SUMMARY
Humans and songbirds are among the rare animal groups that exhibit socially learned vocalizations: speech and song, respectively. These vocal-learning capacities share a reliance on audition and cortico-basal ganglia circuitry, as well as neurogenetic mechanisms. Notably, the transcription factors Forkhead box proteins 1 and 2 (FoxP1, FoxP2) exhibit similar expression patterns in the cortex and basal ganglia of humans and the zebra finch species of songbird, among other brain regions. Mutations in either gene are associated with language disorders in humans. Experimental knock-down of FoxP2 in the basal ganglia song control region Area X during song development leads to imprecise copying of tutor songs. Moreover, FoxP2 levels decrease naturally within Area X when zebra finches sing. Here, we examined neural expression patterns of FoxP1 and FoxP2 mRNA in adult Bengalese finches, a songbird species whose songs exhibit greater sequence complexity and increased reliance on audition for maintaining their quality. We found that FoxP1 and FoxP2 expression in Bengalese finches is similar to that in zebra finches, including strong mRNA signals for both factors in multiple song control nuclei and enhancement of FoxP1 in these regions relative to surrounding brain tissue. As with zebra finches, when Bengalese finches sing, FoxP2 is behaviorally downregulated within basal ganglia Area X over a similar time course, and expression negatively correlates with the amount of singing. This study confirms that in multiple songbird species, FoxP1 expression highlights song control regions, and regulation of FoxP2 is associated with motor control of song.
KEY WORDS: Bengalese finch, basal ganglia, birdsong, variability, zebra finch
INTRODUCTION
The importance of the FOXP subfamily of transcription factors in the brain was not clear until FOXP2 was identified as the monogenetic locus of a speech and language abnormality. Half of the members of a British pedigree, known as the KE family, suffer from a rare communication disorder. Affected members share a single mutation in FOXP2 that causes a severe impairment in the selection and sequencing of fine orofacial movements (Lai et al., 2001; Vargha-Khadem et al., 1998). In addition to articulatory problems, affected individuals have profound deficits in production and comprehension of word inflections and syntactical structure (Alcock et al., 2000; Watkins et al., 2002). The phenotype resulting from its mutation indicates that FOXP2 is linked to neural pathways underlying speech and language.
FOXP1 is the closest forkhead family member to FOXP2, with which it shares high similarity at the amino acid level (68% identity and 80% similarity between the two human sequences). FOXP1 can heterodimerize with FOXP2 and can repress transcription of similar groups of genes (Li et al., 2004; Shu et al., 2001; Wang et al., 2003). FOXP1 is also associated with speech and language through multiple cases (Carr et al., 2010; Hamdan et al., 2010; Horn et al., 2010). For example, a patient with a genetic deletion restricted to FOXP1 exhibits difficulties with verbal expression resembling the phenotype of affected KE family members (Pariani et al., 2009). Besides humans (Homo sapiens), no taxon of primates is capable of substantially modifying its vocal repertoire in response to experience. Moreover, most laboratory animals, including rodents, do not learn a substantial portion of their vocalizations (Kikusui et al., 2011; Arriaga et al., 2012; Mahrt et al., 2013). In striking contrast, thousands of songbird species share the trait of vocal learning with humans, enabling comparison of brain–behavior relationships among these taxa. Zebra finches (Taeniopygia guttata Reichenbach 1862) are a well-studied songbird species in which song learning is sexually dimorphic: juvenile males learn their courtship songs from adult male conspecifics (tutors) whereas females do not produce learned songs. Zebra finch song is composed of notes, syllables, motifs and bouts. Notes are the smallest unit of song and are defined as a region of a syllable that maintains a temporally continuous frequency pattern. Syllables are composed of one or more notes bounded by a brief period of silence. Motifs are repeated sequences of syllables lasting ~1 s with multiple motifs in succession organized in a bout. Bouts are composed of several motifs bounded by a longer period of silence (Brenowitz et al., 1997; Price, 1979).
Male, but not female, zebra finches possess the full and interconnected suite of cortico-basal ganglia nuclei that underlies song learning and production. Song control circuitry includes the anterior forebrain pathway (AFP), which is important for song learning in juveniles and song maintenance and plasticity in adults, and the posterior descending pathway, which is required for song production (Scharff and Nottebohm, 1991; Brainard and Doupe, 2000; Kao et al., 2005). Neurons in the HVC (acronym used as a proper name), a premotor vocal control nucleus, directly project to the robust nucleus of the arcopallium (RA) (Nottebohm et al., 1976; Nottebohm, 2005) and indirectly project to the RA through basal ganglia nucleus Area X, the medial nucleus of the dorsolateral thalamus, and the lateral magnocellular nucleus of anterior nidopallium (LMAN) in the AFP. The AFP is homologous to basal ganglia-thalamo-cortical circuit loops in mammals. Area X shares many features characteristic of the mammalian striatum and pallidum, including cell types and connectivity (Gale and Perkel, 2010).
Songbirds and humans also share neurogenetic mechanisms that underlie their vocal learning capacities. FoxP1 and FoxP2 exhibit similar expression patterns in the cortex and basal ganglia of humans and zebra finches (Teramitsu et al., 2004). Knock-down of FoxP2 in Area X of juvenile zebra finches leads to imprecise copying of the tutor song, suggesting that FoxP2 is involved in the normal process of vocal learning (Haesler et al., 2007). Moreover, Area X FoxP2 is behaviorally and socially regulated. Non-singing zebra finches have high levels of Area X FoxP2 that decline acutely when males practice their songs alone (termed undirected singing) in the morning, but not when they sing to females (directed singing) (Teramitsu and White, 2006; Hilliard et al., 2012). The downregulation of FoxP2 during undirected singing is particularly robust in juvenile zebra finches undergoing sensorimotor learning: the more they practice, the lower their Area X FoxP2 levels. Interestingly, hearing is required to maintain this negative correlation (Teramitsu et al., 2010). Moreover, coincident with decreased FoxP2, vocal variability increases after 2 h of undirected singing in both juvenile and adult zebra finches (Hilliard et al., 2012; Miller et al., 2010). These observations have led us to hypothesize that singing-driven decreases in Area X FoxP2 levels promote vocal variability and motor exploration whereas high levels promote song stabilization (Miller et al., 2010).
Here, we further test the relationship between learned vocal behaviors and FoxP1 and FoxP2 gene expression by examining another songbird species, the Bengalese finch (Lonchura striata domestica Linnaeus 1758), in which song learning and song control circuitry are also sexually dimorphic, but whose song exhibits features that are distinct from zebra finch song. Adult male zebra finches sing a linear song sequence and thus exhibit a very simple birdsong ‘syntax’, whereas male Bengalese finches generate songs with greater syntactical complexity (Okanoya, 2004). After deafening, the songs of Bengalese finches degrade faster than those of zebra finches (Okanoya and Yamaguchi, 1997; Woolley and Rubel, 1997), indicating a greater reliance on audition for their song maintenance. These observations suggest that singing-driven decreases in Area X FoxP2 levels might be more robust in Bengalese finches than in zebra finches. As a consequence, increases in song variability following song practice might be evident in adult male Bengalese finches.
We therefore tested the following hypotheses: (1) Bengalese finches and zebra finches share similar FoxP1 and FoxP2 gene expression patterns; (2) FoxP2 mRNA is behaviorally regulated in male Bengalese finches; (3) downregulation of FoxP2 within Area X is correlated with the amount of undirected singing in both species; (4) the singing-driven regulation of FoxP2 within Area X of Bengalese finches is more profound than in zebra finches; and (5) vocal practice promotes song variability in adult male Bengalese finches.
MATERIALS AND METHODS
We conducted in situ hybridization on brain tissue from Bengalese finches and zebra finches of both sexes under different behavioral conditions to investigate FoxP gene expression patterns, the time course of downregulation of FoxP2, and the relationship between amount of singing and FoxP2 levels within Area X. A separate group of adult male Bengalese finches was used to investigate song variability following two different behavioral conditions known to alter Area X FoxP2 levels (Fig. 1).
Fig. 1.
Timelines for the behavioral groups used in this study. (A) Experimental design for time-course analysis of FoxP1 and FoxP2 behavioral regulation. On the day of the experiment, female birds remained alone in sound attenuation chambers for 2 h (green bar). NS males were discouraged from singing by the experimenter sitting nearby for 2 h (black bar). UD males sang alone in the isolation chamber for variable periods of time (red bars). Arrows indicate the time points at which birds were killed. (B) Experimental design for song variability analysis. Songs sung after the 2 h time point were analyzed for song variability. Birds were not killed in this experiment.
Animals and tissues
All animal use was in accordance with National Institutes of Health guidelines for experiments involving vertebrate animals and approved by the Institutional Animal Care and Use Committee of the University of California, Los Angeles. Adult male and female zebra finches and Bengalese finches (age >120 days) were taken from our breeding colony (where they were kept under a 13 h:11 h light:dark cycle). After behavioral monitoring (see below), birds were decapitated for collection of brains, which were rapidly extracted and frozen on aluminum floats on liquid nitrogen, then stored at −80°C until use.
Riboprobe preparation and in situ hybridization analysis
FoxP genes are highly conserved among such disparate avian species as zebra finches and chickens (FoxP1: zebra finch versus chicken, identities=95%; FoxP2: zebra finch versus chicken, identities=97%). Although the genome of Bengalese finches is not yet available, the similarity of their FoxP genes to zebra finch sequences is expected to be even higher based on their closer phylogenetic relationship. We therefore used riboprobes directed against zebra finch FoxP1 and FoxP2 (Teramitsu et al., 2004) to detect these transcripts in both species. The FoxP1 probe was designed to hybridize to the coding region upstream of the zinc finger domain of zebra finch FoxP1, corresponding to 661–998 bp of human FOXP1 relative to the start codon. The FoxP2 probe was designed to hybridize to 1870–2127 bp of the zebra finch FoxP2 relative to the start codon. pCR4-TOPO vector (Invitrogen, Carlsbad, CA, USA) with zebra finch FoxP cDNA fragments was used for in vitro transcription to generate sense and antisense RNA probes labeled with [33P]UTP (Perkin-Elmer, Foster City, CA, USA) using the Riboprobe Combination System-T3/T7 (Promega, Madison, WI, USA).
Frozen brains were cryosectioned in either the sagittal or coronal plane at 20 μm and adjacent sections were mounted onto 25×75 mm slides (Superfrost, Fisher Scientific, Pittsburgh, PA, USA) in a manner that created seven replicate sets. One set was stained with thionin to enable identification of neuroanatomical structures. The adjacent four sets were exposed to the FoxP1 sense, FoxP1 antisense, FoxP2 sense or FoxP2 antisense probes. In situ hybridizations were performed and signals from different brain regions were quantified as previously described (Teramitsu et al., 2004; Teramitsu and White, 2006; Teramitsu et al., 2010). Sections of Bengalese finches were run aligned with sections of zebra finches from the same behavioral conditions to enable direct comparisons. Preliminary analysis of Bengalese finch sections revealed that: (1) the distinct expression patterns between brains exposed to either FoxP1 or FoxP2 antisense probes were as expected based on prior studies, (2) signals from antisense probes were robust whereas those from sense probes were negligible, and (3) signals were consistent across adjacent brain sections. These results provide confidence that riboprobes designed from zebra finch cDNA also specifically detect FoxP1 and FoxP2 in Bengalese finch brain.
Behavioral monitoring and sound recording
Birds were housed individually in sound attenuation chambers (Acoustic Systems, Austin, TX, USA) for 2–3 days prior to the behavioral experiments to enable acclimation to the recording environment. Sounds were recorded using Countryman EMW omnidirectional lavalier microphones (Countryman Associates, Menlo Park, CA, USA) and digitized using a PreSonus Firepod (44.1 kHz sampling rate, 24 bit depth; Baton Rouge, LA, USA). Recordings were acquired using Sound Analysis Pro (SAP) 2011 software (Tchernichovski et al., 2000).
Behavioral experiments were conducted between 08:00 and 11:00 h, starting at lights on. For FoxP gene analysis, birds were killed following the completion of different behavioral paradigms, which are illustrated in Fig. 1A and described as follows. Female birds were left alone and undisturbed inside the chamber for 2 h after lights on. Non-singing males (referred to as NS; Fig. 1A) were also left alone for 2 h after lights on, but with the door to the chamber ajar. If they appeared to attempt to sing, they were distracted by the experimenter. Those that sang more than five motifs despite the experimenter's presence were excluded from this group. Of note, we previously found that the non-singing paradigm did not lead to detectable changes in zebra finch stress levels as measured by serum corticosterone values (Miller et al., 2008). In addition, Area X gene expression patterns from birds that were distracted from singing by an experimenter clustered together with patterns from birds that sang very little by their own volition. This suggests that singing behavior – and not the absence or presence of the experimenter – is the more crucial determinant of gene expression in Area X (Hilliard et al., 2012). Males singing undirected song (referred to as UD; Fig. 1A) were allowed to sing alone inside the chamber for a pre-determined period of time – 1, 1.5 or 2 h after the first song in the morning. For analysis of song variability, a separate set of birds was used for which the behavioral conditions are illustrated in Fig. 1B. One group of male birds (N=6) was kept from singing for 2 h and then allowed to sing undirected songs. Songs sung during the subsequent 20 min (termed NS-UD songs) were analyzed. On another day, the same group of male birds was allowed to sing undisturbed for 2 h, and then songs that were sung in the subsequent 20 min (termed UD-UD) were analyzed.
Quantification of the amount of singing
Audio files generated by SAP were edited with Audacity 1.3 Beta (http://audacity.sourceforge.net) by manual removal of cage noise and calls, leaving only songs. In our previous study on zebra finches, the amount of singing was quantified by counting the number of motifs (Teramitsu and White, 2006). However, there is considerable variability in phonology and macroscopic song structure both within and between the two songbird species studied here (Fig. 2A). The greater syntactical variability in Bengalese finch song makes it challenging to identify their motifs (Fig. 2B). Moreover, the length of the motifs varies among different Bengalese finches and between the two songbird species. To minimize error and avoid introducing bias by manually identifying song motifs, we used SAP to automatically measure the length of each song syllable. Syllables were segmented using experimenter-derived amplitude thresholds in SAP, and then run through the ‘Feature Batch’ module, which computes the duration of each syllable in the batch. The total amount of singing was then defined as the sum of the durations of all syllables identified for a given time period.
Fig. 2.
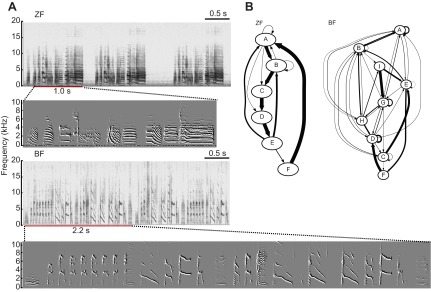
Representative exemplars of zebra and Bengalese finch song. (A) Spectrograms from a male zebra finch (ZF, top) and a male Bengalese finch (BF, middle) are shown. The red bar underneath each spectrogram indicates the length of one motif. Spectral derivatives of these motifs are shown underneath each spectrogram. (B) Markov chains generated from zebra finch and Bengalese finch songs. Letters denote syllables. Arrows represent the probability of syllable transitions. Thicker arrows indicate greater probabilities.
Quantification of FoxP gene expression
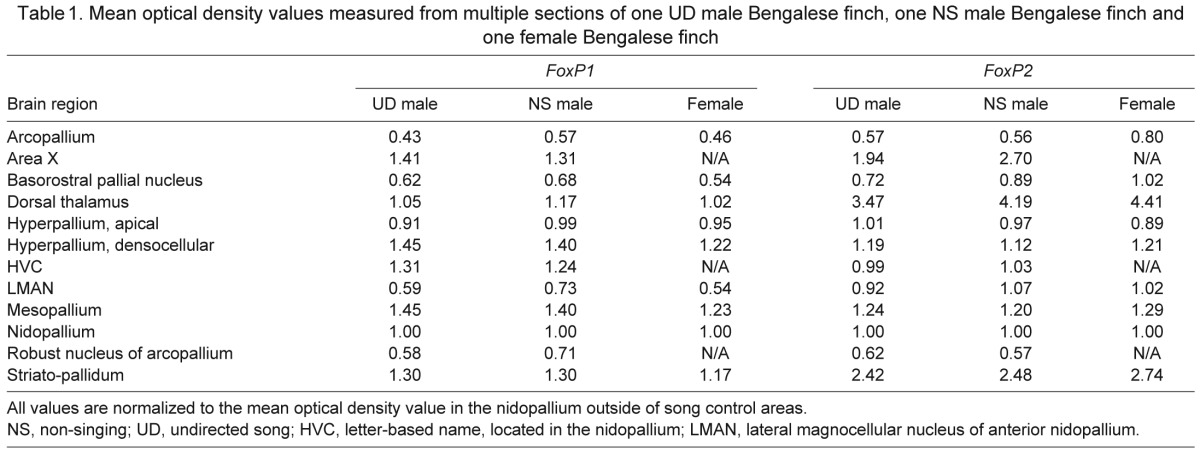
For semi-quantitative and quantitative analyses, optical density (OD) measurements of FoxP signals were obtained from digitized images of autoradiograms using Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA, USA). First, to provide a qualitative comparison of gene expression levels across brain regions, OD values from each region were calculated from multiple sagittal sections of the brains of one 2 h UD Bengalese male, one 2 h NS Bengalese male (shown in Fig. 3) and one Bengalese female (shown in Fig. 4). All OD values were normalized to those from a nidopallial area of the same section that did not contain any song control nuclei. Values are reported in Table 1. For quantitative analysis of Area X FoxP2 levels, OD values from within Area X were normalized to those from the ventral striato-pallidum (VSP), as previously described (Teramitsu and White, 2006). To determine the statistical significance of the Area X FoxP2 levels, a resampling procedure was employed as follows: 10,000 hypothetical data sets of the same size were resampled from the actual normalized OD values and the amount of singing in the experiments. For each resampled data set, a slope of the linear regression of these variables (OD versus amount of singing) was calculated, generating a distribution of 10,000 slopes for each species. A correlation was determined to be significantly negative if the upper and lower boundaries of the 95% confidence interval for the distribution of slopes were negative.
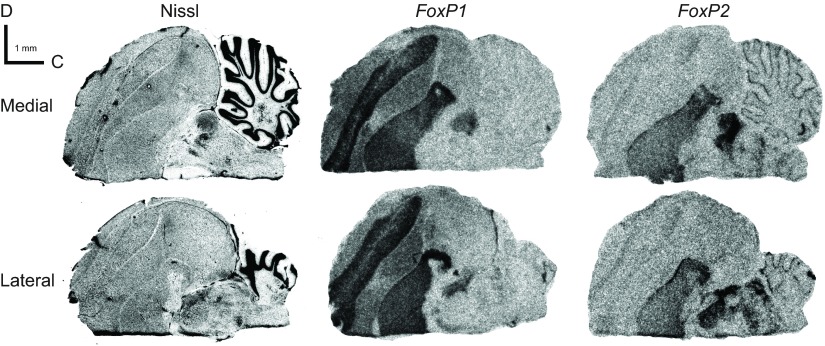
Fig. 3.

Representative brightfield photomicrographs of FoxP1 and FoxP2 mRNA expression patterns in a series of sagittal sections from one 2 h NS (left) and one 2 h UD (right) adult male Bengalese finch brain. Both medial and lateral sections are shown to enable display of the song control nuclei investigated here. (A) Nissl-stained sagittal sections. Locations of medial and lateral sections correspond to the level of sagittal plates 6 and 11, respectively, in the zebra finch atlas of Nixdorf-Bergweiler and Bischof (Nixdorf-Bergweiler and Bischof, 2007). (B) Schematic drawings based on the Nissl stains. A, arcopallium; Bas, basorostral pallial nucleus; HA, apical part of the hyperpallium; HD, densocellular part of the hyperpallium; LMAN, lateral magnocellular nucleus of anterior nidopallium; M, mesopallium; N, nidopallium; NC, caudal nidopallium; RA, robust nucleus of arcopallium; StL, lateral striatum; StM, medial striatum. (C) FoxP1 mRNA signals. (D) FoxP2 mRNA signals. Medial sections in A, C and D were adjacent or near adjacent to one another; similarly, lateral sections were adjacent or near adjacent. D, dorsal; C, caudal.
Fig. 4.
Representative brightfield photomicrographs of FoxP1 and FoxP2 mRNA expression patterns in a pair of sagittal sections from adult female Bengalese finch brain. Locations of medial and lateral sections correspond to the level of sagittal plates 6 and 11, respectively, in the zebra finch atlas of Nixdorf-Bergweiler and Bischof (Nixdorf-Bergweiler and Bischof, 2007). The medial plate shows the HVC and LMAN, and the lateral plate shows the HVC and RA in corresponding sections from male birds (Fig. 3).
Table 1.
Mean optical density values measured from multiple sections of one UD male Bengalese finch, one NS male Bengalese finch and one female Bengalese finch
Syllable identification and clustering
All syllable clustering and sequence analysis was performed in the freely available R programming language (http://www.r-project.org) using custom-written clustering and syntax entropy scripts, available at the White laboratory website (https://www.ibp.ucla.edu/research/white/code.html).
To group syllables in an unbiased fashion and subsequently calculate syntax entropy, a hierarchical clustering and automated tree-trimming algorithm was utilized. Raw acoustic recordings from the first 20 min following NS or UD for each bird were subjected to SAP's ‘Feature Batch’, using experimenter-derived amplitude thresholds to segment syllables. A number of filtration steps were then applied to the ‘Feature Batch’ output to identify song syllables from non-song sounds (wing flaps, cage noise, etc.) captured by the recording software. An initial filtration step implemented user-defined duration thresholds above and below which all sounds were removed from the data set. Next, a maximum inter-syllable interval was determined by the experimenter for all remaining prospective syllables in the data set. Syllables that fell below this inter-syllable interval were grouped into prospective motifs/bouts. A filter to remove all motifs/bouts composed of two and/or three syllables was then applied. WAV files representing each motif/bout were generated and presented to the user for visual inspection, at which point motifs consisting of calls or non-song sounds in the recordings were removed from the data set if present. Finally, individual WAV files for all remaining syllables were generated.
Individual WAV files for both behavioral sessions for each animal were run against themselves in SAP's ‘Similarity Batch’ module in an M × N symmetric similarity batch. Upon completion of the batch, the product of the similarity and accuracy score for each syllable–syllable comparison was calculated and stored in a square matrix with rows and columns representing individual syllables and the elements of the matrix representing the product of the similarity and accuracy scores for a given syllable–syllable comparison. A distance matrix was then created by calculating the Euclidean distance between the product of similarity and accuracy scores for all syllable–syllable pairs. This distance matrix was used as the input to a hierarchical clustering function in the WGCNA R package (Langfelder and Horvath, 2008), generating a dendrogram. Branches of the dendrogram were then pruned using the dynamic tree-trimming algorithm, also in the WGCNA R package, a novel method for detecting clusters within hierarchical trees by considering the shape of the branches when trimming them into groups (Langfelder et al., 2008). Upon completion of cluster detection, each cluster was described by an ‘eigensyllable’, defined as the first principal component of the cluster as determined by singular value decomposition. The Pearson correlation between all module eigensyllables was then computed and clusters whose eigensyllables correlated above a user-defined threshold (in this case, 0.75) were merged, generating the final number of clusters/syllable types in each bird's song.
Final inspection of cluster homogeneity was performed by visual inspection of syllable spectrograms within each cluster. Syllables inappropriately assigned to a cluster were manually reassigned.
Syntax entropy
The syllable syntax, defined as the sequence in which the bird orders its syllables, was determined based on syllable cluster assignment in the preceding step. Syntax entropy was then calculated as described in Miller et al. (Miller et al., 2010). A string-based approach was utilized for syntax analysis, as motifs were often difficult to identify in Bengalese finch songs. Values for syllable syntax entropy reported are weighted entropy scores, which are adjusted for the frequency of occurrence of each syllable type when determining its contribution to overall syntactical entropy. A resampling paired t-test was utilized to assess the significance of change in syntax entropy scores between behavioral conditions for all birds as a group.
Similarity, accuracy, identity and syllable acoustic features
Upon completion of clustering, syllables within each cluster were divided into NS-UD and UD-UD groups. All syllable types that did not have at least 20 renditions sung in both behavioral contexts were removed from consideration in analysis of acoustic features. The range in the number of renditions for the remaining syllables that were analyzed was 55–762. A bootstrap one-way ANOVA was performed on similarity, accuracy and identity scores and all acoustic features within each bird to determine whether syllables were independent of one another. For all acoustic measures, the between-syllable difference P-value was less than 0.05, thus syllables were treated as independent of one another.
Resampling two-way ANOVAs were performed for each acoustic measure using syllables and behavioral condition as the two independent factors. F-statistics were generated for the actual data set and then compared with a distribution of 10,000 F-statistics calculated by resampling the original data under assumption of the null hypothesis to determine whether a syllable effect, a behavioral effect and/or an interaction between the two variables were present for each measure.
RESULTS
FoxP1 expression in Bengalese finch brain
FoxP1 mRNA signals indicated high expression levels in the densocellular part of the hyperpallium, the mesopallium, the striato-pallidum and the dorsal thalamus in both male (Fig. 3) and female (Fig. 4) Bengalese finches. In the basorostral pallial nucleus (Bas) and song control nucleus LMAN, FoxP1 expression was lower than in the surrounding nidopallium region regardless of sex (Fig. 3C, Fig. 4). In contrast, sexually dimorphic FoxP1 expression was observed in song control nuclei HVC, RA and striato-pallidal Area X, as the signals were greater in these nuclei relative to the respective surrounding brain tissue only in male Bengalese finches (Fig. 3C). In females, signals were similar across these sub-regions (Fig. 4). FoxP1 did not appear to be regulated by undirected singing in male Bengalese finches. Expression patterns from sagittal sections containing multiple song control regions were broadly similar between the 2 h NS and UD groups (Fig. 3C). A semi-quantitative summary of these observations is presented in Table 1. Coronal sections from a separate set of birds were used to focus on Area X and LMAN (Fig. 5), but again, no behavioral regulation of FoxP1 was observed.
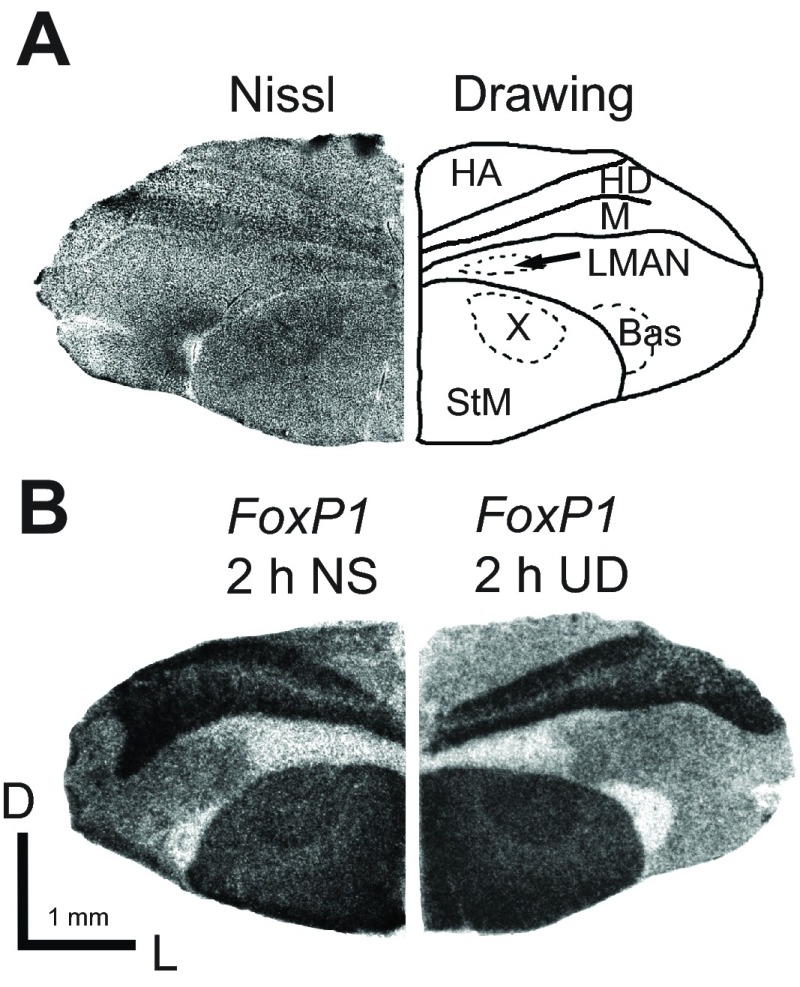
Fig. 5.
FoxP1 mRNA expression in Area X of adult male Bengalese finches. (A) Brightfield photomicrograph of Nissl-stained hemi-coronal section with schematic drawing highlights song nuclei LMAN and Area X. Abbreviations as in Fig. 3B. (B) Representative images of FoxP1 mRNA expression at the level of Area X in 2 h NS (left) and 2 h UD (right) adult male Bengalese finch brain. There is no apparent effect of singing on expression levels. Location of sections corresponds to the level of transverse plate 11 in the zebra finch atlas of Nixdorf-Bergweiler and Bischof (Nixdorf-Bergweiler and Bischof, 2007). D, dorsal; L, lateral.
FoxP2 expression in Bengalese finch brain
FoxP2 signals were lightly and uniformly distributed in cortical areas whereas they were robust in the striato-pallidum, the dorsal thalamus and the Purkinje cell layer of the cerebellum in both male (Fig. 3) and female (Fig. 4) Bengalese finches. No sexual dimorphism of FoxP2 expression was observed in any of the song control nuclei except for Area X. FoxP2 expression within Area X in female Bengalese finches was similar as that of the surrounding striato-pallidum (Fig. 4). FoxP2 expression in Area X of male Bengalese finches has reported to be lower than the surrounding striato-pallidum (Haesler et al., 2004). However, the behavioral condition of the birds used in that experiment was not specified. Here we present evidence that FoxP2 within Area X is comparable to or slightly higher than in the surrounding striato-pallidum in 2 h NS Bengalese finches but lower than in 2 h UD Bengalese finches (Fig. 3D). A semi-quantitative summary of these observations is presented in Table 1.
Behavioral regulation of FoxP2 within Bengalese finch Area X
In zebra finches, FoxP2 expression levels decline specifically within Area X when males engage in 2 h of UD singing in the morning (Hilliard et al., 2012; Teramitsu and White, 2006; Teramitsu et al., 2010). To determine whether similar singing-driven changes occur in a related songbird species with distinct song features, we examined FoxP2 expression in Area X of male Bengalese finches, in parallel with that in zebra finches, and compared levels between UD and NS conditions. To confirm the behavioral regulation of FoxP2 suggested in Fig. 2D, additional 2 h NS and 2 h UD male Bengalese finches were killed and brain tissues were sectioned coronally to display Area X bilaterally in the same section. The additional time points of 1 h UD and 1.5 h UD groups were utilized to track the time course of downregulation of FoxP2 within Area X during singing. We found that Area X FoxP2 levels were significantly downregulated at the 1.5 h UD and 2 h UD time points for both species (Fig. 6).
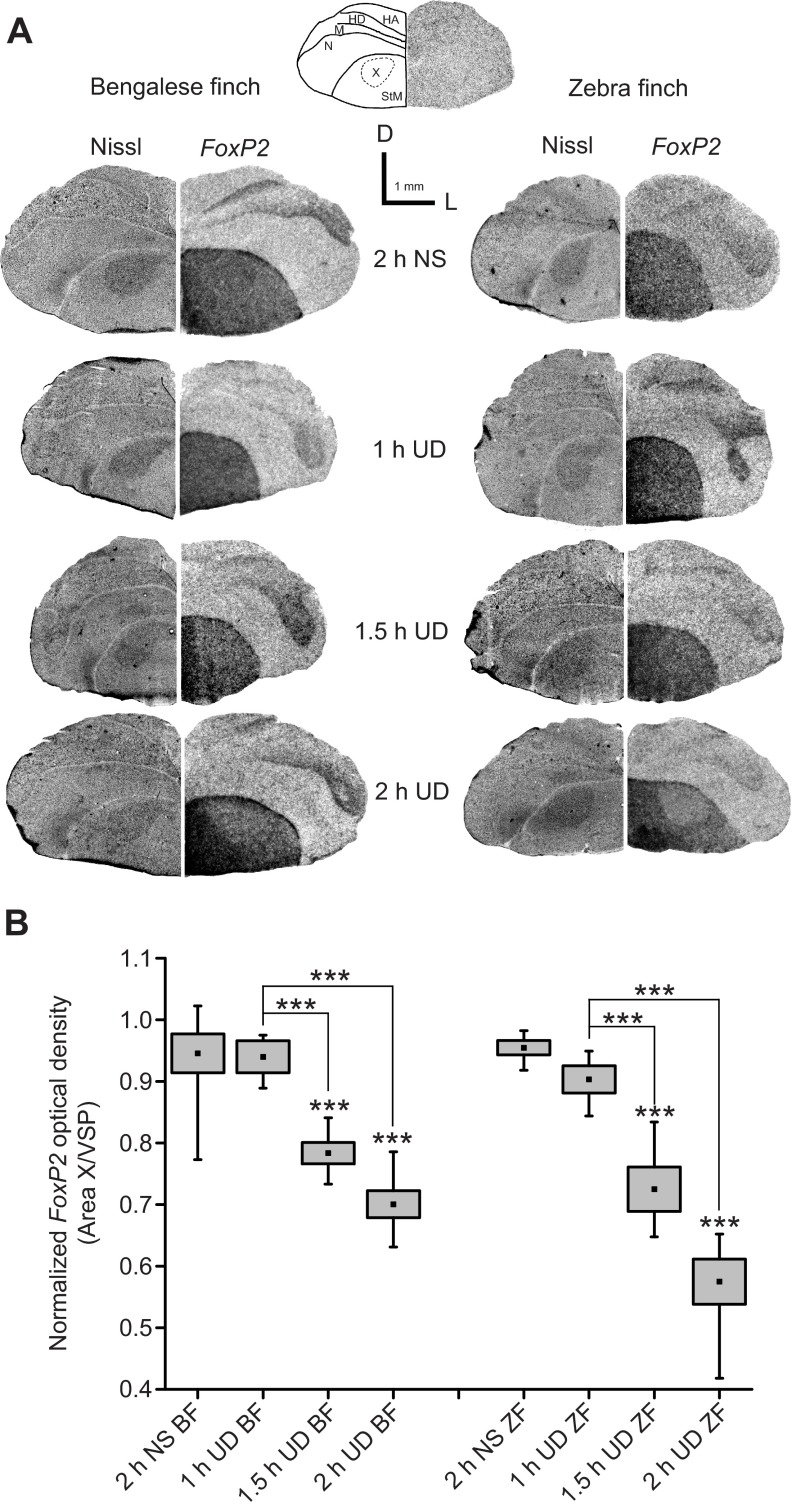
Fig. 6.
FoxP2 mRNA expression within Area X diminishes after birds sing undirected songs. (A) Top: schematic drawing based on a Nissl-stained hemi-coronal section shown with a control hemi-section incubated with sense RNA. Abbreviations as in Fig. 3B. Bottom: representative brightfield photomicrographs of FoxP2 mRNA expression patterns in hemi-coronal sections from Bengalese finches (left) and zebra finches (right) of different behavioral groups shown with corresponding Nissl-stained hemi-sections. D, dorsal; L, lateral. (B) Quantitative results of FoxP2 mRNA expression level within Area X relative to the ventral striato-pallidum (VSP). Boxes indicate s.e.m., points in boxes indicate means and whiskers indicate maximum and minimum values (2 h NS BF: N=7; 1 h UD BF: N=3; 1.5 h UD BF: N=6; 2 h UD BF: N=6; 2 h NS ZF: N=5; 1 h UD ZF: N=4; 1.5 h UD ZF: N=5; 2 h UD ZF: N=6; Kruskal–Wallis nonparametric ANOVA, ***P<0.001).
FoxP2 levels within Area X in 2 h UD Bengalese finches were significantly higher than those found in 2 h UD zebra finches (P<0.01). In order to interpret this difference, we measured the amount of singing in both groups. We found that zebra finches in our study sang more than Bengalese finches did (means ± s.e.m., Bengalese finch 351±53 s versus zebra finch 758±166 s, Kruskal–Wallis nonparametric test, P=0.040). Thus, the difference in FoxP2 levels between 2 h UD Bengalese finches and 2 h UD zebra finches could reflect the difference in the amount of singing. To explore this possibility, the relationship between the amount of singing and FoxP2 levels was further examined.
Correlation between FoxP2 levels and amount of singing
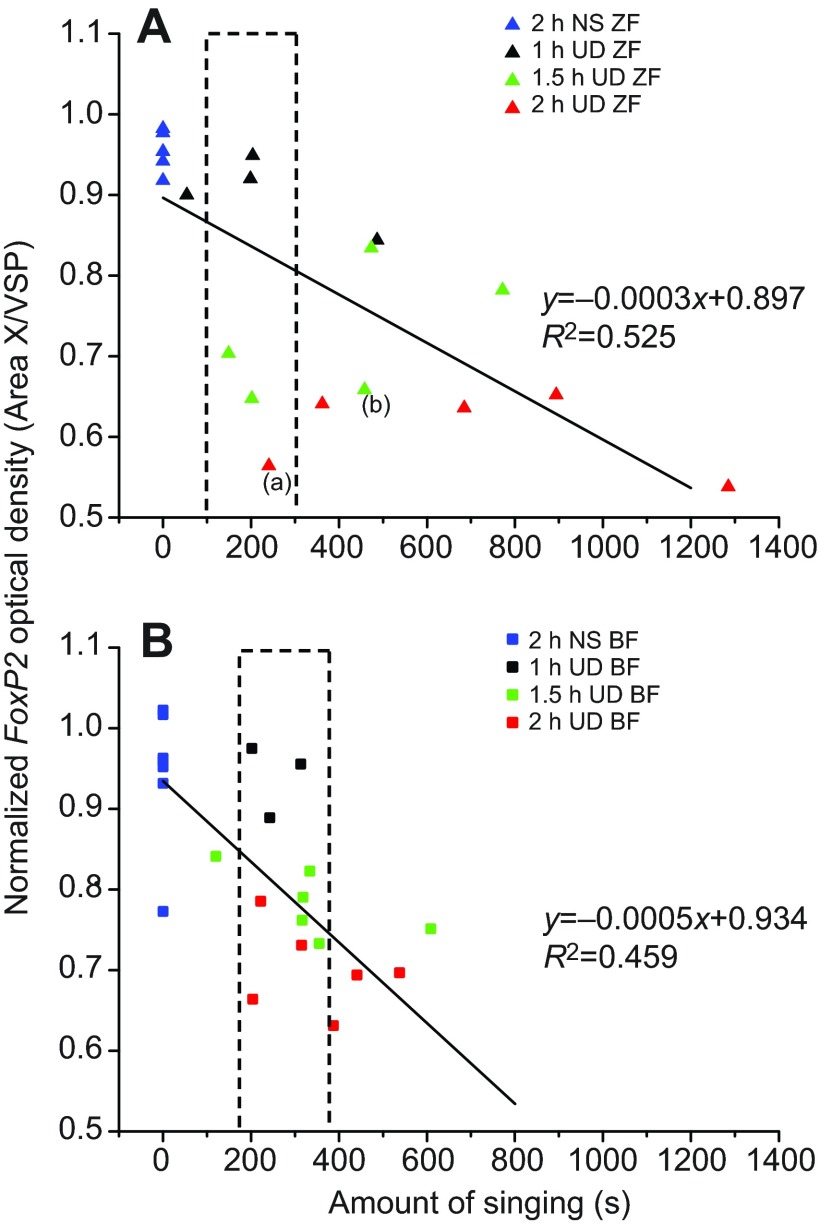
Area X FoxP2 levels were negatively correlated with the amount of singing in both zebra and Bengalese finches, as illustrated by the negative slope of the linear regression lines that were fit to the data from each species (zebra finch: P<0.0002; Bengalese finch: P<0.0003; Fig. 7). These results indicate that the more a given bird sang, the lower its Area X FoxP2 level. There was no statistically significant difference between the slopes of the two regression lines (P>0.05, see below), indicating that, contrary to our prediction, Bengalese finch FoxP2 levels within Area X are not more responsive to singing than those in zebra finches.
Fig. 7.
Correlation between FoxP2 and amount of singing. (A) In zebra finches, FoxP2 levels decrease as the amount of singing increases (P<0.0002). (B) FoxP2 levels also decrease as the amount of singing increases in Bengalese finches (P<0.0003). There is no significant difference between the two regression lines (P>0.05). The dotted rectangle indicates data from those birds that sang similar amounts of song for each species (see Discussion).
Song variability after vocal practice
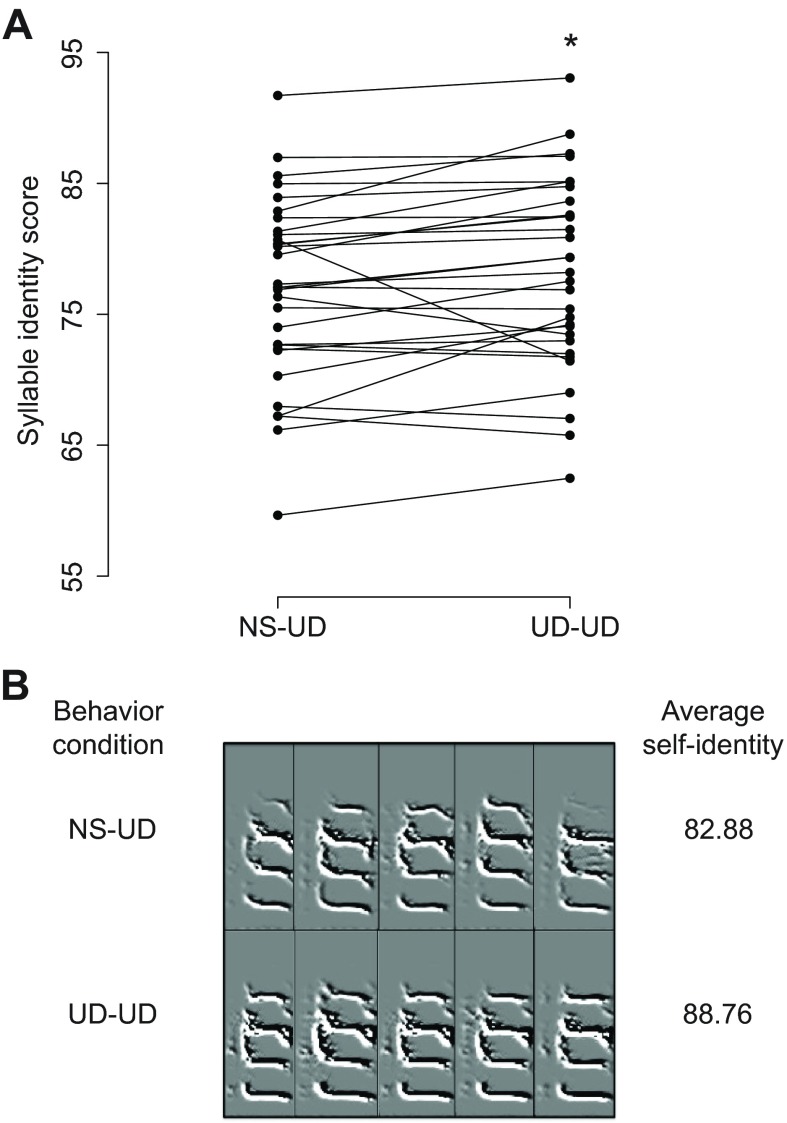
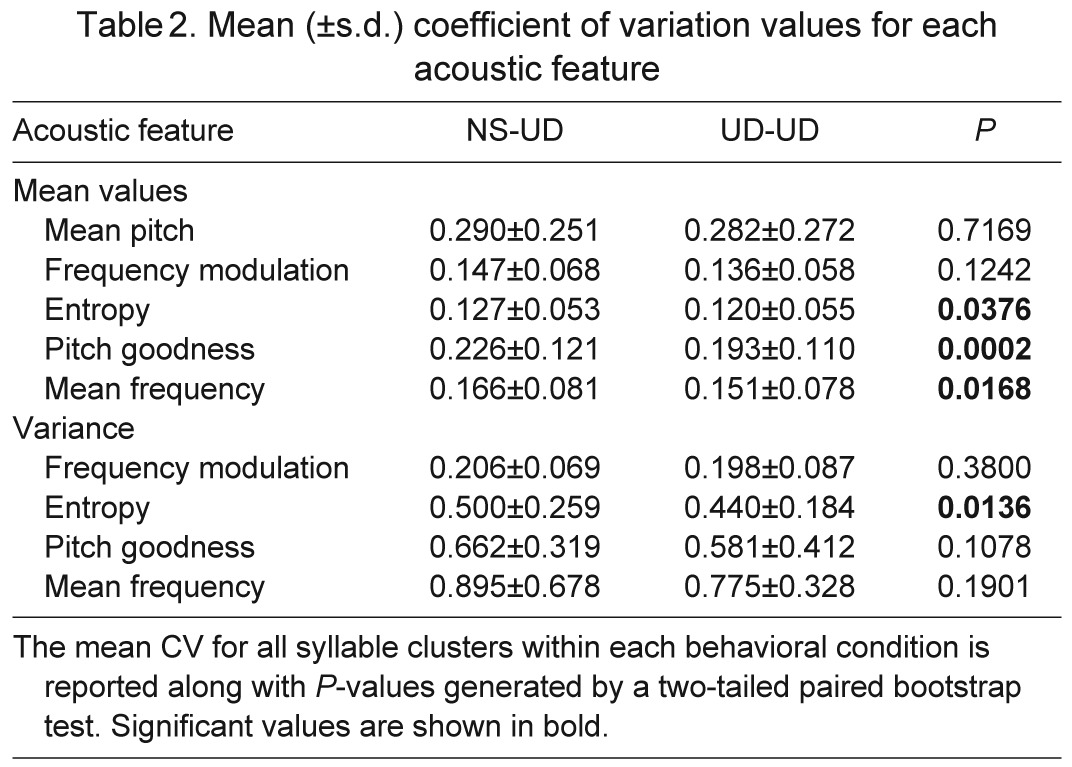
Songs that were sung by adult male Bengalese finches in the 20 min period immediately following a 2 h period of UD singing (UD-UD) were compared with those sung following 2 h of non-singing (NS-UD). One expectation is that there would be no difference between the behavioral conditions, based on prior work in zebra finches in which a difference was only observed in juveniles (Miller et al., 2010). The other expectation is that variability after UD-UD singing would be increased relative to the NS-UD conditions, based on the overall greater variability in Bengalese song and its strong dependence on hearing. In line with a majority of our predictions, we found that for many phonological and sequential measures of song variability there were no differences between the two conditions. However, on certain measures, a slight decrease in variability was observed in the UD-UD condition relative to the NS-UD condition, in contrast to our predictions. To describe syllable variability, we examined the average within-group similarity, accuracy and syllable identity (similarity × accuracy/100) of all syllables within a cluster analyzed as a function of behavioral condition. The variability of syllable identity (P=0.034; Fig. 8) was lower in the UD-UD condition, reflecting similar trends in similarity (P=0.080) and accuracy (P=0.075). We next examined the mean coefficient of variance (CV) for all syllables within a cluster. Again contrary to our predictions, the CV was lower in the UD-UD condition for individual syllable features of pitch goodness (P=0.0002), Wiener entropy (P=0.004) and mean frequency (P=0.017; Table 2). A two-way ANOVA revealed that there was no effect of behavioral condition on the mean values for any of these features. Finally, we utilized entropy-based methods similar to those of Miller et al. (Miller et al., 2010) to measure syntax variability, investigating all syllables produced during the 20 min following each behavioral condition using a string-based analysis described in that study. The results indicate no significant difference in syntax entropy between the two behavioral conditions (average NS-UD entropy=0.185, average UD-UD entropy=0.168; P>0.05), similar to our prior findings in adult zebra finches.
Fig. 8.
Behavioral changes in syllable self-identity. (A) Paired plot of syllable cluster self-identity in NS-UD and UD-UD conditions. The UD-UD condition had higher mean self-identity (*P=0.034, two-tailed paired bootstrap). (B) Representative spectral derivatives of five syllables from one cluster in the NS-UD and UD-UD conditions with self-identity scores reported.
Table 2.
Mean (±s.d.) coefficient of variation values for each acoustic feature
DISCUSSION
Sexually dimorphic expression of FoxP1 in songbirds
In line with our expectations, the brain expression patterns of FoxP1 and FoxP2 in Bengalese finches are broadly consistent with those previously described in zebra finches (Teramitsu et al., 2004), including strong mRNA signals for both factors in multiple song control nuclei and enhancement of FoxP1 in HVC and Area X relative to surrounding brain tissue. One apparent difference between the two species was in the arcopallial song control region, the RA. FoxP1 in the RA of female zebra finches is higher relative to the surrounding brain tissue (Teramitsu et al., 2004), but this enhancement was not prominent in coronal sections of a female Bengalese finch brain (data not shown) and was not detected in sagittal sections of another female Bengalese finch (Fig. 4). Whether this is a true species difference is unclear because we were unable to detect RA in the Nissl-stained female Bengalese finch sections, despite its visibility in sections from male brains subjected to the same staining conditions (Fig. 3). As previously reported in zebra finches (Teramitsu et al., 2004), the RA of male Bengalese finches exhibited FoxP1 signals that were slightly higher than those of the surrounding arcopallium. Projection neurons of the RA synapse directly onto the motor neurons that innervate the muscles of phonation, similar to direct projections of layer V motor cortical neurons onto laryngeal motor neurons in humans, and are thought to enable the capacity for vocal learning (Jürgens, 2009; Arriaga et al., 2012). In the spinal cord, FOXP1 plays a crucial role in defining the columnar identity of motor neurons at each axial position, as well as organizing motor axon projections (Rousso et al., 2008). Similarly, FoxP1 may organize the RA cortical motor neuron projection to syringeal and respiratory motor neurons in songbirds.
With regard to other telencephalic song control regions, enhanced expression of FoxP1 in the HVC and Area X in male, but not female, Bengalese finches mirrors the zebra finch expression pattern. There is no evidence for singing-driven regulation for FoxP1 expression in either adult Bengalese or zebra finch brains (Figs 3, 5, Table 1). The sexually dimorphic expression of FoxP1 in song control areas (HVC, male RA, Area X), together with the speech and language deficits associated with its mutation in humans (Carr et al., 2010; Hamdan et al., 2010; Horn et al., 2010; Pariani et al., 2009), suggest that FoxP1 plays a role in the formation of song circuitry dedicated to singing behavior.
The expression of FoxP1 within the LMAN and Bas in Bengalese finches is low relative to the surrounding tissue, and does not exhibit sexually dimorphic patterns or singing-driven regulation. The Bas is involved in feeding and oral-manipulative behaviors other than vocalization and does not anatomically connect to the vocal control system in songbirds (Wild and Farabaugh, 1996). Because both male and female finches engage in oral movements related to feeding behavior, it is not surprising that FoxP1 levels in the Bas are similar in both sexes. In contrast, the LMAN plays a key role only in male song learning and maintenance (Bottjer et al., 1984; Brainard and Doupe, 2000), yet FoxP1 mRNA expression was not sexually dimorphic in this nucleus. Further investigation may determine whether the FoxP1 protein exhibits sexual dimorphism in the LMAN, as differences between transcriptional and translational levels have been observed for other transcription factors in song control circuitry (Whitney and Johnson, 2005). Although FOXP1 mutations in humans are accompanied by language disorders, the impact of FoxP1 on song learning and production remains to be determined. Given that we did not observe behavioral regulation of FoxP1 in either species, it seems likely that its role may be in promoting the developmental differentiation of neural structures, consistent with the general role of Fox transcription factors during embryogenesis (Carlsson and Mahlapuu, 2002).
Behavioral regulation of FoxP2 in songbirds
Unlike FoxP1, FoxP2 expression in male songbirds was not enriched in the HVC or the RA, and appeared similar to levels in the HVC and RA of female brains (Figs 3, 4, Table 1). In Area X, FoxP2 was slightly higher or comparable to levels in the adjacent VSP in NS adult male songbirds. FoxP2 expression is enhanced in the striato-pallidum of hatchling zebra finches and increases in Area X during development (Teramitsu et al., 2004). This observation, together with the structural deficits in the basal ganglia of affected KE family members, is consistent with a role for FoxP2 in contributing to the structural organization of basal ganglia regions critical for vocal learning. Post-embryogenesis, Area X FoxP2 levels are downregulated after undirected singing in juvenile and adult zebra finches (Teramitsu and White, 2006; Teramitsu et al., 2010). Lentiviral-mediated FoxP2 knockdown in Area X of juvenile zebra finches results in inaccurate copying of the tutor song (Haesler et al., 2007). Together, these findings suggest that FoxP2 is involved not only in forming neural structures for vocal learning during embryogenesis, but also in the ongoing use of such structures during vocal learning and adult song maintenance, including in adult male Bengalese finches.
Correlation between Area X FoxP2 levels and undirected singing in two species of songbird
We investigated the time course over which FoxP2 levels are first observed to decrease in Area X during singing in both Bengalese and zebra finches. We found that levels became significantly downregulated at the 1.5 h time point in both species (Fig. 6). Contrary to our prediction, Area X FoxP2 downregulation in Bengalese finches was not more robust than in zebra finches. This outcome is qualified by the recognition that experimental quantification of the amount of singing is not always proportional to the time spent singing. For example, one zebra finch sang for 241 s within 2 h, whereas another sang for 487 s within 1 h. We observed a negative correlation between the amount of singing and FoxP2 levels within Area X of zebra finches, which confirms results from our prior studies (Hilliard et al., 2012; Teramitsu and White, 2006; Teramitsu et al., 2010). We now report a similar negative correlation in Bengalese finches (Fig. 7B). Thus, singing may promote FoxP2 mRNA degradation, possibly through miRNA regulation (Clovis et al., 2012), or inhibit mRNA synthesis following song onset. In either case, this regulation of FoxP2 takes time, only producing significant decreases 1.5 h following song onset in this study (Fig. 6). It is difficult to disentangle the effects of time and the amount of singing on FoxP2 levels because we cannot control the amount and timing of singing once birds start. For each species, in birds that did sing similar amounts of song (Fig. 7), there is a trend that the longer they were given before being killed, the lower their Area X FoxP2 levels.
FoxP2 downregulation within Area X in Bengalese finches and zebra finches
When all birds are considered, the downregulation of FoxP2 did not occur on a faster time scale in Bengalese finches than in zebra finches, as demonstrated by the lack of a statistically significant difference in the slopes of regression lines plotted to the data (Fig. 7). The lack of a detectable difference between the two species may be due to a lack of sensitivity in the in situ hybridization. However, in pilot experiments, we compared FoxP2 levels obtained with quantitative reverse transcriptase PCR from cDNA obtained from unilateral punches of Area X with those obtained from in situ hybridization of the remaining hemi-sections from the same bird (J. Liu, unpublished). The sensitivity was comparable across methods, indicating the suitability of our approach, which also enables us to compare our current findings with past studies that employed in situ analyses. The relationship between FoxP2 and singing in Bengalese finches may be underestimated here simply because they sang less as a group. A broader range of singing might enable detection of more subtle differences between the species. Alternatively, the dependence of FoxP2 levels on singing may indeed be similar in both species, despite differences in features of their songs.
Vocal variability after vocal practice
We previously found that in juvenile (75 days of age) zebra finches, vocal practice for 2 h in the morning leads to increased vocal variability (Miller et al., 2010) and that in adult zebra finches, the amount of singing is correlated with increased spectral entropy (Hilliard et al., 2012). Thus, we predicted that vocal practice might lead to increased vocal variability in adult Bengalese finches. To our surprise, we found that despite similar behavioral regulation of FoxP2 in Bengalese and zebra finches, periods of low FoxP2 are associated with slight decreases in variability of multiple features in Bengalese finch song (Miller et al., 2010; Hilliard et al., 2012). Thus, it is possible that FoxP2 downregulation may decrease vocal variability or that changes in FoxP2 levels are unrelated to changes in vocal variability in this species. Arguing against these possibilities is the observation that viral knockdown of FoxP2 in Area X is sufficient to increase variability in both juvenile (Haesler et al., 2007) and adult zebra finches (Murugan and Mooney, 2012). Multiple factors could contribute to the observed difference in these select song features, and are detailed below.
The amount of singing performed by each species could influence whether song is more or less variable in the UD-UD condition. Bengalese finches in our study sang roughly half as much as the zebra finches and the corresponding downregulation of FoxP2 is about half the magnitude. It is possible that FoxP2 levels must drop below a critical threshold in order to de-repress gene transcription and initiate molecular changes that lead to increased variability, or that the amount of singing by Bengalese finches was sufficient to downregulate FoxP2 mRNA but not the protein (Miller et al., 2008). These possibilities could be supported by examining Bengalese song after more extended bouts (~4 h) of UD singing; however, this may be confounded by the fact that FoxP2 levels vary as a function of both the amount of singing and the total time allotted for singing (Fig. 7).
The age of the Bengalese finches used here (>300 days) may present another confounding factor in our ability to detect differences in vocal variability between NS-UD and UD-UD birds. Increased song variability was previously observed to be correlated to the amount of song in younger adult zebra finches [N=18 between 120 and 200 days old (Hilliard et al., 2012)]. Both Bengalese and zebra finches undergo age-related changes in vocal quality and the ability to exhibit vocal plasticity (Brainard and Doupe, 2001; Cooper et al., 2012), thus they may undergo age-related changes in how molecular microcircuits impact behavior. Further, age- and species-related differences in basal vocal variability may have statistically limited our ability to detect these changes. In zebra finches, our ability to detect acute regulation of vocal variability was limited to 75-day-old juvenile birds, as 65-day-old birds and a group of six adult birds showed too much and too little variability, respectively, to derive adequate statistical power (Miller et al., 2010). A follow-up study found that statistical power was achieved when the number of adult zebra finches was increased to 18 UD singers with higher numbers of motifs uttered in the 2 h being correlated with increased song variability (Fig. 3B) (Hilliard et al., 2012).
In summary, these data indicate that FoxP1 is enriched in most song control nuclei of male Bengalese finches, with the notable exception of the LMAN, similar to its expression pattern in zebra finches. No singing-driven regulation of this transcription factor was observed in either species, suggesting a sexually dimorphic role in the formation of brain structures that support vocal learning in songbirds. In contrast, FoxP2 levels in Area X do exhibit singing-driven decreases in both species, with a similar dependence on both the amount of singing and the time since song onset, with the caveat that Bengalese finches in our study sang less than zebra finches. The impact of this downregulation in zebra finches appears to be to increase vocal motor exploration, particularly during song learning and as evidenced by multiple prior studies. Here, in Bengalese finches, we did not observe a similar relationship, which could reflect a true species difference. We deem it more likely that the differences in age and amount of singing of the Bengalese finches in our study relative to the zebra finches precluded detection of this relationship. Future work in songbirds to examine protein expression of these factors as well as to genetically intervene in their expression promise to illuminate organizational versus activational functions of these molecules related to human language.
ACKNOWLEDGEMENTS
Lily Sung assisted in the preparation of brain sections. The authors thank Dr Julie E. Miller and two anonymous reviewers for their constructive comments on the manuscript.
LIST OF ABBREVIATIONS
- A
arcopallium
- AFP
anterior forebrain pathway
- Bas
basorostral pallial nucleus
- HA
apical part of the hyperpallium
- HD
densocellular part of the hyperpallium
- HVC
letter-based name, located in nidopallium
- LMAN
lateral magnocellular nucleus of anterior nidopallium
- M
mesopallium
- N
nidopallium
- NC
caudal nidopallium
- NS
non-singing
- OD
optical density
- RA
robust nucleus of arcopallium
- StL
lateral striatum
- StM
medial striatum
- UD
undirected singing
- VSP
ventral striato-pallidum
FOOTNOTES
COMPETING INTERESTS
No competing interests declared.
FUNDING
Supported by the Chinese Scholarship Council (Q.C.) and the National Institutes of Health [RO1 MH070712 to S.A.W.]. Deposited in PMC for release after 12 months.
REFERENCES
- Alcock K. J., Passingham R. E., Watkins K. E., Vargha-Khadem F. (2000). Oral dyspraxia in inherited speech and language impairment and acquired dysphasia. Brain Lang. 75, 17-33 [DOI] [PubMed] [Google Scholar]
- Arriaga G., Zhou E. P., Jarvis E. D. (2012). Of mice, birds, and men: the mouse ultrasonic song system has some features similar to humans and song-learning birds. PLoS ONE 7, e46610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottjer S. W., Miesner E. A., Arnold A. P. (1984). Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science 224, 901-903 [DOI] [PubMed] [Google Scholar]
- Brainard M. S., Doupe A. J. (2000). Auditory feedback in learning and maintenance of vocal behaviour. Nat. Rev. Neurosci. 1, 31-40 [DOI] [PubMed] [Google Scholar]
- Brainard M. S., Doupe A. J. (2001). Postlearning consolidation of birdsong: stabilizing effects of age and anterior forebrain lesions. J. Neurosci. 21, 2501-2517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz E. A., Margoliash D., Nordeen K. W. (1997). An introduction to birdsong and the avian song system. J. Neurobiol. 33, 495-500 [PubMed] [Google Scholar]
- Carlsson P., Mahlapuu M. (2002). Forkhead transcription factors: key players in development and metabolism. Dev. Biol. 250, 1-23 [DOI] [PubMed] [Google Scholar]
- Carr C. W., Moreno-De-Luca D., Parker C., Zimmerman H. H., Ledbetter N., Martin C. L., Dobyns W. B., Abdul-Rahman O. A. (2010). Chiari I malformation, delayed gross motor skills, severe speech delay, and epileptiform discharges in a child with FOXP1 haploinsufficiency. Eur. J. Hum. Genet. 18, 1216-1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clovis Y. M., Enard W., Marinaro F., Huttner W. B., De Pietri Tonelli D. (2012). Convergent repression of Foxp2 3′UTR by miR-9 and miR-132 in embryonic mouse neocortex: implications for radial migration of neurons. Development 139, 3332-3342 [DOI] [PubMed] [Google Scholar]
- Cooper B. G., Méndez J. M., Saar S., Whetstone A. G., Meyers R., Goller F. (2012). Age-related changes in the Bengalese finch song motor program. Neurobiol. Aging 33, 564-568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale S. D., Perkel D. J. (2010). Anatomy of a songbird basal ganglia circuit essential for vocal learning and plasticity. J. Chem. Neuroanat. 39, 124-131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haesler S., Wada K., Nshdejan A., Morrisey E. E., Lints T., Jarvis E. D., Scharff C. (2004). FoxP2 expression in avian vocal learners and non-learners. J. Neurosci. 24, 3164-3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haesler S., Rochefort C., Georgi B., Licznerski P., Osten P., Scharff C. (2007). Incomplete and inaccurate vocal imitation after knockdown of FoxP2 in songbird basal ganglia nucleus Area X. PLoS Biol. 5, e321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdan F. F., Daoud H., Rochefort D., Piton A., Gauthier J., Langlois M., Foomani G., Dobrzeniecka S., Krebs M. O., Joober R., et al. (2010). De novo mutations in FOXP1 in cases with intellectual disability, autism, and language impairment. Am. J. Hum. Genet. 87, 671-678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard A. T., Miller J. E., Fraley E. R., Horvath S., White S. A. (2012). Molecular microcircuitry underlies functional specification in a basal ganglia circuit dedicated to vocal learning. Neuron 73, 537-552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn D., Kapeller J., Rivera-Brugués N., Moog U., Lorenz-Depiereux B., Eck S., Hempel M., Wagenstaller J., Gawthrope A., Monaco A. P., et al. (2010). Identification of FOXP1 deletions in three unrelated patients with mental retardation and significant speech and language deficits. Hum. Mutat. 31, E1851-E1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jürgens U. (2009). The neural control of vocalization in mammals: a review. J. Voice 23, 1-10 [DOI] [PubMed] [Google Scholar]
- Kao M. H., Doupe A. J., Brainard M. S. (2005). Contributions of an avian basal ganglia-forebrain circuit to real-time modulation of song. Nature 433, 638-643 [DOI] [PubMed] [Google Scholar]
- Kikusui T., Nakanishi K., Nakagawa R., Nagasawa M., Mogi K., Okanoya K. (2011). Cross fostering experiments suggest that mice songs are innate. PLoS ONE. 6, e17721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. S., Fisher S. E., Hurst J. A., Vargha-Khadem F., Monaco A. P. (2001). A forkhead-domain gene is mutated in a severe speech and language disorder. Nature 413, 519-523 [DOI] [PubMed] [Google Scholar]
- Langfelder P., Horvath S. (2008). WGCNA: an R package for weighted correlation network analysis. Bioinformatics 9, 559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langfelder P., Zhang B., Horvath S. (2008). Defining clusters from a hierarchical cluster tree: the Dynamic Tree Cut package for R. Bioinformatics 24, 719-720 [DOI] [PubMed] [Google Scholar]
- Li S., Weidenfeld J., Morrisey E. E. (2004). Transcriptional and DNA binding activity of the Foxp1/2/4 family is modulated by heterotypic and homotypic protein interactions. Mol. Cell. Biol. 24, 809-822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahrt E. J., Perkel D. J., Tong L., Rubel E. W., Portfors C. V. (2013). Engineered deafness reveals that mouse courtship vocalizations do not require auditory experience. J. Neurosci. 33, 5573-5583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. E., Spiteri E., Condro M. C., Dosumu-Johnson R. T., Geschwind D. H., White S. A. (2008). Birdsong decreases protein levels of FoxP2, a molecule required for human speech. J. Neurophysiol. 100, 2015-2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. E., Hilliard A. T., White S. A. (2010). Song practice promotes acute vocal variability at a key stage of sensorimotor learning. PLoS ONE. 5, e8592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugan M., Mooney R. (2012). Deficits in motor performance and corticostriatal transmission following FoxP2 knockdown in adult songbirds. Program No. 273.11/II9. 2012 Neuroscience Meeting Planner. New Orleans, LA: Society for Neuroscience; Online [Google Scholar]
- Nixdorf-Bergweiler B. E., Bischof H. (2007). A Stereotaxic Atlas of the Brain of the Zebra Finch, Taeniopygia guttata: with Special Emphasis on Telencephalic Visual and Song System Nuclei in Transverse and Sagittal Sections. Kiel, Germany: University of Kiel; [Google Scholar]
- Nottebohm F. (2005). The neural basis of birdsong. PLoS Biol. 3, e164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottebohm F., Stokes T. M., Leonard C. M. (1976). Central control of song in the canary, Serinus canarius. J. Comp. Neurol. 165, 457-486 [DOI] [PubMed] [Google Scholar]
- Okanoya K. (2004). The Bengalese finch: a window on the behavioral neurobiology of birdsong syntax. Ann. NY Acad. Sci. 1016, 724-735 [DOI] [PubMed] [Google Scholar]
- Okanoya K., Yamaguchi A. (1997). Adult Bengalese finches (Lonchura striata var. domestica) require real-time auditory feedback to produce normal song syntax. J. Neurobiol. 33, 343-356 [PubMed] [Google Scholar]
- Pariani M. J., Spencer A., Graham J. M., Jr, Rimoin D. L. (2009). A 785 kb deletion of 3p14.1p13, including the FOXP1 gene, associated with speech delay, contractures, hypertonia and blepharophimosis. Eur. J. Med. Genet. 52, 123-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price P. H. (1979). Developmental determinants of structure in zebra finch song. J. Comp. Physiol. Psychol. 93, 260-277 [Google Scholar]
- Rousso D. L., Gaber Z. B., Wellik D., Morrisey E. E., Novitch B. G. (2008). Coordinated actions of the forkhead protein Foxp1 and Hox proteins in the columnar organization of spinal motor neurons. Neuron 59, 226-240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff C., Nottebohm F. (1991). A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: implications for vocal learning. J. Neurosci. 11, 2896-2913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu W., Yang H., Zhang L., Lu M. M., Morrisey E. E. (2001). Characterization of a new subfamily of winged-helix/forkhead (Fox) genes that are expressed in the lung and act as transcriptional repressors. J. Biol. Chem. 276, 27488-27497 [DOI] [PubMed] [Google Scholar]
- Tchernichovski O., Nottebohm F., Ho C. E., Pesaran B., Mitra P. P. (2000). A procedure for an automated measurement of song similarity. Anim. Behav. 59, 1167-1176 [DOI] [PubMed] [Google Scholar]
- Teramitsu I., White S. A. (2006). FoxP2 regulation during undirected singing in adult songbirds. J. Neurosci. 26, 7390-7394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramitsu I., Kudo L. C., London S. E., Geschwind D. H., White S. A. (2004). Parallel FoxP1 and FoxP2 expression in songbird and human brain predicts functional interaction. J. Neurosci. 24, 3152-3163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramitsu I., Poopatanapong A., Torrisi S., White S. A. (2010). Striatal FoxP2 is actively regulated during songbird sensorimotor learning. PLoS ONE 5, e8548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargha-Khadem F., Watkins K. E., Price C. J., Ashburner J., Alcock K. J., Connelly A., Frackowiak R. S., Friston K. J., Pembrey M. E., Mishkin M., et al. (1998). Neural basis of an inherited speech and language disorder. Proc. Natl. Acad. Sci. USA 95, 12695-12700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Lin D., Li C., Tucker P. (2003). Multiple domains define the expression and regulatory properties of Foxp1 forkhead transcriptional repressors. J. Biol. Chem. 278, 24259-24268 [DOI] [PubMed] [Google Scholar]
- Watkins K. E., Dronkers N. F., Vargha-Khadem F. (2002). Behavioural analysis of an inherited speech and language disorder: comparison with acquired aphasia. Brain 125, 452-464 [DOI] [PubMed] [Google Scholar]
- Whitney O., Johnson F. (2005). Motor-induced transcription but sensory-regulated translation of ZENK in socially interactive songbirds. J. Neurobiol. 65, 251-259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild J. M., Farabaugh S. M. (1996). Organization of afferent and efferent projections of the nucleus basalis prosencephali in a passerine, Taeniopygia guttata. J. Comp. Neurol. 365, 306-328 [DOI] [PubMed] [Google Scholar]
- Woolley S. M., Rubel E. W. (1997). Bengalese finches Lonchura striata domestica depend upon auditory feedback for the maintenance of adult song. J. Neurosci. 17, 6380-6390 [DOI] [PMC free article] [PubMed] [Google Scholar]