Abstract
Ectopic ACTH secretion in the setting of breast cancer is extremely rare but when present affects both the tumor’s behavior and the incidence of complications. The patient, a 58-year-old woman, first presented with a mass in her left breast as well as multiple osseous metastases and a right femur fracture. Laboratory data revealed a hypokalemic alkalosis. Her plasma ACTH level was elevated. She was diagnosed with breast cancer with ectopic ACTH secretion, and underwent a left mastectomy and axillary lymph node dissection. Histological examination demonstrated a poorly differentiated neuroendocrine carcinoma with ectopic ACTH secretion. Although the signs and symptoms of ectopic ACTH secretion from a breast cancer are frequently subtle, the recognition of ectopic ACTH secretion from breast cancer is important for patient management.
Keywords: adrenocorticotropic hormone, breast cancer, neuroendocrine cancer
The ectopic secretion of ACTH in the setting of cancer is well demonstrated in small-cell lung carcinoma, neuroendocrine tumors of the lung, thymus, gastrointestinal tract, islet cell carcinoma of the pancreas, pheochromocytoma and medullary thyroid carcinoma (Isidori and Lenzi, 2007). Many breast cancers are steroid hormone dependent and may influence the pathological course of the disease by stimulating the initial phase of steroid hormone biosynthesis converting cholesterol to pregnenolone in the adrenal cortex (Wigg et al., 1999). Especially, in post-menopausal patients, most of the circulating estrogen is produced by the aromatization of adrenal androgens in peripheral tissues. Therefore, it is speculated that ACTH would impact the behavior of breast cancer. However, ectopic ACTH secretion from breast cancer is rare, and is associated with less than 1% of tumors (Isidori and Lenzi, 2007). The incidence rate of neuroendocrine cells in breast cancer has been reported to be 21.0% (17/81 patients); however, none of the 17 expressed ACTH (Yao et al., 2003). Furthermore, it is subtle and produces few signs of clinically recognizable Cushing’s syndrome. Consequently, assessment for ectopic ACTH expression is not routinely performed in the management of breast cancer. Here, we report a case of immunohistochemically confirmed ectopic ACTH secretion in a breast cancer patient, which was identified when she developed hypokalemic metabolic alkalosis.
Patient Report
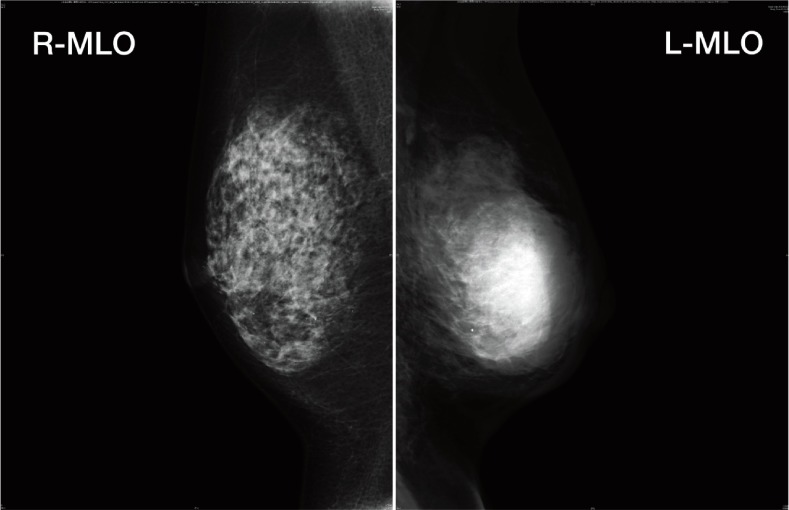
The patient, a 58-year-old woman, was first presented with a 7-month history of a mass in her left breast. On physical examination, she had an ulcerated 7 cm mass on her left breast. She had no Cushingoid features including central abdominal obesity, pigmentation, striae or facial hirsuitism. A mammogram showed a high density, microlobulated, oval mass and pleomorphic segmental calcification (Fig. 1). The tumor was oval and ill-defined with low echogenicity on ultrasound. Aspiration biopsy cytology revealed suspected malignancy, which we presumed to be invasive ductal carcinoma. Computed tomographic scan demonstrated a mass at the left primary site. Lesions consistent with osseous metastases were present in the cranial bone, sterna bone, ribs, vertebras, bilateral ilium and bilateral femurs. These were also evident on a bone scintigram. The right femur demonstrated a pathological fracture. The patient also had a meningeal metastasis. The following tumor markers were elevated: carcinoembryonic antigen (CEA), 135.0 ng/mL; carbohydrate antigen15-3 (CA15-3), 138.5 U/mL; and national cancer center-ST439 (NCC-ST439), 470.0 U/mL. Breast cancer antigen225 (BCA225) [93.6 U/mL] and tissue polypeptide antigen (TPA) [38 U/L] were within the normal range.
Fig. 1.
Bilateral MLO views by mammography. L-MLO view shows a high density, microlobulated, oval mass and pleomorphic segmental calcification, which is highly suggestive of malignancy. L, left; MLO, mediolateral oblique; R, right.
Initial serum chemistry studies revealed a hypokalemic metabolic alkalosis with a serum potassium level of 2.3 mEq/L, bicarbonate of 32.7 mmol/L and pH 7.45. The patient's morning serum cortisol was 11.54 μg/dL (normal range: 6.7–22.6 μg/dL). Her plasma ACTH level was elevated to 83.5 pg/mL (normal range: 7.2–63.3 pg/mL). Her serum aldosterone and renin levels were within the normal range. Her daily urinary secretion of potassium was 26.0 mEq/day, which was within normal range. Her pituitary gland appeared normal on MRI.
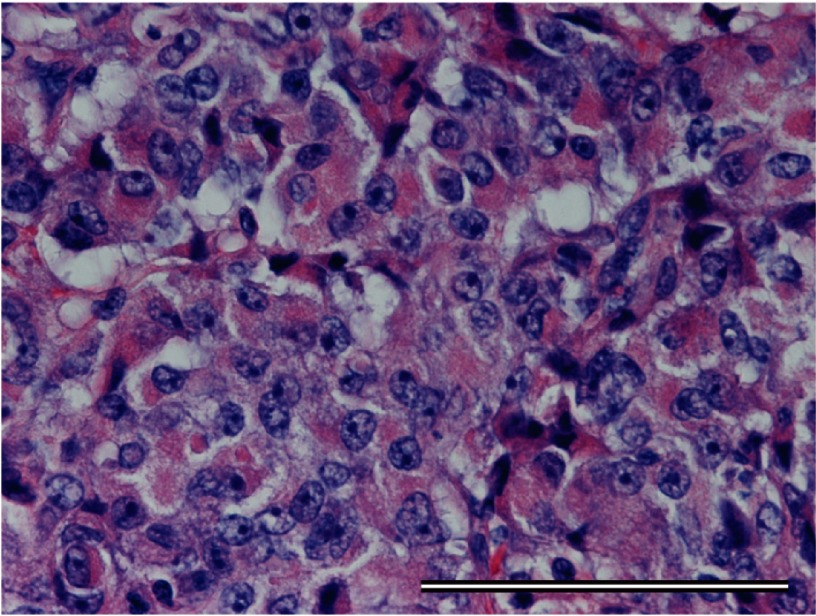
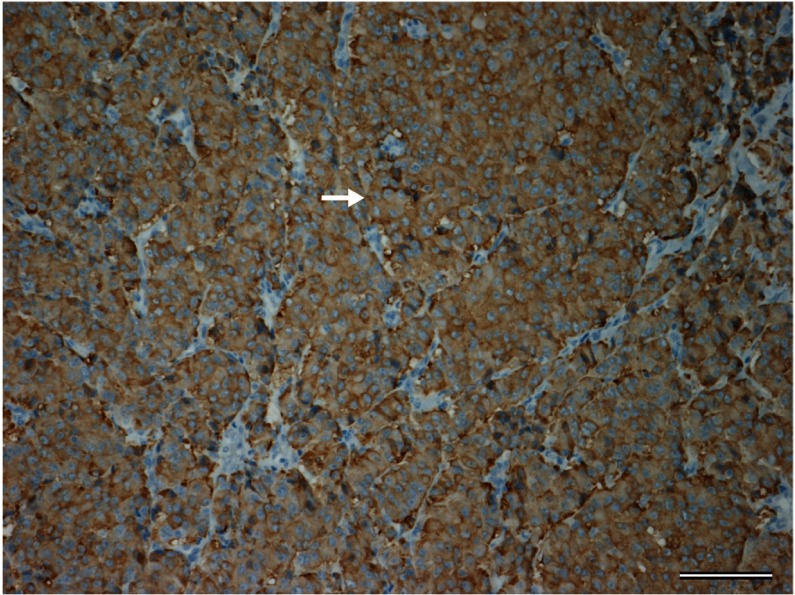
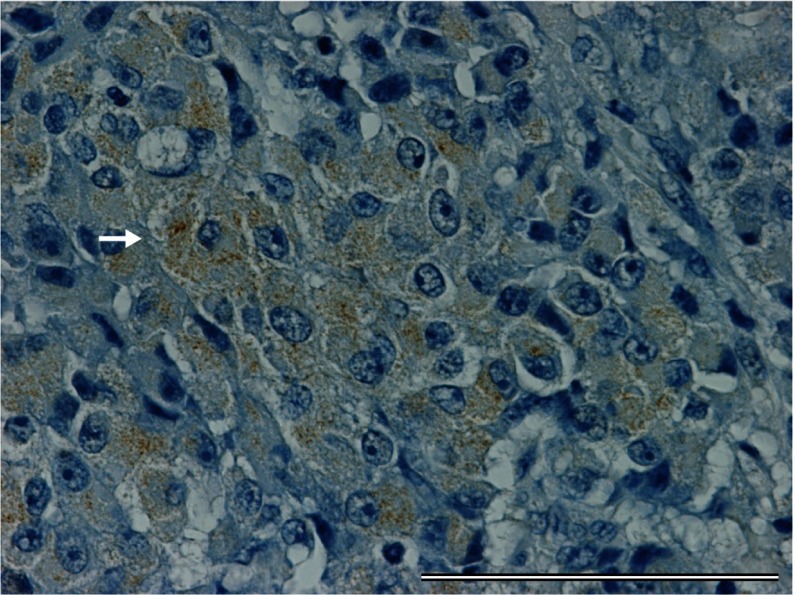
The patient was diagnosed as with presumptive stage IV breast cancer with suspected ectopic ACTH secretion. She underwent a left mastectomy and axillary lymph node dissection for local control. Histological examination of tissue obtained from the primary mass revealed a poorly differentiated neuroendocrine carcinoma (Fig. 2). One of 12 axillary lymph nodes was positive for metastatic carcinoma. The resected tissue was positive for synaptophysin on immunohistochemistry (Fig. 3), neuron-specific enolase, chromogranin A and ACTH (Fig. 4). The tumor also expressed both estrogen and progesterone receptors. Human epidermal growth factor receptor type 2 expression was negative on immunostaining and fluorescence in situ hybridization. Ki-67 staining revealed that 42% of the cells were positive.
Fig. 2.
Micrograph of the primary breast tumor. Eosinophilic tumor cells are shown (hematoxylin-eosin stain). Bar = 100 μm.
Fig. 3.
Tumor cells with synaptophysin positivity are diffusely present. Arrow shows a representative one (synaptophysin staining). Bar = 100 μm.
Fig. 4.
Tumor cells with ACTH positivity are diffusely present. Arrow shows a representative one (ACTH staining). Bar = 100 μm.
Postoperatively, the patient was initially treated with letrozol for 2 months; however, her tumor marker values gradually increased, and administration of tamoxifen was initiated. Her plasma ACTH level was decreased to 36.4 pg/mL at 3 postoperative months. She also received radiation therapy to her vertebral metastasis. Her hypokalemia was treated with potassium supplement. She survives 3 months after surgery, remaining stable, and has been followed as an outpatient.
Discussion
In the breast, there are two main types of neuroendocrine tumors: neuroendocrine differentiated carcinoma and breast carcinoma with neuroendocrine differentiation. The latter is identified in 10 to 15% of breast cancers. In the former, the majority of cells (more than 50%) display a neuroendocrine phenotype, often with a visible endocrine morphology and expression of synaptophysin, neuron-specific enolase and /or chromogranin A by immunohistochemistry. In our patient, the majority of cells were positive for synaptophysin, neuron-specific enolase and chromogranin A. Furthermore, immunostaining of ACTH was positive. Therefore, we made the diagnosis of a neuroendocrine differentiated carcinoma with ectopic ACTH secretion.
In ectopic ACTH-producing breast cancer, few patients show symptoms of Cushing's syndrome (Poddar et al., 2005). It is contrastive to other ectopic ACTH-producing cancers such as lung and bronchial carcinoid tumors, in which the majority of patients present classical signs and symptoms of Cushing's syndrome (Isidori et al., 2006). It is probably because the amount of ACTH secreted by the tumors was insufficient to cause clinical manifestations of hormonal hyperactivity. To our knowledge, only 5 cases of breast cancer with Cushing's syndrome were previously published (Cohle et al., 1979; Woodard et al., 1981; Parker and Jackson, 1984; Wigg et al., 1999; Pelte et al., 2004) (Table 1). Reported patients had mean plasma ACTH levels as 188 to 540 ng/L, while our patient had a mean plasma ACTH level of 83.5 ng/L (Table 1). These suggest that the clinical presentation of ectopic ACTH-producing breast cancer varies and reflects the underlying severity of the ACTH secretion.
Table 1. Cases of breast cancer with ACTH secretion.
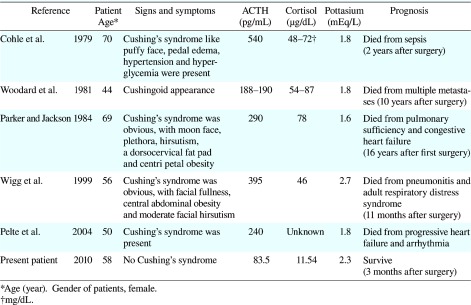
Although our patient lacked the classic stigmata of Cushing's syndrome, ACTH-producing breast cancer was identified by hypokalemic metabolic alkalosis enough to be required to correct one. Hypokalemia has been reported to be present in 70% of the patients with ectopic ACTH secretion (Isidori et al., 2006). Reported patients with ACTH-producing breast cancer along with Cushing’s syndrome had severe hypokalemia (Table 1). These suggest that ectopic ACTH secretion should be included in the differential of breast cancer patients with significant, unexplained hypokalemia.
Even though the ACTH level in our patient was low, ACTH could continuously stimulate the adrenal cortex in a sub-clinical manner to produce excess androgens, thereby modifying the course of the disease. A recent finding is that up to 50% of patients with ectopic ACTH secretion may present osteoporosis or fractures (Ilias et al., 2005). Our patient had a fracture of her right femur associated with a metastatic lesion. It is possible that ectopic ACTH secretion not only influences disease progression but also increases the rate of associated complications. Therefore, awareness of covert ectopic ACTH secretion is important in the management of breast cancers with neuroendocrine features.
The treatment of ectopic ACTH secretion requires a multimodal approach including surgery and medications to control tumor growth and associated symptoms. If a single source is located, surgery is associated with complete remission in more than 80% of patients (Isidori et al., 2006). Patients with overt, severe ectopic ACTH secretion are treated with ketoconazole or metyrapone (Isidori and Lenzi, 2007; Pelte et al., 2004; Wigg et al., 1999).
The prognosis of cancers with ectopic ACTH secretion depends on the primary tumor histology and the presence of metastases (Isidori and Lenzi, 2007). The survival of patients with an occult source of ACTH, but adequately controlled hypercortisolemia, is reported to be favorable (Isidori et al., 2006). In our patient, the tumor histology of a poorly differentiated neuroendocrine carcinoma with a high Ki-67 index and several metastases predicts a poor prognosis irrespective of the patient's ACTH level.
In conclusion, ectopic ACTH secretion from a breast cancer is rare and the signs and symptoms are frequently subtle; however, recognition of ectopic ACTH secretion is important for patient management.
References
- 1.Cohle SD, Tschen JA, Smith FE, Lane M, McGavran MH. ACTH-secreting carcinoma of the breast. Cancer 1979; 43: 2370–2376 [DOI] [PubMed] [Google Scholar]
- 2.Ilias I, Torpy DJ, Pacak K, Mullen N, Wesley RA, Nieman LK. Cushing's syndrome due to ectopic corticotropin secretion: twenty years' experience at the National Institutes of Health. J Clin Endocrinol Metab 2005; 90: 4955–4962 [DOI] [PubMed] [Google Scholar]
- 3.Isidori AM, Kaltsas GA, Pozza C, Frajese V, Newell-Price J, Reznek RH.The ectopic adrenocorticotropin syndrome: clinical features, diagnosis, management, and long-term follow-up. J Clin Endocrinol Metab 2006; 91: 371–377 [DOI] [PubMed] [Google Scholar]
- 4.Isidori AM, Lenzi A. Ectopic ACTH syndrome. Arq Bras Endocrinol Metabol 2007; 51: 1217–1225 [DOI] [PubMed] [Google Scholar]
- 5.Parker MS, Jackson R. Ectopic ACTH syndrome associated with breast carcinoma. South Med J 1984; 77: 518–520 [DOI] [PubMed] [Google Scholar]
- 6.Pelte MF, Schwaller J, Cerrato C, Meier CA. Pro-opiomelanocortin expression in a metastatic breast carcinoma with ectopic ACTH secretion. Breast J 2004; 10: 350–354 [DOI] [PubMed] [Google Scholar]
- 7.Poddar NK, Saha R, Hedau S, Ray A. Adrenocorticotropic hormone production in breast cancer. Indian J Exp Biol 2005; 43: 35–39 [PubMed] [Google Scholar]
- 8.Wigg SJ, Ehrlich AR, Fuller PJ. Cushing's syndrome secondary to ectopic ACTH secretion from metastatic breast carcinoma. Clin Endocrinol 1999; 50: 675–678 [DOI] [PubMed] [Google Scholar]
- 9.Woodard BH, Eisenbarth G, Wallace NR, Mossler JA, McCarty KS., Jr Adrenocorticotropin production by a mammary carcinoma. Cancer 1981; 47: 1823–1827 [DOI] [PubMed] [Google Scholar]
- 10.Yao GY, Zhou JL, Lai MD, Chen XQ, Chen PH. Neuroendocrine markers in adenocarcinomas: an investigation of 356 cases. World J Gastroenterol 2003; 9: 858–861 [DOI] [PMC free article] [PubMed] [Google Scholar]