Abstract
Human islet research is crucial to understanding the cellular biology of the pancreas in developing therapeutic options for diabetes patients and in attempting to prevent the development of this disease. The national Islet Cell Resource Center Consortium provides human pancreatic islets for diabetes research while simultaneously addressing the need to improve islet isolation and transplantation technologies. Since its inception in 2001, the consortium has supplied 297.6 million islet equivalents to 151 national and international scientists for use in clinical and laboratory projects. Data on the volume, quality, and frequency of shipments substantiate the importance of human islets for diabetes research, as do the number of funded grants for beta-cell projects and publications produced as a direct result of islets supplied by this resource. Limitations in using human islets are discussed, along with the future of islet distribution centers. The information presented here is instructive to clinicians, basic science investigators, and policy makers who determine the availability of funding for such work. Organ procurement coordinators also may find the information useful in explaining to donor families why research consent is so valuable.
The goal of this article is to highlight the use of human pancreatic islets as a critical resource for investigators studying diabetes mellitus. The national Islet Cell Resource (ICR) Center Consortium1 is the first and largest organized cooperative effort in the world to provide human islet preparations to researchers while simultaneously addressing the need to improve isolation and transplantation technologies. According to the National Institutes of Health Research Portfolio Online Reporting Tool,2 in fiscal years 2001 through 2008, $67.2 million was invested in establishing and maintaining the ICR consortium. Data collected by the ICR’s Administrative and Bioinformatics Coordinating Center (ABCC) offer information on the scope, direction, and challenges of diabetes research involving human pancreatic islets from basic laboratory science and clinical perspectives.
Possibly the greatest value of islet research is in the prospect of better understanding the uniqueness of human islet biology in the native pancreas, and other environments, to expand therapeutic options for diabetes patients and prevent development of the disease. Herein, we discuss the need for human pancreatic islets, including the various approaches and tools used to study the production, regulation, and endocrine action of islets, as well as the scientific impact of the research. The major limitations in using human islets are discussed, including the supply and demand dilemma and factors limiting consistent and widespread availability of these preparations for medical research purposes. The sustained support of islet distribution centers as a resource for diabetes investigators is considered. We conclude by examining alternatives to using human islets and discussing relevant clinical applications. The use and support of human pancreatic islets in the study of diabetes is a timely and critical topic for scientific and therapeutic advances.
Need for Human Pancreatic Islets
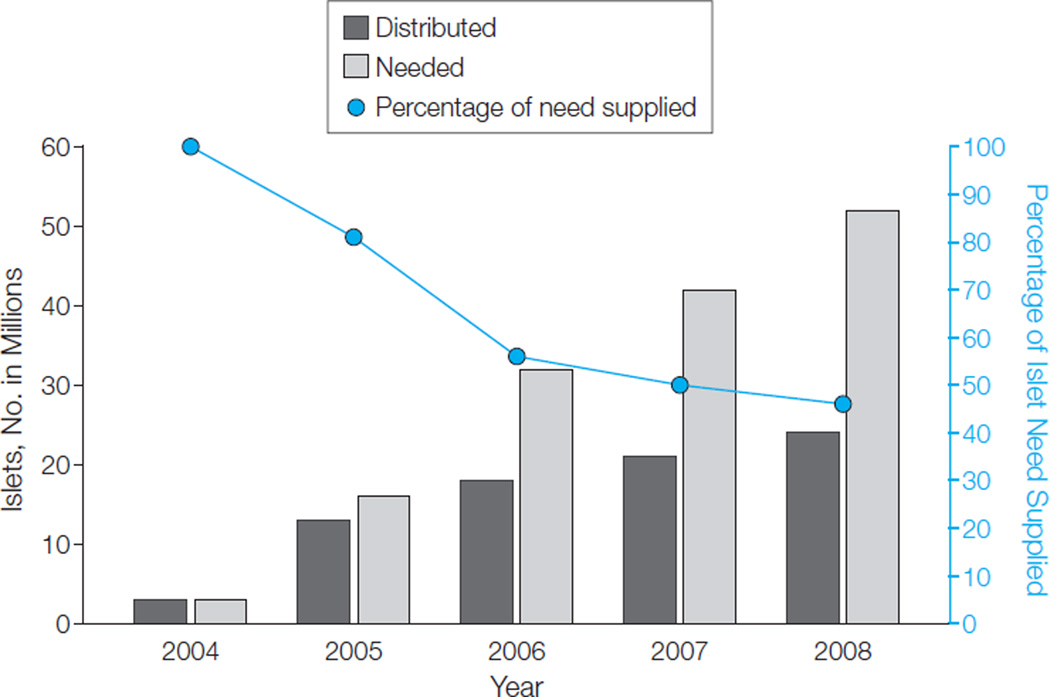
Information available through PubMed and Computer Retrieval of Information on Scientific Projects databases shows that there is an increasing reliance by investigators worldwide on the use of human pancreatic islets to conduct research studies. Perhaps the best way to demonstrate this need is to examine the requested volume, quality, and frequency of islet preparations. These data are available through the ICR ABCC and show that, for ICR-approved investigators, requests for human pancreatic islets for use in basic science research have increased substantially over the past several years, with a demand of 52.3 million islets equivalents (IEQs) in 2008 (Figure). (The word islet is used interchangeably with the term .IEQ. One IEQ represents an islet of a typical size equal to 150 µm in diameter. This scale was introduced nearly 20 years ago to account for islets of different sizes within a pancreas and adopted by all islet isolation laboratories as a standard convention.3)
Figure. Islet Demand of Investigators Approved by the Islet Cell Resource Program.
Through August 2008, 156 diabetes laboratory research projects were approved by the Islet Cell Resource (ICR) basic science human islet distribution program. The first shipment of human islets through the program occurred on February 12, 2004.
Although there was at least a 15% increase in production each year in response to the burgeoning demand, the ICR centers decreased from supplying 81% of the requested total in 2005 to only 46% in 2008. In early 2007, an automated Web-based optimization algorithm for the matching and distribution of pancreatic islet preparations to waiting approved investigators was implemented to improve allocation and demonstrate the efficiency of a central islet coordinating center,4 yet the disparities between supply and demand remain.
From its inception in 2001, a total of 14 academic institutions across the United States have been named as ICR centers with 8 currently active and funded. Prior to providing human pancreatic islets, each ICR had to demonstrate compliance with current good-manufacturing-practice facility requirements via an external audit; although all centers certified as having current good manufacturing practices were allowed to provide islets to approved investigators for basic research purposes, only a limited number of highly qualified centers, who demonstrated additional stringent clinical proficiency, supplied islets for clinical transplantation.
The ICR laboratories are responsible for providing human pancreatic islets to qualified investigators for use in transplantation protocols approved by institutional review boards and the Food and Drug Administration, or for basic biological research studies. Eligibility for receipt of ICR islets is determined through an application process designed to minimize the unnecessary review of mature research studies while helping to constructively develop those that may require additional assistance. Applications for islets can be found on the ICR consortium Web site.5
Through August 2008, a total of 182 applications (156 basic science, 26 clinical transplant) were processed and approved by the ICR consortium, representing an increase from only 10 applications in 2001. Among the clinical protocols, 15 involved the transplantation of islets alone into eligible type 1 diabetic patients, in 5 studies islets were transplanted into patients following a kidney transplant, 3 protocols involved simultaneous islet and kidney transplantation, and 3 were double–intervention group studies comparing more than 1 approach (Table 1). Clinical trials outcome data from these and other islet transplantation protocols have been recently published in the most complete collection of human islet transplants reported to date.6
Table 1.
Clinically Transplanted Islet Preparations Supplied by the ICR Consortium
| Treatment Protocols | Approved Studiesa (n = 26) |
Pancreata Processed for Transplantb (n = 186) |
Patients Receiving ICR Islets (n = 99) |
Transplants Performed (n = 170) |
|---|---|---|---|---|
| Islet alone | 15 | 124 | 69 | 116 |
| Islet after kidney | 5 | 62 | 30 | 54 |
| Simultaneous islet and kidney | 3 | NR | NR | NR |
| Double intervention group Islet alone/islet after kidney |
2 | NR | NR | NR |
| Islet after kidney/simultaneous islet and kidney | 1 | NR | NR | NR |
Abbreviations: ICR, Islet Cell Resource; NR, none reported.
Three applications not reported. Of those, 1 was submitted and denied based on lack of preliminary clinical data and concerns about study feasibility and experimental design. The remaining 2 studies were deferred for further development.
Through August 2008, a total of 201 (169 ICR + 32 non-ICR) pancreata were processed by the ICR consortium for clinical transplantation purposes. These records were linked to available transplant recipient data provided by the Collaborative Islet Transplant Registry (CITR). Using the United Network for Organ Sharing identification number to merge data sets, 186 pancreata were matched to 99 patients receiving 170 transplants. Of the 15 isolations that were not matched to CITR data, 5 preparations were reported for use in simultaneous islet and kidney, 9 islet alone, and 1 islet after kidney.
The approved basic science applications using human islets provided by the ICR centers have been categorized into major areas and subareas of research (Table 2). Categorization of proposals was self-reported, and multiple categories could be chosen. The fact that 59 of these proposals (38%) spanned more than 1 major area or subarea of research highlights the interdisciplinary nature of these studies. Of 182 ICR-supported studies, 119 (65%) were sponsored by the US government, internationally recognized foundations, or both, including the National Institutes of Health (n=65), Juvenile Diabetes Research Foundation (n=36), American Diabetes Association (n=4), National Aeronautics and Space Administration (n=2), National Science Foundation (n=1), or a combination of 1 or more sponsors (n=11). Publicly available funding data2,7,8 were available for 93 of 119 studies and exceeded $83 million.
Table 2.
Diabetes Research Projects Supported by the ICR Consortium by Major Area of Researcha
| Islets Distributed |
Extramural Fundingb |
|||
|---|---|---|---|---|
| Subarea of Research | Studies No. (n = 156) |
Islets, No (%) (n = 71134 199) |
Studies No. (n = 93) |
Grant Amount, $ (n = $83 229 753) |
| Prevention and Treatment | ||||
| Preservation of beta-cell mass and function | 15 | 7407 644 (10.41) | 7 | 4 502412 |
| Autoimmunity (beta-cell destruction and transplant rejection) | 8 | 4 135 665 (5.81) | 6 | 7 793257 |
| Clinical interventions (islet transplantation, beta-cell replacement, gene therapy) | 13 | 4 346 700 (6.11) | 8 | 8820193 |
| Generation of beta cells from stem cells | 10 | 4 740 999 (6.66) | 5 | 5 103901 |
| Beta-cell growth/differentiation | 17 | 6 506 151 (9.15) | 15 | 17 122272 |
| Other | ||||
| Islet/beta-cell imaging | 2 | 948 658 (1.33) | 2 | 1 229342 |
| Islet/beta-cell assessment | 3 | 4 055 264 (5.70) | 1 | 532017 |
| In vitro islet preservation | 0 | 0 | 0 | 0 |
| Pharmaceutical research and drug testing | 4 | 80 000 (0.11) | 1 | 3 274021 |
| ≥2 Prevention and treatment subareas | 14 | 7 441 596 (10.46) | 9 | 6 336554 |
| PathoPhysiology | ||||
| Genetics | 3 | 992 500 (1.40) | 2 | 759870 |
| Insulin (structure, function, action, resistance) | 0 | 0 | 0 | 0 |
| Metabolism | 0 | 0 | 0 | 0 |
| Glucose homeostasis | 3 | 868 750 (1.22) | 3 | 2 463496 |
| Endocrine pancreas | 1 | 290 500 (0.41) | 1 | 548440 |
| Cell signaling and regulation | 6 | 541 800 (0.76) | 2 | 427328 |
| Other | ||||
| Islet/beta-cell imaging | 0 | 0 | 0 | 0 |
| Beta-cell regeneration, survival, and death | 2 | 0 | 1 | 193 600 |
| Virology and autoimmunity | 1 | 1 501 790 (2.11) | 0 | 0 |
| ≥2 Pathophysiology subareas | 2 | 2 861 290 (4.02) | 2 | 1 529560 |
| Both Major Areas or Not Reported | ||||
| ≥2 Any subareas | 43 | 22289 392(31.33) | 25 | 21 958061 |
| Not reported | 9 | 2 125 500 (2.99) | 3 | 635429 |
Abbreviation: ICR, Islet Cell Resource.
Investigators were asked to categorize their research by selecting 1 or more subareas from the list here. One hundred fifty-six proposals were approved between February 12, 2004, and August 31, 2008. Six applications were not approved because of unaddressed concerns about the experimental design (n=5) or excessive islet use (n=1).
One hundred nine of 156 applications were peer-reviewed and funded prior to being submitted to the ICR consortium. Information on funding was publicly available for grants partially or exclusively funded by the National Institutes of Health (NIH),2 Juvenile Diabetes Research Foundation,7 and National Science Foundation8 (n=97) but not for those solely supported by the American Diabetes Association (n=4), National Aeronautics and Space Administration (n=2), or intramural NIH awards (n=6). Of the 97 projects with grant funding data, 1 application was excluded because it could not be matched to NIH dollar amount and 3 because they shared the same grant number with another project (eg, collaborative studies).
Islet Use
High-volume distribution of human pancreatic islets is necessary to establish an adequate supply for repeated experimentation. Data from the ICR ABCC show that 297.6 million islets were produced by 14 ICR laboratories between September 1, 2001, and August 31, 2008 (Table 3). Of those, 199.4 million islets (67%) were used for basic science research, 91.6 million islets (31%) for clinical purposes, and 6.5 million (2%) were not used for various reasons ranging from consent limitations to poor-quality pancreata or islets.
Table 3.
Islet Production by the ICR Consortium According to Islet Use, 2001 –2008
| No. (%) |
||||
|---|---|---|---|---|
| Total, No. | Basic Science Research |
Clinical Transplant |
Not Useda | |
| Pancreatab | 1072 | 805 (75) | 201 (19) | 66 (6) |
| Total islets | 297563 676 | 199 427 452 (67) | 91 633 202 (31) | 6 503 022 (2) |
Abbreviation: ICR, Islet Cell Resource.
Reasons that islets from a pancreas were not used include lack of consent to use islets for research, poor-quality organ or islets, extended cold ischemia time, and pancreas processing problems.
For data reported to the ICR Administrative and Bioinformatics Coordinating Center from September 1, 2001, through August 2008.
Data from the ICR distribution program indicate that 71.1 million of the 199.4 million islets were distributed within 2593 shipments for use in ICR-approved studies requiring human islets for basic research (Table 2). The remaining 128.3 million islets were used for ICR intramural studies to optimize human islet production and study the parameters that affect the quality and performance of islets for transplantation and research. The 91.6 million islets produced for clinical purposes represented 201 islet isolations used for 170 human transplants. Although a majority of these transplants were performed in hospitals with an ICR facility, 5 of these isolations were processed and sent to geographically remote facilities to perform 3 clinical transplants.
Scientific Importance
The importance of human pancreatic islets, clinically or for basic science research, is substantiated by the number and quality of studies being performed that rely on these preparations. Data available through the ICR as of August 2008 indicate that a total of 151 national and international scientists received human islets for use in both intramural research performed by the consortium as well as 182 clinical and basic science projects submitted to the consortium for support. These studies have resulted in 201 publications directly attributable to the use of human islets supplied by ICR laboratories (maintained on the consortium Web site).5 An Institute for Scientific Information Web of Knowledge search9 yielded information on 186 of these citations and showed that articles appeared in more than 50 different journals, which had a median impact factor of 22, and were cited 2771 times from 2002 to 2008. According to all available data on human islet–based research, these preparations are clearly an essential and critical resource for medical and scientific investigators.
Supply and Demand Issues Surrounding Human Islets
Pancreas Donations
The production of human islets is contingent on the availability of pancreata. According to information from the Organ Procurement and Transplantation Network database, there were 56 888 deceased organ donors in the United States between January 1, 2001, and November 30, 2008.10 Of those, 11 204 of the 15 197 recovered pancreata (74%) were used for whole organ transplantation. Although information was not specified on the use of the remaining 3993 pancreata, ICR ABCC data for the same time frame indicate that at least 1212 (30%) were used to isolate human islets.
Table 4 and Table 5 outline the general organ donor characteristics available for 1142 of 1212 ICR-processed pancreata. Several articles have described what constitutes optimal donor criteria for human pancreata used by isolation laboratories.11,12 A wide range of values exist for many of these parameters, which may not have been previously appreciated by medical professionals responsible for referring organ donors or those in charge of coordinating the donation process.
Table 4.
Anthropometric and Laboratory Measurements of Organ Donors Contributing Pancreata for Human Islet Isolationa
| Continuous Variable | Median (Range)b | No. of Donors Without Missing Data |
|---|---|---|
| Age, y | 47.2 (1.3–70.8) | 1141 |
| Weight, kg | 85 (8–200) | 1142 |
| Body mass indexc | 28.1 (14.7–69.2) | 1142 |
| Number of donated organsd | 4 (2–6) | 1142 |
| Serum creatinine, mg/dLe | 1 (0–20) | 1123 |
| Blood urea nitrogen, mg/dLe | 13 (0–110) | 1123 |
| Total bilirubin, mg/dLe | 1 (0–12) | 1112 |
| Aspartate aminotransferase, U/Le | 38 (7–3886) | 1116 |
| Alanine aminotransferase, U/Le | 31 (3–3440) | 1116 |
| Serum lipase, U/Le | 27 (0–1292) | 1042 |
| Serum amylase, U/Le | 58 (0–3875) | 1052 |
SI conversion factors: To convert serum creatinine to µmol/L, multiply by 88.4; urea nitrogen to mmol/L, multiply by 0.357; bilirubin to µmol/L, multiply by 17.104; aspartate aminotransferase, alanine aminotransferase, lipase, and amylase to µkat/L, multiply by 0.0167.
A total of 1212 pancreas islet isolation records were entered into the Islet Cell Resource (ICR) Administrative and Bioinformatics Coordinating Center database from December 3, 2004, to January 5, 2009, for human pancreata obtained from July 22, 2001, through December 31, 2008, from 14 ICR laboratories. Of those, 1142 records were linked to pancreas donor data obtained from the United Network for Organ Sharing.
Median values were reported exclusively for all continuous variables to establish general ranges but do not necessarily indicate skewness in the data.
Calculated as weight in kilograms divided by height in meters squared.
Organs that were donated included kidney (left, right, or both), lung (left, right, or both), pancreas, heart, liver, and intestine.
Values obtained from the donor less than 24 hours prior to cross-clamp and reported as terminal laboratory data.
Table 5.
Gender and Medical History of Organ Donors Contributing Pancreata for Human Islet Isolation (n=1142)a
| No. (%) of Donors Without Missing Data |
||
|---|---|---|
| Categorical Variable | Yes | No |
| Male sex | 636 (55.7) | 506 (44.3) |
| Donation after cardiac death | 39 (3.4) | 1103 (96.6) |
| History of diabetes | 26 (2.3) | 1114 (97.7) |
| Insulin dependence | 7 (30.4) | 16 (69.6) |
| History of hypertension | 418 (36.9) | 714 (63.1) |
| Heavy alcohol use | 176 (18.9) | 755 (81.1) |
| History of cigarette use | 435 (38.4) | 698 (61.6) |
| History of cocaine use | 117 (10.5) | 1002 (89.5) |
| History of other drug use | 273 (24.4) | 844 (75.6) |
| Cause of death | ||
| Cerebrovascular/stroke | 648 (56.9) | |
| Head trauma | 378 (33.2) | |
| Otherb | 113 (9.9) | |
A total of 1212 pancreas islet isolation records were entered into the Islet Cell Resource (ICR) Administrative and Bioinformatics Coordinating Center database from December 3, 2004, to January 5, 2009, for human pancreata obtained from July 22, 2001, through December 31, 2008, from 14 ICR laboratories. Of those, 1142 records were linked to pancreas donor data obtained from the United Network for Organ Sharing.
Other reported causes of death include anoxia (n=92), central nervous system tumor (n=8), bacterial meningitis (n=2), spontaneous cranial bleed (n=2), hydrocephalus (n=2), cerebral edema (n=2), cerebral aneurysm (n=1), brain abscess (n=1), acute respiratory distress syndrome (n=1), left carotid dissection (n=1), and drug overdose (n=1).
Facility Operations
The production of human pancreatic islets requires a pancreas-processing step prior to their use in clinical or basic laboratory research. This creates a unique set of challenges,13 akin to assembly line manufacturing, that require standardization and optimization to minimize disruption of the islet supply for therapeutic and translational research purposes. Each isolation facility must have an infrastructure in place to address regulatory compliance and other factors, including personnel qualifications and training; facility design; reagent, equipment, and supply verification; production and product controls; process validation; adequate documentation; and environmental monitoring. In the ICR model, with the exception of reagents for clinical transplantation, all of the infrastructure expense associated with islet production was paid for by consortium funds.
Moreover, isolation centers manufacturing human islets for clinical purposes must do so under current good-manufacturing-practice regulatory guidelines.14 The cost to establish a current good-manufacturing-practice– compliant facility can range from $1 million to $7 million and annual maintenance from $0.8 million to $3 million,15 highlighting the need for regional laboratories. Even in a facility compliant with current good manufacturing practices, unforeseen circumstances can compromise a laboratory, as was the case in 2007 when nearly all human islet transplants were halted because of concern over the risk of bovine spongiform encephalopathy in Liberase HI (Roche Diagnostics, Indianapolis, Indiana), a critical and specialized collagenase enzyme blend used by nearly all islet isolation facilities to digest pancreata.16
Organ Acquisition Costs
The cost to procure a pancreas represents a formidable fiscal barrier to islet isolation laboratories.17 Data from the ICR consortium show that for 665 pancreata acquired from 2001 to 2008, standard acquisition charges ranged remarkably from a low of $600 to a high of $39 800 (Table 6). Several actions have been suggested to address the discrepancy in cost17; however, this remains an important consideration that limits the ability of facilities wanting to isolate human pancreatic islets.
Table 6.
Standard Acquisition Charges for Pancreata Acquired by the ICR Consortium According to Islet Use, 2001–2008
| Basic Science Research |
Clinical Transplant | Not Used | |
|---|---|---|---|
| Pancreata, No.a | 561 | 86 | 18 |
| Charge, mean (SD), $ | 3665 (2983) | 18 965 (9891)9) | 4349(3087 |
| Charge, median (range), $ | 3000 (600–39 800) | 20 350 (1500–39 800) | 4000 (1600–16 000) |
Abbreviation: ICR, Islet Cell Resource.
Through October 29, 2008, pancreas acquisition charges for 665 islet preparations were available and reported to the ICR Administrative and Bioinformatics Coordinating Center. Fourteen ICR centers reported receiving pancreata from 58 organ procurement organizations throughout the United States during this time.
Islet Shipment
Investigators using human islets are often located far from the pancreas processing facility producing them, necessitating standardized shipping strategies that preserve the mass, quality, and function of the preparation while avoiding contamination. Although shipment protocols for clinical transplantation purposes are still being developed,18 those created for basic science islet distribution have been successfully standardized and are available through the ICR ABCC.5 The fact that only 3 ICR centers have been prepared to ship islets to remote locations for clinical use,19 and only 1 has established an ongoing collaboration with a distant transplant facility, underscores in part the complex requirements for both the islet isolation facility producing the islets and the transplant center using the graft.20 In contrast, 2593 basic science islet shipments have been made by the consortium. Data available on 2124 shipments show that investigators found the quality of islets, by subjective evaluation, to be excellent in 681 shipments (32%), good in 1118 (53%), fair in 218 (10%), and poor in 117 (5%).
Sustained Production
While the number of studies requiring human islets is at a 5-year high, so are the observations that human islets differ considerably from their nonhuman counterparts.21,22 Nearly 20 years after automating the isolation procedure for human pancreatic islets,23 and through the collaborative efforts of government and private foundation sponsorship, an islet distribution infrastructure has been built and is increasingly in demand by investigators worldwide for both basic science research and clinical transplantation. Responding to this demand will require confronting the escalating costs and complexities of maintaining and improving an islet infrastructure capable of responding to the changing needs of the diabetes community. For the ICR consortium, this has meant addressing facility and production challenges, such as those with personnel, reagents, and equipment, as well as attempts to continually improve islet isolation and transplantation technologies, such as pancreas preservation, organ procurement and processing, predictive islet potency testing, and shipping conditions of human islets for either clinical or basic research purposes.
Alternatives to Using Human Islet Preparations
For more than 30 years, pancreatic islets have been used as experimental clinical and laboratory models in diabetes research.24,25 Islets have been obtained from and examined in diverse animal models, including monkey, pig, rat, mouse, hamster, rabbit, and dog.26–28 In addition, islet-like insulin-producing cells have been generated and studied from germ-cell precursors and mature tissues and organs other than the pancreas. Considerable molecular, cellular, and genetic data have been collected from these studies.29 The various approaches pursued are described here.
Surrogates for Human Beta Cells
Several strategies to restore human beta-cell function have been explored to reduce or abolish the need for pancreas-derived isolated human islets.30,31 The first focuses on provoking beta-cell regeneration in vivo through the use of pharmacological and other beta-cell stimulatory agents. This approach is supported by the observations that beta-cell mass is increased in human obesity32 and pregnancy33 and that beta cells can be stimulated to grow in mice.34
The second and third strategies, respectively, seek to induce ex vivo expansion of pancreatic beta cells by either exploiting the proposed models of human beta-cell mass maintenance through differentiation of a stem cell or progenitor population or by beta-cell self-replication. Literature supporting these mechanisms primarily have relied on the use of rodent and other non-human models, resulting in controversy when attempting to validate these findings using human pancreata.35
A fourth approach is based on the transformation of one cell to another using mature tissues or organs other than the pancreas.36 Such transdifferentiation has been performed using bone marrow, intestinal, and liver cells and results in cells that are capable of producing insulin.31 A recent study combining several of these approaches demonstrated for the first time in vivo the ability to convert pancreatic exocrine tissue from adult mice into cells that mimic beta cells by introduction of 3 key transcription factors into the splenic lobe of the dorsal pancreas.37 Yet many fundamental clinical questions remain unanswered regarding surrogate beta cells, such as the pleiotropic effects, oncogenic potential, autoimmune properties, and long-term metabolic function.
Cell Lines
Given the innate variability of biological samples and the cost of human islet isolation, a common renewable reference standard is desirable for investigational and therapeutic purposes. Unlike the cancer model where tumor-derived immortal cell lines are used for preliminary experimentation, no such acceptable substitute yet exists for human islets. A limited number of human beta-cell lines, together with numerous rodent-derived insulinoma cell lines, have proven useful as models for certain aspects of human beta-cell maturation and function.38,39 Although promising, these models have not yet reached the point of therapeutic potential. The main obstacles are similar to those for surrogate beta cells,40 namely long-term cell survival, responsiveness to glucose, and oncogenicity.
Nonhuman Islets
Nonhuman pancreas–derived isolated islets have been used therapeutically41–43 and have been in widespread use experimentally for decades. Islets from swine, primates, and rodents have been used primarily with the rodent model predominating in basic research and preclinical studies of diabetes. Benefits of the rodent model include short generational times, control of environment, homogeneity of genetic background, ease of genomic manipulations, ability to selectively breed, and the relative inexpensiveness of husbandry. Compared with human islets, rodent islets can be isolated in less time, at a fraction of the cost, with a robust insulin secretory response, and in sufficient quantities for experimentation.
Limitations of Alternate Models
Although rodent islets have been used extensively to study various aspects of islet function, basic science studies have shown important differences between human and rodent islets. Early work demonstrated that the beta-cell cytotoxic effects of agents seen in rodent islets was not observed in human preparations,21 suggesting a species-specific response to beta-cell injury. Independent laboratories established that the proportion of beta cells in a human islet are nearly one-third less than that found in the mouse and that, although cell types within the mouse islet cluster together, the same was not true of the human counterpart.44,45
This difference in beta-cell composition accounts for the robust insulin secretion seen in rodent islets, and it raises the possibility that there is an intra-islet mediated concomitant increase of cytokine secretion not seen in human preparations. For example, while vascular endothelial growth factor has been shown to be an important regulator of islet vascularization in rodents,46 recent evidence now implicates human duct cells, but not islets, as the main source of production of vascular endothelial growth factor.22 Taken together with the differences in islet architecture between human and rodents, it is not surprising that there exists species-specific models of islet vascularization and blood flow.47 Recently, Parnaud et al48 showed that, unlike those purified from rats, beta cells from fully differentiated human islets were not capable of proliferating in vitro when grown using extracellular matrix and various growth factor stimulants. These observations illustrate the ongoing critical requirement for human pancreatic islets.
Clinical Use of Human Islets
The primary objective of islet-based research is to cure diabetes. Perhaps the most prominent clinical application of this research is currently in the form of cell replacement therapy. With the exception of 1 report in a type 2 diabetic cohort,47 islet transplantation has been used exclusively for a subset of individuals with type 1 diabetes mellitus and was shown, at least temporarily, to improve glucose control and, in a few cases, to lead to insulin independence. One-year insulin independence success rates of 10% were seen prior to the introduction of the Edmonton protocol in 2000, subsequently improving to 80% to 90% when this protocol was performed at established centers.49 Although the insulin independence rate declined to approximately 10% at 5 years after transplantation, 80% of the recipients continued to have islet graft function with near-normal glycosylated hemoglobin and were protected from severe hypoglycemia, the most common indication for islet transplantation.50
Illustrating the complexity of the procedure,49 success rates vary greatly across transplantation centers, with 1 facility reporting a 4-year insulin independence rate exceeding 50% in single-donor transplants.51 A recent study of 325 adult islet transplant recipients concluded that the procedure significantly improved metabolic control for individuals with previously labile disease.6
Although islet transplantation has been shown to offer both protection against long-term complications of the disease and significant improvement in quality of life,52,53 several obstacles remain, such as limited engraftment,54–57 chronic immunosuppression, and inconsistent supply of human islets. These issues must be addressed if the procedure is to be used as a standard of care for qualified individuals.
CONCLUSIONS
The use of human pancreatic islets can serve as a gold standard for the assessment of beta-cell function in the future, as clinical research advances toward a cure for diabetes. Investigators seeking to understand the biology of human islets have approached the problem in a variety of ways. Some have used surrogate beta cells and cell lines while others have focused on pancreas-derived isolated islets from human or nonhuman sources. Islets and islet-like cells have been used experimentally and clinically to increase beta-cell mass. Basic research studies have shown species-specific differences in islets, especially between human and rodent models, when examining proliferative potential, differentiation capacity, response to injury, composition and organization of cell clusters, and type and amount of secreted products. Clinical studies have been promising, but the challenges continue to include limited engraftment of the islet preparation after transplant, chronic immunosuppression of the individual, and availability of human islets.
Islet sharing networks have been established to help address the supply and demand issues faced by islet isolation laboratories and clinical and laboratory scientists. These distribution networks have had a global influence on diabetes research but face economic obstacles in preserving the availability of human islets in a growing research community. In the 5-year history of the ICR basic science distribution program, islets were provided free of charge for all but 11 months of this initiative. Had a subscription fee been maintained throughout the program, it is conceivable that yearly production may have increased further. On the other hand, investigators faced with the prospect of paying for islets, and unable to secure long-term funding to do so, may have reduced the total number of islets requested.
Human pancreatic islets will be critical for restoration of beta-cell function in patients with diabetes. Even given adequate funding levels, the ongoing challenges to supplying human islets must be addressed for the successful exploration of therapeutic options for this chronic and debilitating disease.
Acknowledgments
Funding/Support: This work was funded by the cooperative efforts of the National Center for Research Resources (NCRR) and the National Institute of Diabetes and Digestive and Kidney Diseases, a component of the US National Institutes of Health (NIH), in conjunction with the generous contributions of the Juvenile Diabetes Research Foundation. Messrs Kaddis and Cravens, Mss Olack and Sowinski, and Dr Niland are supported by grants U42 RR 017673 ( J.C.N.) and U42 RR 023246 (J.L.C.) from the NCRR.
Role of the Sponsor: The funding organizations had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Mr Kaddis had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kaddis, Olack, Sowinski, Niland.
Acquisition of data: Kaddis, Cravens.
Analysis and interpretation of data: Kaddis, Cravens, Contreras, Niland.
Drafting of the manuscript: Kaddis, Olack, Sowinski, Contreras, Niland.
Critical revision of the manuscript for important intellectual content: Kaddis, Olack, Sowinski, Cravens, Niland.
Statistical analysis: Kaddis, Cravens, Niland.
Obtained funding: Niland.
Administrative, technical, or material support: Kaddis, Olack, Sowinski, Contreras, Niland.
Study supervision: Niland.
Financial Disclosures: Mr Cravens reported owning an American Depositary Receipt (ADR) in Roche Diagnostics. No other disclosures were reported.
Disclaimer: The interpretation and reporting of organ donor data are the responsibilities of the authors and in no way should be seen as an official policy of or interpretation by the Organ Procurement and Transplantation Network (OPTN) or the US government.
Additional Information: Additional related materials are available from the author, including a list of all ICR Consortium members (past and present), a statistical comparison of articles and grants using human vs mouse vs rat islets, a map of cities around the world using human islets for research, a narrative on the history of islet-sharing networks, and a list of publications attributable to the use of human islets provided by the ICR Consortium.
Additional Contributions: Organ donor information was supplied by the United Network for Organ Sharing as the contractor for the OPTN. Portions of the clinical data were supplied by the Collaborative Islet Transplant Registry (CITR). The CITR is a voluntary effort comprising 33 islet transplant centers in North America, Europe, and Australia and is supported by NIH contract N01-DK-1–2472 to the EMMES Corporation, Rockville, Maryland. Martha Antler and Tracey Stiller, MS, City of Hope National Medical Center, assisted in gathering some of the data for this article. Alvin Powers, MD, Vanderbilt University; Andrew F. Steward, MD, University of Pittsburgh; and Ali Naji, MD, PhD, University of Pennsylvania, participated in discussions leading to the genesis of this article. Dixon Kaufman, MD, Northwestern University, and Daniel Rosenblum, MD, NCRR, provided helpful comments during the revision process prior to manuscript submission. None of these individuals received compensation for their contributions.
REFERENCES
- 1.Knazek RA. The human pancreatic islet cell resource consortium. Diabetes Technol Ther. 2002;4(4):551–552. doi: 10.1089/152091502760306652. [DOI] [PubMed] [Google Scholar]
- 2. [Accessed October 30, 2008];National Institutes of Health Research Portfolio Online Reporting Tool (RePORT) http://report.nih.gov/index.aspx.
- 3.Ricordi C. Quantitative and qualitative standards for islet isolation assessment in humans and large mammals. Pancreas. 1991;6(2):242–244. doi: 10.1097/00006676-199103000-00018. [DOI] [PubMed] [Google Scholar]
- 4.Qian D, Kaddis J, Niland JC. A matching algorithm for the distribution of human pancreatic islets. Comput Stat Data Anal. 2007;51(12):5494–5506. doi: 10.1016/j.csda.2007.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. [Accessed December 3, 2008];Islet Cell Resource Center Consortium Web site. http://icr.coh.org/.
- 6.Alejandro R, Barton FB, Hering BJ, Wease S. Collaborative Islet Transplant Registry Investigators. 2008 Update from the Collaborative Islet Transplant Registry. Transplantation. 2008;86(12):1783–1788. doi: 10.1097/TP.0b013e3181913f6a. [DOI] [PubMed] [Google Scholar]
- 7. [Accessed November 17, 2008];Juvenile Diabetes Research Foundation International funded research database. http://onlineapps.jdfcure.org/AbstractSearchEngine.cfm.
- 8. [Accessed November 9, 2008];National Science Foundation award search. http://www.nsf.gov/awardsearch/.
- 9.Web of science and journal impact report tools. Institute for Scientific Information (ISI) Web of Knowledge; [Accessed February 18, 2009]. http://www.isiwebofknowledge.com/. [Google Scholar]
- 10. [Accessed February 20, 2009];Organ Procurement and Transplantation Network (OPTN) national data reports. http://www.optn.org/latestData/step2.asp?.
- 11.Lakey JR, Warnock GL, Rajotte RV, et al. Variables in organ donors that affect the recovery of human islets of Langerhans. Transplantation. 1996;61(7):1047–1053. doi: 10.1097/00007890-199604150-00010. [DOI] [PubMed] [Google Scholar]
- 12.Hanley SC, Paraskevas S, Rosenberg L. Donor and isolation variables predicting human islet isolation success. Transplantation. 2008;85(7):950–955. doi: 10.1097/TP.0b013e3181683df5. [DOI] [PubMed] [Google Scholar]
- 13.Pileggi A, Cobianchi L, Inverardi L, Ricordi C. Overcoming the challenges now limiting islet transplantation: a sequential, integrated approach. Ann N Y Acad Sci. 2006;1079:383–398. doi: 10.1196/annals.1375.059. [DOI] [PubMed] [Google Scholar]
- 14.Zoon KC. [Accessed March 17, 2009];Letter to transplant centers: allogeneic pancreatic islets for Transplantation. http://www.fda.gov/cber/ltr/allpan090800.htm.
- 15.Lakey JR, Ricordi C, Hering BJ. Update and new features in cGMP facility, equipment and structure of the clinical islet transplant program. Paper presented at: Human Islet Isolation and Transplantation Techniques (HIITT) 6th Training Workshop; April 9–12, 2006; Snowbird, Utah. [Google Scholar]
- 16. [Accessed September 17, 2008];Risk of bovine spongiform encephalopathy (BSE) in collagenase enzymes. International Society of Cellular Therapy Web site. http://www.celltherapysociety.org/files/PDF/Resources/Risk_BSE_in_Collagenase_Enzymes.pdf.
- 17.Markmann JF, Kaufman DB, Ricordi C, Schwab PM, Stock PG. Financial issues constraining the use of pancreata recovered for islet transplantation: a white paper. Am J Transplant. 2008;8(8):1588–1592. doi: 10.1111/j.1600-6143.2008.02305.x. [DOI] [PubMed] [Google Scholar]
- 18.Ichii H, Sakuma Y, Pileggi A, et al. Shipment of human islets for transplantation. Am J Transplant. 2007;7(4):1010–1020. doi: 10.1111/j.1600-6143.2006.01687.x. [DOI] [PubMed] [Google Scholar]
- 19.Yang Z, Chen M, Deng S, et al. Assessment of human pancreatic islets after long distance transportation. Transplant Proc. 2004;36(5):1532–1533. doi: 10.1016/j.transproceed.2004.04.075. [DOI] [PubMed] [Google Scholar]
- 20.Goss JA, Goodpastor SE, Brunicardi FC, et al. Development of a human pancreatic islet-transplant program through a collaborative relationship with a remote islet-isolation center. Transplantation. 2004;77(3):462–466. doi: 10.1097/01.TP.0000100397.86756.A3. [DOI] [PubMed] [Google Scholar]
- 21.Eizirik DL, Pipeleers DG, Ling Z, Welsh N, Hellerstrom C, Andersson A. Major species differences between humans and rodents in the susceptibility to pancreatic beta-cell injury. Proc Natl Acad Sci U S A. 1994;91(20):9253–9256. doi: 10.1073/pnas.91.20.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Movahedi B, Gysemans C, Jacobs-Tulleneers-Thevissen D, Mathieu C, Pipeleers D. Pancreatic duct cells in human islet cell preparations are a source of angiogenic cytokines interleukin-8 and vascular endothelial growth factor. Diabetes. 2008;57(8):2128–2136. doi: 10.2337/db07-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37(4):413–420. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- 24.Ballinger WF, Lacy PE. Transplantation of intact pancreatic islets in rats. Surgery. 1972;72(2):175–186. [PubMed] [Google Scholar]
- 25.Bhonde R, Shukla RC, Kanitkar M, Shukla R, Banerjee M, Datar S. Isolated islets in diabetes research. Indian J Med Res. 2007;125(3):425–440. [PubMed] [Google Scholar]
- 26.Dufrane D, D’Hoore W, Goebbels RM, Saliez A, Guiot Y, Gianello P. Parameters favouring successful adult pig islet isolations for xenotransplantation in pig-to-primate models. XenoTransplantation. 2006;13(3):204–214. doi: 10.1111/j.1399-3089.2006.00275.x. [DOI] [PubMed] [Google Scholar]
- 27.Rees DA, Alcolado JC. Animal models of diabetes mellitus. Diabet Med. 2005;22(4):359–370. doi: 10.1111/j.1464-5491.2005.01499.x. [DOI] [PubMed] [Google Scholar]
- 28.O’Neil JJ, Tchipashvili V, Parent RJ, et al. A simple and cost-effective method for the isolation of islets from nonhuman primates. Cell Transplant. 2003;12(8):883–890. doi: 10.3727/000000003771000110. [DOI] [PubMed] [Google Scholar]
- 29.Oliver-Krasinski JM, Stoffers DA. On the origin of the beta cell. Genes Dev. 2008;22(15):1998–2021. doi: 10.1101/gad.1670808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonner-Weir S, Weir GC. New sources of pancreatic beta-cells. Nat Biotechnol. 2005;23(7):857–861. doi: 10.1038/nbt1115. [DOI] [PubMed] [Google Scholar]
- 31.Efrat S. Beta-cell replacement for insulin-dependent diabetes mellitus. Adv Drug Deliv Rev. 2008;60(2):114–123. doi: 10.1016/j.addr.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 32.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52(1):102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 33.Sorenson RL, Brelje TC. Adaptation of islets of Langerhans to pregnancy: beta-cell growth, enhanced insulin secretion and the role of lactogenic hormones. Horm Metab Res. 1997;29(6):301–307. doi: 10.1055/s-2007-979040. [DOI] [PubMed] [Google Scholar]
- 34.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429(6987):41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 35.Hanley NA, Hanley KP, Miettinen PJ, Otonkoski T. Weighing up beta-cell mass in mice and humans: self-renewal, progenitors or stem cells? Mol Cell Endocrinol. 2008;288(1–2):79–85. doi: 10.1016/j.mce.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 36.Meivar-Levy I, Ferber S. New organs from our own tissues: liver-to-pancreas transdifferentiation. Trends Endocrinol Metab. 2003;14(10):460–466. doi: 10.1016/j.tem.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 37.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455(7213):627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hohmeier HE, Newgard CB. Cell lines derived from pancreatic islets. Mol Cell Endocrinol. 2004;228(1–2):121–128. doi: 10.1016/j.mce.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 39.Narushima M, Kobayashi N, Okitsu T, et al. A human beta-cell line for transplantation therapy to control type 1 diabetes. Nat Biotechnol. 2005;23(10):1274–1282. doi: 10.1038/nbt1145. [DOI] [PubMed] [Google Scholar]
- 40.Hohmeier HE, Newgard CB. Islets for all? Nat Biotechnol. 2005;23(10):1231–1232. doi: 10.1038/nbt1005-1231. [DOI] [PubMed] [Google Scholar]
- 41.Elliott RB, Escobar L, Garkavenko O, et al. No evidence of infection with porcine endogenous retrovirus in recipients of encapsulated porcine islet xenografts. Cell Transplant. 2000;9(6):895–901. doi: 10.1177/096368970000900616. [DOI] [PubMed] [Google Scholar]
- 42.Groth CG, Korsgren O, Tibell A, et al. Transplantation of porcine fetal pancreas to diabetic patients. Lancet. 1994;344(8934):1402–1404. doi: 10.1016/s0140-6736(94)90570-3. [DOI] [PubMed] [Google Scholar]
- 43.Valdes-Gonzalez RA, Dorantes LM, Garibay GN, et al. Xenotransplantation of porcine neonatal islets of Langerhans and Sertoli cells: a 4-year study. Eur J Endocrinol. 2005;153(3):419–427. doi: 10.1530/eje.1.01982. [DOI] [PubMed] [Google Scholar]
- 44.Brissova M, Fowler MJ, Nicholson WE, et al. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem. 2005;53(9):1087–1097. doi: 10.1369/jhc.5C6684.2005. [DOI] [PubMed] [Google Scholar]
- 45.Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A. 2006;103(7):2334–2339. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brissova M, Shostak A, Shiota M, et al. Pancreatic islet production of vascular endothelial growth factor–a is essential for islet vascularization, revascularization, and function. Diabetes. 2006;55(11):2974–2985. doi: 10.2337/db06-0690. [DOI] [PubMed] [Google Scholar]
- 47.Ballian N, Brunicardi FC. Islet vasculature as a regulator of endocrine pancreas function. World J Surg. 2007;31(4):705–714. doi: 10.1007/s00268-006-0719-8. [DOI] [PubMed] [Google Scholar]
- 48.Parnaud G, Bosco D, Berney T, et al. Proliferation of sorted human and rat beta cells. Diabetologia. 2008;51(1):91–100. doi: 10.1007/s00125-007-0855-1. [DOI] [PubMed] [Google Scholar]
- 49.Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355(13):1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 50.Ryan EA, Shandro T, Green K, et al. Assessment of the severity of hypoglycemia and glycemic lability in type 1 diabetic subjects undergoing islet transplantation. Diabetes. 2004;53(4):955–962. doi: 10.2337/diabetes.53.4.955. [DOI] [PubMed] [Google Scholar]
- 51.Hering B, Parkay J, Kandaswamy R, et al. Long-term survival of islet allografts in type 1 diabetes. Am J Transplant. 2007;7(suppl 2):205. [Google Scholar]
- 52.Cure P, Pileggi A, Froud T, et al. Improved metabolic control and quality of life in seven patients with type 1 diabetes following islet after kidney transplantation. Transplantation. 2008;85(6):801–812. doi: 10.1097/TP.0b013e318166a27b. [DOI] [PubMed] [Google Scholar]
- 53.Toso C, Shapiro AM, Bowker S, et al. Quality of life after islet transplant: impact of the number of islet infusions and metabolic outcome. Transplantation. 2007;84(5):664–666. doi: 10.1097/01.tp.0000280550.01028.89. [DOI] [PubMed] [Google Scholar]
- 54.Barshes NR, Wyllie S, Goss JA. Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: implications for intrahepatic grafts. J Leukoc Biol. 2005;77(5):587–597. doi: 10.1189/jlb.1104649. [DOI] [PubMed] [Google Scholar]
- 55.Huang X, Moore DJ, Ketchum RJ, et al. Resolving the conundrum of islet transplantation by linking metabolic dysregulation, inflammation, and immune regulation. Endocr Rev. 2008;29(5):603–630. doi: 10.1210/er.2008-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Korsgren O, Lundgren T, Felldin M, et al. Optimising islet engraftment is critical for successful clinical islet transplantation. Diabetologia. 2008;51(2):227–232. doi: 10.1007/s00125-007-0868-9. [DOI] [PubMed] [Google Scholar]
- 57.Westermark GT, Westermark P, Berne C, Korsgren O. Nordic Network for Clinical Islet Transplantation. Widespread amyloid deposition in transplanted human pancreatic islets. N Engl J Med. 2008;359(9):977–979. doi: 10.1056/NEJMc0802893. [DOI] [PubMed] [Google Scholar]