Abstract
Ghrelin O-acyltransferase (GOAT) is responsible for catalyzing the attachment of the eight-carbon fatty acid octanoyl to the Ser3 side chain of the peptide ghrelin to generate the active form of this metabolic hormone. As such, GOAT is viewed as a potential therapeutic target for the treatment of obesity and diabetes mellitus. Here, we review recent progress in the development of cell and in vitro assays to measure GOAT action and the identification of several synthetic GOAT inhibitors. In particular, we discuss the design, synthesis, and characterization of the bisubstrate analog GO-CoA-Tat and its ability to modulate weight and blood glucose in mice. We also highlight current challenges and future research directions in our biomedical understanding of this fascinating ghrelin processing enzyme.
1. INTRODUCTION
Acyl ghrelin is a 28-amino acid peptide hormone that has been shown to modulate body weight and blood glucose. Discovered in 1999 by Kojima and colleagues, acyl ghrelin is produced primarily in the stomach, pancreas, and duodenum. Acyl ghrelin contains an unusual eight-carbon fatty acid posttranslational modification, which is essential for its biological function (Date et al., 2000; Heller et al., 2005; Kojima et al., 1999; Prado et al., 2004; Wierup et al., 2002).
The amino acid sequence of acyl ghrelin is highly conserved among mammals, differing only at two residues between humans and rodents. Acyl ghrelin homologs have also been identified in all vertebrates examined, including bullfrogs, chicken, and tilapia. Acyl ghrelin is the endogenous ligand of the growth hormone secretagogue receptor (GHS-R1a) (Kojima et al., 1999), a G-protein-coupled receptor that is present in the brain and other tissues. Upon acyl ghrelin binding to GHS-R1a, cellular phospholipase C is activated to generate inositol triphosphate (IP3) and diacylglycerol, which in turn increases intracellular level of Ca2+, resulting in growth hormone release (Korbonits et al., 1999). This pathway is distinct from that of the growth hormone-releasing hormone (GHRH), where binding to the GHRH receptor results in increase in cAMP levels.
GHS-R1a is predominantly expressed in the arcuate nucleus of the hypothalamus but is also found in the pituitary, the ventromedial nuclei, the hippocampus, and vagal afferent neurons, with lower levels of expression seen in nonneuronal cell types in the periphery, including the pancreas (Chen et al., 2004; Cowley et al., 2003; Guan et al., 1997; Howard et al., 1996). Acyl ghrelin may exert appetite stimulation as well as modulate metabolism via a variety of mechanisms (Chen et al., 2004; Kamegai et al., 2001; Morton and Schwartz, 2001; Willesen et al., 1999); its action is mediated at least in part by the uncoupling protein UCP2 (Andrews et al., 2008).
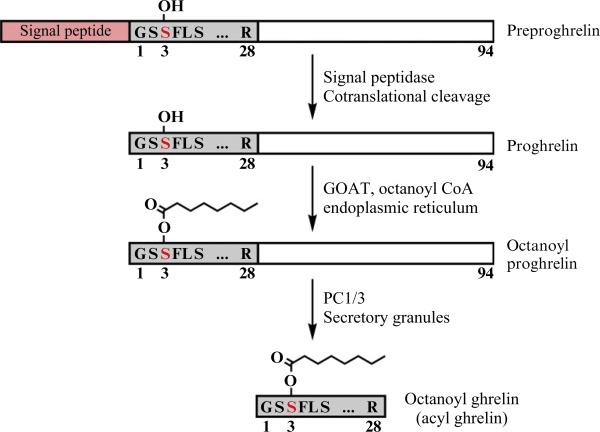
In order to bind to GHS-R1a, acyl ghrelin requires a unique posttranslational modification in that the serine at position 3 is n-octanoylated (Kojima et al., 1999). Intriguingly, ghrelin acylation may be governed by the level of medium-chain triglycerides in the diet (Nishi et al., 2005). The biosynthesis of acyl ghrelin is outlined in Fig. 13.1. The 117-amino acid preprohormone contains a signal peptide and is cotranslationally cleaved, releasing the 94-amino acid proghrelin into the lumen of the endoplasmic reticulum (ER). Attachment of the octanoate group to Ser3 of proghrelin occurs in the ER and is catalyzed by ghrelin O-acyltransferase (GOAT). In secretory granules or perhaps as early as the trans-Golgi, prohormone convertase 1/3 (PC1/3) then cleaves at the C-terminus of acyl proghrelin to produce mature acyl ghrelin (Zhou et al., 1999; Zhu et al., 2006).
Figure 13.1.
Ghrelin biosynthetic pathway. Ghrelin is synthesized as a 117-amino acid precursor, preproghrelin, containing a signal peptide, the 28-amino acid ghrelin sequence, and a 66-amino acid C-terminal peptide. The signal peptide is cotranslationally cleaved, releasing the 94-amino acid proghrelin into the lumen of the endoplasmic reticulum (ER). Attachment of the octanoate group to Ser3 of proghrelin occurs in the ER and is catalyzed by GOAT. In secretory granules, prohormone convertase 1/3 (PC1/3) then cleaves at the C-terminus of acyl proghrelin to give the mature acyl ghrelin.
The ghrelin-octanoylating enzyme GOAT was molecularly identified by two groups and reported in 2008 (GOAT) (Gutierrez et al., 2008; Yang et al., 2008a). A 45-kDa multispanning transmembrane protein, GOAT is a member of the family of membrane-bound O-acyltransferases (MBOAT), which includes heghehog acyltransferase (Hhat) and porcupine (Porc), the substrates of which are the secretory proteins sonic hedgehog (Shh) and Wnt, respectively (Buglino and Resh, 2008; Chamoun et al., 2001; Hofmann, 2000; Kadowaki et al., 1996; Takada et al., 2006). Ghrelin and Wnt3a are the only proteins known to contain acylated serine residues. MBOAT family members have diverse substrates including sterols, phospholipids, and proteins, but as integral membrane proteins the enzymatic properties of these proteins are poorly understood. To date, ghrelin is the only established substrate for GOAT, and is the only known octanoylated protein. In addition to the metabolic roles for acyl ghrelin, this hormone has also been implicated in many areas including but not limited to learning and memory, as well as food-related behavior (Carlini et al., 2002; Diano et al., 2006) and gastrointestinal motility (de la Cour Dornonville et al., 2004; Ogiso et al., 2011). These and other aspects of ghrelin physiology have been recently reviewed (Castaneda et al., 2010). Recent studies on GOAT knockout mice have suggested that this enzyme is important for survival under prolonged, severe starvation, although this characteristic may be context dependent (Sun et al., 2008; Yi et al., 2012; Zhao et al., 2010a). The potential therapeutic benefits of exploiting the ghrelin–GOAT system in managing obesity and diabetes are attractive but not yet fully explored. Much drug discovery work has been focused on GHS-R1a receptor modulators, but the discovery of GOAT offers new potential for the generation of GOAT-selective inhibitors; however, the potential scope of pharmacologic actions of such modulators is not yet known. This chapter discusses the practical issues involved in developing GOAT inhibitors, the initial progress in this area, as well as future challenges.
2. ANALYSIS OF ACYL GHRELIN LEVELS AND GOAT ACTIVITY
2.1. Assays measuring acyl and des-acyl ghrelin from blood and cells
In order to develop effective GOAT inhibitors, an important technical hurdle to overcome is reliable measurement of acyl and des-acyl ghrelin. A challenge in designing assays to measure ghrelin acylation is the instability of acyl ghrelin in biological systems, predominantly due to hydrolysis of the acyl group by esterases in plasma, cell culture medium, and cell extracts. Any assay must measure typically dilute concentrations of the hormone, preserve acylation through isolation, and then detect the acylation in the context of what is usually a larger amount of des-acyl ghrelin. A comparison of literature reports suggests wide ranges (10–100-fold or more) in concentrations of blood acyl ghrelin in studies on normal humans and rodents, underscoring the complexity of the measurements (Groschl et al., 2004; Liu et al., 2008a).
Early acyl ghrelin assays used the functionality of the hormone on the GHS-R1a receptor. While useful, these assays are rather complex and lack precision. Combinations of reversed-phase HPLC and immunoassays have proved increasingly reliable. Acyl ghrelin measurements relying on mass spectrometry have also been reported, although our lab has had limited success with this approach (Gutierrez et al., 2008; Satou et al., 2010). The current state of the art for ease of use and reproducibility seems to be sandwich ELISA assays.
Two-site immunoassays are generally more sensitive and specific than single-antibody assays and also do not cross-react with peptide fragments (Nussbaum et al., 1987). Some early publications used a two-site sandwich ELISA developed inhouse (Barkan et al., 2003), but this was not widely adopted. Liu et al. (2008a) developed two novel sandwich ELISAs specific for acyl and des-acyl full-length ghrelin. Capture was achieved using N-terminal acyl- and des-acyl-specific antibodies, and detection for both assays used an affinity-purified antibody to ghrelin's C-terminal amino acids 21–27. They also detailed an improved collection protocol in which blood is collected directly into chilled tubes preloaded with the protease inhibitor AEBSF (4-(2-aminoethyl)benzenesulfonyl fluoride), maintained on ice until prompt centrifugation, and then immediately acidified with 20% (v/v) 1 N HCl to protect the ghrelin ester from hydrolysis. These combined improvements represent the current state of the field, although the esterase inhibitor PHMB (p-hydroxymercuribenzoic acid) can be substituted for AEBSF and adding 1 M NaCl improves plasma separation.
Commercial two-site sandwich ELISA kits by Spi-Bio (now Bertin Pharma) are now available, sold through Cayman Chemical and Alpco Diagnostics. These kits have been used in recent studies (Barnett et al., 2010; Zhao et al., 2010b). The kits include wells coated with a C-terminal capture antibody and a modification-specific N-terminal antibody conjugated to acetylcholinesterase. The kits from the two companies are apparently identical except for the color of their packaging. We have validated their modification-specificity and sensitivity against both homemade standards and those supplied by the manufacturer. We also tested kits from Millipore with similar results. Other two-site kits are available from Mitsubishi Kagaku Iatron (Tokyo, Japan), using N-terminal-modification-specific antibody and C-terminal capture antibody, although we have not tested them.
2.2. Measuring acyl and des-acyl ghrelin levels in cell-based model systems
To establish a model system for ghrelin acylation, the field first turned to cell lines. The first cell line established was the TT cell, a medullary thyroid carinoma line (Kanamoto et al., 2001). Ghrelin production from these cells was similar to that in rat intestinal production, and approximately 20% of the ghrelin produced was found to be acylated. Ghrelin was secreted into the culture medium as well; the vast majority of secreted material was found to be des-acyl , and the different ratios between intracellular and secreted pools were attributed to degradation. This cell line was used to discover GOAT by Gutierrez et al. (2008) (see below). They demonstrated that the amount of acyl ghrelin in the medium could be increased by the addition of octanoic acid or protection of the acyl group with a modification-specific antibody and that octanoylation occurred only at Ser3.
The human erythroleukemia cell line (De Vriese et al., 2005) also produces acyl ghrelin, which was shown to be part of an autocrine loop leading to cell proliferation. Interestingly, they also demonstrate that the half-life of acyl ghrelin in culture medium is approximately 1 h. However, these cells are of limited utility as a model because the amount of acyl ghrelin produced is very low and ghrelin production is unstable (Takahashi et al., 2009).
Yang et al. (2008a) tested a number of cell lines for the ability to process proghrelin to ghrelin, measuring the retained intracellular ghrelin in cell lysates. HEK-293 and CHO-7 lysates contained only proghrelin, but the endocrine cell lines AtT-20, INS-1, and MIN-6 all contain mixtures of proghrelin and mature, processed ghrelin. Transfection of candidate acyltransferases into the INS-1 cell line was then used to independently discover GOAT, and all three cell lines were able to produce mature, octanoyl ghrelin when GOAT was transfected in.
Gutierrez et al. have transiently transfected plasmids expressing ghrelin and GOAT into GripTite HEK-293 MSR cells and, combined with addition of fatty acids in the medium, showed production of mature acyl ghrelin at much higher levels than seen in TT cells (Gutierrez et al., 2008). Interestingly, they detect mature, processed ghrelin in the medium of the precise expected mass. This demonstrates that HEK-293 cells can in fact process proghrelin to ghrelin, but the cleaved form may be promptly secreted. Enhanced production of acyl ghrelin was subsequently reported in three cell lines, namely, TT, AtT20, and COS-7, by cotransfecting a plasmid expressing ghrelin with either or both plasmids expressing GOAT and one of five proteases (Takahashi et al., 2009).
Three improved cell lines were recently isolated from ghrelinomas in ghrelin-promoter SV40-T-antigen transgenic mice: MGN3-1, PG-1, and SG-1 cells. These cell lines all express ghrelin, GOAT, and PC1/3, and have been shown to recapitulate physiologic ghrelin signaling to some extent, so they should be useful model systems going forward. MGN3-1 (Mouse Ghrelinoma 3-1) cells produce 5000 times more ghrelin than TT cells (Iwakura et al., 2010). Approximately 6–14% of the ghrelin produced in this cell line is acylated when octanoic acid is added to the medium, depending on the experiment, and acyl ghrelin is secreted into the culture medium in mature, cleaved form. siRNA knockdown of GOAT slightly depressed the ratio of acyl to des-acyl ghrelin produced.
The similar cell lines PG-1 and SG-1 were derived from pancreatic and stomach ghrelinomas, respectively (Zhao et al., 2010b). These lines produce comparably high levels of ghrelin to MGN3-1, although direct comparison is difficult because of different measurement techniques (single-site RIA vs. 2-site ELISA) and distinct normalizations (per cell vs. per microgram cellular protein). In PG-1 and SG-1 cells, up to 30% of secreted ghrelin is octanoylated when sodium octanoate-albumin is added to the culture medium.
We recently established the vector phPPG-mGOAT for stable expression of ghrelin and GOAT in the widely available 293T and HeLa cell lines (Barnett et al., 2010). This episomally maintained vector has a CMV promoter (Cyotomegalovirus immediate early promoter), ghrelin, internal ribosome entry site, and then GOAT, in that order, providing substantial production and a high substrate-to-enzyme ratio. By measuring intracellular ghrelin by ELISA, we then used this system to test structure–activity relationships for GOAT inhibitors. Note that this approach does not explicitly distinguish proghrelin from mature ghrelin forms and avoids the issues of ester hydrolysis that appear to accompany secretion into the medium. We have also found it most advantageous to consistently measure acyl and des-acyl ghrelin from the same samples and express the percentage of acyl ghrelin as a key parameter to circumvent the fluctuations of total ghrelin that may be related to cellular conditions or isolation procedures.
2.3. In vitro direct GOAT activity assays using microsomes
Yang et al. established an in vitro GOAT acyl transfer assay using membranes enriched for ER (microsomes) from insect cells infected with baculovirus encoding mouse GOAT to transfer 3H-labeled octanoate onto proghrelin-His8 (Yang et al., 2008b). Mouse GOAT microsomes were prepared as follows: GOAT was cloned into pFastBac HT-A, giving it an N-terminal His10-TEV tag, and the baculovirus infected SF9 cells from which the microsomes were harvested. As a negative control, the MBOAT fingerprint residue H338 was mutated to alanine and control virus was also employed. Proghrelin-His8 and mutant proghrelins were produced in bacteria using an N-terminal GST-TEV tag, such that TEV cleavage produced the authentic N-terminal sequence of proghrelin.
The in vitro octanoylation assay of Yang et al. was performed using 1 μM 3H-octanoyl-CoA (high specific activity), 5 μg proghrelin-His8 (8.6 μM), and 50 μg GOAT-containing microsomes. Reactions were quenched with buffer containing 0.1% SDS, labeled proghrelin was separated from the reaction mixture using nickel-affinity chromatography, and 3H-octanoylproghrelin was quantified using liquid scintillation counting. Yang et al. found that addition of long-chain fatty coenzyme A (CoA) conjugates stimulate the reaction up to 3.5-fold by preventing hydrolysis of octanoyl-CoA to octanoate and therefore 50 μM palmitoyl-CoA was present in all further reactions.
The enzyme kinetics observed in these studies was complex and nonlinear, probably because of the presence of esterases, palmitoyl-CoA, and product inhibition. The apparent Km values for octanoyl-CoA and proghrelin were found by Yang et al. to be 0.6 and 6 μM, respectively. As a control, it was shown that the S3A mutant ghrelin could not be octanoylated by GOAT. Octanoylation of ghrelin mutants G1S, G1A, and F4A was dramatically reduced, indicating the importance of residues G1, S3, and F4 in this recognition. L5A and S6A ghrelin mutations had smaller effects, indicating a lesser contribution of these side chains to recognition, and there was no effect of S2A or P7A mutations. Also, the addition of the two N-terminal residues Ser-Ala, which would be present were the signal peptide not cleaved, markedly reduced octanoylation. Together with the finding of acylated proghrelin in transfected INS-1 cells, this evidence suggests that the natural substrate for GOAT is proghrelin after signal peptide cleavage (Yang et al., 2008b). Yang et al. also demonstrated that truncated ghrelin pentapeptides could be acylated by microsomal GOAT, although they showed weaker apparent affinity for the enzyme.
Our group has developed a related assay for studying recombinant microsomal GOAT, but it has been prepared from human cells rather than insect cells (Barnett et al., 2010). HEK293T GnTI-cells were transfected with mouse GOAT containing a C-terminal 3xFlag tag cloned into a mammalian expression vector (CAG promoter). Microsomes were prepared in a manner similar to that reported by Yang et al., and GOAT assays were performed using a synthetic ghrelin tagged with a C-terminal biotin (Ghrelin27-Biotin), taking advantage of the robustness of streptavidin–biotin affinity. Assays were carried out with 3H-octanoyl-CoA, with streptavidin beads used to isolate radiolabeled octanoyl ghrelin. Although signal was detected in this human cell expression system, there appears to be significantly greater signal using the insect cell expression system.
Garner and Janda (2010) have developed an elegant nonradioactive GOAT assay exploiting click chemistry. Replacing octanoyl-CoA with octynoyl-CoA containing a carbon–carbon triple bond at the 7–8 position, Garner and Janda reacted this with microsomal GOAT and bead-immobilized ghrelin pentapeptide, affording octynoyl ghrelin. This immobilized octynoyl ghrelin was then conjugated using copper-catalyzed cycloaddition to azido-HRP (horseradish peroxidase), and detection was achieved using the amplex red fluorogenic substrate. A strong signal-to-noise ratio was achieved, and the apparent Km values measured for n-octynoyl-CoA and immobilized ghrelin (1–5) pentapeptide were 68 and 100 nM, respectively. The fact that the peptide is immobilized on a solid surface while the enzyme is still membrane-bound may explain the 1000-fold lower apparent Km for the peptide and 10-fold lower apparent Km for octynoyl-CoA in these conditions. The triple bond in the octynoyl-CoA may also affect its interaction with GOAT. This click assay appears to be potentially more amenable to high-throughput screening compared to the radioactive assay (Garner and Janda, 2010, 2011).
3. GOAT INHIBITOR DISCOVERY
Three classes of GOAT inhibitors have been described so far: product acyl-peptide analogs, a small molecule detected in a high-throughput screen, and a rationally designed bisubstrate analog.
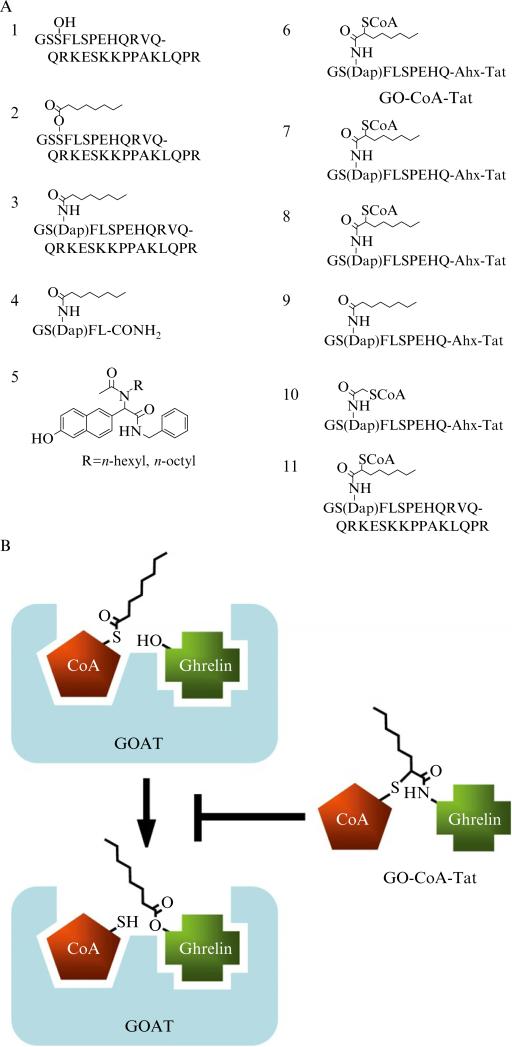
When Ser3 in ghrelin (Fig. 13.2A, Compound 2) was replaced with DAP ((S)-2,3-diaminopropionic acid) creating an octanoyl-amide in place of ester (Fig. 13.2A, Compounds 3 and 4), both the 28-mer and 5-mer acyl ghrelins were potent GOAT inhibitors with IC50 values of 0.2 and 1 μM, respectively. It is likely that these compounds correspond to the strong product inhibition of GOAT, but the lack of hydrolytic sensitivity of the amide linkage confers greater stability.
Figure 13.2.
GOAT inhibitors. (A) Chemical structures of ghrelin and GOAT inhibitors. 1: Des-acyl ghrelin. 2: Acyl ghrelin. 3: Amide-linked octanoyl ghrelin. 4: Amide-linked 5-mer octanoyl ghrelin with C-terminus amidated. 5: Inhibitors discovered by Garner and Janda (2011). 6: GO-CoA-Tat. 7,8: Bisubstrate compounds with five and three amino acids of ghrelin. 9: GO-Tat: an octanoyl-amide Tat-tagged product analog. 10: Bisubstrate inhibitor with two-carbon acyl group. 11: Ghrelin28-Oct-CoA, a bisubstrate compound. (B) Mechanism-based design strategy of GO-CoA-Tat. The lipid–enzyme interaction is not shown but may be important. Also, the form of ghrelin acylated by GOAT is likely proghrelin; the smaller version is shown for clarity.
While showing high potency, product analogs have pharmacologic challenges for in vivo applications. As peptide compounds, their ability to penetrate cell membranes may be limited. Perhaps more importantly, they are likely potent agonists of GHS-R1a. Four residues of ghrelin functionally activate GHS-R1a about as efficiently as full-length ghrelin (Bednarek et al., 2000). We have also found that a Tat-conjugated 10mer-amide is also a potent GHS-R1a agonist (see below).
Garner and Janda (2011) carried out compound screening using their click assay. The assay's Z′ factor was determined to be 0.63, indicating high assay quality (Zhang et al., 1999). A small “credit card” library of drug-like small molecules was then screened for inhibition of GOAT, and two related small molecule inhibitors were discovered (IC50 = 7.5 and 13 μM, respectively, see Fig. 13.2, Compound 5). Interestingly, these compounds contain six- and eight-carbon alkyl chains, suggesting that they possibly compete for the octanoic acid binding site on GOAT. Although these compounds have not yet been explored in depth pharmacologically, they appear to represent attractive leads.
3.1. Bisubstrate analogs
It is now well established that mimics of the transition state of an enzyme-catalyzed reaction can serve as high-affinity inhibitors based on the premise that most enzymes have evolved to bind tightly to the transition state. For enzymes that use two substrates in a ternary complex mechanism, an attractive approach to rational inhibitor design involves covalent linkage of the two substrates to generate a bisubstrate analog, as shown schematically in Fig. 13.2B. Such compounds can show energetically favorable interactions with enzymes because dual occupancy of the substrate binding pockets is facilitated without the entropic penalty incurred with random collision of the individual substrate molecules. To be most effective, it is understood that a tether for the linkage must be able to approximate a mechanistically relevant orientation of the two substrates, ideally capturing elements of the transition state. In the best cases, bisubstrate analogs can show binding free energies to an enzyme that are equal to or greater than the sum of the binding energies of the individual substrate components to the same protein. Successful examples have been recorded of bisubstrate analogs for protein kinases and protein acetyltransferases inspired by enzyme mechanism considerations. By placing an acetyl bridge between ATP and peptide substrate sequences for kinases, compounds that show low micromolar to subnanomolar affinities have been achieved for the insulin receptor kinase, protein kinase A, Csk tyrosine kinase, cyclin-dependent kinase, Abl tyrosine kinase, and the epidermal growth factor tyrosine kinase (Bose et al., 2006; Cheng et al., 2006; Hines and Cole, 2004; Hines et al., 2005; Jencks, 1981; Levinson et al., 2006; Medzihradszky et al., 1994; Parang et al., 2001; Shen and Cole, 2003).
A related linker worked effectively for the histone acetyltransferase enzymes PCAF/GCN5 and p300/CBP which contain a CoA and peptide substrate fragments bridged by an acetyl spacer (Lau et al., 2000; Sagar et al., 2004). Several of these bisubstrate analogs have been useful in structural analysis of the enzyme reaction mechanism and substrate binding features (Liu et al., 2008b). On the other hand, these analogs have suffered from limited pharmacologic utility because of their large size, polarity, and the challenges of cell membrane penetration. However, the discovery of cell-penetrating peptide sequences derived from the HIV Tat protein have allowed for cell and in vivo applications for the bisubstrate analog HAT inhibitors (Bricambert et al., 2010; Cerchietti et al., 2010; Cleary et al., 2005; Guidez et al., 2005; Liu et al., 2008c; Marek et al., 2011; Oussaief et al., 2009; Spin et al., 2010; Wang et al., 2011; Zheng et al., 2005).
3.2. Development of GO-CoA-Tat, a potent and selective bisubstrate inhibitor of GOAT
Following the bisubsrate analog approach described above, we reported the development of GO-CoA-Tat (Barnett et al., 2010). GO-CoA-Tat (Fig. 13.2A, Compound 6) uses nonhydrolyzable amide and thioether linkages to combine octanoyl-CoA with the first 10 amino acids of ghrelin, which are 100% conserved in mammals. An HIV Tat-derived peptide sequence was attached to the C-terminus using a flexible linker to allow cell penetration. GO-CoA-Tat and a set of related analogs and control compounds (Fig. 13.2A, Compounds 6–11) were synthesized by a solid-phase strategy.
We then tested these compounds in HEK and HeLa cells expressing ghrelin and GOAT stably transfected with our phPPG-mGOAT vector. Cells were maintained in a medium supplemented with octanoic acid, pre-incubated with the compound for 24 h, and then lysed. Intracellular acyl and des-acyl proghrelin were measured by ELISA, with values validated using kits from two manufacturers and standards made inhouse. We first tested GO-CoA-Tat in these models, and the mean inhibitory concentration was ~5 μM; control compound D4-Tat had no effect. Interestingly, maximum inhibition was achieved only after 24 h of incubation with GO-CoA-Tat. This could be due to the atypical behavior of the enzyme or inhibitor or due to preexisting intracellular stores of acyl ghrelin. To test this, we used the radioassay described in Section 2.3 and found substantial inhibition occurred within 5 min with 100 nM GO-CoA-Tat. This too suggests that there are intracellular stores of acyl ghrelin in these cells.
We examined structure–activity relationships required for inhibition with compounds used at 6 μM. Consistent with what was seen in other assays, five residues of ghrelin were sufficient for inhibition but three residues were not. Inclusion of 10 residues increased potency, with a maximum of ~75% inhibition of acylation seen. CoA was also required for inhibition; this finding is discussed further in Section 4.3, and a version of the bisubstrate compound with a truncated two-carbon acyl group still showed some inhibition. Tat was required for inhibition, consistent with its role in entry into the cells and ruling out action on a surface receptor. None of the compounds was toxic to the cells in the low micromolar concentration range. GO-CoA-Tat's specificity is also reflected in its lack of inhibition of three acetyl-CoA-utilizing enzymes.
To further analyze GO-CoA-Tat's inhibition of GOAT, we developed a direct binding assay for GOAT, taking advantage of photocrosslinking technology. We first synthesized two chemically modified versions of our bisubstrate inhibitor, namely, GO-CoA-Tat-F4BP and GO-CoA-Tat-L5BP, in which Phe4 or Leu5, respectively, is replaced with a photoreactive amino acid benzoyl-phenylalanine and each is tagged with a biotin group (Barnett et al., 2010). We showed that this compound could covalently crosslink to recombinant solubilized or microsomal GOAT. This crosslinking could be blocked by an excess of unlabeled GO-CoA-Tat, providing evidence for specificity and demonstrating direct binding of GO-CoA-Tat to GOAT.
For these experiments, we used GOAT with a C-terminal 3xFlag tag, produced in SF9 cells using baculovirus. Microsomes were prepared as above, and the reaction was performed either in the microsome membranes or with GOAT purified to homogeneity using anti-Flag affinity chromatography and the Fos-Choline-16 detergent (Anatrace). This detergent was chosen because of its high ability to solubilize GOAT and because we reasoned that the long alkyl chain is less likely to interfere with the octanoic acid binding site on GOAT.
Photocrosslinking reactions were performed in a small water-jacketed quartz cuvette, custom made for this purpose by Quark Glass. The cuvette was connected to the water line and suspended above a magnetic stir plate. A small teflon stir bar was added, with medium agitation. A mercury UV lamp with a ~360-nm long-wave filter, such as UVP #B-100AP, was positioned with the center of the lamp approximately 2 cm from the cuvette, positioning the sample at the position of peak intensity. A time course experiment (not shown) demonstrated that the reaction had neared completion by 30 min. Crosslinked membranes were then solubilized and immunoprecipitated. Biotinylation was visualized using SDS-PAGE and streptavidin–HRP or, for more sensitivity, streptavidin followed by polyclonal anti-streptavidin.
3.3. Glucose and weight control in mice with GO-CoA-Tat
Treatment of C57BL6 mice on medium-chain triglyceride (MCT) diets (Kirchner et al., 2009) with GO-CoA-Tat at 40 mg/kg dose, but not with the control compound D4-Tat or vehicle, decreased plasma acyl ghrelin levels without changing the des-acyl ghrelin levels. Maximum inhibition was seen after 6 h, but some acyl ghrelin suppression was still detectable 24 h after GO-CoA-Tat treatment. Because of the daily fluctuations between animals and ad lib feeding, we found that the acyl to des-acyl ghrelin ratio was a more sensitive and specific measure of inhibition.
We explored the effect on weight gain over a 1-month period in mice placed on an MCT diet. Daily IP injections of GO-CoA-Tat as above reduced the weight gain seen in vehicle-treated mice. As measured by QMR spectroscopy, the difference in weight was due to significantly reduced fat mass in the GO-CoA-Tat-treated animals. In contrast, GO-CoA-Tat- versus vehicle-treated ghrelin-knockout mice showed no statistically significant difference in weight or body composition. To investigate the potential for GO-CoA-Tat toxicity, we examined the blood chemistries and cell counts in the mice after 1 month of treatment with the agent. There was no apparent untoward effect on normal blood chemistries or cell counts under these conditions. Interestingly, WT mice treated with GO-CoA-Tat showed reduced IGF-1 and lower blood glucose, consistent with suppression of ghrelin-mediated somatotroph signaling.
To investigate the role of acute pharmacologic inhibition of acyl ghrelin in insulin signaling and glucose homeostastis, we pretreated with GO-CoA-Tat and then measured the response to a glucose challenge, first in isolated pancreatic islets and then in mice. The insulin response was increased in islets and mice, where the response was accompanied by reduced blood glucose. In contrast, there was no effect when the studies were repeated in ghrelin-knockout animals, suggesting that GO-CoA-Tat's effects on insulin are due to the inhibition of ghrelin acylation. Finally, we showed by quantitative PCR that islets isolated from mice pretreated with GO-CoA-Tat had a 20-fold reduction in expression of uncoupling protein 2 mRNA (UCP2, which suppresses insulin secretion), but there was no change in UCP2 expression in the gastric fundus. Together, these data show a tissue-specific role for GOAT inhibition in augmentation of insulin secretion. Regulation of UCP2 also highlights the importance of ghrelin acylation in obesity and type 2 diabetes, underscoring the need for more drug-like GOAT inhibitors (Andrews et al., 2008; Dezaki et al., 2008; Joseph et al., 2002; Sun et al., 2006; Tong et al., 2010; Zhang et al., 2001).
4. CHALLENGES AND FUTURE DIRECTIONS
While there has been significant progress in GOAT enzymology and inhibition reported in the past few years, many challenges remain.
4.1. Targeting GOAT versus GHS-R1a
One hurdle in developing therapeutic agents to target GOAT is the extensive overlap in the pharmacophore recognized by GOAT and GHS-R1a. We have recently tested a number of compounds using a version of the GHS-R1a assay reported by Kojima et al. (Barnett et al., 2010; Kojima et al., 1999) (Table 13.1). Dose–response traces from individual wells treated with acyl ghrelin are shown in Fig. 13.3A. The responses to selected compounds are shown in Fig. 13.3B. Acyl ghrelin and amide ghrelin (Ser3 Dap-Octanoyl-Amide, Fig. 13.2 compounds 2 and 3, respectively) are indistinguishable at the receptor. In contrast, GO-CoA-Tat does not activate GHS-R1a at the concentrations tested, which include concentrations higher than those used in mice. We also showed that the activity of 1 μM or 100 nM ghrelin at GHS-R was not inhibited by GO-CoA-Tat at 60 nM, 600 nM, or 6 μM. Surprisingly, a 28-mer bisubstrate compound Ghrelin28-Oct-CoA (Compound 11) could activate the receptor with reduced affinity.
Table 13.1.
EC50 values in the GHS-R1a assay
| Compound | EC50 |
|---|---|
| Acyl ghrelin | 18 ± 6 nM |
| Des–acyl ghrelin | > 10 μM |
| Amide ghrelin | 19 ± 8 nM |
| GO–Tat | 23 ± 4 nM |
| GO–CoA–Tat | > 10 μM |
| Ghrelin28–Oct–CoA | 270 ± 70 nM |
| D4–Tat | > 10 μM |
| GO–Tat S2Oct | > 10 μM |
| GO–Tat S6Oct | > 10 μM |
Figure 13.3.
GHS-R1a assay in stably transfected HEK-293T-GHS-R1a cells. (A) Typical dose–response traces for acyl ghrelin, with concentrations on half-log scale from 1 μM to 100 pM, with buffer-only control. (B) Agonism for acyl ghrelin, GO-Tat (Fig. 13.2, Compound 9), and the bisubstrate compound Ghrelin28-Oct-CoA (Fig. 13.2, Compound 11). EC50 values are reported in Table 13.1.
4.2. Moving toward studies of purified GOAT
Studying the activity and mechanism of purified GOAT is critical for improved inhibitor development. To date, all reported GOAT assays, with the exception of one example of our photocrosslinking-based binding assay, have been carried out in complex microsome mixtures containing thousands of other proteins. GOAT is only a small fraction of the total protein in these experiments, and reactions are usually carried out in the presence of relatively high concentrations of palmitoyl-CoA to inhibit esterases and other CoA-utilizing enzymes in these mixtures. Further, only very low conversion percentages are achievable. However, GOAT has not yet been solubilized in an active form. Progress in these areas will be critical to developing better inhibitors and for structural studies of GOAT.
4.3. Structural and mechanistic studies of GOAT and GOAT topology
Currently, we know very little about the structure and mechanism of GOAT. The specific and potent binding of the bisubstrate inhibitor GO-CoA-Tat argues for a ternary complex mechanism, but other mechanisms are still formally possible and further studies in this area are needed. We do not know where proghrelin and the octanoyl-donor bind, and the identity of the octanoyl donor has not been proven. Also, the identity of the active site has not been confirmed.
A map of the topology of GOAT will be helpful to answer some of these questions. Based on sliding-window Kyte–Doolittle hydropathy plots, mouse GOAT was predicted to contain eight transmembrane helices (TM) (Yang et al., 2008a); however, no experiments have yet been reported to further probe GOAT's topology. To acylate ghrelin, GOAT's active site should face the ER lumen where ghrelin is localized; this logic also applies to other MBOATs that acylate secreted and GPI-anchored proteins. The two most conserved residues in the MBOAT fingerprint are N307 and H338 in mouse GOAT, and only H338 is conserved throughout the entire MBOAT family. The homologous histidine in another MBOAT, the human cholesterol acyltransferase ACAT1, was mapped to the lumenal boundary of a TM or perhaps in a short-loop ER lumen (Guo et al., 2005). Like GOAT, mutation of this histidine in ACAT1 abolished catalytic activity. The topology of distantly related yeast MBOAT members Ale1p, Are1p, and Gup1p was recently studied in detail (Pagac et al., 2011); these enzymes acylate lipids, cholesterol, and GPI-anchored proteins, respectively, and Are1p is the yeast ortholog of ACAT1. The conserved histidine in all cases was shown to be in the ER lumen, but none of the active sites has yet been mapped.
These conserved residues have been called “catalytic residues”; however, there is a lack of mechanistic data that firmly establishes this point. Although GOATs with alanine mutants of these conserved His and Asn residues are inactive, the homologous histidine residue was recently shown to be dispensable for palmitoylation of Shh by Hhat. In this case, the H379A caused reduced binding affinity of Shh as reflected in an increased Km without changing Vm (Buglino and Resh, 2010). Therefore, at this point, the identity of the active site of GOAT and other MBOATs is unclear.
With the active site of GOAT in the ER lumen, one question raised by the success of the microsomal GOAT assays is, how do the ghrelin peptides and octanoyl group reach the active site of GOAT? Microsomes are believed to be sealed bilayer vesicles, so in order to octanoylate ghrelin, some of the microsomes are presumably inside-out, with normally lumenal contents exposed to the assay buffer.
Another unanswered question is, if octanoyl-CoA is the correct acyl donor, how do acyl-CoAs, localized predominantly in the cytoplasm, gain access to the ER lumen? Although it has been hypothesized that GOAT might have a role in transport of the octanoyl group across the membrane, there is no specific evidence yet reported. GO-CoA-Tat appears to rely on Tat-mediated delivery of the agent into the cytoplasm (Potocky et al., 2003; Schwarze et al., 1999), but interestingly GO-Tat does not appear to block cellular GOAT even though it is homologous to potent product inhibitors that block GOAT in vitro. Perhaps, the CoA moiety in GO-CoA-Tat is crucial for ER entry through the proposed transport properties of GOAT. In addition to the possibility of GOAT participating in substrate transport, other enzyme mechanisms are also formally possible. Octanoate may first be transferred from octanoyl-CoA to an intermediate host, GOAT, another protein, or a lipid. It is even possible that an additional protein collaborates with GOAT to effect acyl transfer.
4.4. Exploring other MBOATs
The MBOATs porcupine and Hhat, acylating Wnt, and hedgehog proteins, respectively, share many features in common with GOAT. A detailed understanding of these pathways is critical for the progress in understanding how these signaling ligands modulate development, stem cell renewal and differentiation, and initiation and maintenance of cancer (Clevers, 2006; Pasca di Magliano and Hebrok, 2003; Reya and Clevers, 2005; Zhao et al., 2009). Techniques learned in studying the enzymology, structure, and function of the GOAT/ghrelin system should be readily translated to these related cases, leading to new inhibitors and insights into structure and function. Porcupine, in particular, is the only other protein known to acylate a serine and should therefore be most mechanistically like GOAT. A family of small molecules targeting porcupine was recently reported (Zhao et al., 2009); analogs of these compounds may also inhibit GOAT or other MBOATs and should be investigated.
4.5. Toward potent small-molecule inhibitors of GOAT
With the development of the first small-molecule inhibitor of GOAT and a high-throughput-ready screen (ELCCA), the prospect of a potent, specific small-molecule inhibitor of GOAT is exciting (Garner and Janda, 2011). Additionally, new in vitro and cell-based assay systems will surely lead to new mechanistic and structural insights and may also be amenable to screening approaches (Barnett et al., 2010; Iwakura et al., 2010; Yang et al., 2008b). With new model systems for GOAT inhibition now established in cells and mice, the efficacy of new compounds can now be evaluated. These compounds could be very promising leads in the treatment of obesity, diabetes, and other metabolic disorders and may provide much needed tools to map out a complete understanding of GOAT in biology.
ACKNOWLEDGMENTS
We thank Brad Barnett, Dan Leahy, Jun Liu, Paul Pfluger, Henriette Kirchner, Matthias Tschöp, and Mehboob Hussain for contributions to these studies and for a critical reading of this chapter. We also thank Don Steiner for helpful discussion. Further, we thank the NIH and Pfeiffer Foundation, Kaufman Foundation, and Keck Foundation for financial support.
REFERENCES
- Andrews ZB, et al. UCP2 mediates ghrelin's action on NPY/AgRP neurons by lowering free radicals. Nature. 2008;454:846–851. doi: 10.1038/nature07181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan AL, et al. Ghrelin secretion in humans is sexually dimorphic, suppressed by somatostatin, and not affected by the ambient growth hormone levels. J. Clin. Endocrinol. Metabol. 2003;88:2180–2184. doi: 10.1210/jc.2002-021169. [DOI] [PubMed] [Google Scholar]
- Barnett BP, et al. Glucose and weight control in mice with a designed ghrelin O-acyltransferase inhibitor. Science. 2010;330:1689–1692. doi: 10.1126/science.1196154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarek MA, et al. Structure-function studies on the new growth hormone-releasing peptide, ghrelin: minimal sequence of ghrelin necessary for activation of growth hormone secretagogue receptor 1a. J. Med. Chem. 2000;43:4370–4376. doi: 10.1021/jm0001727. [DOI] [PubMed] [Google Scholar]
- Bose R, et al. Protein tyrosine kinase-substrate interactions. Curr. Opin. Struct. Biol. 2006;16:668–675. doi: 10.1016/j.sbi.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Bricambert J, et al. Salt-inducible kinase 2 links transcriptional coactivator p300 phosphorylation to the prevention of ChREBP-dependent hepatic steatosis in mice. J. Clin. Invest. 2010;120:4316–4331. doi: 10.1172/JCI41624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buglino JA, Resh MD. Hhat is a palmitoylacyltransferase with specificity for N-palmitoylation of Sonic Hedgehog. J. Biol. Chem. 2008;283:22076–22088. doi: 10.1074/jbc.M803901200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buglino JA, Resh MD. Identification of conserved regions and residues within Hedgehog acyltransferase critical for palmitoylation of Sonic Hedgehog. PLoS One. 2010;5:e11195. doi: 10.1371/journal.pone.0011195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlini VP, et al. Ghrelin increases anxiety-like behavior and memory retention in rats. Biochem. Biophys. Res. Commun. 2002;299:739–743. doi: 10.1016/s0006-291x(02)02740-7. [DOI] [PubMed] [Google Scholar]
- Castaneda TR, et al. Ghrelin in the regulation of body weight and metabolism. Front. Neuroendocrinol. 2010;31:44–60. doi: 10.1016/j.yfrne.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Cerchietti LC, et al. BCL6 repression of EP300 in human diffuse large B cell lymphoma cells provides a basis for rational combinatorial therapy. J. Clin. Invest. 2010;120:4569–4582. doi: 10.1172/JCI42869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamoun Z, et al. Skinny hedgehog, an acyltransferase required for palmitoylation and activity of the hedgehog signal. Science. 2001;293:2080–2084. doi: 10.1126/science.1064437. [DOI] [PubMed] [Google Scholar]
- Chen HY, et al. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology. 2004;145:2607–2612. doi: 10.1210/en.2003-1596. [DOI] [PubMed] [Google Scholar]
- Cheng KY, et al. The role of the phospho-CDK2/cyclin A recruitment site in substrate recognition. J. Biol. Chem. 2006;281:23167–23179. doi: 10.1074/jbc.M600480200. [DOI] [PubMed] [Google Scholar]
- Cleary J, et al. p300/CBP-associated factor drives DEK into interchromatin granule clusters. J. Biol. Chem. 2005;280:31760–31767. doi: 10.1074/jbc.M500884200. [DOI] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Cowley MA, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Date Y, et al. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255–4261. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- de la Cour Dornonville C, et al. Ghrelin stimulates gastric emptying but is without effect on acid secretion and gastric endocrine cells. Regul. Pept. 2004;120:23–32. doi: 10.1016/j.regpep.2004.02.008. [DOI] [PubMed] [Google Scholar]
- De Vriese C, et al. Ghrelin is produced by the human erythroleukemic HEL cell line and involved in an autocrine pathway leading to cell proliferation. Endocrinology. 2005;146:1514–1522. doi: 10.1210/en.2004-0964. [DOI] [PubMed] [Google Scholar]
- Dezaki K, et al. Ghrelin is a physiological regulator of insulin release in pancreatic islets and glucose homeostasis. Pharmacol. Ther. 2008;118:239–249. doi: 10.1016/j.pharmthera.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Diano S, et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nat. Neurosci. 2006;9:381–388. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- Garner AL, Janda KD. cat-ELCCA: a robust method to monitor the fatty acid acyltransferase activity of ghrelin O-acyltransferase (GOAT). Angew. Chem. Int. Ed Engl. 2010;49:9630–9634. doi: 10.1002/anie.201003387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner AL, Janda KD. A small molecule antagonist of ghrelin O-acyltransferase (GOAT). Chem. Commun. (Camb.) 2011;47:7512–7514. doi: 10.1039/c1cc11817j. [DOI] [PubMed] [Google Scholar]
- Groschl M, et al. Evaluation of the comparability of commercial ghrelin assays. Clin. Chem. 2004;50:457–458. doi: 10.1373/clinchem.2003.025429. [DOI] [PubMed] [Google Scholar]
- Guan XM, et al. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res. Mol. Brain Res. 1997;48:23–29. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- Guidez F, et al. Histone acetyltransferase activity of p300 is required for transcriptional repression by the promyelocytic leukemia zinc finger protein. Mol. Cell. Biol. 2005;25:5552–5566. doi: 10.1128/MCB.25.13.5552-5566.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo ZY, et al. The active site His-460 of human acyl-coenzyme A: cholesterol acyltransferase 1 resides in a hitherto undisclosed transmembrane domain. J. Biol. Chem. 2005;280:37814–37826. doi: 10.1074/jbc.M508384200. [DOI] [PubMed] [Google Scholar]
- Gutierrez JA, et al. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc. Natl. Acad. Sci. USA. 2008;105:6320–6325. doi: 10.1073/pnas.0800708105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller RS, et al. Genetic determinants of pancreatic epsilon-cell development. Dev. Biol. 2005;286:217–224. doi: 10.1016/j.ydbio.2005.06.041. [DOI] [PubMed] [Google Scholar]
- Hines AC, Cole PA. Design, synthesis, and characterization of an ATP-peptide conjugate inhibitor of protein kinase A. Bioorg. Med. Chem. Lett. 2004;14:2951–2954. doi: 10.1016/j.bmcl.2004.03.039. [DOI] [PubMed] [Google Scholar]
- Hines AC, et al. Bisubstrate analog probes for the insulin receptor protein tyrosine kinase: molecular yardsticks for analyzing catalytic mechanism and inhibitor design. Bioorg. Chem. 2005;33:285–297. doi: 10.1016/j.bioorg.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Hofmann K. A superfamily of membrane-bound O-acyltransferases with implications for wnt signaling. Trends Biochem. Sci. 2000;25:111–112. doi: 10.1016/s0968-0004(99)01539-x. [DOI] [PubMed] [Google Scholar]
- Howard AD, et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–977. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- Iwakura H, et al. Establishment of a novel ghrelin-producing cell line. Endocrinology. 2010;151:2940–2945. doi: 10.1210/en.2010-0090. [DOI] [PubMed] [Google Scholar]
- Jencks WP. On the attribution and additivity of binding energies. Proc. Natl. Acad. Sci. USA. 1981;78:4046–4050. doi: 10.1073/pnas.78.7.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JW, et al. Uncoupling protein 2 knockout mice have enhanced insulin secretory capacity after a high-fat diet. Diabetes. 2002;51:3211–3219. doi: 10.2337/diabetes.51.11.3211. [DOI] [PubMed] [Google Scholar]
- Kadowaki T, et al. The segment polarity gene porcupine encodes a putative multitransmembrane protein involved in Wingless processing. Genes Dev. 1996;10:3116–3128. doi: 10.1101/gad.10.24.3116. [DOI] [PubMed] [Google Scholar]
- Kamegai J, et al. Chronic central infusion of ghrelin increases hypothalamic neuropeptide Y and Agouti-related protein mRNA levels and body weight in rats. Diabetes. 2001;50:2438–2443. doi: 10.2337/diabetes.50.11.2438. [DOI] [PubMed] [Google Scholar]
- Kanamoto N, et al. Substantial production of ghrelin by a human medullary thyroid carcinoma cell line. J. Clin. Endocrinol. Metabol. 2001;86:4984–4990. doi: 10.1210/jcem.86.10.7891. [DOI] [PubMed] [Google Scholar]
- Kirchner H, et al. GOAT links dietary lipids with the endocrine control of energy balance. Nat. Med. 2009;15:741–745. doi: 10.1038/nm.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Korbonits M, et al. The growth hormone secretagogue receptor. Growth Horm. IGF Res. 1999;9:93–99. doi: 10.1016/s1096-6374(99)80019-7. [DOI] [PubMed] [Google Scholar]
- Lau OD, et al. HATs off: selective synthetic inhibitors of the histone acetyltransferases p300 and PCAF. Mol. Cell. 2000;5:589–595. doi: 10.1016/s1097-2765(00)80452-9. [DOI] [PubMed] [Google Scholar]
- Levinson NM, et al. A Src-like inactive conformation in the abl tyrosine kinase domain. PLoS Biol. 2006;4:e144. doi: 10.1371/journal.pbio.0040144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, et al. Novel ghrelin assays provide evidence for independent regulation of ghrelin acylation and secretion in healthy young men. J. Clin. Endocrinol. Metabol. 2008a;93:1980–1987. doi: 10.1210/jc.2007-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, et al. The structural basis of protein acetylation by the p300/CBP transcriptional coactivator. Nature. 2008b;451:846–850. doi: 10.1038/nature06546. [DOI] [PubMed] [Google Scholar]
- Liu Y, et al. A fasting inducible switch modulates gluconeogenesis via activator/ coactivator exchange. Nature. 2008c;456:269–273. doi: 10.1038/nature07349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek R, et al. Paradoxical enhancement of fear extinction memory and synaptic plasticity by inhibition of the histone acetyltransferase p300. J. Neurosci. 2011;31:7486–7491. doi: 10.1523/JNEUROSCI.0133-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzihradszky D, et al. Solid-phase synthesis of adenosine phosphopeptides as potential bisubstrate inhibitors of protein-kinases. J. Am. Chem. Soc. 1994;116:9413–9419. [Google Scholar]
- Morton GJ, Schwartz MW. The NPY/AgRP neuron and energy homeostasis. Int. J. Obes. Relat. Metab. Disord. 2001;25:S56–S62. doi: 10.1038/sj.ijo.0801915. [DOI] [PubMed] [Google Scholar]
- Nishi Y, et al. Ingested medium-chain fatty acids are directly utilized for the acyl modification of ghrelin. Endocrinology. 2005;146:2255–2264. doi: 10.1210/en.2004-0695. [DOI] [PubMed] [Google Scholar]
- Nussbaum SR, et al. Highly sensitive two-site immunoradiometric assay of parathyrin, and its clinical utility in evaluating patients with hypercalcemia. Clin. Chem. 1987;33:1364–1367. [PubMed] [Google Scholar]
- Ogiso K, et al. Ghrelin: a gut hormonal basis of motility regulation and functional dyspepsia. J. Gastroenterol. Hepatol. 2011;26:67–72. doi: 10.1111/j.1440-1746.2011.06630.x. [DOI] [PubMed] [Google Scholar]
- Oussaief L, et al. Phosphatidylinositol 3-kinase/Akt pathway targets acetylation of Smad3 through Smad3/CREB-binding protein interaction: contribution to transforming growth factor beta1-induced Epstein-Barr virus reactivation. J. Biol. Chem. 2009;284:23912–23924. doi: 10.1074/jbc.M109.036483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagac M, et al. Topology of 1-acyl-sn-glycerol-3-phosphate acyltransferases SLC1 and ALE1 and related membrane-bound O-acyltransferases (MBOATs) of Saccharomyces cerevisiae. J. Biol. Chem. 2011;286:36438–36447. doi: 10.1074/jbc.M111.256511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parang K, et al. Mechanism-based design of a protein kinase inhibitor. Nat. Struct. Biol. 2001;8:37–41. doi: 10.1038/83028. [DOI] [PubMed] [Google Scholar]
- Pasca di Magliano M, Hebrok M. Hedgehog signalling in cancer formation and maintenance. Nat. Rev. Cancer. 2003;3:903–911. doi: 10.1038/nrc1229. [DOI] [PubMed] [Google Scholar]
- Potocky TB, et al. Cytoplasmic and nuclear delivery of a TAT-derived peptide and a beta-peptide after endocytic uptake into HeLa cells. J. Biol. Chem. 2003;278:50188–50194. doi: 10.1074/jbc.M308719200. [DOI] [PubMed] [Google Scholar]
- Prado CL, et al. Ghrelin cells replace insulin-producing beta cells in two mouse models of pancreas development. Proc. Natl. Acad. Sci. USA. 2004;101:2924–2929. doi: 10.1073/pnas.0308604100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- Sagar V, et al. Bisubstrate analogue structure-activity relationships for p300 histone acetyltransferase inhibitors. Bioorg. Med. Chem. 2004;12:3383–3390. doi: 10.1016/j.bmc.2004.03.070. [DOI] [PubMed] [Google Scholar]
- Satou M, et al. Identification and characterization of acyl-protein thioesterase 1/lysophospholipase I as a ghrelin deacylation/lysophospholipid hydrolyzing enzyme in fetal bovine serum and conditioned medium. Endocrinology. 2010;151:4765–4775. doi: 10.1210/en.2010-0412. [DOI] [PubMed] [Google Scholar]
- Schwarze SR, et al. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- Shen K, Cole PA. Conversion of a tyrosine kinase protein substrate to a high affinity ligand by ATP linkage. J. Am. Chem. Soc. 2003;125:16172–16173. doi: 10.1021/ja0380401. [DOI] [PubMed] [Google Scholar]
- Spin JM, et al. Chromatin remodeling pathways in smooth muscle cell differentiation, and evidence for an integral role for p300. PLoS One. 2010;5:e14301. doi: 10.1371/journal.pone.0014301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, et al. Ablation of ghrelin improves the diabetic but not obese phenotype of ob/ob mice. Cell Metab. 2006;3:379–386. doi: 10.1016/j.cmet.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Sun Y, et al. Characterization of adult ghrelin and ghrelin receptor knockout mice under positive and negative energy balance. Endocrinology. 2008;149:843–850. doi: 10.1210/en.2007-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada R, et al. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev. Cell. 2006;11:791–801. doi: 10.1016/j.devcel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Takahashi T, et al. Production of n-octanoyl-modified ghrelin in cultured cells requires prohormone processing protease and ghrelin O-acyltransferase, as well as n-octanoic acid. J. Biochem. 2009;146:675–682. doi: 10.1093/jb/mvp112. [DOI] [PubMed] [Google Scholar]
- Tong J, et al. Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes. 2010;59:2145–2151. doi: 10.2337/db10-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, et al. The leukemogenicity of AML1-ETO is dependent on site-specific lysine acetylation. Science. 2011;333:765–769. doi: 10.1126/science.1201662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierup N, et al. The ghrelin cell: a novel developmentally regulated islet cell in the human pancreas. Regul. Pept. 2002;107:63–69. doi: 10.1016/s0167-0115(02)00067-8. [DOI] [PubMed] [Google Scholar]
- Willesen MG, et al. Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology. 1999;70:306–316. doi: 10.1159/000054491. [DOI] [PubMed] [Google Scholar]
- Yang J, et al. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008a;132:387–396. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Yang J, et al. Inhibition of ghrelin O-acyltransferase (GOAT) by octanoylated pentapeptides. Proc. Natl. Acad. Sci. USA. 2008b;105:10750–10755. doi: 10.1073/pnas.0805353105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi CX, et al. The GOAT-ghrelin system is not essential for hypoglycemia prevention during prolonged calorie restriction. PLoS One. 2012;7:e32100. doi: 10.1371/journal.pone.0032100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JH, et al. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- Zhang CY, et al. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell. 2001;105:745–755. doi: 10.1016/s0092-8674(01)00378-6. [DOI] [PubMed] [Google Scholar]
- Zhao C, et al. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature. 2009;458:776–779. doi: 10.1038/nature07737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao TJ, et al. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc. Natl. Acad. Sci. USA. 2010a;107:7467–7472. doi: 10.1073/pnas.1002271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao TJ, et al. Ghrelin secretion stimulated by {beta}1-adrenergic receptors in cultured ghrelinoma cells and in fasted mice. Proc. Natl. Acad. Sci. USA. 2010b;107:15868–15873. doi: 10.1073/pnas.1011116107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, et al. Synthesis and evaluation of a potent and selective cell-permeable p300 histone acetyltransferase inhibitor. J. Am. Chem. Soc. 2005;127:17182–17183. doi: 10.1021/ja0558544. [DOI] [PubMed] [Google Scholar]
- Zhou A, et al. Proteolytic processing in the secretory pathway. J. Biol. Chem. 1999;274:20745–20748. doi: 10.1074/jbc.274.30.20745. [DOI] [PubMed] [Google Scholar]
- Zhu X, et al. On the processing of proghrelin to ghrelin. J. Biol. Chem. 2006;281:38867–38870. doi: 10.1074/jbc.M607955200. [DOI] [PubMed] [Google Scholar]