Abstract
Objectives
We hypothesized that RV stroke work index (RVSWI) and pulmonary capacitance (PC) would increase after treatment for pulmonary arterial hypertension (PAH) and that prostanoids would have a stronger effect than oral therapy.
Background
Right ventricular (RV) function is a major determinant of outcome in patients with PAH. Little is known about the response of RV function or its hemodynamic determinants to PAH-specific therapy.
Methods
We reviewed hemodynamic and health data on 58 patients from an institutional registry and analyzed changes in hemodynamics between diagnostic and first repeat catheterization after initiation of therapy for PAH.
Results
RVSWI and PC increased significantly after therapy (p = 0.007 and 0.02, respectively). Improvement in RV function was limited to patients treated with prostanoid-only therapy (p = 0.04); no improvement was found in patients treated with oral therapy (p = 0.25). Patients with the poorest baseline RV function (lowest tertile) had the greatest improvement post-therapy (p = 0.005 and < 0.001 vs. middle and highest tertiles). The major determinant of RVSWI was change in stroke volume (rs = 0.54, p < 0.001), indicating RVSWI is an accurate reflection of RV function.
Conclusion
RV function improves after therapy with regimens including prostanoids but not oral-only regimens. Patients with the least compensated RV function at diagnosis may derive the most benefit from therapy. Larger studies are needed to determine whether changes in RVSWI after therapy are associated with outcomes.
INTRODUCTION
Pulmonary arterial hypertension (PAH) is an incurable disease characterized by progressive pulmonary vascular obliteration, right ventricular (RV) failure, and death (1). Evidence suggests that outcomes in PAH more closely mirror changes in RV function than improvement in pulmonary hemodynamics (2–4). There is limited data that a direct beneficial effect of PAH therapy on right ventricular function might occur (3), but differences among treatment regimens have not been studied. Availability of an accurate measure of RV function at the time of catheterization and knowledge of which medications are likely to improve RV function may influence clinicians’ choice of therapy.
Conventional hemodynamic markers of RV function such as right atrial pressure (RAP), cardiac output (CO) and pulmonary pressure (PAP) can be integrated into a measure of RV function, the right ventricular stroke work index (RVSWI). Lower RVSWI is associated with worse outcome in PAH, left ventricular failure, and left ventricular assist device patients (5–7). Invasive hemodynamics can also be used to measure pulmonary capacitance (PC), a measure of vascular resistance and elastic recoil. Depressed PC is a strong prognostic indicator of adverse outcome in idiopathic PAH (IPAH) (8). We have previously shown that RVSWI and PC are prognostic indicators in a cohort of familial (FPAH) and idiopathic PAH (IPAH)(9). However, the effect of different PAH-specific therapies on RV function and pulmonary capacitance has not been studied.
We studied the effect of therapy for PAH on RVSWI and PC from the time of diagnostic catheterization to the first repeat RHC. We hypothesized that right ventricular function and pulmonary capacitance would improve in response to therapy and that prostanoids would have a stronger effect than oral therapy.
METHODS
Study population
Data for this study was retrospectively analyzed from an institutional registry. Patients in this study are consecutive patients seen in the Vanderbilt University Center for Pulmonary Vascular Disease and enrolled in the Vanderbilt Pulmonary Hypertension Research Cohort (VPHRC). The VPHRC also includes patients evaluated at outside institutions but only patients seen at Vanderbilt were included in this study. To avoid confounding by treatment era, cases were restricted to those with diagnostic hemodynamic and clinical data between January 1, 1996 (when intravenous prostaglandins became commercially available) and March 1, 2011.
The diagnosis of PAH was made by experienced physicians according to consensus guidelines (10) including mean pulmonary artery pressure (mPAP) ≥ 25mmHg, pulmonary vascular resistance (PVR) > 3 wood units (WU), and pulmonary wedge pressure (PWP) ≤ 15mmHg. Only patients with IPAH, FPAH, and connective tissue disease-associated PAH were included in the analysis. Patients were diagnosed with FPAH if they had at least one other family member within their bloodline confirmed with PAH.
Only patients who were treatment-naïve at the time of evaluation were included. Treatment regimens were categorized as prostanoid (intravenous or inhaled), oral (monotherapy or in combination), mixed prostanoid and oral therapy, and vasodilator (calcium channel blocker)-responsive. For purposes of analysis, vasodilator-responsive patients were not included in the oral therapy group given the well-recognized favorable hemodynamic response in this group (11).
Hemodynamics
Heart rate (HR), RAP, PAP (mean, systolic, and diastolic), pulmonary wedge pressure (PWP), and CO were recorded from the patient’s diagnostic catheterization. Cardiac index (CI), PVR, and stroke volume (SV) were calculated from standard formulas.
The physiologic rationale for the calculation of PC has been described in detail elsewhere(8). PC and RVSWI were calculated using the following formulas:
We included only patients who underwent repeat RHC within 3 years of diagnostic catheterization to allow enough time on therapy for pulmonary vascular and right ventricular remodeling while providing a relatively homogenous cohort with regards to length of therapy. Pre- and post-treatment six minute walk test distance (6MWD) and New York Heart Association (NYHA) functional class were also recorded.
Statistical Analysis
Continuous data are expressed as mean ± standard deviation. The Mann Whitney U or Kruskal Wallis test was used to compare differences in continuous demographic, hemodynamic, and outcome variables depending on the number of groups. Paired measurements of RVSWI and PC were compared using the Wilcoxon-signed rank test. Categorical clinical and demographic variables were compared between groups using the chi-square test. Spearman correlation was used to show the relationship between continuous variables. A p value of < 0.05 was considered statistically significant. Statistical analyses were performed using Prism 5.0 software (Graph Pad Software Inc, La Jolla, CA) and SPSS 20 software (SPSS Inc, Chicago, IL).
RESULTS
Demographic and clinical characteristics
At the time of analysis, the VPHRC contained 616 unique cases, 183 of whom were seen at Vanderbilt, treatment-naïve and had diagnostic and repeat RHC data available. Of those, 70 patients had a repeat RHC within 3 years of diagnostic catheterization during the time frame of the study; 12 of those 70 patients had either incomplete RHC data (n = 4) or PWP > 15mmHg (n=8). Fifty-eight patients were included in the analysis representing three subtypes: IPAH (n = 33), FPAH (n = 16), and connective tissue-associated PAH (n = 9; CTD-PAH).
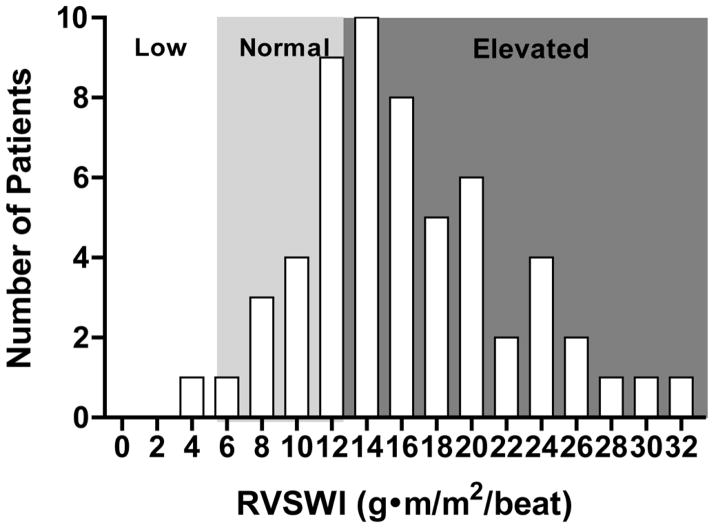
Demographic and clinical characteristics of the fifty-eight study patients divided into treatment regimen are shown in Table 1. The distribution of baseline RVSWI is shown in Figure 1. At the time of presentation, the majority (40/58, 69%) of patients had supra-normal RVSWI, whereas 18/58 (31%) fell into the low or normal range.
Table 1.
Demographic and Clinical Characteristics by Therapy
| Oral Only (n = 21) | Prostanoid Only (n = 17) | Mixed Therapy (n = 7) | Vasodilator Responsive (n = 7) | p value | |
|---|---|---|---|---|---|
| Age (years) | 47.3±14.2 | 36.4±14.1 | 43.0±15.1 | 47.9±14.7 | 0.11 |
| Gender, % female | 88 | 76 | 100 | 86 | 0.55 |
| Functional Class (NYHA) | 2.1±0.6 | 2.1±1.0 | 2.0±0.8 | 1.9±0.9 | 0.94 |
| 6MWD (meters) | 387±111 | 370±117 | 321±136 | 377±93 | 0.59 |
| BMI (kg/m2) | 33.6±12.1 | 30.1±7.3 | 33.0±7.8 | 35.5±11.9 | 0.70 |
| Medical Therapy % (dose range) | n/a | ||||
| Epoprostenol (ng/kg/min) | 0 | 86 (11–30) | 57 (14.2–30) | 0 | |
| Treprostinil (IV or inhaled) (mg/kg/min) | 0 | 14 (12.5–100) | 43 (14–28) | 0 | |
| Ambrisentan (mg/day) | 18 (5) | 0 | 0 | 0 | |
| Bosentan (mg/day) | 35 (250) | 0 | 14 (250) | 0 | |
| Sildenafil (mg/day) | 47 (38–150) | 0 | 86 (60–150) | 0 | |
| Tadalafil (mg/day) | 12 (40) | 0 | 0 | 0 | |
| Amlodipine (mg/day) | 18 (5–10) | 0 | 0 | 42 (5–10) | |
| Diltiazem (mg/day) | 12 (180–360) | 0 | 0 | 29 (240–360) | |
| Nifedipine (mg/day) | 0 | 0 | 0 | 29 (30–120) | |
| Adjuvant Therapy | |||||
| Furosemide (mg/day) | 64 (20–160) | 66 (20–160) | 86 (20–160) | 71 (20–160) | 0.77 |
| Oxygen (L/min) | 6 (2) | 24 (2–4) | 29 (2–3) | 14 (2) | 0.42 |
| Warfarin (mg/day) | 47 (2.5–7.5) | 76 (1.25–11) | 49 (4–7.5) | 49 (5–9) | 0.17 |
BMI = body mass index; IV = intravenous; WHO = World Health Organization.
Figure 1. Distribution of Baseline RVSWI.
The majority (69%) of patients had supra-normal RVSWI at indicating RV compensation in the setting of elevated afterload.
Demographics and clinical characteristics of the cohort divided into tertiles of baseline RV function are shown in Table 2. No differences in age or subtype of PAH were found among the tertiles. However, the lowest tertile contained a higher proportion of men compared to the other tertiles (p = 0.037). There was a strong trend toward better functional class in the tertile with the highest RVSWI (p = 0.07).
Table 2.
Demographic and Clinical Characteristics by Tertile of Right Ventricular Stroke Work Index
| Lowest Tertile (n = 19) | Middle Tertile (n=20) | Highest Tertile (n=19) | p value | |
|---|---|---|---|---|
| Age at Diagnosis | 42.0 ± 15.5 | 41.5 ± 13.5 | 43.5 ± 15.6 | 0.94 |
| Sex (% female) | 12 (63) | 19 (95) | 16 (84) | 0.04 |
| Diagnosis | 0.38 | |||
| IPAH | 10 (53) | 12 (60) | 11 (58) | |
| HPAH | 8 (42) | 4 (20) | 4 (21) | |
| CTD-PAH | 1 (5) | 4 (20) | 4 (21) | |
| Functional Class | 2.2 ± 0.9 | 2.4 ± 0.8 | 1.8 ± 0.6 | 0.07 |
| 6MWD (meters) | 361 ± 125 | 337 ± 126 | 330 ± 98 | 0.56 |
| Diabetes (%) | 5 (26) | 6 (30) | 6 (26) | 0.96 |
| BMI (kg/m2) | 31.4 ± 7.0 | 33.3 ± 13.1 | 30.1 ± 9.2 | 0.66 |
CTD-PAH = connective tissue disease-associated pulmonary arterial hypertension; IPAH = idiopathic pulmonary arterial hypertension; HPAH = heritable pulmonary arterial hypertension.
Medical Therapy and Hemodynamics
The median time from starting medical therapy to the first follow-up clinic visit was 2.7 months (IQR 1.9–5.2 months). The median time between diagnostic and repeat RHC was 15.6 months (IQR 12.0–32.0 months). Seventeen patients were started on oral therapy (monotherapy or any combination) and 21 patients were started on prostanoid (intravenous or inhaled) therapy. Seven patients were started on combination oral and prostanoid therapy and an additional seven patients were found to be vasodilator responsive and started on calcium channel blockers; six patients were not started on therapy due to patient or physician preference.
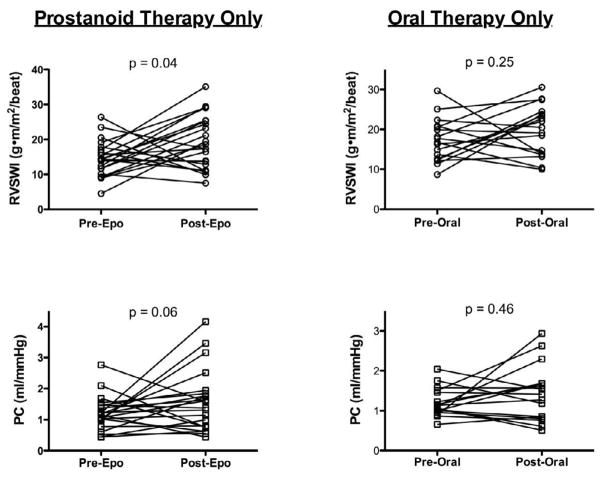
Hemodynamic data at the time of diagnostic catheterization according to treatment regimen are shown in Table 3. The only difference among treatment groups was lower mixed venous oxygen saturation in the prostanoid group compared to the oral therapy group (58.2±10.7 vs. 66.6±5.6, respectively; p = 0.01). For the entire cohort, we found a significant increase in RVSWI (mean increase 3.4 ± 9.5 g-m/m2/beat, p = 0.007) and PC (mean increase 0.4 ± 1.0 mL/mmHg, p = 0.02) after medical therapy (Figure 2). In the prostanoid group there was a significant increase in RVSWI (p = 0.04) and a trend toward improvement in PC (p = 0.06). However, in the 17 patients started on oral therapy neither RVSWI nor PC increased significantly after treatment (p = 0.25 and 0.46, respectively; Figure 3). In the seven patients who were treated with calcium channel blockers, RVSWI (15.7 ± 4.0 vs. 19.4 ± 3.2 g·m/m2/beat; p = 0.02) and PC (1.2 ± 0.3 vs. 2.3 ± 1.1 mL/mmHg; p = 0.03) both increased after therapy. Because only prostanoids and calcium channel blockers were available between 1996 and 2001, we repeated our analysis using a cutoff diagnosis date of January 2001 (n = 50). Improvement in RVSWI and PC remained significant in the prostanoid group (p = 0.04 and 0.01, respectively) and did not reach significance in the oral therapy group (p = 0.23 and 0.30, respectively).
Table 3.
Hemodynamics at Diagnosis by Therapy
| Parameter | Prostanoid Only | Oral Only | Mixed Therapy | Vasodilator Responsive | P |
|---|---|---|---|---|---|
| Heart rate (beats per minute) | 80.8±11.7 | 77.0±10.1 | 81.4±6.1 | 77.3±9.1 | 0.76 |
| Mean RAP (mmHg) | 9.6±6.8 | 7.3±6.2 | 7.7±5.6 | 7.3±3.3 | 0.69 |
| PA systolic pressure (mmHg) | 82.4±18.5 | 81.9±20.3 | 75.3±14.1 | 80.1±14.1 | 0.91 |
| Mean PA pressure (mmHg) | 54.5±12.6 | 49.8±11.0 | 48.3±9.5 | 47.7±9.3 | 0.49 |
| Pulmonary Wedge Pressure (mmHg) | 8.8±2.5 | 7.8±3.6 | 9.5±3.6 | 8.3±3.7 | 0.85 |
| Pulmonary Vascular Resistance (WU) | 13.6±7.7 | 9.7±2.8 | 10.0±3.3 | 10.0±2.6 | 0.49 |
| Cardiac Index (l/min/m2) | 2.0±0.6 | 2.2±0.3 | 2.0±0.8 | 2.2±0.5 | 0.28 |
| Mixed venous oxygen saturation (%) | 58.2±10.7* | 66.6±5.6 | 57.2±9.7† | 63.7±9.4 | 0.04 |
| RVSWI (g·m/m2/beat) | 14.8±5.0 | 17.1±5.6 | 15.8±8.9 | 15.7±4.0 | 0.63 |
| PC (mL/mmHg) | 1.2±0.6 | 1.2±0.3 | 1.2±0.5 | 1.2±0.3 | 0.98 |
PA = pulmonary artery; PC = pulmonary capacitance; RAP = right atrial pressure; RVSWI = right ventricular stroke work index; WU = Wood units.
p = 0.01 for prostanoid vs. oral therapy group.
n =5
Figure 2. Right Ventricular Stroke Work Index and Pulmonary Capacitance Improve after PAH Therapy.
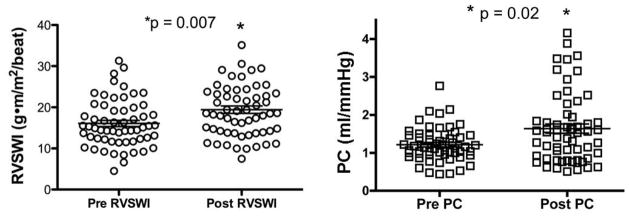
RVSWI and PC increase after administration of PAH-specific therapy. PC = pulmonary capacitance; RVSWI = right ventricular stroke work index. Bars represent mean ± standard deviation.
Figure 3. Effect of Prostanoid or Oral Therapy on RVSWI and PC.
RV function improves after treatment with prostanoids, but not after therapy with oral PAH medications. There is a trend toward improvement in PC with prostanoids and no improvement after oral therapy. PC = pulmonary capacitance; RVSWI = right ventricular stroke work index.
Determinants of Right Ventricular Function
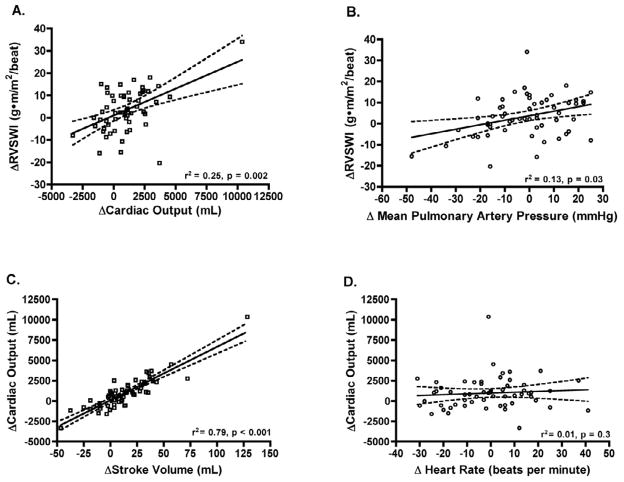
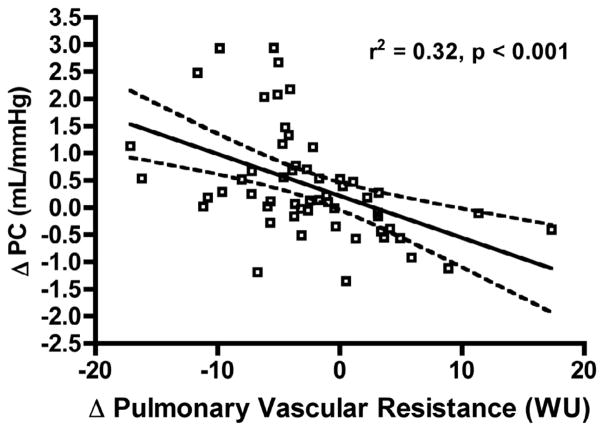
Change in RVSWI after therapy was driven more by change in cardiac output (rs = 0.5, p = 0.002) than change in mPAP (rs = 0.36, p = 0.03; Figure 4A, B). The major determinant of RVSWI was change in stroke volume (rs = 0.54, p < 0.001). Increase in cardiac output after therapy was almost entirely due to an increase in SV (rs = 0.89, p < 0.001) with no contribution from change in heart rate (rs = 0.1, p = 0.3; Figure 4C, D). We found that change in PC was strongly influenced by change in PVR (rs = −0.6, p < 0.001; Figure 5).
Figure 4. Determinants of Response of Right Ventricular Stroke Work Index to Therapy.
Change in RVSWI after therapy is strongly influenced by change in cardiac output with less contribution from change in mean pulmonary artery pressure (A, B). Improvement in cardiac output after therapy is due to change in stroke volume with no influence from change in heart rate (C, D). Solid line represents best-fit line; dashed lines are 95% confidence interval.
Figure 5. Pulmonary Capacitance is a Measure of Pulmonary Vascular Resistance in Patients with Pulmonary Arterial Hypertension.
In patients with PAH, change in PC after therapy is primarily influenced by change in pulmonary vascular resistance. Solid line represents best-fit line; dashed lines are 95% confidence interval.
Change in PVR was investigated as a possible explanation for the difference in RV function response between oral and prostanoid therapy groups. We found no difference in delta PVR between the oral-only and prostanoid groups (−0.4±4.6 WU vs. −4.5±7.9WU, respectively; p = 0.07), though there was a strong trend toward statistical significance. Change in PVR and change in RVSWI did not correlate significantly in either the oral only (rs = −0.12, p = 0.66) or prostanoid group (rs = −0.20, p = 0.44).
When comparing the response to therapy by RVSWI tertile, we found a stepwise response with the highest improvement in RVSWI in the lowest tertile (Figure 6). There was no association between tertile of baseline RVSWI and likelihood of being treated with prostanoid therapy (p = 0.67; 95% CI 0.4 to 1.7). A similar stepwise pattern in PC was seen in response to therapy with the lowest baseline tertile improving the most (Figure 6).
Figure 6. RVSWI and PC at the Time of Diagnosis Predict Hemodynamic Response to PAH Therapy.
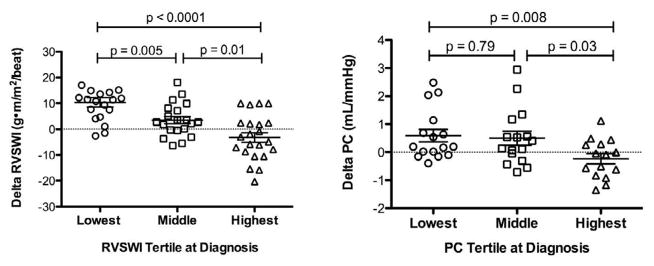
RVSWI and PC change in a step-wise fashion after therapy with the greatest improvement found in the patients in the lowest baseline tertiles. Bars represent mean ± standard deviation.
Outcomes
Differences in change in 6MWD and functional class by group are shown in Table 4. Improvement in both 6MWD and functional class was significantly better in the prostanoid group compared to the oral therapy group. For the cohort as a whole, no correlation was found between RVSWI at diagnosis and 6MWD (rs = −0.08, p = 0.59) or functional class (rs = −0.19, p = 0.16) at diagnosis although improvement in RVSWI (net increase 3.4±9.5 g·m/m2/beat) was associated with improvement in both functional class (net decrease −0.8±0.8; rs = −0.32, p = 0.016) and change in 6MWD (net increase 46±103m; rs = 0.52, p = 0.04) at first follow-up visit after initiation of therapy. Change in stroke volume was an independent predictor of improvement in functional class when controlling for mPAP and HR with an odds ratio of 1.04 per mL (p = 0.03; 95%CI 1.004 to 1.09).
Table 4.
Outcome Differences among Treatment Groups
| Outcome | Prostanoid (n = 21) | Oral (n = 17) | Mixed Therapy (n = 7) | Vasodilator Responsive (n = 7) | p |
|---|---|---|---|---|---|
| Delta 6MWD | 124±106* | 4±109 | 40±57 | 32±35 | 0.013 |
| Delta Functional Class | −1.3±0.6† | −0.5±0.5 | −0.7±0.8 | −1.0±0.8 | 0.001 |
Negative value indicates improvement in functional class. 6MWD = six minute walk distance.
p = 0.006 vs. Oral therapy group.
p < 0.001.
DISCUSSION
In this study of 58 mixed-etiology PAH patients, we report the response of RV function and pulmonary capacitance before and after PAH-specific therapy. For the entire cohort, we found that improvement in RV function was driven by patients treated with prostanoid therapy. Patients with the poorest baseline RV function had the greatest improvement post-therapy, a finding that may have implications for identifying patients with the greatest potential benefit from therapy. Improvement in PC was limited to patients treated with prostanoid therapy and vasodilator-responsive patients treated with calcium channel blockers. Finally, we showed that improvement in RVSWI predicts improvement in post-therapy 6MWD and functional class.
RVSWI is a measure of RV function which can be readily calculated from the usual components of a diagnostic RHC. It is used clinically in patients with LV failure, but little is known about its natural history or ability to prognosticate in patients with PAH (12). We previously showed that, as judged by RVSWI, FPAH patients are less compensated at the time of diagnosis compared to IPAH patients. In addition, RVSWI is lower in FPAH patients who die or undergo lung transplant within 5 years of diagnosis (13). In our cohort, the majority (69%) of patients had supra-normal RVSWI at baseline despite limited functional capacity measured by NYHA functional class and exercise capacity. This discrepancy may be explained by the subjective nature of NYHA class and variations in effort on 6MW testing, particularly in relation to obese patients as in our cohort (14,15).
In order to better understand why RVSWI changes in response to therapy, we analyzed the relationship between individual components (mPAP and CO) and the composite parameter. CO had a stronger influence than mPAP in the response of RVSWI to PAH therapy, but both components provided a significant influence. Change in cardiac output was almost entirely driven by improvement in stroke volume, supporting our hypothesis that improvement in RVSWI in response to therapy is due to improved RV contractility, not increase in heart rate. The importance of RV function in PAH is further supported in our study by the independent value of SV in predicting improvement in functional class. RVSWI may be superior to either CO or mPAP alone and may have a previously unrecognized role to play in the interpretation of invasive hemodynamics in PAH.
Little is known about the response to therapy of RV function in PAH. Although RVSWI and PC increased post-therapy in the cohort as a whole, this finding was driven by an increase in RVSWI and PC in the 21 patients started on prostanoid only therapy. Potential explanations for this difference include greater efficacy of prostanoids in therapy of PAH pulmonary vascular disease, a direct effect of prostaglandins on the right ventricle (16,17), or differences in treatment as a function of disease severity reflected in RVSWI or other unfavorable hemodynamic predictors. The strong influence we found of stroke volume on change in RVSWI suggests that prostanoids may exert an inotropic effect on the RV, but this requires further study.
Patients in the lowest tertile at diagnosis had the greatest improvement in RVSWI after treatment, reflecting the well-described ability of the right ventricle to recover function with removal of load stress (18,19). Although signs and symptoms of RV dysfunction at diagnosis are often recognized by treating physicians, quantification of low RVSWI at diagnosis may help clinicians identify patients at risk for poor outcomes and the greatest potential benefit from aggressive therapy.
PC measures the ability of the pulmonary vasculature to receive blood during RV systole and then expel blood from the pulmonary tree during diastole. In the normal pulmonary circulation, resistance is nearly zero (≤ 1 Wood unit) and therefore elastic recoil is primarily responsible for capacitance. In contrast, in PAH, there is reduction in lumen size, dropout of vessels, and thickening of large arteries such that compliance is low and PVR probably accounts for most of capacitance data. This is supported by our data showing that PVR has a strong inverse association with PC in our cohort. The prognostic value of PC, measured at RHC or echocardiography, in patients with IPAH is well-described (8,20). However, the response of PC to PAH therapy has not previously been studied. The increase in PC after therapy in our study was driven by the presence of prostanoids in the treatment regimen (either alone or in combination with oral therapy).
In patients with PAH, a decrease in PVR is thought to drive improvement in RV function by decreasing RV afterload; however, improvement or decline in RV function is often independent of the change in PVR after therapy (3). This is supported by our finding of no difference in change in PVR between patients with oral-only regimens and those treated with prostanoids; however, our study may have been underpowered to detect this difference given that the p value was nearly significant (p = 0.07). Tedford et al recently showed that the influence of PVR on RV afterload is governed by the hyperbolic relationship between PVR and PC (21). The fixed relationship between PVR and PC and the flatness of the curve at elevated PVR means that patients with high baseline PVR require significant decreases in PVR (not often produced with current PAH therapy) to achieve a decrease in RV afterload. Thus, our findings suggest that improvement in RV function on prostanoid therapy may represent a direct effect on RV contractility, not simply a decrease in resistance.
Limitations
Our study has a relatively small sample size compared to contemporary studies of hemodynamics in PAH, but it is the first to report the response of RVSWI and PC to therapy. Patients who died or were lost from the study before follow-up catheterization were excluded from this analysis which may limit the prognostic value of our findings and bias to the null hypothesis.
RVSWI is an imperfect measure of RV function and reflects influences from both afterload and preload. However, it is used clinically in patients with left heart disease and has prognostic value in patients with PAH (13,22). In addition, our detailed analysis suggests that it may add incremental value to interpretation of its individual components. Echocardiographic data would have provided an additional measure of RV function but were not available for analysis in our cohort over the extended period of the study.
CONCLUSIONS
In this study of serial hemodynamic measurements in patients with PAH we have shown that RV function improves after prostanoid therapy but not after therapy with oral medications. Patients with the least compensated RV function at diagnosis had the greatest post-therapy improvement in RV function and may derive the most benefit from therapy. Larger studies are needed to validate these findings and determine the clinical utility of RVSWI and PC versus conventional hemodynamic parameters. Further study is also needed to explore the potential of prostanoid therapy to directly improve RV function.
Acknowledgments
Funding Sources: This work was supported by National Institutes of Health [K08 HL093363 (Hemnes), K23 HL0987431 (Austin), 1PO HL108800-01A1 (Loyd, Newman, Hemnes, Austin), and NCRR/NIH 1 UL1 RR024975 (Vanderbilt)] and the American College of Cardiology Foundation/Merck Fellowship (Brittain).
ABBREVIATIONS
- PAH
pulmonary arterial hypertension
- RV
right ventricle
- RVSWI
right ventricular stroke work index
- PC
pulmonary capacitance
- RAP
right atrial pressure
- CO
cardiac output
- mPAP
mean pulmonary artery pressure
- RHC
right heart catheterization
- PVR
pulmonary vascular resistance
- IPAH
idiopathic pulmonary arterial hypertension
- FPAH
familial pulmonary arterial hypertension
- CTD-PAH
connective tissue disease associated pulmonary arterial hypertension
- VPHRC
Vanderbilt Pulmonary Hypertension Research Cohort
- PWP
pulmonary wedge pressure
- HR
heart rate
- BMI
body mass index
- NYHA
New York Heart Association
- 6MWD
Six minute walk distance
- SV
stroke volume
Footnotes
Disclosures: Anna Hemnes, MD – Grants: Pfizer, NIH; Consultant: Pfizer, United Therapeutics Meredith Pugh – Consultant: Gilead; Ivan Robbins: Advisory Board Member: Actelion, Gilead, United Therapeutics; Grants: Actelion, Gilead, United Therapeutics, Geno, Novartis
All other authors report no disclosures.
All other authors report no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Voelkel NF, Quaife RA, Leinwand LA, et al. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation. 2006;114:1883–91. doi: 10.1161/CIRCULATIONAHA.106.632208. [DOI] [PubMed] [Google Scholar]
- 2.Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation. 2010;122:156–63. doi: 10.1161/CIRCULATIONAHA.109.911818. [DOI] [PubMed] [Google Scholar]
- 3.van de Veerdonk MC, Kind T, Marcus JT, et al. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol. 2011;58:2511–9. doi: 10.1016/j.jacc.2011.06.068. [DOI] [PubMed] [Google Scholar]
- 4.Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) Circulation. 2010;122:164–72. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- 5.Fukamachi K, McCarthy PM, Smedira NG, Vargo RL, Starling RC, Young JB. Preoperative risk factors for right ventricular failure after implantable left ventricular assist device insertion. Ann Thorac Surg. 1999;68:2181–4. doi: 10.1016/s0003-4975(99)00753-5. [DOI] [PubMed] [Google Scholar]
- 6.La Vecchia L, Varotto L, Zanolla L, Spadaro GL, Fontanelli A. Right ventricular function predicts transplant-free survival in idiopathic dilated cardiomyopathy. Journal of cardiovascular medicine. 2006;7:706–10. doi: 10.2459/01.JCM.0000243006.90170.ce. [DOI] [PubMed] [Google Scholar]
- 7.Campo A, Mathai SC, Le Pavec J, et al. Hemodynamic predictors of survival in scleroderma-related pulmonary arterial hypertension. Am J Respir Crit Care Med. 2010;182:252–60. doi: 10.1164/rccm.200912-1820OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahapatra S, Nishimura RA, Sorajja P, Cha S, McGoon MD. Relationship of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial hypertension. J Am Coll Cardiol. 2006;47:799–803. doi: 10.1016/j.jacc.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 9.Brittain EL, Pugh ME, Wheeler LA, et al. Shorter Survival in Familial Versis Idiopathic Pulmonary Arterial Hypertension is Associated with Hemodynamic Markers of Impaired Right Ventricular Function. Pulm Circ. 2013 doi: 10.1086/674326. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Badesch DB, Champion HC, Sanchez MA, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S55–66. doi: 10.1016/j.jacc.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Rich S, Kaufmann E, Levy PS. The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension. N Engl J Med. 1992;327:76–81. doi: 10.1056/NEJM199207093270203. [DOI] [PubMed] [Google Scholar]
- 12.Slaughter MS, Pagani FD, Rogers JG, et al. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant. 2010;29:S1–39. doi: 10.1016/j.healun.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Brittain E, Pugh M, Wheeler L, et al. Heritable Pulmonary Arterial Hypertension is Associated with Worse Survival Compared to Idiopathic Pulmonary Arterial Hypertension (abstr) Am J Respir Crit Care Med. 2012;185:A3825. [Google Scholar]
- 14.Troosters T, Gosselink R, Decramer M. Six minute walking distance in healthy elderly subjects. Eur Respir J. 1999;14:270–4. doi: 10.1034/j.1399-3003.1999.14b06.x. [DOI] [PubMed] [Google Scholar]
- 15.Hulens M, Vansant G, Claessens AL, Lysens R, Muls E. Predictors of 6-minute walk test results in lean, obese and morbidly obese women. Scand J Med Sci Sports. 2003;13:98–105. doi: 10.1034/j.1600-0838.2003.10273.x. [DOI] [PubMed] [Google Scholar]
- 16.Pavlovic M, Petkovic D, Cvetkovic M, Zdjelar K, Starcevic V, Bosnic O. Study of the mechanism of prostacyclin (PgI2) action on myocardial contractility. Agents Actions Suppl. 1992;37:171–5. doi: 10.1007/978-3-0348-7262-1_23. [DOI] [PubMed] [Google Scholar]
- 17.Montalescot G, Drobinski G, Meurin P, et al. Effects of prostacyclin on the pulmonary vascular tone and cardiac contractility of patients with pulmonary hypertension secondary to end-stage heart failure. Am J Cardiol. 1998;82:749–55. doi: 10.1016/s0002-9149(98)00439-1. [DOI] [PubMed] [Google Scholar]
- 18.Reesink HJ, Marcus JT, Tulevski, et al. Reverse right ventricular remodeling after pulmonary endarterectomy in patients with chronic thromboembolic pulmonary hypertension: utility of magnetic resonance imaging to demonstrate restoration of the right ventricle. J Thorac Cardiovasc Surg. 2007;133:58–64. doi: 10.1016/j.jtcvs.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 19.Brittain E, Hemnes AR, Keebler M, Lawson M, Byrd B, DiSalvo T. Right ventricular plasticity and functional imaging. Pulm Circ. 2012;2:309–326. doi: 10.4103/2045-8932.101407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahapatra S, Nishimura RA, Oh JK, McGoon MD. The prognostic value of pulmonary vascular capacitance determined by Doppler echocardiography in patients with pulmonary arterial hypertension. J Am Soc Echocardiogr. 2006;19:1045–50. doi: 10.1016/j.echo.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Tedford RJ, Hassoun PM, Mathai SC, et al. Pulmonary capillary wedge pressure augments right ventricular pulsatile loading. Circulation. 2012;125:289–97. doi: 10.1161/CIRCULATIONAHA.111.051540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forfia PR, Fisher MR, Mathai SC, et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med. 2006;174:1034–41. doi: 10.1164/rccm.200604-547OC. [DOI] [PubMed] [Google Scholar]