Abstract
Purpose
To examine the protective effects of antioxidants in cultured trabecular meshwork (TM) cells exposed to oxidative stress.
Methods
Primary porcine TM cells were pretreated with 50 μM resveratrol, 0.2 mM urate, 1 mM ascorbate, 1 mM reduced glutathione (rGSH), or 1 mM ρ-coumarate followed by exposure to hydrogen peroxide (0.5-4 mM). Cell metabolism was determined by mitochondrial enzyme activity and cell viability by uptake of the vital dye calcein, a fluorescent calcium binding dye. Reactive oxygen species (ROS), which may reflex oxidative damage, were determined by 2′7′-dichlorofluorescein diacetate.
Results
Trabecular meshwork cell metabolism was reduced to 72 ± 5% of control levels with 1 mM hydrogen peroxide (H2O2) treatment. TM cells that co-incubated with ascorbate (85% ± 5%), ρ-coumarate (98 ± 11%) or rGSH (103 ± 17%) had significantly increased metabolism compared to 1 mM H2O2 treatment. Resveratrol significantly increased TM cell metabolism at both 2 mM (102 ± 14% live) and 4 mM H2O2 (27 ± 12% live), with H2O2-treated cultures containing mostly metabolically inactive cells (3% at 2 mM; 2% at 4 mM). Similar results were obtained in cell viability assays. Ascorbate and resveratrol, but not ρ-coumarate or rGSH, decreased ROS levels in TM cells exposed to a sublethal dose of H2O2 (0.5 mM). Urate had no protective effect against H2O2 damage in any of the assays.
Conclusions
Increased oxidative damage was demonstrated in the TM of patients with primary open angle glaucoma. The antioxidants (resveratrol, ascorbate, ρ-coumarate) and the antioxidant enzyme cofactor (rGSH) protected TM cells from H2O2-induced damage.
Translational Relevance
Future experiments are needed to determine whether addition of antioxidants may maintain TM cell viability in vivo. Antioxidants could be applied either topically or coupled with extended-release vehicles for intraocular injection to reduce free radical formation leading to enhanced therapeutic outcomes. Ultimately, studies using animal models could determine whether application of antioxidants can ameliorate progression in diseases such as glaucoma and macular degeneration.
Keywords: glaucoma, trabecular meshwork, antioxidants, oxidative stress
Introduction
Several lines of evidence suggest that a reduction in the antioxidant properties of the trabecular meshwork (TM) region of the eye correlates to the progression of primary open angle glaucoma (POAG). Exposure to oxidative stress induces the glucocorticoid-response protein myocilin1 and long-term induction of myocilin is associated with steroid induced glaucoma. Short-term treatment of cultured TM cells with sublethal doses of hydrogen peroxide (H2O2) (1 mM) reduces TM cell adhesion to the growth matrix, and repeated exposure might potentially lead to cell loss, compromised tissue integrity, and subsequent impaired outflow.2 It has also been shown that the circulating plasma levels of the powerful antioxidant reduced glutathione (rGSH) were consistently lower in patients with POAG as compared to age-matched controls.3 Long-term exposure of TM cultures to low doses of (0.2 mM) H2O2 induced expression of inflammatory markers associated with TM from glaucoma donors.4 Superoxide dismutase, a glutathione (GSH)-utilizing enzyme required for neutralizing superoxide radicals, shows an age-dependent decline in the TM.5 Finally, three studies found statistically higher levels of oxidative DNA damage to both nuclear and mitochondrial DNA in TM tissue taken from glaucoma versus healthy human eyes.6–8 Prior studies on human TM specimens have shown that glaucomatous tissue shows significantly fewer TM cells than age-matched tissue from normal eyes.9 Taken together, cumulative oxidative damage to TM cells by chronic exposure to H2O2 leading to cell death may be one component of the age-dependent increase in incidence of POAG.
In previous work, multi-photon microscopy was used to analyze the real-time reduced nicotinamide adenine dinucleotide phosphate (NADPH) levels in human cultured TM cells exposed to different concentrations of H2O2. Findings show that concentrations of H2O2 of 20 mM and above cause a significant dose- and time-dependent decrease in NADPH fluorescence over a 30-minute exposure.10 NADPH is a cofactor necessary for regenerating levels of rGSH, which is itself a cofactor for the glutathione peroxidases that catalyze H2O2 neutralization. Finally, other studies show that the phenolic compound resveratrol reduces reactive oxygen species (ROS) and inhibits expression of inflammatory markers in TM cells exposed to H2O2.11 In the present study an investigation was undertaken to determine the ability of several antioxidants to prevent metabolic damage and cell death in cultured human TM cells in response to H2O2 insult.
Material and Methods
Assay and Cell Culture Reagents
Dulbecco's modified Eagle medium (DMEM), calcium and magnesium-free Dulbecco's phosphate buffered saline (DPBS), qualified fetal bovine serum (FBS), penicillin-streptomycin (100× solution), and recombinant human basic fibroblast growth factor (bFGF) were purchased from Invitrogen/Life Technologies (Grand Island, NY). The metabolic activity indicator 3-(4,5-dimethyl-2-thiazoyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) was purchased from Sigma Aldrich Corporation (St. Louis, MO). The ROS indicator 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) and the Live/Dead Viability/Cytotoxicity Kit for Mammalian Cells were purchased from Invitrogen/Life Technologies.
The antioxidants sodium ascorbate, rGSH, uric acid, ρ-coumaric acid, and resveratrol as well as H2O2 (30% solution) were purchased from Sigma Aldrich Corporation. 100× stock solutions of ascorbate (100 mM), rGSH (100 mM), urate (20 mM) and ρ-coumarate (100 mM), a phenolic compound found in cinnamon, were prepared in DMEM and, when necessary, the pH of the solutions were adjusted to 7.4 by addition of sodium hydroxide. A 500× stock solution of resveratrol (25 mM) was prepared in ethanol. Aliquots of the antioxidant stock solutions were stored at −80°C and used immediately after thawing.
Primary Cell Culture
Trabecular meshwork cells were isolated from porcine eyes (Visiontech Inc., Mesquite, TX) within 24 hours after death. After the cornea was removed with a razor blade the ciliary body, iris, and lens were removed using forceps. Under a stereo microscope, tissue strips containing the TM region were carefully dissected free with forceps. Individual TM cells were isolated by digesting the tissue strips in serum-free DMEM containing 5 mg/mL collagenase type IV (Worthington Biochemical Corporation, Lakewood, NJ) at 37°C for 20 to 30 minutes. TM cells were pelleted by centrifugation at 250 × g for 5 minutes and plated onto gelatin-coated dishes in DMEM containing 10% FBS, 10 ng/mL bFGF, penicillin, and streptomycin. Third passage porcine TM cells (approximately 3 × 104) were plated into each well of a gelatin coated 96-well plate. Fluorescent assays were plated in black opaque plates (Cellstar; Greiner Bio-One North America Inc., Monroe, NC), while absorbance-based assays were plated in clear plates (Greiner Bio-One North America Inc.).
Hydrogen Peroxide Treatment of TM Cells
Trabecular meshwork cells were assayed upon reaching confluence, usually 2 to 3 days post-plating. Antioxidant solutions were diluted in fresh culture media at the following final concentrations: 50 μM resveratrol, 1 mM ascorbate, 1 mM rGSH, 1 mM ρ-coumarate, or 0.2 mM urate. TM cells were incubated with 100 μL of antioxidant solutions at 37°C and 5% carbon dioxide (CO2) for 24 hours. After pretreatment, the antioxidant solutions were aspirated and replaced with fresh antioxidant solutions including a range of H2O2 (0–4 mM) and returned to the 37°C/5% CO2 incubator. After 24 hours, TM cells were assayed for cell viability and ROS. In one set of experiments, TM cells were pretreated with antioxidant solutions for 24 hours followed by a 24-hour exposure to a range of H2O2 (0–4 mM) in the absence of antioxidants.
TM Cell Viability/Metabolic Assays
Following exposure to H2O2, the mitochondrial activity of the TM cells was determined by MTT assay. After aspiration of media, TM cells were washed twice in DPBS to remove excess H2O2 that could interfere with the biological reduction of MTT. TM cells were then incubated in 100 μL of 0.5 mg/mL MTT dissolved in culture media for 1 hour at 37°C. The MTT media was aspirated and the purple formazan reaction product was solubilized by addition of 100 μL dimethyl sulfoxide. The absorbance of each sample was read at 540 nm in a Synergy 4 Multi-Mode Microplate Reader using the Gen5 Reader Control and Data Analysis Software (BioTek, Winooski, VT). Data were normalized to the MTT absorbance of control TM cells (100% live) and reported as the mean ± SD of n = 6 experiments.
TM Cell Live-Cell Assays
Following exposure to H2O2, the membrane integrity of TM cells was assayed by uptake and retention of the fluorescent calcium-binding dye calcein-AM. After aspiration of media, TM cells were washed twice in DPBS to remove excess H2O2. TM cells were then incubated in 100 μL of 2 μM calcein-AM prepared in DPBS for 20 minutes at room temperature. Calcein fluorescence (F528) was quantified using band-pass filters (excitation = 485 ± 20 nm, emission = 528 ± 20 nm) in a Synergy 4 Multi-Mode Microplate Reader using the Gen5 Reader Control and Data Analysis Software (BioTek). Fluorescent data were analyzed as outlined in the manufacturer's instructions. Briefly, all F528 fluorescence was corrected by first subtracting the F528 fluorescence from cells not treated with calcein, and then normalized to the corrected F528 fluorescence from control cells (100% live). Data for each treatment are reported as the mean ± SD of n = 3 experiments.
Assay of Reactive Oxygen Species in TM Cells
The ROS that accumulate in TM cells following exposure to H2O2 was assayed by conversion of the nonfluorescent H2DCFDA into a fluorescent oxidized form. TM cells were washed twice in DPBS to remove excess H2O2 and then incubated in 10 μM H2DCFDA in culture media for 30 minutes at 37°C and 5% CO2. TM cells were washed once in DPBS, and fluorescence was determined in a Synergy 4 Multi-Mode Microplate Reader using the Gen5 Reader Control and Data Analysis Software (BioTek) in single wavelength mode (493 nm excitation, 521 nm emission). Fluorescent data were first corrected by subtracting fluorescence from cells without H2DCFDA, and then reported as a percent of ROS fluorescence of control TM cells (0 mM H2O2). Data represent the mean ± SD of n = 3 experiments.
Statistical Analysis
Mean values for each concentration were analyzed by the Student's t-test (Excel; Microsoft, Redmond, WA); the level of significance was set at 0.05.
Results
Antioxidants Protect TM Cell Metabolism Against H2O2-Induced Injury
Cultured porcine TM cells were incubated with growth media containing H2O2 for 24 hours and mitochondrial enzymes activity was determined via MTT assays. No significant metabolic effect was seen with a 24-hour exposure to 0.5 mM H2O2 (106.2% of control) (Table 1). A significant reduction in TM cell mitochondrial function was determined to occur after exposure to concentrations of 1 mM H2O2 (72.3% of control) and above. Mitochondrial function was further reduced to near zero levels at both 2 mM H2O2 (2.5% of control) and 4 mM H2O2 (1.7%) exposures.
Table 1.
Co-Incubation of TM Cells with Antioxidants Protects Against Peroxide-Induced Metabolic Injury
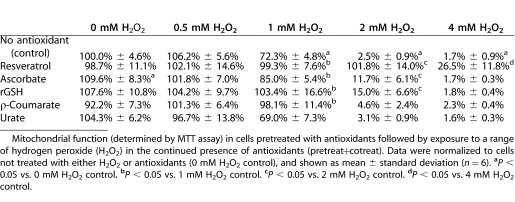
TM cells treated with 0.2 mM urate for 24 hours followed by H2O2 exposure in the continued presence of fresh urate (“pretreat+cotreat”) showed no differences in mitochondrial function compared to control cells not treated with urate (Table 1). Under pretreat+cotreat conditions, 1 mM ρ-coumarate improved mitochondrial function in TM cells exposed to 1 mM H2O2 (98.1% vs. 72.3%) but was not protective at higher concentrations of H2O2. With both 1 mM H2O2 (103.4% vs. 72.3%) and 2 mM H2O2 (15.0% vs. 2.5%) exposures, 1 mM rGSH was found to be protective.
Under pretreat+cotreat conditions, only the antioxidant ascorbate (1 mM) improved TM cell metabolism in cells not exposed to H2O2 (109.6% vs. 100%), likely due to the fact that ascorbate is a mitochondrial enzyme substrate. With 1 mM H2O2 (98.1% vs. 72.3%) and 2 mM H2O2 (11.7% vs. 2.5%) exposures, 1 mM ascorbate was also found to be protective. Finally, 50 μM resveratrol was determined to be the most powerful antioxidant tested in pretreat+cotreat conditions. Compared to H2O2-treated TM cells, resveratrol improved mitochondrial function at 1 mM H2O2 (99.3% vs. 72.3%), 2 mM H2O2 (101.8% vs. 2.5%), and 4 mM H2O2 (26.5% vs. 1.7%) exposures (Table 1).
Since many of the antioxidants were used at concentrations (0.2–1 mM) similar to the concentration of H2O2 (0.5–4 mM), it is hypothetically possible that these molecules neutralized the H2O2 in the growth media via a simple oxidation-reduction reaction. In order to test whether the protection shown in Table 1 was due to a biological change within the TM cells, cells were pretreated with antioxidants for 24 hours followed by H2O2 exposure for 24 hours without inclusion of antioxidants (“pretreat-only”).
As with pretreat-cotreat conditions, a significant reduction in TM cell mitochondrial function occurred after exposure to concentrations of 1 mM H2O2 (79.4% of control) and above (Table 2). Mitochondrial function was further reduced to near zero levels at both 2 mM H2O2 (2.5% of control) and 4 mM H2O2 (1.2%) exposures. Under pretreat-only conditions, both ascorbate (117.4%) and rGSH (122.0%) improved TM cell metabolism in cells not exposed to H2O2 (100%).
Table 2.
Pretreatment of TM Cells with Antioxidants Affords Lesser Protection Against H2O2-Induced Injury
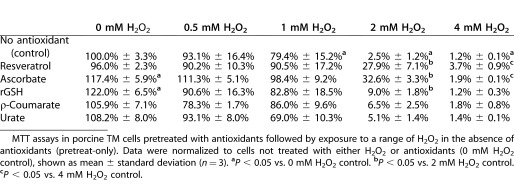
Similar to the results from pretreat+cotreat conditions, urate had no protective effect against subsequent H2O2 damage under pretreat-only conditions (Table 2). Overall, the data presented in Table 2 demonstrate that the metabolic protective effect of antioxidants under pretreat-only conditions is reduced compared to pretreat+cotreat conditions. Unlike pretreat+cotreat conditions, no antioxidant showed a statistically significant protective effect at 1 mM H2O2 exposure. Also, under pretreat-only conditions, ρ-coumarate did not show a statistical improvement of mitochondrial function in TM cells exposed to H2O2. rGSH had a small protective effect at 2 mM H2O2 (9.0% vs. 2.5%) exposure. Under pretreat-only conditions, ascorbate (32.6%) and resveratrol (27.9%) had larger protective effects on TM cell metabolism after 2 mM H2O2 (2.5%) exposure. Finally, ascorbate (3.7%) and resveratrol (1.9%) showed an extremely small but statistically significant protective effect after 4 mM H2O2 (1.2%) exposure.
Antioxidants Decrease H2O2-Induced TM Cell Death
Cultured porcine TM cells were incubated with growth media containing H2O2 for 24 hours and cell membrane integrity and cell viability were assayed using calcein. No statistically significant decrease in viability was seen with 1 mM H2O2 (94.9% of control) (Table 3). A significant reduction in TM cell viability was seen with 2 mM H2O2 (43.0% of control) and 4 mM H2O2 (6.7% of control) exposures.
Table 3.
Co-Incubation of TM Cells with Antioxidants Also Protects Against H2O2-Induced Cell Death
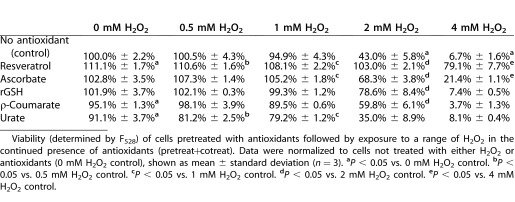
Viability assays were performed in TM cells treated with the pretreat+cotreat conditions outlined above for the metabolic assays shown in Table 1. While ascorbate increased metabolic activity in cells not exposed to H2O2 (Table 1), it showed no increase in TM cell viability as assayed by F528 (102.8% vs. 100%). This supported the hypothesis that this was a mitochondrial and not a cell proliferation effect. However, it should be noted that resveratrol increased calcium fluorescence in TM cells not exposed to H2O2 (111.1% vs. 100.0%).
While urate had no effect on H2O2-induced metabolic damage, under the same pretreat+cotreat conditions urate was shown to actually reduce TM cell viability. Under pretreat+cotreat conditions, rGSH increased TM cell viability in cells exposed to 2 mM H2O2 (78.6% vs. 43.0%). While ρ-coumarate was metabolically protective only at 1 mM H2O2 (Table 1), ρ-coumarate was shown to increase TM cell viability with 2 mM H2O2 (78.6% vs. 43.0%) exposure.
Ascorbate increased cell viability in TM cells exposed to 2 mM H2O2 (68.3% vs. 43.0%), and 4 mM H2O2 (21.4% vs. 6.7%). Again, resveratrol was determined to be the most powerful antioxidant tested in pretreat+cotreat conditions. Compared to H2O2-treated TM cells, resveratrol increased TM cell viability at 2 mM H2O2 (103.0% vs. 43.0%) and 4 mM H2O2 (79.1% vs. 6.7%) exposures (Table 3).
Co-Incubation of TM Cells With Antioxidants Decrease Intracellular Reactive Oxygen Species
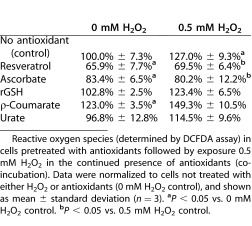
In order to determine a possible mechanism of action for the protective effect of antioxidants to H2O2 exposure, the intracellular reactive oxygen species (determined by DCFDA assay) in TM cells was determined. Cells were pretreated with antioxidants for 24 hours followed by 24-hour exposure to a sublethal concentration of H2O2 (0.5 mM H2O2) in the continued presence of antioxidants (pretreat+cotreat conditions). Higher levels of H2O2 were not tested since the death of TM cells complicated the analysis of ROS levels.
In TM cells exposed to 0 mM H2O2, resveratrol and ascorbate significantly reduced ROS to 66.9% and 83.4%, respectively, of the levels seen in control cells (100%). ρ-coumarate, on the other hand, significantly increased the levels of ROS to 123.0% of the levels seen in control cells (Table 4). In TM cells exposed to 0.5 mM H2O2, levels of ROS in resveratrol and ascorbate remained essentially unchanged (Table 4), while the levels of ROS in TM cells not treated with antioxidants increased to 127.0%. Treatment of TM cells with any of the other antioxidants failed to prevent the rise of intracellular ROS upon exposure to 0.5 mM H2O2.
Table 4.
Co-Incubation of TM Cells with Antioxidants Reduces Radical Oxygen Species in TM Cells Exposed to a Sub-Lethal Dose of H2O2
Discussion
The TM region of the eye is exposed to a constant low level of oxidative insult. The ability of TM cells to counter oxidative damage is critical to their survival since H2O2 in the human aqueous humor (AH) may be as high as 25 μM.12 H2O2 is believed to be constantly generated through a light-dependent reaction with iris melanin.13,14 In the current study, previous H2O2 studies10 were expanded upon using a longer exposure time (24 hours vs. 30 minutes) and lower concentrations of H2O2 (<4 mM vs. >20 mM) in order to mimic the chronic low-level oxidative stress that TM cells experience in vivo. The studies were performed in low-passage primary cultures of TM cells to eliminate the complication of analyzing data from TM cells with different metabolic capabilities, as is noted with older TM cultures.15 Antioxidants were chosen to include both metabolic (intrinsic) agents as well as dietary agents. For the latter, coumaric acid was selected because of its ability to lessen UV damage in cornea, and resveratrol because of its anti-inflammatory effects in TM cells. For the former, ascorbate, urate, and GSH were chosen because these antioxidants had been detected in the AH.
For metabolic agents, biological molecules with intrinsic antioxidant or chemical-reducing abilities that had been detected in the AH of the eye were selected. The TM region of the eye contains high intracellular levels of rGSH,16 a cofactor that supplies reducing equivalents to glutathione peroxidase (H2O2 neutralizing enzymes). Submillimolar levels of rGSH have been reported in the AH, and these are reduced in patients with pseudoexfoliation syndrome.17 Ascorbate (vitamin C) is a mitochondrial enzyme substrate that can act as an antioxidant either indirectly by generating reducing equivalents (NADPH) via the citric acid cycle or directly through the action of ascorbate peroxidases. Ascorbate levels in the AH range between 0.86 and 1.3 mM.14,18,19 Like ascorbate, urate is a powerful antioxidant and reducing agent detectable in the AH in a range between 0.15-0.21 mM.18 However, urate (but not ascorbate) is higher in the AH of patients with poor trabeculectomy outcomes,18 and serum hyperuricemia is noted in patients with POAG.20 Ascorbate (1 mM) and urate (0.2 mM) were used at their AH concentrations, while rGSH (1 mM), because of the question of permeability, was used at a higher concentration (1 mM) than its AH concentration (∼0.15 mM).
For the selection of exogenous agents, antioxidant molecules were selected that had been shown to be beneficial to countering oxidative stress in ocular tissues. ρ-coumarate has been shown to lessen ultraviolet (UV) damage to the cornea when applied topically to rabbits.21 Resveratrol, a phenolic compound from grapes, has been shown to reduce ROS and inhibit expression of inflammatory markers in TM cells exposed to H2O2.11 Extensive dose-response curves were not performed for coumarate/resveratrol, but the concentrations used were verified to be nontoxic.
To determine the extent of H2O2 damage on TM cells, both the calcein uptake to assay plasma membrane integrity (Table 3) and MTT (Tables 1 and 2) to assay mitochondrial function were used. Care was taken to wash cells thoroughly between H2O2 exposure and subsequent assays. The results demonstrated rescue of H2O2-induced TM injury by resveratrol, ascorbate, and rGSH (in that rank order of effectiveness). All three compounds mitigated the H2O2-induced loss of mitochondrial activity as well as cell death. Ascorbate and resveratrol were also effective at reducing production of intracellular oxygen species, a metric for oxidative damage. The effectiveness of these compounds was greatest when administered during H2O2 challenge. However, pretreating cultured TM cells with these antioxidants also was effective at preventing subsequent H2O2-induced changes, which suggested that these compounds had a lasting metabolic or intracellular effect. The protective effect of rGSH resulted from three possible mechanisms. Either the organic ion transporting polypeptides (organic anion-transporting polypeptide [OATP]) or the multi-drug resistance-associated protein transporter have been hypothesized to import rGSH.22 OATP subtypes are present in endothelial cells of the human TM and Schlemm canal where they transport prostaglandins from the AH.23 Secondly, rGSH could act extracellularly either by directly neutralizing hydrogen H2O2 or by reducing disulfide bonds in transmembrane proteins that had been oxidized. Alternatively, rGSH could have reduced another metabolite present in the media (e.g., lactate to pyruvate) that could itself have been easily transported in the TM cells.
Further investigation of these compounds is needed to determine their long-term effectiveness at reducing H2O2 damage in TM cells and to better understand how these in vitro findings could translate to in vivo experiments. Possible studies would involve showing that application of antioxidants can help ameliorate disease progression in animal models of glaucoma and macular degeneration (and other eye diseases linked with oxidative stress). The use of antioxidants to treat eye diseases, as illustrated by ρ-coumarate's ability to lessen UV damage to the cornea when applied topically,21 indicated a possible dosing mechanism that bypassed issues with absorption and bioavailability of oral dosing. Furthermore, coupling these agents with extended release vehicles for intraocular injection could result in a consistent antioxidant influence on local free radical formation leading to enhanced therapeutic outcomes.
Acknowledgments
Disclosure: D.A. Ammar, None; K.M. Hamweyah, None; M.Y. Kahook, None
Footnotes
david.ammar@ucdenver.edu
khamweyah@alfaisal.edu
malik.kahook@gmail.com
Mitochondrial function (determined by MTT assay) in cells pretreated with antioxidants followed by exposure to a range of hydrogen peroxide (H2O2) in the continued presence of antioxidants (pretreat+cotreat). Data were normalized to cells not treated with either H2O2 or antioxidants (0 mM H2O2 control), and shown as mean ± standard deviation (n = 6). aP < 0.05 vs. 0 mM H2O2 control. bP < 0.05 vs. 1 mM H2O2 control. cP < 0.05 vs. 2 mM H2O2 control. dP < 0.05 vs. 4 mM H2O2 control.
MTT assays in porcine TM cells pretreated with antioxidants followed by exposure to a range of H2O2 in the absence of antioxidants (pretreat-only). Data were normalized to cells not treated with either H2O2 or antioxidants (0 mM H2O2 control), shown as mean ± standard deviation (n = 3). aP < 0.05 vs. 0 mM H2O2 control. bP < 0.05 vs. 2 mM H2O2 control. cP < 0.05 vs. 4 mM H2O2 control.
Viability (determined by F528) of cells pretreated with antioxidants followed by exposure to a range of H2O2 in the continued presence of antioxidants (pretreat+cotreat). Data were normalized to cells not treated with either H2O2 or antioxidants (0 mM H2O2 control), shown as mean ± standard deviation (n = 3). aP < 0.05 vs. 0 mM H2O2 control. bP < 0.05 vs. 0.5 mM H2O2 control. cP < 0.05 vs. 1 mM H2O2 control. dP < 0.05 vs. 2 mM H2O2 control. eP < 0.05 vs. 4 mM H2O2 control.
Reactive oxygen species (determined by DCFDA assay) in cells pretreated with antioxidants followed by exposure 0.5 mM H2O2 in the continued presence of antioxidants (co-incubation). Data were normalized to cells not treated with either H2O2 or antioxidants (0 mM H2O2 control), and shown as mean ± standard deviation (n = 3). aP < 0.05 vs. 0 mM H2O2 control. bP < 0.05 vs. 0.5 mM H2O2 control.
References
- 1.Polansky JR, Fauss DJ, Chen P, et al. Cellular pharmacology and molecular biology of the trabecular meshwork inducible glucocorticoid response gene product. Ophthalmologica. 1997;211:126–139. doi: 10.1159/000310780. [DOI] [PubMed] [Google Scholar]
- 2.Zhou L, Li Y, Yue BY. Oxidative stress affects cytoskeletal structure and cell-matrix interactions in cells from an ocular tissue: the trabecular meshwork. J Cell Physiol. 1999;180:182–189. doi: 10.1002/(SICI)1097-4652(199908)180:2<182::AID-JCP6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 3.Gherghel D, Griffiths HR, Hilton EJ, Cunliffe IA, Hosking SL. Systemic reduction in glutathione levels occurs in patients with primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2005;46:877–883. doi: 10.1167/iovs.04-0777. Available at: http://www.iovs.org/content/46/3/877. [DOI] [PubMed] [Google Scholar]
- 4.Li G, Luna C, Liton PB, Navarro I, Epstein DL, Gonzalez P. Sustained stress response after oxidative stress in trabecular meshwork cells. Mol Vis. 2007;13:2282–2288. Available at: http://www.iovs.org/content/37/9/1849. [PMC free article] [PubMed] [Google Scholar]
- 5.De La Paz MA, Epstein DL. Effect of age on superoxide dismutase activity of human trabecular meshwork. Invest Ophthalmol Vis Sci. 1996;37:1849–1853. Available at: http://www.iovs.org/content/37/9/1849. [PubMed] [Google Scholar]
- 6.Izzotti A, Sacca SC, Cartiglia C, De Flora S. Oxidative deoxyribonucleic acid damage in the eyes of glaucoma patients. Am J Med. 2003;114:638–646. doi: 10.1016/s0002-9343(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 7.Sacca SC, Pascotto A, Camicione P, Capris P, Izzotti A. Oxidative DNA damage in the human trabecular meshwork: clinical correlation in patients with primary open-angle glaucoma. Arch Ophthalmol. 2005;123:458–463. doi: 10.1001/archopht.123.4.458. [DOI] [PubMed] [Google Scholar]
- 8.Izzotti A, Sacca SC, Longobardi M, Cartiglia C. Mitochondrial damage in the trabecular meshwork of patients with glaucoma. Arch Ophthalmol. 2010;128:724–730. doi: 10.1001/archophthalmol.2010.87. [DOI] [PubMed] [Google Scholar]
- 9.Alvarado J, Murphy C, Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 1984;91:564–579. doi: 10.1016/s0161-6420(84)34248-8. [DOI] [PubMed] [Google Scholar]
- 10.Masihzadeh O, Ammar DA, Lei TC, Gibson EA, Kahook MY. Real-time measurements of nicotinamide adenine dinucleotide in live human trabecular meshwork cells: effects of acute oxidative stress. Exp Eye Res. 2011;93:316–320. doi: 10.1016/j.exer.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luna C, Li G, Liton PB, et al. Resveratrol prevents the expression of glaucoma markers induced by chronic oxidative stress in trabecular meshwork cells. Food Chem Toxicol. 2009;47:198–204. doi: 10.1016/j.fct.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spector A, Garner WH. Hydrogen peroxide and human cataract. Exp Eye Res. 1981;33:673–681. doi: 10.1016/s0014-4835(81)80107-8. [DOI] [PubMed] [Google Scholar]
- 13.Pirie A. A light-calalyses reaction in the aqueous humor of the eye. Nature. 1965;205:500–501. doi: 10.1038/205500a0. [DOI] [PubMed] [Google Scholar]
- 14.Wielgus AR, Sarna T. Ascorbate enhances photogeneration of hydrogen peroxide mediated by the iris melanin. Photochem Photobiol. 2008;84:683–691. doi: 10.1111/j.1751-1097.2008.00341.x. [DOI] [PubMed] [Google Scholar]
- 15.Sacca SC, La Maestra S, Micale RT, et al. Ability of dorzolamide hydrochloride and timolol maleate to target mitochondria in glaucoma therapy. Arch Ophthalmol. 2011;129:48–55. doi: 10.1001/archophthalmol.2010.324. [DOI] [PubMed] [Google Scholar]
- 16.Kahn MG, Giblin FJ, Epstein DL. Glutathione in calf trabecular meshwork and its relation to aqueous humor outflow facility. Invest Ophthalmol Vis Sci. 1983;24:1283–1287. Available at: http://www.iovs.org/content/24/9/1283. [PubMed] [Google Scholar]
- 17.Gartaganis SP, Georgakopoulos CD, Patsoukis NE, Gotsis SS, Gartaganis VS, Georgiou CD. Glutathione and lipid peroxide changes in pseudoexfoliation syndrome. Curr Eye Res. 2005;30:647–651. doi: 10.1080/02713680590968367. [DOI] [PubMed] [Google Scholar]
- 18.Jampel HD, Moon JI, Quigley HA, Barron Y, Lam KW. Aqueous humor uric acid and ascorbic acid concentrations and outcome of trabeculectomy. Arch Ophthalmol. 1998;116:281–285. doi: 10.1001/archopht.116.3.281. [DOI] [PubMed] [Google Scholar]
- 19.Ringvold A, Anderssen E, Kjonniksen I. Distribution of ascorbate in the anterior bovine eye. Invest Ophthalmol Vis Sci. 2000;41:20–23. Available at: http://www.iovs.org/content/41/1/20. [PubMed] [Google Scholar]
- 20.Elisaf M, Kitsos G, Bairaktari E, Kalaitzidis R, Kalogeropoulos C, Psilas K. Metabolic abnormalities in patients with primary open-angle glaucoma. Acta Ophthalmol Scand. 2001;79:129–132. doi: 10.1034/j.1600-0420.2001.079002129.x. [DOI] [PubMed] [Google Scholar]
- 21.Lodovici M, Caldini S, Morbidelli L, Akpan V, Ziche M, Dolara P. Protective effect of 4-coumaric acid from UVB ray damage in the rabbit eye. Toxicology. 2009;255:1–5. doi: 10.1016/j.tox.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 22.Ballatori N, Hammond CL, Cunningham JB, Krance SM, Marchan R. Molecular mechanisms of reduced glutathione transport: role of the MRP/CFTR/ABCC and OATP/SLC21A families of membrane proteins. Toxicol Appl Pharmacol. 2005;204:238–255. doi: 10.1016/j.taap.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Kraft ME, Glaeser H, Mandery K, et al. The prostaglandin transporter OATP2A1 is expressed in human ocular tissues and transports the antiglaucoma prostanoid latanoprost. Invest Ophthalmol Vis Sci. 2010;51:2504–2511. doi: 10.1167/iovs.09-4290. Available at: http://www.iovs.org/content/51/5/2504. [DOI] [PubMed] [Google Scholar]


