Abstract
Purpose
Depression, anxiety, and poor sleep are associated with increased risks of cardiovascular diseases. Previous studies have demonstrated the relationship between negative emotion and retinal microvascular changes among adults, yet no study has been done in pregnant women so far. This study aims to examine the association of antenatal mental health and retinal vascular caliber among Asian pregnant women.
Methods
Nine hundred and fifty two Asian pregnant women aged 18 to 46 years were included in this study, who were recruited from two Singapore cohort studies, the Growing Up in Singapore Towards Healthy Outcomes (GUSTO) study and the In Vitro Fertilization (IVF) study conducted from 2009 onwards. A total of 835 Asian pregnant women underwent retinal photography at 26 weeks follow up, of whom 800 had gradable photographs. Symptoms of depression, anxiety, and sleep quality were assessed with self-administered questionnaires.
Results
In multiple linear regression models adjusted for age, ethnicity, household income, pregnancy outcome history, means of conception, hypertension history, diabetes history, cigarette smoking history, mean arterial blood pressure, body mass index, and spherical equivalent, each standard deviation (SD) increase in the Edinburgh Postnatal Depression Scale (EPDS) (4.49 scores) and in the Pittsburgh Sleep Quality Index (PSQI) (2.90 scores) was associated with a 0.80 μm (P = 0.03) and a 1.22 μm (P = 0.01) widening in retinal arteriolar caliber, respectively.
Conclusions
Our study demonstrates relationships of antenatal depressive symptoms and poor sleep quality with retinal arteriolar widening in pregnant women.
Translational Relevance
We speculate that this might possibly indicate an effect of antenatal depression and poor sleep on the microcirculation during pregnancy.
Keywords: antenatal mental health, retinal vascular caliber, pregnancy, antenatal depression, poor sleep quality
Introduction
Depression, anxiety, and poor sleep have been well established to be associated with cardiovascular diseases both in general population and clinical patients with obesity or coronary heart disease.1–5 Growing evidence also shows that depression and anxiety during pregnancy is related to adverse maternal outcome such as pre-eclampsia6 by possibly sharing similar pathophysiology with cardiovascular disease (CVD).7 Since antenatal depression is commonly accompanied by anxiety and poor sleep8,9 and it affects up to 12.8% of pregnant women10 in the Caucasian population and 12.2% in the Singapore population11 during the second trimester, antenatal mental health has become an important public health issue for its influence on further vascular complications. Therefore, it is necessary to study the precise pathophysiological mechanisms to enlighten the association of antenatal depression, anxiety, and poor sleep with hemodynamic circulation.
The retinal circulation is a “window” to the human microvascular system.12 Many studies have shown that retinal vascular changes (e.g., retinal arteriolar narrowing and venular widening) are associated with vascular risk such as hypertension 13 and diabetes,14 as well as coronary heart disease,15,16 cardiovascular mortality,17 and stroke.18 In the general population, several studies have reported a relationship between psychosocial risk factors and retinal vascular signs, suggesting the underlying influence of negative emotion on microvascular process.19–23 However, the findings of negative emotion and retinal vasculature are inconsistent. For example, Jensen et al. reported a significant relationship between lack of emotional support, increased trait anxiety, more depressive symptoms,21 and retinopathy, whereas Kim et al. reported a null relationship between depressive mood and retinopathy.22 Regarding retinal vascular caliber, Jensen et al. reported patients with higher trait anger and anxiety tended to have narrower retinal arteriolar caliber than those with lower scores in both index,21 whereas Nguyen et al. found that patients with type 2 diabetes and depression tended to have wider retinal arteriolar caliber than the control group and patients with type 2 diabetes, but without depression.23
To our knowledge, there are no studies on the relationship between symptoms of depression, anxiety, and poor sleep and retinal vascular caliber in pregnant women. Therefore, our study aims to investigate the association between antenatal mental health and retinal vascular caliber at 26 week gestation among a large number of multi-ethnic Asian pregnant women.
Methods
Study Population
The Growing Up in Singapore Towards Healthy Outcomes (GUSTO) birth cohort (natural conceiving) and the In Vitro Fertilization (IVF) cohort study (conceiving assisted reproductive techniques) consisted of 1248 pregnant women recruited from 2009 to 2010. The inclusion criteria were same for both studies: Singapore citizens or Singapore permanent residents of age 18 years and above, attending the first trimester antenatal dating ultrasound scan clinic at the two major public maternity units of the National University Hospital (NUH) and Kedang Kerbau Women's and Children's Hospital (KKH), intending to eventually deliver in the above named hospitals and to reside in Singapore for the next 5 years. For this study, we only analyzed participants from KKH due to logistic constraints. Among the 1248 total participants at baseline who agreed to join the study, 952 were from KKH, of which 835 participants (response rate = 87.71%) underwent retinal photography. A total of 800 out of 835 pregnant women had gradable retinal photographs (95.81%) taken at the 26 weeks follow up.
Participants were recruited by research coordinators, who received training in conducting the interview and consent process. After a detailed explanation, written informed consent of our retinal sub study was obtained at 26 weeks follow up. This study was approved by both Singhealth Centralized institutional review board and the National Health Group's Domain Specific Review Board and was conducted according to the tenets of the Declaration of Helsinki.
Evaluation of Antenatal Mental Health
Symptoms of antenatal depression were measured with the Edinburgh Postnatal Depression Scale (EPDS).24 Symptoms of antenatal anxiety were measured with the State-Trait Anxiety Inventory (STAI),25 only the state anxiety subscale was used for the analyses. Antenatal sleep quality was measured with the Pittsburg Sleeping Quality Index (PSQI).26 All three assessments were taken at the 26 weeks visit. The EPDS is a widely used, self-administered 10 item screening method for depression in the postnatal period. It has proven to be reliable and sensitive in detecting perinatal depression and has also been used to screen antenatal depression in pregnant women.24 Based on Spitzer's Research Diagnostic Criteria, a cut off of 12/13 and 14/15 is generally used as its prediction on possible postnatal depression and possible antenatal depression for most women, respectively.27
The STAI consists of 40 questions with two subscales, state-anxiety and trait-anxiety, and each summated separately. It is a widely used and accepted measure for anxiety in adults. It differentiates between temporary condition of “state anxiety” (a temporary acute anxiety state) and the more general and long standing quality of “trait anxiety” (a propensity for general anxiety).25 In our study, STAI raw scorings and categorized scores were analyzed. Cut off for high STAI score was determined by top the 75 percentile suggested by Nasreen et al.28 and Teixeira et al.29
The validated PSQI consists of 19 self-rated questions to assess a wide variety of factors relating to sleep quality and the frequency and severity of specific sleep-related problems. The PSQI has seven subscales each of a score of 0 to 3, which together adds up to a total PSQI score with a range of 0 to 21.26 Participants with PSQI total score of 6 and above are considered as poor sleep quality.26
Retinal Photography and Measurements of Retinal Vascular Caliber
Nondilated, right eye digital retinal photographs were taken by using a Canon 45° nonmydriatic retinal camera (Model CR-1 Mark II with a 40D SLR digital retinal camera backing; Canon Inc., Tokyo, Japan). Retinal fundus images centered on the optic disc were used for the measurement of retinal vascular parameters according to standard protocols as described in previous reports in adults and children.17,30,31 We used a newly developed semi-automated computer–assisted program (Singapore I Vessel Assessment [SIVA], version 3.0; National University of Singapore, Singapore) to quantitatively measure the retinal vascular parameters 0.5- to 2.0-disc diameters away from the optic disc margin (Fig.). All visible vessels larger than 25 μm crossing through this zone were measured. Estimates of the average diameters of arterioles and venules were calculated based on the revised Knudtson-Parr-Hubbard formula and summarized as central retinal arteriolar caliber and central retinal venular caliber.32 A single grader was masked to measure the retinal vascular parameters using SIVA. Intragrader reliability was assessed in 90 randomly selected retinal photographs from the same pool, and the intraclass correlation coefficient was 0.942 for retinal arteriolar caliber and 0.965 for retinal venular caliber, respectively.
Figure. .
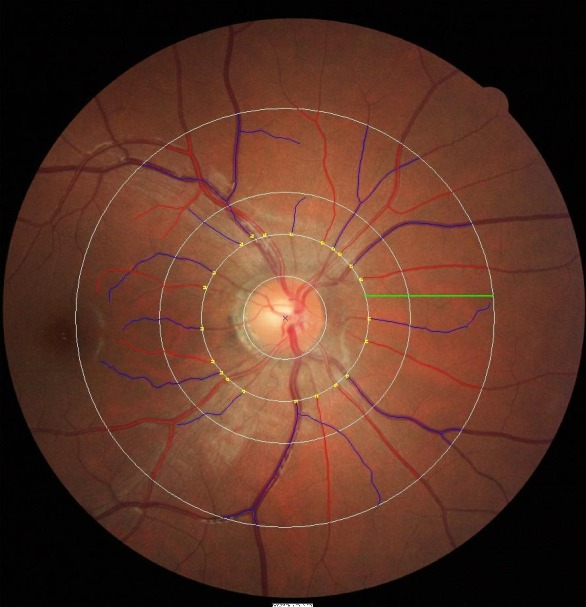
Retinal vessel measurements are performed 0.5- to 2.0-disc diameters away from the optic disc margin (zone C) by SIVA software, shown as a green line in the Figure. Estimates of central retinal arteriolar equivalent and central retinal venular equivalent, which represented the average diameters of retinal arterioles and retinal venules, were calculated by the revised Knudtson-Parr-Hubbard formula. Arterioles are marked in red and venules are marked in blue.
Other Measurements and Covariates
Blood pressure was measured during the clinic visit with a standard protocol, using the automatic Omron sphygmomanoter (Omron HEM 705 LP; Omron Healthcare Inc., Lake Forest, IL) after 5 minutes of rest.22 Three separate measurements were taken and their average was calculated. Mean arterial blood pressure (MABP) equals diastolic pressure minus one third of the difference between the systolic and diastolic pressures (MABP = DBP + 1/3 [P systolic − P diastolic]).
Standing height was measured by SECA model 213 (Seca, Hamburg, Germany), while weight was measured by SECA model 803 according to standard protocols.31 Both measurements were separately taken with bare feet two times. If the first two measurements differed by 1.0 cm or 200 g for height or weight, a third measurement was taken for the average calculation. Body mass index (BMI) was calculated as weight divided by the height squared (kilograms per meter squared).
Autorefraction was performed using a Canon Autorefractor RK-F1 (Canon Inc.), where the average from five consecutive readings of sphere and cylinder were obtained. Readings were considered acceptable if the difference between the lowest and highest reading was 0.25 diopters or less. Spherical equivalent was calculated as the sphere plus half of negative cylinder.
Questions on sociodemographic information were completed by parents in either English, Chinese, Malay, or Tamil questionnaires. Household income was classified into five categories as follows: (1) Singapore dollar (SGD) 0 to 999 per month, (2) SGD 1,000 to 1,999 per month, (3) SGD 2,000 to 3,999 per month, (4) SGD 4,000–5,999 per month, and (5) More than or equal to SGD 6,000 per month. Pregnancy outcome history was classified into two categories as follows: (1) No live birth given before, and (2) at least one live birth given. Ethnicity, life style (e.g., alcohol intake and cigarette smoking), and past medical history (e.g., hypertension, diabetes, and depression) were obtained from the clinic interview.
Statistical Analysis
Independent variables of interests as EPDS, STAI, and PSDI scores, were all analyzed continuously and categorized into quintiles. EPDS and PSDI scores were further classified into two or four groups by the respective clinical cut offs. Dependent variables as retinal arteriolar and venular caliber were estimated based on two models: model 1: age and ethnicity-adjusted; and model 2: multivariate-adjusted including variables in model 1, and later adjusted for household income, pregnancy outcome history, means of conception, hypertension history, diabetes history, cigarette smoking history, MABP, BMI, and spherical equivalent. Backward stepwise modeling was conducted to determine the best-fitting and most parsimonious model. Considering a history of infertility and the stress of fertility treatment would be expected to increase dysphoric mood and disturb sleep, we, therefore, treated means of conception as one of the possible confounders in current analysis. Test of trend was determined by treating quintiles and clinical categories of EPDS, STAI, and PSQI as continuous variables in ordinal scale. Multiple regression models were used to estimate the changes in retinal vascular caliber with every standard deviation (SD) increase in EPDS/STAI/PSQI scores.
Potential interactions of EPDS/STAI/PSQI scores by ethnicity, EPDS/STAI/PSQI scores by household income, EPDS/STAI/ PSQI scores by means of conception, EPDS/STAI/PSQI scores by cigarette smoking history, EPDS/STAI/PSQI scores by MABP, and EPDS/STAI/PSQI scores by BMI were examined in stratified analyses and as interaction terms in the models. A significant P value (two-tailed) was defined as less than 0.05. All statistical analyses were performed using PASW 19.0 (SPSS Inc., Chicago, IL).
Results
Table 1a presents the baseline characteristics of pregnant women with and without gradable retinal photographs. Overall, there were no significant differences between these two groups. Table 1b compares the means of major variables between pregnant women with normal conception and those assisted with IVF productive techniques. Again, there was no difference found across items representing antenatal mental health status or retinal vascular caliber.
Table 1a. .
Comparison of Basic Characteristics between the Group with Retinal Photographs Taken and the Group without Retinal Photographs Taken among GUSTO Participants Attending KKH Clinic
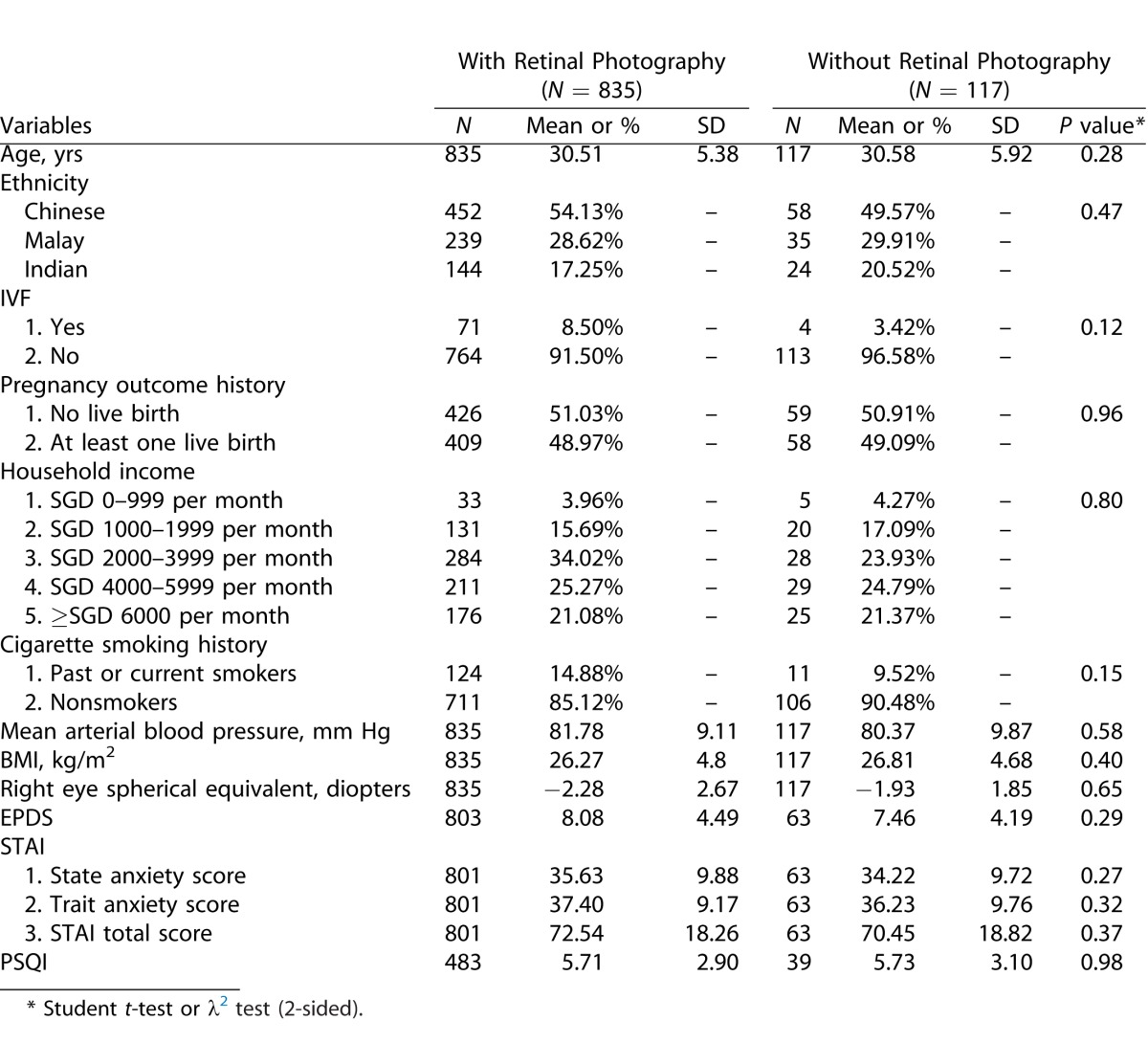
Table 1b. .
Comparison of Major Variables between Participants with Normal Conception and Participants Assisted with Productive Techniques in KKH Clinic

Table 2 shows the association of depression scores by EPDS with retinal vascular caliber. In multivariate model, each SD increase in EPDS score (4.49 scores) was significantly associated with a 0.80-μm larger retinal arteriolar caliber (P = 0.03). A similar direction was found for the association between higher quintiles of EPDS total depression score and retinal arteriolar caliber (P trend = 0.03). Given the higher value of correlation coefficient (R2), we used a cut off of 14 as possible symptoms of antenatal depression in our participants. Subjects who were classified into doctor referral depression group showed a significant wider retinal arteriolar (123.91 vs. 121.67 μm; P = 0.04) than those classified into non doctor referral depression group. Furthermore, higher EPDS total depression score was marginally associated with retinal venular widening both in linear regression model and test of trend. However, there was no significant correlation between the STAI total scale and retinal arteriolar (P = 0.32) or venular caliber (P = 0.34).
Table 2. .
Multivariate Analysis of Association between Depression Scores and Retinal Vascular Caliber in GUSTO Participants with Retinal Photography Taken during a 26 Week Visit
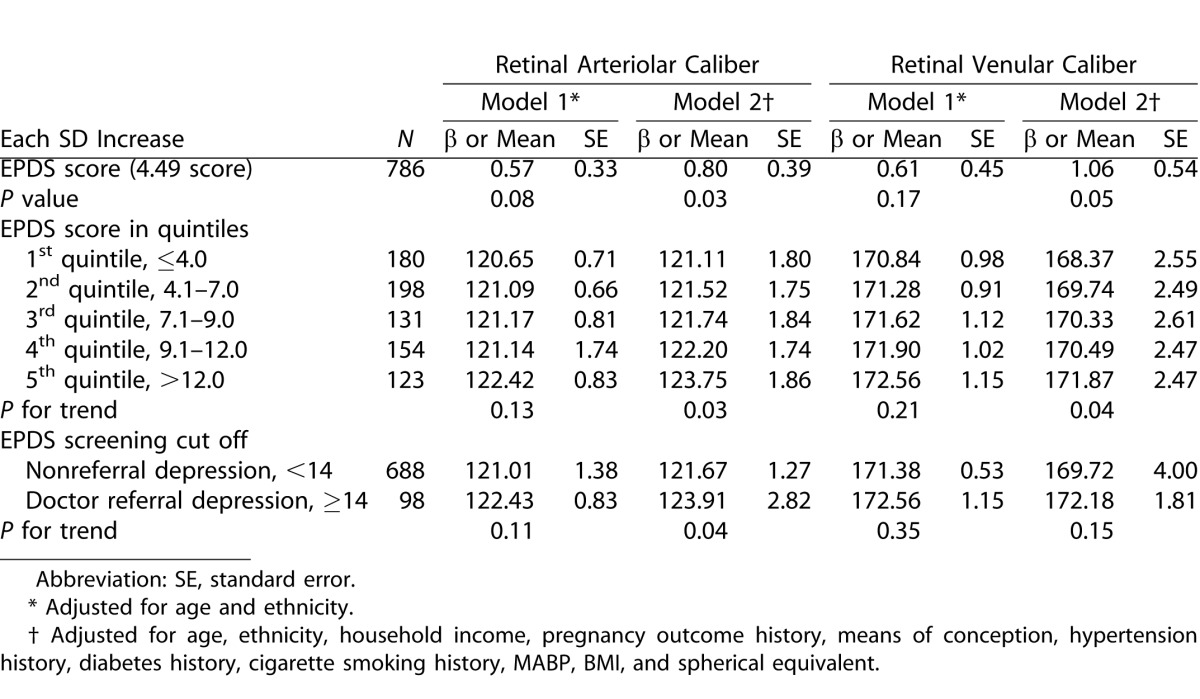
Table 3 showed the multivariate analysis of sleep quality and retinal vascular caliber. Each SD increase in PSQI score (2.90 scores) was associated with a 1.22-μm larger retinal arteriolar caliber (P = 0.01). A significant association was found in participants with higher PSQI total score quintile and retinal arteriolar widening (P trend = 0.02). Compared with participants clinically classified into normal sleeping quality, those clinically classified into abnormal sleeping quality had a wider retinal arteriolar caliber (125.23 vs. 122.88 μm, P trend = 0.01) and a marginally larger retinal venular caliber (172.31 vs. 174.80 μm, P trend = 0.05). There was no association found between PSQI score and retinal venular caliber.
Table 3. .
Multivariate Analysis between Sleeping Quality Index and Retinal Vascular Caliber in GUSTO Participants with Retinal Photography Taken during a 26 Week Visit

Finally, we evaluated the relationship of EPDS/STAI/PSQI scores with retinal vascular caliber by evaluating the potential effect modifiers. No significant interactions were found in EPDS/STAI/PSQI scores by ethnicity, EPDS/STAI/PSQI scores by household income, EPDS/STAI/ PSQI scores by means of conception, EPDS/STAI/PSQI scores by cigarette smoking history, EPDS/STAI/PSQI scores by MABP, or EPDS/STAI/PSQI scores by BMI.
Discussion
Our study showed that antenatal depressive symptoms and poor sleep quality were significantly associated with wider retinal arteriolar caliber, but not with retinal venular caliber in Asian pregnant women. Antenatal anxious symptoms were not associated with retinal vascular caliber.
Thus far, several previous studies have reported inconsistent results. Nguyen et al. found a trend of widening retinal arteriolar caliber across the control group, the group of type 2 diabetic patients without depression and the group of type 2 diabetic patients with major depression (P trend = 0.02),23 while Cheung et al. reported that “vital exhaustion”, which was measured by Maastricht questionnaire, to capture symptoms of unusual fatigue and feelings of rejection, was associated with generalized retinal venular widening.19 Two other cross-sectional studies also reported an association between retinopathy signs with depressive moods.21,22 However, prospective data from the Rotterdam Study consisting of 3,605 participants who were followed for an average 9.0 years, reported that retinal vascular calibers were associated neither with incident depressive symptoms nor depressive syndrome.20
We have previously reported that elevated blood pressure and maternal obesity during pregnancy were associated with abnormal retinal vascular signs, such as retinal arteriolar narrowing, retinal venular widening, and also accompanied with other changes on retinal arteriolar fractal dimension, retinal arteriolar branching angle, and retinal venular curvature tortuosity in the same cohort.33,34 In the current study, we provided new data on antenatal depressive symptoms and maternal microcirculation. This study focused on antenatal depression, which refers to both major and minor episodes during pregnancy.35 Antenatal depression is reported to be most common in the second trimester of the pregnancy.36 Several mechanisms may hypothetically underlie the associations between mental problems during pregnancy and wider retinal arteriolar caliber, including inflammatory response and endothelial dysfunction. First, Gold et al. showed that hypercortisolism in patients with major depression could contribute to increases in visceral fat mass, portal and peripheral free fatty acids, which further promotes endothelial inflammation in terms of higher level of tumor necrosis factor α (TNF-α), interleukin 6 (IL-6), and C-reactive protein (CRP).8 In the early phase of an inflammatory response, hyperemia is often noted and it probably reflects an initial reaction of arterioles.37 Endothelial cells may be the main factor contributing to the early dilatory response of arterioles to inflammation, by means of producing more nitric oxide (NO) to further relax vascular smooth muscle.37 Second, impaired brachial artery flow-mediated dilation (FMD) and depletion of circulating CD34/KDR+ endothelial progenitor cells (EPC) has also been found to be associated with higher depression and anxiety scores in a recent study, examining the link between vascular disease and depression.38 Last, a number of mechanisms have been proposed for the pathophysiology of maternal depression by the hormones (e.g., estrogen and progesterone) involved in the pathway.35 Estrogen-mediated endothelial NO synthase (eNOS) upregulation modulates eNOS expression in the fetal pulmonary endothelium, optimizing the capacity for NO-mediated pulmonary vasodilation at birth.39 It might imply possible eNOS-mediated effects on maternal vasodilation due to the rapid increase of gestational estrogen. Despite the study by Mariak Z et al. reporting that estrogens have a great vasodilatation effect on retinal arterioles in healthy women,40 we are not able to further explain whether this association between antenatal depressive symptoms and retinal arteriolar widening is specific for pregnant women, due to the fact that we do not have non pregnant Singapore women as a control group to evaluate the baseline association between depression and retinal vascular caliber.
Although anxiety is often a comorbid disorder with depression during pregnancy, and we did not observe any significant associations between anxiety and retinal vascular caliber. This is in agreement with Chen et al.41 in Hong Kong Chinese, yet in disagreement with Jensen et al. in multi-ethnic Americans.21 The possible explanation for this might be due to difference in the nature of studied population such as age, ethnicities, duration of anxiety, and specific hormone level in vivo. Thus, it requires further studies to investigate the relationship between antenatal anxiety and retinal microvasculation.
As poor sleep is prevalent in patients with major depression with up to 90% of patients reporting frequent insomnia symptoms,4 it is not surprising to find that sleep was also associated with changes in retinal arteriolar caliber in our study. It has been shown that sleep is crucial for the maintenance and restoration of homeostasis through the regulation of energy, repair and infection control, and is important for neuronal activity.3 Consequently, deprivation of sleep or sleep disorders may be associated with CVD disease.42,43 Similarly to antenatal depression, sleep quality may affect susceptibility to infection with increased levels of high-sensitivity CRP, TNF-α, IL-6, and inflammatory cytokines.44,45 Our study showed that poorer sleep quality was associated with wider retinal arteriolar caliber, which might imply the same influence of early and acute state of inflammation on arteriolar blood flow.
The strengths of our study include retinal vessel caliber measurements in a large cohort of pregnant women, using a standardized protocol for retinal vascular caliber measurement, widely used reliable measures for symptoms of antenatal depression, anxiety, and sleep quality assessments, and detailed information on a wide range of potential confounders. There are limitations of our study. First, the cross-sectional nature of the 26 week gestational age measures will not be able to establish the temporal sequence of antenatal depression and sleep quality with retinal vascular caliber. Second, the self-administered psychological measures (EPDS, STAI, and PSQI) have not been validated in our local pregnant women, and no structured diagnostic interviews were conducted. As the questionnaires were all filled in a single sitting, there could have been a response set bias. Last, selection bias may have affected our results, since only participants recruited in KKH with gradable photographs were studied. Our findings on association of antenatal mental health and retinal vascular caliber still need to be examined in further studies, especially in longitudinal studies.
Since retinal and cerebral vessels shared similar anatomic, embryologic, and functional features, retinal vascular abnormalities were shown to be closely correlated with cerebral circulation.36 Our finding that pregnant women with antenatal depressive symptoms and poor sleep quality had greater retinal arteriolar vasodilatation might not only help to provide pathophysiological mechanism in how antenatal depression and poor sleep quality interact with maternal microhemodynamic changes, but also imply possible changes of cerebral blood flow in pregnant women with higher depression scores. Thus, the translational relevance reflected from our study is that retinal vasculature may shed lights on the correlation between mental health and microvasculature.
In conclusion, our study showed associations of antenatal depressive symptoms and poor sleep quality with retinal vascular caliber changes (arteriolar widening) among pregnant women. No association was found between antenatal anxiety and retinal vascular caliber. The findings suggest a possible impact of symptoms of antenatal depression and poor sleep on microcirculation in vivo during pregnancy.
Acknowledgments
Grant information: This study is funded by the National Health and Medical Research Council, Singapore (NMRC/TCR/004-NUS/2008, NMRC/STaR/0003/2008 and NMRC/CG/SERI/2010).
Disclosure: L.-J. Li, None; M.K. Ikram, None; L. Broekman, None; C.Y.-L. Cheung, None; H. Chen, None; J.J. Gooley, None; S.-E. Soh, None; P. Gluckman, None; K. Kwek, None; Y.-S. Chong, None; M. Meaney, None; T.-Y. Wong, None; S.-M. Saw, None
Footnotes
ephssm@nus.edu.sg
References
- 1.Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. 2006;27:2763–2774. doi: 10.1093/eurheartj/ehl338. [DOI] [PubMed] [Google Scholar]
- 2.Carney RM, Freedland KE. Depression, mortality, and medical morbidity in patients with coronary heart disease. Biol Psychiatry. 2003;54:241–247. doi: 10.1016/s0006-3223(03)00111-2. [DOI] [PubMed] [Google Scholar]
- 3.Miller MA, Cappuccio FP. Inflammation, sleep, obesity and cardiovascular disease. Curr Vasc Pharmacol. 2007;5:93–102. doi: 10.2174/157016107780368280. [DOI] [PubMed] [Google Scholar]
- 4.Motivala SJ. Sleep and Inflammation: psychoneuroimmunology in the context of cardiovascular disease. Ann Behav Med. 2011;42(2):141–152. doi: 10.1007/s12160-011-9280-2. [DOI] [PubMed] [Google Scholar]
- 5.Fiedorowicz JG, He J, Merikangas KR. The association between mood and anxiety disorders with vascular diseases and risk factors in a nationally representative sample. J Psychosom Res. 2011;70:145–154. doi: 10.1016/j.jpsychores.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qiu C, Sanchez SE, Lam N, Garcia P, Williams MA. Associations of depression and depressive symptoms with preeclampsia: results from a Peruvian case-control study. BMC Womens Health. 2007;7:15. doi: 10.1186/1472-6874-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodie VA, Freeman DJ, Sattar N, Greer IA. Pre-eclampsia and cardiovascular disease: metabolic syndrome of pregnancy? Atherosclerosis. 2004;175:189–202. doi: 10.1016/j.atherosclerosis.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 8.Gold PW, Chrousos GP. The endocrinology of melancholic and atypical depression: relation to neurocircuitry and somatic consequences. Proc Assoc Am Physicians. 1999;111:22–34. doi: 10.1046/j.1525-1381.1999.09423.x. [DOI] [PubMed] [Google Scholar]
- 9.Kop WJ, Synowski SJ, Gottlieb SS. Depression in heart failure: biobehavioral mechanisms. Heart Fail Clin. 2011;7:23–38. doi: 10.1016/j.hfc.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Bennett HA, Einarson A, Taddio A, Koren G, Einarson TR. Prevalence of depression during pregnancy: systematic review. Obstet Gynecol. 2004;103:698–709. doi: 10.1097/01.AOG.0000116689.75396.5f. [DOI] [PubMed] [Google Scholar]
- 11.Chee CY, Chong YS, Ng TP, Lee DT, Tan LK, Fones CS. The association between maternal depression and frequent non-routine visits to the infant's doctor–a cohort study. J Affect Disord. 2008;107:247–253. doi: 10.1016/j.jad.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Wong TY, Klein R, Klein BE, Tielsch JM, Hubbard L, Nieto FJ. Retinal microvascular abnormalities and their relationship with hypertension, cardiovascular disease, and mortality. Surv Ophthalmol. 2001;46:59–80. doi: 10.1016/s0039-6257(01)00234-x. [DOI] [PubMed] [Google Scholar]
- 13.Wong TY, Klein R, Sharrett AR, et al. Retinal arteriolar diameter and risk for hypertension. Ann Intern Med. 2004;140:248–255. doi: 10.7326/0003-4819-140-4-200402170-00006. [DOI] [PubMed] [Google Scholar]
- 14.Wong TY, Klein R, Sharrett AR, et al. Retinal arteriolar narrowing and risk of diabetes mellitus in middle-aged persons. JAMA. 2002;287:2528–2533. doi: 10.1001/jama.287.19.2528. [DOI] [PubMed] [Google Scholar]
- 15.McGeechan K, Liew G, Macaskill P, et al. Meta-analysis: retinal vessel caliber and risk for coronary heart disease. Ann Intern Med. 2009;151:404–413. doi: 10.7326/0003-4819-151-6-200909150-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong TY, Klein R, Sharrett AR, et al. Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The Atherosclerosis Risk in Communities Study. JAMA. 2002;287:1153–1159. doi: 10.1001/jama.287.9.1153. [DOI] [PubMed] [Google Scholar]
- 17.Wong TY, Klein R, Nieto FJ, et al. Retinal microvascular abnormalities and 10-year cardiovascular mortality: a population-based case-control study. Ophthalmology. 2003;110:933–940. doi: 10.1016/S0161-6420(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 18.McGeechan K, Liew G, Macaskill P, et al. Prediction of incident stroke events based on retinal vessel caliber: a systematic review and individual-participant meta-analysis. Am J Epidemiol. 2009;170:1323–1332. doi: 10.1093/aje/kwp306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheung N, Rogers S, Mosley TH, Klein R, Couper D, Wong TY. Vital exhaustion and retinal microvascular changes in cardiovascular disease: atherosclerosis risk in communities study. Psychosom Med. 2009;71:308–312. doi: 10.1097/PSY.0b013e318190f009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ikram MK, Luijendijk HJ, Hofman A, et al. Retinal vascular calibers and risk of late-life depression: the Rotterdam Study. Am J Geriatr Psychiatry. 2010;18:452–455. doi: 10.1097/jgp.0b013e3181d69250. [DOI] [PubMed] [Google Scholar]
- 21.Jensen RA, Shea S, Ranjit N, et al. Psychosocial risk factors and retinal microvascular signs: the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2010;171:522–531. doi: 10.1093/aje/kwp414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim DH, Newman AB, Hajjar I, et al. Retinal microvascular signs and functional loss in older persons: the cardiovascular health study. Stroke. 2011;42:1589–1595. doi: 10.1161/STROKEAHA.110.605261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen TT, Wong TY, Islam FM, et al. Evidence of early retinal microvascular changes in patients with type 2 diabetes and depression. Psychosom Med. 2010;72:535–538. doi: 10.1097/PSY.0b013e3181da90f4. [DOI] [PubMed] [Google Scholar]
- 24.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh postnatal depression scale. Br J Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 25.Kendall PC, Finch AJ, Jr, Auerbach SM, Hooke JF, Mikulka PJ. The State-Trait Anxiety Inventory: a systematic evaluation. J Consult Clin Psychol. 1976;44:406–412. doi: 10.1037//0022-006x.44.3.406. [DOI] [PubMed] [Google Scholar]
- 26.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 27.Gibson J, McKenzie-McHarg K, Shakespeare J, Price J, Gray R. A systematic review of studies validating the Edinburgh Postnatal Depression Scale in antepartum and postpartum women. Acta Psychiatr Scand. 2009;119:350–364. doi: 10.1111/j.1600-0447.2009.01363.x. [DOI] [PubMed] [Google Scholar]
- 28.Nasreen HE, Kabir ZN, Forsell Y, Edhborg M. Low birth weight in offspring of women with depressive and anxiety symptoms during pregnancy: results from a population based study in Bangladesh. BMC Public Health. 2010;10:515. doi: 10.1186/1471-2458-10-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teixeira JM, Fisk NM, Glover V. Association between maternal anxiety in pregnancy and increased uterine artery resistance index: cohort based study. BMJ. 1999;318:153–157. doi: 10.1136/bmj.318.7177.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li LJ, Cheung CY, Chia A, et al. The relationship of body fatness indices and retinal vascular caliber in children. Int J Pediatr Obes. 2011;6(3–4):267–274. doi: 10.3109/17477166.2011.583657. [DOI] [PubMed] [Google Scholar]
- 31.Wong TY, Islam FM, Klein R, et al. Retinal vascular caliber, cardiovascular risk factors, and inflammation: the multi-ethnic study of atherosclerosis (MESA) Invest Ophthalmol Vis Sci. 2006;47:2341–2350. doi: 10.1167/iovs.05-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knudtson MD, Lee KE, Hubbard LD, Wong TY, Klein R, Klein BE. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res. 2003;27:143–149. doi: 10.1076/ceyr.27.3.143.16049. [DOI] [PubMed] [Google Scholar]
- 33.Li LJ, Cheung CY, Ikram MK, et al. Blood pressure and retinal microvascular characteristics during pregnancy: Growing Up in Singapore Towards Healthy Outcomes (GUSTO) Study. Hypertension. 2012;60:223–230. doi: 10.1161/HYPERTENSIONAHA.112.195404. [DOI] [PubMed] [Google Scholar]
- 34.Li LJ, Ikram MK, Cheung CY, et al. Effect of maternal body mass index on the retinal microvasculature in pregnancy. Obstet Gynecol. 2012;120:627–635. doi: 10.1097/AOG.0b013e3182639577. [DOI] [PubMed] [Google Scholar]
- 35.Leung BM, Kaplan BJ. Perinatal depression: prevalence, risks, and the nutrition link–a review of the literature. J Am Diet Assoc. 2009;109:1566–1575. doi: 10.1016/j.jada.2009.06.368. [DOI] [PubMed] [Google Scholar]
- 36.Wong TY, Mitchell P. The eye in hypertension. Lancet. 2007;369:425–435. doi: 10.1016/S0140-6736(07)60198-6. [DOI] [PubMed] [Google Scholar]
- 37.Granger DN, Senchenkova E. Impaired vasomotor responses. In: Granger DN, Granger JP, editors. Morgan & Claypool Publishers; 2012. pp. 15–18. In. eds. Inflammation and the Mictrocirculation. San Rafael, CA. [Google Scholar]
- 38.Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry. 1998;55:580–592. doi: 10.1001/archpsyc.55.7.580. [DOI] [PubMed] [Google Scholar]
- 39.MacRitchie AN, Jun SS, Chen Z, et al. Estrogen upregulates endothelial nitric oxide synthase gene expression in fetal pulmonary artery endothelium. Circ Res. 1997;81:355–362. doi: 10.1161/01.res.81.3.355. [DOI] [PubMed] [Google Scholar]
- 40.Mariak Z, Rakowski G, Krejza J. The influence of the estrogens on a retinal blood vessels. Klin Oczna. 2005;107:401–404. [PubMed] [Google Scholar]
- 41.Chen H, Yiu KH, Tse HF. Relationships between vascular dysfunction, circulating endothelial progenitor cells, and psychological status in healthy subjects. Depress Anxiety. 2011;28:719–727. doi: 10.1002/da.20839. [DOI] [PubMed] [Google Scholar]
- 42.Kasasbeh E, Chi DS, Krishnaswamy G. Inflammatory aspects of sleep apnea and their cardiovascular consequences. South Med J. 2006;99:58–67. doi: 10.1097/01.smj.0000197705.99639.50. ; quiz 68-59, 81. [DOI] [PubMed] [Google Scholar]
- 43.Resnick HE, Howard BV. Diabetes and cardiovascular disease. Annu Rev Med. 2002;53:245–267. doi: 10.1146/annurev.med.53.082901.103904. [DOI] [PubMed] [Google Scholar]
- 44.Clearfield MB. C-reactive protein: a new risk assessment tool for cardiovascular disease. J Am Osteopath Assoc. 2005;105:409–416. [PubMed] [Google Scholar]
- 45.Teramoto S, Yamamoto H, Ouchi Y. Increased C-reactive protein and increased plasma interleukin-6 may synergistically affect the progression of coronary atherosclerosis in obstructive sleep apnea syndrome. Circulation. 2003;107:E40–40. doi: 10.1161/01.cir.0000053956.46188.5f. [DOI] [PubMed] [Google Scholar]


