Abstract
Rapamycin is an immunosuppressive drug that binds simultaneously to the 12-kDa FK506- and rapamycin-binding protein (FKBP12, or FKBP) and the FKBP-rapamycin binding domain (FRB) of the mammalian target of rapamycin (mTOR) kinase. The resulting ternary complex has been used to conditionally perturb protein function, and one such method involves perturbation of a protein of interest through its mislocalization. We synthesized two rapamycin derivatives that possess large substituents at the C16 position within the FRB-binding interface, and these derivatives were screened against a library of FRB mutants using a three-hybrid assay in Saccharomyces cerevisiae. Several FRB mutants responded to one of the rapamycin derivatives, and twenty of these mutants were further characterized in mammalian cells. The mutants most responsive to the ligand were fused to yellow fluorescent protein, and fluorescence levels in the presence and absence of the ligand were measured to determine stability of the fusion proteins. Wild-type and mutant FRB domains were expressed at low levels in the absence of the rapamycin derivative, and expression levels rose up to ten-fold upon treatment with ligand. The synthetic rapamycin derivatives were further analyzed using quantitative mass spectrometry, and one of the compounds was found to contain contaminating rapamycin. Furthermore, uncontaminated analogs retain the ability to inhibit mTOR, albeit with diminished potency relative to rapamycin. The ligand-dependent stability displayed by wildtype FRB and FRB mutants as well as the inhibitory potential and purity of the rapamycin derivatives should be considered as potentially confounding experimental variables when using these systems.
A common approach for studying complex biological processes is to perturb a protein of interest and observe subsequent changes to the biological system. The classical approach of disrupting a gene and studying the resultant phenotype is problematic for examining essential genes. As a result, several conditional techniques have been developed including the tet/dox and Cre/lox systems, where control is exerted at the transcriptional level (1, 2). At the posttranscriptional level RNA interference is becoming rapidly used as a method of gene silencing (3), but RNAi lacks tunability and does not affect existing protein levels. An ideal perturbation method would directly affect the protein with a cell-permeable probe that rapidly and reversibly targets a specific protein of interest.
Small molecules have been very useful as conditional perturbants, however the lack of specificity of many of these reagents can make it difficult to unambiguously interpret results. One approach to ensure specificity is to chemically modify a small molecule with a large substituent, and this technique has been widely used to interrogate specific protein tyrosine kinases that possess a complementary cavity-forming mutation (4, 5). An alternative system has recently been developed using a cell-permeable small molecule to regulate the stability of specific proteins in a rapid, reversible, and dose-dependent manner (6).
A third approach for directly controlling a protein's function is by perturbing its subcellular localization. Conditional regulation of protein localization has been achieved using the FKBP•rapamycin•FRB complex (7-12). With this method a protein of interest is fused to either FKBP1, the 12-kDa FK506- and rapamycin-binding protein or FRB, the FKBP-rapamycin binding domain of the protein kinase, mTOR. The other rapamycin-binding protein is modified to include a domain that regulates subcellular localization (e.g., nuclear export or plasma membrane-associated). The addition of rapamycin induces heterodimerization of the FRB and FKBP fusions and rapid formation of a ternary complex, which causes the protein of interest to be rapidly rerouted within the cell. One disadvantage of this system is that rapamycin has biological activity; it binds to endogenous mTOR kinase, inhibition of which may confound interpretation of the experiment (13, 14).
In order to engineer biologically silent ligands to induce dimerization, a “bump-hole” strategy has been pursued in which rapamycin derivatives were designed to possess bulky substituents within the FRB/mTOR binding region. These derivatives bind poorly to mTOR, and thus are relatively nontoxic to cells, but can bind to FRB domains that contain compensatory cavity-forming mutations. One such derivative contains a methallyl addition at the C20 position in rapamycin (MaRap) and binds a triple mutant of FRB (K2095P, T2098L, W2101F) designated FRB* (15). Fusing the essential gene GSK-3β to FRB* and a nuclear export sequence to FKBP, the addition of MaRap should allow the formation of a ternary complex and a resultant ligand-dependent translocation of GSK-3β to the cytoplasm would be expected. However, in the absence of MaRap levels of GSK-3β expressed as a fusion to FRB* are significantly decreased with respect to the native protein, indicating that the FRB* domain confers instability (16).
To conditionally mislocalize a protein of interest in a rapid and reversible fashion, the protein domains that comprise the ternary complex should not be inherently unstable, and the small molecule should be easy to prepare and possess desirable pharmacokinetics in vivo. We set out to develop a conditional mislocalization system that would have these characteristics. To this end we synthesized two rapamycin derivatives that possess large substituents at the C16 position within the FRB-binding interface, C16-(R)-trimethoxyphenyl rapamycin (TMOP-rap) and C16-(S)-3-methylindolerapamycin (iRap) (8, 10). Screening a library of FRB mutants using a three-hybrid assay in yeast (17) provided many FRB mutants that activated reporter gene expression in response to iRap. Further analyses in mammalian cells revealed that these FRB mutants, as well as wtFRB, significantly reduced expression levels of fusion proteins, and addition of iRap caused a dose-dependent increase in expression levels. An awareness of this ligand-dependent behavior should aid investigators in interpreting experiments based on this conditional dimerization technology.
Experimental Procedures
FRB Library Generation
The FRB gene used for these studies encodes residues 2015-2114 of mTOR. Diversity in the FRB sequence was generated using a combination of error-prone PCR and nucleotide analog mutagenesis. Primers for mutagenic PCR were designed to contain 50 bases of homology to the yeast expression vector pGAD-T7 (Clontech, Palo Alto, CA) including the desired restriction site and 18 bases complementary to either the 5′- or 3′-terminus of the FRB gene. For the first library wtFRB was used as the PCR template for mutagenesis. One set of library conditions utilized 10 ng wtFRB template, 0.5 μM of each oligonucleotide primer, 8 mM MgCl2, 0.2 mM dATP and dGTP, 1.0 mM dCTP and dTTP, 2.5 units Taq polymerase and either 0.5 or 0.05 mM MnCl2. For the second set of conditions, the non-natural nucleotides 8-oxo-dGTP and dPTP were utilized to encourage misincorporation (18). For the second FRB library a mixed template was generated, consisting of equimolar ratios of wtFRB, FRB21, FRB22 as well as a FRB21/FRB22 double mutant. Three independent condition sets were utilized to generate diversity within the FRB coding sequence and are described in (6).
Synthesis of Rapamycin Analogs
C16-(R)-trimethoxyphenyl rapamycin (TMOP-rap) and C16-(S)-3-methylindolerapamycin (iRap) were synthesized as described (8, 10). AP21967 (C16-(S)-7-methylindolerapamycin) was obtained from Ariad Pharmaceuticals, Inc., Cambridge, MA.
Yeast Strains
YSE2 (Matα leu2-3,112 ura3-52 trp1-901 his3-Δ200 ade2-101 gal4Δ gal80A fpr1 Δ∷ADE2 TOR1-1 LYS2∷GAL1 →HIS3 GAL1 → LacZ ura3∷GAL1 →yeGFP GAL1 →hph HO∷ADH1 →Gal4BD-FKBP ADH1 →kanR) was derived by transforming YSB7 (19) with the full-length GAL1/10 promoter driving yeast-enhanced GFP (yeGFP) and the selectable marker hph into the ura3 locus and the full-length ADH1 promoter driving a GAL4BD-FKBP fusion and the selectable marker kanR into the HO locus.
Yeast Transformations
The protocols for both high efficiency and library transformations were followed (20) with the following exceptions. A 50-mL culture (100-mL for library transformations) of the YSE2 strain was grown overnight in YPD for 8 generations of doubling to a final OD600 of 1.0. For the library transformation 100 μg of library DNA along with 10 μg of digested pGAD-T7 library vector was added to the cells. DMSO (1/10 volume of the PEG/lithium acetate mixture) was added to the cells before a 5-minute heat shock at 42 °C. Cells were pelleted, resuspended in YPD and allowed to recover for 3 h at 30 °C, shaking, before being plated on selective dropout media.
Flow Cytometry with Saccharomyces cerevisiae and Collection of FRB Mutants
YSE2 cells carrying the FRB library in selective media were treated with ligand (5 μM TMOP-rap for library #1 and 1 μM iRap for library #2) 16 h prior to analysis and the top 1% of fluorescent cells were collected and grown at 30 °C for 48 h before retreatment. Cells were analyzed (with 10,000 events collected for analysis) and sorted at Stanford Shared FACS Facility. Library DNA was recovered with a yeast plasmid rescue kit (Zymo Research, Orange, CA) and transformed into electrocompetent TOP10 cells (BioRad Gene Pulser: 25 μF, 2.5 kV, 0.2 cm cuvette).
Cell Culture
All cell lines were cultured in DMEM supplemented with 2 mM glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin and 10% heat-inactivated donor bovine serum (NIH3T3 cells), 10% heat-inactivated fetal bovine serum (ΦNX, 293T and COS-1 cell lines) or 10% heat-inactivated horse serum (HEK293 cells).
Secreted Alkaline Phosphatase (SeAP) Reporter Assays
COS-1 cells were electroporated (7) with three vectors: pG5IL2SX to drive transcription of bacterial SeAP by 5 Gal4 DNA binding elements and a human interleukin-2 minimal promoter, pBJ5-GF3E, which expresses Gal4BD fused to three copies of FKBP, and pBJ5-FRBVE, which expresses an FRB fusion, wild-type or mutant, to the herpes simplex virus VP16 activation domain. Cells were treated as described (16, 21), and data were analyzed with Microsoft Excel and KaleidaGraph.
Transfections and Transductions
FRB mutants were cloned into the pBMN retroviral expression vector as N-terminal fusions to YFP. Either the ΦNX ecotropic packaging cell line or 293T cells were transfected with the pBMN vectors using standard Lipofectamine 2000 protocols. For a transient transfection, 293T cells were cultured in growth media for 24 h. To generate transduced NIH3T3 cells, viral supernatants were harvested from the ΦNX cells 48 h post-transfection and NIH3T3 cells were incubated with the retroviral supernatants supplemented with 4 mg/mL polybrene for 4 h at 37 °C. Cells were cultured in growth media for 24 h to allow for viral integration before analysis.
Flow Cytometry with Mammalian Cells
Cells were plated at 1 × 105 cells per well of a 12-well plate and treated with iRap. For the stability assay, cells were treated with 1 μM iRap for 24 h and then harvested. For the dose response assay, cells were treated with iRap (0-10 μM) and harvested at each time point. Cells were trypsinized from the plate, washed once with PBS, and resuspended in 200 μL PBS. Cells were analyzed at the Stanford Shared FACS Facility with 10,000 events collected for analysis.
Immunoblotting for the mTOR Kinase Assay
HEK293 cells were plated at 2-2.5 × 105 cells per well of a 12-well plate and serum-starved for 24 h in DMEM only. Cells were mock-treated or treated with rapamycin (0.05-50 nM), iRap (0.5-500 nM), or AP21967 (0.5-500 nM) for 15 minutes at 37 °C. Serum was added to a final concentration of 20% for 30 minutes at 37 °C. Cells were lysed as described (22) and cell lysates were separated by SDS-PAGE. Resolved proteins were transferred to a PVDF membrane and immunoblotted with a phosphospecific primary antibody against Thr389 of p70 S6 kinase (Cell Signaling Technology, Inc. #9205, Danvers, MA). Data were analyzed using ImageQuant and KaleidaGraph.
Results
Screening FRB Mutants in Yeast
To identify FRB mutants able to accommodate either TMOP-rap or iRap we used a three-hybrid transcriptional switch assay in S. cerevisiae. In this assay FKBP is fused to the Gal4 DNA-binding domain (Gal4BD) and FRB is fused to the Gal4 activation domain (Gal4AD). Addition of rapamycin leads to formation of the FKBP•rapamycin•FRB complex, reconstituting the Gal4 transcription factor. We engineered a yeast strain in which yeGFP is placed under control of the GAL1/10 promoter, and reporter gene expression indicates successful formation of the ternary complex (Fig. 1A). The rapamycin derivatives TMOP-rap and iRap were synthesized with modifications at the C16 position within the FRB-binding region to abrogate binding to the native target mTOR (Fig. 1B).
Fig. 1.
Three-Hybrid Transcriptional Switch Model and Structures of Rapamycin Compounds.
A, The Gal4 DNA-binding domain is fused to FKBP and the Gal4 activation domain is fused to an FRB library. The fusion proteins are colocalized only in the presence of a rapamycin analog, reconstituting the transcription factor and activating expression of the reporter gene.
B, The molecular structures of rapamycin and its analogs with the FRB- and FKBP-binding regions highlighted. Me = methyl.
To accommodate the added steric bulk of the rapamycin analog, a compensatory cavity in FRB must be introduced, and we used error-prone PCR and nucleotide analog mutagenesis to prepare a library of FRB mutants. The FRB library was designed and cloned into the destination vector by in vivo ligation, a process in which yeast cells ligate a library of PCR-derived inserts into a vector through homologous recombination. Successful recombination allows cells to grow on selective dropout media. Transformed yeast were subjected to multiple rounds of FACS-based sorting, enriching for cells that express yeGFP.
A library containing ∼106 members was subjected to six rounds of sorting using flow cytometry. For each round the transformed cells were induced with 5 μM TMOP-rap 16 h prior to sorting and the fluorescent cells were collected and further cultured from 1 to 7 d between sorts. The library population contained ∼2% positives upon initial analysis and was enriched to ∼80% by the final sort. These fluorescent cells were collected, and the plasmid DNA isolated and sequenced. Sequence analysis of 33 clones revealed only two mutations – the first was I2021T and the second involved a mutation of the stop codon to one encoding leucine. The construct encoding FRB possesses a second in-frame stop codon four residues beyond the original stop codon, thus the second mutant has a four-residue extension (LDSS) at the C-terminus.
To validate the screening method for selecting an FRB mutant that could bind TMOP-rap and to eliminate false positives, both FRB mutants in the pGAD-T7 vector were transformed into YSE2 and plated onto selective dropout media. Cells were treated with 5 μM TMOP-rap and analyzed by flow cytometry for expression of the reporter gene yeGFP. Surprisingly, the two FRB mutants responded weakly to TMOP-rap treatment and the yeGFP expression levels did not reach those of the library population. To find an FRB mutant more responsive to ligand treatment a second library was generated in a similar method to the first. The two mutations derived from the first screen were included in the library generation, so the mixed FRB template included wild-type FRB, both single FRB mutants discovered from the first library and the corresponding double mutant. Additionally, another rapamycin derivative, iRap, was chosen for the screen.
A second FRB library containing ∼106 members was subjected to two rounds of sorting with flow cytometry. Transformed cells were treated with 1 μM iRap 16 h prior to sorting, and the top 1% of cells were collected and grown in the absence of ligand for 48 h. These cells were then treated with iRap 16 h prior to sorting. At this time ∼55% of the population was fluorescent (Fig. 2A). The top 1% of cells were collected, and the plasmid DNA isolated and sequenced. Sequence analysis of 54 clones revealed 29 new FRB mutants.
Fig. 2.
FRB Library Screen and Validation of FRB Mutants in S. cerevisiae.
A, The library of FRB mutants expressed in yeast was treated with 1 μM iRap for 16 h and assayed by flow cytometry for yeGFP expression. The population was enriched from 3% to 55% positives with one round of sorting.
B, Each FRB mutant was individually transformed into yeast, treated with 1 μM iRap for 16 h and assayed by flow cytometry for yeGFP expression. Data are displayed as fold increase in fluorescence level over background, which was normalized to one. Experiments were performed in duplicate and the average of both experiments is shown.
For validation, these FRB mutants were all individually transformed into YSE2 as described above. Two colonies expressing each mutant were treated with 1 μM iRap, and yeGFP expression levels were measured by flow cytometry. Fluorescence levels of the protein varied from barely above background to six-fold higher (Fig. 2B). Twenty of these clones, all expressing high levels of yeGFP, were next tested in mammalian cells using a three-hybrid transcriptional switch.
Further Characterization of FRB Mutants in Mammalian Cells
COS-1 cells were co-transfected by electroporation with the vectors pG5IL2SX, pBJ5-GF3E and pBJ5-FRBVE (encoding either wild-type or mutant sequences of FRB) (21). Mutant FRB sequences included the twenty clones from the iRap library as well as both of the mutants isolated from the first TMOP-rap-dependent screen. Supplemental table S1 lists the sequences of these 22 FRB mutants. Transfected cells were treated for 16 h in quadruplicate with rapamycin or iRap (0.5-500 nM). Fluorescence levels, representative of SeAP activity, were measured for all FRB constructs and a representative sample is shown in Fig. 3. FRB mutants show varying response to iRap, and the EC50 values for the mutants range from 14.4 to 250 nM (Table 1). Additionally, iRap induces expression with an EC50 value of ∼20 nM, indicating that both wtFRB and the FRB mutants isolated from the library screen respond similarly to iRap in this transcriptional switch assay.
Fig. 3.
Secreted Alkaline Phosphatase (SeAP) activity of the FRBs in COS-1 cells.
COS-1 cells were co-transfected with Gal4BD-FKBP, VP16AD-FRB and reporter constructs and treated with iRap from 0.5-500 nM or rapamycin from 0.05-50 nM. Cells were treated with the quenched fluorescent substrate methylumbelliferylphosphate and assayed for SeAP expression. Data are shown with open symbols for rapamycin samples and filled symbols for iRap samples. Data for wtFRB and representative mutants are presented as the average (± SEM) for an experiment performed in quadruplicate.
Table 1. FRB Mutants Treated with iRap Exhibit a Range of EC50 values by the SeAP Assay.
Fluorescence data generated by the SeAP assay were fit and EC50 values derived from the curve fits. Data are presented as the mean value (±SEM) for quadruplicate measurements. Identities of mutants are listed in supplemental Table S1.
| FRB Mutant | EC50 (±SEM) nM |
|---|---|
| WT FRB | 20.5 (0.6) |
| FRB19 | 14.4 (0.4) |
| FRB22 | 18.4 (0.8) |
| FRB11 | 19.3 (1.1) |
| FRB10 | 20.4 (1.2) |
| FRB20 | 22.1 (2.0) |
| FRB14 | 23.0 (0.6) |
| FRB2 | 24.9 (2.1) |
| FRB21 | 26.0 (1.1) |
| FRB18 | 28.1 (2.0) |
| FRB1 | 29.7 (2.2) |
| FRB6 | 31.4 (1.1) |
| FRB17 | 32.6 (1.5) |
| FRB9 | 33.4 (2.1) |
| FRB4 | 39.0 (5.3) |
| FRB8 | 39.1 (2.3) |
| FRB5 | 41.7 (4.6) |
| FRB16 | 42.7 (9.2) |
| FRB13 | 45.1 (10.5) |
| FRB3 | 67.4 (10.0) |
| FRB7 | 88.8 (19.3) |
| FRB12 | 100 (55) |
| FRB15 | 250 (140) |
Examining the Stability of FRB Mutants
An important characteristic for a conditional mislocalization system that operates in a rapid fashion is that all ligand-binding proteins are stable in the absence of ligand. This was not the case for the original MaRap-sensitive FRB mutant (16). To evaluate the stability of the FRB mutants isolated from the library screen, FRB mutants #2, 10, 11, and 19, as well as wtFRB, were fused to the N-terminus of YFP, and the constructs were expressed in mammalian cells. These four FRB mutants were chosen because they displayed the lowest EC50 values for iRap in the SeAP assay.
NIH3T3 cells were individually transduced with all five constructs and mock-treated or treated with 1 μM iRap 24 h prior to FACS analysis. 293T cells were transiently transfected with only the wtFRB construct and treated as above. YFP fluorescence levels were measured in the presence and absence of iRap (Fig. 4A). In the absence of iRap, YFP fluorescence levels for the FRB constructs are only slightly above background, showing that the FRB proteins are unstable when expressed in mammalian cells. Moreover, as for FRB* treated with MaRap, the FRB proteins appear to be stabilized when treated with iRap. A ten-fold increase in fluorescence is observed when cells stably expressing wtFRB fused to YFP are treated with iRap (Fig. 4A). Similar iRap-dependent stability is observed when measuring YFP expression levels for 293T cells transiently transfected with the wtFRB-YFP construct. All of the FRB mutants demonstrate similar increases in fluorescence (four- to eight-fold) upon treatment with iRap.
Fig. 4.
Characterization of FRB Mutants in Mammalian Cells.
A, NIH3T3 cells were transduced with YFP, wtFRB-YFP or mutant FRB-YFP constructs, treated with 1 μM iRap for 24 h and assayed for YFP fluorescence by flow cytometry. 293T cells were transiently transfected with a wtFRB-YFP construct and treated as above. Data are presented as the mean fluorescence intensity (MFI) for each FRB construct with error bars for triplicate measurements.
B, NIH3T3 cells stably expressing wtFRB-YFP were treated with concentrations of iRap from 10 nM to 10 μM and YFP expression was monitored by flow cytometry for 24 h. Data are presented as the mean fluorescence intensity relative to the overall highest value. Experiment was performed in duplicate.
Characterization of FRB-Induced Instability
To determine both the time required to achieve maximum fluorescence as well as the minimum dose required to reach this fluorescence level, a kinetic study of NIH3T3 cells stably expressing the wtFRB-YFP construct was carried out. Transduced cells were treated with various concentrations of iRap, and YFP fluorescence was monitored as a function of time (Fig. 4B). We observed that YFP fluorescence increased at approximately the same rate for the cell populations treated with 100 nM, 1 μM and 10 μM iRap, whereas the cell population treated with 10 nM iRap showed decreased fluorescence levels at all time points. All cell populations achieved maximum fluorescence at 12 h and levels dropped off slightly by 24 h, indicating that maximum fluorescence is reached by 12 h but not maintained in the absence of further dosing.
A dose-response assay was performed to determine the concentration of iRap required to stabilize the wtFRB-YFP fusion protein. NIH3T3 cells transduced with wtFRB-YFP were treated with iRap (0-1 μM) and YFP fluorescence levels measured. Cells achieve maximum fluorescence with 1 μM iRap and the EC50 value is ∼10 nM (supplemental Fig. S2).
To confirm that YFP fluorescence levels correlate to protein expression levels, NIH3T3 cells transduced with the wtFRB-YFP construct and treated with 1 μM iRap for 24 h were harvested and immunoblotted with a primary antibody against YFP. The protein expression levels are consistent with fluorescence levels (data not shown).
mTOR Kinase Assays
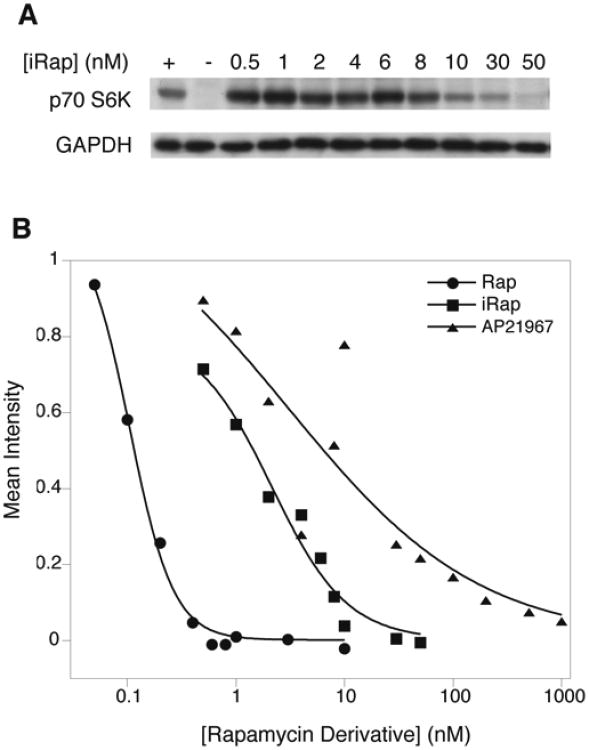
The experiments carried out thus far utilized FRB, which is a relatively small domain within the mTOR kinase. In order to determine whether the rapamycin derivatives bind with the same affinity to mTOR as they bind to FRB, a series of S6 kinase assays was performed. The mTOR protein kinase phosphorylates p70 S6 kinase (p70 S6K) at threonine 389, so the ability of rapamycin and its derivates to inhibit endogenous mTOR can be probed using a phosphospecific antibody for this residue. HEK293 cells were treated with rapamycin (0.05-50 nM), iRap (0.5-500 nM), or the derivative C16-(S)-7-methylindolerapamycin (AP21967) (0.5-500 nM) under conditions that stimulate mTOR kinase activity. Cell lysates were resolved by SDS-PAGE and immunoblotted with a phosphospecific antibody for p70 S6K (T389). A representative Western blot is shown in Fig. 5A, in which the signal for phosphorylation of p70 S6K T389 disappears as iRap inhibits mTOR. All three compounds were found to inhibit endogenous mTOR, with IC50 values of ∼0.1 nM for rapamycin, ∼5 nM for iRap and ∼10 nM for AP21967 (Fig. 5B).
Fig. 5.
Inhibition of mTOR Kinase Activity.
HEK293 cells serum-starved 24 h were mock-treated or treated with rapamycin (0.05-50 nM), iRap (0.5-500 nM), or AP21967 (0.5-500 nM) for 15 min. Serum was added for 30 min and cell lysates were immunoblotted with a phosphospecific antibody against p70 S6K T389. GAPDH serves as a loading control.
A, Representative blot showing levels of phosphorylated T389 of p70 S6K in response to iRap treatment. The plus sign indicates the positive control lane (mock-treated cells) lane and the minus sign indicates the negative control lane (cells treated with 10 nM rapamycin).
B, Intensities of the signal from each sample were quantified with respect to the loading control and normalized to phosphorylation levels in untreated cells. Data were then plotted and fit to derive IC50 values.
Discussion
We set out to discover a ternary complex comprising FKBP, an FRB mutant and a rapamycin derivative that could be used to conditionally mislocalize proteins to rapidly and reversibly probe their function. The FRB library screened with the rapamycin derivative iRap revealed twenty mutants that expressed yeGFP above background levels (Fig. 2B). In a mammalian three-hybrid system using the reporter gene SeAP, these FRB mutants exhibited a range of expression levels. Some mutants were slightly more potent than wtFRB (EC50 values < 20 nM) but most mutants were moderately weaker (EC50 values > 20 nM) (Table 1).
The iRap sample was pure by 1H NMR analysis (95%), but we became concerned that trace quantities of rapamycin might be responsible for the behavior of some of the mutants isolated from the library screen. Upon analysis by quantitative mass spectrometry we determined that the iRap sample contains ∼2.6% rapamycin. Rapamycin was not detected (< 0.1%) in the sample of AP21967. Our findings show that the bumped rapamycin analogs retain the ability to bind to wtFRB (Fig. 3) and full-length mTOR (Fig. 5), but trace quantities of rapamycin may improve the potency of the analogs. Given this fact, it is difficult to determine how much activity (i.e., isolation of mutants, activation of SeAP) is due to the rapamycin analog.
The fusion protein of wtFRB to YFP was examined in both stably transduced (NIH3T3) and transiently transfected (293T) cells and the ten-fold increase in expression levels in the presence of iRap is consistent for both protocols. Additionally, wtFRB and the FRB mutant proteins are only partially rescued by ligand, as the fluorescence levels in the presence of iRap are still 1-2 orders of magnitude lower than a construct expressing YFP only. Thus, although the FRB proteins exhibit ligand-dependent stability, the degree of stabilization in the presence of ligand is modest relative to unfused YFP.
The destabilization behavior of FRB* was originally attributed to the cavity-forming mutations, but it appears that even the wild-type FRB protein is destabilizing. To create a technology to control protein function based on rapid subcellular localization, the ligand-binding proteins should be stable. Waiting 12-24 hours for protein levels to rise largely removes the temporal advantage of ligand-induced mislocalization. Additionally, a mislocalization system built upon domains that display ligand-dependent stability may produce ambiguous results, as it would be difficult to ascertain how much of an observed phenotype is due to mislocalization versus changes in protein expression levels.
We found by monitoring fluorescence growth that NIH3T3 cells transduced with a wtFRB-YFP fusion and treated with 10 nM iRap reach 50% of the maximum value that the populations treated with higher concentrations of iRap reach and have lower expression levels at every time point. Thus when treating cells with a ligand at low concentrations it is important to note that maximum protein expression levels are never achieved.
Upon examining the activity of rapamycin, iRap, and AP21967 in mTOR kinase assays both iRap and AP21967 were found to inhibit mTOR with IC50 values in the low nanomolar range (Fig. 5). Our studies to quantitate rapamycin levels in these samples revealed that rapamycin was not detected in AP21967 (< 0.1%). Since the C16 substituent of iRap is nearly identical to that of AP21967, this finding suggests that iRap's ability to more potently inhibit mTOR (Fig. 5) or translocate fusion proteins (10) is likely due to traces of contaminating rapamycin.
In our search for a ternary complex that could be used to rapidly mislocalize proteins of interest we have discovered that the FRB domain of the mTOR kinase is inherently unstable. When FRB is fused to the fluorescent protein YFP and studied in the presence and absence of ligand the expression levels of the fusion protein increase from four- to ten-fold for all of the FRB proteins examined. Both the ligand-dependent stability of FRB fusion proteins as well as the purity of rapamycin derivatives should be considered as potentially confounding variables when using rapamycin-based ligand-mediated colocalization systems.
Supplementary Material
Footnotes
We thank the Crabtree and Davis labs for yeast strains and plasmids, Joshua Grimley for the synthesis of iRap, Laura Banaszynski for the synthesis of TMOP-rap, and Angela Chu for helpful discussions. This work was supported by the NIH (GM068589).
The following abbreviations are used: FKBP, 12-kDa FK506- and rapamycin-binding protein; FRB, FKBP12-rapamycin binding domain; Gal4AD, Gal4 activation domain; Gal4BD, Gal4 binding domain; iRap, C16-(S)-3-methylindolerapamycin; mTOR, mammalian target of rapamycin; p70 S6K, p70 S6 kinase; SeAP, secreted alkaline phosphatase; TMOP-rap, C16-(R)-trimethoxyphenyl rapamycin; wtFRB, wild-type FRB; YFP, yellow fluorescent protein; YPD, yeast peptone dextrose; yeGFP, yeast-enhanced green fluorescent protein.
References
- 1.Furth PA, St Onge L, Boeger H, Gruss P, Gossen M, Kistner A, Bujard H, Hennighausen L. Proc Natl Acad Sci U S A. 1994;91:9302–9306. doi: 10.1073/pnas.91.20.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryding ADS, Sharp MGF, Mullins JJ. J Endocrin. 2001;171:1–14. doi: 10.1677/joe.0.1710001. [DOI] [PubMed] [Google Scholar]
- 3.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 4.Shah K, Liu Y, Deirmengian C, Shokat KM. Proc Natl Acad Sci U S A. 1997;94:3565–3570. doi: 10.1073/pnas.94.8.3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bishop AC, Shah K, Liu Y, Witucki L, Kung CY, Shokat KM. Curr Biol. 1998;8:257–266. doi: 10.1016/s0960-9822(98)70198-8. [DOI] [PubMed] [Google Scholar]
- 6.Banaszynski LA, Chen LC, Maynard-Smith LA, Ooi AGL, Wandless TJ. Cell. 2006;126:995–1004. doi: 10.1016/j.cell.2006.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho SN, Biggar SR, Spencer DM, Schreiber SL, Crabtree GR. Nature. 1996;382:822–826. doi: 10.1038/382822a0. [DOI] [PubMed] [Google Scholar]
- 8.Luengo JI, Yamashita DS, Dunnington D, Beck AK, Rozamus LW, Yen HK, Bossard MJ, Levy MA, Hand A, Newman-Tarr T, Badger A, Faucette L, Johnson RK, D'Alessio K, Porter T, Shu AYL, Heys R, Choi J, Kongsaeree P, Clardy J, Holt DA. Chem Biol. 1995;2:471–481. doi: 10.1016/1074-5521(95)90264-3. [DOI] [PubMed] [Google Scholar]
- 9.Klemm JD, Beals CR, Crabtree GR. Curr Biol. 1997;7:638–644. doi: 10.1016/s0960-9822(06)00290-9. [DOI] [PubMed] [Google Scholar]
- 10.Inoue T, Heo WD, Grimley JS, Wandless TJ, Meyer T. Nat Meth. 2005;2:415–418. doi: 10.1038/nmeth763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heo WD, Inoue T, Park WS, Kim ML, Park BO, Wandless TJ, Meyer T. Science. 2006;314:1458–1461. doi: 10.1126/science.1134389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suh BC, Inoue T, Meyer T, Hille B. Science. 2006;314:1454–1457. doi: 10.1126/science.1131163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, Schreiber SL. Nature. 1994;369:756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- 14.Vilella-Bach M, Nuzzi P, Fang Y, Chen J. J Biol Chem. 1999;274:4266–4272. doi: 10.1074/jbc.274.7.4266. [DOI] [PubMed] [Google Scholar]
- 15.Liberles SD, Diver ST, Austin DJ, Schreiber SL. Proc Natl Acad Sci U S A. 1997;94:7825–7830. doi: 10.1073/pnas.94.15.7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stankunas K, Bayle JH, Gestwicki JE, Lin YM, Wandless TJ, Crabtree GR. Mol Cell. 2003;12:1615–1624. doi: 10.1016/s1097-2765(03)00491-x. [DOI] [PubMed] [Google Scholar]
- 17.Licitra EJ, Liu JO. Proc Natl Acad Sci U S A. 1996;93:12817–12821. doi: 10.1073/pnas.93.23.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zaccolo M, Williams DM, Brown DM, Gherardi E. J Mol Biol. 1996;255:589–603. doi: 10.1006/jmbi.1996.0049. [DOI] [PubMed] [Google Scholar]
- 19.Biggar SR, Crabtree GR. J Biol Chem. 2000;275:25381–25390. doi: 10.1074/jbc.M002991200. [DOI] [PubMed] [Google Scholar]
- 20.Gietz RD, Woods RA. Methods Enzymol. 2002;350:87–96. doi: 10.1016/s0076-6879(02)50957-5. [DOI] [PubMed] [Google Scholar]
- 21.Bayle JH, Grimley JS, Stankunas K, Gestwicki JE, Wandless TJ, Crabtree GR. Chem Biol. 2006;13:99–107. doi: 10.1016/j.chembiol.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 22.Park IH, Erbay E, Nuzzi P, Chen J. Exp Cell Res. 2005;309:211–219. doi: 10.1016/j.yexcr.2005.05.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.