Abstract
Purpose
To examine whether automated visual field (VF) testing may exert a short-term influence on subsequent intraocular pressure (IOP) measurement during the same visit.
Methods
We reviewed patients with primary open-angle glaucoma whose most recent visit occurred at a major academic institution from July to December 2009 and who had 3 visits without intervening changes in glaucoma management within the previous 5 years. Exclusion criteria were patient admittance of nonadherence with medical therapy and documented difficulty of IOP measurement. One hundred nine right eyes from 109 patients were included. IOP obtained within 30 minutes after VF testing was compared with IOP from the previous and next visits without VF testing. Subgroup analyses included the role of reliability of VF test performance, surgical versus medical IOP control, and different topical medications.
Results
The average IOP measured after VF testing was 14.9± 4.7 mm Hg, higher than both the previous (13.7±4.4 mm Hg, P<0.001) and next visits without VF examination (13.8±4.4 mm Hg, P<0.001). A total of 22.9% of patients experienced a more than 20% increase of IOP. Eyes with surgical control had less IOP elevation than eyes with medical control (3.1%±15.9% vs. 11.7%±17.4%, P=0.009). Users of β-blockers or α-2-agonists had less IOP elevation than eyes controlled with prostaglandins or carbonic anhydrase inhibitors (0.9%±15.1% vs. 9.0%±12.3%, P=0.030).
Conclusions
This retrospective study suggests that patients with primary open-angle glaucoma experience a small and transient increase in IOP after VF testing and that this effect may be lower after surgical pressure control.
Keywords: glaucoma, automated visual field testing, intraocular pressure
In our practice, intraocular pressure (IOP) is measured after automated visual field (VF) testing to have results available for better decision making should therapy have to be advanced and to avoid tear film disruption that may lead to interference with VF testing. We were concerned that we may have detected a pattern of increased IOP after VF analysis in some glaucomatous eyes with previously within-target pressures, suggesting that treatment may need to be intensified, only to measure at the next visit pressures that were similar to before VF testing without having changed management. We hypothesized that VF testing may cause a transient elevation in IOP through an unidentified mechanism that could unnecessarily prompt the treating physician to adjust treatment.
Currently, there is no consensus in the literature about the effects of VF testing on IOP, with 1 prospective study of 49 primary open-angle glaucoma (POAG) patients suggesting a transient 1-hour increase in IOP of 2.38±3.49 mm Hg,1 and other smaller studies reporting no difference.2,3 Proposed factors include the stress of the testing conditions,4,5 personal tolerance of environmental stressors,6 and decrease in visual accommodation during VF testing.7,8
To examine whether our observation of IOP increase after VF testing was truly present, we conducted a retrospective study of patients with POAG. We compared IOP measured directly after VF testing with IOP measured at 1 preceding and 1 subsequent visit and tested for correlating factors.
METHODS
This study adhered to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board. A retrospective chart review was performed of all patients with POAG treated by 2 glaucoma specialists J.C.T. and M.B.S. at the Yale Eye Center at the Yale University Medical School whose most recent clinic visit took place from July to December 2009. Patients selected for inclusion were those who exhibited unchanged glaucoma management during a period spanning at least 3 consecutive office visits within the previous 5 years. VF testing visit took place during the second of the 3 visits for each patient, whereas the first and third visits did not include this examination. Exclusion criteria were changes in disease management during the data collection period, patient admittance of nonadherence with medical therapy, documented difficulty taking pressure measurements and IOP measured after dilation. All patients followed the same algorithm of VF testing followed by IOP measurement within 30 minutes and discussion with the responsible physician. The VF analysis was performed with a Humphrey 630 VF analyzer (HFA II model 750 operating system 12.6; Carl Zeiss Meditec, Jena, Germany) and the 24-2 or 10-2 Swedish Interactive Threshold Algorithm standard program on undilated eyes. All were examined using the same VF machines, examination rooms, and tonometers operated by the same personnel; no specific technicians performed a larger proportion of measurements during perimetry visits. IOP was measured with the same standard Goldmann tonometers that were within 4 months of calibration by 1 of 2 technicians or 2 attendings, all of whom have worked in a glaucoma clinic for more than 15 years and assumed to have accurate, repeatable techniques. Measurements of IOP had all been taken from the right eyes first. Only data from 1 eye in each individual were used for analysis of IOP change from visit to visit to reduce the effect of confounding. Left eye data were used only when right eye data were not available or incomplete.
For each patient and each time period included, the most recent visit during which VF examination was performed was identified. IOP from the VF visit was compared with IOP from the visit immediately preceding and immediately after VF testing using analysis of variance (ANOVA) for repeated measures and 2-tailed paired t tests for repeat measurements in the same patients. The IOP measurements of the first and the last visit, both without VF examination, were used to compute the baseline average IOP. VF testing-associated IOP change was expressed as a percent change in IOP measured during the VF visit compared with this baseline average IOP. A simple linear regression of IOP change with respect to age was performed to analyze whether IOP change varies predictably with the age of the patient. Subanalyses were performed to examine the impact of unreliable VF test performance, medical versus surgical IOP control, and different topical glaucoma medications on IOP after VF testing. When the subanalysis of medication groups showed a clinically significant difference in mean IOP but an insufficient number of patients, both right and left eyes were included. A nested ANOVA analysis was used to account for intereye correlation in multieye data. IOP change in unreliable VF test takers was compared with that of reliable VF test takers using a 2-tailed t test. Reliability is a binary categorical indicator reported by the Humphrey analyzer’s SITA statistical software.9 Similarly, IOP change in eyes that were only treated with topical glaucoma medications (medical group) were compared with those that had undergone successful glaucoma surgery in the form of trabeculectomy (surgical group). Successful surgical control was defined as achieving the individually set target pressure at which disease was not expected to progress, with or without additional medications. VF examination effect on IOP of different topical glaucoma medications was analyzed the same way by dividing them into 2 groups: eyes using β-blockers (BB) or α-2-agonists (AA) only, and eyes using neither BB nor AA. These 2 drug classes were combined, as they are both mediators of the sympathetic response with identical intracellular mechanisms that result in reduced activity of membrane-bound adenylyl cyclase.10-15
RESULTS
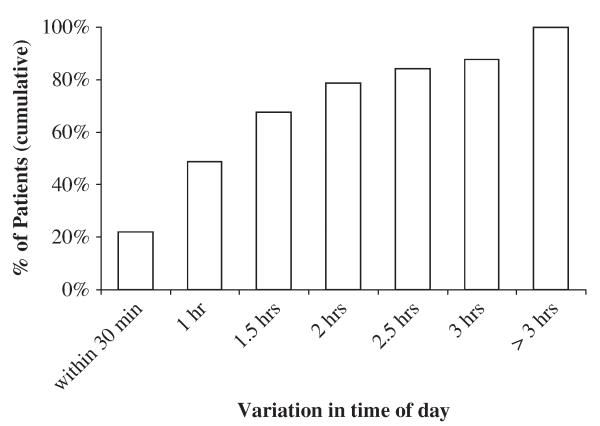
Four hundred twenty-seven patient records were reviewed, and 109 patients (109 eyes) were included based on the predetermined criteria. Thirty patients were from the first glaucoma specialist’s clinic and 79 patients were from the second clinic. Forty patients were male and 69 were female, with an average age of 72.0±9.5 years and 77.0±9.3 years, respectively. The average time span between the first and third visits was 229±79 days. All IOPs were obtained within 1.3±1.4 hours of the same time of day for each patient. Patients (84.4%) had measurements obtained within 3 hours of the same time of day. In 21% of patients, all 3 measurements were taken before 10 AM; in 43% of patients, all measurements were taken after 10 AM. Across all patients, the clock hour of IOP measurement spanned between 8:00 AM and 4:45 PM. There was no preferential time for VF visits and glaucoma clinics were equally distributed to AM and PM slots. There were no substantial time-of-day differences in IOP measurement on VF testing days versus non-VF testing days; across all patients, 80% of IOP measurements on VF testing days occurred within 2 hours of IOP measurements on non-VF testing days (Fig. 1). VF testing results exhibited low reliability in 27 patients (24.8%). At the time of data collection, all patients were controlled medically with an average of 2 glaucoma drugs. Patients with trabeculectomy required an average of 1 glaucoma drug. Patients (67%) were taking a prostaglandin drug, 46% of patients were taking a carbonic anhydrase inhibitor, 59% of patients were taking a BB, and 30% of patients were taking an α-2 adrenergic agonist (Table 1).
FIGURE 1.
Cumulative distribution of time-of-day difference between intraocular pressure (IOP) measurements on visual field (VF) testing days compared with non-VF testing days. Cumulative percentages shown. Time-of-day difference was calculated as time of IOP measurement on VF testing day minus the average time of IOP measurement on non-VF testing days.
TABLE 1.
Patient Characteristics
| Average | SD | |
|---|---|---|
| Age | 75.2 | 9.6 |
| Male | 72.0 | 9.5 |
| Female | 77.0 | 9.3 |
| Male:female ratio | 40:69 | |
| Average no. glaucoma drugs taken | 2 | |
| Patients taking prostaglandin (%) | 67.0 | |
| Patients taking carbonic anhydrase inhibitor (%) |
45.9 | |
| Patients taking β-blocker (%) | 58.7 | |
| Patients taking α-2-agonist (%) | 30.3 | |
| Average | SD | |
| Days between first and third visit | 229 | 79 |
| Range in time of IOP measurement (h) | 1.33 | 1.37 |
| Percent with low reliability on VF testing | 24.8% | |
IOP indicates intraocular pressure; VF, visual field.
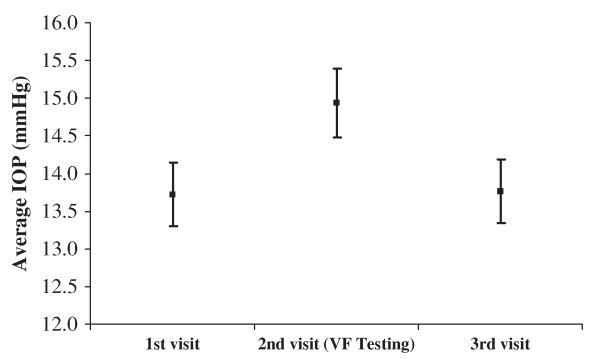
Patients experienced a significant increase in IOP from the previous visit when Humphrey VF analysis was performed before tonometry; this elevation reversed upon the next visit: the average IOP measured after VF testing was 14.9±4.7, compared with 13.7±4.4 during the previous visit, and 13.8±4.4 during the next visit (Table 2, Fig. 2). The IOP measured during the VF visit was higher than both the previous and the next visit (P<0.001, P<0.001). The IOP of the first and the third visits were not significantly different (P=0.846). The average change in IOP during the visit with VF testing compared with the first visit was 10.6%±20.1%, whereas the change during the third visit compared with the first visit was 1.9%±19.8% (Table 2). There was no IOP difference between the 2 different glaucoma clinics at the day of VF analysis (15.4±5.2 vs. 14.8±4.6, P=0.560).
TABLE 2.
IOP Measurements During 3 Consecutive Visits
| Average IOP |
SD | % Change Compared With First Visit |
SD | |
|---|---|---|---|---|
| First visit IOP | 13.7 | 4.4 | — | — |
| Second visit (VF testing visit) IOP |
14.9 | 4.7 | 10.6% | 20.1% |
| Third visit IOP | 13.8 | 4.4 | 1.9% | 19.8% |
| ANOVA for repeated measures |
P = 0.042 | |||
| Paired t tests: | ||||
| First and second visit |
P< 0.001 | |||
| Third and Second visit |
P< 0.001 | |||
| First and Third visit |
P = 0.846 |
ANOVA indicates analysis of variance; IOP, intraocular pressure; VF, visual field.
FIGURE 2.
Intraocular pressure (IOP) during 3 consecutive visits with standard error of the mean (SEM). Square points represent average IOP for each visit; error bars indicate SEM for each visit. IOP measured during the second visit (with visual field testing) was higher than both the first and third visits. IOP measured during the first and third visits were not different.
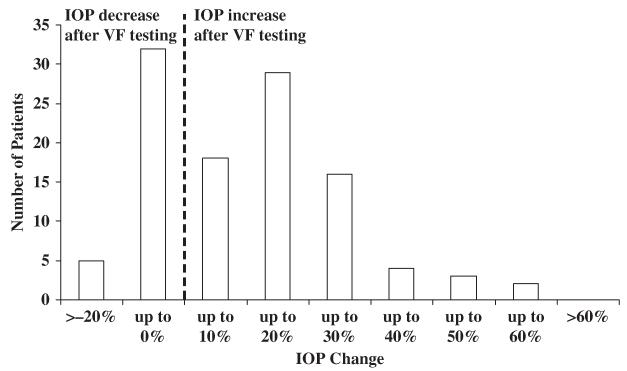
As there was no difference in IOP between the first and third visits, the average of these 2 measurements was taken as the baseline IOP. IOP change was then calculated as the percent change of the VF test visit IOP compared with this baseline average. The average IOP change after VF testing was 10.6%±20.1%, with 22.9% of all patients experiencing more than 20% increase and 8.3% experiencing more than 30% increase (Fig. 3). A simple linear regression with IOP change as the dependent variable and age as the independent variable showed no significant relationship between the 2 parameters. Patients whose test performance was reliable did not exhibit different IOP change when compared with patients whose tests were not reliable (9.3%±17.1% vs. 10.2%±15.4%, P=0.805). Eyes with surgically controlled glaucoma experienced lower IOP after VF testing compared with medically managed eyes (3.1%±15.9% vs. 11.7%±17.4%, P=0.009; Table 3). Within the medically managed group, eyes using BB or AA were compared with eyes using neither BB nor AA. There was no difference in IOP change between these 2 groups. In the pooled analysis of both eyes using nested ANOVA, eyes treated with BB or AA exhibited lower IOP change than eyes managed with neither BB nor AA (P=0.030, Table 4).
FIGURE 3.
Distribution of intraocular pressure (IOP) change after visual field (VF) testing. VF testing-associated IOP change was expressed as a percent change in IOP measured during the VF visit compared with the baseline average IOP measured during the previous and next visits. The mean IOP change was 10.6% ± 20.1%; 22.9% of all patients experienced >20% increase, 8.3% of all patients experienced >30% increase.
TABLE 3.
IOP Change Associated With VF Testing in Patients With Medical Versus Successful Surgical Glaucoma Treatment
| IOP Change, OD Only | N | Average mm Hg | SD | Average % | SD |
|---|---|---|---|---|---|
| Medically treated eyes | 81 | 1.5 | 1.8 | 11.7% | 17.4% |
| Surgically treated eyes | 28 | 0.3 | 2.3 | 3.1% | 15.9% |
| T test | P = 0.009 |
VF testing-associated IOP change was expressed as a percent change in IOP measured during the VF visit compared with the baseline average IOP measured during the previous and next visits.
IOP indicates intraocular pressure; OD, oculus dexter; VF, visual field.
TABLE 4.
IOP Chanae Associated With VF Testina in Patients With Different Medical Treatments. OD Only and Pooled OD+OS
| N | Average mm Hg | SD | Average % | SD | |
|---|---|---|---|---|---|
| IOP Change, OD Only | |||||
| Medically treated eyes without BB or AA | 22 | 1.0 | 2.2 | 7.4% | 14.2% |
| Medically treated eyes with BB or AA only | 8 | −0.4 | 2.7 | −0.4% | 12.7% |
| T test | P = 0.155 | ||||
| IOP Change, Pooled OD+OS | |||||
| Medically treated eyes without BB or AA | 44 | 1.3 | 1.9 | 9.0% | 12.3% |
| Medically treated eyes with BB or AA only | 16 | −0.2 | 2.3 | 0.9% | 15.1% |
| Nested ANOVA | P = 0.030 |
VF testing-associated IOP change was expressed as a percent change in IOP measured during the VF visit compared with the baseline average IOP measured during the previous and next visits.
AA indicates α-2-agonist; ANOVA, analysis of variance; BB, β-blocker; IOP, intraocular pressure; OD, oculus dexter; OS, oculus siniter; VF, visual field.
DISCUSSION
We performed tonometry after VF examination because both IOP and VF results can be discussed with the patient and treatment may be readily adjusted. Tear film disruption and a decreased blinking frequency could also potentially reduce the quality of VF testing if pressure is measured before.
Before this study, we observed that in some patients IOP repeatedly exceeded their usual range if IOP was measured after VFs were obtained. We wanted to further clarify whether this was a true effect from VF examination, which may convince the treating physicians to advance treatment. In this retrospective study applying conservative statistical methods, we found that patients with POAG indeed had a higher IOP after VF examination that was not detectable at the subsequent visit. Although this IOP increase was small on average (10.6%), 22.9% of patients experienced a change of >20%, and 8.3% experienced a change of >30%. Twenty percent to 30% lowering of IOP may be regarded as a treatment goal to prevent glaucoma progression or development16,17; conversely, an increase in IOP of the same magnitude may lead the physician to advance treatment.
Several earlier studies have examined the effect of VF testing on IOP, but with contradictory results. Recupero et al1 first reported a transient 1-hour increase in IOP of 2.38 mm Hg after VF testing in 94 POAG eyes. We report the first confirmatory study of these results. Recupero et al1 hypothesize that decreased accommodation with age may be a factor that reduces outflow facility. In our study, regression analysis shows no significant relationship between age and IOP variation after VF testing. Two other studies failed to show increased IOP after VF testing. However, patient numbers were low, 27 and 21, respectively.2,3
Successful surgical treatment of glaucoma has been shown to decrease diurnal and 24-hour variation in IOP.18,19 A recent prospective study of 30 patients treated by trabeculectomy and 30 medically treated patients showed that the medical group had a higher 24-hour IOP range than the surgical group (4.8±2.3 mm Hg vs. 2.3± 0.8 mm Hg, P<0.0001).18 Our subgroup analysis similarly showed that eyes with surgically controlled glaucoma exhibited lower IOP variation after VF testing than did eyes with medical management alone.
It is possible that VF testing is perceived by some patients as stressful, leading to a sympathetic response that transiently elevates IOP. IOP is highly influenced by sympathetic input. When cervical sympathetic nerves are directly stimulated, norepinephrine (NE) is released into the aqueous humor and a significant IOP elevation can be observed. Gallar et al20 found that a 1.5 mm Hg increase may occur as early as within 15 minutes in laboratory rabbits, approximating the IOP increase seen in our study.21 Serum cortisol, a sensitizer to β-adrenergic stimulation of structures including the eye,22-25 is further correlated with the diurnal pattern of IOP variation observed in both normal and glaucomatous eyes.26
Our observation that patients using topical BB or AA do not experience the same IOP elevation after VF testing may represent a clinical correlate to these animal studies. We grouped these 2 classes of drugs together because they have the same intracellular mechanism of action on the ciliary body and in blunting the NE-mediated sympathetic response. NE is postulated to act on both postsynaptic β-receptors and presynaptic α-2 receptors to increase aqueous flow.10 Both agents are modulators of this response, which may explain the observed lack of IOP elevation after VF examination if indeed this test represents a stress to patients. Both decrease cyclic adenosine monophosphate by acting on G-protein-linked receptors to decrease the activity of adenylyl cyclase.10 There is evidence of β-receptor-linked Gs proteins within the ciliary body cell membrane which activate adenylyl cyclase.12 There also exist presynaptic α-2 receptor-linked Gi protein in ciliary body, which inhibit the activity of adenylyl cyclase.11,14,15
There is some evidence in the literature to suggest that environmental stressors may be associated with increased IOP in POAG patients.27 Prospective studies of healthy controls demonstrated that exposure to a 5 to 10 minutes psychological stressor of performing mental arithmetic can lead to a 1.3 to 1.6 mm Hg increase in IOP.4,5,28 The effect was not attributed to eye muscle tension and declined with repeated testing and relaxation training. A small prospective study of 15 healthy controls exposed to both physical stress and a computer-game psychological stress showed that only the mental stressor resulted in an increase in IOP; moreover, computer game performance correlated with IOP change.29 The process of VF testing may present a similar achievement-based stressor in which poor performance corresponds with experiencing a higher level of achievement-based stress. However, in our analysis, patients with reliable test results exhibited as similar level of IOP fluctuation as patients whose tests were not reliable. The reliability of VF results may not be a good proxy for performance-induced stress levels during the test.
A limitation of this study is its retrospective nature that makes sources of error due to confounding and bias more common when compared with prospective studies. This was necessary to increase number of participants and the power of the study. As we required 3 consecutive visits without change in management, many patients had to be excluded leaving only few for the subgroup analyses. In addition, different providers may have obtained IOP measurements at the 3 clinic visits. However, there was no systematic bias in who would obtain IOP measurements during VF examination visits. Another potential concern is that patients with high IOP are invited back to the glaucoma clinic sooner, and regression to the mean may account for their lower IOP on the third (non-VF) visit. However, all patients regardless of IOP are invited back to the glaucoma clinic for the next visit instead of referred back to their general ophthalmologists, therefore selection bias is likely to be low. More importantly, regression to the mean cannot explain the statistically higher IOP on the second (VF) visit compared with the preceding visit, as the time interval between non-VF visits (visit 1) and VF visits (visit 2) is independent of IOP. Nonetheless, patients could also have changed their behavior between visits by increasing short-term adherence to therapy upon being informed that their pressures are higher than on previous visits. Although we do not recommend patients in our practice withhold their drops, there could also be a subset of patients with selective nonadherence to drops on VF testing days, therefore increasing their IOP measurement.
The size of the IOP effect attributed to VF-testing seems to be small (+1.1 to 1.2 mm Hg) and transient in average. This study did not determine the length of this elevation. The clinical significance of this study is that VF examination seems to be associated with significant IOP increase in some patients. This highlights the need to obtain further pressure measurements before treatment is changed in these individuals. Only a prospective and ideally randomized study can test the hypothesis that VF examination truly increases IOP. The effect of BB before VF examination could be tested in a double-blinded manner to evaluate the idea that an adrenergic pathway may be responsible for this.
CONCLUSIONS
We performed a retrospective study to examine the effect of VF testing on IOP measured during the same visit. We found a 10.6% increase from the previous visit that reversed upon the subsequent visit. However, 22.9% of patients experienced a change in IOP of >20%, and 8.3% experienced a change of >30% during the VF visit, which may lead physicians to advance treatment. The magnitude of this change was not correlated with patient age or the reliability of the VF test. Although the hypothesis that VF testing increases IOP requires a prospective study, physicians are cautioned to obtain further measurements before changing treatment for increased IOP after VF examination.
ACKNOWLEDGMENT
The authors thank Dr John H. Liu for critically reviewing our article.
Supported in part by an unrestricted Departmental Challenge Grant from Research to Prevent Blindness, Inc., New York, NY.
Footnotes
Disclosure: Dr M. Bruce Shields serves as a consultant for Opko Health. No other authors have any financial/conflicting interests to disclose for this study.
Contributions of authors: design of the study (J.C.T., N.A.L., N.N.); data collection (N.N., J.C.T., M.B.S.), conduct of the study (N.N., N.A.L.); manuscript writing and revision (N.N., J.C.T., M.B.S., N.A.L.). This study adhered to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of Yale University, also known as the Human Investigations Committee.
REFERENCES
- 1.Recupero S, Contestabile M, Taverniti L, et al. Open-angle glaucoma: variations in the intraocular pressure after visual field examination. J Glaucoma. 2003;12:114. doi: 10.1097/00061198-200304000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Rebolleda G, Rodríguez-Villace C, Anton M. Variations in intraocular pressure after visual field examination. J Glaucoma. 2004;13:178–179. doi: 10.1097/00061198-200404000-00017. [DOI] [PubMed] [Google Scholar]
- 3.Martin L. Intraocular pressure before and after visual field examination. Eye. 2007;21:1479–1481. doi: 10.1038/sj.eye.6702552. [DOI] [PubMed] [Google Scholar]
- 4.Brody S, Erb C, Veit R, et al. Intraocular pressure changes: the influence of psychological stress and the Valsalva maneuver. Biol psychol. 1999;51:43–57. doi: 10.1016/s0301-0511(99)00012-5. [DOI] [PubMed] [Google Scholar]
- 5.Kaluza G, Strempel I, Maurer H. Stress reactivity of intraocular pressure after relaxation training in open-angle glaucoma patients. J Behav Med. 1996;19:587–597. doi: 10.1007/BF01904906. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto K, Sakamoto Y, Irie M, et al. The relationship between IMPS-measured stress score and intraocular pressure among public school workers. J Physiol Anthropol. 2008;27:43–50. doi: 10.2114/jpa2.27.43. [DOI] [PubMed] [Google Scholar]
- 7.Armaly MF, Jepson NC. Accommodation and the dynamics of the steady-state intraocular pressure. Invest ophthalmol. 1962;1:480–483. [PubMed] [Google Scholar]
- 8.Greene PR. Mechanical considerations in myopia: relative effects of accommodation, convergence, intraocular pressure, and the extraocular muscles. Am J Optome Physiol Opt. 1980;57:902–914. [PubMed] [Google Scholar]
- 9.Katz J, Sommer A, Gaasterland DE, et al. Comparison of analytic algorithms glaucomatous visual field loss detecting. Arch Ophthalmol. 1991;109:1684–1689. doi: 10.1001/archopht.1991.01080120068028. [DOI] [PubMed] [Google Scholar]
- 10.Insel PA. Adrenergic receptors—evolving concepts and clinical implications. N Eng JMed. 1996;334:580–585. doi: 10.1056/NEJM199602293340907. [DOI] [PubMed] [Google Scholar]
- 11.Bausher LP, Horio B. Regulation of cyclic AMP production in adult human ciliary processes. Exp Eye Res. 1995;60:43–48. doi: 10.1016/s0014-4835(05)80082-x. [DOI] [PubMed] [Google Scholar]
- 12.Neufeld AH, Bartels SP, Liu JH. Laboratory and clinical studies on the mechanism of action of timolol. Surv Ophthalmol. 1983;28(suppl):286–292. doi: 10.1016/0039-6257(83)90152-2. [DOI] [PubMed] [Google Scholar]
- 13.Walters TR. Development and use of brimonidine in treating acute and chronic elevations of intraocular pressure: a review of safety, efficacy, dose response, and dosing studies. Surv Ophthalmol. 1996;41(suppl 1):S19–S26. doi: 10.1016/s0039-6257(96)82028-5. [DOI] [PubMed] [Google Scholar]
- 14.Jumblatt JE, Ohia SE, Hackmiller RC. Prejunctional modulation of norepinephrine release in the human iris-ciliary body. Invest Ophthalmolo. 1993;34:2790–2793. [PubMed] [Google Scholar]
- 15.Liu JH, Dacus AC, Bartels SP. Adrenergic mechanism in circadian elevation of intraocular pressure in rabbits. Invest Ophthalmol. 1991;32:2178–2183. [PubMed] [Google Scholar]
- 16.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–13. doi: 10.1001/archopht.120.6.701. discussion 829-830. [DOI] [PubMed] [Google Scholar]
- 17.Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–1279. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- 18.Konstas AG, Topouzis F, Leliopoulou O, et al. 24-hour intraocular pressure control with maximum medical therapy compared with surgery in patients with advanced open-angle glaucoma. Ophthalmology. 2006;113:761–765. doi: 10.1016/j.ophtha.2006.01.029. e1. [DOI] [PubMed] [Google Scholar]
- 19.Medeiros FA, Pinheiro A, Moura FC, et al. Intraocular pressure fluctuations in medical versus surgically treated glaucomatous patients. J Ocul Pharmacol Ther. 2002;18:489–498. doi: 10.1089/108076802321021036. [DOI] [PubMed] [Google Scholar]
- 20.Gallar J, Liu JH. Stimulation of the Cervical Sympathetic Nerves Increases Intraocular Pressure. Invest Ophthalmol. 1993;34:596–605. [PubMed] [Google Scholar]
- 21.Zhan G, Ohia SE, Camras CB, et al. Superior cervical ganglionectomy-induced lowering of intraocular pressure in rabbits: role of prostaglandins and neuropeptide Y. Exp Eye Res. 1999;32:189–194. doi: 10.1016/s0306-3623(98)00187-6. [DOI] [PubMed] [Google Scholar]
- 22.Hadcock JR, Malbon CC. Regulation of beta-adrenergic receptors by “permissive” hormones: glucocorticoids increase steady-state levels of receptor mRNA. Proc Natl Acad Sci U S A. 1988;85:8415–8419. doi: 10.1073/pnas.85.22.8415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakaue M, Hoffman BB. Glucocorticoids induce transcription and expression of the alpha 1B adrenergic receptor gene in DTT1 MF-2 smooth muscle cells. J Clin Invest. 1991;88:385–389. doi: 10.1172/JCI115315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jampol LM, Weinreb R, Yannuzzi L. Involvement of corticosteroids and catecholamines in the pathogenesis of central serous chorioretinopathy: a rationale for new treatment strategies. Ophthalmology. 2002;109:1765–1766. doi: 10.1016/s0161-6420(02)01303-9. [DOI] [PubMed] [Google Scholar]
- 25.Ullian M. The role of corticosteroids in the regulation of vascular tone. Cardiovasc Res. 1999;41:55–64. doi: 10.1016/s0008-6363(98)00230-2. [DOI] [PubMed] [Google Scholar]
- 26.Kersey J, Broadway D. Corticosteroid-induced glaucoma: a review of the literature. Eye. 2006;20:407–416. doi: 10.1038/sj.eye.6701895. [DOI] [PubMed] [Google Scholar]
- 27.Ripley H, Wolff H. Life situations, emotions, and glaucoma. Psychosom Med. 1950;12:215–224. doi: 10.1097/00006842-195007000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Sauerborn GA, Schmitz MA, Franzen UA, et al. Stress and intraocular pressure in myopes. J Psychol Health. 1992;6:61–68. [Google Scholar]
- 29.Erb C, Brody S, Rau H. Effect of mental and physical stress on intraocular pressure-a pilot study. Klin Monatsbl Augenheilk. 1998;212:270–274. doi: 10.1055/s-2008-1034878. [DOI] [PubMed] [Google Scholar]