56brightCD16− dNK cells, minority CD56brightCD16− pNK cells, and majority CD56dimCD16+ pNK cells. B: Mean fluorescence intensity (MFI) ± SD of chemokine receptor expression in NK cell from three donors.
Effects of TNF-α, IL-1β, and IFN-γ on First Trimester Decidual Cell IP-10 and I-TAC Expression
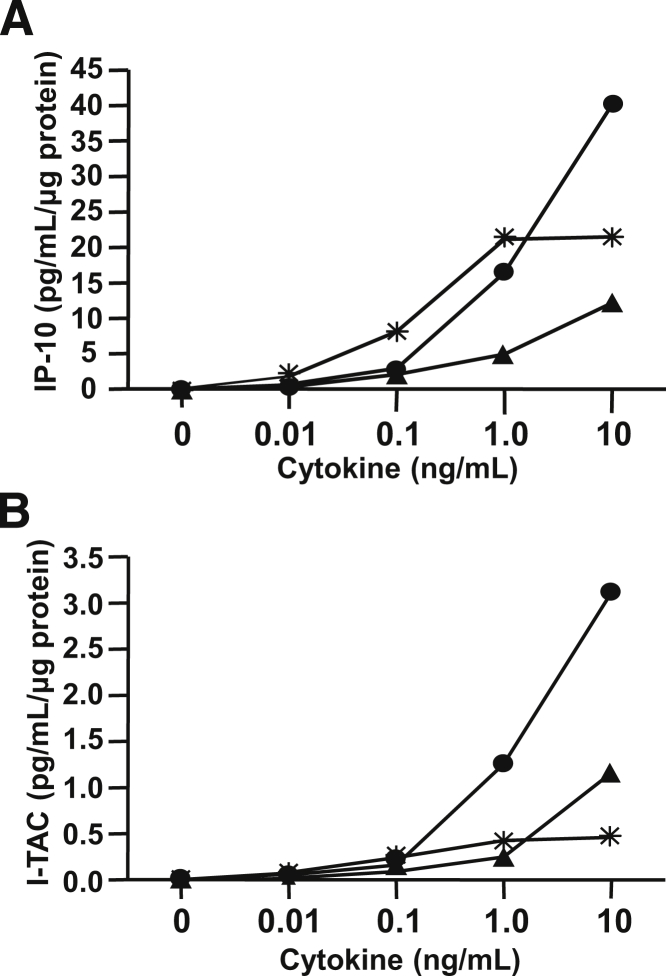
The microarray screening results displayed in Table 2 indicate that in cultured first trimester decidual cells incubated with E2 + MPA, to mimic the hormonal milieu of early pregnancy, addition of either TNF-α or IL-1β significantly up-regulates steady-state levels of mRNAs for the cognate CXCR3 ligands, IP-10, and I-TAC. Figure 2 displays the effects of incubating first trimester leukocyte-free decidual cell monolayers over a physiological range of concentrations up to 10 ng/mL TNF-α or IL-1β or IFN-γ on secreted levels of immunoreactive IP-10 (Figure 2A) and I-TAC (Figure 2B). The responses are linear over the entire concentration range for TNF-α and IFN-γ, but attain a maximum effect at 1.0 ng/mL for IL-1β.
Table 2.
Microarray Results for IP-10 and I-TAC mRNA in First Trimester Decidual Cells
| First trimester |
Fold change |
|||
|---|---|---|---|---|
| Chemokine | Cognate receptor | Alternate name | EM vs EMI | EM vs EMTNF |
| CXCL10 | CXCR3 | IP-10 | 239.33 | 174.86 |
| CXCL11 | CXCR3 | I-TAC | 154.34 | 58.65 |
Leukocyte-free first trimester decidual cells were primed in E2 + MPA (Control) and then switched to defined medium with E2 + MPA ± 10 ng/mL IL-1β (EMI) or TNF-α (EMTNF) for 6 hours. mRNA levels were measured by microarray. Results are shown as fold-change versus control.
Figure 2.
Concentration-dependent effects of TNF-α or IL-1β or IP-10 and I-TAC protein levels in cultured first trimester decidual cells. Leukocyte-free first trimester decidual cells were primed in E2 + MPA then switched to a defined medium with E2 + MPA ± IL-1β (asterisk) or TNF-α (black circle) or IFN-γ (black triangle) at the concentrations indicated on the abscissa for 24 hours. Levels of IP-10 (A) and I-TAC (B) were measured by ELISA in conditioned medium supernatants and normalized to total cell protein (see Materials and Methods for details) (average of two separate experiments).
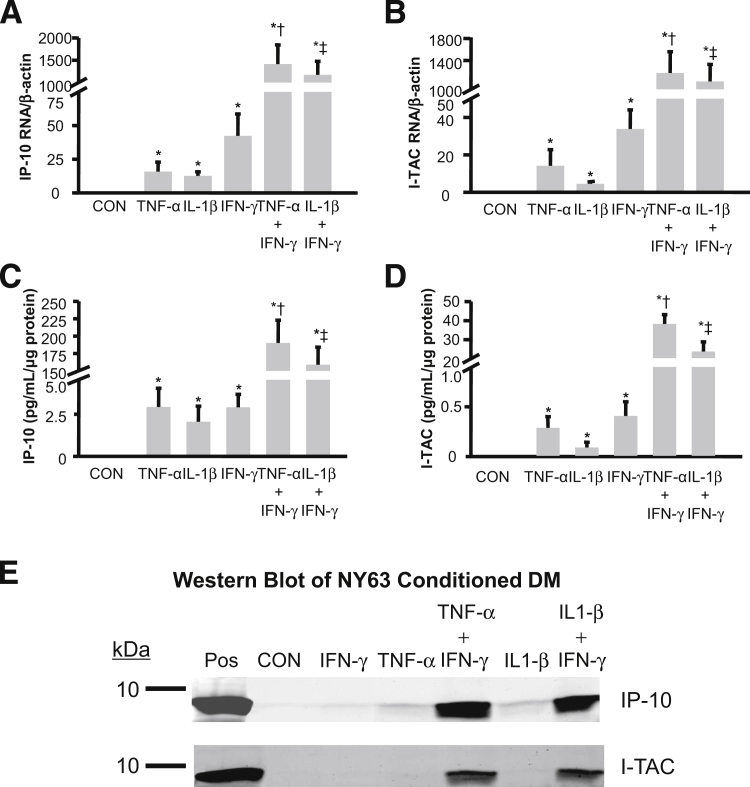
The individual effects of 1.0 ng/mL each of TNF-α or IL-1β or IFN-γ and the combined effects of IFN-γ with either TNF-α or IL-1β on mRNA expression are displayed for IP-10 (Figure 3A) and for I-TAC (Figure 3B). For IP-10 mRNA levels (mean ± SEM): IFN-γ, TNF-α, and IL-1β produced increases over basal output of 922 ± 430-fold, 581 ± 381-fold, and 508 ± 219-fold, respectively. For I-TAC mRNA levels (mean ± SEM): IFN-γ, TNF-α, and IL-1β produced increases over basal output of 897 ± 508-fold, 202 ± 97-fold, and 131 ± 64-fold, respectively. The combination of IFN-γ with TNF-α or IL-1β resulted in IP-10 mRNA levels of more than 40,000-fold greater than basal output and about 30-fold greater than the sum of each cytokine used alone. For I-TAC, the combination of IFN-γ with TNF-α or IL-1β results in mRNA expression levels that are about 36,000-fold greater than basal output, and about 40-fold greater when each cytokine was used alone.
Figure 3.
Separate and interactive effects of TNF-α or IL-1β or IFN-γ on IP-10 and I-TAC protein and mRNA expression in cultured first trimester decidual cells. Leukocyte-free first trimester decidual cells were primed in E2 + MPA (CON). Cultures were then switched to defined medium (DM) with the steroids and either IL-1β or TNF-α or IFN-γ or TNF-α + IFN-γ or IL-1β+ IFN-γ for 6 hours for mRNA or 24 hours for protein analysis. The mRNA levels for IP-10 (A) and I-TAC (B) were measured by RT-qPCR on RNA extracts and normalized to β-actin, a reference gene (n = 8, mean ± SEM). Protein levels of IP-10 (C) and I-TAC (D) were measured by ELISA in conditioned medium supernatants and normalized to total cell protein (n = 10, mean ± SEM). See Materials and Methods for details. *P < 0.05 versus CON; †P < 0.05 versus TNF-α alone; ‡P < 0.05 versus IL-1β alone. Western blotting (E) was carried out for IP-10 and I-TAC on 24 hours conditioned medium. Recombinant human IP-10 or I-TAC (2.5 ng per lane) are shown as positive controls (Pos) (see Materials and Methods for details).
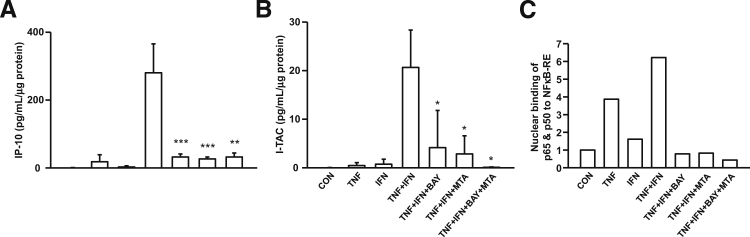
Figure 3 also displays the individual effects of 1.0 ng/mL TNF-α or IL-1β or IFN-γ and the combined effects of IFN-γ with either TNF-α or IL-1β on secreted IP-10 (Figure 3C) and I-TAC (Figure 3D) concentrations. For IP-10 levels (mean ± SEM): IFN-γ stimulation induces 3.20 ± 0.84 pg/mL/μg protein, an increase of 468 ± 179-fold over basal output (0.012 ± 0.003 pg/mL/μg protein); TNF-α induces 3.22 ± 1.22 pg/mL/μg protein, an increase of 356 ± 115-fold over basal output; and IL-1β induces 2.26 ± 1.02 pg/mL/μg protein, an increase of 256 ± 104-fold over basal output. For I-TAC (mean ± SEM): IFN-γ induces 0.45 ± 0.16 pg/mL/μg protein, an increase of 278 ± 120-fold over basal output (0.002 ± 0.0003 pg/mL/μg protein); TNF-α induces 0.32 ± 0.13 pg/mL/μg protein, an increase of 179 ± 64-fold over basal output; and IL-1β induces 0.10 ± 0.06 pg/mL/μg protein, an increase of 52 ± 29-fold over basal output. Note that basal and induced levels of IP-10 are approximately 5 to 10 times greater than the corresponding levels for I-TAC. Co-incubation of IFN-γ with either TNF-α or IL-1β produced striking synergistic augmentation of IP-10 and I-TAC outputs. Combinations of IFN-γ with TNF-α or IL-1β produced IP-10 levels of 190.1 ± 32.7 and 158.8 ± 25.3 pg/mL/μg protein, respectively. These represent increases of about 24,000-fold over basal output, and 35-fold greater than the levels induced by each cytokine added individually. In parallel measurements for I-TAC, IFN-γ added with TNF-α or IL-1β produces levels of 38.0 ± 4.9 and 23.5 ± 5.1 pg/mL/μg protein, respectively, which represent a >14,000-fold increase over basal output and an 80-fold increase over each cytokine used alone.
The Western blots depicted in Figure 3E confirm the ELISA results for IP-10 (Figure 3C) and for I-TAC (Figure 3D). Specifically, the antibodies used recognize individual bands in the decidual cell–conditioned medium that migrate with the mobility of IP-10 (8.5 kDa) and I-TAC (8.3 kDa). Compared with the corresponding control incubation, the magnitude of each band is modestly augmented during incubation with TNF-α or IL-1β or IFN-γ, with marked synergistic augmentation evident in co-incubations with IFN-γ plus TNF-α or IL-1β.
Effects of TNF-α, IL-1β, and IFN-γ on First Trimester Decidual Cell MCP-1, IL-6, IL-8, and IL-11 Expression
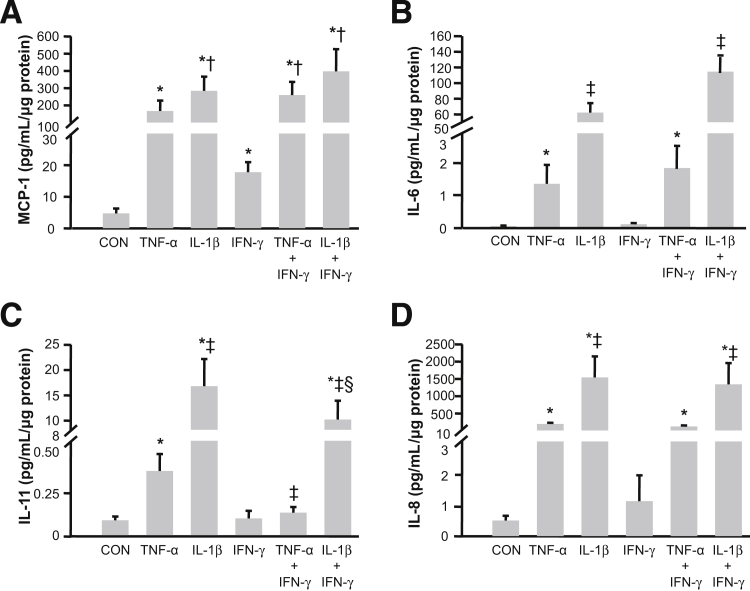
As shown in Figure 4, both TNF-α, and IL-1β significantly increased secreted levels of each of the evaluated chemokines. Specifically, for MCP-1 compared with basal output of 4.7 ± 1.6 pg/mL/μg cell protein (mean ± SEM), TNF-α elicits a 48.9 ± 13.5-fold increase and IL-1β elicits a 79.6 ± 15.5-fold increase (mean ± SEM), respectively (Figure 4A). Figure 4B indicates basal IL-6 production of 0.06 ± 0.02 pg/mL/μg cell protein, with TNF-α eliciting a 32.5 ± 5.1-fold increase and IL-1β eliciting a 2871 ± 994-fold increase, respectively. Figure 4C shows basal IL-11 output of 0.08 ± 0.02 pg/mL/μg cell protein, with TNF-α eliciting a 7.4 ± 2.1-fold increase and IL-1β eliciting a 618.9 ± 309.6-fold increase, respectively. Figure 4D shows basal IL-8 output of 0.50 ± 0.15 pg/mL/μg cell protein, with TNF-α eliciting a 547.0 ± 116.2-fold increase and IL-1β eliciting a 2850 ± 385-fold increase (mean ± SEM), respectively. Unlike the marked responses to TNF-α and IL-1β, IFN-γ is either ineffective or much less effective at elevating secreted levels of each chemokine. Only for MCP-1 did IFN-γ up-regulation achieve statistical significance (5.9 ± 1.5-fold increase, mean ± SEM, P < 0.05). Moreover, in contrast to the striking synergistic increases in secreted levels of IP-10 and I-TAC displayed in Figure 3, C and D, during co-incubation of IFN-γ with either TNF-α or IL-1β, Figure 4 indicates that co-incubation of IFN-γ with either cytokine produces less than additive effects for MCP-1 (Figure 4A), IL-6 (Figure 4B), and IL-8 (Figure 4D), and a subtractive effect for IL-11 (Figure 4C).
Figure 4.
Separate and interactive effects of TNF-α or IL-1β or IFN-γ on monocyte, neutrophil, and NK cell recruiting chemokine expression by first trimester decidual cell monolayers. Leukocyte-free first trimester decidual cells were primed in E2 + MPA [control (CON)]. Cultures were then switched to defined medium with the steroids and either IL-1β or TNF-α or IFN-γ or TNF-α + IFN-γ or IL-1β+ IFN-γ for 24 hours. Levels of MCP-1 (A), IL-6 (B), IL-11 (C), and IL-8 (D) measured by ELISA in conditioned medium supernatants and normalized to total cell protein (see Materials and Methods for details) (n = 8, mean ± SEM). *P < 0.05 versus CON; †P < 0.05 versus IFN-γ alone; ‡P < 0.05 versus TNF-α alone; and §P < 0.05 versus IL-1β alone.
Effects of Knockdown of IFN-γR1 and -R2 mRNA on IP-10 and I-TAC mRNA Expression
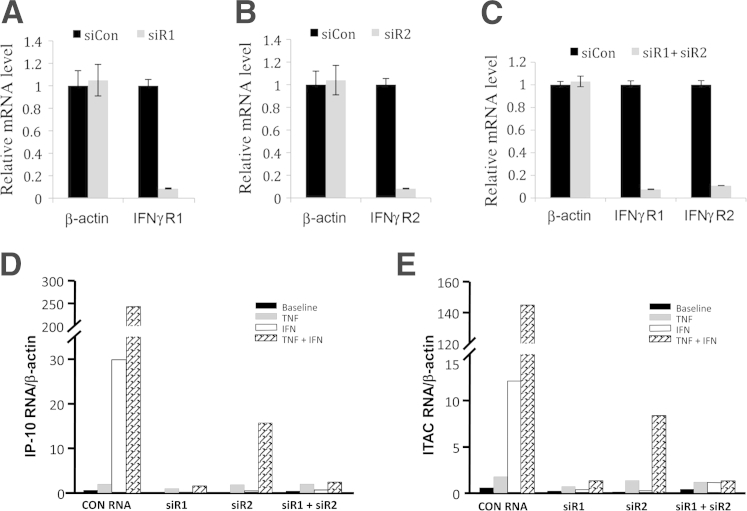
As shown in Figure 5A, 92% knockdown of IFN-γR1 mRNA was obtained 48 hours after siR1 transfection versus control siRNA. Importantly, the siR1 does not affect the nontargeted β-actin mRNA, confirming the specificity of the IFN-γR1 knockdown. Similarly, transfection of siR2 leads to a 95% down-regulation of IFN-γR2 (Figure 5B). Furthermore, the combination of siR1 and siR2 (double knockdown) elicits an ∼90% knockdown efficiency with each gene (Figure 5C). Consistent with the RT-qPCR results shown in Figure 3, A and B, results obtained with control siRNA indicate that IFN-γ induces higher mRNA levels for IP-10 (Figure 5D) and I-TAC (Figure 5E) than TNF-α added alone and that co-incubation with TNF-α plus IFN-γ synergistically enhances both IP-10 and I-TAC. Individual siRNA-mediated knockdown of IFN-γR1 and -R2 neutralizes augmentation of IP-10 (Figure 5D) and I-TAC (Figure 5E) elicited by IFN-γ, but only marginally inhibits TNF-α–induced IP-10 and I-TAC mRNA levels. In co-incubation with TNF-α plus IFN-γ, siRNA-mediated knockdown of IFN-γR1 is more effective than IFN-γR2 in blocking synergistic up-regulation of IP-10 and I-TAC. Simultaneous silencing of both receptors eliminates the synergistic up-regulation of both IP-10 and I-TAC (Figure 5, D and E).
Figure 5.
Effects of siRNA-mediated knockdown of IFN-γR1 and IP-10, I-TAC, IFN-γR1, and IFN-γR2 mRNA expression. A–C: Gene knockdown of human IFN-γR1, or IFN-γR2, or both. Levels of β-actin are shown as a nontargeted negative control. A: Single IFN-γR1 gene knockdown. Cells were transfected with control siRNA (siCon) or siR1, and the levels of IFN-γR1 mRNAs are shown on the ordinate. B: Single IFN-γR2 gene knockdown. Cells were transfected with siCon or siR2, and the levels of IFN-γR2 mRNAs are shown on the ordinate. C: Double knockdown of IFN-γR1 and IFN-γR2. Cells were transfected with siCon, or a combination of siR1 and siR2, and the levels of IFN-γR1 and IFN-γR2 mRNAs are shown. The levels of the indicated mRNAs from siCon-transfected cells were arbitrarily set as 1. Error bars indicate mean ± SD (n = 3). D and E: Human leukocyte-free first trimester decidual cells were transfected with siRNAs specific for siR1, siR2, or siCon for 24 hours and then incubated with either 1 ng/mL TNF-α or IFN-γ or both for 6 hours (n = 1). Levels of mRNA were measured using real-time and RT-qPCR for IP-10 (D) and I-TAC (E).
Immunostaining of IP-10, I-TAC, IFN-γR1, and -R2 in First Trimester Human Decidua
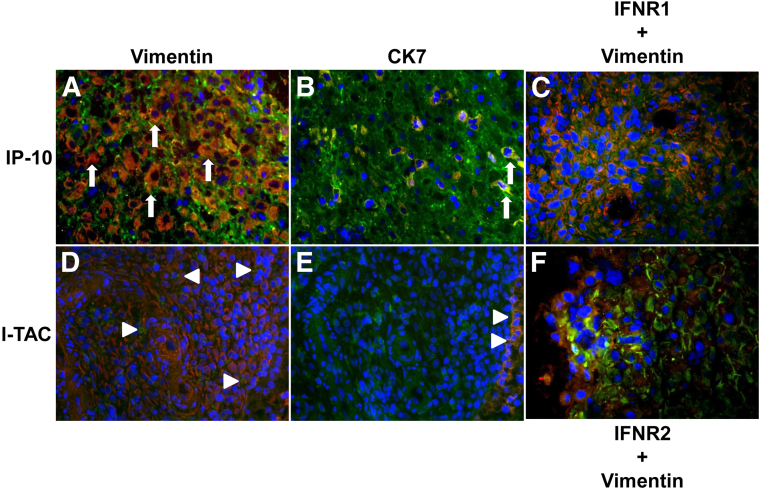
Cryosections of first trimester deciduae were sequentially immunostained for either IP-10 (Figure 6, A and B) or I-TAC (Figure 6, D and E) and then with either vimentin as a decidual cell marker (Figure 6, A and D) or CK7 as an EVT marker (Figure 6, B and E). The sections were also stained for DAPI to identify nuclei. Overlap between red and green immunostaining appears as yellow-to-orange staining. For IP-10, inspection of Figure 6A indicates that the overwhelming majority of cells demonstrate colocalization of IP-10 in vimentin-positive decidual cells, evident by the resulting yellow-orange staining. The pure green staining in the majority of the cells in Figure 6B indicates that cells expressing IP-10 are mainly non-EVT. Figure 6B indicates that only a few CK7-positive EVT cells express IP-10. Inspection of Figure 6D indicates that virtually all of the decidual cells also express I-TAC, albeit at lower intensity than IP-10. As with IP-10, very few EVTs express I-TAC as seen in Figure 6E. These in situ findings are consistent with the higher levels of basal IP-10 than I-TAC mRNA (Figure 3, A and B) and secreted protein (Figure 3, C and D) measured in cultured decidual cells. Cryosections were also immunostained for IFN-γR1 or -R2 and then vimentin. Positive staining for IFN-γR1 (Figure 6C) and -R2 (Figure 6F) was primarily localized to decidual cells, with blue staining denoting nuclei.
Figure 6.
Immunostaining for IP-10, I-TAC, IFN-γR1, and IFN-γR2 in first trimester decidua. Serial cryosections of first trimester decidua were immunostained with IP-10 (A and B) or I-TAC (D and E) (green fluorescence) and then with either the decidual cell marker vimentin (A and D) or the EVT marker cytokeratin 7 (CK7) (B and E) (red fluorescence). Sections were also immunostained for vimentin (red) and either IFN-γR1 (C) or IFN-γR2 (F) (green). DAPI (blue fluorescence) denotes nuclear immunostaining. Overlap between red and green immunostaining is seen as yellow or orange. Arrows indicate colocalization of IP-10 in vimentin-positive decidual cells (A) and indicate CK7-positive EVT cells expressing IP-10 (B). Arrowheads indicate decidual cells expressing I-TAC (D) and EVTs expressing I-TAC (E). Original magnification of representative sections, ×400.
Signaling Pathway Mediation of Synergistic Enhancement of IP-10 and I-TAC Expression
Figure 7 demonstrates that TNF-α + IFN-γ significantly enhances IP-10 (Figure 7A) and I-TAC (Figure 7B) expression compared with control or TNF-α alone or IFN-γ alone. Pretreatment of cultured decidual cells with either BAY (NFκB inhibitor) or MTA (JAK/STAT inhibitor) alone or in combination blocks the synergistic effects of TNF-α + IFN-γ on IP-10 or I-TAC expression.
Figure 7.
Signaling pathway mediation of IP-10 and I-TAC expression. A: IP-10 and B: I-TAC secretion by decidual cells treated with vehicle, TNF-α, IFN-γ, and TNF-α + IFN-γ with or without NFκB inhibitor BAY, or JAT/STAT inhibitor (MTA) or BAY + MTA (n = 3, mean ± SEM). *P < 0.05, **P < 0.01, and ***P < 0.005. (C) Representative binding of decidual cell–derived nuclear NFκB subunits p65 and p50 to NFκB-RE by E-EMSA (n = 2).
The E-EMSA analysis displayed in Figure 7C shows that nuclear extracts obtained from decidual cells treated with TNF-α alone or IFN-γ alone exhibit higher binding of NFκB subunits p65 and p50 to NFκB-RE compared with control. This binding to NFκB-RE increased further in nuclear extracts from decidual cells treated with TNF-α + IFN-γ. Moreover, pretreatment of decidual cells with either BAY or MTA alone or with BAY + MTA for 1 hour significantly reduced NFκB-RE binding of p65 and p50 in nuclear extracts compared with decidual cells treated with TNF-α + IFN-γ (Figure 7C).
The Effects of IP-10 and I-TAC on CXCR3 Expression by and Migration of pNK Cells
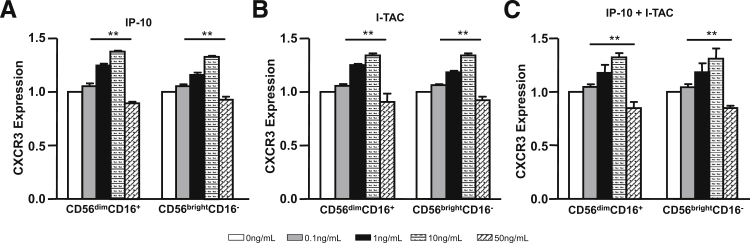
Figure 8 demonstrates the effects of incubating CD56brightCD16− or CD56dimCD16+ pNK cells with IP-10 or I-TAC on CXCR3 expression. Incubation of both NK cell subsets with either IP-10 (Figure 8A) or I-TAC (Figure 8B) elicits a concentration-dependent increase in CXCR3 that peaks at 10 ng/mL. However, either 50 ng/mL IP-10 or I-TAC down-regulates CXCR3 expression. Figure 8C demonstrates that the combination of equal concentrations of IP-10 and I-TAC elicits a similar concentration-dependent biphasic effect on CXCR3 expression. Parallel concentration-dependent biphasic effects are also observed on the migration of both pNK cell subtypes (Figure 9). Thus, either IP-10 (Figure 9A) or I-TAC (Figure 9B) or the combination of IP-10 and I-TAC (Figure 9C) progressively enhances cell migration at concentrations of 0.1 to 10 ng/mL, whereas 50 ng/mL impedes migration. Importantly, the biphasic effects observed are donor-independent.
Figure 8.
Effects of IP-10 and I-TAC on CXCR3 expression by pNK cells. The lymphocyte population from healthy human donor blood was enriched by Ficoll density gradient centrifugation, followed by purification of pNK cells using NK cell isolation kits (see Materials and Methods). Enriched (91% to 95%) pNK cells were incubated with 0, 0.1, 1, 10, or 50 ng/mL IP-10 (A) or I-TAC (B) or IP-10 plus I-TAC (C) for 48 hours. After incubation, CXCR3 expression was analyzed on gated CD56+CD3− NK cells. Data are presented as means ± SEM (n = 7), with CXCR3 expression levels of cells incubated with 0 ng/mL IP-10 and I-TAC arbitrarily set as 1. The bar groups represent CD56dimCD16+ and CD56brightCD16− subsets, respectively. ∗∗P < 0.01 0 ng/mL versus 0.1, 1, 10, or 50 ng/mL IP-10 and I-TAC, by Student's t-test.
Figure 9.
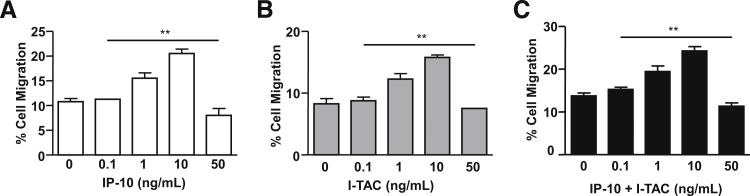
Effects of IP-10 and I-TAC on migration of pNK cells. pNK cells isolated from healthy donors were pre-incubated with IP-10 (A), I-TAC (B), or both (C) at a final concentration of 0, 0.1, 1, 10, or 50 ng/mL, each for 48 hours. Migration assays were carried out in the presence of 100 ng/mL IP-10 or I-TAC or both as chemoattractants. Results are presented as the mean ± SEM (n = 3) of the percentage of migrated cells. ∗∗P < 0.01 0 ng/mL versus 0.1, 1, 10, or 50 ng/mL IP-10 and I-TAC, by Student's t-test.
CD56brightCD16− NK Cells in Preeclamptic Decidua
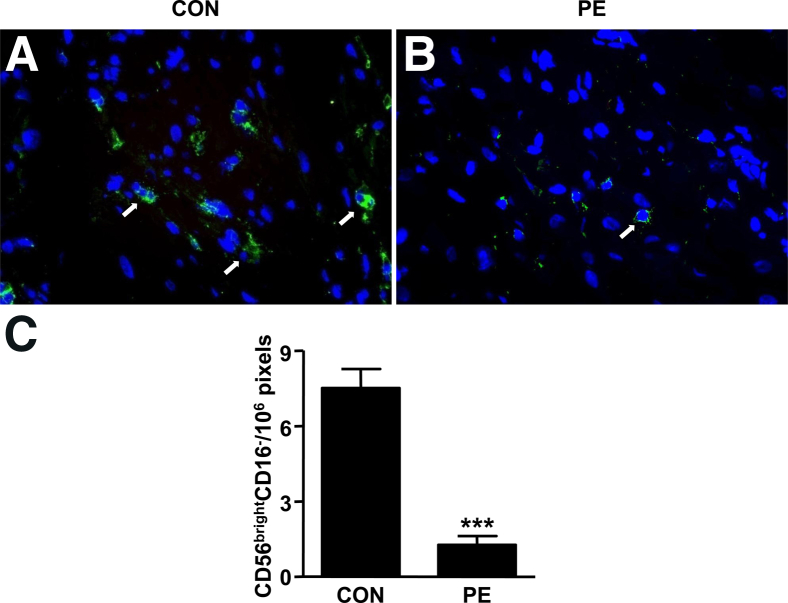
Decidual sections from women with preeclampsia versus gestational age–matched control specimens were immunostained sequentially for CD56 (green fluorescence) and CD16 (red fluorescence). The absence of CD16 and the presence of strong staining for CD56 in control and preeclamptic specimens indicate that virtually all NK cells in decidua are CD56brightCD16−. The more abundant green staining in Figure 10A compared with Figure 10B suggests that preeclamptic decidua contains fewer CD56brightCD16− NK cells. As indicated in Figure 10C, lower NK cell numbers in preeclamptic versus gestational age–matched control decidua attained statistical significance.
Figure 10.
CD56brightCD16− NK cells in preeclamptic (PE) versus control (CON) decidua. Decidua from patients with preeclampsia (A) and gestational age–matched controls (B) were stained with anti-human CD56 (green) and CD16 (red) antibodies. Arrows indicate CD56brightCD16− cells. Representative slides from eight independent experiments are shown. Original magnification, ×400. C: Comparison of CD56brightCD16− cells between preeclamptic and control decidua. Results are presented as mean ± SEM (n = 8) of the CD56brightCD16− cells. ***P < 0.001.
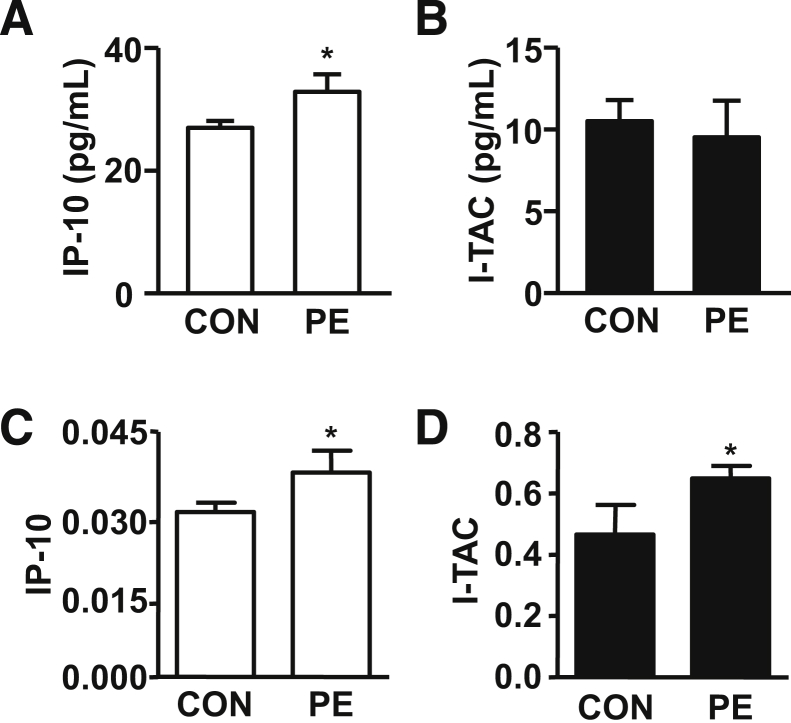
Measurement of IP-10 and I-TAC Levels in First Trimester Sera and Term Deciduae of Patients With and Without Subsequent Preeclampsia
In Figure 11A, a statistically significant increase in IP-10 levels in first trimester serum predicts later development of preeclampsia. By contrast, no such correlation was observed for the serum levels of I-TAC (Figure 11B). Tissue ELISA analyses for decidual extracts from patients with preeclampsia find significantly higher levels of IP-10 (Figure 11C) and I-TAC (Figure 11D) than age-matched controls.
Figure 11.
Comparison of immunoreactive IP-10 and I-TAC expression in serum from first trimester of controls (CON) versus patients subsequently developing preeclampsia (PE) and decidual tissues from preeclampsia versus gestational age-match third trimester decidua. IP-10 (A) and I-TAC (B) levels in serum obtained from first trimester gestation measured by ELISAs. Results are presented as mean ± SEM (n = 30 for preeclampsia, n = 90 for control) of IP-10 and I-TAC serum levels. *P < 0.05. IP-10 (C) and I-TAC (D) levels in deciduae obtained from patients with and without preeclampsia measured by ELISAs. Results are presented as means ± SEM (n = 7). *P < 0.05.
Discussion
In NK cell–deficient mice, the decidual vasculature lacks characteristic mid-gestation dilation, elongation, and loss of smooth muscle associated with increased blood flow, similar to lesions seen in human preeclampsia.22 These effects are ameliorated by treatment with mouse IFN-γ or with the IFN-γ–regulated cytokine-binding protein, α-2 macroglobulin, which enhances decidual remodeling and produces normal histological changes in decidual blood vessels.23 Ninety percent of mouse uterine IFN-γ is dNK cell–derived, indicating that IFN-γ is necessary and sufficient to mediate normal uterine vascular remodeling in pregnant mice.24 Close proximity between dNK cells and blood vessels suggests that dNK cell–derived angiogenic factors may augment the effects of IFN-γ to promote normal vascular remodeling.25 Extrapolation of studies in NK cell–depleted mice to humans26,27 suggests that the minority CD56brightCD16− pNK cells traffic from the circulation to the decidua in early pregnancy.
Transformation of spiral arteries and arterioles into high-capacity vessels with markedly increased uteroplacental blood flow to the developing fetal–placental unit has been attributed to replacement of vascular smooth muscle and endothelial cells by fibrinoid-embedded endovascular EVTs.28 Spiral arterial lining endovascular EVTs express stromal cell–derived factor-1 (SDF-1) (alias CXCL12), which binds to CCR4-expressing CD56brightCD16− pNK cells to mediate their recruitment to the decidua.28 In these specimens, infiltrating dNK cells and macrophages were identified in the vascular smooth muscle layers accompanied by the appearance of apoptotic markers in vascular smooth muscle and endothelial cells. This induction of apoptosis was attributed to dNK cell– and macrophage-expressed matrix metalloproteinase (MMP)-7 and MMP-9.29 Such trophoblast-independent decidualization-associated vascular remodeling is also indicated by early signs of vascular transformation in decidua from ectopic tubal pregnancies or in late luteal phase nonpregnant endometrium.30,31 In the early pregnant golden hamster, dNK cells invade the vascular wall before trophoblast invasion and are implicated in directly mediating endometrial vascular remodeling.32
The origin of dNK cells in humans is alternatively ascribed to: i) differentiation of resident endometrial NK cells7,11; ii) self-renewal from local progenitor stem cells33; and iii) trafficking of pNK cells.34 In support of the last, the majority circulating CD56dimCD16+ pNK cells were shown to be recruited to the decidua, then differentiate into CD56brightCD16− NK cells by decidual cell–derived transforming growth factor-β.35,36 However, abundant evidence points to the minority circulating CD56brightCD16− pNK cells as the major contributors to the dNK cell population. Their preferential recruitment is suggested by elevated expression of L-selectin, which mediates interactions with vascular endothelium37 by CD56brightCD16− pNK cells, but not by CD56dimCD16+ pNK cells. Moreover, the current study confirms that CD56brightCD16− pNK cells express high levels of CXCR314 (Figure 1, A and B), a receptor for decidual cell–derived IP-10 and I-TAC that can preferentially promote trafficking to the decidua. Recently, IP-10 secreted by various human endometrial, but not NK cells, was shown to chemoattract blastocyst-derived CXCR3-expressing trophectoderm.38
Human maternal–fetal interactions create a mild systemic inflammatory state initiated in the luteal phase of the menstrual cycle characterized by vascular endothelial activation, leukocytosis, increased monocyte activity, and elevated circulating chemokine and cytokine levels that become most apparent in the third trimester of uncomplicated human pregnancies.39 Decidual cell–mediated recruitment of NK cells in normal pregnancy is indicated by a report that progesterone enhances mRNA expression of NK cell-recruiting chemokines, ie, fractalkine (alias CX3CL1) and MCP-1 (alias CCL2), as well as IP-10 in primary first trimester human decidual cells, and NK cell migration is enhanced by using these decidual cells cultured with progesterone as a substrate.40 Extending these observations to better mimic the decidual milieu of pregnancy, the current study used E2 + MPA to maintain decidualization of leukocyte-free first trimester decidual cell monolayers in combination with proinflammatory cytokines IFN-γ, a primary NK cell product, and/or TNF-α or IL-1β, primary macrophage products. The resulting marked synergistic increase in IP-10 and I-TAC mRNA and protein expression suggests that decidual cell–mediated crosstalk between NK cells and macrophages leads to preferential recruitment and retention of additional CDbrightCD16– NK cells to the decidua.28
Previously, expression profiling of several genes revealed striking differences between the minority peripheral versus decidual CD56brightCD16− NK cell populations, suggesting that dNK cells represent a unique NK cell subset.17 Evidence that the decidual milieu induces differentiation of recruited CD56brightCD16− NK cells is provided by observations that incubation of peripheral CD56brightCD16− NK cells with decidual cell–derived cytokines, particularly IL-15, induces a chemokine receptor repertoire similar to that expressed by dNK cells.28 Progestins and NK cell–derived IFN-γ enhance decidual cell IL-15 production.41 These observations complement a report that dNK cells express the IL-2 receptor,42 which mediates IL-15 activation of NK cells.43 Like the majority CD56dimCD16+ pNK cells, dNK cells express KIRs and display granules containing cytolytic molecules, such as perforin and granzymes A and B.17 However, dNK cells exhibit markedly reduced cytotoxicity compared with the majority CD56dimCD16+ pNK cells and are even less cytotoxic than the relatively nontoxic minority CD56brightCD16− pNK cells.11,44 Although dNK cells form conjugates and activating immune synapses with target cells, their failure to polarize their microtubule organizing centers and transport perforin-containing granules to the synapse impedes cytotoxicity.44
Paradoxically, dNK cells express multiple pregnancy-promoting factors. Like the minority circulating CD56brightCD16− pNK cells, dNK cells produce growth factors, such as GM-CSF, leukemia inhibitory factor, TNF-α, and IFN-γ.7,17,45–47 In addition, dNK cells express EVT invasion-enhancing IL-8 and IP-10, as well as angiogenic factors, such as vascular endothelial growth factor, placental-derived growth factor, angiopoietin 2, and NKG5.7,45,46 Taken together, these observations indicate that human dNK cells differ phenotypically from both pNK cells and NK cells in cycling endometrium to form a unique NK cell subtype that supports normal pregnancy by promoting trophoblast invasion, vascular remodeling, and in view of their lack of cyototoxicity, immune tolerance of the fetal semiallograft.
The current study employs a multitiered approach to reveal that targeting of first trimester human decidual cells48 by paracrine effectors released by the predominant decidual immune cell types, dNK cells and macrophages, recruits additional NK cells from the circulation. Initially, flow cytometric analysis demonstrated high expression of CXCR3 on peripheral and decidual CD56brightCD16− NK cells with lower CXCR3 levels on the majority circulating CD56dimCD16+ pNK cells. Subsequently, incubation of leukocyte-free first trimester decidual cells with either of the primary macrophage-derived cytokines, TNF-α or IL-1β,15 or primary dNK cell–derived cytokine, IFN-γ,12 markedly elevated IP-10 and I-TAC mRNA and protein levels. Co-incubation of IFN-γ with either TNF-α or IL-1β produced striking synergistic increases in IP-10 and I-TAC mRNA and protein levels far exceeding the sum of the individual responses. In parallel incubations, expression of other monocyte, neutrophil, and NK cell chemoattractants and/or activators, ie, MCP-1, IL-6, -8, and -11 (Figure 4) were at best additive, thereby emphasizing specificity of the synergistic up-regulation of IP-10 and I-TAC expression in human decidual cells. Consistent with in situ hybridization observations for IP-10 and I-TAC,49 we observed preferential immunolocalization of IP-10 and I-TAC to vimentin-positive decidual cells versus CK7-positive trophoblasts in first trimester decidual sections.
The individual and interactive contribution of IFN-γ to the marked synergistic enhancement of IP-10 and I-TAC expression by first trimester decidual cells revealed by the current study provides strong evidence that IFN-γ is involved in CD56brightCD16− cell recruitment to the decidua. Two receptors, IFN-γR1 and IFN-γR2, mediate IFN-γ effects in target cells. Binding of cell membrane expressed IFN-γR1 initiates IFN-γR1/R2 heterodimerization, which mediates intracellular signaling.16 Use of specific siRNAs to silence each receptor separately and together neutralized the contribution of IFN-γ, but did not affect that of TNF-α to enhanced IP-10 and I-TAC mRNA expression. In situ observations complementing in vitro results provide the first indication that at the implantation site, immunoreactive IFN-γR1 and -R2 are preferentially localized to vimentin-positive decidual cells (Figure 6).
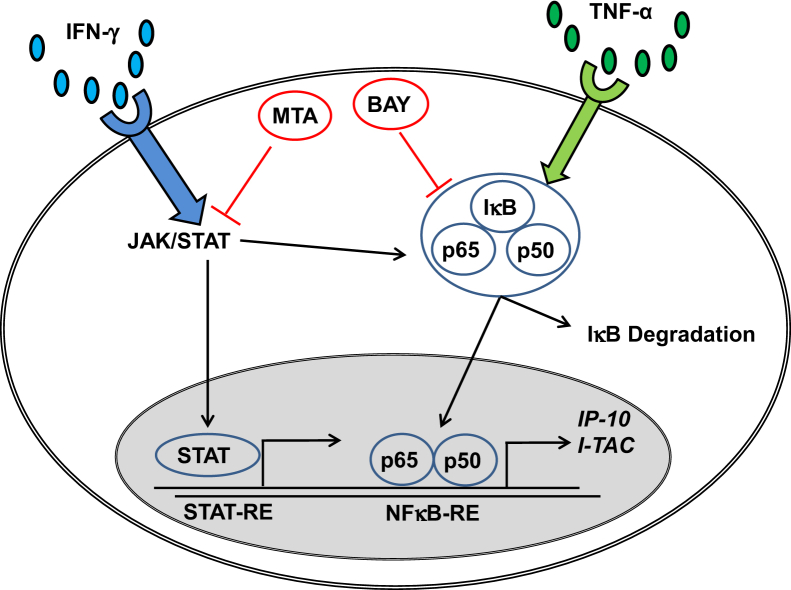
Elucidation of the potential mechanisms underlying the striking synergistic up-regulation of IP-10 and I-TAC expression induced by TNF-α and IFN-γ focused on NFκB and JAK/STAT signaling pathways, which have been shown to mediate cellular effects of TNF-α and IFN-γ, respectively.50,51 Pre-incubation of first trimester decidual cells with either BAY or MTA was sufficient to block the synergistic increase of TNF-α + IFN-γ–induced IP-10 and I-TAC expression measured by ELISAs or E-EMSA (Figure 7). Signaling pathway mediation of these effects is depicted by the scheme in Figure 12. Specifically, it indicates that binding of TNF-α to its receptor results in dissociation of NFκB p65 and p50 subunits from NFκB inhibitory molecule IκB, which is degraded by proteasomes. The resulting p65 and p50 homo- and/or heterodimerization is followed by translocation to the nucleus. The p65/p50 heterodimer then binds to NFκB-RE in the IP-10 and I-TAC promoters. IFN-γ activates the IFN-γ receptor and its downstream signaling JAK/STAT pathway. Activation of JAK/STAT results in nuclear translocation of STAT where it binds to its response element in the IP-10 and I-TAC promoters. Because the JAK/STAT inhibitor MTA also suppresses NFκB binding to NFκB-RE, IFN-γ can also activate further NFκB binding via the JAK/STAT pathway, thereby contributing to synergistic up-regulation.
Figure 12.
Schematic representation of the contribution of JAK/STAT and NFκB signaling pathways to TNF-α + IP-10 and II-TAC secretion in first trimester decidual cells.
The current observations on primary human first trimester decidual cells and immunostaining of decidual sections are complemented by parallel assessment of CXCR3 expression and in vitro migration of CD56brightCD16− and CD56dimCD16+ pNK cells during incubation with IP-10 or I-TAC or both (Figures 8 and 9). Taken together, our findings have translational implications for normal recruitment of both pNK cell subsets to the decidua and aberrant pNK cell trafficking during preeclampsia. Specifically, incubation of both pNK cell subsets with IP-10 and/or I-TAC elicits concentration-dependent enhancement of CXCR3 expression and NK cell migration that peaked at 10 ng/mL, with 50 ng/mL inhibiting both endpoints. These observations suggest that secreted IP-10 and I-TAC levels resulting from co-stimulation of decidual cells by IFN-γ with either TNF-α or IL-1β cause the recruitment of additional NK cells to the early decidua, but that overstimulation by these cytokines, as would be observed in the inflammatory milieu of preeclampsia, inhibits CXCR3 expression by, and in vitro migration of, both pNK cell subsets. This would account for the reduced number of NK cells observed in preeclamptic decidua (Figure 10) and why elevated IP-10 levels in first trimester serum predict the subsequent development of preeclampsia. Indeed, although elevated circulating levels of angiogenesis mediators, endoglin and soluble fms-like tyrosine kinase receptor-1 (sFlt-1), in second or third trimester serum52 also predict preeclampsia, IP-10 appears to be an earlier indicator of risk. The importance of our observations is highlighted by a recent review article53 that states “Currently, no reliable markers exist to identify patients before the development of symptoms. The early identification of women with risk of developing preeclampsia is critical, because careful monitoring and referral to a specialized perinatal care center could substantially improve outcomes for both the mother and fetus.”53,p957
The causes of preeclampsia-related decidual inflammation are yet to be fully discerned. Placental oxidative and endoplasmic reticulum stress mediated by trophoblast-derived growth factors, activin-A, corticotrophin-releasing hormone, leptin, and placental microvesicles may play a role.54,55 Our laboratory and others have observed excess dendritic cells and macrophages in the preeclamptic decidua.56 Moreover, we found that incubation of first trimester decidual cells with either IL-1β or TNF-α markedly enhances expression of several monocyte/macrophage-recruiting and -activating cytokines19, and that activated macrophages treated with conditioned medium obtained from first trimester decidual cells after incubation with IL-1β or TNF-α induce EVT apoptosis and inhibit EVT invasiveness.51,57
Other studies evaluating the relationship between preeclampsia and dNK cell numbers and phenotype have produced conflicting results.58 However, as noted by Lash and Bulmer,58 the lack of clarity among various studies as to whether dNK cell numbers are reduced in preeclampsia and intrauterine growth restriction likely reflects differences in sampling, analysis, and severity of the disease. Beyond altered dNK cell numbers, preeclampsia, as well as other adverse pregnancy outcomes, such as fetal growth restriction and recurrent miscarriage, are associated with mismatched KIR haplotype (KIR AA) expression by dNK cells and EVTs.59
In summary, we postulate that enhanced periconceptional decidual inflammation contributes to the genesis of shallow placentation in preeclampsia by promoting excess decidual cell IP-10 and I-TAC production, which blunts pNK cell recruitment and leads to deficient dNK cell numbers. Coupled with excess decidual macrophages, these changes impede EVT-mediated remodeling of spiral arteries, the pathological sine qua non of preeclampsia.
Acknowledgments
We thank Drs. Jack L. Strominger for the initial, and crucial, flow cytometry observations made in his laboratory and Mizanur Rahman for his expert cell culture work.
Footnotes
Supported by NIH grants R01HD33937-12 (C.J.L.), Project 2 PO1HD054713-01A1 (C.J.L.), and R01HD056123-04 (S.J.H.).
References
- 1.Zhou Y., Fisher S.J., Janatpour M., Genbacev O., Dejana E., Wheelock M., Damsky C.H. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J Clin Invest. 1997;99:2139–2151. doi: 10.1172/JCI119387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pijnenborg R., Vercruysse L., Brosens I. Deep placentation. Best Pract Res Clin Obstet Gynaecol. 2011;25:273–285. doi: 10.1016/j.bpobgyn.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Young B.C., Levine R.J., Karumanchi S.A. Pathogenesis of preeclampsia. Annu Rev Pathol. 2010;5:173–192. doi: 10.1146/annurev-pathol-121808-102149. [DOI] [PubMed] [Google Scholar]
- 4.Steegers E.A., von Dadelszen P., Duvekot J.J., Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631–644. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 5.Silasi M., Cohen B., Karumanchi S.A., Rana S. Abnormal placentation, angiogenic factors, and the pathogenesis of preeclampsia. Obstet Gynecol Clin North Am. 2010;37:239–253. doi: 10.1016/j.ogc.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Trundley A., Moffett A. Human uterine leukocytes and pregnancy. Tissue Antigens. 2004;63:1–12. doi: 10.1111/j.1399-0039.2004.00170.x. [DOI] [PubMed] [Google Scholar]
- 7.Manaster I., Mandelboim O. The unique properties of uterine NK cells. Am J Reprod Immunol. 2010;63:434–444. doi: 10.1111/j.1600-0897.2009.00794.x. [DOI] [PubMed] [Google Scholar]
- 8.Bryceson Y.T., Chiang S.C., Darmanin S., Fauriat C., Schlums H., Theorell J., Wood S.M. Molecular mechanisms of natural killer cell activation. J Innate Immun. 2011;3:216–226. doi: 10.1159/000325265. [DOI] [PubMed] [Google Scholar]
- 9.Caligiuri M.A. Human natural killer cells. Blood. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trotta R., Chen L., Ciarlariello D., Josyula S., Mao C., Costinean S., Yu L., Butchar J.P., Tridandapani S., Croce C.M., Caligiuri M.A. miR-155 regulates IFN-gamma production in natural killer cells. Blood. 2012;119:3478–3485. doi: 10.1182/blood-2011-12-398099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalkunte S., Chichester C.O., Gotsch F., Sentman C.L., Romero R., Sharma S. Evolution of non-cytotoxic uterine natural killer cells. Am J Reprod Immunol. 2008;59:425–432. doi: 10.1111/j.1600-0897.2008.00595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lash G.E., Robson S.C., Bulmer J.N. Review: Functional role of uterine natural killer (uNK) cells in human early pregnancy decidua. Placenta. 2010;31(Suppl):S87–S92. doi: 10.1016/j.placenta.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 13.Kopcow H.D., Karumanchi S.A. Angiogenic factors and natural killer (NK) cells in the pathogenesis of preeclampsia. J Reprod Immunol. 2007;76:23–29. doi: 10.1016/j.jri.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper M.A., Fehniger T.A., Caligiuri M.A. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 15.Singh U., Nicholson G., Urban B.C., Sargent I.L., Kishore U., Bernal A.L. Immunological properties of human decidual macrophages: a possible role in intrauterine immunity. Reproduction. 2005;129:631–637. doi: 10.1530/rep.1.00331. [DOI] [PubMed] [Google Scholar]
- 16.Trinchieri G. Type I interferon: friend or foe? J Exp Med. 2010;207:2053–2063. doi: 10.1084/jem.20101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koopman L.A., Kopcow H.D., Rybalov B., Boyson J.E., Orange J.S., Schatz F., Masch R., Lockwood C.J., Schachter A.D., Park P.J., Strominger J.L. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med. 2003;198:1201–1212. doi: 10.1084/jem.20030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arici A., Marshburn P.B., MacDonald P.C., Dombrowski R.A. Progesterone metabolism in human endometrial stromal and gland cells in culture. Steroids. 1999;64:530–534. doi: 10.1016/s0039-128x(99)00029-x. [DOI] [PubMed] [Google Scholar]
- 19.Huang S.J., Schatz F., Masch R., Rahman M., Buchwalder L., Niven-Fairchild T., Tang C., Abrahams V.M., Krikun G., Lockwood C.J. Regulation of chemokine production in response to pro-inflammatory cytokines in first trimester decidual cells. J Reprod Immunol. 2006;72:60–73. doi: 10.1016/j.jri.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Zhang M., Guller S., Huang Y. Method to enhance transfection efficiency of cell lines and placental fibroblasts. Placenta. 2007;28:779–782. doi: 10.1016/j.placenta.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 21.ACOG Committee on Practice Bulletins—Obstetrics ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol. 2002;99:159–167. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 22.Greenwood J.D., Minhas K., di Santo J.P., Makita M., Kiso Y., Croy B.A. Ultrastructural studies of implantation sites from mice deficient in uterine natural killer cells. Placenta. 2000;21:693–702. doi: 10.1053/plac.2000.0556. [DOI] [PubMed] [Google Scholar]
- 23.He H., McCartney D.J., Wei Q., Esadeg S., Zhang J., Foster R.A., Hayes M.A., Tayade C., Van Leuven F., Croy B.A. Characterization of a murine alpha 2 macroglobulin gene expressed in reproductive and cardiovascular tissue. Biol Reprod. 2005;72:266–275. doi: 10.1095/biolreprod.104.029835. [DOI] [PubMed] [Google Scholar]
- 24.Ashkar A.A., Croy B.A. Functions of uterine natural killer cells are mediated by interferon gamma production during murine pregnancy. Semin Immunol. 2001;13:235–241. doi: 10.1006/smim.2000.0319. [DOI] [PubMed] [Google Scholar]
- 25.Chantakru S., Wang W.C., van den Heuvel M., Bashar S., Simpson A., Chen Q., Croy B.A., Evans S.S. Coordinate regulation of lymphocyte-endothelial interactions by pregnancy-associated hormones. J Immunol. 2003;171:4011–4019. doi: 10.4049/jimmunol.171.8.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bilinski M.J., Thorne J.G., Oh M.J., Leonard S., Murrant C., Tayade C., Croy B.A. Uterine NK cells in murine pregnancy. Reprod Biomed Online. 2008;16:218–226. doi: 10.1016/s1472-6483(10)60577-9. [DOI] [PubMed] [Google Scholar]
- 27.Guimond M.J., Wang B., Croy B.A. Engraftment of bone marrow from severe combined immunodeficient (SCID) mice reverses the reproductive deficits in natural killer cell-deficient tg epsilon 26 mice. J Exp Med. 1998;187:217–223. doi: 10.1084/jem.187.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanna J., Wald O., Goldman-Wohl D., Prus D., Markel G., Gazit R., Katz G., Haimov-Kochman R., Fujii N., Yagel S., Peled A., Mandelboim O. CXCL12 expression by invasive trophoblasts induces the specific migration of CD16- human natural killer cells. Blood. 2003;102:1569–1577. doi: 10.1182/blood-2003-02-0517. [DOI] [PubMed] [Google Scholar]
- 29.Smith S.D., Dunk C.E., Aplin J.D., Harris L.K., Jones R.L. Evidence for immune cell involvement in decidual spiral arteriole remodeling in early human pregnancy. Am J Pathol. 2009;174:1959–1971. doi: 10.2353/ajpath.2009.080995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pijnenborg R., Vercruysse L., Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27:939–958. doi: 10.1016/j.placenta.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Craven C.M., Morgan T., Ward K. Decidual spiral artery remodelling begins before cellular interaction with cytotrophoblasts. Placenta. 1998;19:241–252. doi: 10.1016/s0143-4004(98)90055-8. [DOI] [PubMed] [Google Scholar]
- 32.Pijnenborg R., Robertson W.B., Brosens I. The arterial migration of trophoblast in the uterus of the golden hamster, Mesocricetus auratus. J Reprod Fertil. 1974;40:269–280. doi: 10.1530/jrf.0.0400269. [DOI] [PubMed] [Google Scholar]
- 33.Lynch L., Golden-Mason L., Eogan M., O'Herlihy C., O'Farrelly C. Cells with haematopoietic stem cell phenotype in adult human endometrium: relevance to infertility? Hum Reprod. 2007;22:919–926. doi: 10.1093/humrep/del456. [DOI] [PubMed] [Google Scholar]
- 34.Male V., Hughes T., McClory S., Colucci F., Caligiuri M.A., Moffett A. Immature NK cells, capable of producing IL-22, are present in human uterine mucosa. J Immunol. 2010;185:3913–3918. doi: 10.4049/jimmunol.1001637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keskin D.B., Allan D.S., Rybalov B., Andzelm M.M., Stern J.N., Kopcow H.D., Koopman L.A., Strominger J.L. TGFbeta promotes conversion of CD16+ peripheral blood NK cells into CD16- NK cells with similarities to decidual NK cells. Proc Natl Acad Sci U S A. 2007;104:3378–3383. doi: 10.1073/pnas.0611098104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allan D.S., Rybalov B., Awong G., Zuniga-Pflucker J.C., Kopcow H.D., Carlyle J.R., Strominger J.L. TGF-beta affects development and differentiation of human natural killer cell subsets. Eur J Immunol. 2010;40:2289–2295. doi: 10.1002/eji.200939910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamaguchi T., Kitaya K., Daikoku N., Yasuo T., Fushiki S., Honjo H. Potential selectin L ligands involved in selective recruitment of peripheral blood CD16(-) natural killer cells into human endometrium. Biol Reprod. 2006;74:35–40. doi: 10.1095/biolreprod.105.045971. [DOI] [PubMed] [Google Scholar]
- 38.Sela H.Y., Goldman-Wohl D.S., Haimov-Kochman R., Greenfield C., Natanson-Yaron S., Hamani Y., Revel A., Lavy Y., Singer O., Yachimovich-Cohen N., Turetsky T., Mandelboim O., Reubinoff B., Yagel S. Human trophectoderm apposition is regulated by interferon gamma-induced protein 10 (IP-10) during early implantation. Placenta. 2013;34:222–230. doi: 10.1016/j.placenta.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 39.Sargent I.L., Borzychowski A.M., Redman C.W. NK cells and human pregnancy—an inflammatory view. Trends Immunol. 2006;27:399–404. doi: 10.1016/j.it.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 40.Carlino C., Stabile H., Morrone S., Bulla R., Soriani A., Agostinis C., Bossi F., Mocci C., Sarazani F., Tedesco F., Santoni A., Gismondi A. Recruitment of circulating NK cells through decidual tissues: a possible mechanism controlling NK cell accumulation in the uterus during early pregnancy. Blood. 2008;111:3108–3115. doi: 10.1182/blood-2007-08-105965. [DOI] [PubMed] [Google Scholar]
- 41.Dunn C.L., Critchley H.O., Kelly R.W. IL-15 regulation in human endometrial stromal cells. J Clin Endocrinol Metab. 2002;87:1898–1901. doi: 10.1210/jcem.87.4.8539. [DOI] [PubMed] [Google Scholar]
- 42.Verma S., Hiby S.E., Loke Y.W., King A. Human decidual natural killer cells express the receptor for and respond to the cytokine interleukin 15. Biol Reprod. 2000;62:959–968. doi: 10.1095/biolreprod62.4.959. [DOI] [PubMed] [Google Scholar]
- 43.Carson W.E., Giri J.G., Lindemann M.J., Linett M.L., Ahdieh M., Paxton R., Anderson D., Eisenmann J., Grabstein K., Caligiuri M.A. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180:1395–1403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kopcow H.D., Allan D.S., Chen X., Rybalov B., Andzelm M.M., Ge B., Strominger J.L. Human decidual NK cells form immature activating synapses and are not cytotoxic. Proc Natl Acad Sci U S A. 2005;102:15563–15568. doi: 10.1073/pnas.0507835102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalkunte S.S., Mselle T.F., Norris W.E., Wira C.R., Sentman C.L., Sharma S. Vascular endothelial growth factor C facilitates immune tolerance and endovascular activity of human uterine NK cells at the maternal-fetal interface. J Immunol. 2009;182:4085–4092. doi: 10.4049/jimmunol.0803769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hanna J., Goldman-Wohl D., Hamani Y., Avraham I., Greenfield C., Natanson-Yaron S., Prus D., Cohen-Daniel L., Arnon T.I., Manaster I., Gazit R., Yutkin V., Benharroch D., Porgador A., Keshet E., Yagel S., Mandelboim O. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12:1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- 47.Hanna J., Mandelboim O. When killers become helpers. Trends Immunol. 2007;28:201–206. doi: 10.1016/j.it.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 48.Dunn C.L., Kelly R.W., Critchley H.O. Decidualization of the human endometrial stromal cell: an enigmatic transformation. Reprod Biomed Online. 2003;7:151–161. doi: 10.1016/s1472-6483(10)61745-2. [DOI] [PubMed] [Google Scholar]
- 49.Red-Horse K., Drake P.M., Fisher S.J. Human pregnancy: the role of chemokine networks at the fetal-maternal interface. Expert Rev Mol Med. 2004;6(11):1–14. doi: 10.1017/S1462399404007720. [DOI] [PubMed] [Google Scholar]
- 50.Horvath CM: The Jak-STAT pathway stimulated by interferon gamma. Sci STKE 2004, 260:tr8. http://dx.doi.org/10.1126/stke.2602004tr8 [DOI] [PubMed]
- 51.Li M., Wu Z.M., Yang H., Huang S.J. NFkappaB and JNK/MAPK activation mediates the production of major macrophage- or dendritic cell-recruiting chemokine in human first trimester decidual cells in response to proinflammatory stimuli. J Clin Endocrinol Metab. 2011;96:2502–2511. doi: 10.1210/jc.2011-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hagmann H., Thadhani R., Benzing T., Karumanchi S.A., Stepan H. The promise of angiogenic markers for the early diagnosis and prediction of preeclampsia. Clin Chem. 2012;58:837–845. doi: 10.1373/clinchem.2011.169094. [DOI] [PubMed] [Google Scholar]
- 53.Zhao Z., Moley K.H., Gronowski A.M. Diagnostic potential for miRNAs as biomarkers for pregnancy-specific diseases. Clin Biochem. 2013;46:953–960. doi: 10.1016/j.clinbiochem.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 54.Redman C.W., Sargent I.L. Immunology of pre-eclampsia. Am J Reprod Immunol. 2010;63:534–543. doi: 10.1111/j.1600-0897.2010.00831.x. [DOI] [PubMed] [Google Scholar]
- 55.Burton G.J., Yung H.W., Cindrova-Davies T., Charnock-Jones D.S. Placental endoplasmic reticulum stress and oxidative stress in the pathophysiology of unexplained intrauterine growth restriction and early onset preeclampsia. Placenta. 2009;30(Suppl A):S43–S48. doi: 10.1016/j.placenta.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang S.J., Chen C.P., Schatz F., Rahman M., Abrahams V.M., Lockwood C.J. Pre-eclampsia is associated with dendritic cell recruitment into the uterine decidua. J Pathol. 2008;214:328–336. doi: 10.1002/path.2257. [DOI] [PubMed] [Google Scholar]
- 57.Wu Z.M., Yang H., Li M., Yeh C.C., Schatz F., Lockwood C.J., Di W., Huang S.J. Pro-inflammatory cytokine-stimulated first trimester decidual cells enhance macrophage-induced apoptosis of extravillous trophoblasts. Placenta. 2012;33:188–194. doi: 10.1016/j.placenta.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lash G.E., Bulmer J.N. Do uterine natural killer (uNK) cells contribute to female reproductive disorders? J Reprod Immunol. 2011;88:156–164. doi: 10.1016/j.jri.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 59.Hiby S.E., Apps R., Sharkey A.M., Farrell L.E., Gardner L., Mulder A., Claas F.H., Walker J.J., Redman C.W., Morgan L., Tower C., Regan L., Moore G.E., Carrington M., Moffett A. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. J Clin Invest. 2010;120:4102–4110. doi: 10.1172/JCI43998. [DOI] [PMC free article] [PubMed] [Google Scholar]