SUMMARY
Unidirectional, continuous airflow through the avian lung is achieved through an elaborate air sac system with a sequential, posterior to anterior ventilation pattern. This classical model was established through various approaches spanning passively ventilated systems to mass spectrometry analysis of tracer gas flow into various air sacs during spontaneous breathing in restrained ducks. Information on flow patterns in other bird taxa is missing, and these techniques do not permit direct tests of whether the basic flow pattern can change during different behaviors. Here we use thermistors implanted into various locations of the respiratory system to detect small pulses of tracer gas (helium) to reconstruct airflow patterns in quietly breathing and behaving (calling, wing flapping) songbirds (zebra finch and yellow-headed blackbird). The results illustrate that the basic pattern of airflow in these two species is largely consistent with the model. However, two notable differences emerged. First, some tracer gas arrived in the anterior set of air sacs during the inspiration during which it was inhaled, suggesting a more rapid throughput through the lung than previously assumed. Second, differences in ventilation between the two anterior air sacs emerged during calling and wing flapping, indicating that adjustments in the flow pattern occur during dynamic behaviors. It is unclear whether this modulation in ventilation pattern is passive or active. This technique for studying ventilation patterns during dynamic behaviors proves useful for establishing detailed timing of airflow and modulation of ventilation in the avian respiratory system.
KEY WORDS: bird, respiration, airflow pattern, modulation, timing
INTRODUCTION
In contrast to the tidal ventilation of the alveoli in the mammalian lung, air flows continuously and unidirectionally through the parabronchi of the avian lung. This unidirectional airflow pattern is thought to be maintained through the combined action of airway constrictions and aerodynamic valves at crucial confluences between large bronchial conduits and the air sacs (e.g. Scheid and Piiper, 1989; Duncker, 2000; Maina, 2005). As a result, the lung is perfused with oxygenated air during both respiratory phases. This feature is thought to contribute to the superior gas exchange efficiency of the avian lung compared with that of mammals, particularly in hypoxic or high-altitude environments (e.g. Tucker, 1968; Lasiewski and Calder, 1971; Maina, 2000; Scott et al., 2011).
Ventilation of the avian lung is driven by the bellows-like action of the air sacs, which are functionally grouped into a posterior set that is connected to the posterior part of the lung and an anterior set that receives air from the anterior part of the lung (Fig. 1). During inhalation, air moves from the trachea, through the extrapulmonary primary bronchi into the intrapulmonary bronchi, bypassing the sharp turn that connects the anterior set of air sacs to the bronchi (Butler et al., 1988; Wang et al., 1992; Maina and Africa, 2000). As air enters the posterior part of the intrapulmonary primary bronchi, a portion moves through the dorsobronchi into the parabronchi. The majority of the inhaled volume, however, moves into the posterior set of air sacs, comprised of posterior thoracic (PTAS) and abdominal (AAS) air sacs. Part or all of the residential air in the lung is drawn into the anterior set of air sacs, the interclavicular (ICAS), anterior thoracic (ATAS) and cervical air sacs, as they expand, thereby making room in the lung for a newly arriving portion of inhaled air. Upon exhalation, air from the posterior set of air sacs is routed through action of an aerodynamic valve (Brown et al., 1995) into the lung rather than through the low-resistance pathway into the intrapulmonary primary bronchi, and air from the anterior set of air sacs flows into the primary bronchi and exits through the trachea (e.g. Duncker, 1971; Duncker, 2000; Scheid and Piiper, 1989; Maina, 2005).
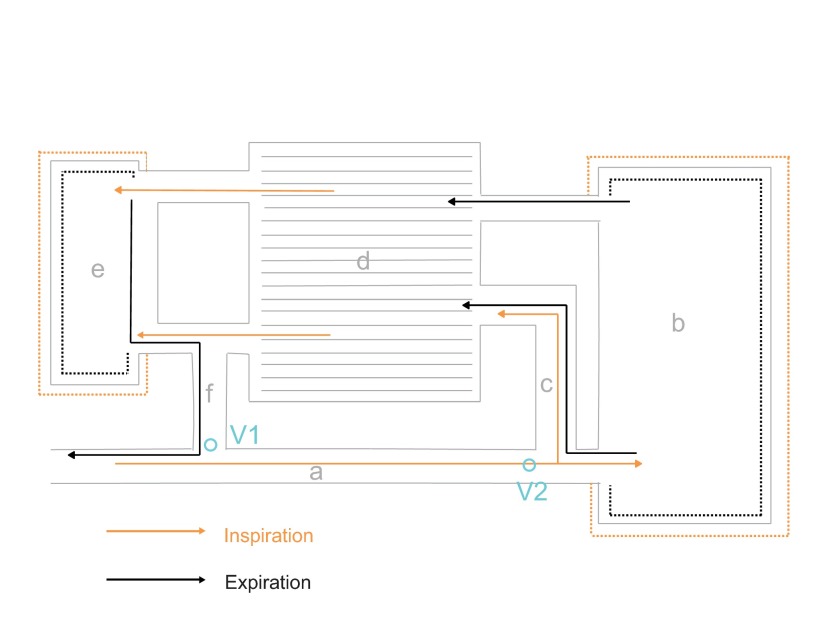
Fig. 1.
Schematic of the avian respiratory system illustrating the model for ventilation of the air sac system and lung. During inspiration (orange arrows), air is thought to flow through the mesobronchus (a) toward the posterior set of air sacs (b), bypassing the opening of the medioventral secondary bronchus (f), which connects to the anterior set of air sacs (e). A portion of air is thought to enter the lung (d) directly through the mediodorsal secondary bronchus (c). An ‘inspiratory aerodynamic’ valve (V1) is thought to regulate this inspiratory flow pattern. On expiration (black arrows), air moves from the posterior air sac system into the lung. A second aerodynamic valve (V2) is thought to prevent outflow through the mesobronchus. At the same time, air from the anterior set of air sacs is moved into the extrapulmonary primary bronchi and is exhaled through the trachea.
This flow pattern is the widely accepted model for lung ventilation in birds. As a consequence of this ventilatory pattern, each bolus of air entering the respiratory system resides there for two breath cycles before it is again exhaled. The most direct evidence for establishing this model of ventilation comes from airflow measurements at various locations in the respiratory system in ducks that were suspended in an upright position. Flow measurements were made during normal breathing, under anesthesia and during panting (Bretz and Schmidt-Nielsen, 1971). In addition, the time course of air movement through the various compartments in relation to breath cycles was analyzed by injecting a tracer gas (argon) and sampling gas in various air sacs for mass spectrometry analysis (Bretz and Schmidt-Nielsen, 1972). Argon entered the posterior set of air sacs during the inspiration when it was inhaled, and it was registered in the anterior set during the following expiration. Limited temporal resolution did not allow a detailed investigation of precise arrival times or their dependence on respiratory variables (Bretz and Schmidt-Nielsen, 1972).
Although it is assumed that the airflow pattern found in ducks is the general pattern in all birds, comparative data across avian taxa are not available. Furthermore, it is not known how ventilation patterns of the lung may change during different dynamic behaviors (Bretz and Schmidt-Nielsen, 1971). Although the unidirectional flow pattern is present in fixed and artificially ventilated lungs (Scheid et al., 1972), this passive pattern might be influenced by active control mechanisms. For example, the air sac pressure differential between the posterior and anterior sets of air sacs (e.g. King and Molony, 1971; Brackenbury, 1971; Brackenbury, 1972; Brackenbury, 1973; Kuethe, 1988) is not constant for all dynamic behaviors. The pressure differential between anterior and posterior air sacs may be subject to dynamic modulation by activities such as running (Boggs et al., 2001), flying (Boggs et al., 1997) and diving (Boggs et al., 1998) or vocalization (Beckers et al., 2003). These small differences in pressure may indicate either a local regulation of air sac pressure through additional muscles used in the dynamic behaviors or a more active regulation of directing airflow, which in turn would be reflected in these pressure differences. In either case, different pressure conditions must affect detailed airflow patterns, but the degree to which the basic pattern is altered is not known.
Aside from panting, data on the ventilation pattern do not exist for behavioral activities that are intimately connected to respiration. For example, vocal behavior is typically associated with remarkable changes in respiratory patterns (for reviews, see Suthers and Goller, 1998; Suthers et al., 1999; Goller and Cooper, 2008; Suthers and Zollinger, 2008). Call series and songs are often elaborate and rapid series of expiratory pulses of varying duration that alternate with short deep inspirations (mini-breaths). Although mini-breaths replenish the air used during the preceding sound syllable, gas exchange may be different from that during quiet breathing. Periods of apnea after long song bouts suggest that in some male canaries (Serinus canaria) and zebra finches (Taeniopygia guttata), hyperventilation occurs during song (Hartley and Suthers, 1989; Franz and Goller, 2003). Active control of ventilation patterns may allow birds to reduce respiratory alkalosis during vocal behavior. However, it is unknown whether such control occurs or is even physiologically possible. Data from panting ducks suggest that no major changes in ventilation pattern take place (Bretz and Schmidt-Nielsen, 1971).
Here we explore airflow patterns in the respiratory system of songbirds using a new technique for sensing a tracer gas, allowing us to track the flow of an inhaled bolus of helium into the air sacs during dynamic behaviors. The results confirm that lung ventilation in songbirds follows the basic avian pattern, but also show deviations in the timing of airflow between air sacs and substantial deviation in residence time of air in the respiratory system.
MATERIALS AND METHODS
Six female zebra finches (Taeniopygia guttata Gould 1837) and four male yellow-headed blackbirds [Xanthocephalus xanthocephalus (Bonaparte 1825)] were used in this experiment. To compare breathing under anesthesia with that of freely behaving birds, two of the zebra finches were studied while anesthetized, and four after recovery from anesthesia and as they were perched in their cage. Blackbirds were studied one to several days after they had recovered from anesthesia and were freely behaving in their cage. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Utah. Birds were accustomed to the experimental situation 2 days before surgical procedures by fitting an elastic belt around their thorax with a Velcro tab on the back. This belt did not restrict respiration as it was loosely fitted such that its ventral portion rested over the furcula. Birds were tethered to a counterbalanced tether arm that allowed free movement in the cage. In preparation for surgical implantation of transducers, birds were deprived of food and water for 1 h (zebra finch) or 2 h (yellow-headed blackbird).
Surgical procedures
Birds were anesthetized with isoflurane for implantation of air sac cannulae, helium sensor probes and the tracheal flow probe. Flow probes and helium sensors were custom-made with microbead thermistors (Thermometrics BB05JA202 thermistors, Edison, NJ, USA). Cannulae, containing a helium sensor probe in the tip, were inserted into the PTAS, ATAS and ICAS. The PTAS and ATAS were accessed by inserting the cannula tip through the body wall below the last rib and secured to the rib cage with a suture. Medial placement of the cannula gave access to the ATAS, and lateral placement near the free ribs to the PTAS. A flow probe was inserted into the distal portion of the trachea slightly above the attachment site of the ICAS. A small hole was made into the connective tissue between two tracheal rings through which the thermistor bead was placed into the center of the tracheal lumen. The bead was held in place with tissue cement and a suture around a tracheal ring and the flow probe wires. The wires from the thermistor probes were routed subcutaneously or in the cannula to the backpack, where connections to the signal conditioning units (Hector Engineering, Ellettsville, IN, USA) were made through stronger wire in the tether. A feedback circuit heated the thermistor beads to ~60°C, thus increasing the sensitivity to gas with different thermal properties.
Collection of experimental data
A controlled volume of helium was delivered into the mouth of the bird with a picospritzer (model III; Parker Hannifin Corp., Fairfield, NJ, USA). In anesthetized zebra finches, a small tube (outer diameter 1.5 mm) was inserted into the beak to deliver the helium pulses. In experiments with awake zebra finches, helium was delivered through an air channel that was formed out of dental cement to route helium from the head to a small groove in the upper mandible. A flexible tube was attached to this air channel on the head and helium pulses were delivered into the mouth through this system. Helium was administered to yellow-headed blackbirds by clamping a small steel tube to the lower mandible during the recording session. One end of the steel tube extended approximately 1 cm into the mouth to deliver helium near the glottis. A flexible tube from the picospritzer was connected to the other end of the steel cannula.
Three data sets were collected for this study. First, we recorded data from two anesthetized zebra finches in the supine position to explore the feasibility of the method for detecting the flow of helium through the respiratory system. Next, we collected data from four conscious zebra finches during normal breathing while they were perched in their cages. At irregular intervals, a pulse of helium lasting 100–300 ms was administered with the picospritzer. Helium traces were allowed to wash out before another pulse was administered. Finally, recordings from freely behaving yellow-headed blackbirds were made during different respiratory rhythms that accompanied quiet breathing, calling and wing flapping. Full free flight could not be accomplished as the birds were tethered. Wing flapping was induced by holding the tether and removing the perch or hands from beneath the bird.
Monitoring of helium
The anterior sac cannula was connected to a small piezoresistive pressure transducer (Fujikura FPM-02PG, Tokyo, Japan) mounted on the backpack. Air sac pressure was used to monitor respiratory rhythm and determine the respiratory phase because airflow signals from the tracheal flow probe do not distinguish between expiratory and inspiratory flow. The thermistor probes detected the presence of helium in the air sacs because the different thermal constant of helium induces a marked DC shift in output voltage of the thermistor probes. Although a gas that more closely matches the density of normal air would be less likely to alter the aerodynamic mechanisms or introduce density-dependent distribution effects, the detection of the tracer gas based on its thermal properties ruled out the use of argon or other gases.
Data analysis
Data files were analyzed with Signal software (Engineering Design, Belmont, MA, USA). The appearance of helium in the trachea was determined by a distinct DC shift of the output voltage of the tracheal flow probe. The delay from the arrival of helium in the trachea to its appearance in each air sac was measured and related to the respiratory cycle. We measured the elapsed time from the onset of the helium-induced DC shift registered by the tracheal flow probe to a distinct DC shift in the various air sacs. To quantify the amount of helium entering the respiratory system and each air sac, we integrated the voltage trace over a respiratory period (first inspiration, expiration, etc.). Changes in flow patterns as determined through arrival in an air sac were compared for the different behaviors, ‘normal’ breathing at rest (from here on called ‘quiet breathing’), calling and wing flapping.
RESULTS
Methodological observations
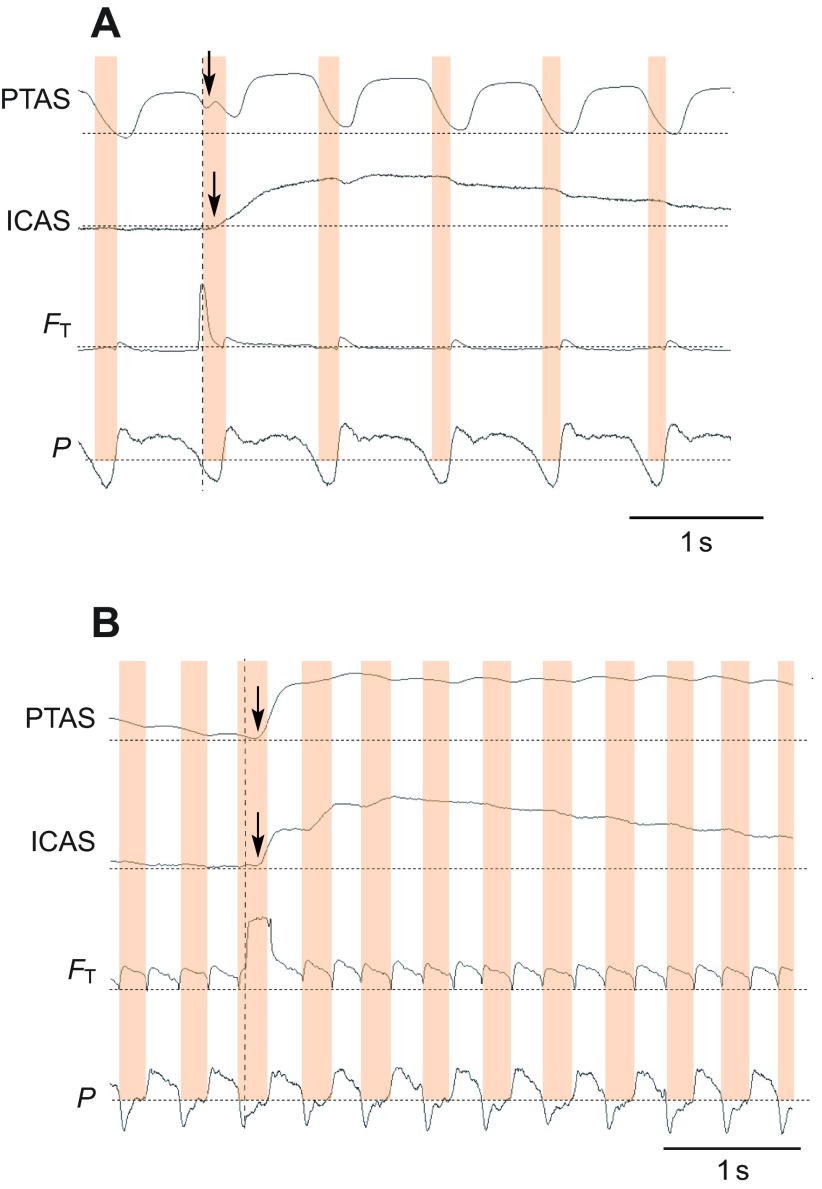
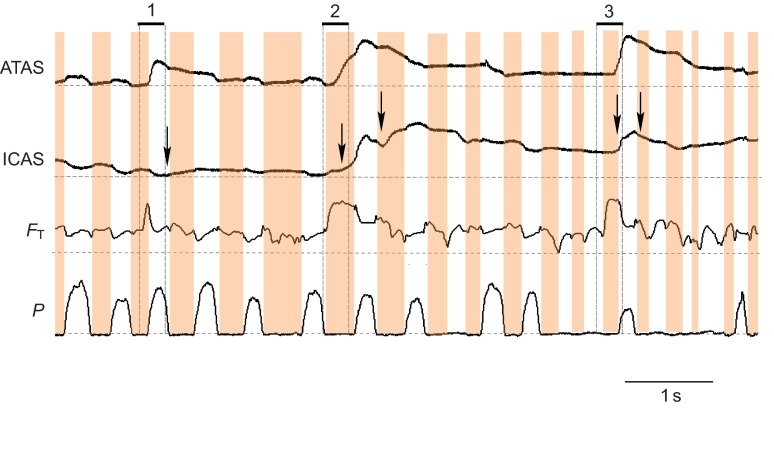
The arrival of helium pulses could be clearly detected in the various air sacs with the thermistor technique (Fig. 2). However, other events that also cause a DC shift in the output voltage did occasionally generate signals that are difficult to interpret. The tracheal probe was secured and its DC shift in the output voltage was clearly associated with the passage of heliox. However, the specific placement of the thermistor probes and their shifting during respiratory movements in the various air sacs could lead to complex output signals. Fluctuations, presumably caused by movement of the thermistor (Fig. 2A), were sometimes superimposed on the registration of helium, specifically in calling and wing flapping data sets. If this occurred, a small error in determining the arrival time of the helium pulse might be introduced. In attempt to reduce error, we removed from our data set all obvious instances (10 out of 80 events) in which accurate timing and movement of helium could not be confidently measured. These events could be clearly identified with DC shifts in voltage that were unrelated in time to the inhalation of helium as confidently monitored with the tracheal probe. Additionally, we focus here on reporting only the major timing events that are not substantially influenced by these methodological problems and do not discuss or test fine-scale differences in timing.
Fig. 2.
The movement of a helium pulse through the respiratory system can be traced with thermistors. (A) Example for recordings from an anesthetized zebra finch shows that helium is first registered by the tracheal flow probe (FT; dashed vertical line) and enters (arrow) the posterior thoracic air sac (PTAS) and, with another delay (arrow), the interclavicular air sac (ICAS) during the same inspiration as indicated by subsyringeal air sac pressure measurements (P; dotted horizontal lines indicate atmospheric pressure; inspirations are highlighted in orange). The PTAS thermistor also responds to movement during respiration that is unrelated to the presence of helium. The slow washout of helium is illustrated by elevated output voltage for several respiratory cycles in both air sacs. (B) Example for recordings during quiet breathing in a perched, awake zebra finch. Again, helium is registered in the air sacs during the same inspiration in which it enters the trachea, and the washout takes several respiratory cycles.
A second experimental issue concerned the lifetime of the thermistor probes in the air sacs. In some cases thermistor signals were lost after 1 day, or failed to return any signal during testing, thus making it difficult to achieve complete data sets from all targeted air sacs within one individual. This difficulty explains the partial data sets for some individuals in Tables 1, 2, 3, 4 and 5. Despite these methodological problems, this technique is suitable for studying airflow patterns in the avian respiratory system during dynamic behaviors.
Table 1.
Delay in the appearance of the helium pulse between the trachea and air sacs in the zebra finch
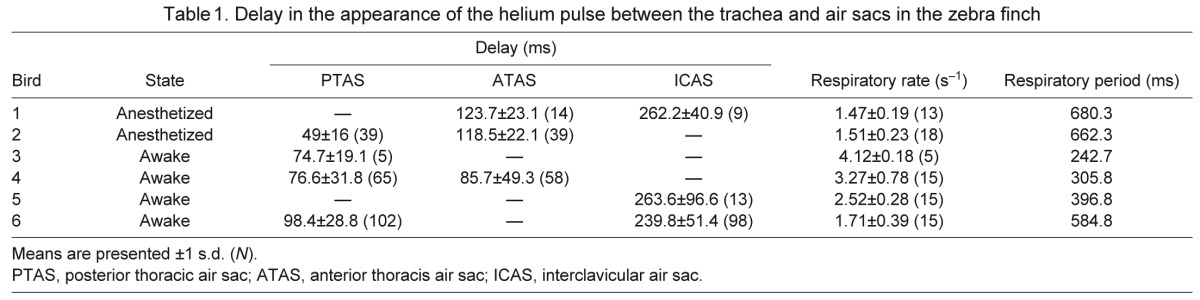
Table 2.
Delay in the appearance of the helium pulse between the trachea and air sacs in the yellow-headed blackbird (perched or at rest)
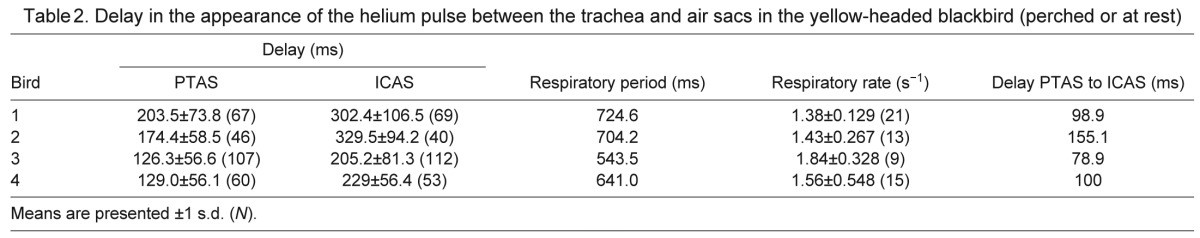
Table 3.
Residence time of helium in air sacs of the yellow-headed blackbird
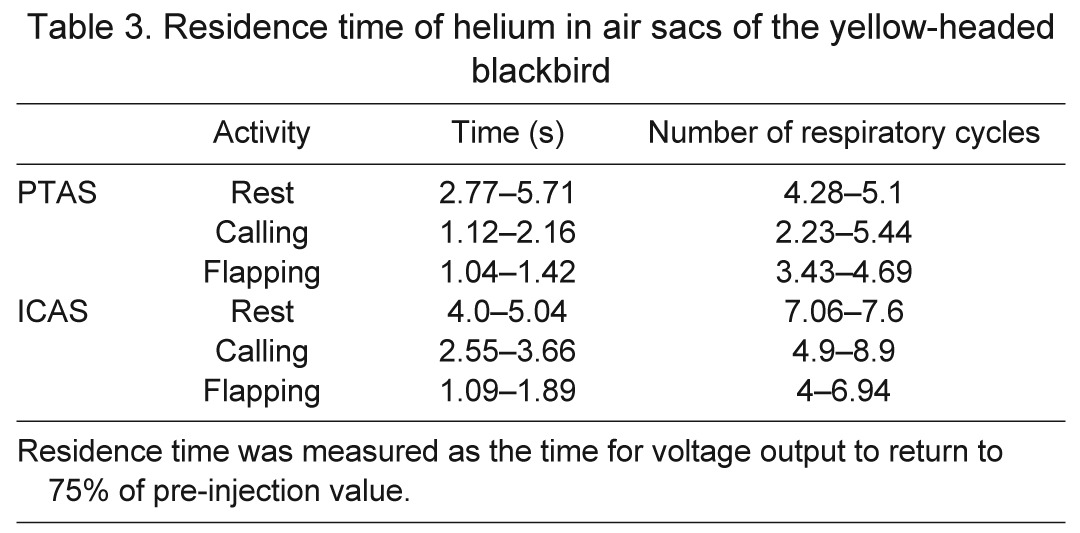
Table 4.
Delay in the appearance of the helium pulse between the trachea and air sacs in calling yellow-headed blackbirds
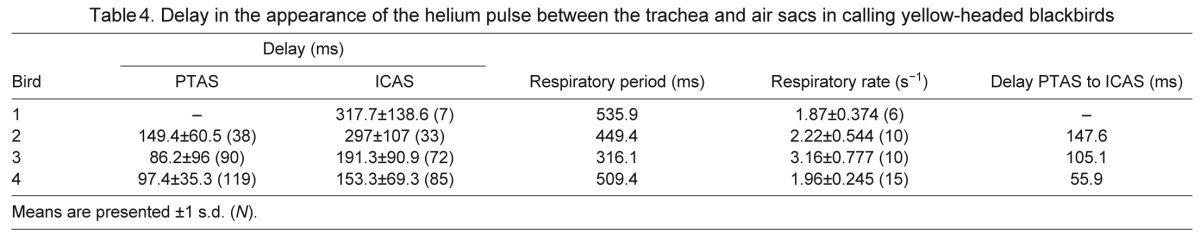
Table 5.
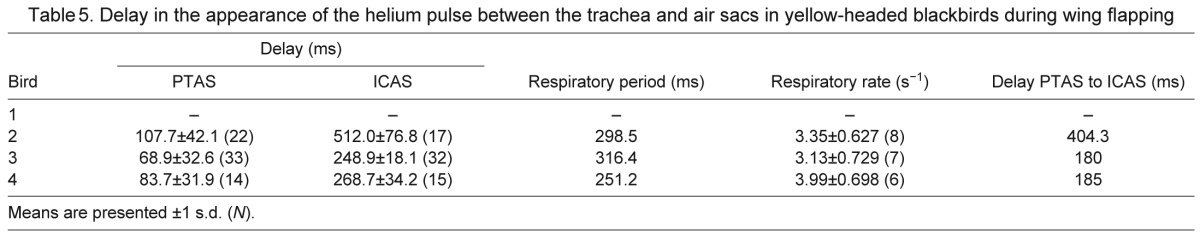
Delay in the appearance of the helium pulse between the trachea and air sacs in yellow-headed blackbirds during wing flapping
Ventilation during breathing while resting
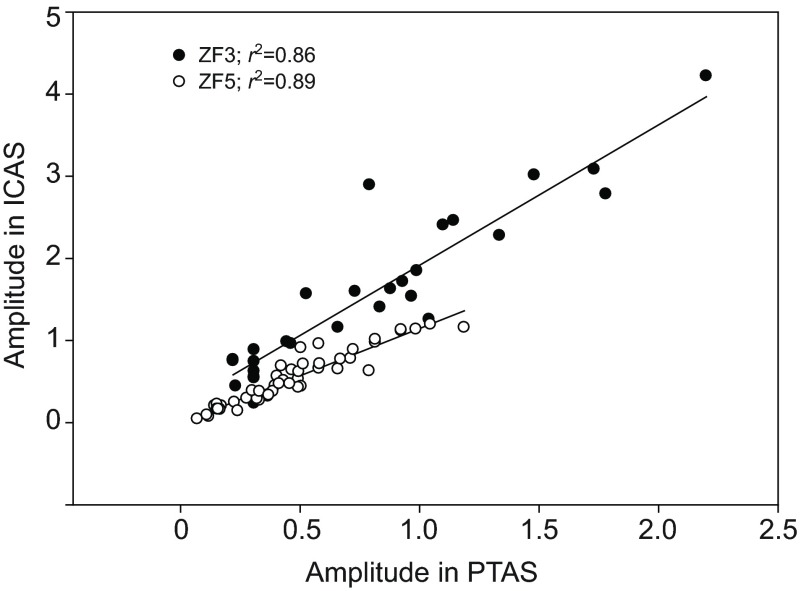
The examples in Fig. 2 indicate a similar flow pattern of the helium pulse in anesthetized and awake, quietly breathing zebra finches. Once the helium pulse passed the tracheal flow probe, it arrived with a short delay first in the PTAS and then in the ICAS. In both cases, helium arrived in the ICAS during the ongoing inspiration, and continued to increase during the subsequent expiration and inspiration. Helium stayed in both air sacs for several respiratory cycles, and the slow washout is confirmed by only slightly and progressively less DC-shifted output voltage of the tracheal probe during expirations following the helium injection (Fig. 2). The amount of helium arriving in the PTAS was predictive of the amount registered in the ICAS (Fig. 3).
Fig. 3.
The arrival of helium induces a voltage shift in thermistor output, and the amplitude of this shift in the posterior thoracic (PTAS) and interclavicular air sacs (ICAS) is positively correlated. Two examples for different, quietly perched zebra finches are shown (filled and open circles). The r2 values indicate that a substantial portion of the variance is explained by this relationship.
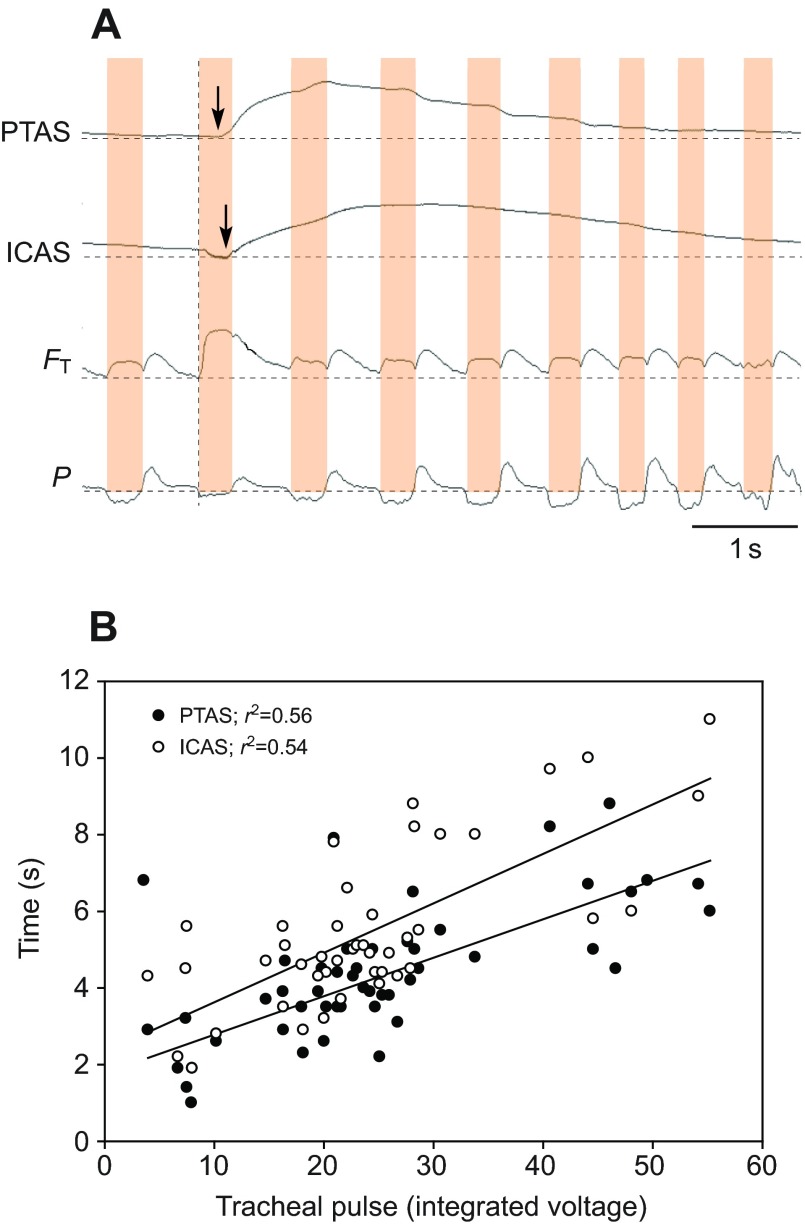
The same basic pattern of flow was observed in perched, quietly breathing yellow-headed blackbirds (Fig. 4A). In this example, the probes in the PTAS and ICAS indicated rising helium levels during at least two respiratory cycles after the injection, with an earlier decline in the PTAS (after the second inspiration). The amount of helium registered in the trachea was positively correlated with the time helium was registered in the air sacs, but this relationship did not explain more than 56% of the variation in residence time (Fig. 4B). The slow washout was evident in the only slightly elevated voltage of the tracheal flow probe after the first respiratory cycle (Fig. 4A).
Fig. 4.
(A) Example of arrival of helium in the trachea and the posterior thoracic (PTAS) and interclavicular air sacs (ICAS) in a quietly breathing yellow-headed blackbird. After passing the tracheal flow probe, helium arrives with a delay first in the PTAS (arrow) and then in the ICAS (arrow). Abbreviations and labels as in Fig. 2. (B) The more helium (quantified as integrated voltage of the tracheal flow signal during the passage of helium) enters the respiratory system, the longer its presence in the PTAS and ICAS. Data show one example for a yellow-headed blackbird.
In the zebra finch, the helium pulse was registered by the thermistor in the PTAS 50–100 ms after it passed the tracheal flow probe. This delay was 85–125 ms in the ATAS and 240–260 ms in the ICAS. The ranges of means were similar between anesthetized birds in the supine position and awake, perched birds. Considering the large variation and the small number of animals, we did not test for differences in the measured delays between the two conditions (Table 1). In yellow-headed blackbirds, the delay between arrival in the trachea and arrival in the PTAS was longer (range 126–203 ms) than in the smaller zebra finch. In the ICAS, however, helium was registered between 205 and 330 ms after it passed the tracheal probe (Table 2), which overlaps with the values measured in the zebra finch.
The duration of inspirations was >120 ms in the zebra finch and >270 ms in the yellow-headed blackbird (Tables 1, 2). The delays in arrival of helium between the trachea and the anterior set of air sacs indicate clearly that in both species the latter received helium during the ongoing inspiration (Figs 2, 3). In the zebra finch, the mean delays between the PTAS and the ATAS tended to be shorter than those between the PTAS and the ICAS (Table 1) and ranged from 9 to 141 ms. The delay between registration of helium in the trachea and the ICAS (79–155 ms) in the yellow-headed blackbird (Table 2) also places the arrival of helium in the anterior set during the inspiration when helium passed the tracheal flow probe (Fig. 4).
Whereas the injected helium pulse was only registered by the tracheal flow probe during one or two inspirations, it was registered in each air sac for several breaths in both species. As helium entered an air sac, the thermistor output voltage peaked, either during the first or second respiratory cycle and then decreased continuously, indicating a slow washout of helium (Figs 2, 4). In quiet, perched yellow-headed blackbirds some helium resided in the PTAS for four to five respiratory cycles and in the ICAS for seven to eight respiratory cycles (Table 3).
Changes in timing during dynamic behaviors
Compared with regular, quiet breathing, respiratory patterns changed drastically during calling and wing flapping. Respiratory rates increased overall, but became less rhythmic (Fig. 5, Tables 3, 4). The mean respiratory rate recorded during calling was 35% higher than that during quiet breathing (increase from 1.5 to 2.3 breaths s−1), and the delay of helium arrival in the trachea to arrival in the PTAS decreased from a mean of 143.2 to 111 ms. The delay in the ICAS also decreased in three of the four yellow-headed blackbirds, with a mean for all four birds of 239.8 ms, as compared with 266.5 ms during quiet breathing (Table 4). The delay in arrival between the PTAS and the ICAS in the three individuals with data for both air sacs increased by almost 30 ms in one bird, whereas it decreased in the two other birds by 26 and 44 ms, respectively (Tables 2, 4).
Fig. 5.
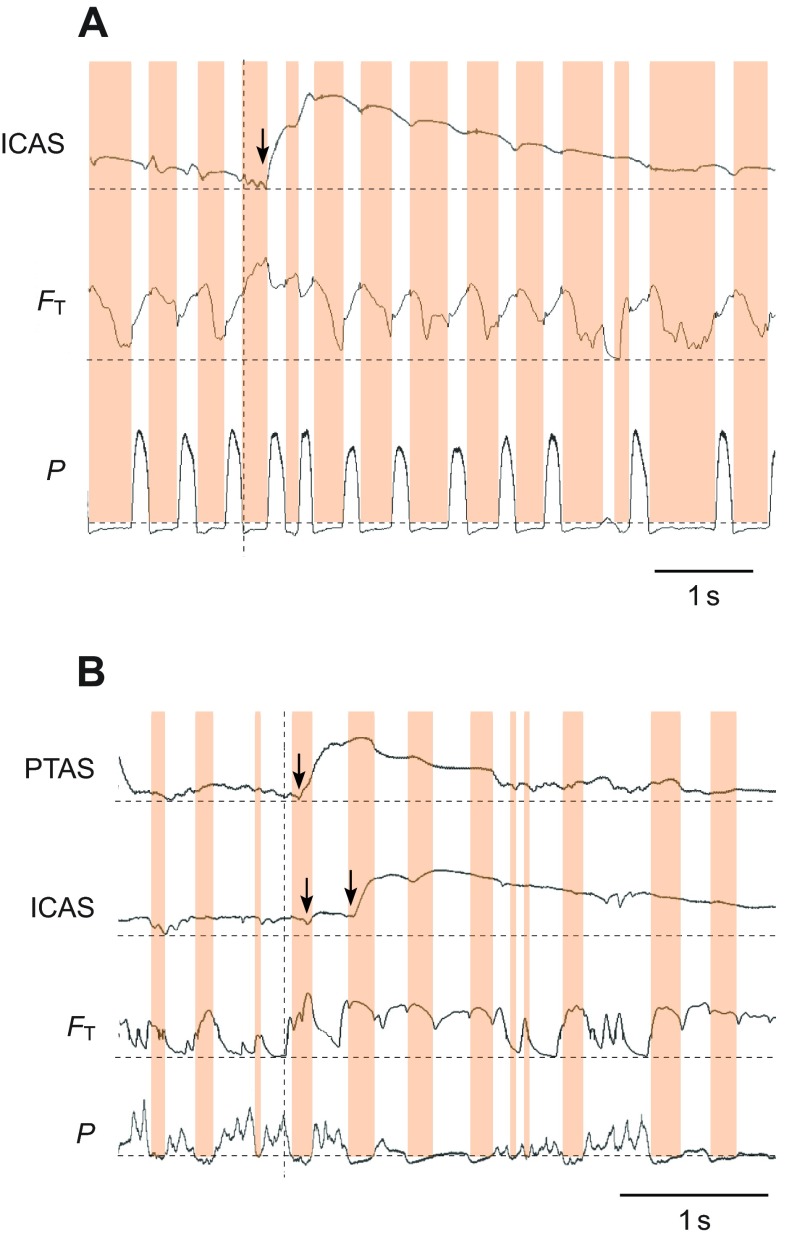
Dynamic behaviors change the respiratory rhythm and the airflow pattern. (A) Example for a series of calls produced by a yellow-headed blackbird. Calls are generated during expiratory pulses with increased amplitude (P) and the inter-call interval consists of short inspirations of variable duration (orange highlights). In this example, the helium pulse arrives in the ICAS at the end of the inspiration during which it is inhaled (arrow). (B) Wing flapping is accompanied by irregular modulation of the subsyringeal air sac pressure (P) and a different arrival pattern of helium in the air sacs. Although some helium arrives in the ICAS during the first inspiration (left arrow), the increase during the second inspiration (right arrow) is much more pronounced, indicating that a larger volume of helium enters the air sac during this second inspiration. Labels for traces as in Fig. 2.
During wing flapping, the respiratory rate increased to 3.5 breaths s−1, and the delay between the tracheal sensor and the PTAS decreased to 87 ms. Interestingly, the mean delay between the PTAS and ICAS increased consistently from 111 to 256.4 ms (Table 5, Fig. 6), suggesting a change in flow pattern compared with that during quiet breathing. This increase in mean delay arose from a shift of the arrival of helium in the ICAS from the first inspiration during quiet breathing (Fig. 7A) to the second inspiration during flapping (Fig. 7B). At the same time, the arrival in the ATAS still occurred during the first inspiration.
Fig. 6.
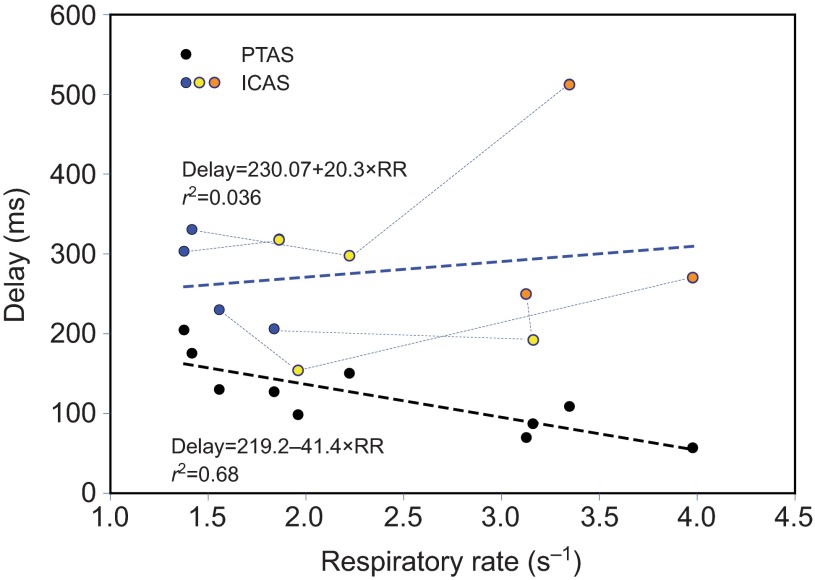
The delay between the arrival of helium in the trachea to that in the PTAS and ICAS is variable. Whereas this delay steadily decreases with respiratory rate in the PTAS (dashed black line), it shows a decrease in the ICAS for the elevated respiratory rate of calling (yellow symbols; data for different individuals are connected with dotted lines), but an increase for wing flapping (orange symbols) relative to that of quiet breathing (blue symbols; overall trend is indicated by dashed blue line). This increase results from the fact that during some wing flapping episodes helium did not arrive in the ICAS until the inspiration that followed the one when it passed the tracheal flow probe.
Fig. 7.
The arrival of helium in the anterior air sacs is not always synchronous. Whereas helium arrives in the anterior thoracic (ATAS) and interclavicular air sacs (ICAS) simultaneously during quiet breathing before wing flapping (A), during wing flapping it arrives during the first inspiration in the ATAS and during the following inspiration in the ICAS (B). Other labels as in Fig. 2.
Qualitative observations of changing airflow patterns during calling and wing flapping
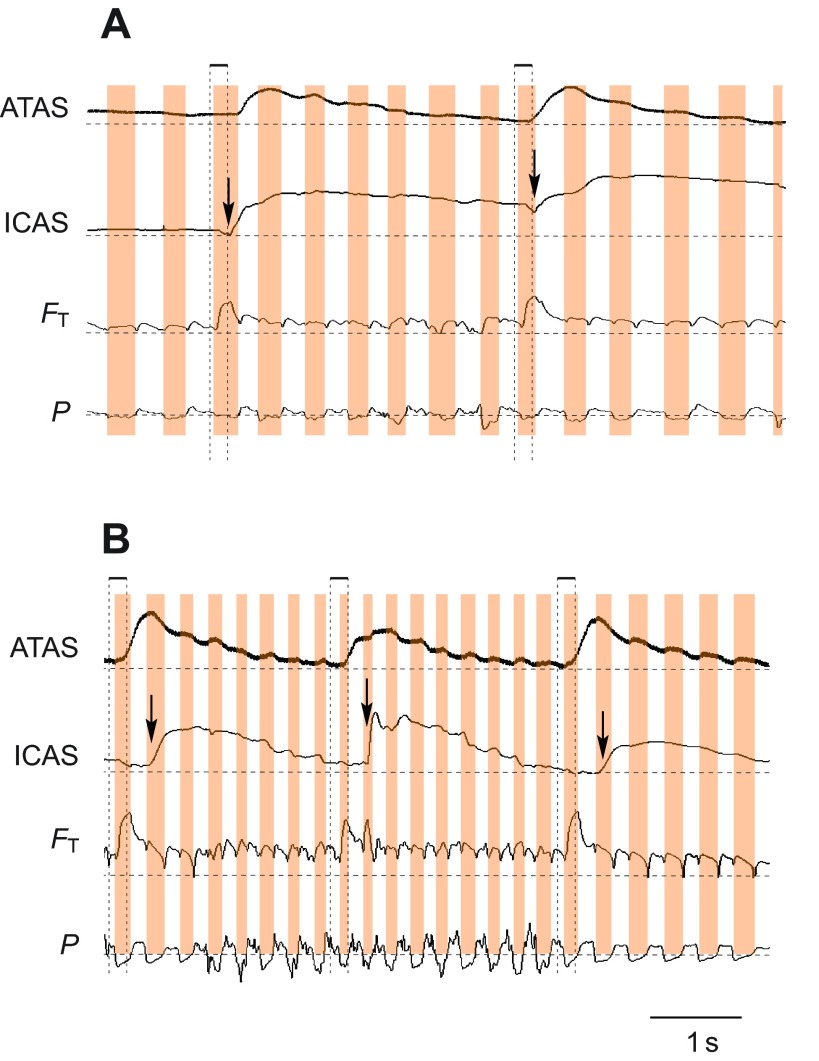
During calling (Fig. 8A), but not quiet respiration (Fig. 8B), helium sometimes exited the respiratory system on the first expiration following its inhalation. The peak voltage in the tracheal flow probe (FT) occurred at the end of the expiratory pulse for calling (Fig. 8A, marked with 2). This peak voltage indicates an abnormally high concentration of helium in the excurrent air, and is not a result of higher airflow. Airflow during call production is typically lower than during the mini-breaths (Fig. 5A, Fig. 8A, Fig. 9). This unusual flow pattern coincided with a short deep mini-breath within a call series. The inspiration was substantially shorter (105 ms) than others (>200 ms) and helium arrived in the ATAS during the expiration for the next call (Fig. 8C). Helium was not registered in the ICAS until the next inspiration. The washout of helium from the ATAS was also much faster than from that from the ICAS. This unusual flow pattern was observed in five out of 30 cases where helium was delivered during a call series and always coincided with a shorter than usual inter-call inspiration.
Fig. 8.
During calling, an unusual flow pattern occurred during short inter-call inspirations. (A) Helium inhaled during a short inspiration (labeled 1 on the left) is immediately exhaled at the end of the following call (expiration, labeled 2) This pattern was not present during a later helium pulse when calling stopped (right label 1). (B) During quiet breathing following the production of the call series the flow of helium also followed the typical pattern. (C) Expanded view of the unusual, rapid exhalation of helium during calling. The peak voltage output of the tracheal thermistor (FT) occurs at the end of the call, and this voltage is higher than during the initial inhalation, indicating a high concentration of helium in the excurrent air.
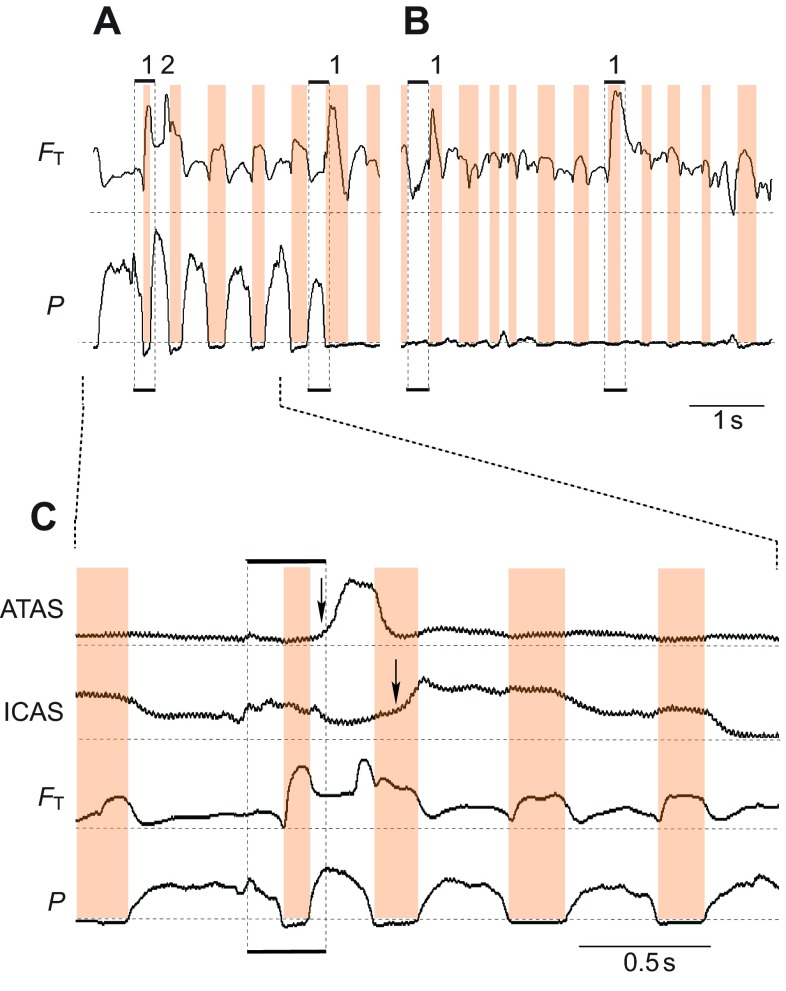
Fig. 9.
During calling, the arrival of helium in the anterior air sacs is also not synchronized. Three deliveries of helium (marked 1–3) show consistent arrival in the ATAS during the first inspiration and following expiration. In the ICAS, different arrival patterns are present for each delivery. Whereas very little, if any (arrow), helium enters the ICAS during the first delivery, during the second some helium enters the ICAS during the first inspiration (first arrow) followed by much higher helium concentration during the following expiration and subsequent second inspiration (second arrow). The third delivery of helium enters the ICAS only during the call produced by the expiration following the initial inhalation (first arrow) and is washed out or diluted rapidly (second arrow).
Arrival of helium and amount of helium in the two anterior air sacs (ATAS and ICAS) differed between quiet breathing and dynamic behaviors. As already pointed out above, during quiet breathing helium arrived in the ICAS and ATAS during the same inspiration when it passed the tracheal flow probe. During wing flapping, however, helium entered the ICAS only on the subsequent inspiration (Fig. 7), while it still arrived at the first inspiration in the ATAS. The situation was even more complex during calling. Within one call series, an inconsistent pattern emerged of how and when helium entered the two anterior air sacs. Whereas helium always entered the ATAS during the inhalation when it passed the tracheal probe, it did not always arrive at the ICAS (1 in Fig. 9). During subsequent helium pulses, both anterior air sacs received helium, but the amount and timing were different (2, 3 in Fig. 9). In the segment marked with 2 in Fig. 9, the probe in the ICAS registered less helium on the first inspiration than the probe in the ATAS, and both received additional helium during the following expiratory pulse associated with a call. However, more helium entered the ICAS on the subsequent breaths than the ATAS, as indicated by increasing voltage in the former and decreasing output voltage in the latter air sac. At the end of the calling series, both air sacs again showed a more uniform pattern of arrival and washout of helium (3 in Fig. 9).
DISCUSSION
We show here that thermistor probes positioned in various locations throughout the respiratory system permit reconstruction of flow patterns during dynamic behavior through use of a tracer gas with different thermal properties (helium) from those of normal air. The technique provides sufficient temporal resolution for measuring accurately when tracer gas appears in different air sacs. The data are the first for small birds and largely confirm the basic pattern of airflow through the avian air sac/lung system that was obtained in larger birds. However, the measurements also suggest differences from the model and illustrate distinct changes with dynamic behaviors. In the following, we discuss methodological issues as well as similarities and differences with the textbook model of airflow in birds.
Critique of the technique
The technique of monitoring arrival and volume of helium through the shift in voltage output of a thermistor provides a sensitive assay for airflow patterns with excellent temporal resolution. It is particularly useful in studying flow patterns during different behaviors and can be applied to small birds such as the zebra finch (mass range 10–14 g). However, some methodological difficulties did arise. The viability of thermistors placed into the air sacs was limited. A further complication arose from unrelated DC shifts that might have reflected movement of the thermistor bead in the air sac with each respiratory movement. Finally, the use of helium may influence the flow pattern as discussed below.
Timing of air sac ventilation
The data of this study provide the first accurate measurements of the time air takes to travel from the trachea to various air sacs in active birds. Previous studies using oxygen pulses and tracer gases with mass spectrometry did not have similar temporal resolution (Schmidt-Nielsen et al., 1969; Bretz and Schmidt-Nielsen, 1972). As expected, the timing depends on the size of the bird and, within one individual, the airflow. Air reaches the PTAS in the smaller zebra finch in less than 100 ms during quiet breathing and in the yellow-headed blackbird in less than 200 ms. Within the latter species, higher ventilation rates during singing (mini-breaths) and wing flapping decrease this delay. Mean arrival in the anterior set of air sacs differed between the ATAS and the ICAS. In the zebra finch, the former received helium ~100 ms earlier than the latter. However, the arrival of helium in the anterior set of air sacs during the inspiration at which helium entered the trachea contradicts the main model and will be discussed in detail below.
Helium resided in the air sacs for a fairly long time after the initial inhalation. The numbers in the yellow-headed blackbird correspond well with those reported for ducks (Bretz and Schmidt-Nielsen, 1972). As expected, the washout time decreased with increasing respiratory rates during dynamic behaviors. Helium resided in the ICAS longer than in the PTAS. This longer period arises in part from the main flow pattern that predicts a delay of one respiratory cycle for the ICAS and takes into account that helium arrives in the anterior set of air sacs on the first inspiration. However, the difference is larger than one respiratory cycle in many cases, suggesting that the ICAS is slightly less ventilated than the PTAS (Zeuthen, 1942; Schmidt Nielsen et al., 1969; Bretz and Schmidt-Nielsen, 1972). In the duck, it was assumed that the tracer gas did not arrive in the anterior air sacs before the second inspiration. The residual time of argon, estimated as the number of breathing cycles, shows overlapping values between the PTAS and the ICAS. Because they were measured at different respiratory rates, however, these data also suggest a longer residual time in the ICAS (Bretz and Schmidt-Nielsen, 1972). Nevertheless, both studies (Bretz and Schmidt-Nielsen, 1972; present study) indicate clearly that tracer gas of different density can be detected in air sacs well beyond the second expiration and therefore indicate that only part of the volume of each air sac is exchanged with each breath cycle.
Is the deviation from the model real or an artifact?
In both zebra finches and yellow-headed blackbirds, helium arrived in the PTAS during the inspiration in which the helium pulse was administered. This finding is in good agreement with the general avian model. However, helium also arrived in the anterior set of air sacs during this same inspiration. This was consistently the case during quiet breathing and also occurred during most, but not all, recordings of calling and wing flapping in the yellow-headed blackbirds. According to the model, helium should arrive in the anterior set of air sacs during the inspiration following its initial inhalation. This early arrival requires explanation.
Early arrival of helium in the anterior set of air sacs could be explained by two possible events. First, sufficient helium passes all the way through the lung during the first inspiration to be registered in the anterior air sacs. Although this possibility contrasts the model, there is some support in the classical study for this scenario. Bretz and Schmidt-Nielsen occasionally detected argon in the anterior set of air sacs with their mass spectrometry technique, but interpreted this finding as an artifact of the gas sampling procedure (Bretz and Schmidt-Nielsen, 1972). Our results with helium confirm the possibility of arrival of a small portion of the tracer gas in the anterior set of air sacs during the inspiration when it was delivered. This interpretation is also consistent with the timing of arrival. Helium was always registered toward the end of the inspiration and well after helium arrived in the PTAS.
Alternatively, the arrival of helium in the anterior set of air sacs during the first inspiration might indicate a failure of the aerodynamic valve that routes air through the mesobronchus and prevents flow into the ventrobronchus. The effectiveness of the valve is thought to depend on gas density and velocity. Failure could therefore be induced by the difference in density between the tracer gas and nitrogen. Nitrogen is approximately sevenfold more dense than helium, and this difference can lead to failure of the aerodynamic valve when helium replaces nitrogen (Banzett et al., 1987). However, several lines of evidence suggest that failure of the valve might not be the explanation for the early arrival of helium in the anterior set of air sacs. First, in the current experiment only a small pulse of helium was used, thus inducing only a small change in density, unlike in the case where helium replaced nitrogen altogether (Banzett et al., 1987). Furthermore, the early arrival of argon in the duck could not be explained by a failure of the aerodynamic valve as the density of nitrogen and argon are much more similar and argon is not thought to induce failure (Banzett et al., 1987). Second, the timing of arrival after helium has passed into the PTAS requires additional circumstances for inducing failure at this point. Perhaps a reduction in velocity toward the end of the inspiration, combined with the density change, could affect the aerodynamic valve at this point. However, such reduction is not observed consistently in all cases (e.g. Figs 2, 4, 7, 9). Furthermore, the fact that the amount of helium registered in the PTAS is highly correlated with that arriving in the ICAS (Fig. 3) also speaks against this latter scenario.
We therefore suggest that the helium arriving in the anterior air sacs toward the end of the inspiration during which it was administered represents gas that has passed through the lung and is not a consequence of failure of the aerodynamic valve.
Changes in flow patterns with dynamic behaviors
Although the observations on ventilation during wing flapping and calling are mostly qualitative, they show unambiguously that flow patterns through the avian respiratory system can change during different behaviors. The two main deviations were: (1) that ventilation of the anterior air sacs was not uniform and (2) that during calling helium was sometimes exhaled very rapidly.
Helium arrived in the ATAS during the first inspiration (or the following expiration in the case of Fig. 8) consistently, but the pattern for the ICAS was more variable. During wing flapping and calling helium was registered later during the second inspiration. In addition to this timing difference, the amount of helium that was registered for a given inspiration was also not the same in the ATAS and the ICAS. It is unclear whether this differential ventilation pattern of the anterior air sacs is actively controlled or arises from pressure differentials generated by the behavioral activities. Pressure differentials in various air sacs have been recorded during locomotion and vocalization in other birds (Boggs et al., 1997; Boggs et al., 1998; Boggs et al., 2001; Beckers et al., 2003). Especially during phonation, the pressure conditions in the ICAS are important. ICAS pressure generates a force on the vibrating labia and membranes that influences gating of airflow and acoustic parameters such as frequency. If birds are able to regulate the ICAS independently of the subsyringeal pressure, this would constitute a new active control mechanism for vocal control. However, it is unknown how such active control might be exerted.
The unusual flow pattern indicated by early arrival of exhaled helium at the tracheal flow probe during calling (Fig. 8) could result from two possible differences from the typical flow pattern. A larger portion of the air–helium mixture could move through the lung than is typical, or a significant portion of air could pass through the mesobronchus rather than the lung on exhalation. The unusual timing and magnitude of the voltage peak suggest that the latter mechanism is the likely explanation. Because airflow during calling is typically below that of normal respiration, the former interpretation is unlikely. It is more likely that toward the end of the expiratory pulse generating the call, gas exits directly through the mesobronchus. If this interpretation is correct, birds may have active control over the efficiency of the expiratory aerodynamic valve. Such a mechanism could be useful in avoiding hyperventilation during the rapid breathing cycles of song (Hartley and Suthers, 1989; Franz and Goller, 2003).
FOOTNOTES
COMPETING INTERESTS
No competing interests declared.
FUNDING
This work was supported by National Institutes of Health grants [DC 04390, DC 06876 to F.G.]. Deposited in PMC for release after 12 months.
REFERENCES
- Banzett R. B., Butler J. P., Nations C. S., Barnas G. M., Lehr J. L., Jones J. H. (1987). Inspiratory aerodynamic valving in goose lungs depends on gas density and velocity. Respir. Physiol. 70, 287-300 [DOI] [PubMed] [Google Scholar]
- Beckers G. J. L., Suthers R. A., ten Cate C. (2003). Mechanisms of frequency and amplitude modulation in ring dove song. J. Exp. Biol. 206, 1833-1843 [DOI] [PubMed] [Google Scholar]
- Boggs D. F., Seveyka J. J., Kilgore D. L., Jr, Dial K. P. (1997). Coordination of respiratory cycles with wingbeat cycles in the black-billed magpie (Pica pica). J. Exp. Biol. 200, 1413-1420 [DOI] [PubMed] [Google Scholar]
- Boggs D. F., Butler P. J., Wallace S. E. (1998). Differential air sac pressures in diving tufted ducks Aythya fuligula. J. Exp. Biol. 201, 2665-2668 [DOI] [PubMed] [Google Scholar]
- Boggs D. F., Baudinette R. V., Frappell P. B., Butler P. J. (2001). The influence of locomotion on air-sac pressures in little penguins. J. Exp. Biol. 204, 3581-3586 [DOI] [PubMed] [Google Scholar]
- Brackenbury J. (1971). Airflow dynamics in the avian lung as determined by direct and indirect methods. Respir. Physiol. 13, 319-329 [DOI] [PubMed] [Google Scholar]
- Brackenbury J. H. (1972). Lung-air-sac anatomy and respiratory pressures in the bird. J. Exp. Biol. 57, 543-550 [DOI] [PubMed] [Google Scholar]
- Brackenbury J. H. (1973). Respiratory mechanics in the bird. Comp. Biochem. Physiol. 44A, 599-611 [DOI] [PubMed] [Google Scholar]
- Bretz W. L., Schmidt-Nielsen K. (1971). Bird respiration: flow patterns in the duck lung. J. Exp. Biol. 54, 103-118 [DOI] [PubMed] [Google Scholar]
- Bretz W. L., Schmidt-Nielsen K. (1972). The movement of gas in the respiratory system of the duck. J. Exp. Biol. 56, 57-65 [Google Scholar]
- Brown R. E., Kovacs C. E., Butler J. P., Wang N., Lehr J., Banzett R. B. (1995). The avian lung: is there an aerodynamic expiratory valve? J. Exp. Biol. 198, 2349-2357 [DOI] [PubMed] [Google Scholar]
- Butler J. P., Banzett R. B., Fredberg J. J. (1988). Inspiratory valving in avian bronchi: aerodynamic considerations. Respir. Physiol. 72, 241-255 [DOI] [PubMed] [Google Scholar]
- Duncker H.-R. (1971). The lung air sac system of birds. A contribution to the functional anatomy of the respiratory apparatus. Ergeb. Anat. Entwicklungsgesch. 45, 7-171 [PubMed] [Google Scholar]
- Duncker H.-R. (2000). Der Atemapparat der Vögel und ihre lokomotorische und metabolische Leistungsfähigkeit. J. Ornithol. 141, 1-67 [Google Scholar]
- Franz M., Goller F. (2003). Respiratory patterns and oxygen consumption in singing zebra finches. J. Exp. Biol. 206, 967-978 [DOI] [PubMed] [Google Scholar]
- Goller F., Cooper B. (2008). Peripheral sensorimotor mechanisms and the control of song. In The Neuroscience of Birdsong (ed. Zeigler H. P., Marler P.), pp. 99-114 Cambridge: Cambridge University Press; [Google Scholar]
- Hartley R. S., Suthers R. A. (1989). Airflow and pressure during canary song: direct evidence for mini-breaths. J. Comp. Physiol. A 165, 15-26 [Google Scholar]
- King A. S., Molony V. (1971). The anatomy of respiration. In Physiology and Biochemistry of the Domestic Fowl (ed. Bell D. J., Freeman B. M.), pp. 93-169 New York, NY: Academic Press; [Google Scholar]
- Kuethe D. O. (1988). Fluid mechanical valving of air flow in bird lungs. J. Exp. Biol. 136, 1-12 [DOI] [PubMed] [Google Scholar]
- Lasiewski R. C., Calder W. A., Jr (1971). A preliminary allometric analysis of respiratory variables in resting birds. Respir. Physiol. 11, 152-166 [DOI] [PubMed] [Google Scholar]
- Maina J. N. (2000). Comparative respiratory morphology: themes and principles in the design and construction of the gas exchangers. Anat. Rec. 261, 25-44 [DOI] [PubMed] [Google Scholar]
- Maina J. N. (2005). The Lung-Air Sac System of Birds. Berlin: Springer; [Google Scholar]
- Maina J. N., Africa M. (2000). Inspiratory aerodynamic valving in the avian lung: functional morphology of the extrapulmonary primary bronchus. J. Exp. Biol. 203, 2865-2876 [DOI] [PubMed] [Google Scholar]
- Scheid P., Piiper J. (1989). Respiratory mechanics and air flow in birds. In Form and Function in Birds (ed. King A. S., McLelland J.), pp. 369-391 London: Academic Press; [Google Scholar]
- Scheid P., Slama H., Piiper J. (1972). Mechanisms of unidirectional flow in parabronchi of avian lungs: measurements in duck lung preparations. Respir. Physiol. 14, 83-95 [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielsen K., Kanwisher J., Lasiewski R. C., Cohn J. E., Bretz W. L. (1969). Temperature regulation and respiration in the ostrich. Condor 71, 341-352 [Google Scholar]
- Scott G. R., Meir J. U., Hawkes L. A., Frappell P. B., Milsom W. K. (2011). Point: high altitude is for the birds! J. Appl. Physiol. 111, 1514-1515 [DOI] [PubMed] [Google Scholar]
- Suthers R. A., Goller F. (1998). Motor correlates of vocal diversity in songbirds. In Current Ornithology, Vol. 14 (ed. Nolan V., Ketterson E. D., Thompson C. F.), pp. 235-288 New York, NY: Plenum Press; [Google Scholar]
- Suthers R. A., Zollinger S. A. (2008). From brain to song: the vocal organ and vocal tract. In Neuroscience of Birdsong (ed. Zeigler H. P., Marler P.), pp. 78-98 Cambridge: Cambridge University Press; [Google Scholar]
- Suthers R. A., Goller F., Pytte C. (1999). The neuromuscular control of birdsong. Philos. Trans. R. Soc. B 354, 927-939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker V. A. (1968). Respiratory physiology of house sparrows in relation to high-altitude flight. J. Exp. Biol. 48, 55-66 [DOI] [PubMed] [Google Scholar]
- Wang N., Banzett R. B., Nations C. S., Jenkins F. A., Jr (1992). An aerodynamic valve in the avian primary bronchus. J. Exp. Zool. 262, 441-445 [DOI] [PubMed] [Google Scholar]
- Zeuthen E. (1942). The ventilation of the respiratory tract in birds. K. Danske Vidensk. Selsk. Biol. Medd. 17, 1-50 [Google Scholar]