Abstract
Introduction
Obesity increases the risk of death from many adverse health outcomes and has also been linked with cancer outcomes. The impact of obesity on outcomes of advanced non-small cell lung cancer patients is unclear.
Methods
The authors evaluated the association of body mass index and outcomes in 2585 eligible patients enrolled to three consecutive first-line trials conducted by the Eastern Cooperative Oncology Group. Body mass index was categorized as underweight (BMI < 18.5 kg/m2), normal weight (BMI: 18.5 to < 25 kg/m2), overweight (BMI: 25 to < 30 kg/m2) and obese (BMI ≥ 30 kg/m2). In addition to analyzing overall and progression-free survival, reasons for treatment discontinuation were also assessed by BMI group.
Results
4.6% of patients were underweight, 44.1% were normal weight, 34.3% of patients were classified as overweight, and 16.9% were obese. Non-proportional hazards existed for obese patients relative to the other three groups of patients, with a change in OS hazard occurring at approximately 16 months. In multivariable Cox models, obese patients had superior outcomes earlier on study compared to normal/overweight patients 0.86 (p=0.04, 95% CI: 0.75–0.99), but later experienced increased hazard 1.54 (p<0.001, 95% CI: 1.22–1.94), indicating a time effect while undergoing treatment.
Conclusion
Data from these three trials suggest differential outcomes associated with body mass index, and additional studies of the mechanisms underlying this observation, as well as dietary and lifestyle interventions, are warranted to help optimize therapy.
INTRODUCTION
Elevated body mass index (BMI), defined as weight in kilograms divided by the square of the height in meters, increases the risk of death from many adverse health outcomes and continues to remain a significant public health problem in developed nations such as the United States, Canada and Europe.1 BMI-defined overweight and obesity, which affect nearly two-thirds of the U.S. population and continue to increase in prevalence, are associated with increased risk of cardiovascular disease, diabetes, arthritis and asthma, as well as colon, breast, endometrial and renal cancers. 2–6 With respect to lung cancer, however, many investigations have demonstrated an inverse association between BMI and risk of fatal lung cancers. 7–18
Despite the wealth of literature detailing the association between BMI and lung cancer incidence, studies evaluating the relationship of BMI on outcomes for patients with lung cancer are somewhat limited.19 To our knowledge these studies have not focused on lung cancer patients enrolled on clinical trials, which select for patients with fewer co-morbidities by way of their eligibility criteria; trials typically require good performance status, adequate organ function, and limited exposure to major surgery or treatments within a reasonable timeframe of study entry. Increased BMI has also been associated with improved outcomes for patients with renal cell cancer and diffuse large B-cell lymphoma, but with poorer prognosis in patients with colon, prostate, and breast cancers. 3, 20–22 It is therefore of interest to study whether or not the association between BMI and clinical outcomes can be validated in this setting.
The current study presents results from an analysis of the clinical course of advanced non-small cell lung cancer (NSCLC) patients enrolled to the most recent three front-line phase III trials conducted by the Eastern Cooperative Oncology Group in this patient population: E5592, E1594, and E4599. Statistical endpoints included overall survival, progression-free survival, best objective response, toxicity, and time to treatment discontinuation. To our knowledge, this study is the first to analyze these data using prospectively collected treatment and eligibility criteria and to include detailed information on underweight patients.
MATERIALS AND METHODS
Study Population
During the period from 1993 to 2004, the Eastern Cooperative Oncology Group enrolled 2684 patients to three phase III trials of first line systemic chemotherapy for advanced NSCLC. In brief, eligible patients had stage IIIB, IV or recurrent disease, ECOG performance status 0–1, no prior systemic chemotherapy and adequate bone marrow, hepatic and renal function. Per protocol, all patients were dosed based on actual weight. Additional details regarding eligibility, treatment, and results have been reported elsewhere and are summarized in Table 1; E1594 enrolled 65 eligible patients with PS 2 prior to a protocol amendment restrict to ECOG PS of 0 or 1 only.23–25 The primary endpoint of these trials was overall survival, and the primary analyses were conducted among all eligible patients. Each participant gave informed consent. These studies were conducted in accordance with the Declaration of Helsinki, current Food and Drug Administration Good Clinical Practices, and local institutional review board requirements.
Table 1.
Eastern Cooperative Oncology Phase III First-Line Trials in Advanced Non-Small Cell Lung Cancer, 1993–2004
| Study | Regimens | Accrual period | No. patients with BMI data |
|---|---|---|---|
| E5592: Bonomi et al., 2000 | Cisplatin (75 mg/m2) + Etoposide (100 mg/m2) Cisplatin (75 mg/ m2) + Paclitaxel (250 mg/m2) Cisplatin (75 mg/ m2) + Paclitaxel (135 mg/m2) |
1993–94 | 574 |
| E1594: Schiller et al., 2002 | Cisplatin (75 mg/ m2) + Paclitaxel (135 mg/m2) Cisplatin (100 mg/ m2) + Gemcitabine (1000 mg/ m2) Cisplatin (75 mg/ m2) + Docetaxel (75 mg/ m2) Carboplatin, AUC 6.0 mg/ml/min + Paclitaxel (225 mg/ m2) |
1996–99 | 1161 |
| E4599: Sandler et al., 2006 | Carboplatin, AUC 6.0 mg/ml/min + Paclitaxel (200 mg/m2) + Bevacizumab (15 mg/kg) Carboplatin, AUC 6.0 mg/ml/min + Paclitaxel (200 mg/m2) |
2001–04 | 850 |
Statistical Methods
Baseline patient demographics and disease characteristics were compared using Fisher’s exact test. Overall survival, the primary endpoint considered, was defined as time interval in months from randomization to death from any cause. Progression-free survival was defined as the time interval in months from randomization to documented progression or death. Patients not experiencing an event were censored at the last date of follow-up for OS and the last date of disease assessment for PFS. Time-to-event distributions were estimated using the Kaplan-Meier method, and comparisons of them were made using the logrank test.26 Multivariable piecewise Cox proportional hazards models were used to estimate hazard ratios for OS and PFS.27 Response and toxicity on protocols E5592 and E1594 were assessed using ECOG criteria; for E4599, the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0 and Common Terminology Criteria for Adverse Events (CTCAE) version 2.0 were used. The cumulative incidence function of time to treatment discontinuation due to toxicity, adjusted for death, progression and withdrawal/other as competing events was constructed using the method of Kalbfleish and Prentice.28 All p-values are two-sided, confidence intervals are at the 95% level, and no adjustments have been made for multiple comparisons.
BMI at the time of randomization was defined as weight in kilograms divided by the square of the height in meters. Patients were stratified into BMI groups defined by the World Health Organization: underweight (BMI < 18.5 kg/m2), normal weight (BMI: 18.5 to < 25 kg/m2), overweight (BMI: 25 to < 30 kg/m2) and obese (BMI ≥ 30 kg/m2). 20, 29
RESULTS
At a median follow-up of 64.9 months, 2585 of the 2684 patients (96.3%) randomized on these trials were declared eligible and constituted the primary analysis population; all had BMI measurements at the time of study registration. Table 2 displays the baseline patient demographics and disease characteristics of the study cohort by BMI group. Consistent with the general population, 4.6% of patients were underweight, 44.1% were normal weight, 34.3% of patients were classified as overweight, and 16.9% were obese. Most of the baseline demographics and disease characteristics were significantly imbalanced by BMI group, with the exception of stage, histology, prior surgery, pleural involvement, liver metastases and baseline serum albumin. Underweight patients were more likely to be younger, African American, female, worse ECOG performance status, have more weight loss prior and RT to study enrollment, and be enrolled on the more recent trials.
Table 2.
Distribution of baseline patient demographics and disease characteristics by body mass index.
| Underweight BMI<18.5 kg/m2 (n=123) | Normal 18.5≤BMI<25 kg/m2 (n=1139) | Overweight 25≤BMI<30 kg/m2 (n=887) | Obese BMI≥30 kg/m2 (n=436) | Total (n=2585) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Protocol (p=0.004) | ||||||||||
| E5592 | 34 | 27.6% | 276 | 24.2% | 191 | 21.5% | 73 | 16.7% | 574 | 22.2% |
| E1594 | 59 | 48.0% | 514 | 45.1% | 391 | 44.1% | 197 | 45.2% | 1161 | 44.9% |
| E4599 | 30 | 24.4% | 349 | 30.6% | 305 | 34.4% | 166 | 38.1% | 850 | 32.9% |
| Age (p=0.003) | ||||||||||
| median, range | 59 | 35–83 | 61 | 29–85 | 62 | 25–86 | 63 | 31–88 | 62 | 25–88 |
| Race (p=0.01) | ||||||||||
| White | 94 | 76.4% | 971 | 85.5% | 779 | 87.8% | 376 | 86.4% | 2220 | 86.0% |
| Black | 21 | 17.1% | 93 | 8.2% | 62 | 7.0% | 33 | 7.6% | 209 | 8.1% |
| Other | 8 | 6.5% | 72 | 6.3% | 46 | 5.2% | 26 | 6.0% | 152 | 5.9% |
| Sex (p=0.001) | ||||||||||
| Male | 58 | 47.2% | 678 | 59.5% | 572 | 64.5% | 249 | 57.1% | 1557 | 60.2% |
| Female | 65 | 52.8% | 461 | 40.5% | 315 | 35.5% | 187 | 42.9% | 1028 | 39.8% |
| ECOG PS (p<0.001) | ||||||||||
| 0 | 27 | 22.0% | 347 | 30.6% | 336 | 37.9% | 163 | 37.5% | 873 | 33.9% |
| 1 | 89 | 72.4% | 761 | 67.0% | 532 | 60.0% | 259 | 59.5% | 1641 | 63.6% |
| 2 | 7 | 5.7% | 27 | 2.4% | 18 | 2.0% | 13 | 3.0% | 65 | 2.5% |
| Prior Weight Loss (p<0.001) | ||||||||||
| <5% | 44 | 35.8% | 697 | 61.2% | 687 | 77.5% | 366 | 83.9% | 1794 | 69.4% |
| ≥5% | 79 | 64.2% | 441 | 38.8% | 200 | 22.5% | 70 | 16.1% | 790 | 30.6% |
| Unknown | 1 | 1 | ||||||||
| Stage (p=0.13) | ||||||||||
| IIIB | 11 | 8.9% | 152 | 13.3% | 114 | 12.9% | 68 | 15.6% | 345 | 13.4% |
| IV | 97 | 78.9% | 839 | 73.7% | 647 | 73.0% | 294 | 67.4% | 1877 | 72.6% |
| Recurrent | 15 | 12.2% | 148 | 13.0% | 125 | 14.1% | 74 | 17.0% | 362 | 14.0% |
| Histology (p=0.43) | ||||||||||
| Squamous | 16 | 13.0% | 161 | 14.2% | 120 | 13.5% | 58 | 13.3% | 355 | 13.7% |
| Adenocarcinoma | 66 | 53.7% | 693 | 60.9% | 548 | 61.8% | 277 | 63.5% | 1584 | 61.3% |
| Other | 41 | 33.3% | 283 | 24.9% | 219 | 24.7% | 101 | 23.2% | 644 | 24.9% |
| Prior RT (p=0.02) | ||||||||||
| Yes | 25 | 20.3% | 234 | 20.5% | 143 | 16.1% | 67 | 15.4% | 469 | 18.2% |
| No | 98 | 79.7% | 905 | 79.5% | 743 | 83.9% | 368 | 84.6% | 2114 | 81.8% |
| Prior Surgery (p=0.84) | ||||||||||
| Yes | 42 | 34.4% | 415 | 36.6% | 319 | 36.1% | 166 | 38.2% | 942 | 36.6% |
| No | 80 | 65.6% | 719 | 63.4% | 564 | 63.9% | 268 | 61.8% | 1631 | 63.4% |
| Pleura involvement (p=0.94) | ||||||||||
| Yes | 37 | 30.1% | 352 | 30.9% | 263 | 29.7% | 131 | 30.0% | 783 | 30.3% |
| No | 86 | 69.9% | 787 | 69.1% | 624 | 70.3% | 305 | 70.0% | 1802 | 69.7% |
| Liver mets (p=0.19) | ||||||||||
| Yes | 30 | 24.4% | 237 | 20.8% | 164 | 18.5% | 80 | 18.3% | 511 | 19.8% |
| No | 93 | 75.6% | 902 | 79.2% | 723 | 81.5% | 356 | 81.7% | 2074 | 80.2% |
| Serum Albumin (p=0.89) | ||||||||||
| median, range | 3.6 | 2.1–5.1 | 3.7 | 0–8.4 | 3.8 | 1.7–8.2 | 3.9 | 2.1–5.0 | 3.8 | 0–8.4 |
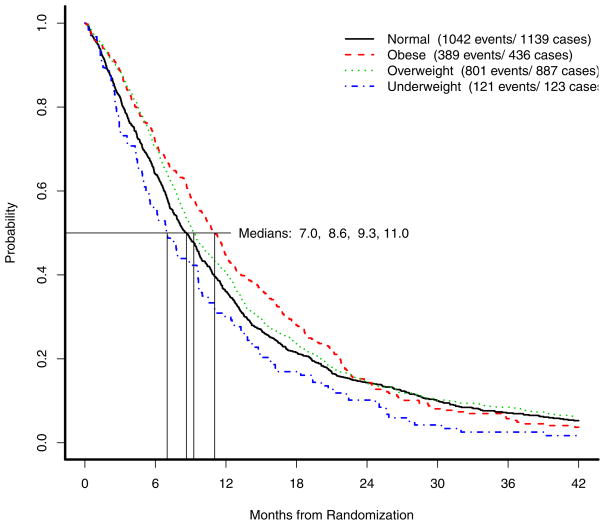
Figure 1 displays the results of the OS analysis by BMI group. Of 2585 patients, 2353 (91%) had died at the time of this analysis. The median OS estimated among underweight patients was 7.0 months (95% CI: 5.5–9.6), among normal-weight patients was 8.6 months (95% CI: 8.0–9.4), among overweight patients was 9.3 months (95% CI: 8.6–10.1), and among obese patients was 11.0 months (95% CI: 10.2–11.9). A test for the equality of these four OS distributions was statistically significant (p=0.005), though it is important to note that there was no statistically significant difference in OS between normal-weight and overweight patients (p=0.11). Visual inspection of the OS Kaplan-Meier curves as well as a formal test for proportional hazards using a test based on Schoenfeld residuals leads to the conclusion that non-proportional hazards exist for obese patients relative to the other three groups of patients; specifically, the hazard for obese patients appears to begin increasing at approximately 16 months post-randomization. 30–32 At this timepoint, a total of 656 patients remained in followup: 23 underweight patients, 266 normal weight patients, 225 overweight patients, and 142 obese patients. To account for this in the analysis of OS, piecewise Cox models estimating the hazard ratio of obese patients relative to the combined group of normal and overweight patients adjusting for time as a time-varying covariate were fitted, stratified by protocol to account for any potential trends in BMI over time, as well as protocol effects; the hazard ratio comparing underweight patients to normal/overweight patients was also estimated. In a model unadjusted for other baseline prognostic factors, the estimated OS hazard ratio comparing underweight patients to normal/overweight patients was 1.26 (p=0.01, 95% CI: 1.05–1.51); the estimated OS hazard ratio comparing obese patients whose days on study was less than 16 months from randomization to normal/overweight patients was 0.81 (p=0.001, 95% CI: 0.71–0.92). When time on-study exceeded 16 months, obese patients experienced a significant increase in their OS hazard rate relative to normal/overweight patients, with an estimated OS hazard ratio of 1.31 (p=0.02, 95% CI: 0.62–0.95). After adjusting for gender (female vs. male, HR=0.83, p<0.001), ECOG performance status (1/2 vs. 0, HR=1.40, p<0.001), stage (IV/recurrent vs. IIIB, HR=1.37, p<0.001), presence of liver metastases (HR=1.39, p<0.001), weight loss (> 5% in the previous 6 months, HR=1.26, p<0.001) and elevated baseline albumin (> 3.8, HR=0.67, p<0.001), all of which were chosen using backwards stepwise selection and statistically significant, the estimated OS hazard ratio comparing underweight patients to normal/overweight patients was 1.12 (p=0.29, 95% CI: 0.91–1.37), and comparing obese patients whose days post-registration were less than 16 months to normal/overweight patients was 0.86 (p=0.04, 95% CI: 0.75–0.99). When time on-study exceeded 16 months, obese patients experienced a significant increase in their OS hazard rate relative to normal/overweight patients, with an estimated OS hazard ratio of 1.54 (p<0.001, 95% CI: 1.22–1.94).
Figure 1.
Overall survival by body mass index.
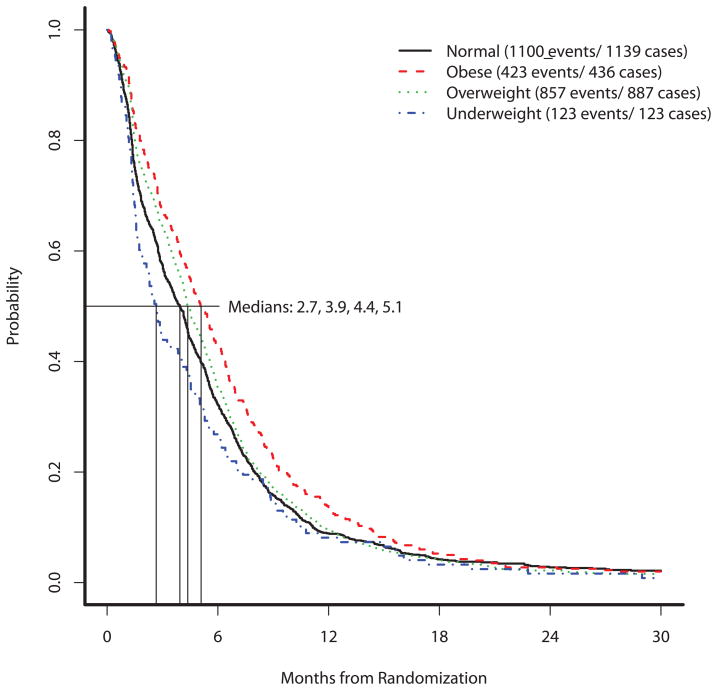
At the time of last follow-up, 2503 (97%) patients had experienced a PFS event. The median PFS estimated among underweight patients was 2.7 months (95% CI: 2.0–4.0), among normal-weight patients was 3.9 months (95% CI: 3.6–4.3), among overweight patients was 4.4 months (95% CI: 4.2–4.8), and among obese patients was 5.1 months (95% CI: 4.5–5.7). A test for the equality of these four PFS distributions was statistically significant (p=0.001); the results are displayed in Figure 2. In an unadjusted Cox model stratified on protocol, the estimated PFS hazard ratio comparing underweight patients to normal/underweight patients was 1.19 (p=0.06, 95% CI: 0.99–1.43); comparing obese patients to normal/underweight patients, the estimated PFS hazard ratio was 0.85 (p=0.002, 95% CI: 0.76–0.94). After adjusting for the same prognostic variables included in the multivariable OS Cox models (gender (HR=0.91, p=0.03), ECOG performance status (HR=1.24, p<0.001), stage HR=1.42, p<0.001), presence of liver metastases (HR=1.19, p=0.002), weight loss HR=1.21, p<0.001) and elevated baseline albumin HR=0.76, p<0.001), the adjusted hazard ratios were 1.04 (p=0.73, 95% CI: 0.85–1.27) for underweight patients and 0.92 (p=0.13, 95% CI: 0.82–1.03) for obese patients, when comparing each of these two groups to normal/overweight patients.
Figure 2.
Progression-free survival by body mass index.
We next assessed the best objective response rates for patients treated on these three protocols across the 4 BMI groups. Among underweight patients, the response rate was 13.8% compared to 20.5% among normal weight patients, 22.5% among overweight patients, and 21.3% among obese patients (p=0.15). There were also no significant differences observed in the rates of grade 3 or higher hematological toxicities across BMI groups: 39.3%, 45.1%, 44.6%, and 40.0% within the underweight, normal weight, overweight, and obese groups, respectively (p=0.20). Similarly, were no significant imbalances observed in the rates of grade 3 or higher non-hematological toxicities: 61.5%, 62.4%, 62.2%, and 67.4% within the underweight, normal weight, overweight, and obese groups, respectively (p=0.24).
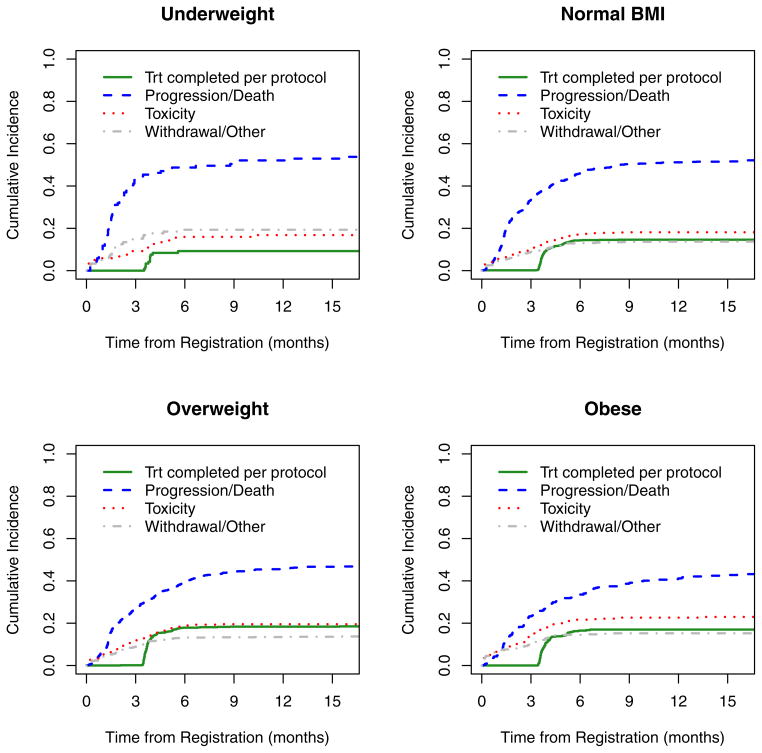
We next explored the time to treatment discontinuation to assess whether or not the differences in outcome by BMI group could be explained in part by protocol compliance. The 15-month point estimates for the cumulative incidence rates and their corresponding standard deviations are reported beneath the cumulative incidence curves in Figure 3. The percent of patients discontinuing treatment due to progression or death was highest among underweight patients (52.3%) and decreased with BMI to 42.3% among obese patients; the rate of discontinuation due to patient withdrawal and other reasons followed a similar inverse trend with BMI. The rate of treatment discontinuation per protocol increased with BMI, however; it was estimated to be 9.2% among underweight patients and increased to 17.0% among obese patients. Obese patients were also more likely to discontinue treatment due to adverse events (23.0%) than those with lower BMI, with a rate of 16.8% among underweight patients. In a regression model of subdistribution functions in competing risks, these results were significantly different across BMI (p=0.004).
Figure 3.
Cumulative incidence of treatment discontinuation by body mass index, as well as 15-month point estimates and their corresponding standard deviations.
DISCUSSION
In this retrospective analysis of 2585 patients with advanced non-small cell lung cancer enrolled on three Eastern Cooperative Oncology Group clinical trials, we assessed the relationship between BMI and clinical outcomes. In multivariable models, obese patients had significantly different overall survival when compared to normal and overweight patients; however their risk of death from any cause increased dramatically once they had been on study longer than 16 months. This indicates that the protective effect of obesity in lung cancer patients is for a limited time, after which the ultimate impact of obesity on survival from all causes supersedes. Though not statistically significant, there was a trend toward worse outcomes for underweight patients when compared to normal/overweight patients.
These results are consistent with the limited number of previous studies evaluating the role of BMI on outcomes for NSCLC patients and on risk of lung cancer, but this report addresses several of the limitations of previous analyses by including prospectively defined and collected study data, uniform staging, and pre-selecting for patients with good performance status, cardiac/organ function, and otherwise lower symptom burden and complicating co-morbid illness. In our study, we have also evaluated the reasons why patients stopped protocol treatment by BMI group and found that the outcomes associated with higher BMI occur in accordance with lower rates of withdrawal from study and despite a higher rate of treatment discontinuation due to toxicity. One reason for this observation may be due to differential pharmacokinetics resulting in higher chemotherapy dosing among BMI groups; however, it is also possible that patients with lower BMI at the time of enrollment have more aggressive disease and worse nutritional status, subjecting them to more cachexia and subsequently more rapid cancer cell growth.
The time-dependence of obesity on outcome relative to patients with normal or underweight status at the time of diagnosis is an interesting observation. One biological reason contributing to this observation may lie in the synergy that exists between peroxisome proliferator-activated receptor (PPAR) ligands, which include natural compounds such as fatty acids and anti-diabetic drugs, and platinum-based agents which has been shown to increase the efficacy of platinum by as much as 4-fold in preclinical studies.33 Because second-line therapies for advanced NSCLC do not include platinum agents as standard of care, the rapid decline in obese patients late in their course of follow-up on these trials could be attributed to the absence of cisplatin or carboplatin after progression, thereby decreasing the synergy with PPAR ligands. Other research has implied that metabolic drugs such as phenformin and metformin induce apoptosis in LKB1-deficient NSCLC; given that LKB1 is inactivated approximately 20% of NSCLC, and that obese patients have a reasonable likelihood of receiving anti-diabetic drugs, the superior outcomes early on in their course of cancer treatment could be driven largely by interactions with these concomitant medications. We unfortunately do not have details on the prevalence of diabetes, concomitant medications, or on differences in treatment at progression, to address these hypotheses. 33–35 It is important to note that our results contradict evidence that IGF-1, a hormone associated with obesity, promotes tumor growth.36
A limitation of our study is that smoking status, a confounding variable known to be associated with lower BMI, was not collected on any of these clinical trials, but it was recently reported that residual confounding due to smoking status did not contribute to the inverse relationship between BMI and risk of lung cancer in a prospective cohort study.7 Despite eligibility criteria that select for good prognosis patients, we do not have details on patient comorbidities, which may supersede the effects of treatment and expose the patients to excess risk later in their disease trajectory and thereby explaining the change in hazard for obese patients.
Our results are consistent with observational studies demonstrating an inverse association between BMI and risk of fatal lung cancers, as well as with outcomes for patients with lymphoma and renal cancer.7–18, 20–21 This obesity paradox has also been described in other areas of medicine including acute lung injury, septic shock, heart failure, and HIV. 37–40 Because obesity is associated with more co-morbidities and other adverse health conditions, the inverse association between BMI and survival seems intuitively discordant; one explanation for this may be in how our studies and others have defined overweightness and obesity. Body mass index is a numerical measure of both fat mass and muscle mass, and is therefore not the most accurate measure of body fatness. Other methods of evaluating true body fat, such as waist circumference and weight to height ratio, as well as cardiorespiratory and muscular fitness have suggested an important role in the obesity paradox, but these methods were unfortunately not available for analysis in our study.41
The ideal situation would be conducting an analysis of serial weight measurements over the entire post-randomization timeframe, since the rapid decline of the obese patients could be because they have lost so much weight that they have become normal or underweight at that point in time. Unfortunately, these data are not available for our studies. This type of information is traditionally collected on the treatment forms, which are submitted at each treatment cycle while a patient remains on protocol treatment. A limitation of our analysis is that some of the treatment regimens were administered for as short a timeframe as 6 3-week cycles or until documented disease progression, at which time protocol therapy would be discontinued and treatment forms would no longer be collected – this is actually fairly standard for clinical trials reporting. With a median PFS of approximately 4 months in this population, this leaves us with scarce data to conduct a robust analysis of BMI over time until death. It is important to note, however, that analyses of weight change over time do not allow for clinical decision making about best course of therapy for patients at baseline, when they present with untreated metastatic disease. That being said, weight gain has been shown to be an important factor in outcomes for patients with locally advanced NSCLC, and serial weight measurements may inform other studies, such as of renal function. 42–43
In summary, higher body mass index among patients with advanced NSCLC enrolled to three NCI-funded Cooperative Group clinical trials is associated with significantly differential survival; however, further studies of the mechanisms underlying this observation, as well as dietary and lifestyle interventions, are warranted to help optimize therapy.
Table 3.
| Underweight | Normal BMI | Overweight | Obese | |||||
|---|---|---|---|---|---|---|---|---|
| % | SD | % | SD | % | SD | % | SD | |
| Per protocol | 9.2 | 2.7 | 14.6 | 1.1 | 18.4 | 1.3 | 17.0 | 1.8 |
| Progression/death | 52.3 | 4.7 | 51.6 | 1.5 | 46.7 | 1.7 | 42.3 | 2.5 |
| Toxicity | 16.8 | 3.5 | 18.1 | 1.2 | 19.5 | 1.4 | 23.0 | 2.1 |
| Withdrawal/Other | 19.3 | 3.7 | 13.7 | 1.0 | 13.6 | 1.2 | 15.2 | 1.8 |
Acknowledgments
Research support:
This study was coordinated by the Eastern Cooperative Oncology Group (Robert L. Comis, M.D., Chair) and supported in part by Public Health Service Grants CA23318, CA66636, CA21115, CA49957, CA21076, CA49883, CA16116 and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
References
- 1.Flegal KM, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 3.Halabi SH, Ou S-S, Vogelzang, et al. Inverse correlation between body mass index and clinical outcomes in men with advanced castration-recurrent prostate cancer. Cancer. 2007;110:1478–1484. doi: 10.1002/cncr.22932. [DOI] [PubMed] [Google Scholar]
- 4.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 5.Renehan AG, Soerjomataram I, Leitzmann MF. Interpreting the epidemiological evidence linking obesity and cancer: a framework for population-attributable risk estimations in Europe. Eur J Cancer. 2010;46:2581–2592. doi: 10.1016/j.ejca.2010.07.052. [DOI] [PubMed] [Google Scholar]
- 6.Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 7.Smith L, Brinton LA, Spitz MR, et al. Body mass index and risk of lung cancer among never, former, and current smokers. JNCI. 2012;104:1–12. doi: 10.1093/jnci/djs179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andreotti G, Hou L, Beane Freeman LE, et al. Body mass index, agricultural pesticide use, and cancer incidence in the Agricultural Health Study cohort. Cancer Causes Control. 2010;21:1759–1775. doi: 10.1007/s10552-010-9603-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 10.Henley SJ, Flanders WD, Manatunga A, et al. Leanness and lung cancer risk: fact or artifact? Epidemiology. 2002;13:268–276. doi: 10.1097/00001648-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Kabat GC, Kim M, Hunt JR, et al. Body mass index and waist circumference in relation to lung cancer risk in the Women’s Health Initiative. Am J Epidemiol. 2008;168:158–169. doi: 10.1093/aje/kwn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kabat GC, Miller AB, Rohan TE. Body mass index and lung cancer risk in women. Epidemiology. 2007;18:607–612. doi: 10.1097/ede.0b013e31812713d1. [DOI] [PubMed] [Google Scholar]
- 13.Knekt P, Heliovaara M, Rissanen A, et al. Leanness and lung-cancer risk. Int J Cancer. 1991;49:208–213. doi: 10.1002/ijc.2910490211. [DOI] [PubMed] [Google Scholar]
- 14.Leung CC, Lam TH, Yew WW, et al. Lower lung cancer mortality in obesity. Int J Epidemiol. 2011;40:174–182. doi: 10.1093/ije/dyq134. [DOI] [PubMed] [Google Scholar]
- 15.Olson JE, Yang P, Schmitz K, et al. Differential association of body mass index and fat distribution with three major histologic types of lung cancer: evidence from a cohort of older women. Am J Epidemiol. 2002;156:606–615. doi: 10.1093/aje/kwf084. [DOI] [PubMed] [Google Scholar]
- 16.Samanic C, Chow WH, Gridley G, et al. Relation of body mass index to cancer risk in 362,552 Swedish men. Cancer Causes Control. 2006;17:901–909. doi: 10.1007/s10552-006-0023-9. [DOI] [PubMed] [Google Scholar]
- 17.Tsai SP, Donnelly RP, Wendt JK. Obesity and mortality in a prospective study of a middle-aged industrial population. J Occup Environ Med. 2006;48:22–27. doi: 10.1097/01.jom.0000184866.49000.e5. [DOI] [PubMed] [Google Scholar]
- 18.Yang L, Yang G, Zhou M, et al. Body mass index and mortality from lung cancer in smokers and nonsmokers: a nationally representative prospective study of 220,000 men in China. Int J Cancer. 2009;125:2136–2143. doi: 10.1002/ijc.24527. [DOI] [PubMed] [Google Scholar]
- 19.Yang R, Cheung MC, Pedroso FE, et al. Obesity and weight loss at presentation of lung cancer are associated with opposite effects on survival. J Surg Res. 2011;170:e75–e83. doi: 10.1016/j.jss.2011.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carson KR, Bartlett NL, McDonald JR, et al. Increased body mass index is associated with improved survival in United States Veterans with diffuse large B-cell lymphoma. J Clin Oncol. 2012;30:3217–3222. doi: 10.1200/JCO.2011.39.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waalkes S, Merseburger AS, Kramer MW, et al. Obesity is associated with improved survival in patients with organ-confined clear-cell kidney cancer. Cancer Causes Control. 2010;21:1905–1910. doi: 10.1007/s10552-010-9618-2. [DOI] [PubMed] [Google Scholar]
- 22.Sparano JA, Wang M, Zhao F, et al. Obesity at diagnosis is associated with inferior outcomes in hormone receptor-positive operable breast cancer. Cancer. 2012;118:5937–5946. doi: 10.1002/cncr.27527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonomi P, Kim K, Fairclough D, et al. Comparison of survival and quality of life in advanced non-small-cell lung cancer patients treated with two dose levels of paclitaxel combined with cisplatin versus etoposide with cisplatin: results of an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2000;18:623–31. doi: 10.1200/JCO.2000.18.3.623. [DOI] [PubMed] [Google Scholar]
- 24.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–8. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 25.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 27.Cox DR, Oates D. Regression models and life tables. J Royal Stat Soc B. 1972;34:187–220. [Google Scholar]
- 28.Klabfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York: Wiley; 1980. [Google Scholar]
- 29.World Health Organization. [Accessed November 1, 2012];Global database on body mass index. http://apps.who.int/bmi/index.jsp?introPage=intro_3.html.
- 30.Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–26. [Google Scholar]
- 31.Schoenfeld D. Chi-squared goodness of fit tests for the proportional hazards regression model. Biometrika. 1980;67:145–53. [Google Scholar]
- 32.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69:239–241. [Google Scholar]
- 33.Girnun GD, Naseri E, Vafai SB, et al. Synergy between PPARgamma ligands and platinum-based drugs in cancer. Cancer Cell. 2007;11:395–406. doi: 10.1016/j.ccr.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu N, Gu C, Gu H, et al. Metformin induces apoptosis of lung cancer cells through activating JNK/p38 MAPK pathway and GADD153. Neoplasma. 2011;58:482–90. doi: 10.4149/neo_2011_06_482. [DOI] [PubMed] [Google Scholar]
- 35.Shackelford DB, Abt E, Gerken L, et al. LKB1 inactivation dictates therapeutic response of non-small cell lung cancer to the metabolism drug phenformin. Cancer Cell. 2013 Jan 15; doi: 10.1016/j.ccr.2012.12.008. pii: S1535–6108(12)00518–1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moschos SJ, Mantzoros CS. The role of the IGF system in cancer: from basic to clinical studies and clinical applications. Oncology. 2002;63:317–32. doi: 10.1159/000066230. [DOI] [PubMed] [Google Scholar]
- 37.O’Brien JM, Phillips GS, Ali NA, et al. Body mass index is independently associated with hospital mortality in mechanically ventilated adults with acute lung injury. Crit Care Med. 2006;34(3):738–44. doi: 10.1097/01.CCM.0000202207.87891.FC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wurzinger B, Dünser MW, Wohlmuth C, et al. The association between body-mass index and patient outcome in septic shock: a retrospective cohort study. Wien Klin Wochenschr. 2010;122(1–2):31–6. doi: 10.1007/s00508-009-1241-4. [DOI] [PubMed] [Google Scholar]
- 39.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 40.Hanrahan CF, Golub JE, Mohapi L, et al. Body mass index and risk of tuberculosis and death. AIDS. 2010;24:1501–1508. doi: 10.1097/QAD.0b013e32833a2a4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lavie CJ, De Schutter A, Patel DA, Milani RV. Body composition and fitness in the obesity paradox-Body mass index alone does not tell the whole story. Prev Med. 2013 Mar 29; doi: 10.1016/j.ypmed.2013.03.010. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 42.Sher DJ, Gielda BT, Liptay MJ, et al. Prognostic significance of weight gain during definitive chemoradiotherapy for locally advanced non-small-cell lung cancer. Clin Lung Cancer. 2012 Dec 20; doi: 10.1016/j.cllc.2012.10.009. pii: S1525–7304(12)00263-X [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 43.Gielda BT, Mehta P, Khan A, et al. Weight gain in advanced non-small-cell lung cancer patients during treatment with split-course concurrent chemoradiotherapy is associated with superior survival. Int J Radiat Oncol Biol Phys. 2011;15;81(4):985–91. doi: 10.1016/j.ijrobp.2010.06.059. [DOI] [PubMed] [Google Scholar]
- 44.Kutluk Cenik B, Sun H, Gerber DE. Impact of renal function on treatment options and outcomes in advanced non-small cell lung cancer. Lung Cancer. 2013 Mar 14; doi: 10.1016/j.lungcan.2013.02.011. pii: S0169–5002(13)00070–6 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]