Abstract
An efficient catalytic method has been developed for aerobic oxidation of primary amines to the corresponding nitriles. The reactions proceed at room temperature and employ a catalyst consisting of (4,4′-tBu2bpy)CuI/ABNO (ABNO = 9-azabicyclo[3.3.1]nonan-3-one N-oxyl). The reactions exhibit excellent functional group compatibility and substrate scope, and are effective with benzylic, allylic and aliphatic amines. Preliminary mechanistic studies suggest that aerobic oxidation of the Cu catalyst is the turnover-limiting step of the reaction.
Keywords: aerobic oxidation, copper, nitroxyl, nitrile, amine oxidation
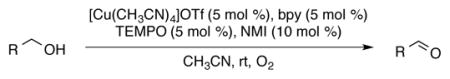
Homogeneous (bpy)Cu/TEMPO and related catalyst systems have emerged as some of the most effective catalysts for aerobic oxidation of alcohols and amines.1,2 Catalyst systems of this type can be traced to the 1960s,3 but they have become the focus of extensive investigation and development in recent years.4,5,6 In 2011, we reported a (bpy)CuI/TEMPO catalyst system that mediates efficient aerobic oxidation of primary alcohols to aldehydes (eq 1).4i,j The reactions exhibit broad substrate scope and are compatible with benzylic, allylic and aliphatic alcohols bearing diverse functional groups. We envisioned that amine oxidation (i.e., C–N dehydrogenation) could be achieved using a similar Cu/nitroxyl-based catalyst system. Several other groups recently reported important progress in this area. For example, Kerton,5b Kanai,5c and Xu5d demonstrated that various Cu/nitroxyl catalyst systems promote aerobic oxidation of primary amines to the corresponding homocoupled imines 3 (Scheme 1).7 The production of homocoupled imines suggests that condensation of second amine with the primary imine intermediate A is more facile than oxidation of A to the corresponding nitrile 2. Here, we report a Cu/nitroxyl system that enables selective formation of nitriles from diverse primary amines. The room temperature reaction conditions are very convenient and much more mild than other catalytic methods for oxidation of primary amines to nitriles,8,9 and the results highlight the ability to tune product selectivity by variation of the catalyst components. Preliminary mechanistic studies provide insights into the catalytic reactions.
Scheme 1.
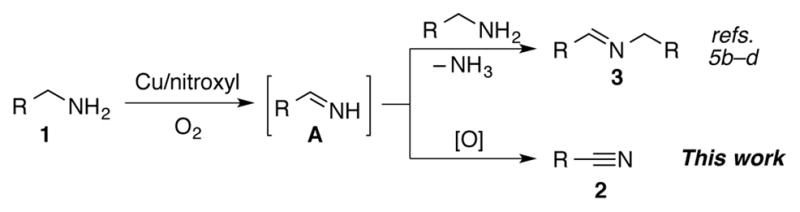
The selectivity of Cu/nitroxyl catalyzed primary amine oxidation.
 |
(1) |
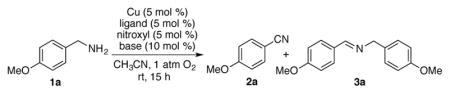
We initiated the present study with 4-methoxybenzylamine as a model substrate using 5 mol % of Cu and a nitroxyl radical source in acetonitrile (Table 1). Our previously reported alcohol oxidation conditions gave only 25% of nitrile 2a with 50% of homocoupled imine 3a (entry 1). Use of CuI led to better selectivity for 2a (entry 2) albeit with lower yield. Replacement of TEMPO with the sterically less hindered nitroxyl species such as ABNO and AZADO resulted in a significantly improved yield and selectivity of the nitrile 2a (entries 3 and 4).10 Use of ketoABNO, which is a stronger oxidant but sterically the same as ABNO, led to inferior results (entry 5). Extensive screening of ligands and bases was carried out (Table 1, entries 6–9; for full screening data, see Table S2) and the best yield and selectivity for 2a was achieved with reactions containing 4,4′-tBu2bpy (4,4′-di-tert-butyl-2,2′-bipyridyl) and DMAP (4-dimethylamino-pyridine) (entry 9). This optimized catalyst system was evaluated in solvents other than acetonitrile. Good substrate conversion was observed in THF, dioxane, DMF and toluene; however, the selectivity for nitrile 2a was significantly lower (Table S1). Use of air as the oxidant led to lower yield and selectivity (entry 10).
Table 1.
Optimization of Cu/nitroxyl catalyzed aerobic oxidation of primary amine to nitrile.a
| ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| entry | Cu | ligand | nitroxyl | base | yield (%)b | |
|
| ||||||
| 2a | 3a | |||||
| 1 | CuOTf | bpy | TEMPO | NMI | 25 | 50 |
| 2 | CuI | bpy | TEMPO | NMI | 27 | 34 |
| 3 | CuI | bpy | ABNO | NMI | 78 | 22 |
| 4 | CuI | bpy | AZADO | NMI | 77 | 23 |
| 5 | CuI | bpy | ketoABNO | NMI | 53 | 46 |
| 6 | CuI | 4,4′-tBu2bpy | ABNO | NMI | 87 | 13 |
| 7 | CuI | 4,4′-Me2bpy | ABNO | NMI | 80 | 14 |
| 8 | CuI | 4,4′-OMe2bpy | ABNO | NMI | 74 | 26 |
| 9 | CuI | 4,4′-tBu2bpy | ABNO | DMAP | 90 | 4 |
| 10c | CuI | 4,4′-tBu2bpy | ABNO | DMAP | 75 | 24 |
Conditions: 1a (0.5 mmol), Cu, ligand, nitroxyl, and base in CH3CN (2.0 mL) under O2 balloon at room temperature for 15 h.
Yield determined by 1H NMR (internal standard: 1,1,2,2-tetrachloroethane).
Carried out under air.
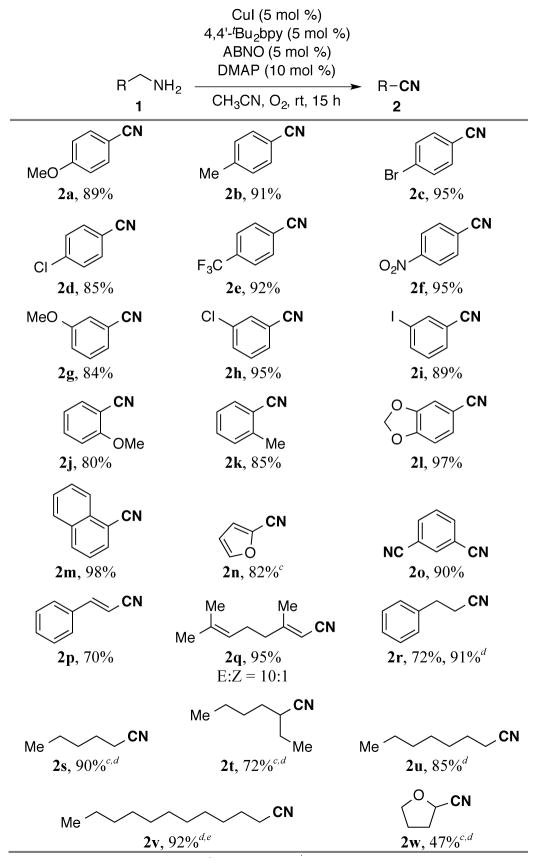
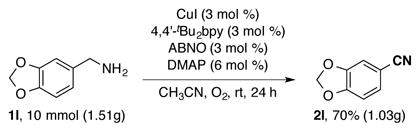
The optimized reaction conditions (Table 1, entry 9) were applied to diverse substrates to assess the reaction scope and limitations (Table 2). Primary benzylic amines with a variety of substituents (1a–1o) reacted to afford the corresponding nitriles in good to excellent yields. Electron-donating and electron-withdrawing groups, ranging from -OMe to -CF3 and -NO2 groups, were all well tolerated in the reactions, and the method was compatible with aryl chlorides, bromides and iodides that can be used in subsequent coupling reactions. Polycyclic or heteroaromatic methylamines, such as 1-naphthylmethylamine (1m) and 2-furyl-methylamine (1n), underwent effective conversion into the nitriles, and oxidation of m-xylenediamine (1o) afforded 1,3-dicyanobenzene in high yield. The allylic amines, cinnamylamine (1p) and geranylamine (1q), afforded α,β-unsaturated nitriles in good yields. As has been observed in alcohol oxidation reactions, aliphatic substrates were less reactive, but good yields of the corresponding nitriles (2r–2v) were obtained by increasing the CuI/ABNO catalyst loading to 10 mol %. A reduced yield of the nitrile was observed from oxidation of tetrahydrofurfurylamine (1w), possibly reflecting inhibition by chelation of the adjacent ether group. This amine oxidation method was effective on larger scale (eq 2); amine 1l underwent efficient oxidation on 10 mmol scale, resulting in a 70% yield of the nitrile, even with decreased catalyst loading (3 mol %).
Table 2.
|
Conditions: 1 (0.5 mmol), CuI, 4,4′-tBu2bpy, ABNO, and DMAP in CH3CN (2.0 mL) under O2 (balloon) at room temperature for 15 h.
Isolated yield.
Yield determined by 1H NMR spectroscopy.
10 mol % of CuI, 4,4′-tBu2bpy, ABNO, and 20 mol % of DMAP were employed.
Carried out at 40 °C
 |
(2) |
Some limitations of substrate scope were observed in the oxidation of 4-bromophenethylamine, which formed a complex mixture of products, and 4-hydroxybenzylamine, which produced no 4-hydroxybenzonitrile (Figure 1). These limitations resemble those of analogous alcohol oxidation reations in which homobenzylic alcohols afford complex product mixtures and phenols inhibit catalytic turnover. Overall, these amine oxidation reactions exhibit a broad scope that closely resembles the alcohol oxidation reactions.
Figure 1.
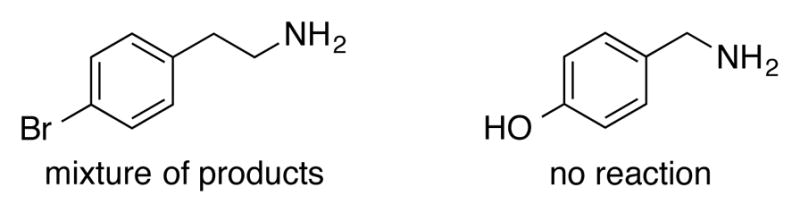
Problematic substrates for of CuI/ABNO-catalyzed amine oxidation.
The reaction profile for the oxidation of p-methoxybenzyl amine, monitored by gas uptake methods and GC analysis of the nitrile product, revealed an O2:product stoichiometry of 1:1 (Figure 2).11 This ratio is consistent with that expected for four-electron oxidation of a primary amine to a nitrile with concomitant four-electron reduction of O2 to two equivalents of water. Only a small amount of the homocoupled imine 3a is detected, with the majority formed early in the reaction when the amine substrate concentration is highest.
Figure 2.
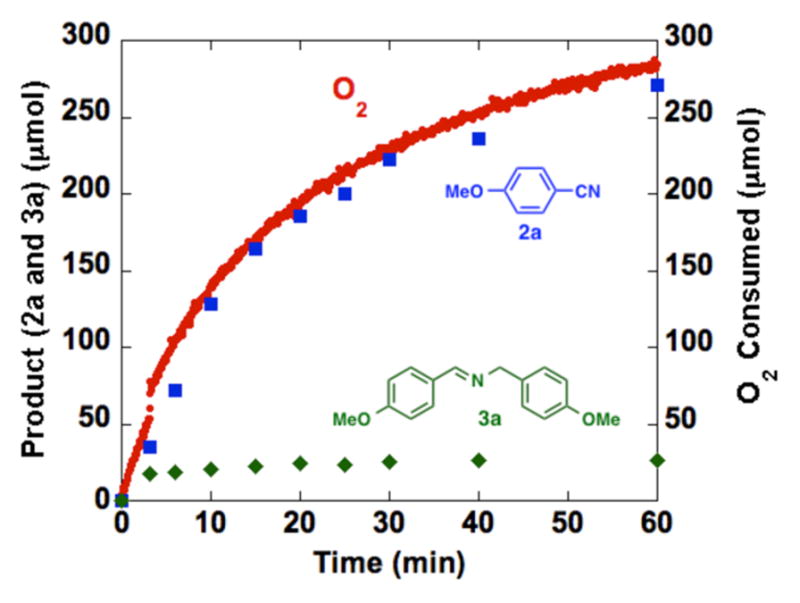
Reaction time course, monitored by gas uptake (O2) and GC analysis of the organic products (nitrile and imine), showing the 1:1 relationship between O2 consumption and nitrile formed. See supporting information for details.
A kinetic isotope effect (KIE) was obtained for oxidation of p-methoxybenzylamine and 4-MeOPhCD2NH2 by comparing the rate of the protio substrate with that of the deuterated derivative (Figure 3A). The small observed KIE, kH/kD = 1.18 ± 0.02, indicates that C-H bond cleavage is not turnover limiting in this present reaction. Similarly, a Hammett study with different p-substituted benzylamines reveals the absence of a significant electronic effect on the reaction rate (ρ = −0.07; Figure 3B). On the other hand, the reaction rate exhibits a first-order dependence on the oxygen pressure (Figure 4 and S3). These observations, together with insights from our recently reported mechanistic study of Cu/TEMPO-catalyzed alcohol oxidation,12 are consistent with a two-stage catalytic mechanism consisting of (i) aerobic oxidation of the reduced catalyst, (tBu2bpy)CuI and ABNO–H, and (ii) dehydrogenation of the amine substrate by (tBu2bpy)CuII species and ABNO (Scheme 2). Both the primary amine and the primary imine can serve as substrates in the second stage of the reaction. The kinetic data suggest that catalyst oxidation by O2, not substrate dehydrogenation, is turnover limiting. These preliminary results, together with our previous study of Cu/TEMPO-catalyzed alcohol oxidation,12 provide a foundation for a more thorough comparison of the relative reactivity of alcohols and amines and similarities and differences between the reaction mechanisms.
Figure 3.
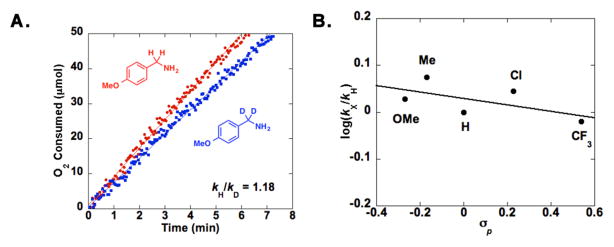
Isotope-effect (A) and Hammett (B) data from CuI/ABNO-catalyzed aerobic oxidation of p-methoxybenzyl-amine to p-methoxybenzonitrile.
Figure 4.
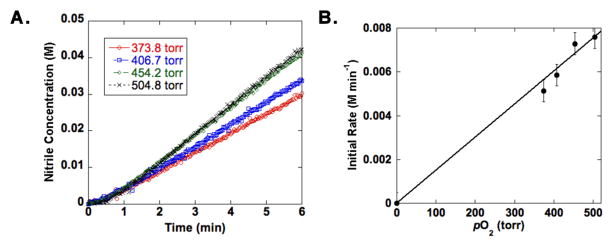
O2-dependence kinetic data from CuI/ABNO-catalyzed aerobic oxidation of p-methoxybenzylamine to p-methoxy-benzonitrile.
Scheme 2.
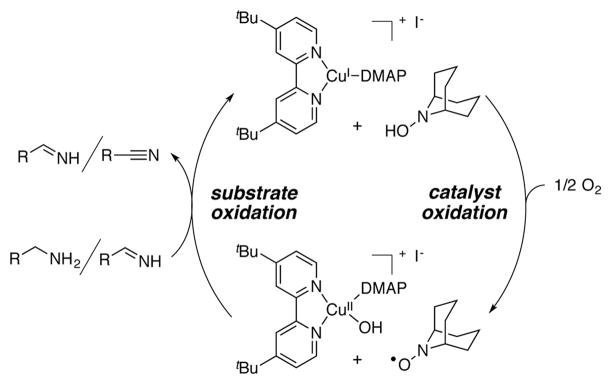
Simplified catalytic cycle for (tBu2bpy)CuI/-ABNO catalyzed aerobic amine oxidation.
The time course in Figure 2 suggests that the homocoupled imine 3a does not convert into the nitrile once it forms, and it is obtained in approximately 5% yield after completion of the reaction. Control experiments were performed to assess the reversibility of imine formation under the reaction conditions. Imine 3a was independently prepared and subjected to the typical reaction conditions. Some conversion of 3a into nitrile 2a could be achieved by adding ammonia to the reaction mixture (Scheme 3), and increasing the ammonia equivalents increases the nitrile yield. Nevertheless, the relatively slow rate and the quantity of ammonia required to promote this reaction suggest that the homocoupled imine will, at best, convert slowly into nitrile under the amine oxidation reactions. These observations further suggest that nitrile formation with this catalyst system primarily proceeds via direct oxidation of primary imine A, without diverting to the homocoupled imine.
Scheme 3.
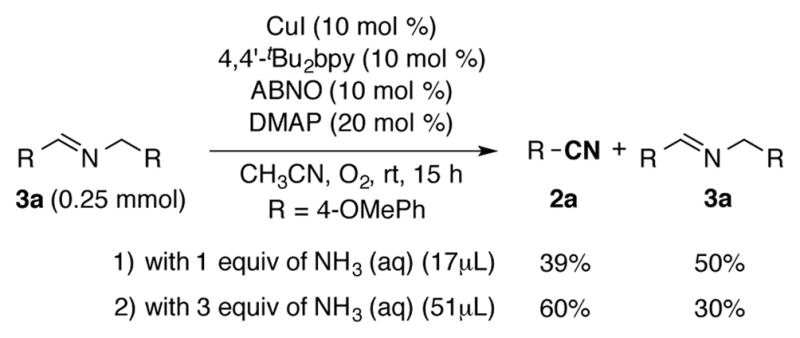
Investigation of the possiblities of homocoupled imine intermediates.
The compatibility of this catalyst system with aqueous ammonia raised the possibility of converting alcohols to nitriles via oxidative coupling with ammonia (Scheme 4). Upon oxidation of the alcohol to the aldehyde, condensation with ammonia would afford the same primary imine intermediate (A) involved in amine oxidation reactions. In this case, however, the reaction would not be susceptible to formation of the homocoupled imine. Initial screening studies revealed that Cu(OTf)2/TEMPO catalyzes efficient conversion of benzylic alcohols into nitriles in high yield with 2.2 equiv of aqueous ammonia in DMSO at 60 °C (Scheme 5; see supporting information for full details). While this effort was in progress, the groups of Huang,6c Tao,6d and Muldoon6e reported similar catalyst systems for this transformation. The work of Huang et al. is particularly noteworthy because their (bpy)CuI/TEMPO catalyst system enables conversion of a broad range of allylic, benzylic and aliphatic alcohols.
Scheme 4.
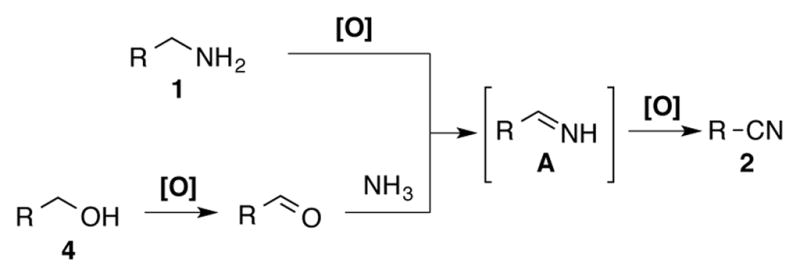
Complementary pathways for the preparation of nitriles from 1° amines or 1° alcohols/ammonia.
Scheme 5.
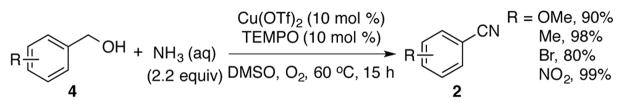
Preliminary results of aerobic oxidative coupling of alcohols and ammonia to form nitriles.
In conclusion, we have identified (tBu2bpy)CuI/ABNO as an efficient catalyst system with broad substrate scope for oxidative dehydrogenation of primary amines to nitriles. The identity of the nitroxyl reagent is a key factor in achieving selectivity for nitrile rather than the homocoupled imine. Elucidation of the mechanistic origin of this nitroxyl-dependent selectivity, together with characterization of the mechanistic similarities and differences between reactions of alcohols and amines is an important focus of ongoing work.
Supplementary Material
Acknowledgments
We are grateful to the NIH (R01-GM100143), together with a consortium of pharmaceutical companies (Eli Lilly, Pfizer, and Merck) for financial support of this work. NMR spectroscopy facilities were partially supported by the NSF (CHE-1048642).
ABBREVIATIONS
- ABNO
9-azabicyclo [3.3.1] nonane N-oxyl
- bpy
2,2′-bipyridyl
- DMAP
4-dimethylaminopyridine
- DTBNO
di-tert-butylnitroxide
- ketoABNO
9-azabicyclo [3.3.1] nonan-3-one N-oxyl
- NMI
N-methylimidazole
- TEMPO
2,2,6,6-tetramethyl-1-piperidinyloxyl
Footnotes
ASSOCIATED CONTENT
Detailed experimental procedures, kinetic data, and additional experiment data are included. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
References
- 1.For reviews of aerobic alcohol oxidation, see: Arends IWCE, Sheldon RA. In: In Modern Oxidation Methods. Bäckvall JE, editor. Wiley-VCH Verlag Gmb & Co.; Weinheim: 2004. pp. 83–118.Sheldon RA, Arends IWCE, ten Brink GJ, Dijksman A. Acc Chem Res. 2002;35:774. doi: 10.1021/ar010075n.Schultz MJ, Sigman MS. Tetrahedron. 2006;62:8227.Parmeggiani C, Cardona F. Green Chem. 2012;14:547.
- 2.For a review of aerobic oxidation of amines, see: Schümperli MT, Hammond C, Hermans I. ACS Catal. 2012;2:1108.
- 3.(a) Brackman W, Gaasbeek C. Rec Trav Chim. 1966;85:221. [Google Scholar]; (b) Brackman W, Gaasbeek C. Rec Trav Chim. 1966;85:257. [Google Scholar]
- 4.For leading references to Cu/TEMPO-mediated aerobic alcohol oxidation, see the following: Semmelhack MF, Schmid CR, Cortés DA, Chou CS. J Am Chem Soc. 1984;106:3374.Ragagnin G, Betzemeier B, Quici S, Knochel P. Tetrahedron. 2002;58:3985.Gamez P, Arends IWCE, Reedijk J, Sheldon RA. Chem Commun. 2003:2414. doi: 10.1039/b308668b.Gamez P, Arends IWCE, Sheldon RA, Reedijk J. Adv Synth Catal. 2004;346:805.Geisslmeir D, Jary WG, Falk H. Monatsh Chem. 2005;136:1591.Jiang N, Ragauskas AJ. J Org Chem. 2006;71:7087. doi: 10.1021/jo060837y.Mannam S, Alamsetti SK, Sekar G. Adv Synth Catal. 2007;349:2253.Kumpulainen ETT, Koskinen AMP. Chem Eur J. 2009;15:10901. doi: 10.1002/chem.200901245.Hoover JM, Stahl SS. J Am Chem Soc. 2011;133:16901. doi: 10.1021/ja206230h.Hoover JM, Steves JE, Stahl SS. Nat Protoc. 2012;7:1161. doi: 10.1038/nprot.2012.057.Könning D, Hiller W, Christmann M. Org Lett. 2012;14:5258. doi: 10.1021/ol302420k.
- 5.For leading references to Cu/TEMPO and related catalyst systems for amine oxidation, see the following: Han B, Yang XL, Wang C, Bai YW, Pan TC, Chen X, Yu W. J Org Chem. 2012;77:1136. doi: 10.1021/jo2020399.Hu Z, Kerton FM. Org Biomol Chem. 2012;10:1618. doi: 10.1039/c2ob06670j.Sonobe T, Oisaki K, Kanai M. Chem Sci. 2012;3:3249.Huang B, Tian H, Lin S, Xie M, Yu X, Xu Q. Tetrahedron Lett. 2013;54:2861.
- 6.For leading references to Cu/TEMPO and related catalyst systems for oxidative coupling of alcohols and amines, see: Tian H, Yu X, Li Q, Wang J, Xu Q. Adv Synth Catal. 2012;354:2671.Flanagan JCA, Dornan LM, McLaughlin MG, McCreanor NG, Cook MJ, Muldoon MJ. Green Chem. 2012;14:1281.Yin W, Wang C, Huang Y. Org Lett. 2013;15:1850. doi: 10.1021/ol400459y.Tao C, Liu F, Zhu Y, Liu W, Cao Z. Org Biomol Chem. 2013;11:3349. doi: 10.1039/c3ob00002h.Dornan LM, Cao Q, Flanagan JCA, Crawford JJ, Cook MJ, Muldoon MJ. Chem Commun. 2013;49:6030. doi: 10.1039/c3cc42231c.
- 7.For Cu-catalyzed oxidative homocoupling of amines in the absence of a nitroxyl cocatalyst, see: Patila RD, Adimurthy S. Adv Synth Catal. 2011;353:1695.
- 8.Cu-mediated oxidation of primary amines to nitriles in the absence of a nitroxyl cocatalyst require stoichiometric Cu, more-forcing reaction conditions and/or promote competitive formation of homocoupled imine: Capdevielle P, Lavigne A, Maumy M. Synthesis. 1989:453.Capdevielle P, Lavigne A, Sparfel D, Baranne-Lafont J. Tetrahedron Lett. 1990;31:3305.Maeda Y, Nishimura T, Uemura S. Bull Chem Soc Jpn. 2003;76:2399.
- 9.For Ru catalyzed amine oxidation for nitrile, see: Yamaguchi K, Mizuno N. Angew Chem, Int Ed. 2003;42:1480. doi: 10.1002/anie.200250779.Li F, Chen J, Zhang Q, Wang Y. Green Chem. 2008;10:553.Zhang Y, Xu K, Chen X, Hu T, Yu Y, Zhang J, Huang J. Catal Commun. 2010;11:951.Venkatesan S, Kumar AS, Lee JF, Chan TS, Zen JM. Chem Eur J. 2012;18:6147. doi: 10.1002/chem.201103913.
- 10.(a) Shibuya M, Tomizawa M, Suzuki I, Iwabuchi Y. J Am Chem Soc. 2006;128:8412. doi: 10.1021/ja0620336. [DOI] [PubMed] [Google Scholar]; (b) Shibuya M, Tomizawa M, Sasano Y, Iwabuchi Y. J Org Chem. 2009;74:4619. doi: 10.1021/jo900486w. [DOI] [PubMed] [Google Scholar]
- 11.When 0.415 mmol of nitrile is produced, 0.411 mmol of oxygen was consumed. For the detailed gas uptake procedure, see the supporting information.
- 12.Hoover JM, Ryland BL, Stahl SS. J Am Chem Soc. 2013;135:2357. doi: 10.1021/ja3117203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


