Abstract
Laparosocpy-assisted pylorus-preserving gastrectomy (LAPPG) is a widely accepted surgical procedure for the treatment of early gastric cancer in the middle third of the stomach. We have been performing this operation since 2007. Compared with traditional distal gastrectomy, LAPPG has postoperative nutritional benefits for patients. However, this procedure preserves only the pyloric branch of the vagus nerve and not the celiac branch. We found that patients retain a large amount of residual food in the gastric remnant, which interferes with the detection of secondary cancer on endoscopic follow-up. To improve the pyloric function and postoperative gastrointestinal motility, we changed our procedure during 2009 to preserve both the pyloric and celiac branches of the vagus nerve, and we named this new procedure laparoscopy-assisted vagus nerve and pylorus-preserving gastrectomy (LAVNPPG). From 2009 to 2011, 11 patients underwent LAVNPPG at our hospital. Retrospective comparison of the safety of operation, postoperative complications, and condition of the gastric remnant between LAPPG (n = 13) and LAVNPPG (n = 11) found that the occurrence of postprandial stasis and food residue in the gastric remnant tended to be lower following LAVNPPG, though the differences were not significant. These findings indicate that LAVNPPG may be an operative procedure that could replace LAPPG.
Keywords: gastrectomy, gastric cancer, laparoscopy-assisted, pylorus, vagus nerve
Distal gastrectomy is the most common surgical procedure for treating gastric cancer. However, this procedure often leads to nutritional disadvantages, such as weight loss, dumping syndrome, and esophageal regurgitation (Katsube et al., 2008). Pylorus-preserving gastrectomy (PPG) was originally used in gastric ulcer surgery to prevent dumping syndrome and duodenal juice reflux (Maki et al., 1967). Recently, this procedure has been recognized as a treatment options for patients with early gastric cancer located in the middle third of the stomach (Morita et al., 2008). We have previously demonstrated that PPG can provide nutritional and immunological benefits over distal gastrectomy in patients with early gastric cancer (Ikeguchi et al., 2010a, 2010b).
As early gastric cancer has a low recurrence rate and a long survival time after surgical treatment, the current focus is on developing function-preserving and less invasive operations to improve postoperative quality of life. Laparoscopy is being used with the aim of achieving minimally invasive surgery for early gastric cancer. Laparoscopy-assisted PPG (LAPPG) is widely accepted for the surgical treatment of patients diagnosed with early gastric cancer in the middle third of the stomach (Tanaka et al., 2011). Our data and other previous studies have demonstrated that PPG patients have a large amount of food residue in the gastric remnant on endoscopy (Imada et al., 1998; Nunobu et al., 2007; Ikeguchi et al., 2010b). This food residue may interfere with the detection of secondary cancer in the gastric remnant (Nagao et al., 2004).
To retain pyloric function and improved postoperative gastrointestinal motility including motility of the residual stomach, we performed LAPPG with preservation of both the pyloric and celiac branches of the vagus nerve. The clinical benefits of this new operative technique have been previously reported (Tsujii et al., 2003).
In the present study, we introduce the operative procedure for this vagus nerve preserving LAPPG, named laparoscopy-assisted vagus nerve and pylorus-preserving gastrectomy (LAVNPPG). We also retrospectively compare the occurrence of stasis and the amount of food residue in the gastric remnant between LAPPG and LAVNPPG.
Subjects and Methods
Patients
The indications for PPG have previously been described as follows: i) a diagnosis of early gastric cancer, ii) tumor located in the middle third of the stomach, iii) no lymph node metastasis and iv) tumor less than 5.0 cm in diameter (Ikeguchi et al., 2010a). We used LAPPG during 2007 and 2008 and performed the procedure in 13 patients. During 2009, we changed to using LAVNPPG and performed the new procedure in 11 patients with a preoperative diagnosis of early gastric cancer in the middle third of the stomach and no lymph node metastasis. The diagnosis was established by endoscopic and histopathological examinations and the depth of invasion was evaluated by endosonography.
Surgical procedure
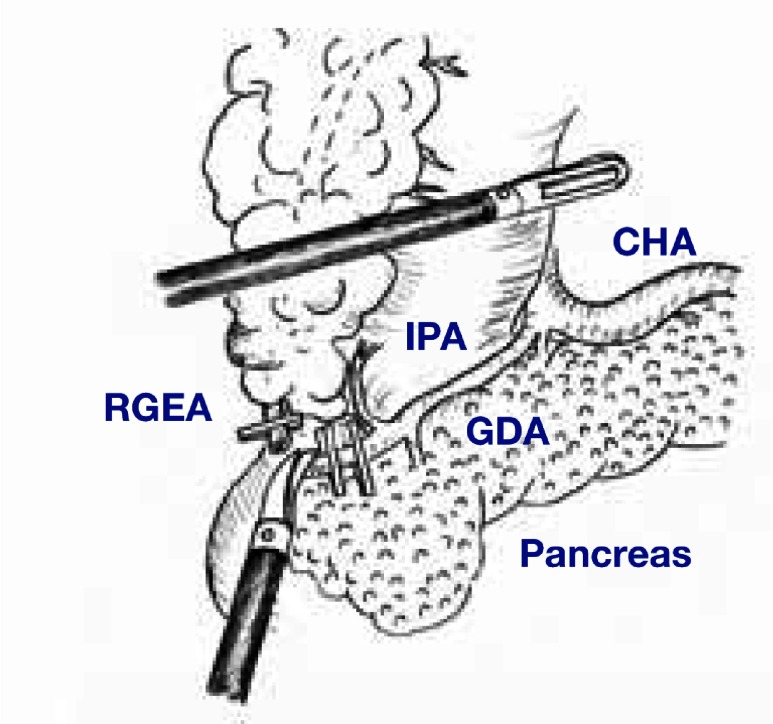
A pneumoperitoneum was created by injection of carbon dioxide insufflation at 8 to 10 mmHg, and the laparoscope was inserted through the umbilical port. Four 5 to 12-mm ports were inserted under direct vision: one in each of the left upper, left lower, right upper and right lower quadrants. The gastrocolic ligament was divided 4 cm distal to the epiploic arcade towards the lower pole of the spleen by laparoscopic coagulating shears (LCS). During dissection of the left gastroepiploic vein and artery above the pancreatic tail, the station 4Sb lymph nodes were dissected. Station 6 nodes were then dissected with preservation of the infrapyloric artery. The right gastroepiploic artery was clipped and dissected from the gastroduodenal artery (Fig. 1).
Fig. 1.
Dissection of station 6 lymph nodes with preservation of the IPA. CHA, common hepatic artery; IPA, infrapyoric artery; GDA, gastroduodenal artery; RGEA, right gastroepiploic artery.
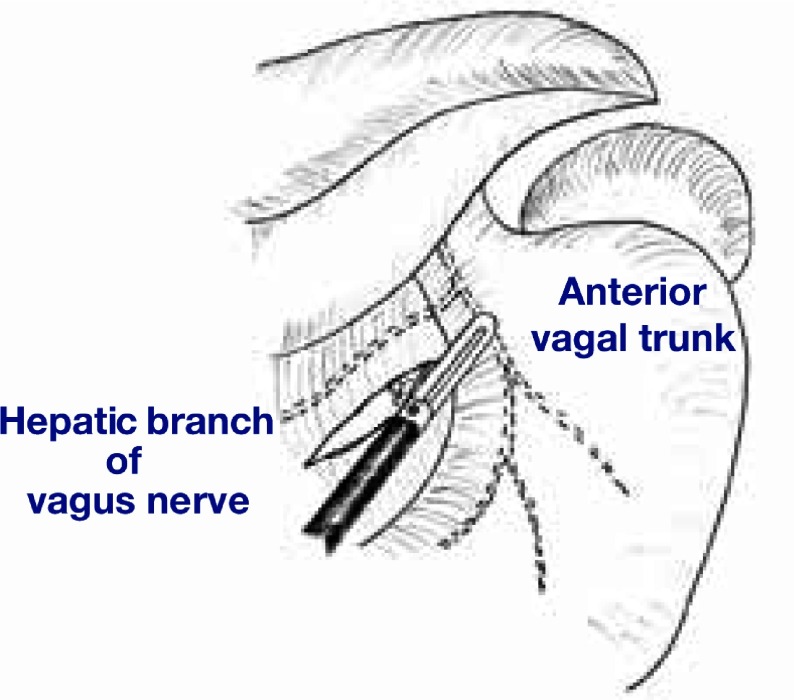
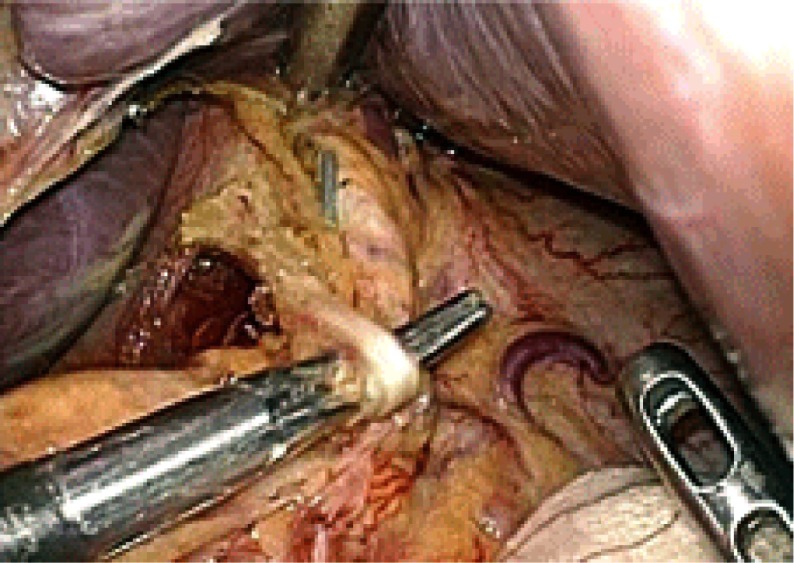
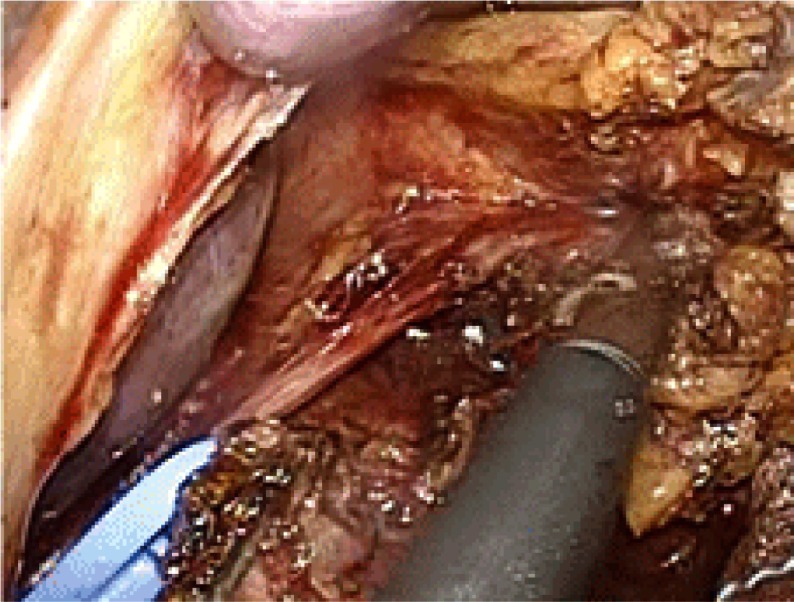
In order to preserve pyloric function and to retain the blood flow to the remnant antral segment, the pyloric and hepatic branches of the vagus nerve and the right gastric artery were preserved (Fig. 2). As a result, the station 5 and 12 lymph nodes were not dissected in this procedure. A 3-cm length of antral segment of the distal stomach was preserved. The common hepatic artery was exposed towards the root of the left gastric artery. The lymph nodes at stations 7, 8a, 9 and 11p were dissected. During this procedure, the celiac branch of the posterior vagal trunk was identified near the left gastric artery (Fig. 3). The celiac branch was taped and separated from the left gastric artery, and the left gastric artery was clipped and cut by LCS. The cardiac lymph nodes (station 1) and the lymph nodes along the lesser curvature of the stomach (station 3) were removed. The celiac branch of the vagus nerve was left intact by this procedure (Fig. 4).
Fig. 2.
The hepatic branch of the vagus nerve is preserved.
Fig. 3.
The celiac branch of the posterior vagal trunk is identified.
Fig. 4.
The celiac branch of the posterior vagal trunk is taped, and separated from the left gastric artery. Station 1 lymph nodes are then dissected.
The proximal stomach was divided approximately 3 cm distal to the primary tumor. End-to-end anastomosis was performed using the Gambee suture technique.
Clinicopathological findings
The histopathological findings, stage classification, depth of tumor invasion, lymph node grouping, and curability of gastric resection were reported according to the Japanese Classification of Gastric Carcinoma (1998).
Endoscopic evaluation
Gastrointestinal endoscopy was performed at 6 or 12 months after gastrectomy for these patients. The Los Angeles Classification was used to diagnose and describe erosive esophagitis (Lundell et al., 1999). The degree of residual gastritis and the amount of food residue in the gastric remnant were classified according to a previous report (Kubo et al., 2002).
Statistical analysis
Chi-squared and Fisher's exact probability tests were used to compare the distribution of individual variables between the patient groups. Differences in the data between the two groups were evaluated using the Mann-Whitney U test. P < 0.05 was regarded as statistically significant.
Results
During 2007 and 2008, we undertook LAPPG in patients with early gastric cancer located in the middle third of the stomach. We introduced this LAVNPPG for these in early 2009. The safety of surgery, postoperative complications, and amount of food residue in the gastric remnant were compared between the LAPPG and LAVNPPG groups (Table 1). There were no differences in operation time, intraoperative blood loss, or postoperative hospital stay between the groups. Postoperative complications, such as anastomotic leakage or intraabdominal abscess, were not detected in either group. Food intake disturbances, such as stasis or a sense of abdominal fullness were more frequent in the LAPPG group than the LAVNPPG group, but these differences were not significant.
Table 1. Clinical differences between LAPPG and LAVNPPG.
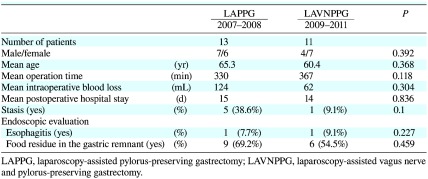
We investigated the postoperative condition of the esophagus and the gastric remnant in all 24 cases by endoscopy. Mild esophagitis was detected in 1 of 11 patients in the LAVNPPG group (9.1%). Sever reflux esophagitis was found in 1 of 13 (7.7%) patients in the LAPPG group, and a Roux-en-Y reconstruction was performed in this case 14 months after LAPPG. Food residue in the gastric remnant was observed more frequently in the LAPPG group (69.2%) than in the LAVNPPG group (54.5%), however this difference was not significant.
Discussion
The nutritional benefits of PPG compared with conventional distal gastrectomy have been widely recognized. However, some PPG patients occasionally experience a sensation of gastric fullness after food intake, and some have long-term retention of food in the gastric remnant. In PPG, the pyloric branch of the vagus nerve is preserved to retain pyloric function. However, our data and other previous studies have demonstrated that these patients have a large amount of residual food in the gastric remnant on endoscopy (Ikeguchi et al., 2010b). In a scintigraphic demonstration, gastric emptying was delayed following PPG compared to distal gastrectomy, even with preservation of the pyloric branch of the vagus nerve (Nishikawa et al., 2002). This indicates that preservation of the pyloric branch of the vagus nerve may not be sufficient to maintain normal pyloric function. Food residue may interfere with the detection of secondary cancer in the gastric remnant on endoscopic follow-up after PPG.
According to Tsujii et al. (2003), preservation of the celiac branch of the vagus nerve at gastrectomy improved postoperative gastrointestinal motility, including motility of the gastric remnant. However, the functional benefit of preservation of the celiac branch of the vagus nerve in PPG has not been clearly demonstrated. In our present study, we demonstrated that operation time, intraoperative blood loss, and the incidence of postoperative complications were almost the same in LAVNPPG and LAPPG. These findings may indicate that LAVNPPG is a safe procedure. Although the sample size of our study is small, the safety and the quality of operation may be based on progression of technique of surgeons. Our study was a retrospective study, in which the occurrence of postprandial stasis and food residue in the gastric remnant were lower with LAVNPPG compared with LAPPG.
In the present study, we introduce the operative procedure for LAVNPPG for early gastric cancer. Further studies will be required to conclude that the LAVNPPG procedure may be of use for resolving the limitation of LAPPG.
References
- 1.Ikeguchi M, Hatada T, Yamamoto M, Miyake T, Matsunaga T, Fukuda K.Evaluation of a pylorus-preserving gastrectomy for patients preoperatively diagnosed with early gastric cancer located in the middle third of the stomach. Surg Today 2010a; 40: 228–233 [DOI] [PubMed] [Google Scholar]
- 2.Ikeguchi M, Kuroda H, Kihara K, Hatata T, Matsunaga T, Fukuda K.Nutritional assessment of patients after pylorus-preserving gastrectomy for early gastric cancer. Indian J Surg 2010b; 72: 453–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imada T, Rino Y, Takahashi M, Hatori S, Tanaka J, Shiozawa M.Gastric emptying after pylorus-preserving gastrectomy in comparison with conventional subtotal gastrectomy for early gastric carcinoma. Surg Today 1998; 28: 135–138 [DOI] [PubMed] [Google Scholar]
- 4.Japanese Gastric Cancer Association Japanese classification of gastric carcinoma. 2nd English ed. Gastric Cancer 1998; 1: 10–24 [DOI] [PubMed] [Google Scholar]
- 5.Katsube T, Konnno S, Murayama M, Kuhara K, Sagawa M, Yoshimatsu K.Changes of nutritional status after distal gastrectomy in patients with gastric cancer. Hepatogastroenterology 2008; 55: 1864–1867 [PubMed] [Google Scholar]
- 6.Kubo M, Sasako M, Gotoda T, Ono H, Fujishiro M, Saito D.Endoscopic evaluation of the remnant stomach after gastrectomy: proposal for a new classification. Gastric Cancer 2002; 5: 83–89 [DOI] [PubMed] [Google Scholar]
- 7.Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP.Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut 1999; 45: 172–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maki T, Shiratori T, Hatafuku T, Sugawara K. Pylorus-preserving gastrectomy as an improved operation for gastric ulcer. Surgery 1967; 61: 838–845 [PubMed] [Google Scholar]
- 9.Morita S, Katai H, Saka M, Fukagawa T, Sano T, Sasako M. Outcome of pylorus-preserving gastrectomy for early gastric cancer. Br J Surg 2008; 95: 1131–1135 [DOI] [PubMed] [Google Scholar]
- 10.Nagano H, Ohyama S, Sakamoto Y, Ohta K, Yamaguchi T, Muto T.The endoscopic evaluation of gastric remnant residue, and the incidence of secondary cancer after pylorus-preserving and transverse gastrectomies. Gastric Cancer 2004; 7: 54–59 [DOI] [PubMed] [Google Scholar]
- 11.Nishikawa K, Kawahara H, Yumiba T, Nishida T, Inoue Y, Ito T.Functional characteristics of the pylorus in patients undergoing pylorus-preserving gastrectomy for early gastric cancer. Surgery 2002; 131: 613–624 [DOI] [PubMed] [Google Scholar]
- 12.Nunobu S, Sasako M, Saka M, Fukagawa T, Katai H, Sano T. Symptom evaluation of long-term postoperative outcomes after pylorus-preserving gastrectomy for early gastric cancer. Gastric Cancer 2007; 10: 167–172 [DOI] [PubMed] [Google Scholar]
- 13.Tanaka N, Katai H, Saka M, Morita S, Fukagawa T. Laparoscopy-assisted pylorus-preserving gastrectomy: a matched case-control study. Surg Endosc 2011; 25: 114–118 [DOI] [PubMed] [Google Scholar]
- 14.Tsujii H, Andoh S, Sakakibara K. The clinical evaluation of vagus nerve preserving gastric operation with D2 lymph node dissection for early and advanced gastric cancer. Nippon Shokakigeka Gakkai Zasshi 2003; 36: 78–84(in Japanese with English abstracf) [Google Scholar]