Abstract
The landscape of treatment for advanced/metastatic renal cell carcinoma (mRCC) has advanced significantly in the last decade and continues to evolve with the approval of new drugs targeting the vascular endothelial growth factor (VEGF) and its receptors and mammalian target of rapamycin (mTOR). Currently available oral VEGF tyrosine kinase inhibitors (TKIs) approved for treatment of mRCC include sorafenib, sunitinib, pazopanib, and axitinib. This review focuses on pazopanib, a multikinase VEGF TKI indicated for patients with treatment-naïve and cytokine-refractory mRCC. This article describes the preclinical and clinical evolution of pazopanib, with an emphasis on its development and role in mRCC. Pivotal trials are discussed that demonstrate the efficacy and safety of pazopanib and its important role in the treatment of patients with mRCC in comparison to other available treatment options. The clinical path of pazopanib continues to develop further, with several ongoing studies exploring its role in neoadjuvant and adjuvant RCC. Furthermore, its potential role in sequential and combination studies with other VEGFR and non-VEGFR targeted agents is discussed. Overall, pazopanib is a unique VEGF TKI, with a different and more favorable safety profile compared with other members of the VEGF TKI family and represents an attractive alternative for patients with mRCC.
Keywords: kidney cancer, pazopanib, renal cell carcinoma, targeted therapy, tyrosine kinase inhibitors, vascular endothelial growth factor
Introduction
Renal cell carcinoma (RCC) accounts for approximately 2–3% of all cancers and is the sixth leading cause of death in the United States, with an estimated 65,150 new cases and 13,680 deaths in 2013 (http://www.cancer.org). About 30% of patients with RCC present with advanced/metastatic disease at time of presentation, portending an overall poor prognosis.
RCC is refractory to conventional systemic therapeutic agents and radiotherapy. Until the development of targeted angiogenesis inhibitors, cytokine-based therapies such as interferon and high-dose interleukin 2 (HD IL-2) were the only systemic agents readily available despite limited clinical efficacy [Atkins et al. 2004]. HD IL-2 is the only systemic treatment that can potentially result in a cure in some patients with metastatic RCC (mRCC), producing a 14% response rate (RR) with some durable responses seen in phase II trials; it was approved by the US Food and Drug Administration (FDA) for use in first-line therapy for mRCC in 1992 [Fyfe et al. 1995]. Recent evidence from a multicenter study suggests that HD IL-2 may produce a RR of 29%, significantly higher than the historical experience [McDermott et al. 2010]. However, the higher RR could have been due to better patient selection; namely, the majority of patients with prior nephrectomy, clear-cell histology, Eastern Cooperative Oncology Group Performance Status (ECOG PS) of 0, and Memorial Sloan–Kettering Cancer Center (MSKCC) intermediate risk category [Motzer et al. 1999]. In summary, while HD IL-2 can result in a cure in mRCC, given the significant toxicity and limited efficacy, its application is limited to selected patients with mRCC.
Loss or mutation of the von Hippel–Lindau gene leads to overexpression of hypoxia-inducible factor (HIF) and HIF target genes such as vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) as well as their receptors, thus making them attractive therapeutic targets in our therapeutic armamentarium [Mandriota et al. 2002]. Significant advances in our understanding of RCC tumor biology including the role of VEGF and mammalian target of rapamycin (mTOR) pathway have led to the clinical development of multitargeted tyrosine kinase inhibitors (TKIs) and mTOR inhibitors for the treatment of advanced RCC [Hanna et al. 2008; Rini, 2007]. Seven agents have been approved by the FDA in the last 7 years for the treatment of mRCC, including VEGF TKIs sorafenib [Escudier et al. 2007a], sunitinib [Motzer et al. 2007], pazopanib [Sternberg et al. 2010] and axitinib [Rini et al. 2011], a humanized monoclonal antibody targeting VEGF, bevacizumab [Escudier et al. 2007b], and mTOR inhibitors temsirolimus [Hudes et al. 2007] and everolimus [Motzer et al. 2008], transforming our current treatment paradigm.
Pazopanib (GW786034; Votrient, GlaxoSmithKline, Research Triangle Park, NC, USA), a VEGF TKI, was approved by the FDA in October 2009 and by the European Medicines Agency (EMA) in June 2010 for the treatment of mRCC. Herein, the clinical development, current scope, and future insights regarding the use of pazopanib in advanced RCC are reviewed.
Pharmacology
Pazopanib is an orally bioavailable indazolylpyrimidine 5-[[4-{(2, 3-dimethyldimethyl-2H-indazol-6-yl)methylamino}-2-pyrimidinyl]amino]-2-methylbenzenesulfonamide [Harris et al. 2008]. Figure 1 details the chemical structure of pazopanib. Pazopanib is a low nanomolar, highly selective and potent kinase inhibitor of VEGFR, platelet-derived growth factor receptor (PDGFR), and c-kit tyrosine kinases. It inhibits the intracellular tyrosine kinase portion of all the VEGFR subtypes (VEGFR1, VEGFR2, and VEGFR3) and the PDGFR subtypes (PDGFRα and PDGFRß). In addition, it inhibits fibroblast growth factor receptor (FGFR1, FGFR3), and transmembrane glycoprotein receptor tyrosine kinases (c-fms). While the inhibitory concentration 50 of pazopanib for optimal VEGFR2 inhibition was 0.02 μmol/liter, a plasma concentration of around 40 μmol/liter was required for maximal VEGFR2 inhibition in vivo. This discrepancy between in vivo and in vitro requirements was attributed to pazopanib’s significant protein binding [Kumar et al. 2007].
Figure 1.
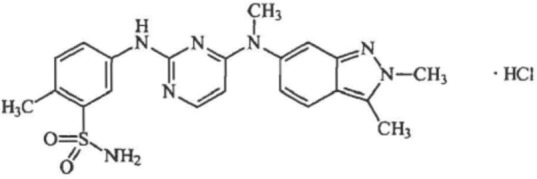
Chemical structure of pazopanib [Harris et al. 2008].
Molecular formula: C21H23N7O2S•HCl.
Chemical name: 5-[[4-{(2,3-dimethyl-2H-indazol-6-yl)methylamino}-2pyrimidinyl]amino]-2-methylbenzenesulfonamide monohydrochloride.
Clinical efficacy
Phase I
Based on the encouraging preclinical activity of pazopanib, a phase I dose escalation clinical trial was undertaken by Hurwitz and colleagues [Hurwitz et al. 2005] in which 63 patients with refractory solid tumors received escalating doses of pazopanib (50 mg three times weekly, 50–2000 mg daily, and 300–400 mg twice daily). The T½ values of pazopanib ranged from 18.1 to 52.3 h, the steady state concentration was achieved at this dose as well as at a 300 mg twice-daily dose. A decrease in tumor perfusion as measured by dynamic contrast-enhanced magnetic resonance imaging was observed in patients receiving 800 mg once daily or at a 300 mg twice-daily dose. Pazopanib was well tolerated, with hypertension, which is a class effect of all VEGF TKIs, being the most frequent grade 3 adverse event. Diarrhea, hair depigmentation, and nausea were other adverse events that occurred. Clinical activity was seen at doses of 800 mg once daily or at 300 mg twice daily; out of 12 patients with mRCC, two patients had partial responses (PRs), four patients had stable disease (SD), and four patients had progressive disease. The dose of 800 mg once daily was selected for evaluation in phase II and III clinical trials with various solid tumor types.
Phase II
The phase II study to evaluate the efficacy and safety of pazopanib consisted of 800 mg administered once daily in patients with mRCC designed as a randomized discontinuation study which enrolled 225 patients at 43 clinical sites across nine countries [Hutson et al. 2010]. After 12 weeks, patients with SD were randomized to pazopanib or placebo. However, an interim analysis done after 60 patients completed 12 weeks of treatment demonstrated a RR of 38% and based on the recommendations by the independent data monitoring committee, randomization was halted, and all continuing patients in the study were treated on an open-label basis until disease progression. Of the 225 patients, 155 (69%) were treatment naïve, and 70 (31%) had received one prior cytokine or bevacizumab containing regimen. A total of 55 patients with SD at week 12 participated in the randomized component of the study, with the remaining 170 patients receiving open-label pazopanib. The RR was 35% [95% confidence interval (CI) 28–41%], when assessed by an independent review committee and 34% (95% CI 28–40%) as determined by investigator assessment. RR was 34% in treatment-naïve patients (95% CI 26–41%) and 37% in patients with refractory disease (95% CI 26–49%). Median duration of response was 68 weeks and median progression-free survival (PFS) was 52 weeks (95% CI 44–60 weeks). This was similar to the RR of 31% and median PFS of 11 months previously reported with sunitinib in patients with mRCC [Motzer et al. 2007]. Overall, pazopanib was well tolerated; the most common treatment-emergent adverse events were diarrhea (63%), fatigue (46%), hair depigmentation (43%), nausea (42%), and hypertension (41%) with the most common grade 3 or 4 treatment-related adverse events being hypertension (8%), increased serum liver function studies [aspartate aminotransferase (ALT) in 6%, alanine aminotransferase (AST) in 4%], diarrhea (4%), and fatigue (4%). Importantly, there were two deaths thought to be treatment related. Elevations in ALT (54%) and AST (53%) were the most common treatment-emergent laboratory abnormalities. Incidences of grade 3 or grade 4 myelosuppression, hand–foot syndrome, and mucositis were low with pazopanib. Based on the efficacy and tolerability of pazopanib in this study, a phase III randomized trial of pazopanib versus placebo was completed, as described below.
Phase III
Pazopanib versus placebo
This pivotal phase III study was a randomized, global, multicenter, double-blind, placebo-controlled study evaluating the efficacy of pazopanib treatment-naive and cytokine-pretreated patients with advanced or metastatic disease [Sternberg et al. 2010a]. This study enrolled 435 patients across 80 centers in Europe, Asia, South America, North Africa, Australia, and New Zealand. Treatment-naïve patients were primarily from countries where there was limited access to the standard systemic therapies at that time or where cytokines were not routinely used as a standard treatment. The majority of patients had clear-cell or predominantly clear-cell histology, and were stratified to the good and intermediate risk category based on MSKCC criteria [Motzer et al. 1999]. Patients were randomized in a 2 to 1 ratio to receive either 800 mg pazopanib daily or placebo, with patients receiving placebo allowed to cross over to the pazopanib arm at the time of progression through an extension trial. The primary endpoint of the study was PFS. Of all 435 patients, 233 were treatment naïve, with the remaining 202 patients cytokine pretreated. Pazopanib significantly prolonged the median PFS compared with the placebo arm [9.2 versus 4.2 months; hazard ratio (HR) 0.46; 95% CI 0.34–0.62; p < 0.0001]. Improvement in PFS was more pronounced in the treatment-naïve subgroup (11.1 versus 2.8 months; HR 0.40; 95% CI 0.27–0.60; p < 0.0001), as opposed to the cytokine-pretreated subgroup (7.4 versus 4.2 months; HR 0.54; 95% CI 0.35–0.84; p < 0.001). The RR in the overall study population was 30%, with a median duration of response of 58.7 weeks, with similar rates seen in the treatment-naive (32%) and cytokine-pretreated (29%) populations. The interim overall survival (OS) analysis showed a benefit to pazopanib (21.1 months versus 18.7 months, HR 0.73, p = 0.02). The updated final OS results showed a median OS of 22.9 months with pazopanib versus 20.5 months with placebo (HR 0.91; 95% CI 0.71–1.16; stratified log-rank p-value = 0.224) [Sternberg et al. 2010b]. It was felt that the crossover from placebo to pazopanib, with more placebo- than pazopanib-treated patients (66% versus 30%) could have constituted a confounding variable. A post hoc inverse probability of censoring weighted statistical analysis was conducted to adjust for crossover and estimate the effect of pazopanib compared with placebo and it demonstrated a 50% reduction in the risk of death with pazopanib (HR 0.50; 95% CI 0.32–0.76; p = 0.002). This study demonstrated superiority of pazopanib over placebo in the frontline setting for mRCC, with its clinical efficacy comparable to other previously approved agents which led to its approval by both the FDA and EMA.
These results also demonstrated that the PFS with pazopanib was comparable to that reported with sunitinib, which has been approved by the FDA for use in mRCC since 2006. However, the toxicity profile of pazopanib is quite distinct from sunitinib, with pazopanib having a lower incidence of hand–foot syndrome, hematologic toxicity, and impact on underlying cardiac function, but it has a reported higher incidence of hepatic dysfunction. The uniqueness of the side effect profile of these respective targeted agents highlights the importance of tailoring therapy based upon individual patient comorbidities and the relative toxicity profile of the VEGF TKI agent being considered.
Pazopanib versus sunitinib
Results from the much awaited pivotal trial comparing pazopanib with sunitinib as a first-line treatment for clear-cell mRCC (COMPARZ trial) were recently presented [Motzer et al. 2012]. This study was designed as a global, phase III, randomized, open-label trial which enrolled 1100 patients, with the primary endpoint of the study being PFS, with secondary endpoints being safety and quality of life which was powered for noninferiority. Patients were randomized to receive pazopanib at 800 mg daily or sunitinib 50 mg daily for 4 weeks, followed by 2 weeks off treatment. Pazopanib demonstrated similar efficacy compared with sunitinib, with a median PFS of 10.5 months versus 10.2 months (HR 0.998; 95% CI 0.863–1.154, not significant) respectively. RR was statistically significantly higher (p = 0.03) with pazopanib (31% versus 25%, respectively). In an interim analysis, OS was similar (28.4 months versus 29.3 months respectively), with a HR of 0.91, 95% CI 0.76–1.08, which was not statistically significant. Pazopanib was associated with a lower incidence of hand–foot syndrome (29% versus 50%), fatigue (55% versus 63%), taste alteration (26% versus 36%) as well as dyspepsia, hypothyroidism, mucositis, and myelosuppression. Notably, hypertension was more frequent among patients receiving pazopanib (46% versus 41%). Other side effects more common with pazopanib were liver function enzyme increases (i.e. ALT) of 31% versus 18% respectively and hair discoloration (30% versus 10%, respectively). Quality of life measures were also in favor of pazopanib. In conclusion, while pazopanib is similar to sunitinib in its clinical efficacy, it has a different toxicity profile, with it being better tolerated by most patients.
Another phase III study (PISCES) assessed patient-reported outcomes with pazopanib and sunitinib in which the investigators assessed whether the tolerability differences were significant enough to result in a patient preferring to continue their treatment with one of these two agents [Escudier et al. 2012]. A total of 168 patients with mRCC were blinded and randomized in a 1:1 manner to receive as first-line treatment either 800 mg of pazopanib for 10 weeks followed by a 2-week washout and then 50 mg of sunitinib for 10 weeks (4/2 weeks on/off schedule) or vice versa. The primary endpoint was patient preference assessed at 22 weeks and compared using Prescott’s statistical methodology (α = 0.10). In the protocol-driven primary analysis, 70% of patients preferred pazopanib, and only 22% of patients preferred sunitinib, with the remaining 8% of patients having no preference. After adjusting for a modest sequence effect, the difference in preference was 49% (90% CI 37.0–61.5%; p < 0.001) in favor of pazopanib.
Combination studies of pazopanib
A rational approach to improve clinical outcomes and potentially overcome drug resistance in mRCC might be to combine different drugs targeting the VEGF receptor and non-VEGF receptor pathways. While theoretically appealing, the combination studies of other VEGF TKIs and mTOR inhibitors were associated with increased toxicity and no added clinical efficacy [Feldman et al. 2009; Mulders, 2009; Negrier et al. 2011; Rini, 2007; Azad et al. 2008; Rini et al. 2011]. However, a combination of bevacizumab and everolimus in treatment-naïve and VEGF-TKI-treated patients with mRCC resulted in a 30% RR [Hainsworth et al. 2010]. An ongoing study is exploring the combination of pazopanib and bevacizumab in treatment-naïve patients with mRCC [ClinicalTrials.gov identifier: NCT01684397]. A combination study of sunitinib, pazopanib or iplimumab, an anticytotoxic T-lymphocyte antigen 4 antibody, with nivolumab, an immunoglobulin G4 antibody, is also ongoing (CheckMate016) [ClinicalTrials.gov identifier: NCT01472081]. The results from these studies are awaited and will help determine the safety and feasibility of combining pazopanib with other targeted agents and immunotherapeutic agents.
Scope of pazopanib in subsequent-line therapy
Sorafenib, a VEGF TKI, is currently indicated for second-line use in patients with mRCC who progress on cytokine therapy but not on targeted therapy [Escudier et al. 2007a]. Everolimus, an mTOR inhibitor, is approved for use in mRCC after failure of first-line VEGF TKIs [Motzer, 2008]. Given the uniqueness of each VEGF TKI and some lack of cross resistance among them, sequential use of VEGF TKIs after failure of distinct initial VEGF TKIs could be a rational therapeutic approach [Rini and Atkins, 2009]. The AXIS trial compared axitinib and sorafenib in patients with mRCC progressing after first-line therapy with cytokines or sunitinib and reported improvement in PFS with axitinib, leading to its approval for this indication [Escudier et al. 2007a; Rini et al. 2011]. There is no consensus over choosing the optimal second-line agent after failure of VEGF-TKI therapy, and clinicians are posed with the conundrum of choosing between axitinib and affinitor.
There are very limited data on the role of pazopanib in this setting; in a phase II study, pazopanib was evaluated in 44 patients with mRCC who had progressed on first-line single-agent sunitinib or bevacizumab [Reeves et al. 2011]. At a median follow up of 9 months, overall RR was 20% (16% among those with prior sunitinib, 33% among those with prior bevacizumab), with the overall disease control rate, that is complete response (CR) + PR + SD, being 77% (66% with prior sunitinib and 83% with prior bevacizumab). In another single-institution retrospective study reported in abstract form, 88 patients had received salvage pazopanib after receiving another VEGF TKI, mTOR inhibitor, or both, with 25% of patients reporting a PR [Matrana et al. 2011].
The Ireland Cooperative Oncology Research Group is conducting a phase II study to evaluate the role of pazopanib following clinical failure of sunitinib alone or when followed by an mTOR inhibitor [ClinicalTrials.gov identifier: NCT01566747] and another phase II study with pazopanib in a second- or third-line setting is ongoing among patients with VEGF-TKI-refractory mRCC. [ClinicalTrials.gov identifier: NCT01157091]. The results from these trials are awaited to demonstrate whether pazopanib may be of benefit as subsequent-line therapy.
Scope of pazopanib in poor-risk and non-clear-cell metastatic renal cell carcinoma
Current National Comprehensive Cancer Network guidelines recommend the use of the mTOR inhibitor temsirolimus for poor-risk patients with mRCC [Motzer et al. 1999; Hudes et al. 2007; National Comprehensive Cancer Network, 2012]. There is lack of data on the role of pazopanib in this setting and ongoing studies in poor-risk mRCC are addressing this question: the FLIPPER study is a single-arm study with first-line pazopanib [ClinicalTrials.gov identifier: NCT01521715] and another randomized phase II study comparing it with temsirolimus [ClinicalTrials.gov identifier: NCT01392183].
There is lack of prospective, randomized data pertaining to the efficacy of VEGF-TKIs in the treatment of non-clear-cell mRCC, although some data from the expanded access programs suggest that the VEGF TKIs sorafenib and sunitinib may have some activity in this subgroup of patients, as these tumors overexpress c-kit and these drugs target c-kit [Gore et al. 2009; Stadler et al. 2010]. Given that pazopanib is an inhibitor of c-kit as well, its efficacy in non-clear-cell mRCC is being explored in a phase II trial [ClinicalTrials.gov identifier: NCT01538238]. The results from these studies would further clarify whether pazopanib has a role in this subgroup of patients with mRCC.
Scope of pazopanib in the neoadjuvant setting
Although there is a lack of randomized studies evaluating the role of presurgical targeted therapy, there is evidence that it imparts a response within primary renal tumors [Amin et al. 2008; Abel et al. 2011; Shuch et al. 2008; van der Veldt et al. 2008] reported primary tumor responses in a single institution series of 168 patients who received targeted therapy with the primary tumor in situ [Abel et al. 2011]. Patients had received various neoadjuvant-targeted agents, including sunitinib, pazopanib, bevacizumab (alone or in combination with erlotinib), or temsirolimus. The overall primary tumor PR was only 6% in this series, and there were no CRs demonstrated. The median maximal response was approximately 7%, occurring at a median time point of 154 days, and a 24.5% response was seen only in a minority of patients at 174 days. Although it is unusual to expect a significant response with the neoadjuvant use of TKIs within primary renal tumors and within the clinical setting of mRCC, encouraging results were reported in a single-arm phase II study (PANTHER 2009-016675-29) evaluating 12–14 weeks of pazopanib prior to planned nephrectomy in 34 untreated patients with mRCC stratified within the MSKCC intermediate- and poor-risk groups [Boleti et al. 2012]. A total of 30 out of 34 (88%) patients derived a clinical benefit [by Response Evaluation Criteria In Solid Tumors (RECIST)] from pazopanib prior to surgery and a 21% PR rate within the primary tumor was observed (by RECIST). None of the patients had inoperable disease due to local disease progression and nephrectomy was successfully performed in 74% of patients. These results suggest upfront pazopanib followed by nephrectomy can be performed safely in patients with mRCC, with disease control occurring in the majority of patients. Another ongoing phase II study is evaluating the merit of 12 weeks of pazopanib among patients with localized RCC, with the hope that it can help further characterize predictive biomarkers of drug activity [ClinicalTrials.gov identifier: NCT01361113].
Scope of pazopanib in the adjuvant setting
The prognosis of patients after nephrectomy for clinically localized RCC depends on the tumor stage, grade, and histology; recurrence rates in patients with localized RCC range from 35% to 65% [Lam et al. 2006]. Several trials have tested interferon α or HD IL-2 post nephrectomy, although they failed to demonstrate any clinical benefit to this therapeutic approach [Clark et al. 2003; Messing et al. 2003; Pizzocaro et al. 2001]. Robust intermediate- to long-term data are currently pending from studies evaluating the role of VEGF TKIs in this setting, including the following: the ASSURE trial comparing adjuvant sorafenib or sunitinib; the S-TRAC trial comparing sunitinib with placebo; and the SORCE trial comparing sorafenib with placebo [Sciarra et al. 2012]. Importantly, pazopanib is also being evaluated in this role as part of the ongoing PROTECT trial, a randomized, double-blind, placebo-controlled phase III study evaluating the efficacy and safety of pazopanib as adjuvant therapy for patients with localized, high-risk or locally advanced RCC following nephrectomy [ClinicalTrials.gov identifier: NCT01235962]; this study is expected to be completed in 2016.
In summary, while there is no established role for adjuvant systemic therapy in RCC following nephrectomy, the results from the aforementioned studies could help establish whether VEGF TKIs, including pazopanib, can improve patient outcomes in this setting.
Selecting the optimal targeted therapy in metastatic renal cell carcinoma
The therapeutic landscape of mRCC continues to evolve significantly, with the availability of numerous agents targeting VEGF and non-VEGFR pathways along with many promising small-molecule agents in the clinical pipeline. With the availability of four approved VEGF TKIs, a VEGFR antibody and two mTOR inhibitors, it is routine to achieve disease control for a period of time in many patients with mRCC. However, selection of the optimal targeted agent for any given patient is quite arbitrary, with direct head-to-head comparisons lacking for most agents and the rapid evolution of newer drugs further complicating our current treatment algorithm. It remains a significant challenge to identify the most effective targeted agent, with the best toxicity profile, and to determine the ideal sequence or combination of drugs that should be employed for individual patients to offer a truly personalized treatment approach. We suspect the characterization of serological and tissue biomarkers predicting optimal responses to specific targeted agents will continue to evolve, which will ultimately lead us to our primary objective of truly seeing consistent CRs with these systemic agents, resulting in durable long-term treatment responses, with meaningful improvements in overall and disease-specific survival.
Conclusion
The approval of pazopanib has been a critical addition to the treatment options of treatment-naïve and cytokine-refractory mRCC (Table 1). The results from pivotal randomized phase III studies provide important evidence that pazopanib has similar efficacy to sunitinib. In addition, due to its different toxicity profile, it is better tolerated and may be preferred over sunitinib in most patients. While its role in the setting of mRCC has been well established for the last few years, several ongoing clinical trials are also evaluating its role in the neoadjuvant and adjuvant setting. Various studies are exploring the optimal sequencing regimen and combination studies with pazopanib in mRCC and the data from these studies are eagerly awaited (Table 2). Some of the key points about pazopanib, its current role and future scope in advanced RCC/mRCC are listed in Table 3. With the therapeutic arena of mRCC becoming increasingly crowded, with the addition of newer agents, the choice of the most appropriate targeted agent for the treatment of mRCC is quite ambiguous and challenging at this time. Lastly, clinicians must carefully choose the appropriate therapy based upon tumor histology, clinical course of disease, prognostic factors, patient comorbidities, preferences, as well as the efficacy and safety profile of the individual agents.
Table 1.
Summary of randomized phase III trials of approved targeted agents in metastatic renal cell cancer.
| Study/author | Targeted agent | Setting | N | Median PFS (months) | Median OS (months) |
|---|---|---|---|---|---|
| [Motzer et al. 2009] | Sunitinib versus IFN-α | First line | 750 | 11.0 versus 5.0 | 26.4 versus 21.8 |
| TARGET [Escudier et al. 2007a] | Sorafenib versus placebo | Second line | 903 | 5.5 versus 2.8 | 17.8 versus 15.2 |
| [Sternberg et al. 2010b] | Pazopanib versus placebo | First, second line | 435 | 9.2 versus 4.2 | 22.9 versus 20.5 |
| AVOREN [Escudier et al. 2007b] | Bevacizumab + IFNα versus placebo + IFNα | First line | 649 | 10.2 versus 5.4 | 23.3 versus 21.3 |
| CALGB 90206 [Rini et al. 2008] | Bevacizumab + IFNα versus IFNα | First line | 732 | 8.5 versus 5.2 | 18.3 versus 17.4 |
| [Hudes et al. 2007] | Temsirolimus versus IFNα | First line | 626 | 3.8 versus 1.9 | 10.9 versus 7.3 |
| [Motzer et al. 2008] | Everolimus versus placebo | Second line | 416 | 4.9 versus 1.9 | 14.8 versus 14.4 |
| [Rini et al. 2011] | Axitinib versus sorafenib | Second line | 723 | 6.7 versus 4.7 | Not reached versus 18 |
| COMPARZ [Motzer et al. 2012] | Pazopanib versus sunitinib | First line | 1100 | 10.5 versus 10.2 | 28.4 versus 29.3 |
IFN, interferon; OS, overall survival; PFS, progression-free survival.
Table 2.
Ongoing clinical trials evaluating pazopanib in renal cell carcinoma.
| Identifier | Phase | Description |
|---|---|---|
| NCT01566747 | II | Sequential treatment of pazopanib in VEGF TKI refractory mRCC |
| NCT01157091 | II | Sequential treatment of pazopanib in VEGF TKI refractory mRCC |
| NCT01684397 | II | Combination study of pazopanib and bevacizumab |
| NCT01472081 | II | Combination study of sunitinib, pazopanib or iplimumab, an anti-CTLA-4 antibody with nivolumab, an IgG4 antibody |
| NCT01521715 | II | Single arm, pazopanib in poor-risk mRCC |
| NCT01392183 | II | Pazopanib versus temsirolimus in poor-risk mRCC |
| NCT01538238 | II | Single arm, pazopanib in non-clear-cell mRCC |
| NCT01361113 | II | Single arm, pazopanib in neoadjuvant localized RCC |
| NCT01235962 | III | Randomized, placebo-controlled study of pazopanib in high-risk localized RCC following nephrectomy |
CTLA-4, cytotoxic T-lymphocyte antigen 4; IgG, immunoglobulin G; mRCC, metastatic renal cell carcinoma; RCC, renal cell carcinoma; TKI, tyrosine kinase inhibitor; VEGF, vascular enothelial growth factor.
Table 3.
Key points and summary for pazopanib in renal cell carcinoma.
| Pazopanib is a TKI with activity against VEGFR, PDGFR and c-KIT |
| Pazopanib significantly improved PFS over placebo in both treatment-naïve and cytokine-treated patients with mRCC; it demonstrated a trend towards improved OS despite crossover of patients in the placebo arm |
| Pazopanib has a favorable toxicity profile, with the most common side effects being grade 1–2 hypertension, diarrhea, hair and skin color changes, nausea, vomiting and anorexia. The most common laboratory abnormalities include grade hepatic enzyme and bilirubin elevations, grade 3 laboratory abnormalities include hepatic enzyme elevations in up to 13% of patients |
| Compared with sunitinib, pazopanib has similar efficacy but a lower incidence of mucositis, hand–foot syndrome, fatigue, and hematologic toxicity, making it a preferred option for most patients |
| Ongoing clinical trials will clarify its role in sequential and combination studies with other targeted agents, as well as poor-risk and patients with non-clear-cell mRCC. |
| Pazopanib is also being tested in neoadjuvant and adjuvant settings in RCC but its role in this regard remains unknown at this time |
mRCC, metastatic renal cell carcinoma; OS, overall survival; PDGFR, platelet-derived growth factor receptor; PFS, progression-free survival; RCC, renal cell carcinoma; TKI, tyrosine kinase inhibitor; VEGFR, vascular enothelial growth factor receptor.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Shilpa Gupta, Department of Genitourinary Oncology, H Lee Moffitt Cancer Center and Research Institute, Tampa FL, USA.
Philippe E. Spiess, Associate Member, Department of Genitourinary Oncology, H Lee Moffitt Cancer Center and Research Institute, 12902 Magnolia Drive, Tampa, FL 33612, USA
References
- Abel E., Culp S., Tannir N., Matin S., Tamboli P., Jonasch E., et al. (2011) Primary tumor response to targeted agents in patients with metastatic renal cell carcinoma. Eur Urol 59: 10–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin C., Wallen E., Pruthi R., Calvo B., Godley P., Rathmell W. (2008) Preoperative tyrosine kinase inhibition as an adjunct to debulking nephrectomy. Urology 72: 864–868 [DOI] [PubMed] [Google Scholar]
- Atkins M., Regan M., McDermott D. (2004) Update on the role of interleukin 2 and other cytokines in the treatment of patients with stage IV renal carcinoma. Clin Cancer Res 10: 6342S–6346S [DOI] [PubMed] [Google Scholar]
- Azad N., Posadas E., Kwitkowski V., Steinberg S., Jain L., Annunziata C., et al. (2008) Combination targeted therapy with sorafenib and bevacizumab results in enhanced toxicity and antitumor activity. J Clin Oncol 26: 3709–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boleti E., Sarwar N., Jones R., Chowdhury S., Crabb S., Shamash J., et al. (2012) The safety and efficacy of pazopanib prior to planned nephrectomy in metastatic clear cell renal cancer. J Clin Oncol 30(Suppl. 5): abstract 427. [Google Scholar]
- Clark J., Atkins M., Urba W., Creech S., Figlin R., Dutcher J., et al. (2003) Adjuvant high-dose bolus interleukin-2 for patients with high-risk renal cell carcinoma: a cytokine working group randomized trial. J Clin Oncol 21: 3133–3140 [DOI] [PubMed] [Google Scholar]
- Escudier B., Eisen T., Stadler W., Szczylik C., Oudard S., Siebels M., et al. (2007a) Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356: 125–134 [DOI] [PubMed] [Google Scholar]
- Escudier B., Pluzanska A., Koralewski P., Ravaud A., Bracarda S., Szczylik C., et al. (2007b) Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet 370: 2103–2111 [DOI] [PubMed] [Google Scholar]
- Escudier B., Porta C., Bono P., Giorgi U., Parikh O., Hawkins R. (2012) Patient preference between pazopanib (Paz) and sunitinib (Sun): Results of a randomized double-blind, placebo-controlled, cross-over study in patients with metastatic renal cell carcinoma (mRCC)—PISCES study, NCT 01064310. J Clin Oncol 30(15 Suppl.): CRA4502 [Google Scholar]
- Feldman D., Baum M., Ginsberg M., Hassoun H., Flombaum C., Velasco S., et al. (2009) Phase I trial of bevacizumab plus escalated doses of sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol 27: 1432–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyfe G., Fisher R., Rosenberg S., Sznol M., Parkinson D., Louie A.(1995) Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol 13: 688–696 [DOI] [PubMed] [Google Scholar]
- Gore M., Szczylik C., Porta C., Bracarda S., Bjarnason G., Oudard S., et al. (2009) Safety and efficacy of sunitinib for metastatic renal-cell carcinoma: an expanded-access trial. Lancet Oncol 10: 757–763 [DOI] [PubMed] [Google Scholar]
- Hainsworth J., Spigel D., Burris H., 3rd, Waterhouse D., Clark B., Whorf R. (2010) Phase II trial of bevacizumab and everolimus in patients with advanced renal cell carcinoma. J Clin Oncol 28: 2131–2136 [DOI] [PubMed] [Google Scholar]
- Hanna S., Heathcote S., Kim W. (2008) mTOR pathway in renal cell carcinoma. Expert Rev Anticancer Ther 8: 283–292 [DOI] [PubMed] [Google Scholar]
- Harris P., Boloor A., Cheung M., Kumar R., Crosby R., Davis-Ward R., et al. (2008) Discovery of 5-[[4-[(2,3-dimethyl-2H-indazol-6-yl)methylamino]-2-pyrimidinyl]amino]-2-methyl-b enzenesulfonamide (pazopanib), a novel and potent vascular endothelial growth factor receptor inhibitor. J Med Chem 51: 4632–4640 [DOI] [PubMed] [Google Scholar]
- Hudes G., Carducci M., Tomczak P., Dutcher J., Figlin R., Kapoor A., et al. (2007) Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 356: 2271–2281 [DOI] [PubMed] [Google Scholar]
- Hurwitz H., Dowlati A., Savage S., Fernando N., Laasalvia S., Whitehead B., et al. (2005) Safety, tolerability, and pharmacokinetics of oral administration of GW780634 in pts with solid tumors. J Clin Oncol 23(Suppl. 16S): 3012 [Google Scholar]
- Hutson T., Davis I., Machiels J., De Souza P., Rottey S., Hong B., et al. (2010) Efficacy and safety of pazopanib in patients with metastatic renal cell carcinoma. J Clin Oncol 28: 475–480 [DOI] [PubMed] [Google Scholar]
- Kumar R., Knick V., Rudolph S., Johnson J., Crosby R., Crouthamel M., et al. (2007) Pharmacokinetic–pharmacodynamic correlation from mouse to human with pazopanib, a multikinase angiogenesis inhibitor with potent antitumor and antiangiogenic activity. Mol Cancer Ther 6: 2012–2021 [DOI] [PubMed] [Google Scholar]
- Lam J., Belldegrun A., Figlin R. (2006) Adjuvant treatment for renal cell carcinoma. Exp Opin Pharmacother 7: 705–720 [DOI] [PubMed] [Google Scholar]
- Mandriota S., Turner K., Davies D., Murray P., Morgan N., Sowter H., et al. (2002) HIF activation identifies early lesions in VHL kidneys: evidence for site-specific tumor suppressor function in the nephron. Cancer Cell 1: 459–468 [DOI] [PubMed] [Google Scholar]
- Matrana M., Atkinson B., Corn P., Jonasch E., Tannir N. (2011). Metastatic renal cell carcinoma treated with pazopanib after progression on other targeted agents: a single-institution experience.J Clin Oncol 29(Suppl. 7): abstract 351. [Google Scholar]
- McDermott D., Ghebremichael M., Signoretti S., Margolin K., Clark J., Sosman J., et al. (2010) The high-dose aldesleukin (HD IL-2) ‘SELECT’ trial in patients with metastatic renal cell carcinoma (mRCC). J Clin Oncol 28(15 Suppl.): 4514 [Google Scholar]
- Messing E., Manola J., Wilding G., Propert K., Fleischmann J., Crawford E., et al. (2003) Phase III study of interferon alfa-NL as adjuvant treatment for resectable renal cell carcinoma: an Eastern Cooperative Oncology Group/Intergroup trial. J Clin Oncol 21: 1214–1222 [DOI] [PubMed] [Google Scholar]
- Motzer R., Escudier B., Oudard S., Hutson T., Porta C., Bracarda S., et al. (2008) Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 372: 449–456 [DOI] [PubMed] [Google Scholar]
- Motzer R., Hutson T., Reeves J., Hawkins R., Guo J., Nathan P., et al. (2012) Randomized, open-lael, phase III trial of pazopanib versus sunitinib in first-line treatment of patients with metastatic renal cell carcinoma (mRCC): results of the COMPARZ trial. Ann Oncol 23(Suppl. 9): abstract 2325. [Google Scholar]
- Motzer R., Hutson T., Tomczak P., Michaelson M., Bukowski R., Oudard S., et al. (2009) Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 27: 3584–3590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzer R., Hutson T., Tomczak P., Michaelson M., Bukowski R., Rixe O., et al. (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356: 115–124 [DOI] [PubMed] [Google Scholar]
- Motzer R., Mazumdar M., Bacik J., Berg W., Amsterdam A., Ferrara J. (1999) Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol 17: 2530–2540 [DOI] [PubMed] [Google Scholar]
- Mulders P. (2009) Vascular endothelial growth factor and mTOR pathways in renal cell carcinoma: differences and synergies of two targeted mechanisms. BJU Int 104: 1585–1589 [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network (2013) V 1 guidelines for management kidney tumor. Available at:http://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf (accessed 11 March 2013).
- Negrier S., Gravis G., Perol D., Chevreau C., Delva R., Bay J., et al. (2011) Temsirolimus and bevacizumab, or sunitinib, or interferon alfa and bevacizumab for patients with advanced renal cell carcinoma (TORAVA): a randomised phase 2 trial. Lancet Oncol 12: 673–680 [DOI] [PubMed] [Google Scholar]
- Pizzocaro G., Piva L., Colavita M., Ferri S., Artusi R., Boracchi P., et al. (2001) Interferon adjuvant to radical nephrectomy in Robson stages II and III renal cell carcinoma: a multicentric randomized study. J Clin Oncol 19: 425–431 [DOI] [PubMed] [Google Scholar]
- Reeves J., Spigel D., Daniel D., Friedman E., Burris H., Hainsworth J., et al. (2011) Pazopanib in patients with metastatic renal cell carcinoma previously treated with sunitinib or bevacizumab: a Sarah Cannon Research Institute phase II trial. J Clin Oncol 29(Suppl.): abstract 4659. [Google Scholar]
- Rini B. (2007) Vascular endothelial growth factor-targeted therapy in renal cell carcinoma: current status and future directions. Clin Cancer Res 13: 1098–1106 [DOI] [PubMed] [Google Scholar]
- Rini B., Atkins M. (2009) Resistance to targeted therapy in renal-cell carcinoma. Lancet Oncol 10: 992–1000 [DOI] [PubMed] [Google Scholar]
- Rini B., Escudier B., Tomczak P., Kaprin A., Szczylik C., Hutson T., et al. (2011) Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 378: 1931–1939 [DOI] [PubMed] [Google Scholar]
- Rini B., Halabi S., Rosenberg J., Stadler W., Vaena D., Ou S., et al. (2008) Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma. CALGB 90206. J Clin Oncol 26(33): 5422–5428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciarra A., Cattarino S., Salciccia S., Alfarone A., Gentilucci A., Parente U., et al. (2012) The emerging role of targeted therapy in renal cell carcinoma (RCC): is it time for a neoadjuvant or an adjuvant approach? Crit Rev Oncol Hematol 81: 151–162 [DOI] [PubMed] [Google Scholar]
- Shuch B., Riggs S., LaRochelle J., Kabbinavar F., Avakian R., Pantuck A., et al. (2008) Neoadjuvant targeted therapy and advanced kidney cancer: observations and implications for a new treatment paradigm. BJU Int 102: 692–696 [DOI] [PubMed] [Google Scholar]
- Stadler W., Figlin R., McDermott D., Dutcher J., Knox J., Miller W., Jr, et al. (2010) Safety and efficacy results of the advanced renal cell carcinoma sorafenib expanded access program in North America. Cancer 116: 1272–1280 [DOI] [PubMed] [Google Scholar]
- Sternberg C., Davis I., Mardiak J., Szczylik C., Lee E., Wagstaff J., et al. (2010a) Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 28: 1061–1068 [DOI] [PubMed] [Google Scholar]
- Sternberg C., Hawkins R., Szczylik C., Davis I., Wagstaff J., McCann L., et al. (2010b) Randomized, double-blind phase III study of pazopanib in patients with advanced/metastatic renal cell carcinoma (MRCC): final overall survival (OS) results. Ann Oncol 21(Suppl. 8): abstract LBA22. [Google Scholar]
- van der Veldt A., Meijerink M., van den Eertwegh A., Bex A., de Gast G., Haanen J., et al. (2008) Sunitinib for treatment of advanced renal cell cancer: primary tumor response. Clin Cancer Res 14: 2431–2436 [DOI] [PubMed] [Google Scholar]


