Abstract
An ultra sensitive method for arsenic (As) speciation analysis based on selective hydride generation (HG) with preconcentration by cryotrapping (CT) and inductively coupled plasma- mass spectrometry (ICP-MS) detection is presented. Determination of valence of the As species is performed by selective HG without prereduction (trivalent species only) or with L-cysteine prereduction (sum of tri- and pentavalent species). Methylated species are resolved on the basis of thermal desorption of formed methyl substituted arsines after collection at −196°C. Limits of detection of 3.4, 0.04, 0.14 and 0.10 pg mL−1 (ppt) were achieved for inorganic As, mono-, di- and trimethylated species, respectively, from a 500 μL sample.
Speciation analysis of river water (NRC SLRS-4 and SLRS-5) and sea water (NRC CASS-4, CASS-5 and NASS-5) reference materials certified to contain 0.4 to 1.3 ng mL−1 total As was performed. The concentrations of methylated As species in tens of pg mL−1 range obtained by HG-CT-ICP-MS systems in three laboratories were in excellent agreement and compared well with results of HG-CT-atomic absorption spectrometry and anion exchange liquid chromatography- ICP-MS; sums of detected species agreed well with the certified total As content.
HG-CT-ICP-MS method was successfully used for analysis of microsamples of exfoliated bladder epithelial cells isolated from human urine. Here, samples of lysates of 25 to 550 thousand cells contained typically tens pg up to ng of iAs species and from single to hundreds pg of methylated species, well within detection power of the presented method. A significant portion of As in the cells was found in the form of the highly toxic trivalent species.
Keywords: Arsenic, Speciation analysis, Hydride generation, Inductively coupled plasma mass spectrometry
INTRODUCTION
Arsenic (As) is a typical example of an element where information on speciation is of paramount importance to understand toxicity and metabolism, or transport processes in the environment. In biological and environmental samples, As is present in a variety of compounds with different toxicities. Most important from the point of toxicology are forms of inorganic arsenic (iAs), arsenite (iAsIII) and arsenate-(iAsV), and the methylated forms, trivalent methylarsonite (MAsIII) and dimethylarsinite (DMAsIII) and pentavalent methylarsonate (MAsV), dimethylarsinate (DMAsV), and trimethylarsine oxide (TMAsVO). These species are generated in the course of iAs metabolism and are found in biological systems either unbound or bound to proteins or other cellular components 1.
A comprehensive review 2 gives an overview of the state of As speciation analysis and its challenges. Although a variety of analytical approaches are used depending on the information desired, quantitative speciation analysis of As now relies heavily on a liquid phase separation followed by a sensitive element specific detection such as inductively coupled plasma mass spectrometry (ICP-MS) or postcolumn HG- atomic fluorescence spectrometry. Ion-exchange and ion-pairing high performance liquid chromatography (HPLC) are, by far, the most frequent separation techniques used in As speciation analysis; separation conditions are reviewed elsewhere. 3,4,5 Advantages of the liquid phase separation approach are selectivity and ability to analyze virtually all As species. The coupling of the separation method to the detector and system operation are usually easy. Unfortunately, this approach is confined by the limitations and practical problems of the separation technique. Complex biological samples require extraction of As species, potentially resulting in low recoveries and species transformation, i.e., oxidation. During the separation step, As species can be lost or transformed on the column. 6,7,8 Moreover, good separation of all arsenic species of interest can be difficult to achieve under one set of separation conditions.
Sensitivity of HPLC-ICP-MS is principally limited by sample volume (typically 100 μl or less for analytical HPLC). Also sample zone broadening during separation in fact means analyte dilution. A significant analyte loss is experienced at the interface of LC with the plasma, i.e., in the nebulizer. Because typical nebulization efficiency is only in the single percent range, the remaining analyte goes to the waste rather than to the plasma. Higher efficiency nebulizers, e.g., ultrasonic, do exist, but the maximum ICP-MS plasma load with solvent/buffer is a limiting factor. High-efficiency micronebulizers that can reach 100% nebulization efficiency are confined to very low effluent flows, offering an advantage only to separations with low applicable sample volume 9. Generally, the published limits of detection (LOD) for HPLC-ICP-MS based analysis range from tens of pg to ng mL−1 5.
An alternative and complementary approach to As speciation analysis is based on the generation of volatile As species, including arsine and methyl-substituted arsines 2,10. Here, arsines are preconcentrated in a liquid nitrogen cooled cryotrap (CT) filled with chromatographic packing and separated upon desorption by their boiling points aided with trap packing properties. Because analytes from a relatively large volume of a sample is collected and focused into a few seconds wide peak shaped signals, excellent LOD can be achieved. The HG-CT designs in combination with atomic absorption spectrometry (AAS) detection have traditionally been used for As speciation analysis. Reports of HG-CT applications in connection with ICP-MS detection are surprisingly scarce. 11,12,13,14,15,16 The principal drawback of the HG-based approach is the limited selectivity, as the CT step can only distinguish four volatile As species, arsine and mono-, di- and trimethylarsine. Therefore, the selectivity of the analysis is aided by the HG step because some As species do not form hydrides under certain conditions. The simplest approach relies on selective HG. Thus, at pH 6, pentavalent iAsV and MAsV do not form volatile hydrides at all and DMAsV only to small extent (5–8%). In contrast, trivalent species (iAsIII, MAsIII and DMAsIII) as well as TMAsVO are converted to hydrides quantitatively. The sum of tri- and pentavalent As species is then determined in a second sample aliquot under different HG conditions 17.
In recent years, the authors’ laboratories have developed a semi-automated setup for HG-CT analysis coupled to an AAS detector with a quartz multiatomizer 17,18. Tri- and pentavalent species are distinguished on the basis of selective HG directly and after L-cysteine (cys) prereduction. Methylation of arsenic species is then determined in the CT step. Good LOD have been achieved even with simple AAS detection: LOD in water and cell culture experiments are in tens of ng mL−1 for iAs and ng mL−1 for methylated species 17,18. Initially developed for analyses of cell culture experiments (cell lysates and culture medium) and urine, this setup has been used for As speciation analysis in a range of samples including, in vitro As methylating enzymatic assays 19, small volume samples of human cells, e.g., bladder epithelial cells (BECs) that defoliate from the bladder epithelium into the urine 20, or antimony compound-based drugs 21. Importantly, the HG process is performed in a very controlled manner and near 100% efficiency is achieved for all As hydride forming species. Additionally, quantitation of all species can be achieved using one or several stable and readily available As compounds. This approach is uniquely suited for the analysis of highly unstable trivalent arsenicals8, both free and protein or thiol-complex bound, because no sample preparation step is required. Even direct analysis of trivalent arsenicals in homogenized liver tissue was successfully accomplished with good recoveries 22,23, which would be impossible by any LC-based approach.
The aim of this study was to further develop the HG-CT technique to achieve the lowest possible LOD. This is achieved by replacing the AAS detector with ICP-MS, currently the benchmark for high sensitivity. To demonstrate the performance, flexibility and potential of this optimized method, standard reference materials and human BECs are examined. First is analysis of river and sea water reference materials containing extremely low concentrations of methylated As species (total As content below ng mL−1). The second is an oxidation state specific analysis of human BECs containing down to pg levels of As species from individuals exposed to iAs through the ingestion of contaminated drinking water.
2. Experimental
2.1 Instrumentation
2.1.1. HG-CT-ICP-MS system
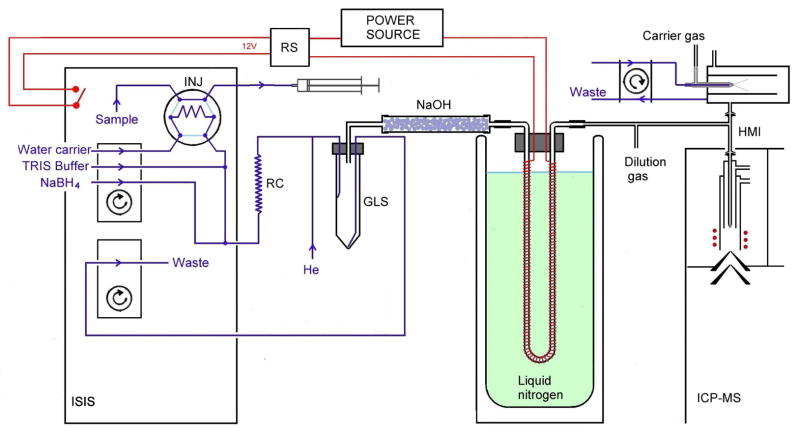
An Agilent 7700x ICP-MS spectrometer equipped with an Integrated Sample Introduction System (ISIS) accessory containing two peristaltic pumps and injection valve was used. The system for HG-CT was, in principle, identical to that previously described 17, and adapted for use with an ISIS flow injection module and ICP-MS detector. A scheme of the system is presented in Fig. 1. The sodium borohydride (NaBH4) and TRIS·HCl buffer solutions and deionized water (DIW) were pumped an ISIS multichannel peristaltic pump (PP1) at flow rates of 1 mL ·min−1. The manifold was built of PEEK T-junctions (Vici Jour, Sweden) and PTFE tubing of 0.75 mm i.d.−1/16′ o.d., except the reaction coil (1 mm i.d.−1/16′ o.d., 1000 mm long, total volume 0.79 mL), He and H2 inlet (0.5 mm i.d.−1/16′ o.d) and tubing connecting the gas-liquid separator (GLS), dryer cartridge, cryogenic trap and torch interface (1.6 mm i.d./1/8′ o.d.). Sample was injected into the flow of DIW by an injection valve. The carrier gas with a flow rate of 75 mL ·min−1 of He, was controlled by mass flow controller (Omega Engineering, inc., Stamford, USA) and added upstream of the GLS. A plastic GLS capable handling overpressure caused by resistance of the U-tube consisted of a 50 mL polypropylene screw-vial with a custom acrylic lid. A forced liquid waste outlet from the GLS was driven by a second ISIS pump (PP2) at an arbitrary flow rate. Gaseous phase was dried by a polyethylene cartridge (100 mm long, 17 mm i.d.) containing approximately 25 g of NaOH (p.a., LachNer, Czech Rep.) in the form of 3 mm pearls 24. The cryogenic trapping device consisted of a 305 mm long glass U-tube with a 2.5 mm i.d. wrapped with a Ni80-Cr20 resistance wire (0.6 mm o.d.; 5.275 Ω·m−1; Omega Engineering, Inc., Stanford, CT, U.S.A.) providing a total resistance of 15 Ω for gradual heating and was filled with approximately 0.9 g of Chromosorb WAW-DMCS 45/60, 15 % OV-3 (Supelco, Bellefonte, USA). After packing, the U-tube was treated with 50 μl REJUV-8 (hexamethyldisilazane, N, O-bis(trimethylsilyl)acetamide, n-trimethylsilyl-imidazole; Supelco, Bellefonte, USA) and flushed with 50 mL·min−1 of He for 4 hours. The liquid nitrogen bath was contained in a 300 mm deep, 1 L Dewar flask (KGW-Isotherm, Karlsruhe, Germany). During the release phase, the U-tube was gradually heated with approximately 20 V, regulated by a laboratory source (Electro-Automatik GmbH & Co. KG, Viersen, Germany) and switched on and off via an internal remote switch in the ISIS module (Agilent). The complete ISIS program is presented in Table S1 (Electronic supplementary material).
Fig. 1.
Scheme of the HG-CT-ICPMS (System 1). INJ: Injection vent. GLS: Phase separator. RS: Relay switch. RC: Reaction coil. NaOH: NaOH pellet dryer.
The gas outlet from the U-tube was mixed within a T-connector into the flow of the dilution gas connected in the quartz high-matrix interface (HMI) between the spray chamber and ICP torch. An internal standard of 100 ng.mL −1 Te was co-nebulized during the experiments to monitor the plasma and provide more robust wet plasma conditions. Measurements were performed in the He collision mode (3.5 mL ·min He); complete plasma settings are in presented in Table 1. The m/z 75 (As, 0.5 s) and 125 (Te, 0.1 s) were monitored. Peak areas are exclusively reported. Integration was performed either directly in Agilent Mass Hunter Chromato software, or exported and integrated in MS Excel.
Tab. 1.
ICP-MS operating parameters
| HG-CT-ICP-MS System 1 | HG-CT-ICP-MS System 2 | HG-CT- ICP-MS System 3 | HPLC-ICP-MS | |
|---|---|---|---|---|
| ICP-MS | Agilent 7700x | Agilent 7500cx | Agilent 7500cx | Agilent 7700x |
| Power, W | 1600 | 1500 | 1500 | 1600 |
| Nebulizer Ar, L min−1 | 0.6 | 1 | 1 | 1 |
| Dilution Ar, L min−1 | 0.5 | 0 | 0 | 0.15 |
| Nebulizer pump, rps | 0.1 | 0.1 | 0.1 | 0.3 |
| i.e., mL min−1 | 0.3 | 0.3 | 0.3 | 1 |
| Nebulized solution | Te 100 ng mL −1 | DIW | DIW | eluent |
| Collision He, mL·min−1 | 3.5 | 4 | 0 | 3.5 |
| Flow injection module | ISIS | FIAS | FIAS | - |
| Injected volume, μL | 500 | 50 | ||
| Reagent flow rates, mL·min−1 each | 1 | |||
| Carrier He, mL ·min−1 | 75 | |||
2.1.2. Alternative HG-CT-ICP-MS systems
Alternative analyses were performed on HG-CT-ICP-MS Systems 2 and 3 in the UNC and NRC laboratories, respectively. In both systems, the HG-CT equipment was built around a FIAS 400 flow-injection unit (Perkin-Elmer, Norwalk, CT) controlled by a laptop; reading of ICP MS instrument was triggered by FIAS via the remote read signal. The HG-CT setup was identical to that previously described 17 except that the cryotrap cooling was operated manually by immersing in liquid nitrogen, and the addition of H2 was omitted. The operating conditions of these systems are presented in Table 1, and a complete FIAS program in detailed in Table S2 (Electronic supplementary material).
2.1.3 HG-CT AAS
The HG-CT-AAS system, build around a Perkin-Elmer FIAS 400 unit in connection with AAnalyst 800 AAS spectrometer (Perkin-Elmer, U.S.A.) with a quartz multiatomizer was identical to that previously described 17. The U-tube was cooled by liquid nitrogen in a semi-automated CT device. Sample volume was 2000 μl, and reagents were pumped at 1.5 mL·min−1. Gases included 75 mL ·min−1 of He premixed with 20 mL·min−1 of H2 as a carrier gas and 35 mL min−1 of air was introduced into the outer shell of the multiatomizer. The AAS signals were exported and integrated in MS Excel.
2.1.4. HPLC-ICP-MS
A simple HPLC-ICP-MS system consisted of an isocratic HPLC pump (Agilent 1200) and a manual injection valve (Rheodyne 7725i, 50 μL loop) triggering the ICP-MS analysis. Agilent anion exchange column for speciation analysis in drinking water (Agilent G3154–65001; 4.6×150 mm) with Hamilton PRP-X100 pre column at the recommended conditions (mobile phase 2.0 mM NaH2PO4/0.2 mM EDTA-2Na, pH 6, 1 mL min−1) were used for As separation. The ICP-MS operational conditions are presented in Table 1. The chromatogram signals were exported and integrated in MS Excel.
2.2 Reagents
Deionized water (<0.2 μS cm-1, ULTRAPURE, Watrex) was used for all solutions. The reducing solution containing 1% (m/v) NaBH4 (Fluka, Buchs, Switzerland) in 0.1% (m/v) KOH (p.a., Lachema, Brno, Czech Rep.) was prepared daily. For analysis of cell lysates, an Antifoam B emulsion (1 mL of 1% (v/v) solution per 200 mL) was added to the reducing solution to prevent foaming. A 0.75 M Tris(hydroxymethyl)aminomethane (TRIS) - HCl buffer (pH 6) was prepared from a reagent grade Trizma® hydrochloride (Sigma) and pH adjusted to 6 with KOH.
A 1000 μg L−1 As AAS standard solution (Merck, Darmstadt, Germany) was used as iAsV stock standard solution. Stock solutions of 1000 μg L−1 As were prepared for all other As species in DIW using following compounds: iAsIII: As2O3, Lachema, Czech republic; MAsV: Na2CH3AsO3.6H2O, Chem. Service, West Chester, PA, USA; DMAsV: H(CH3)2AsO2, Strem Chemicals, Inc., TMAsVO: (CH3)3AsO (obtained courtesy of Dr. William Cullen, University of British Columbia, Vancouver, Canada). Working standards were prepared for individual species by serial dilution of the stock solutions in DIW. Mixed standards were used only in the last dilution, i.e. at the sub-ng mL−1 level.
2.3. HG-CT-ICP-MS Procedure
Solid L-cysteine hydrochloride monohydrate (biochemistry grade, Merck) was added to samples and standards as a pre-reducing agent to a final concentration of 2% (m/v) for at least 1 hour prior to analysis of iAs, MAs and DMAs. TMAsVO was measured separately without prereduction, as it causes reduction and loss of TMAsVO 17,25. The U-tube was immersed in liquid nitrogen before beginning of the cycle and pre-cooled for 30 s while the sampling loop was filled. For water analyses, the sampling loop of the injection vent was filled manually by sucking with a syringe (System 1 with ISIS module) or by run of the PP1 pump (FIAS module). HG then started and the sample was injected into a carrier flow. For analysis of cells, the injection vent was bypassed and sample was pipetted into a funnel made of a pipette tip attached to the carrier pump tubing 22,23, followed by a rinse with 300 μL of DIW. HG was performed for 90 s without removing waste liquid from the GLS, followed by 80 s with pumps off to complete the reaction and transport arsines from the GLS to the U-tube. Then, at the beginning of volatilization stage, just before the heating switched on, the cooling bath with liquid nitrogen was manually removed. Signal recording (60 to 90s read window) started after a 5s delay. As the U-tube was gradually heated, arsines evaporated, separated and entered the atomizer/plasma. At the end of measurement, PP2 was switched on and the waste liquid was removed from the GLS; the heating was left on for an additional 30 s to dry and clean-up the U-tube. Complete programs for ISIS and FIAS units for Systems 1 and 2–3, respectively, are available in Tables S1 and S2 in the Electronic supplementary material.
2.4. Sample preparation
2.4.1. Certified reference materials (CRM)
The following five CRM from NRC Measurement Science and Standards, Ottawa, Canada with certified total As content ranging from 0.41 to 1.27 ng mL −1 were analyzed for As speciation: CASS-4 and CASS-5 Nearshore Seawater, NASS-5 Seawater, and SLRS-4 and SLRS-5 River water reference materials. For TMAsVO analysis, reference materials were sampled directly; for iAs, MAs and DMAs analysis, solid L-cysteine hydrochloride to a concentration of 2% m/v was added at least 1 hour prior to analysis. 500 μL was sampled via the injection valve. For HPLC measurements, pH of CRM was adjusted with a NaOH solution to pH 5–7 prior to analysis. Quantification of As was performed using a 4 point aqueous calibration of mixed standards up to 1250, 63, 125 and 80 pg mL−1 As for iAs, MAs, DMAs and TMAsVO, respectively. Standards and samples were measured in at least triplicate (System 1) or duplicate (Systems 2 and 3).
2.4.2. Bladder Exfoliated Cells (BECs)
Midstream urine samples (~100 mL) were collected from residents of Chihuahua, Mexico, who are exposed to iAs in drinking water. Urine samples were collected to sterile 50-mL Falcon tubes (BD Biosciences, San Jose, CA). Each sample was chilled immediately after collection and centrifuged at 700 x g for 10min at 4°C. The pellets containing BECs were transferred to 1.5-mL tubes, washed with 1mL Dulbecco’s phosphate buffered saline (DPBS, Sigma) and centrifuged at 700 x g for 10 min at 4°C). The supernatant was discarded and the pellet was resuspended in 400uL DPBS. A cell count was performed in 10 μl of the resuspended pellet using 0.4% trypan blue exclusion dye. The number of BECs from one donor ranged from 76,000 to over 1,700,000, with median value of ~300,000. BEC pellets were frozen at −80°C and shipped in dry ice to UNC for analysis.
Here, BEC samples were analyzed by HG-CT-ICP-MS System 2 within 10 to 35 days from sampling. Prior to analysis, cells were lysed in 900 μL ice cold DIW, vortexed. Two aliquots of 300 uL each were taken for analysis, the remaining approx. 300 uL was stored at −80°C for later use. One aliquot was analyzed immediately for trivalent species. This aliquot was only diluted with 200 μl of pre-chilled DIW. For the sum of tri- and pentavalent species, a second 300 μl aliquot was mixed with 50 μl of 20% cys and 150 μl of DIW, and incubated for at least 1 hour at room temperature. Sample aliquots were pipetted into the 1 mL plastic pipette tip connected directly to the peristaltic pump tubing, followed by a 350 μl of DIW microtube rinse; the injection vent was disconnected.
Eleven samples of BEC lysate sample aliquots were shipped in dry ice to IAC in Prague for reference analysis on HG-CT-ICP-MS System 1. In the reference laboratory, 120 μl of 20% cys (m/v) and 780 μl of DIW was added to approximately 300 μl of remaining BEC lysates (exact mass of the aliquot found by differential weighting of the vial) yielding total volume approximately 1200 μl, and measured in duplicate by HG-CT-ICP-MS System 1 as described above.
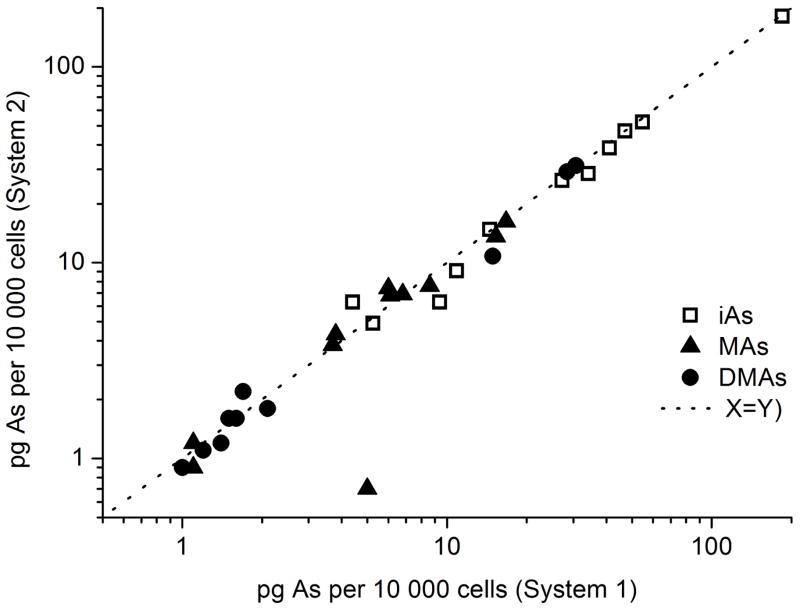
Quantification of As species in UNC and IAC laboratories was performed against external calibration of aqueous mixed standards (10–1000 pg mL −1 of individual species) treated with 2% cys. A single rinse of DIW was run in between measurement of BEC samples to avoid carryover. Uncertainties given as 95% confidence limits in Table S3 were calculated according to Ref. 26 Reported amounts of As species in Fig. 6. are normalized per 10,000 cells.
Fig. 6.
Comparison of results of analyses of BEC cells by HG-CT- ICP-MS Systems 1 and 2.
3. Results and discussion
3.1. HG-CT ICP-MS system development and optimization
The HG-CT system was previously optimized and tested for several matrices 17,18,22,23. Because there are essentially no additional requirements for the ICP-MS, the system was left unchanged except that H2 flow (required by AAS muliatomizer) was omitted. Three principally identical HG-CT units, denoted System 1–3, were built in the course of the study and each paired with an ICP-MS instrument in three laboratories. System 3 at NRC was used for a pilot study testing the feasibility and performance of this approach, while System 1 at IAC was used for optimization and method validation. System 2 at UNC is currently used for routine analytical applications. To assure continuous, trouble-free operation of the HG-CT, e.g., water (partially) blocking the cold trap, a dryer cartridge containing NaOH pellets was included in Systems 1 and 2 between the GLS and the U-tube. This cartridge does not absorb any arsine or methyl-substituted arsines 24.
Of the several options for interfacing the HG-CT to the ICP-MS, an inlet for gas bypassing the spray chamber (called High Matrix Interface, HMI, by the manufacturer) was chosen. An outlet from the U-tube was either directly connected to the HMI inlet (systems 2 and 3), or introduced into the flow of dilution gas via a T-piece (System 1). This approach prevents peak broadening due to the volume of the spray chamber. Because the HG reaction is finished at the time of hydride release from the U-tube, the signal is not disturbed by an unsteady flow of gas caused by fluctuations in the HG reaction coil and production of hydrogen. In System 1, the HG-CT outlet can be introduced or disconnected from the ICP without extinguishing the plasma.
ICP-MS was tuned as for standard liquid introduction procedure using an Agilent tuning solution and As standard solution with the HG He carrier gas on. Tuning with continuous online HG does not reflect the detection conditions; once the HG is running, plasma characteristics change dramatically due to the production of hydrogen. Notably, in HG-CT the HG reaction is completed prior to the release/detection stage and no H2 gas is present in the detection stage. Additionally, this type of tuning would not be practical because the U-tube would have to be inserted after the tune, and relatively high As concentrations would be necessary to perform tune without preconcentration. Actual tune using the cryotrapping peaks is not necessary and definitely not feasible for routine work.
Performance of System 1 with and without the use of the collision cell was compared. Although peak area sensitivity was approximately 3 times higher in the no gas mode compared to 3.5 mL min−1 He as the collision gas, there was no significant difference in the LODs. Collision mode was used for further work even if no signs of chloride interference were detected; any traces of chlorides carried over from the HG system (possibly arising from the TRIS-HCl buffer and cys used for prereduction) are efficiently removed by the NaOH dryer cartridge and/or the U-tube.
3.2. Analytical performance
Similar peak area sensitivity is achieved for all determined species; calibration slopes of methylated species typically differed from the iAs slope by less than 10%; this suggests similar (and the authors suppose near complete) efficiency of HG and hydride trapping/release. Several studies have reported similar sensitivities and good recoveries of individual species for a variety of matrices, including water, urine, cell culture samples 8,17,18,20 and homogenized tissues 22,23 performed using this HG-CT system with AAS detection. Because the HG-CT step is independent of the detector, results of these studies are directly applicable also for HG-CT-ICP-MS.
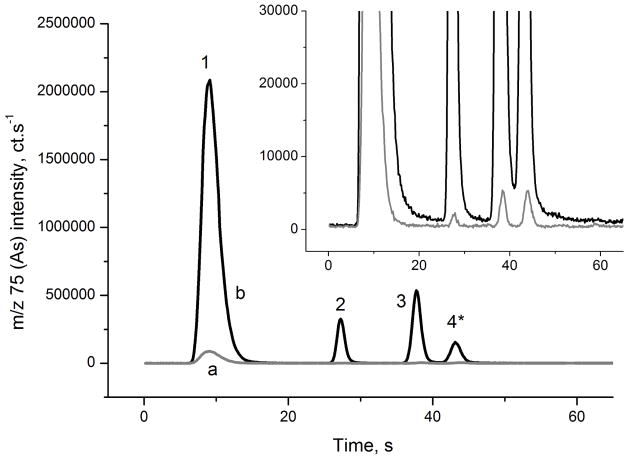
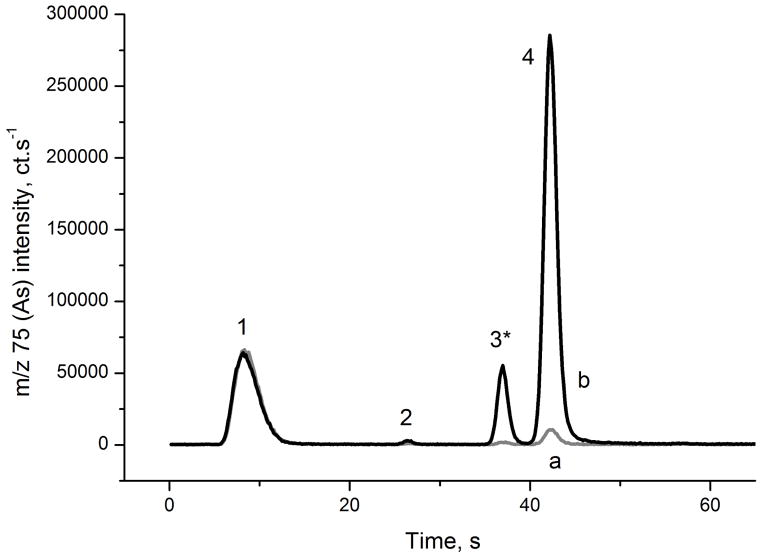
Typical signals obtained in HG-CT-ICP-MS System 1 with and without cys prereduction are presented in Figs. 2 and 3, respectively. Chromatograms for SLRS-4 river water reference material and blank are shown; see Tables 2 and 3 for As content in blanks and reference materials. Baseline resolution of iAs, MAs and DMAs and near baseline resolution between DMAs and TMAsVO is achieved. A short pause in U-tube heating starting at the approximate the time of MAs peak maximum 17 (See Table S1 and S2) improved the DMAs- TMAsVO resolution.
Fig. 2.
Signal of the cys- treated river water (CRM SLRS-4) analyzed by HG-CT-ICP-MS (System 1). a: 2% cys blank, b: CRM SLRS-4. 1: iAs; 2: MAs; 3: DMAs; 4: TMAsVO. * TMAsVO partially lost in cys- prereduction. For species concentrations, see Table 3.
Fig. 3.
Signal of the river water CRM SLRS-4 without prereduction analyzed by HG-CT-ICP-MS (System 1). a: DIW blank, b: SLRS-4 CRM. 1: iAsIII; 2: MAsIII; 3: DMAsIII,*; 4: TMAsVO. * DMAV signal contribution, see Sec. 3.3.
Table 2.
Typical blank, LOD and LOQ values achieved using HG-CT-ICP-MS (System 1).
| LOD | LOQ | ||||
|---|---|---|---|---|---|
| Blank, pg mL−1 | pg mL−1 | pg | pg mL−1 | pg | |
| iAsIII | 11 | 1.2 | 0.6 | 4.1 | 2.1 |
| iAs | 13 | 3.4 | 1.7 | 11.3 | 5.6 |
| MAsIII | <LOD | 0.040 | 0.020 | 0.13 | 0.07 |
| MAs | 0.1 (<LOQ) | 0.055 | 0.027 | 0.18 | 0.09 |
| DMAsIII | 0.2 (<LOQ) | 0.069 | 0.034 | 0.23 | 0.11 |
| DMAs | 0.4 (<LOQ) | 0.14 | 0.071 | 0.47 | 0.24 |
| TMAsVO | 1.8 | 0.10 | 0.049 | 0.33 | 0.16 |
Table 3.
Concentrations of As species found in water CRM (pg mL−1 As); 95 percent confidence limits of the mean are given.
| Method | iAs | MAs | DMAs | TMAsVO | Sum of species | Certified total As | Recovery %a | |
|---|---|---|---|---|---|---|---|---|
| CASS-4 | HG-CT-ICP-MS 3 | NA | 9 ± 4 | 94 ± 1 | 14 ± 1 | NA | 1110 ± 160 | NA |
| Nearshore | HG-CT-ICP-MS 1 | 1046 ± 15 | 9 ± 0.7 | 80 ± 1 | 14 ± 1 | 1149 | 104 | |
| Sea water | HG-CT-AAS | 1083 ± 46 | 3–10b | 95 ± 4 | 10–34b | 1205 | 109 | |
| CASS-5 | HG-CT-ICP-MS 3 | NA | 11 ± 0.3 | 123 ± 1 | 18 ± 1 | NA | 1240 ± 90 | NA |
| Nearshore | HG-CT-ICP-MS 2 | 1054 ± 19 | 9 ± 1.8 | 117 ± 6 | 20 ± 1 | 1200 | 97 | |
| Seawater | HG-CT-ICP-MS 1 | 1014 ± 13 | 10 ± 0.9 | 108 ± 1 | 18 ± 1 | 1151 | 93 | |
| HG-CT-AAS | 1033 ± 25 | 5–18b | 113 ± 6 | 7–23b | 1173 | 95 | ||
| NASS-5 | HG-CT-ICP-MS 3 | NA | 2 ± 0.4 | 47 ± 1 | 13 ± 1 | NA | 1270 ± 120 | NA |
| Seawater | HG-CT-ICP-MS 1 | 1105 ± 13 | 2 ± 0.8 | 40 ± 1 | 12 ± 1 | 1158 | 91 | |
| HG-CT-AAS | 1163 ± 36 | <3c | 46 ± 2 | 10–34b | 1227 | 97 | ||
| SLRS-4 | HG-CT-ICP-MS 3 | NA | 50 ± 1 | 90 ± 1 | 51 ± 4 | NA | 680 ± 60 | NA |
| River | HG-CT-ICP-MS 1 | 483 ± 12 | 39 ± 1 | 72 ± 1 | 56 ± 1 | 651 | 96 | |
| Water | HG-CT-AAS | 527 ± 22 | 42 ± 2 | 86 ± 2 | 63 ± 5 | 718 | 106 | |
| HPLC-ICP-MS | 604 ± 64 | 16–52b | 77 ± 23 | 26–85b | 729 | 107 | ||
| SLRS-5 | HG-CT-ICP-MS 3 | NA | 44 ± 1 | 51 ± 1 | 22 ± 1 | NA | 413 ± 39 | NA |
| River | HG-CT-ICP-MS 2 | 284 ± 12 | 37 ± 2 | 44 ± 4 | 24 ± 1 | 389 | 94 | |
| Water | HG-CT-ICP-MS 1 | 263 ± 11 | 37 ± 1 | 44 ± 1 | 23 ± 1 | 367 | 89 | |
| HG-CT-AAS | 271 ± 8 | 40 ± 4 | 43 ± 3 | 7–23b | 372 | 90 | ||
| HPLC-ICP-MS | 299 ± 90 | 16–52b | 54 ± 13 | 26–85 | 401 | 97 |
Sum of species to certified total As value
Value between LOD and LOQ
< LOD
NA: not available.
The system precision is very good; 17 consecutive measurements of a mixed aqueous As standard (200 pg mL−1 iAsV and 100 pg mL−1 each MAsV and DMAsV) yielded a precision of 1.1%. The same precision was observed for the signal of a co-nebulized internal standard (IS) (Te 100 ng mL−1, signal at m/z 125 averaged over peak integration times). The data are presented in Fig. S1 (electronic supplement). A correction for IS did not improve the precision; this is understandable, as the sources of signal fluctuations from the introduction system are unrelated for HG-CT and IS nebulization. Sensitivity drifts are generally very small because only IS standard solution and analyte in gaseous form enter the plasma. The analyte is separated from the matrix in the HG system, and the aerosol produced in the HG is effectively removed by the dryer cartridge and collection in the U-tube. IS was used as a tool for monitoring performance of the system, however the IS correction was not used in further work.
The carryover effect, i.e. an increase in blank signal following the measurement of a standard at higher concentration level was below 1%. One cycle with DIW as the sample was enough to rinse the system so that a stable blank level is established.
In very sensitive analytical systems, the blank levels are typically the determining factor for the LOD. The great advantage of the HG-CT approach compared to other speciation methods is that it requires very little sample preparation for many matrices- no filtration, or extraction is required, and the danger of sample contamination is minimized. The limits of detection are also greatly influenced by the design of hydride generator. The sample volume, the volume and dilution of reagent solutions, as well as HG efficiency affect the blank values for individual species. LOD (3σ of the blank) and limits of quantitation, LOQ (10σ of the blank) for individual As species obtained for System 1 are shown in Table 2. The performance of Systems 2 and 3 were comparable. The values reported in Table 2 were achieved using 10 vials with individually cys treated DIW samples, corresponding to the whole procedure. The LOD indeed depended on the blank levels observed for individual species. For MAs, where the lowest blank values, typically bellow 0.2 pg mL−1, were observed, a LOD as low as 40 fg mL−1 (ppq) was achieved. This value is close to the instrumental LOD. Conversely, blank values for iAs of approximately 25 pg mL−1 were found, resulting in LOD in single pg mL−1 range. Typical blank values presented in Table 2 were obtained using DIW as a sample; these values combine contribution from both the sample and the reagents. Efforts to trace the source of As in blanks to individual reagents were not successful. Generally, similar blank values were found using all 3 systems in different laboratories (data not shown). The blank contribution of pentavalent species present in reagents (carrier, TRIS buffer and reducing solution) is however greatly reduced because these solutions are not in contact with cys for enough time to be pre-reduced, and therefore, produce hydrides with very low efficiency. The authors are not sure of the TMAsVO blank origin, but it was consistent and present in all three laboratories. We suspect that TMAsVO as non-ionic species may not be efficiently removed from deionized water by ion-exchangers.
LOD data presented in Table 2 are better than published values of other HG-CT-ICP-MS systems. Tseng and associates presented absolute LOD of 50, 2 and 5 pg for iAs, MAs and DMAs, respectively, which are one to two orders of magnitude higher than in this work27. However, the sample volume used in Ref.27. was as high as 500 mL, resulting in better relative LOD values. Elwood and associates reported LOD of 0.15, 1.35 and 5.2 pg for iAs, MAs and DMAs, respectively using a 1 mL sample volume 11.
A total analysis time of 5 to 7 min per sample (see Tables S1 and S2 for time program) is comparable with typical ion chromatography analysis time of approximately 10 minutes and allows for convenient routine use. However, if oxidation state specific As speciation or quantification of TMAsVO is required, two aliquots of the sample (i.e. with and without cys prereduction) need to be measured.
3.3. Analysis of water reference materials
The results of As speciation analysis of sea and river water CRM are presented in Table 3. Analyses were performed on three HG-CT-ICP-MS systems in three different laboratories, with results in excellent agreement. In all cases, methylated species (MAs, DMAs and TMAsVO) were detected at the tens of pg mL−1 levels accounting for 5 to 12% and 25 to 27% of total As in sea water and river water, respectively. Because stability of valency of species in HNO3 conserved CRMs is not assured, only sums of trivalent and pentavalent species are presented in Table 3. However, to quantify TMAsVO, the analysis of an aliquot without cys was performed because TMAsVO is lost in the course of prereduction 17,25. The presence of trivalent species was not observed in this aliquot. The DMAs signal obtained without prereduction (Fig. 3) is likely an artifact due to the partial HG activity of DMAsV; 17 see also Sec. 3.4.
The sum of detected As species (“mass balance”) was in agreement with the certified total As values. These data indicate that non active HG species (e.g., arsenobetaine and arsenocholine), which cannot be detected by the HG-CT method, are not present as major species. Conversely, because the methylated species are present in relatively low quantities, the difference in concentrations of those species would not cause major difference in total As mass balance.
To confirm these results, analysis of the CRM by another methodology was attempted. The HG-CT-AAS method, which is well established in two of our laboratories, provided matching results (see Table 3). Due to lower sensitivities, MAs and TMAsVO values were below or close to the LOQ values in most cases. However, HG-CT-AAS cannot be considered a truly independent method because the HG-CT step was identical.
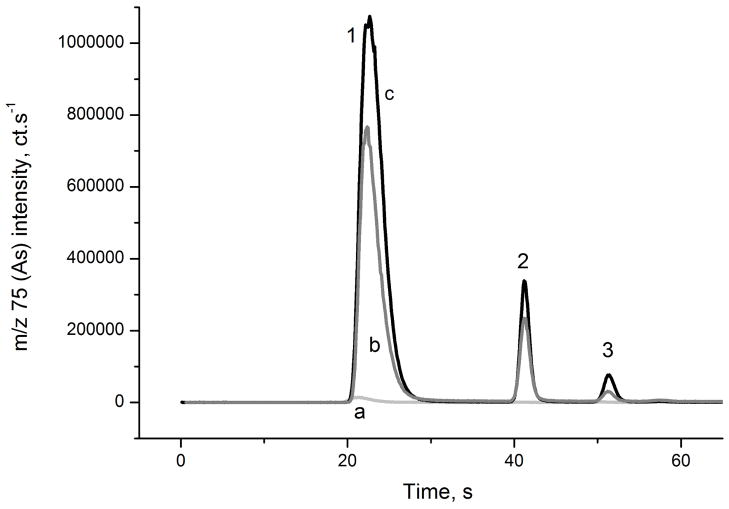
The HPLC-ICP-MS method was used to confirm the accuracy of these results. The chromatograms of the SLRS-4 CRM analysis are presented in Figure 4. The sensitivity and LOQ values of this method are slightly worse than those of HG-CT-AAS, approximately 50 pg mL−1 for MAsV and DMAsV and 80 pg mL−1 for iAsV, iAsIII, and TMAsVO. Therefore, the values of methylated species in CRM samples are below or close to LOQ. However, only freshwater CRM could be analyzed by this method because the high salt content of the seawater matrix is not compatible with the described setup. Additionally, pronounced blanks containing iAsV and TMAsVO or iAsIII, which co-elute, were observed, deteriorating the analytical performance for these species. Figure 4 depicts chromatograms of a solution of 0.025 M ultrapure HNO3 neutralized by NaOH to pH of 5–7 that was used for CRM analysis, yielding approximately 70 pg mL−1 iAsV and 100 pg mL−1 TMAsVO.
Fig. 4.
HPLC-ICP-MS chromatograms
a: 0.025 M HNO3, pH adjusted with NaOH to approx. 6;
b: Standard 400 ng mL−1 iAs V and 200 ng mL−1 each MAsV, DMAsV and TMAsVO;
c: SLRS-4 river water SRM, pH adjusted with NaOH to approx. 6; 1,2- TMAsVO (void volume) and iAsIII coeluting, 3-DMAV, 4- MAV, 5- iAsV, U- unknown Traces shifted vertically for clarity.
Reports on the analysis of methylated As compounds in CRM are not common because of the high sensitivity required for the analysis. However, previous reports are in agreement with the reference total As value, even if, in contrast to others, Hsiung and Wang found approximately 35% in form of iAsIII. 28 Analysis of MAs in SLRS-4 by HG-CT-ICPMS in Ref. 11, 59 pg mL−1, corresponds to the value found in this work (Table 3), but the reported DMAs was below the shown LOD (<5 pg mL−1). 11 Two publications presented the analysis of NASS-5. In a work using a HG-CT-AAS method, 28 MAs and DMAs concentrations were below the LOD, which were reported as 4.5 and 6.3 pg mL−1, respectively. Another report found 30 and 45 pg mL−1 MAs by HPLC-ICP-MS and HPLC-HG-ICP-MS with a MAs LOD of 20 pg mL−1; DMAs was not analyzed because it did not elute from the column. 29
3.4. Analysis of bladder exfoliated cells
It has been hypothesized that analysis of As species in target tissues or cells originated from these tissues, including BECs may be a more effective tool for risk assessment of bladder cancer and other diseases associated with exposures to iAs 20. Currently, BECs are being collected in an ongoing study in Chihuahua from individuals exposed to iAs in drinking water (see Sec. 2.4.2) and analyzed by HG-CT-ICP-MS. A detailed presentation of results of this study and its toxicological implications are not in the scope of this article, and will be published elsewhere. However, the authors would like to demonstrate the performance of the method here.
In previous studies using an identical HG-CT setup with AAS detection, tests in similar matrices, lysates of cultured human urothelial cells and hepatocytes, exhibited similar calibration slopes for all species compared to aqueous standards, as well as high recoveries of As standards spiked into these matrices 17,18,20,23. The improved sensitivity of the ICP-MS detector allows for convenient analysis in small volume samples containing lysate from several tens of thousands of BEC cells. The mass of As species present in the sampled aliquot ranged from tens of pg up to ng of iAs species and from single to hundreds pg of methylated species. Typical signals from the analysis of the samples with As content near the median value are shown in Fig. 5. The As retained in BECs was generally well above the absolute LOD of the method presented in Table 2. In approximately 10% of the samples were found values below LOD for some species, but in none of the samples were all species below LOD. TMAsVO was not determined in those samples as its contents in the samples were very small.
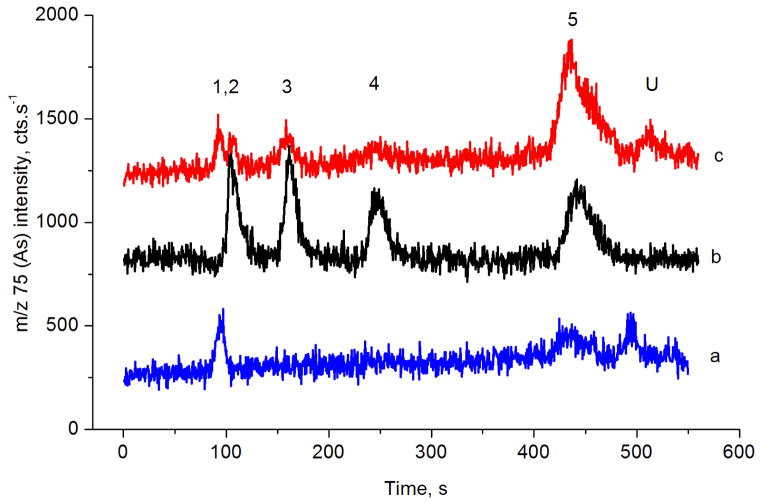
Fig. 5.
Typical signal of the BEC lysate analyzed by HG-CT-ICP-MS (System 2). a: 2% cys blank; b: BEC lysate; c: cys treated BEC lysate. 1: iAs; 2: MAs; 3: DMAs. Species found in aliquots: iAsIII 215 pg; iAs 343pg; MAsIII 32 pg; MAs 43pg; DMAsIII 3.5 pg; DMAs 11.3 pg.
Analytical methodology for analysis of trivalent methylated species based on selective hydride generation, HG properties and stability of these species in cell cultures and tissues were developed, thoroughly studied and verified in our previous works.8,17,18,22,23 In BEC’s, significant portions of iAs and MAs (20 to 100% of total As) were found in the form of trivalent species. In contrast, DMAsIII constituted approximately only up to 21% of total DMAs. The DMAsIII data had to be corrected for the limited selectivity of pH specific HG activity of DMAIII at pH 6 because DMAsV contributes to a small portion of the DMAIII signals 17. The extent of this correction was determined by spiking experiments; the DMAsV generation efficiency at pH 6 without cys prereduction in the BEC matrix was 5.9% (sd 0.7%; n=6). Similar experiment proved that the generation efficiency of MAsV at these conditions was not significant, in accordance with earlier findings.17 The difference in trivalent species portion of iAs and MAs compared to DMAs in BEC’s can be partially explained by low stability of the DMAsIII documented in water and urine 30. However, the MAsIII a DMAsIII species in cells are expected to be bound to cellular structures and proteins 31 and much more resistant to oxidation.23
The accuracy of the method was tested by analyzing Standard Reference Material® 2669 Arsenic Species in Frozen Human Urine Level II (NIST), the closest matrix reference material with certified As speciation available. The SRM was diluted 100 times in DIW, so that the concentration range was similar to that found in the BEC samples. Recoveries of 102%, 95% and 107% for iAs, MAs and DMAs, respectively, were obtained (arsenobetaine and arsenocholine cannot be not analyzed by the HG-CT method).
The reproducibility of these results was verified by repeating analysis on System 1 in the IAC laboratory. Only the sum of tri- and pentavalent species were compared because the oxidation state of species is likely not preserved after freezing of the lysed cells, storage for several weeks, and shipping overseas on dry ice. The results of the reference analyses are presented in Fig. 6 and Table S3, which show a very good agreement between laboratories. The precision of the analysis is very good; the difference between duplicate measurements on System 1 was typically 1.5 – 5% at levels below 10 pg.
Conclusions
The HG-CT-ICP-MS method presented is an ultra sensitive approach for speciation analysis of As. This method can tolerate matrices that are difficult or impossible for HPLC-based methods, including seawater or protein rich biological samples. The main species produced in the course of iAs metabolism, i.e. trivalent and pentavalent methylated species, can be quantified whether they are present in free form or complexed to other molecules. This system has already been successfully applied to As speciation analysis in murine pancreatic islets in a study examining the mechanisms of As-induced diabetes. 16 Additionally, its application to the direct analysis of homogenized rodent tissues is imminent, with sensitive ICP-MS detection allowing for studies involving lower exposure levels then those currently reported using HG-CT-AAS. 22,23
Even if the HG-CT-ICP-MS approach outperforms HPLC-based methods in terms of sensitivity and required sample pretreatment, the authors are aware that it is far from being universal. Interferences can occur if other As species present in the samples are volatilized under the same HG conditions. Thioanalogues of methylated species and arsenosugars are suspected to produce arsines to some, although a small, extent. 15,18,32 Although other volatile by-products than corresponding (methylsubstitted) arsanes can be formed from analytes by condensation and transmethylation reactions of arsanes after HG,33,34 this appears of little concern at (ultra) trace concentration levels and in the presence of L-cysteine.34
The speciation analysis field is now approaching maturity, with reports discovering completely new principles and methodologies giving way to more subtle but no less important efforts in assuring analytical quality and reliability of results 35. In the future, we would like to see more As speciation data supported by two or more independent analytical methods to resolve persisting controversies. Our efforts in the development of HG-CT based methodology aim to provide an additional reliable tool for the As science community.
Supplementary Material
Acknowledgments
This work was supported by the Ministry of Education, Youth and Sports of the Czech Republic Kontakt II program project No. LH12040, the AS CR institutional support RVO:68081715 and by the U.S. National Institutes of Health grant No. 5R01 ES015326-02 to M.S. The authors thank to the staff and students of the Autonomous University of Chihuahua who were in charge of subject recruitment, interviews and examination, and thus made the use of BECs in this study possible.
References
- 1.Rehman K, Naranmandura H. Metallomics. 2012;4:881–892. doi: 10.1039/c2mt00181k. [DOI] [PubMed] [Google Scholar]
- 2.Francesconi KA, Kuehnelt D. Analyst. 2004;129:373–395. doi: 10.1039/b401321m. [DOI] [PubMed] [Google Scholar]
- 3.Gong ZL, Lu XF, Ma MS, Watt C, Le XC. Talanta. 2002;58:77–96. doi: 10.1016/s0039-9140(02)00258-8. [DOI] [PubMed] [Google Scholar]
- 4.Ammann AA. Am J Anal Chem. 2011;2:27–45. [Google Scholar]
- 5.Komorowicz I, Baralkiewicz D. Talanta. 2011;84:247–261. doi: 10.1016/j.talanta.2010.10.065. [DOI] [PubMed] [Google Scholar]
- 6.Raab A, Meharg AA, Jaspars M, Genney DR, Feldmann J. J Anal At Spectrom. 2004;19:183–190. [Google Scholar]
- 7.Šlejkovec Z, Falnoga I, Goessler W, van Elteren JT, Raml R, Podgornik H, Èernelè P. Anal Chim Acta. 2008;607:83–91. doi: 10.1016/j.aca.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 8.Currier J, Saunders JR, Ding L, Bodnar WM, Cable P, Matoušek T, Creed JT, Stýblo M. J Anal At Spectrom. 2013;2013 doi: 10.1039/C3JA30380B. Accepted Manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stefanka Z, Koellensperger G, Stingeder G, Hann S. J Anal At Spectrom. 2006;21:86–89. [Google Scholar]
- 10.Kumar AR, Riyazuddin P. Int J Environ An Ch. 2007;87:469–500. [Google Scholar]
- 11.Ellwood MJ, Maher WA. J Anal At Spectrom. 2002;17:197–203. [Google Scholar]
- 12.Tseng CM, Amouroux D, Brindle ID, Donard OFX. J Environ Monitor. 2000;2:603–612. doi: 10.1039/b007499n. [DOI] [PubMed] [Google Scholar]
- 13.Wuerfel O, Thomas F, Schulte MS, Hensel R, Diaz-Bone RA. Appl Organomet Chem. 2012;26:94–101. [Google Scholar]
- 14.Diaz-Bone RA, Hitzke M. J Anal At Spectrom. 2008;23:861–870. [Google Scholar]
- 15.Regmi R, Milne BF, Feldmann J. Anal Bioanal Chem. 2007;388:775–782. doi: 10.1007/s00216-006-1076-z. [DOI] [PubMed] [Google Scholar]
- 16.Douillet C, Currier J, Saunders J, Bodnar WM, Matoušek T, Stýblo M. Toxicol App Pharm. 2013;267:11–15. doi: 10.1016/j.taap.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matoušek T, Hernández-Zavala A, Svoboda M, Langerová L, Adair BM, Drobná Z, Thomas DJ, Stýblo M, Dědina J. Spectrochim Acta B. 2008;63:396–406. doi: 10.1016/j.sab.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernández-Zavala A, Matoušek T, Drobna Z, Paul DS, Walton FS, Adair BM, Dědina J, Thomas DJ, Stýblo M. J Anal At Spectrom. 2007;23:342–351. doi: 10.1039/b706144g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding L, Saunders RJ, Drobná Z, Walton FS, Xun P, Thomas DJ, Stýblo M. Toxicol App Pharm. 2012;264:121–130. doi: 10.1016/j.taap.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hernández-Zavala A, Valenzuela OL, Matoušek T, Drobná Z, Dědina J, Garcia-Vargas GG, Thomas DJ, Del Razo LM, Stýblo M. Environ Health Persp. 2008;116:1656–1660. doi: 10.1289/ehp.11503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moraes DP, Svoboda M, Matoušek T, Flores EMM, Dědina J. J Anal At Spectrom. 2012;27:1734–1742. [Google Scholar]
- 22.Currier JM, Svoboda M, de Moraes DP, Matoušek T, Dědina J, Stýblo M. Chem Res Toxicol. 2011;24:478–480. doi: 10.1021/tx200060c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Currier JM, Svoboda M, Matoušek T, Dědina J, Stýblo M. Metallomics. 2011;3:1347–1354. doi: 10.1039/c1mt00095k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taurková P, Svoboda M, Musil S, Matoušek T. J Anal At Spectrom. 2011;26:220–223. [Google Scholar]
- 25.Musil S, Matoušek T. Spectrochim Acta B. 2008;63:685–691. doi: 10.1016/j.sab.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Massart DL, Vandeginste BG, Buydens LM, De Jong S, Lewi PJ, Smeyers J. Handbook of Chemometrics, Verbeke. 1999. p. 198. [Google Scholar]
- 27.Tseng CM, Amouroux D, Brindle ID, Donard OFX. J Environ Monitor. 2000;2:603–612. doi: 10.1039/b007499n. [DOI] [PubMed] [Google Scholar]
- 28.Hsiung TM, Wang JM. J Anal At Spectrom. 2004;19:923–928. [Google Scholar]
- 29.Nakazato T, Tao H, Taniguchi T, Isshiki K. Talanta. 2002;58:121–132. doi: 10.1016/s0039-9140(02)00261-8. [DOI] [PubMed] [Google Scholar]
- 30.Gong ZL, Lu XF, Cullen WR, Le XC. J Anal At Spectrom. 2001;16:1409–1413. [Google Scholar]
- 31.Hippler J, Zdrenka R, Reichel RAD, Weber DG, Rozynek P, Johnen G, Dopp E, Hirner AV. J Anal At Spectrom. 2011;26:2396–2403. [Google Scholar]
- 32.Schmeisser E, Goessler W, Kienzl N, Francesconi KA. Anal Chem. 2004;76:418–423. doi: 10.1021/ac034878v. [DOI] [PubMed] [Google Scholar]
- 33.Ulivo AD’, Dědina J, Mester Z, Sturgeon RE, Wang Q, Welz B. Pure Appl Chem. 2011;83:1283–1340. [Google Scholar]
- 34.Ulivo AD’, Meija J, Mester Z, Pagliano E, Sturgeon R. Anal Bioanal Chem. 2012;66:740–747. doi: 10.1007/s00216-011-5503-4. [DOI] [PubMed] [Google Scholar]
- 35.Sturgeon RE, Francesconi KA. Environ Chem. 2009;6:294–297. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.