Abstract
Expression of the costimulatory molecule B7-H4, a member of the inhibitory B7 family, has been reported to be upregulated on tumor-associated macrophages, and this overexpression may be involved in immune evasion in cancer patients. The present study was designed to investigate B7-H4 expression on monocytes/macrophages and its relationship with immune evasion in gastric cancer patients. B7-H4 expression on circulating monocytes and tumor-associated macrophages was evaluated by multicolor flow cytometry. Carboxyfluorescein succinimidyl ester proliferation assays and quantitative interferon-gamma enzyme-linked immunosorbent assays were carried out to determine the inhibitory effect of B7-H4+ monocytes on CD4+ T cells. B7-H4 expression on circulating monocytes was upregulated and these B7-H4+ monocytes showed immunosuppressive properties. B7-H4 expression was closely related to the depth of invasion, as well as the presence of lymphatic and venous invasion. There were significant correlations between B7-H4 expression and B7-H1 or HLA-DR expression on monocytes/macrophages in gastric cancer patients. B7-H4 expression was remarkably upregulated in gastric cancer tissues compared with peripheral blood samples. Complete surgical removal of the tumor decreased B7-H4 expression on circulating monocytes from gastric cancer patients. Cocultures of gastric cancer cell lines and monocytes led to upregulation of B7-H4 expression on monocytes. Increased B7-H4 expression on circulating monocytes and tumor-associated macrophages may be one of the key mechanisms responsible for immune evasion by tumors in gastric cancer.
Keywords: B7-H1, B7-H4, gastric cancer, macrophage, monocyte
The last 2 decades of research in the cancer immunology field have revealed that the majority of cancer cells express antigens that can be recognized by T cells of the host immune system. However, despite the expression of these antigens, spontaneous immune-mediated rejection of cancer cells seems to be a rare event. The failure of spontaneous immune-mediated tumor elimination can generally be explained by poor T-cell priming or inadequate execution of the effector phase of the immune response. In each of these contexts, potential roles for T-cell costimulatory and coinhibitory receptors have been suggested.
T lymphocytes are thought to play an important role in the control of tumors as a result of their cytotoxic activity and by releasing soluble factors. Optimal activation of T cells requires at least 2 signals, namely antigen recognition and costimulation. T cell receptor complexes, which recognize antigenic peptides presented by major histocompatibility complex (MHC) molecules, are critical for maintaining the specificity of the immune response. Costimulation is an antigen-independent signal required for sustained cell proliferation, effector/memory cell generation and prevention of anergy or apoptosis, and can be either positive or inhibitory. Positive costimulation can enhance T and B cell activation, while inhibitory costimulation can just as strongly attenuate these responses. The final outcome of an immune response is likely to depend on the balance between these positive and negative signals (Driessens et al., 2009). The expression of costimulatory molecules is restricted to professional antigen-presenting cells, such as dendritic cells, macrophages and monocytes. The B7 family comprises such costimulatory molecules and consists of both stimulatory and inhibitory molecules (Carreno and Collins, 2002; Greenwald et al., 2002; Liang and Sha, 2002; Chen, 2004; Driessens et al., 2009). Signals mediated by the B7 family members result in T-cell suppression or activation in different settings. B7-1 and B7-2 are 2 identified stimulatory B7 family members, while B7-H1 and B7-H4 are 2 identified inhibitory B7 family members (Chen, 2004). Since the final outcome of an immune response is likely to depend on the balance between these positive and negative signals, it is possible that overexpression of inhibitory B7 family members induces immunosuppression through the suppression of T cell function. In fact, previous studies have demonstrated that both ovarian cancer and ovarian cancer-associated myeloid dendritic cells express B7-H1 and that these B7-H1+ cancer cells and myeloid dendritic cells reduce tumor-associated antigen (TAA)-specific T-cell immunity through distinct mechanisms (Dong et al., 2002; Curiel et al., 2003). With regard to B7-H4, several research groups have shown that human ovarian cancers express high levels of B7-H4 protein (Choi et al., 2003; Salceda et al., 2005; Bignotti et al., 2006; Kryczek, Zou et al., 2006; Simon et al., 2006; Tringler et al., 2006). In addition, high levels of B7-H4 were detected in non-small-cell lung cancer (Sun et al., 2006), ductal and lobular breast cancer (Salceda et al., 2005; Tringler et al., 2005) and renal cell carcinoma (Krambeck et al., 2006). Moreover, Kryczek et al. demonstrated that human ovarian cancer-associated B7-H4+ macrophages inhibited TAA-specific T-cell immunity, in part through B7-H4 (Kryczek et al., 2006). These accumulating lines of evidence suggest the possibility that inhibitory B7 family members, including B7-H1 and B7-H4, are closely related to immune evasion in cancer patients.
Gastric cancer is one of the most common cancers in Asia and its mortality rate still ranks second among all cancer deaths worldwide (Parkin et al., 2005). Despite the expression of tumor rejection antigens such as MAGE 1–3 (Inoue et al., 1995) and the presence of tumor-specific cytotoxic T cells (Hoshino et al., 1997), the immune system fails to exhibit immune responses against gastric carcinoma cells, similar to the case for other cancer cells. However, the mechanisms for how gastric cancer cells overcome the antitumor immunological responses are poorly understood. Therefore, we undertook the present study to determine the expression of B7-H4 as well as B7-H1 on circulating monocytes and tumor-associated macrophages to clarify one of the possible mechanisms responsible for immune evasion in gastric cancer patients.
Materials and Methods
Patients and normal donors
Forty-three patients (29 males and 14 females) who were treated at Tottori University Hospital and pathologically diagnosed as gastric cancer were enrolled in this study. None of the patients received radiotherapy, chemotherapy or other medical interventions before surgery. The patients' characteristics are shown in Table 1. Healthy controls (n = 18; 13 males and 5 females) who were treated at Tottori University Hospital and diagnosed as inguinal hernia or cholelithiasis were age-matched (67.9 ± 10.9 years for the controls versus 67.6 ± 8.9 years for the patients), and each experiment was performed in parallel for the patients and healthy controls. The clinicopathological findings were determined according to the Japanese Classification of Gastric Carcinoma (Japanese Gastric Cancer Association, 1998).
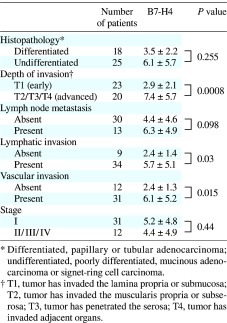
Table 1. B7-H4 expression on circulating monocytes and clinicopathologic characteristics in gastric cancer patients.
Preparation of PBMCs
Peripheral blood mononuclear cells (PBMCs) were prepared by drawing a 30-mL peripheral blood sample from each of the controls or from the patients before surgery and centrifuged through a Ficoll-Paque (Pharmacia, Uppsala, Sweden) gradient. Informed consent was obtained from all the subjects before blood donation.
Isolation of tumor-infiltrating lymphocytes
Freshly excised tumor tissues were minced and then digested with 1.5 mg/mL of collagenase D (Wako Pure Chemical Industries, Osaka, Japan). The resulting cell suspensions were filtered through a mesh filter (BD Falcon, Franklin Lakes, NJ).
Flow cytometry analysis
Fluorescence-activated cell sorting (FACS) analysis was performed using a FACSCalibur (Becton Dickinson, Franklin Lakes, NJ). The following antibodies were used to classify the cells: anti-CD3-PE-Cy5, anti-CD14-FITC, anti-HLA-DE-PE, anti-B7-H1-PE and anti-B7-H4-PE (BD PharMingen, Franklin Lakes, NJ).
Measurement of IFN-gamma concentrations
The interferon (IFN)-gamma concentrations were measured by enzyme-linked immunosorbent assay (ELISA) using a Human IFN-gamma ELISA Kit (R&D Systems, Minneapolis, MN).
Media
A complete culture medium consisting of RPMI 1640 (Gibco-BRL, Grand Island, NY), 1% l-glutamine (Invitrogen, Carlsbad, CA), 1% penicillin/streptomycin (Invitrogen), 2.5% HEPES buffer solution (Gibco-BRL), 1% sodium pyruvate (Sigma-Aldrich, St. Louis, MO), 1% essential amino acids (Sigma-Aldrich) and 10% heat-inactivated fetal calf serum (Thermo Trace, Melbourne, Australia) was used. For T-cell cultures, fetal calf serum was replaced with 10% human AB serum (Gemini Bio-Products, Woodlands, CA).
Cell lines
The gastric cancer cell lines MKN-45 and MKN-74 were purchased from the RIKEN Cell Bank (Tsukuba, Japan).
CSFE labeling and proliferation assay
Cell proliferation was determined using carboxyfluorescein succinimidyl ester (CFSE) dilution assays. CD4+ T cells were positively selected with anti-CD4 microbeads. For staining with CFSE (Invitrogen, Carlsbad, CA), CD4+ T cells at 1 × 106 cells/mL in phosphate-buffered solution were incubated with 2 μM CFSE for 10 min at 37 ˚C. Staining was terminated by adding RPMI 1640 containing 10% human AB serum (Gemini Bio-Products) at 4 ˚C, followed by 1 wash with phosphate-buffered solution. The CFSE-labeled CD4+ T cells were seeded in anti-CD3 antibody-coated 96-well plates (3 × 105 cells/well; Nunc A/S, Roskilde, Denmark). In other experiments, CD14+ cells were depleted of other cells using anti-CD3, anti-CD16, anti-CD19, anti-CD56 and anti-glycophorin A microbeads (Miltenyi, Gladbach, Germany) and sorted based on their CD14+B7-H4+ or CD14+B7-H4– phenotypes. Next, either B7-H4+ or B7-H4– monocytes were added to CD4+ T cells at a ratio of 1:5. The CD4+ T cells were profiled by CFSE labeling after 5 days of incubation. Unstained cells were included in all experiments and were used to set the compensations on the flow cytometer.
Regulation of B7-H4 expression
PBMCs (5 × 106) were cultured for different times with cancer cells, cell-free malignant ascites or serum obtained from gastric cancer patients in 6-well plates. After each period of coculture (12, 24 and 48 h), PBMCs were harvested and analyzed for their B7-H4 expression and supernatants were obtained after centrifuging at 300 × g for 5 min.
Statistical analysis
The statistical significance of differences between 2 groups was determined by the paired t-test or Mann-Whitney U-test. Correlations were analyzed by the Spearman rank correlation coefficient test. The accepted level of significance was P < 0.05. The GraphPad Prism software (GraphPad Software, La Jolla, CA) was used for all statistical analyses.
Results
B7-H4 expression on circulating monocytes and tumor-associated macrophages in patients with gastric cancer
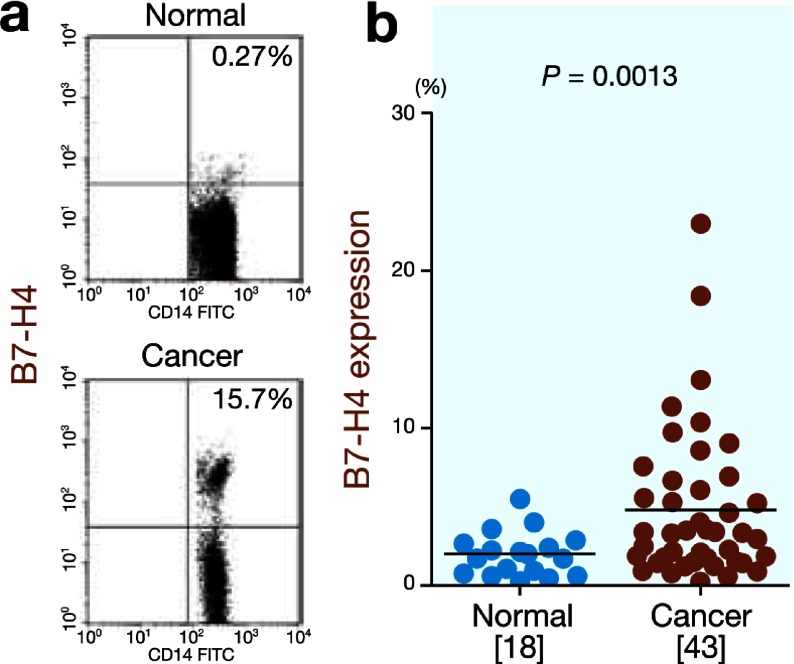
First, we determined the B7-H4 expression on circulating monocytes from the normal controls (n = 18) and gastric cancer patients (n = 43). The B7-H4 expression was significantly higher on circulating monocytes from the gastric cancer patients than on those from the normal controls (4.9 ± 4.8% versus 2.0 ± 1.4%, P = 0.013; Fig. 1). Table 1 shows the correlations between the B7-H4 expression on circulating monocytes and various clinicopathologic factors. The circulating monocytes from advanced gastric cancer patients exhibited higher B7-H4 expression than those from early gastric cancer patients (7.4 ± 5.7% versus 2.9 ± 2.1%, P = 0.0008). The B7-H4 expression was significantly higher in patients with lymphatic invasion than in patients without lymphatic invasion (5.7 ± 5.1% versus 2.4 ± 1.4%, P = 0.03). Moreover, significantly higher B7-H4 expression was observed in patients with venous invasion than in patients without venous invasion (6.1 ± 5.2% versus 2.4 ± 1.3%, P = 0.015). These findings indicate that increased B7-H4 expression on circulating monocytes is closely correlated with disease progression.
Fig. 1.
B7-H4 expression on circulating monocytes.
a: Representative FACS data for B7-H4 expression on circulating monocytes in the normal controls and gastric cancer patients.
b: B7-H4 expression is significantly higher on circulating monocytes from the gastric cancer patients than on those from the normal controls (P = 0.013).
[ ], number of subjects. FACS, fluorescence-activated cell sorting.
Next, we determined the B7-H4 expression on tumor-associated macrophages obtained from gastric cancer tissues. The B7-H4 expression was significantly higher on tumor-associated macrophages in the gastric cancer tissues than on circulating monocytes (13.3 ± 6.8% versus 2.5 ± 2.7%, P = 0.0011; Fig. 2).
Fig. 2.
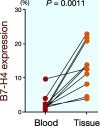
B7-H4 expression on tumor-associated macrophages is significantly higher in gastric cancer tissues than on circulating monocytes (P = 0.0011).
B7-H1 expression on circulating monocytes and tumor-associated macrophages and its relationship with B7-H4 expression in gastric cancer patients
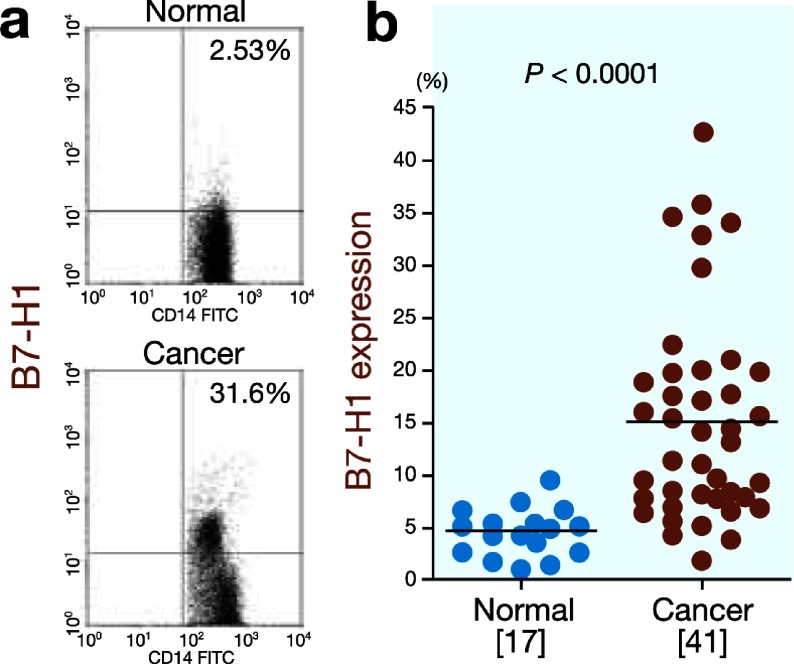
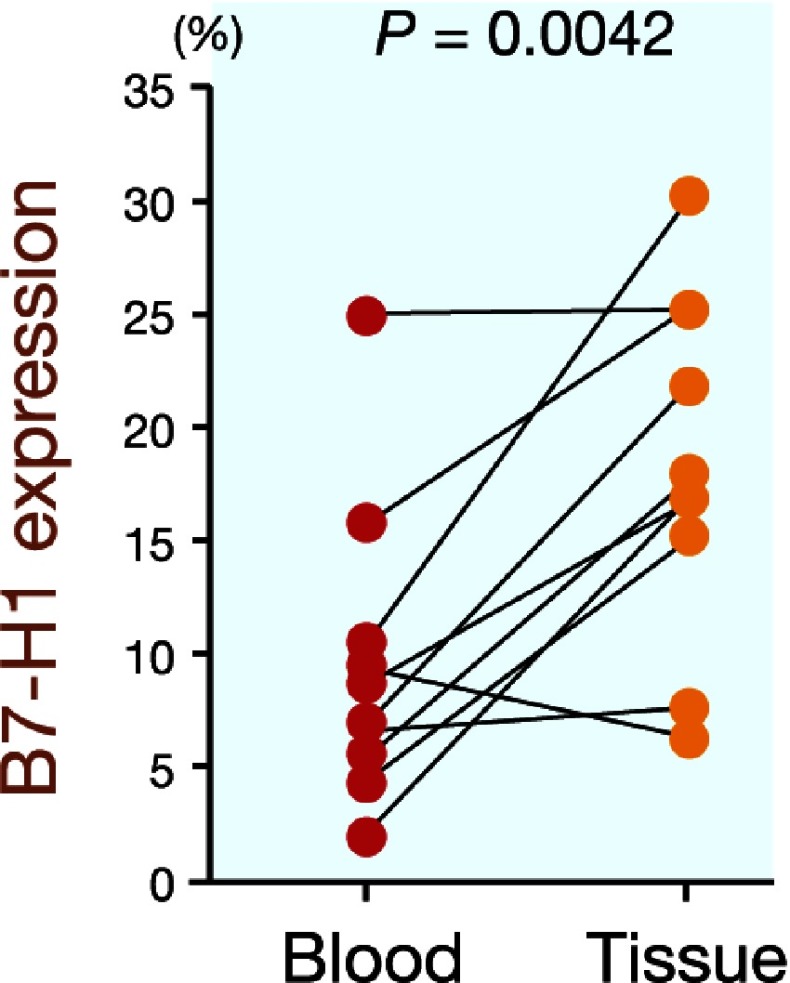
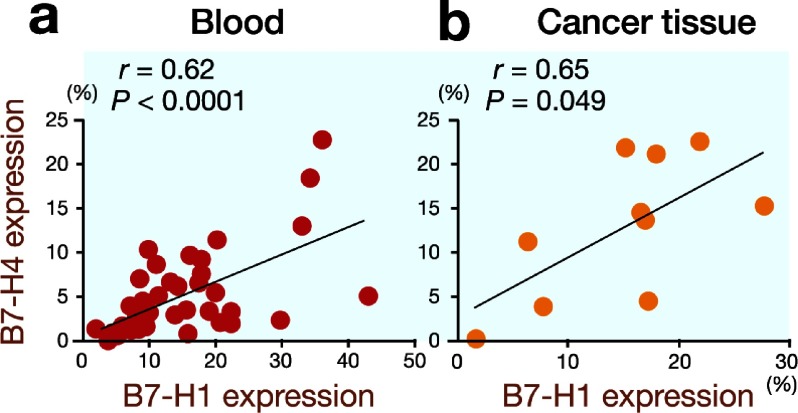
B7-H1 is another inhibitory member of the B7 family. To clarify the correlation between B7-H1 expression and B7-H4 expression on circulating monocytes and tumor-associated macrophages, we determined the B7-H1 expression in the gastric cancer patients and normal controls. The B7-H1 expression was significantly higher on circulating monocytes from the gastric cancer patients than on those from the normal controls (15.2 ± 10.0% versus 4.7 ± 2.3%, P < 0.0001; Fig. 3). Furthermore, the B7-H1 expression was significantly higher on tumor-associated macrophages in the gastric cancer tissues than on circulating monocytes (18.4 ± 7.6% versus 9.5 ± 6.6%, P = 0.0042; Fig. 4). Next, we analyzed the correlation between B7-H1 expression and B7-H4 expression and found that B7-H4 expression was closely correlated with B7-H1 expression in both the peripheral blood samples (r = 0.62, P < 0.00001; Fig. 5a) and gastric cancer tissues (r = 0.65, P = 0.049; Fig. 5b).
Fig. 3.
B7-H1 expression on circulating monocytes.
a: Representative FACS data for B7-H1 expression on circulating monocytes from the normal controls and gastric cancer patients.
b: B7-H1 expression is significantly higher on circulating monocytes from the gastric cancer patients than on those from the normal controls (P < 0.0001).
[ ], number of subjects. FACS, fluorescence-activated cell sorting.
Fig. 4.
B7-H1 expression is significantly higher on tumor-associated macrophages in gastric cancer tissues than on circulating monocytes (P = 0.0042).
Fig. 5.
B7-H4 expression is closely correlated with B7-H1 expression in both peripheral blood samples (r = 0.62, P < 0.0001) and gastric cancer tissues (r = 0.65, P = 0.049).
B7-H4 expression and HLA-DR expression on monocytes/macrophages in gastric cancer patients
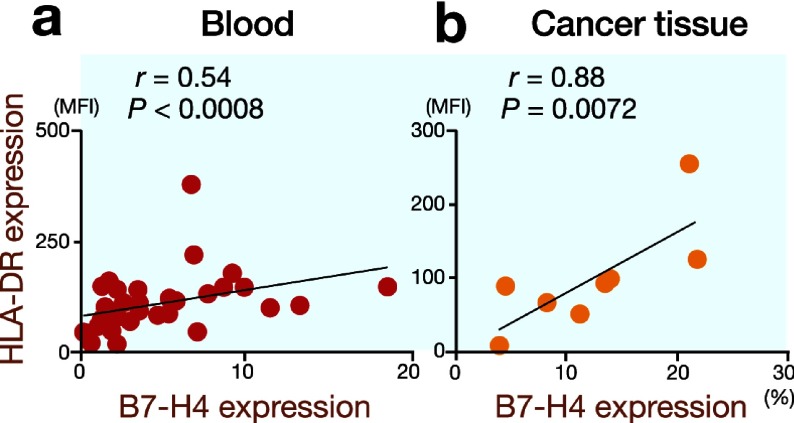
Kuang et al. observed that monocytes in the peritumoral stroma had an activated phenotype with increased expressions of HLA-DR, CD80 and CD86 (Kuang et al., 2009). Furthermore, they found significant correlations between the numbers of B7-H1+ and HLA-DRhigh monocytes in hepatocellular carcinoma tissues. Therefore, we determined the HLA-DR expression on monocytes/macrophages to investigate whether the same phenomenon was observed in gastric cancer patients. Significant correlations between B7-H4 expression and HLA-DR expression were found on both circulating monocytes (Fig. 6a) and tumor-associated macrophages (Fig. 6b).
Fig. 6.
There are significant correlations between B7-H4 expression and HLA-DR expression on circulating monocytes (r = 0.54, P = 0.0008) and tumor-associated macrophages (r = 0.88, P = 0.0072). MFI, mean fluorescence intensity.
B7-H4+ monocytes/macrophages exhibit an immunosuppressive phenotype
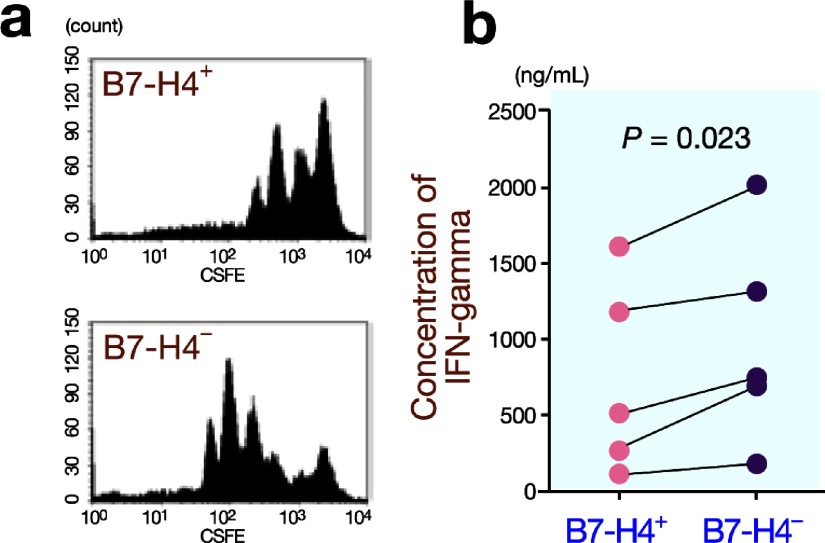
To examine the immunosuppressive function of B7-H4+ monocytes/macrophages, we sorted B7-H4+ monocytes and cocultured the isolated cells with CD4+ T cells in anti-CD3 antibody-coated wells. CFSE assays revealed that B7-H4+ monocytes suppressed the proliferation of CD4+ T cells compared with B7-H4– monocytes (Fig. 7a). Furthermore, B7-H4+ monocytes significantly suppressed IFN-gamma secretion by CD4+ T cells compared with B7-H4– monocytes (750 ± 623 ng/mL versus 996 ± 689 ng/mL) (Fig. 7b).
Fig. 7.
Immunosuppressive effects of B7-H4+ monocytes on CD4+ T cells.
a: B7-H4+ monocytes suppress the proliferation of CD4+ T cells compared with B7-H4– monocytes, as evaluated by CFSE assays.
b: B7-H4+ monocytes significantly suppress IFN-gamma secretion by CD4+ T cells compared with B7-H4– monocytes (P = 0.023).
CFSE, carboxyfluorescein succinimidyl ester.
Decreased B7-H4 expression on circulating monocytes from gastric cancer patients after complete surgical removal of the tumor
B7-H4 expression was detected on 7.9 ± 6.9% and 2.8 ± 1.3% of monocytes (n = 12) before surgery and after surgery (1 month later), respectively, and this difference was statistically significant (P = 0.029; Fig. 8).
Fig. 8.
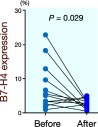
Increased B7-H4 expression on circulating monocytes from gastric cancer patients is downregulated after complete surgical removal of the tumor (P = 0.029).
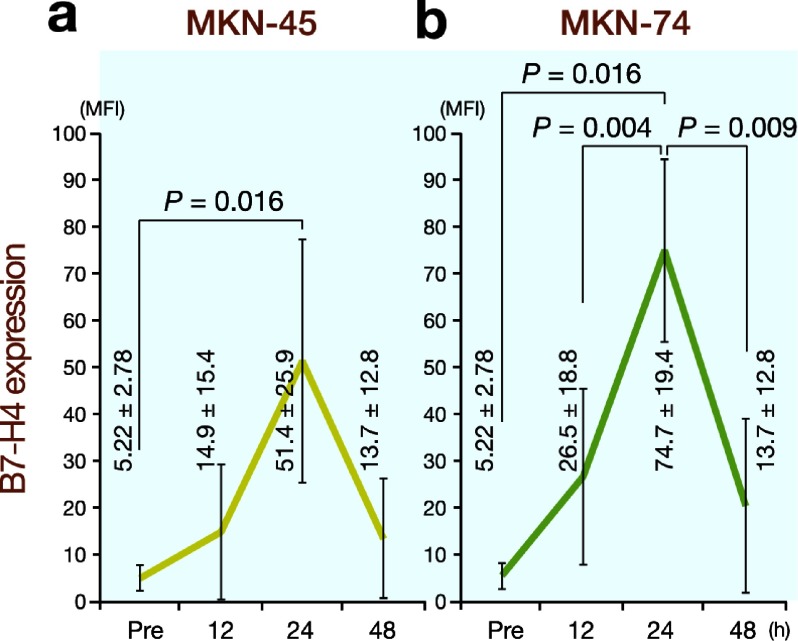
Gastric cancer cell lines induce B7-H4 expression on monocytes
Finally, we carried out experiments using gastric cancer cell lines to examine the effects of cancer cells on B7-H4 expression. We used 2 gastric cancer cell lines, MKN-45 and MKN-74. As shown in Fig. 9, cocultures of the gastric cancer cell lines and PBMCs resulted in remarkable increases in B7-H4 expression on monocytes. This phenomenon was not observed when PBMCs were cultured in the presence of supernatants from the 2 gastric cancer cell lines. Moreover, upregulation of B7-H4 expression was not observed in the presence of serum or ascites obtained from the gastric cancer patients. These results indicate that direct contacts between cancer cells and monocytes, but not soluble factors, are indispensable for B7-H4 overexpression on monocytes.
Fig. 9.
Cocultures of gastric cancer cell lines (MKN-45 and MKN-74) and PBMCs exhibit a remarkable increase in B7-H4 expression on monocytes after 24 h. Seen are mean fluorescence intensity (MFI) (mean ± SD). Pre, before coculture.
Discussion
Immune suppressor cells are currently an area of active research interest. The best-studied immunosuppressive cell population is the well-known, yet still relatively newly described, CD4+CD25+ regulatory T cells (Treg cells) (Sakaguchi et al., 2001; Shevach, 2002; von Herrath and Harrison, 2003). Human Treg cells (Woo et al., 2002; Curiel et al., 2004) disable TAA-specific T-cell immunity (Zou, 2005, 2006). It has been shown that increased numbers of Treg cells are correlated with poor survival in hepatocellular carcinoma patients (Fu et al., 2007) and ovarian cancer patients (Curiel et al., 2004). Furthermore, myeloid-derived suppressor cells (Ochoa et al., 2007; Hoechst et al., 2008) and CD4+NKG2D+ T cells (Groh et al., 2006) can diminish immunity against cancer cells in cancer patients. These accumulating lines of evidence indicate that immunosuppressive cells play important roles in tumor progression.
In the present study, we determined B7-H4 expression on circulating monocytes and tumor-associated macrophages in gastric cancer patients to clarify one of the key mechanisms responsible for immune evasion in gastric cancer. B7-H4 expression was significantly higher on circulating monocytes from the gastric cancer patients than on those from the normal controls. B7-H4 expression was closely related to the depth of invasion, as well as the presence of lymphatic and venous invasion. Furthermore, B7-H4 expression was significantly higher on tumor-associated macrophages in the gastric cancer tissues than on circulating monocytes. The B7-H4+ circulating monocytes exhibited an immunosuppressive phenotype that suppressed CD4+ T-cell proliferation and IFN-gamma secretion by CD4+ T cells. Therefore, B7-H4+ circulating monocytes and tumor-associated macrophages may be related to immune evasion in gastric cancer patients.
In addition to B7-H4, another member of the B7 family, B7-H1, is also selectively expressed by various cellular components in the tumor microenvironment and has been reported to be related to immune evasion in cancer patients (Dong et al., 2002; Curiel et al., 2003). In the present study, B7-H1 expression was also significantly higher on circulating monocytes from the gastric cancer patients than on those from the normal controls. Furthermore, B7-H1 expression was significantly higher on tumor-associated macrophages in the gastric cancer tissues than on circulating monocytes. The B7-H1 expression on circulating monocytes and tumor-associated macrophages was correlated with their HLA-DR expression, indicating that B7-H1+ circulating monocytes and tumor-associated macrophages show activated phenotypes (data not shown). These findings are consistent with those in a previous report (Kuang et al., 2009). Of interest is that B7-H4 expression on circulating monocytes and tumor-associated macrophages was also correlated with their HLA-DR expression. Moreover, B7-H4 expression was closely correlated with B7-H1 expression in both peripheral blood samples and gastric cancer tissues. These findings indicate that circulating monocytes and tumor-associated macrophages were activated and that both B7-H1 and B7H4 expressions were upregulated as a result of this activation in gastric cancer patients. To the best of our knowledge, this is the first study to demonstrate overexpression of B7-H1 and B7-H4 on circulating monocytes and tumor-associated macrophages in gastric cancer patients.
Previous reports have demonstrated that upregulation of B7-H1 and B7-H4 is related to soluble factors, such as IL-6, IL-10 and TNF-alpha (Kryczek et al., 2006; Kuang et al., 2009). However, serum and ascites obtained from the gastric cancer patients did not affect B7-H4 expression on monocytes in the present study (data not shown). Furthermore, supernatants obtained from 2 gastric cancer cell lines also did not affect B7-H4 expression on monocytes (data not shown). These findings indicate that soluble factors are not related to the upregulation of B7-H4 observed in the present study. On the other hand, Kryczek et al. showed that upregulation of B7-H4 expression was correlated with the presence of Treg cells (Kryczek et al., 2007). Therefore, we determined the frequencies of Foxp3+ Treg cells in our blood and cancer tissue samples and found no correlation between B7-H4 expression on monocytes/macrophages and the frequency of Foxp3+ Treg cells (data not shown). In the present study, we observed remarkable upregulation of B7-H4 expression on macrophages in cancer tissues compared with peripheral blood samples. Furthermore, a significant decrease in B7-H4 expression on circulating monocytes from gastric cancer patients was observed after complete surgical removal of the tumor. These findings suggest the possibility that cancer cells themselves may affect the B7-H4 overexpression on monocytes and macrophages in gastric cancer patients. In fact, cocultures of gastric cancer cell lines and monocytes induced upregulation of B7-H4 expression on monocytes. Since the supernatants from the 2 gastric cancer cell lines did not affect B7-H4 expression, it is likely that direct contacts between cancer cells and monocytes/macrophages are indispensable for the upregulation of B7-H4 expression on monocytes/macrophages. Further investigations to clarify which molecules on tumor cells are related to the upregulation of B7-H4 expression on monocytes/macrophages are urgently required.
Since monocytes/macrophages are closely related to immune evasion, they are important targets for cancer immunotherapy. In this regard, Hagemann et al. showed that macrophages are polarized via IL-1R and MyD88 to an immunosuppressive "alternative" phenotype that requires IkappaB kinase beta-mediated nuclear factor (NF)-kappa B activation (Hagemann et al., 2008). When NF-kappa B signaling is specifically inhibited in tumor-associated macrophages, the cells become cytotoxic toward tumor cells and switch to a "classically" activated phenotype of interleukin (IL)-12high and MHC IIhigh, but IL-10low and arginase-1low. Targeting NF-kappa B signaling in tumor-associated macrophages also promotes the regression of advanced tumors in vivo by inducing macrophage tumoricidal activity and activating the antitumor activity through IL-12-dependent NK cell recruitment. Furthermore, Luo et al. demonstrated that DNA vaccines targeting legumain, a member of the asparaginyl endopeptidase family that functions as a stress protein, induce a robust CD8+ T-cell response against tumor-associated macrophages, which dramatically reduced their density in tumor tissues and markedly suppressed tumor growth and metastasis in a murine model (Luo et al., 2006). These findings show that targeting of tumor-associated macrophages is attractive and effective for anticancer strategies. Since B7-H1+ and B7-H4+ monocytes/macrophages with immunosuppressive properties are increased in gastric cancer patients, targeting B7-H1+ and B7-H4+ monocytes/macrophages may provide a breakthrough in the treatment of gastric cancer.
In conclusion, B7-H1+ and B7-H4+ circulating monocytes and tumor-associated macrophages with immunosuppressive activity were increased in gastric cancer patients. This phenomenon may be one of the key mechanisms responsible for immune evasion in gastric cancer patients. Therefore, targeting B7-H1+ and B7-H4+ monocytes/macrophages may provide a breakthrough in the treatment of gastric cancer. Further investigations to clarify which molecules are related to the upregulation of B7-H4 expression on monocytes/macrophages are urgently required.
References
- 1.Bignotti E, Tassi RA, Calza S, Ravaggi A, Romani C, Rossi E.Differential gene expression profiles between tumor biopsies and short-term primary cultures of ovarian serous carcinomas: identification of novel molecular biomarkers for early diagnosis and therapy. Gynecol Oncol 2006; 103: 405–416 [DOI] [PubMed] [Google Scholar]
- 2.Carreno BM, Collins M. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu Rev Immunol 2002; 20: 29–53 [DOI] [PubMed] [Google Scholar]
- 3.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol 2004; 4: 336–347 [DOI] [PubMed] [Google Scholar]
- 4.Choi IH, Zhu G, Sica GL, Strome SE, Cheville JC, Lau JS.Genomic organization and expression analysis of B7-H4, an immune inhibitory molecule of the B7 family. J Immunol 2003; 171: 4650–4654 [DOI] [PubMed] [Google Scholar]
- 5.Curiel TJ, Coukos G, Linhua Z, Alvarez X, Pui C, Mottram P.Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004; 10: 942–949 [DOI] [PubMed] [Google Scholar]
- 6.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P.Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med 2003; 9: 562–567 [DOI] [PubMed] [Google Scholar]
- 7.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB.Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002; 8: 793–800 [DOI] [PubMed] [Google Scholar]
- 8.Driessens G, Kline J, Gajewski TF. Costimulatory and coinhibitory receptors in anti-tumor immunity. Immunol Rev 2009; 229: 126–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B.Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology 2007; 132: 2328–2339 [DOI] [PubMed] [Google Scholar]
- 10.Greenwald RJ, Latchman YE, Sharpe AH. Negative co-receptors on lymphocytes. Curr Opin Immunol 2002; 14: 391–396 [DOI] [PubMed] [Google Scholar]
- 11.Groh V, Smythe K, Dai Z, Spies T. Fas-ligand-mediated paracrine T cell regulation by the receptor NKG2D in tumor immunity. Nat Immunol 2006; 7: 755–762 [DOI] [PubMed] [Google Scholar]
- 12.Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H, Thompson RG."Re-educating" tumor-associated macrophages by targeting NF-kappaB. J Exp Med 2008; 205: 1261–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Krüger C, Manns MP.A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology 2008; 135: 234–243 [DOI] [PubMed] [Google Scholar]
- 14.Hoshino T, Seki N, Kikuchi M, Kuramoto T, Iwamoto O, Kodama I.HLA class-I-restricted and tumor-specific CTL in tumor-infiltrating lymphocytes of patients with gastric cancer. Int J Cancer 1997; 70: 631–638 [DOI] [PubMed] [Google Scholar]
- 15.Inoue H, Mori M, Honda M, Li J, Shibuta K, Mimori K.The expression of tumor-rejection antigen "MAGE" genes in human gastric carcinoma. Gastroenterology 1995; 109: 1522–1525 [DOI] [PubMed] [Google Scholar]
- 16.Japanese Gastric Cancer Association Japanese classification of gastric carcinoma--2nd English Edition-- Gastric Cancer 1998; 1: 10–24 [DOI] [PubMed] [Google Scholar]
- 17.Krambeck AE, Thompson RH, Dong H, Lohse CM, Park ES, Kuntz SM.B7-H4 expression in renal cell carcinoma and tumor vasculature: associations with cancer progression and survival. Proc Natl Acad Sci U S A 2006; 103: 10391–10396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kryczek I, Wei S, Zhu G, Myers L, Mottram P, Cheng P.Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Res 2007; 67: 8900–8905 [DOI] [PubMed] [Google Scholar]
- 19.Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P.B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med 2006; 203: 871–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C.Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med 2009; 206: 1327–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang L, Sha WC. The right place at the right time: novel B7 family members regulate effector T cell responses. Curr Opin Immunol 2002; 14: 384–390 [DOI] [PubMed] [Google Scholar]
- 22.Luo Y, Zhou H, Krueger J, Kaplan C, Lee SH, Dolman C.Targeting tumor-associated macrophages as a novel strategy against breast cancer. J Clin Invest 2006; 116: 2132–2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ochoa AC, Zea AH, Hernandez C, Rodriguez PC. Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin Cancer Res 2007; 13(2 Pt 2): 721S–726S [DOI] [PubMed] [Google Scholar]
- 24.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55: 74–108 [DOI] [PubMed] [Google Scholar]
- 25.Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M.Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev 2001; 182: 18–32 [DOI] [PubMed] [Google Scholar]
- 26.Salceda S, Tang T, Kmet M, Munteanu A, Ghosh M, Macina R.The immunomodulatory protein B7-H4 is overexpressed in breast and ovarian cancers and promotes epithelial cell transformation. Exp Cell Res 2005; 306: 128–141 [DOI] [PubMed] [Google Scholar]
- 27.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol 2002; 2: 389–400 [DOI] [PubMed] [Google Scholar]
- 28.Simon I, Zhuo S, Corral L, Diamandis EP, Sarno MJ, Wolfert RL.B7-h4 is a novel membrane-bound protein and a candidate serum and tissue biomarker for ovarian cancer. Cancer Res 2006; 66: 1570–1575 [DOI] [PubMed] [Google Scholar]
- 29.Sun Y, Wang Y, Zhao J, Gu M, Giscombe R, Lefvert AK.B7-H3 and B7-H4 expression in non-small-cell lung cancer. Lung Cancer 2006; 53: 143–151 [DOI] [PubMed] [Google Scholar]
- 30.Tringler B, Liu W, Corral L, Torkko KC, Enomoto T, Davidson S.B7-H4 overexpression in ovarian tumors. Gynecol Oncol 2006; 100: 44–52 [DOI] [PubMed] [Google Scholar]
- 31.Tringler B, Zhuo S, Pilkington G, Torkko KC, Singh M, Lucia MS.B7-h4 is highly expressed in ductal and lobular breast cancer. Clin Cancer Res 2005; 11: 1842–1848 [DOI] [PubMed] [Google Scholar]
- 32.von Herrath MG, Harrison LC. Antigen-induced regulatory T cells in autoimmunity. Nat Rev Immunol 2003; 3: 223–232 [DOI] [PubMed] [Google Scholar]
- 33.Woo EY, Yeh H, Chu CS, Schlienger K, Carroll RG, Riley JL.Cutting edge: regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol 2002; 168: 4272–4276 [DOI] [PubMed] [Google Scholar]
- 34.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer 2005; 5: 263–274 [DOI] [PubMed] [Google Scholar]
- 35.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol 2006; 6: 295–307 [DOI] [PubMed] [Google Scholar]