Abstract
Purpose:
While prisms are commonly prescribed for homonymous hemianopia to extend or expand the visual field, they cause potentially troubling visual side effects, including nonveridical location of perceived images, diplopia, and visual confusion. In addition, the field behind a prism at its apex is lost to an apical scotoma equal in magnitude to the amount of prism shift. The perceptual consequences of apical scotomas and the other effects of various designs were examined to consider parameters and designs that can mitigate the impact of these effects.
Methods:
Various configurations of sector and peripheral prisms were analyzed, in various directions of gaze, and their visual effects were illustrated using simulated perimetry. A novel “percept” diagram was developed that yielded insights into the patient's view through the prisms. The predictions were verified perimetrically with patients.
Results:
The diagrams distinguish between potentially beneficial field expansion via visual confusion and the pericentrally disturbing and useless effect of diplopia, and their relationship to prism power and gaze direction. They also illustrate the nonexpanding substitution of field segments of some popular prism designs.
Conclusions:
Yoked sector prisms have no effect at primary gaze or when gaze is directed toward the seeing hemifield, and they introduce pericentral field loss when gaze is shifted into them. When fitted unilaterally, sector prisms also have an effect only when the gaze is directed into the prism and may cause a pericentral scotoma and/or central diplopia. Peripheral prisms are effective at essentially all gaze angles. Since gaze is not directed into them, they avoid problematic pericentral effects. We derive useful recommendations for prism power and position parameters, including novel ways of fitting prisms asymmetrically.
Translational Relevance:
Clinicians will find these novel diagrams, diagramming techniques, and analyses valuable when prescribing prismatic aids for hemianopia and when designing new prism devices for patients with various types of field loss.
Keywords: low vision, rehabilitation, prism treatment, perimetry, visual field loss, hemianopia, traumatic brain injury, TBI, stroke
Introduction
Homonymous hemianopia (HH), the loss of half the visual field in both eyes on the same side and to similar extent, results from postchiasmatic lesions typically caused by stroke, tumor, or trauma.1 Hemianopic visual field loss reduces detection of objects in the blind hemifield and impacts the ability to avoid obstacles while walking and driving.2–4
Spectacle-mounted prisms are the most commonly used rehabilitation devices for HH. There are various designs and methods for fitting prism spectacles. All involve tradeoffs between the access to otherwise invisible areas of the visual scene and the various potentially troubling visual side effects the prisms introduce. While the prism apical scotoma has been mentioned in the literature,5–9 its functional significance has not been addressed, nor has its impact on the residual visual field been illustrated. This paper examines common prism configurations used for HH, and primarily analyzes the oft-ignored, but important, effect of prism apical scotomas. Other effects, such as the diplopia and visual confusion induced in some configurations and the nonveridical views created by all designs are also addressed, and their impact is illustrated in a novel way. Careful fitting design based on the parameters and diagrams provided here can mitigate these effects. The analyses will also provide practitioners insights into why some configurations may be accepted and valued more than others by their patients. Novel designs emerged from these analyses, described in the sections on offset sector and peripheral prism placement.
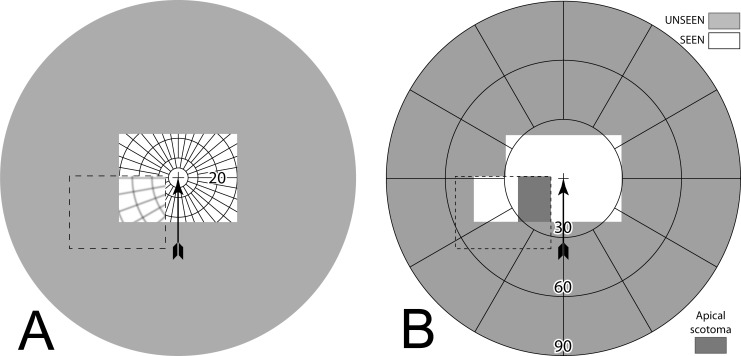
The bending of light rays by a prism creates a gap in the visible field of view between the last undeviated ray just outside the prism, at its apex, and the first ray deviated by the prism.5 The angular extent of that gap is equal to the prism power in degrees (Fig. 1A). That gap is referred to as the apical scotoma. The perceived (retinal) field of view is still fully covered by rays from portions of the external field, but the view from within the scotoma is missing, obscured by the prism itself. Thus, this “scotoma” is a gap in the portion of the external world view that is seen, not a blind retinal area. The retinal image is discontinuous, but has no blanks. (If a prism is replaced by an opaque occluder, visual field equal in width to the visual angle the occluder subtends would be lost behind the occluder. With a prism, the field blocked by the prism beyond the apical scotoma is seen at the prism apex; hence, the apical scotoma is the only loss.) The effect is illustrated photographically by holding a prism in front of a camera lens (Fig. 1B).
Figure 1. .
Apical prism scotoma. (A) Schematic ray diagram of the apical scotoma of a sector prism. The apical scotoma occurs between the last undeviated ray and the first light ray, which is deviated by the prism (prism apex highlighted by yellow star). The angle between these two light rays is equivalent to the prism power in degrees. In this example, the cat is not visible through the prism, since it is located within the prism's apical scotoma, indicated by the gray shading. (B) Photograph taken with a 30Δ prism with base to the left covering most of the lower left quadrant of the frame. The prism extends the camera's field of view to the left, revealing crosswalk left of the lamppost, but loses field at its apex, as the lower half of the man is now missing. Had the prism extended the full height of the image, the man would be lost completely to the apical scotoma. The aperture of the camera lens creates vignetting at the prism edges.
All of the prism configurations to be discussed cause portions of the world view to appear in nonveridical directions; the apparent location through the prism is displaced from the true direction (e.g., the lower portion of the lamppost in Fig. 1B).
When prisms are placed bilaterally for HH, with essentially the same position and coverage for each eye, the prism apical scotomas induce a gap in the visual field. When placed monocularly (or at different relative positions on each carrier lens), it is possible for the view of one eye to include field portions missing to the apical scotoma of the fellow eye. These configurations produce areas of visual confusion, in which two different views are seen at the same apparent direction by the two eyes, and some produce diplopia, wherein a given object or portion of the scene is seen in two different directions simultaneously. We avoid using the term “double vision,” as it has been ambiguously applied in the literature to include or exclude confusion and diplopia.
If the total field area available (at a given direction of gaze) is larger than the hemifield available without prisms, we deem this to be true field expansion. Semitransparent mirrors have been used to provide true expansion,10 but they are bulky, unsightly, and add the disorientation of a reversed view to the visual confusion. They are now rarely used and are not discussed further here. As will be seen below, true expansion is possible with prisms, but only if they are fit asymmetrically, or monocularly, so that different portions of the field are available to each eye (albeit with some visual confusion).
Most bilateral prism designs provide field substitution rather than expansion: The total area visible at any time is no greater than without prisms; the area shifted into view displaces an equal amount of the unaided view, creating an optical scotoma in the field—the apical scotoma. We also avoid the term “field enhancement”, as it is ambiguous with respect to these important distinctions.
If prisms cover the entire field of view (as happens with bilateral full-field yoked prisms6,9,11), field shifting, without expansion or substitution, occurs. Field shifting is of no value for patients with HH, as normal fixation, head, and eye movements will negate it to bring the view straight ahead into primary gaze. The prism configurations discussed here (sector and peripheral prisms) do not span the full field horizontally, so the placement of the prism apices becomes a critical design element, affecting the role the apical scotomas play in the patient's experience, as well as the location of regions of visual confusion and diplopia. (The prism bases are set far into the blind side, where they are rarely in view.) Visual confusion is a necessary consequence of prismatic field expansion. It is more readily tolerated in the periphery than in central vision. Confusion and diplopia are very disturbing centrally (parafoveally), while they are normal in the periphery.12 Prism-induced diplopia anywhere, however, represents a waste of prism power that could be better used for expansion (or substitution), as will become apparent below.
In the subsequent sections, we identify the parameters that affect optimal positioning and power for each of the common methods of fitting prisms, and discuss their implications in terms of field expansion or substitution and troublesome perceptual effects including apical scotomas, diplopia, and visual confusion. Often confusion and diplopia are thought to coexist, but these analyses make clear how independent these phenomena can be. The examples are illustrated using predicted/calculated and actual visual field diagrams from patients, and we introduce a new visual field representation technique, the percept diagram, to describe the patient's binocular view through the prism spectacles.
Methods
Calculations used to predict Goldmann visual field diagrams and the associated percept diagrams for each prism configuration, as well as to manufacture the corresponding spectacles used, assume that the nodal point distance (NPD) between an eye's nodal point and the cornea is 7.1 mm (Gullstrand's Schematic Eye13) and the back vertex distance (BVD) between the cornea and the carrier lens is 13 mm. Formulas used for these calculations are provided below. The visual angle (A°) between primary gaze and the prism apex is given by
where L is the distance (mm) on the carrier lens from the intersection of the visual axis (in primary gaze) to the apex. Nodal point is used when calculating visual field angles, while center of rotation (CR, assumed to be 13.5 mm from the cornea) is substituted for NPD when calculating eye rotations.
The conversion of prism power from prism diopters (PΔ) to degrees (P°) is
A conventional Goldmann visual field diagram provides information about which portions of the external visual field are detected by the visual system (in primary gaze). In this paper, we use Goldmann visual field measurements to identify the portions of the external field that are seen (and unseen) when various prism configurations are used and in various directions of gaze, not to map visual system status. To cover the range of configurations tested (not for between–subject comparisons), prism spectacles were worn by three individuals with complete HH. The spectacles were fit according to common clinical practice for each of the designs.
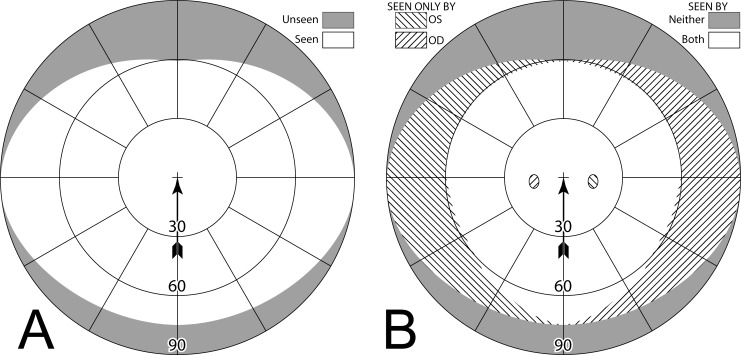
We also introduce a synthetic variant of the Goldmann diagram to illustrate the visual field as it would be seen by the prism wearer. We call this a percept diagram (although, to be precise, it shows what is available on the retinas to be perceived, not necessarily what is perceived, as binocular postretinal factors such as suppression may intercede). A percept diagram illustrates what the world would look like if the world were a Goldmann grid; as if the patient is inside a huge sphere marked with radial lines and concentric circles originating at primary gaze. Figure 2A is the percept diagram for the prism photo in Figure 1B. The percept diagram is a handy way to understand the nonveridical views that prisms introduce. The percept diagrams make it easier to see where visual confusion is introduced and the nonveridical perceived directions of objects, while the Goldmann perimetry diagrams make it easy to note field substitution or expansion, diplopia, and field-of-view losses due to apical scotomas.
Figure 2. .
(A) This percept diagram represents the way a Goldmann grid would be captured by a camera with a 30Δ prism interposed as in Figure 1B. The area outside the camera's field of view is shaded. The arrow points to the center of the field of view (the fixation or “gaze point”). The arrow is not included in subsequent percept diagrams, as fixation is always in the center, although the grid cross will appear off center when diagramming averted gaze views. A dashed line (not part of the percept) outlines the prism location. Its left and lower edges are outside of the camera's field. The base-left prism has allowed a portion of the grid otherwise outside the camera's field on the left to be visible to the camera. The lines in the prism view have been blurred slightly, not just to represent the loss of quality that occurs through prisms, but also as a diagramming aid to differentiate the shifted and unshifted areas. The wider spacing in that area is due to its view of more peripheral grid, not magnification. A 16.7° wide rectangular patch of grid is missing at the prism apex. That is the apical scotoma. (B) The Goldmann diagram that would result if the camera could respond to Goldmann stimuli with a prism similarly interposed. The simulated Goldmann diagram clearly shows the effect of the apical scotoma, shaded in darker gray for emphasis. The prism has substituted the field of view to the left, with a resulting loss of pericentral view.
Figure 2B gives the simulated Goldmann diagram that corresponds to this percept diagram. It is the diagram, which would result if the camera could respond to Goldmann stimuli with the prism configuration of Figure 1B interposed. The simulated Goldmann diagram gives a clear understanding of the field substitution the prism provides as well as the loss of field at the prism apex. The diagrams below illustrating the effects of various prism configurations will, of course, show the limits of a subject's visual fields, not the camera's. Details of many of the diagrams will benefit from the magnification provided by viewing this document at full screen width.
All calculated diagrams assume a pinhole pupil, so they do not include the transition vignetting effect of a larger pupil (exaggerated by the larger camera aperture in Fig. 1B), nor do they include the fringes of color or the other known optical distortions that prisms induce, as these are not generally factors considered when fitting prisms for HH.14,15 The simulated Goldmann diagrams, and those of patients wearing the prisms, are dichoptic, representing separately what each eye sees when both are viewing. Although we have developed perimetry systems that actually measure dichoptically,16,17 most patient diagrams here are actually a synthesis of three separate diagrams, with OD, OS, and OU measured separately and then combined (the OU diagrams were necessary to identify diplopia and confirm binocular scotomas). This technique provided wider plots than are possible when wearing the goggles used in our dichoptic perimeters. Figure 3 illustrates the diagram notations and the distinction between binocular and dichoptic perimetry.
Figure 3. .
Simulated normal Goldmann fields. (A) Binocular (B) Dichoptic (or superimposed separate OD and OS).
All procedures were conducted according to the tenets of the Declaration of Helsinki, and the protocol was approved by the Schepens Eye Research Institute Institutional Review Board. Informed consent was obtained from all subjects.
Sector Prism Spectacles for Hemianopia
Bilateral Sector Prisms
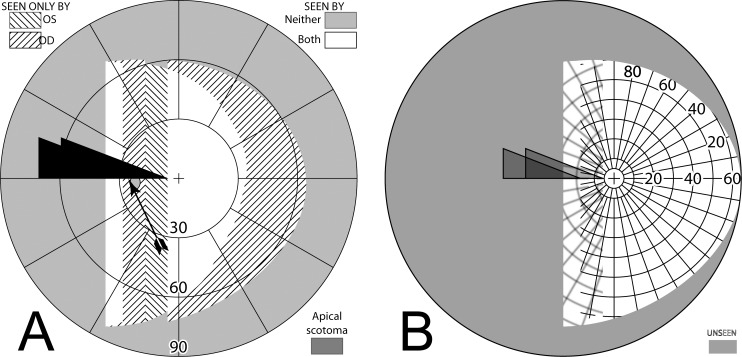
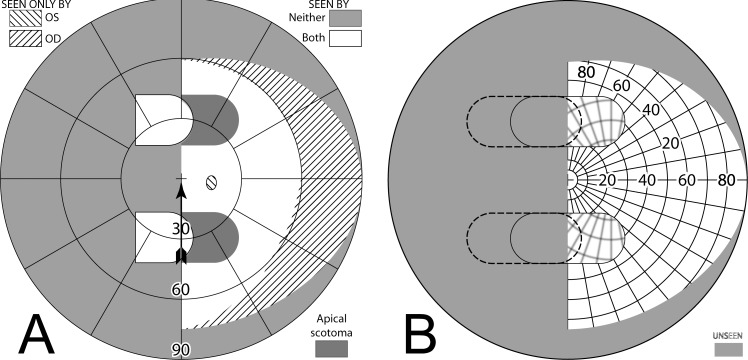
Yoked bilateral sector prisms, up to 20Δ18 (and rarely 30Δ19–21), were one of the first designs of prism spectacles prescribed for hemianopia,22,23 and have been prescribed frequently since.5,7,24–26 In this design, prisms usually extend the full height of the carrier lens, covering about a full half of each lens on the side of the blind hemifield (Fig. 4A, 4B). For this patient with left hemianopia, the prism bases are directed toward the left, so that when looking into the prisms, images of objects in the blind hemifield are shifted into the right seeing field.
Figure 4. .
Bilateral sector prisms. (A) and (B) The prism bases are placed toward the blind side, shown here with base left. The prism apices are placed left of primary gaze by 2.1 mm. (C) Dichoptic simulated Goldmann diagram at primary gaze with these bilateral 20Δ sector prisms. The prisms have no effect, as they are entirely in the blind hemifield, so this is simply the same as a diagram of a left hemianope without prisms. The black triangle is included to indicate the prism orientation and apex location, but not its size. The arrowhead indicates the gaze point. (D) Corresponding percept diagram. It, too, is exactly the same as a diagram of a left hemianope without prisms.
The prism apices are typically offset away from the seeing field, although the recommended offset varies considerably, from 1.5 mm6 to 6 mm.27 Often clinicians offset the prisms, such that a 5° ocular rotation into the blind side is required to reach the prism segment7 (~2.3 mm). As a result of that placement, the prisms are not visible to the wearer at primary gaze (or when gazing in the direction of the seeing hemifield), and do not alter the normal hemianopic Goldmann visual field diagram (Fig. 4C). When the wearer glances into the prisms, objects may be viewed through the prisms with less of an angular eye shift than would otherwise be needed. Since high-power prisms can be quite bulky, prisms are generally limited to 20Δ,7,8 yielding a field shift of about 11°. Less bulky press-on Fresnel prisms are also available, but their poorer optical quality18 and need for frequent replacement limit their application to temporary fitting, allowing a patient to experience prisms before more expensive prism spectacles are prescribed.
Figure 5A simulates the Goldmann diagram that would result if the patient is facing the Goldmann perimeter's fixation target but gazing left 20° into 20Δ (~11°) prisms with apices shifted left 2.1 mm (~6°). In this and all subsequent diagrams illustrating averted gaze, eye rotation would move the eye's nodal point slightly away from the center of the Goldmann bowl. To avoid the complexities in interpretation (and diagramming) that change induces, the diagrams are based on rotations about the nodal point, not the center of rotation. In actuality, only 20.3° of ocular rotation is needed to achieve the 26° view change. We provide diagrams with gaze shifts this large as an upper limit; saccades larger than 15° are infrequent,28,29 and we have found no reports documenting an ability of scan training to alter this. Averted gaze representations are provided as an aid to estimating effects of eye (but not head) movements.
Figure 5. .
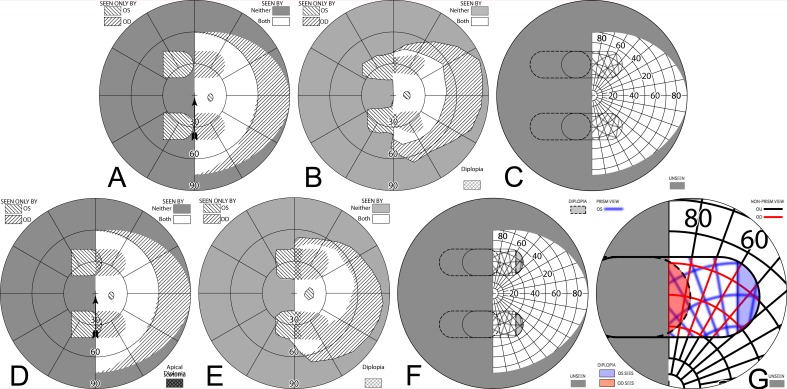
Gazing 20° into bilateral 20Δ sector prisms. (A) Simulated Goldmann diagram with the prism configuration of Figure 4 and the patient facing the fixation target, but gazing left into the prisms by 20°, for a total gaze shift of 26°. The arrow is rotated counterclockwise at the gaze point into the prism to indicate the 26° angle of incidence (from perpendicular) of the gaze. A 20°-wide region of field from 17° into the blind hemifield is visible (6° to the prism apex plus 11° of prism shift), but the 11° just left of the prism apex is missing due to the apical scotomas. Since the gaze is shifted left, some of the right far periphery has been lost from view. (B) The corresponding percept diagram shows that the patient sees 20° of field shifted from 11° farther left, but there is a discontinuity where 11° of pericentral view has been lost to the apical scotomas. The triangle is also shown here to indicate the location of the prism apex. Fixation is at the lower intersection of the triangle and the hemifield margin. (C) In order to provide a fixation reference when gazing into the prism, it is necessary to perform Goldmann perimetry with the patient's head turned right so that the gaze falls on the shifted view of the perimeter's fixation target. A simulation of a field diagram taken that way is shown in (C). We have rotated the prism symbol clockwise about the gaze point to indicate the magnitude of head rotation to the right needed to see the fixation target with a 20° gaze into the prism (37°; 6° to the prism, plus 20° into the prism, plus 11° of prism shift). The arrow rotation indicates the gaze incidence at the prism. (D) An actual Goldmann visual field diagram taken this way. This patient has overall restricted peripheral fields on the seeing side in addition to the hemianopia. The measured apical scotoma is highlighted in dark gray.
To the immediate right of the gaze point the patient sees the 20° wide section of the Goldmann perimeter that is shifted from a region 11° farther left. Eleven degrees of the perimeter to the left of the prism apices is unseen (the apical scotomas) while the 6° between the prism apices and the fixation target are seen directly, as is the remaining portion of the patient's field to the right of that. Since the patient is gazing left a total of 26°, portions of the Goldmann hemifield beyond 64° (nominally) at the extreme right are invisible. The corresponding percept diagram is shown in Figure 5B. The field substitution through the prisms is apparent, as is discontinuity and partial loss of field behind the prisms (due to the apical scotomas).
However, it is not practical to perform actual Goldmann perimetry this way. Since the patient is gazing into the prism, away from the fixation target, there is no reference point on the blank Goldmann dome to serve as a target and stabilize fixation. Instead, we had the patient turn his head (and the prism-bearing spectacles) sufficiently to the right so that the fixation target was viewed through the prisms when gazing 20° (in this example) into the prisms. The calculated Goldmann diagram in that situation is shown in Figure 5C. (It may be easier to think of this as having the patient maintain a fixed head position, while the Goldmann perimeter is rotated until the fixation target is foveated with gaze averted 26°.30 Of course, it is more practical for the patient to turn within the chin rest.) A binocular Goldmann diagram with the patient's head turned in this way is shown in Figure 5D. Note that the measured apical scotoma does not appear as large as the calculated scotoma, likely due to a combination of measurement error, vignetting, and slight misalignment of the prism apices between the eyes when the head is turned in this direction (but see Fig. 9 for a way that “misalignment” can be an advantage).
Figure 9. .
Offset placement of bilateral sector prisms can avoid diplopia. This is illustrated for a gaze 20° into 20Δ sector prisms. The OD prism has the conventional 6° offset from primary gaze, while the OS prism is offset the additional 11° of the prism power. Although field extent is identical, the pericentral scotoma of the conventional bilateral fitting (Fig. 5) is gone, as is the diplopic area of unilateral fitting (Fig. 8), though central confusion remains. (A) Simulated Goldmann (with head straight). (B) The percept diagram shows that a region of visual confusion exists where OD is viewing through a prism and OS is not. The apex of the leftmost triangle in each diagram identifies the location of the OS prism apex.
A similar set of diagrams is given in Figure 6, with gaze shifted only 5° into the prisms (requiring 11° shift from primary gaze). Since the amount of eye movement is smaller, the apical scotoma is closer to the center of gaze, where it may have a more deleterious impact.
Figure 6. .
Diagrams corresponding to those in Figure 5, but with a gaze of only 5° into the prisms. (A) Simulated Goldmann with head straight. (B) Percept diagram. (C) Simulated Goldmann with head turned 22° (6° to the prism, plus 5° into the prism, plus 11° of prism shift), so that a gaze shift of 11° (6° to the prism and 5° into the prism) enables the Goldmann fixation target foveation through the prism. (D) Actual Goldmann visual field measured that way. Although the gaze had to be averted 11° to achieve a 5° sliver of field substitution, 11° are still missed pericentrally (the apical scotoma).
Thus, bilateral sector prisms provide no benefit at primary gaze, but provide access to portions of the blind hemifield with less eye rotation (by the amount of prism power in degrees) than would be required without prisms. However, while gazing into the prisms, a vertical swath of visual field, also the width of the prism shift, is missed between the shifted and unshifted views due to the apical scotomas. The field thus lost includes pericentral areas important for safe mobility.31 The apical scotomas and large gaze shifts needed to achieve field substitution (but not expansion) likely account for the paucity of reports of long-term acceptance of this prism configuration. Some clinicians suggest that these prisms are training the patient to become aware of the blind area “by looking further into the prism or turning their head and viewing the object through the carrier portion of the spectacle,”7 but we are not aware of any studies demonstrating a training effect.
In order to mitigate the effects of the apical scotomas, we next consider two alternatives to the common bilateral fitting of sector prisms. The first, unilateral fitting, has been used previously.6,8,32 The second, offset bilateral placement, is new and as yet untested clinically. It arises directly from our analyses of scotoma effects presented here. Neither of these configurations overcomes the lack of field expansion or substitution at primary gaze (or any gaze in the direction of the sighted hemifield), and they induce central and pericentral visual confusion when gaze is directed into them. Peripheral prisms, discussed later, overcome those limitations.
Unilateral Sector Prisms
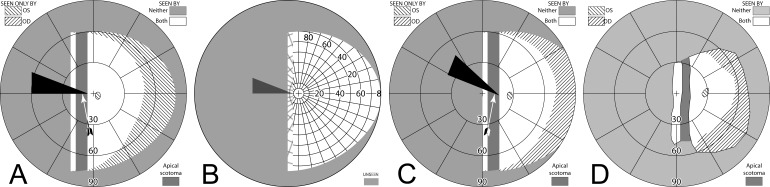
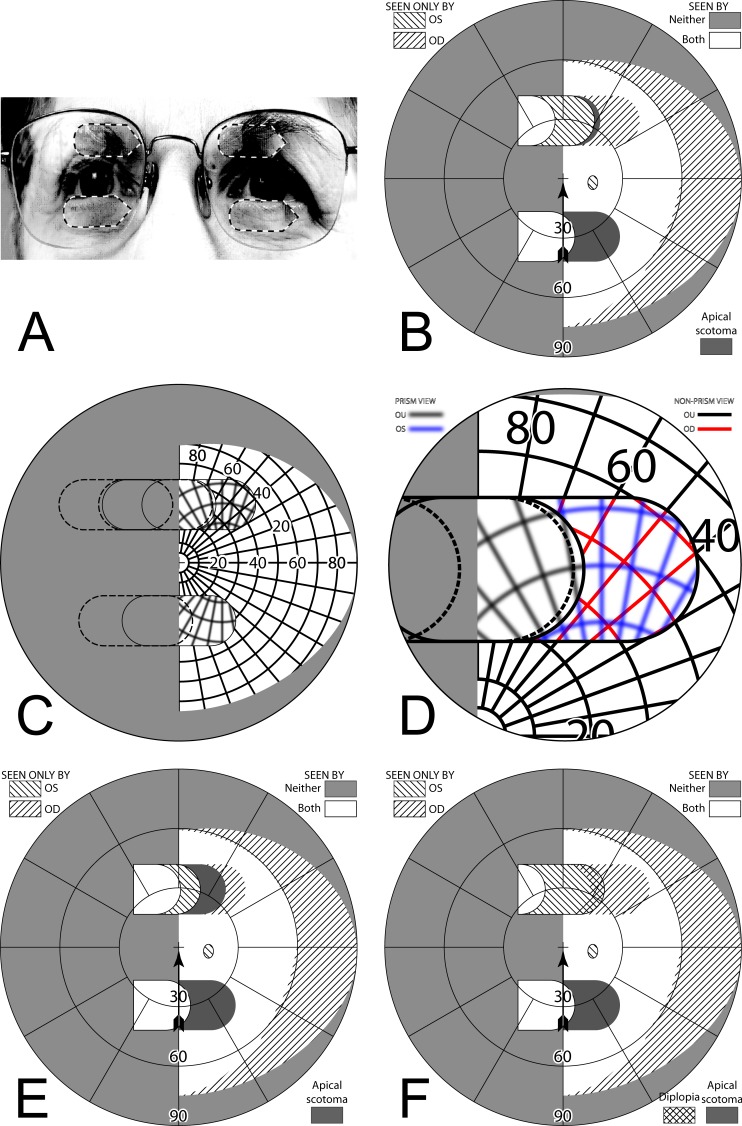
If a sector prism of the type described above is fit on only one carrier lens (typically ipsilateral to the field defect; OS for a patient with left hemianopia), the nonprism eye can see some or all of the area lost to the prism eye's apical scotoma. The region between the gaze point into the prism and the prism apex is seen unblocked by the prism by the nonprism eye, and is, thus, not lost in the binocular field to the scotoma. If the angle of gaze into the prism is less than the prism power, some scotoma will remain. If the angle of gaze into the prism is larger, there is an area of diplopia equal in width to the excess of the gaze angle beyond the prism power. In this and subsequent discussions, we assume that the patient is orthophoric. Because the unilateral prism can dissociate binocularity, the actual binocular field results may vary with patient's phoria and eye dominance. Note, in this unilateral sector design too, the prism has no effect in primary position of gaze or when the patient gazes towards the sighted hemifield.
Figure 7 builds the percept diagram for the case in which the gaze angle into the prism is larger than the prism power. The fitting parameters are as above (gazing 20° into a 20Δ prism offset 6° from primary gaze). The fellow eye now sees the region lost to the apical scotoma of the prism eye, although there is visual confusion in that area and a 9° wide area of diplopia. Confusion and diplopia around the gaze point can make it difficult for the patient to quickly interpret the view, and may result in suppression; thus, eye dominance and the overall binocular status of the patient may be important factors.
Figure 7. .
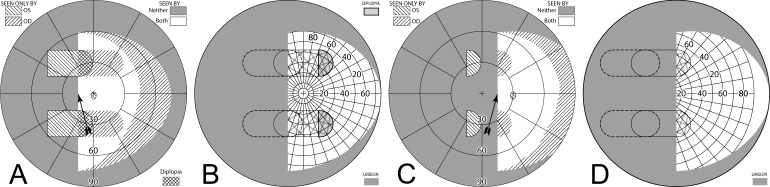
Building a percept diagram with confusion and diplopia. (A) The OD view, when the patient is gazing 20° into a 20Δ OS-only sector prism, is unaffected by the prism. (B) OS view. The view shifted through the prism brings 11° of the grid from the blind hemifield into view, while losing view of an 11° section at the apical scotoma. (C) Combined percept. There is visual confusion in the 20° section where OS sees through the prism and OD does not. Although OS views 20° of field through the prism, only the leftmost 11° provide expansion. The remaining 9° seen by OS at the prism apex side are visible to OD, but are seen in a different apparent direction, and thus diplopic. At the prism apex OD sees the 11° of field lost to OS by the apical scotoma. (The OD optic nerve head is outlined for clarity, but not likely perceived.) The OD-only view in (D) shades the portion of the OD field behind the OS prism that is seen diplopically, and the OS-only view in (E) shades the corresponding portion of the OS field. The field of view covered by the outlined areas in (D) and (E) is identical, but separated in the patient's view by the 11° prism power. In the final diagram (F), the outline for the diplopic region of only the prism view is shown, as that is the area in which diplopia is present without adding any expansion benefit, while the remaining areas of visual confusion are a necessary consequence of true field expansion. Central diplopia and confusion are annoying and confusing and, thus, poorly tolerated.
Figure 8A is the computed Goldmann visual field diagram analogous to Figure 5A, with the patient facing straight at the fixation light and gaze shifted 20° into the prism. Figure 8C gives the calculated diagram analogous to Figure 5C, with the head rotated 26° right, so that the left eye is gazing 20° into the prism when the right eye is gazing at the fixation target. Figure 8D gives the actual patient results under that condition. As noted above, the calculated diagrams are created as if the nodal points of both eyes are at the perimeter origin; clearly impossible to actually achieve. However, in the mobility situations where these diagrams are relevant, the distance to the targets of interest are so large that the distance between the eyes is inconsequential.
Figure 8. .
Unilateral sector prism. With a sector prism (of the type in Fig. 5) fitted on just one eye, the fellow eye sees some of or the entire region lost to the apical scotoma, albeit with visual confusion. (A–D) The calculated head straight Goldmann diagram, percept (from Fig. 7), and calculated head turned and actual Goldmann diagrams, respectively, when gazing 20° into the prism. 20° of field is shifted into view by the prism. The 11° prism scotoma region is visible to the fellow eye, but with 20° of visual confusion and 9° (20°–11°) of diplopia. In the Goldmann diagrams the diplopic area is crosshatched. (E–H) Corresponding diagrams when gazing just 5° into the prism (yet 11° away from primary gaze, so 11° of field at the right periphery is lost); 5° of shifted field are viewed through the prism, and there is 5° of visual confusion, but no diplopia and only 5° of the apical scotoma has become visible to the nonprism eye.
Figures 8E through 8H are the corresponding diagrams when gaze is shifted just 5° into the prism, where the amount of eye movement in degrees is less than the amount of prismatic shift. For a 5° gaze shift into the prism segment (total 6° + 5° = 11° shift from primary gaze), there is no diplopia, but the apical scotoma is present in the binocular view, as the nonprism eye sees only 5° of the 11° apical scotoma region, and that part is seen with visual confusion.
Thus, the unilateral sector prism design can reduce or eliminate the apical scotoma and provide some true binocular field expansion, not just substitution. However, the apical scotoma does appear in the binocular view as the eye crosses the prism segment, until the amount of gaze shift is equivalent to or greater than the prism power in degrees. Furthermore, central and peripheral field confusion around the gaze point do occur, and as the gaze is shifted farther into the prism, central and peripheral diplopia ensue. The percept diagram shows these effects. Since this is the first example with confusion and diplopia, Figure 7 builds the diagram a step at a time, so the component effects are clearer. Subsequent diagrams will only show the compounded final effect. To best represent the situation the patient experiences, we do not use color to distinguish the OD and OS contributions to visual confusion. However, where this may be particularly hard for the reader to interpret, we have also provided magnified detail views with color. Nonetheless, since the perceived location of prism-induced diplopia is not readily identified in the Goldmann diagrams, we highlight it in the percept diagrams, even though that delineation, of course, is not apparent to the patient.
The Gottlieb prism design32 differs from unilateral sector prisms, in that only a circular cutout of the sector prism is used. The prism apex is placed at the limbus, and is, thus, offset by about 6 mm (~13° = tan−1[6/(CR+BVD)]), which is a larger angle than 97% of natural saccades.28,29 The prism is intended to be used during brief glances to aid in locating objects of interest. Its smaller size makes it lighter weight and perhaps less conspicuous (better cosmetics), losing some peripheral areas of a full sector prism that are arguably less important. The main optical effects, however, are identical to those of a unilateral sector prism within the coverage area of the Gottlieb prism.
Offset Bilateral Sector Prisms
To avoid the pericentral and central diplopia that accompanies unilateral sector prisms (when in gaze), bilateral prisms can be offset so that the apical scotoma for each prism lies in a different portion of the binocular visual field. If the offset is exactly equal to the prism power, no portion of the field will be completely lost within the apical scotomas of both prisms simultaneously, as illustrated in Figure 9. The extent of coverage is identical to that provided by the unilateral and conventional bilateral configurations. There is true binocular field expansion when the eyes are turned far enough to be gazing into one or both prisms. Visual confusion is substituted for the apical scotomas of the conventional bilateral configuration and the diplopia of unilateral fitting. There is still no field expansion at primary gaze, making this (as yet untested) configuration less desirable than the peripheral placement of prisms described next. The central confusion that remains with this design, though preferable to diplopia that does not contribute anything to field expansion, is not less annoying or bothersome than central diplopia.
Peripherally-Placed Prism Spectacles for Hemianopia
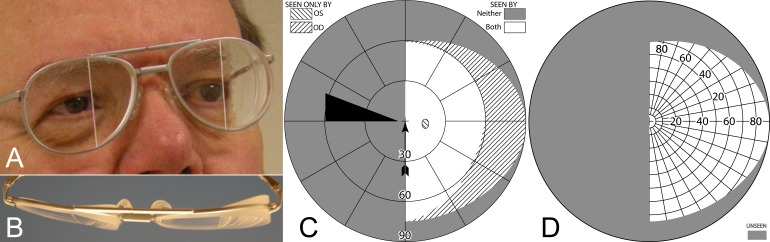
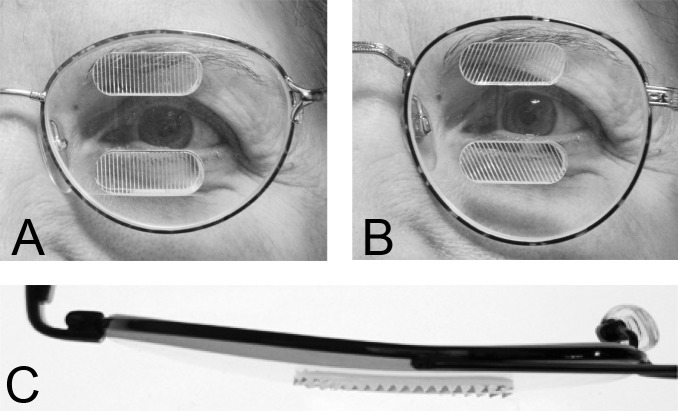
Also known as EP Prisms or Peli Prisms,33 the peripheral prism spectacle design uses high power prism inserts placed in upper and lower peripheral parts of a carrier spectacle lens (usually in Fresnel format), with a clear central area in between (Fig. 10A). The commercially available rigid permanent prism segments extend 22 mm across and 8 mm high, with the center of the prisms generally placed above and below the patient's pupil center when in primary gaze. A typical interprism separation of 12 mm34 leaves a clear central field approximately 17° (tan−1[6/(NPD+BVD)]) above and below primary gaze.
Figure 10. .
57Δ horizontal EP Prism glasses (A) have a horizontal apex-base axis, while 57Δ oblique EP Prism glasses (B) tilt the apex-base axis approximately 25°. (C) A bird's-eye view of the horizontal EP.
Since the prisms extend left and right of primary gaze, prism-shifted images are continuously present in the patient's superior and inferior peripheral vision, providing visual field expansion in these areas at essentially all directions of lateral gaze; thus, augmenting the natural ability of peripheral vision to capture attention on the blind side. This differs from the sector designs, where the prism-shifted images are only visible when the patient's gaze is shifted into the prism segment. Since the prisms do not block pericentral vision (and gaze is not intentionally directed into the prisms), any diplopia, visual confusion, and scotomas that the prisms may induce are confined to the upper and lower peripheral retina, where they are more readily tolerated.35 Binocular foveal fusion is maintained; thus, this design is not affected by patient's phoria or binocular status. The peripheral prisms design was found to be effective in single36,37 and multicenter34,38 clinical trials. Since Fresnel prisms are thinner and lighter than regular ophthalmic sector prisms, and the reduced optical quality is not as disturbing in the periphery as it would be in the fovea, prism powers (and the concomitant benefits) can be much greater, with 40Δ or even 57Δ typically used. They can be fit in either a horizontal33 or in an oblique design39,40 (Fig. 10).
Horizontal Peripheral Prism Spectacles
The bases of horizontal peripheral prisms are placed toward the blind hemifield to provide field access to areas in the blind hemifield horizontally in line with the prisms. (Like the sector prisms, the apex-base axis is horizontal, parallel to the 180° meridian.) Prisms of 40Δ and 57Δ provide shifts of 22° and 30°, respectively, and prisms can be fit unilaterally or bilaterally. While it is preferable to fit patients with the 57Δ peripheral prisms, as they provide more field expansion, press-on temporary prisms are only available up to 40Δ. Thus, 40Δ press-on Fresnel prisms (available precut to the same size as permanent prisms) are recommended for initial prescription by the clinician on a trial basis before prescribing higher-powered permanent Fresnel prisms. Higher prism powers have correspondingly larger apical scotomas, but as shown below for unilateral fitting, that can be turned into an advantage.
Unilateral Horizontal Peripheral Prisms
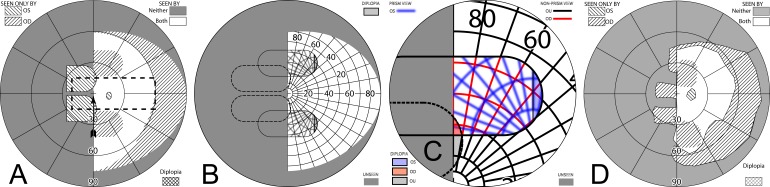
Peripheral prisms are more commonly fit unilaterally, usually placing the prism on the lens ipsilateral to the side of visual field loss and with the base always to that side. One eye has access to portions of the blind hemifield (via the prisms), while the other eye can still see objects within the intact seeing hemifield that may otherwise not be seen due to the apical scotomas.
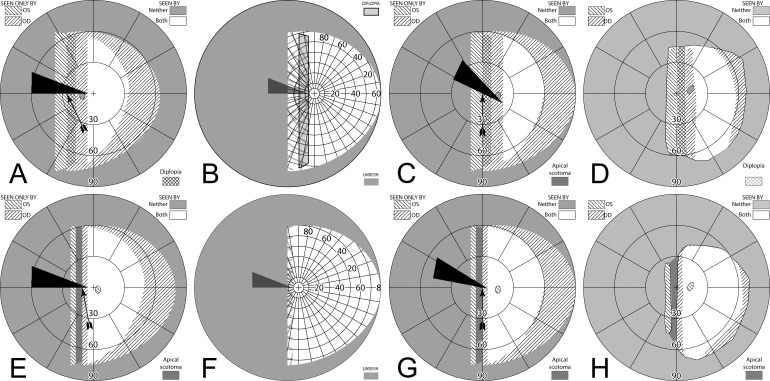
When fitting a patient with unilateral peripheral prisms, an apical scotoma is not present in the binocular visual field. If the prisms are fit as recommended, with the center of the prism segment aligned with the patient's pupil center, the field lost to the apical scotomas will be located within an area of the patient's seeing hemifield; thus, visible to the fellow nonprism eye. The 11 mm half-width of an EP Prism subtends about 29°, closely matching the 57Δ apical scotoma of 29.7°. Figure 11, illustrates the percept and predicted visual field diagrams for a patient with left hemianopia wearing unilateral horizontal peripheral prisms. The lateral extents of the prism expansion areas in the left visual field are determined by the prism power, while the vertical extents are determined by the height of the prism segments. The prisms result in two areas in the seeing hemifield that are seen by the right (nonprism) eye only; the prism apical scotomas. With 40Δ prisms, there is also an area of the visual field in the seeing hemifield that is visible to both the prism eye and the fellow eye, resulting in peripheral diplopia.
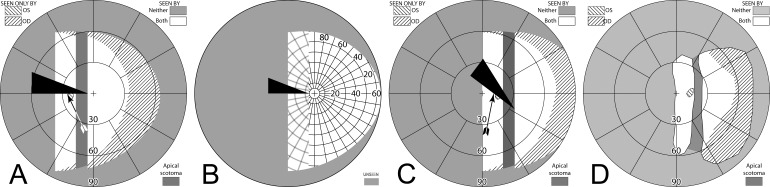
Figure 11. .
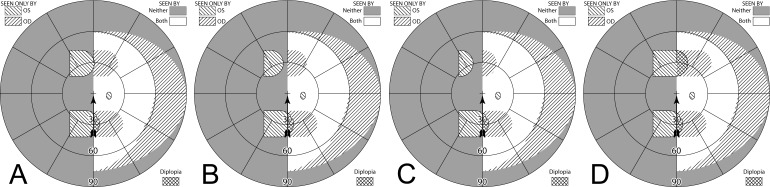
(A–C) Forward gaze with unilateral 57Δ EP Prism glasses. (D–F) The corresponding diagrams with 40Δ prisms. (A) Simulated Goldmann visual field. The left eye sees regions shifted from 30° left, while the right eye maintains a view of the regions blocked by the left eye's prisms' apical scotomas. Since the monocularly-viewed regions do not overlap, there is confusion but no diplopia, and there is a small gap between the expansion area and the normal seeing hemifield. (B) Actual composite field diagram from a patient. Imprecision in measurements or different fitting parameters result in a small region of apparent diplopia. (C) The corresponding percept diagram shows that there is visual confusion in the peripheral prism regions (where it is reasonably tolerated). The patient does not intentionally gaze into the prisms, but activity or objects seen there can alert the user and cause a gaze shift into the blind hemifield, possibly adjusting head direction slightly to view the area of interest centrally and not through the prisms. Dashed outlines indicate the source of the prism views. For simplicity and clarity, we plot rounded rectangles as the projected shape of the prisms. In actuality, they would appear slightly trapezoidal, with slightly curved horizontal edges. (D–F) With the lower-power prisms, the diplopic area, where the prism view includes portions of the normal seeing hemifield, is larger, since the prism power is less than the angular size of the visible portion of the prisms. Diplopia crosshatching in the clinical perimetry is based on the overlap found in monocular OS and OD diagrams, but was not actually reported by the subjects, which we take as another indication of its inconsequence peripherally. Diplopia in the percept diagram is outlined and lightly shaded in prism view locations where it offers no expansion benefit. (G) Annotated detail of the upper prism area of (F), with color coding to identify the contribution of each eye to the visual confusion and diplopia. The area behind the prism shaded in red (and its red grid lines) is seen directly by OD, while the same area (blue shading and blurred blue grid lines) is seen by OS shifted to a different location by the prism (diplopia).
Figures 12A and 12B illustrate the expanding visual field when the patient's gaze is shifted into the blind field (in this case left) by about 15°. Since the gaze of the patient has shifted, the entire field is shifted to the left by 15° and the areas of expansion have also shifted farther to the left by this amount. As the gaze of the patient is shifted toward the blind field, more of the prism segment is now acting in the periphery of the patient's seeing hemifield; thus, there is more diplopia and visual confusion, created by the presence of the prismatically shifted and nonshifted views of the same region. Since the gaze is not directly into the prisms, the diplopia is peripheral and easily tolerated.
Figure 12. .
Unilateral horizontal 57Δ peripheral prisms, with gaze shifted. (A) With gaze shifted left 15°, more of the blind hemifield is visible in the prisms. Since the prism shift is less than the visible width of the prisms, there is diplopia in the overlap regions (crosshatched). (B) The corresponding percept diagram shows the visual confusion in the prism regions, combined with diplopia (outlined and lightly shaded in the prism view location). (C–D) Unlike sector prisms, when gaze is shifted 15° away from the blind side, the prisms still provide field expansion, providing some continued access to activity in the important region ahead of the wearer. Diagrams for 40Δ prisms are included in Supplementary Figure S1. They show more diplopia during gaze to the left and a smaller gap with gaze to the right.
When the patient's gaze is shifted right (away from the blind side), the prisms remain in the seeing periphery and provide field expansion that provides information from areas directly ahead of the patient while the gaze is averted (Figs. 12C–D). This, of course, is in contrast to sector prisms, which provide no expansion at primary gaze, let alone gazes directed away from the blind side. Continuing to monitor the straight ahead direction when looking to the side, as done with normal vision, may be particularly important during mobility.
Considering prism position size and power
Patients sometimes ask for the prisms to be shifted farther to their blind side to provide even greater expansion. At primary gaze, the extent of expansion is determined (and limited by) the prism power, not the prism edge positions. The prism base edge is almost always in the blind hemifield, except for extreme (and uncomfortable) gaze angles. Shifting the prism toward the blind side simply limits the availability of prism on the seeing side, where it is needed when gazing away from the blind side, as shown in Figure 13.
Figure 13. .
Examples of altering the upper 40Δ prism position of Figure 11D. (A–C) Temporal shifts of 5°, 10°, and 15°, respectively, open an increasing gap between the expansion area and the midline, decreasing, not increasing, the expansion. The Table of Contents icon animates this effect. (D) A 5° nasal shift only increases the amount of peripheral diplopia.
At primary gaze, if the prism power is less than the angular extent of the visible portion of the prism, diplopia will be present (cf Figs. 11A, 11D). If the power is greater, the apical scotoma creates a gap between the expansion area and the seeing side at primary gaze (as occurs with averted gaze in Fig. 12C). Peripheral diplopia, while not necessarily disturbing, represents a lost opportunity for further expansion, as the diplopic view through the prism would be more productively directed to a nonoverlapping region. Since it makes sense to optimize the prism effect at primary gaze (where the users spend most of their time,41 Vargas-Martin F, et al. IOVS. 2002;43:ARVO E-Abstract 3809), minimizing diplopia and any gap between the expansion area and the midline strikes the best balance, and this occurs when the prism power equals the prism half-width (when both are expressed in degrees).
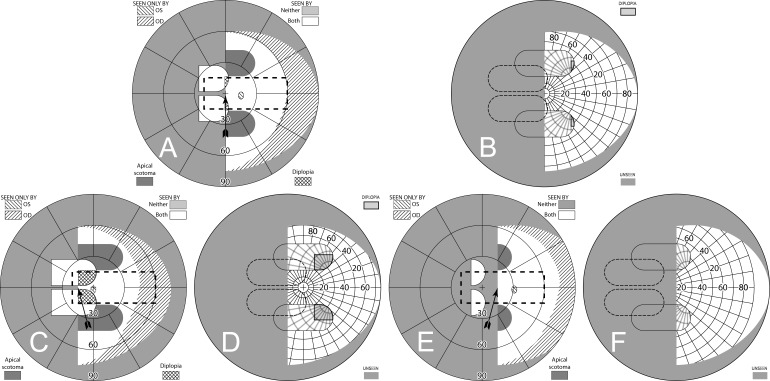
Bilateral Horizontal Peripheral Prisms
Although peripheral prisms are typically applied unilaterally, they can also be fitted bilaterally and yoked, all bases in the direction of the field loss. When each prism center is aligned with the pupil position at primary gaze there is no visual confusion, but apical scotomas are present in the binocular field (Fig. 14), as was the case for sector prisms. This is also the case if a patient with only one functional eye is fitted with peripheral prisms. Unlike sector prisms, however, objects not completely blocked vertically by the prisms can still be detected (as is the upper half of the man in Fig. 1B).
Figure 14. .
Bilateral horizontal 40Δ peripheral prisms. Since both eyes have the same prism views, the prism apical scotomas remain in the binocular view. (A) Simulated Goldmann diagram shows field substitution, not expansion. (B) The corresponding percept diagram shows no visual confusion, as the view from each eye is the same. Images from the blind side are merely shifted to displace areas of the seeing hemifield.
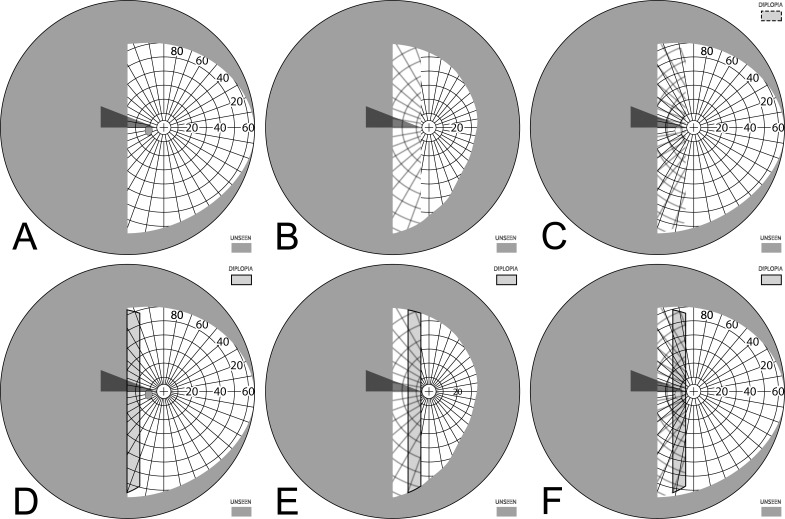
Offset Bilateral Horizontal Peripheral Prisms
The apical scotomas of bilateral fitting can be mitigated by offset placement of the prisms relative to the line of sight. If both eyes' prisms are moved nasally, the left and right apical scotomas can be made to fall in nonoverlapping areas of the visual field (Fig. 15). Since both bases are on the blind side, one prism apex (contralateral to the field loss) moves toward the line of primary gaze, and the other (on the lens ipsilateral to the field loss) moves away. Shifting the prisms relative to each other by an angular shift equal to the prism power accomplishes that (Fig. 15B). The figure also shows that less shift leaves partial scotomas, while more leads to peripheral diplopia at primary gaze. Of course, having different views through each prism does create visual confusion in peripheral regions proportional to the amount of prism offset, but, unlike unilateral placement, the true expansion area is seen binocularly without confusion. Portions of the seeing hemifield are now viewed with visual confusion, but hazards that exceed the prism height would be partially seen binocularly and without confusion on the seeing side. Although as yet untested clinically, this is likely to be preferable over unilateral placement and clearly avoids the apical scotomas of nonoffset bilateral placement.
Figure 15. .
Offset bilateral horizontal 40Δ peripheral prisms. (A) Spectacles with the upper prisms offset nasally (and outlined for clarity), shown for left hemianopia, though only the direction of the base (illustrated with the sharp points) will be different for right hemianopia. While the lower prisms could also be shifted, many spectacle frames do not provide much room for that, as the lens shape accommodates the flaring of the nose at the bottom. (B) Moving each of the upper prisms 10° (3.5 mm) nasally on its carrier lens (for a difference between them of 20°) essentially eliminates the 22° scotomas in the binocular field. (C) The percept diagram shows that even though the upper prisms are placed differently for each eye, they provide the same view where they overlap, since they have the same power. The view lost to the upper OD apical scotoma is seen in the OS prism, while the area of the OS apical scotoma is seen in the OD nonprism view. (D) Magnified detail of the upper prism area has the monocular views coded in color to more clearly identify the area of visual confusion possibly subject to suppression or rivalry. (E) Shifting each prism only 5° nasally (1.8 mm) still leaves significant scotomas. (F) Shifting each prism 15° (5.4 mm) introduces diplopia and needlessly extends the region of visual confusion. A total relative shift equal to the prism power is optimal.
Tilting the Prism Base-Apex Axis: The Oblique Peripheral Prism Design
The field expansion provided by horizontal peripheral prisms provides access to many of the obstacles, hazards, and orientation cues important for pedestrian mobility and common daily activities. Many states do not prohibit hemianopes from driving, in which case the prisms may be of help. However, as the vertical extent of a car's windshield is limited, the area of expansion provided by horizontal peripheral prisms falls mostly outside of the visual field typically used when driving. Moving the prisms vertically closer to primary gaze to increase the view through a windshield is not an acceptable option, as head bobbing due to car motion (that shifts the prisms into the gaze) could create the central confusion and diplopia that plague sector prisms. Leaving the prism location unchanged, but tilting the prism apex-base axes, provides a solution.40 This “oblique” design gives peripheral access to pericentral regions critical for driving without blocking the central view. The apical scotomas (or visual confusion) with this design mostly fall in areas inside the car, on the visor and dashboard. In an on-road study of people with hemianopia, use of the oblique prisms improved hazard detection on the blind side and reduced the number of interventions needed by the driving examiner.39
An oblique prism has the same effect as the superposition of a vertical and horizontal prism, as illustrated in Supplementary Figure S2 (433.8KB, pdf) .
Unilateral Oblique Peripheral Prisms
An apex-base angle of about 30° provides a good compromise between vertical shift and the resulting loss of some horizontal expansion. An oblique prism of 57Δ and 30° tilt provides 26° of horizontal shift and 15° vertical shift. Figure 16 diagrams the effects of this configuration. Supplementary Figure S3 has diagrams illustrating the effects of the same design with gazes to the right and left, and Supplementary Figure S4 has the corresponding diagrams for 40Δ prisms.
Figure 16. .
Unilateral oblique 57Δ peripheral prisms, ±30° apex-base angle at 12 mm interprism separation, provide access to pericentral regions. (A) Simulated Goldmann visual field diagram shows true expansion with no remaining apical scotomas. The dashed rectangle outlines the typical view area through a car's windshield.41 When driving, the prisms' expansion regions fall outside the direct road view, lessening any effect of visual confusion against the bland interior background. (B) The percept diagram shows the areas of visual confusion associated with unilateral fitting. (C) The magnified view of the upper prism area of (B) color-codes the monocular portions and shades the diplopic correspondence as in Figure 11. A portion of the small region of interocular diplopia is also seen as monocular diplopia by OS; inconsequential, but an interesting wrinkle. (Monocular diplopia occurs when the visual angle of the effective prism base-apex distance is less than the prism power.) When gaze is directed to the left (Supplementary Figs. S3A, S3B) the diplopic region increases. Although partially seen foveally, patients have not reported noticing it. (D) A measured patient visual field diagram.
Thus, unilateral oblique peripheral prisms provide awareness of areas closer to the horizontal meridian, as might benefit a driver. Unlike sector prisms, they do this without actually impinging on the pericentral region and, thus, maintain binocular foveal fusion and avoid central confusion. With unilateral fitting there is peripheral confusion, but apical scotomas are avoided in the binocular field. With the bland design of most visors and dashboards, the outside road view on the blind side, with its dynamic nature, is likely to predominate under this rivalrous condition.
Bilateral Oblique Peripheral Prisms
Oblique prisms can be fitted bilaterally (Fig. 17) to avoid the visual confusion associated with unilateral fitting. However, the apical scotomas are significant, and there is a small area of pericentral diplopia that increases as gaze is shifted toward the blind hemifield, rendering this a trade-off of questionable added value. Lower-power prisms increase the diplopia, as shown in Supplementary Figure S5. However, apical scotomas in the driving situation are mostly located outside the field of view through the windshield and, thus, may have lower impact on performance or safety. The view through the prisms is binocular and free of confusion or rivalry. This yet untested design may prove especially useful when driving.
Figure 17. .
Bilateral oblique 57Δ peripheral prisms, ±30° apex-base angle. With gaze forward (A, B) there is a bit of diplopia and significant peripheral apical binocular scotomas. With gaze shifted left (C, D), since the prism views include larger portions of the seeing hemifield, there is a larger area of pericentral diplopia, while areas behind the prisms are still lost to the apical scotomas. With gaze shifted right (E, F) there is some field substitution (but not expansion) without confusion or diplopia. The dashed rectangles in (A), (C), and (E) outline the typical view area through a car's windshield. When driving, most of the peripheral scotoma is inside the car, not detracting from the view of the road.
As with the horizontal prisms, offset placement can provide some mitigation of the apical scotomas in the binocular field. However, since only the horizontal component of the prisms is offset, the vertical component of the scotomas remains. Supplementary Figure S6 has diagrams of the offset bilateral oblique prisms.
Conclusions and Recommendations
Prisms are often prescribed to provide access to portions of the field of view lost to hemianopia. Failure to consider the pericentral view that is lost to prism apical scotomas can lead to deficits potentially more harmful than those the prisms are intended to resolve, yet reports of prism use frequently include discussions or diagrams that omit these scotomas.18,21,43–45 If the fellow eye does not have a scotoma in the same location, the effect can be mitigated. Unilateral fitting, or bilateral fitting of prisms offset so that their apical scotomas block different portions of the binocular visual field, can accomplish that, albeit by introducing regions of visual confusion and possibly also creating diplopia.
Visual confusion and diplopia are particularly objectionable if in central or pericentral view, while they are well tolerated in the periphery, where in some sense they are normal. Visual confusion is the mechanism by which field expansion with prisms is made possible. Without confusion, prisms can only shift the view, trading one scotomatous location with another. Diplopia, on the other hand, has no beneficial effect in these treatments and should be avoided or reduced when possible. Direct foveation through prisms reduces acuity and contrast sensitivity and introduces other disturbing and noticeable spatial and chromatic distortions. Peripherally-placed prisms are much less disturbing in these regards, and yet are effective in attracting attention that can cause the wearer to shift gaze centrally to the alerted direction, similar to the natural role of peripheral vision.
These observations lead us to conclude that traditional sector prisms, which provide no field substitution or expansion at primary gaze and induce central and pericentral scotomas when the gaze is shifted into them, are of questionable value. The claim that they serve to train the users to fixate in their direction7,46 has not been supported with any documentation. The peripheral prisms developed in our lab avoid those shortcomings and have been met with considerable patient acceptance. They have been found useful in single and multicenter clinical trials, both open label34 and randomized controlled.38,39 The peripheral prisms provide true field expansion at most horizontal gaze directions, including primary. Although operating in the periphery, the oblique design can provide pericentral field expansion. This effect is particularly important for patients who are permitted to drive. The prisms access important regions of the road view, while the confusion in unilateral fit and apical scotomas in bilateral fit fall in the bland interior of the car and thus are less likely to be deleterious. Although the peripheral prisms are generally fit unilaterally, as this is easier and less expensive, offset bilateral fitting provides another option for dealing with the apical scotomas. Oblique bilateral fitting may prove more beneficial for driving than unilateral fitting.
Despite the long history of sector prisms, we found only three controlled sector prism trials,18,32,47 and each had limitations. Rossi et al.18 used a parallel arm trial to evaluate bilateral 15Δ sector prisms against a no treatment control in patients undergoing an inpatient stroke rehabilitation program. The study (which found no advantage for the prisms in activities of daily living) recruited patients with either HH or spatial neglect (without HH) during the acute post stroke period, in which spontaneous recovery in neglect and visual field is common. Much of the improvement in the treatment group occurred on spatial neglect tests. However, the way sector prisms may be affecting neglect is not known and beyond the scope of this paper. “Expansion” occurred in both the treatment and control groups and did not include measurements in the seeing hemifield where the apical scotomas lay. As the prisms were fitted 2 mm into the blind hemifield, they should have had no effect on the perimetry results; thus, the improvements recorded were most likely due to spontaneous recovery. Gottlieb et al.32 evaluated their unilateral 18.5Δ sector prisms against a control device, which appeared similar, but included a “plano lens without prism power.” Methodology was not well described, but all subjects tried the real prisms before the shams. They reported increased “awareness” of the visual field ranging from 10° to 45° in binocular (but not monocular) viewing with the real prisms; however, a 45° increase in visual field from a 10.5° prism is physically impossible. Szlyk et al.,47 used a counterbalanced crossover trial to evaluate the Gottlieb unilateral 18.5Δ ophthalmic sector prisms against similarly shaped and positioned press-on 20Δ Fresnel prisms. The aim was to evaluate the relative efficacy of the two types of prisms when combined with an intensive 3-month training program. Unsurprisingly, improvements in performance on a wide range of tests were similar for the two prism types, as they had similar prismatic powers and differed only in optical quality and cosmetics. Unfortunately, the study design did not permit an evaluation of the benefits of the prisms alone (without training) relative to no prisms. The importance of including a sham control treatment in detecting placebo effects was highlighted in our clinical trial of real versus sham peripheral prisms38 in which 26% of subjects selected the sham control over no glasses or the real prisms.
The limitations in the clinical trials of sector prisms do not strengthen or weaken our comparison with peripheral prisms, as our arguments against sector prisms are based upon the physics of the configurations. Peripheral prisms were developed and refined specifically to address the shortcomings of sector prisms. The theoretical benefits of the design have now withstood verification in clinical, single and multicenter, and randomized controlled trials, and their continued use by about 50% of patients after 12 months is a strong result for a low vision rehabilitation aid.
Attention to the principles and diagrams we provided can avoid prescription mistakes. Moving peripheral prisms temporally will not increase their effect; rather it exacerbates the apical scotoma effects and wastes much of the prism extent where it is not likely to be used, robbing it from where it does provide benefit. Similarly, increasing the power of sector prisms increases the region lost to the apical scotomas, requiring even larger gaze shifts for pericentral views into the blind hemifield.
Our analyses and illustrations of the effects of apical scotomas have yielded subtle insights into the many ways prisms can both aid and hinder vision. Conventional wisdom and our own intuition were proved wrong or incomplete on numerous occasions by this analysis and diagramming.
Acknowledgments
Supported by the National Institutes of Health Grants EY12890 and P30EY003790.
Aspects of this study have been presented as: Ross NC, et al. IOVS. 2009;50:ARVO E-Abstract 4734.
Disclosure: H.L. Apfelbaum, None; N.C. Ross, None; A.R. Bowers, None; E. Peli, Schepens Eye Research Institute to Chadwick Optical (P)
References
- 1.Zhang X, Kedar S, Lynn MJ, Newman NJ, Biousse V. Natural history of homonymous hemianopia. Neurology. 2006;66:901–905. doi: 10.1212/01.wnl.0000203338.54323.22. [DOI] [PubMed] [Google Scholar]
- 2.Gall C, Franke GH, Sabel BA. Vision-related quality of life in first stroke patients with homonymous visual field defects. Health Qual Life Outcomes. 2010;8:1–28. doi: 10.1186/1477-7525-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowers AR, Mandel AJ, Goldstein RB, Peli E. Driving with hemianopia: 1. detection performance in a driving simulator. Invest Ophthalmol Vis Sci. 2009;50:5137–5147. doi: 10.1167/iovs.09-3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papageorgiou E, Hardiess G, Schaeffel F, et al. Assessment of vision-related quality of life in patients with homonymous visual field defects. Graefe's Arch Clin Exp Ophthalmol. 2007;245:1749–1758. doi: 10.1007/s00417-007-0644-z. [DOI] [PubMed] [Google Scholar]
- 5.Dickinson C. Low Vision: Principles and Practice. Oxford, England: Butterworth;; 1998. pp. 189–190. [Google Scholar]
- 6.Cotter SA. Uses of Prism in Low Vision. In: Weiss NJ, Brown WL, editors. Clinical Uses of Prism: A Spectrum of Applications. St. Louis: Mosby-Year Book, Inc.;; 1995. pp. 279–300. In. ed. [Google Scholar]
- 7.Perlin RR, Dziadul J. Fresnel prisms for field enhancement of patients with constricted or hemianopic visual fields. J Am Optom Assoc. 1991;62:58–64. [PubMed] [Google Scholar]
- 8.Bailey IL. Prismatic treatment for field defects. Optom Monthly. 1978;69:99–107. [Google Scholar]
- 9.Cohen JM, Waiss B. Visual field remediation. In: Cole RG, Rosenthal BP, editors. Remediation and Management of Low Vision. St. Louis: Mosby; 1996. pp. 1–25. In. eds. [Google Scholar]
- 10.Goodlaw E. Rehabilitating a patient with bitemporal hemianopia. Am J Optom Physiol Opt. 1982;59:617–619. doi: 10.1097/00006324-198207000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Cohen JM. An overview of enhancement techniques for peripheral field loss. J Am Optom Assoc. 1993;64:60–70. [PubMed] [Google Scholar]
- 12.Bishop PO. Binocular vision. In: Moses RA, editor. Adler's Physiology of the Eye: Clinical Applications. St. Louis: C.V. Mosby; 1998. pp. 575–649. In. ed. [Google Scholar]
- 13.Fannin TE, Grosvenor T. Clinical Optics. 2nd ed. Boston: Butterworth-Heinemann; 1996. The Correction of Ametropia; pp. 109–126. In. [Google Scholar]
- 14.Morgan MW. Distortions of ophthalmic prisms. Am J Optom. 1963;40:111. [Google Scholar]
- 15.Katz M. Visual acuity through Fresnel, refractive, and hybrid diffractive/refractive prisms. Optometry. 2004;75:503–508. doi: 10.1016/s1529-1839(04)70175-x. [DOI] [PubMed] [Google Scholar]
- 16.Satgunam P, Apfelbaum HL, Peli E. Volume perimetry: measurement in depth of visual field loss. Optom Vis Sci. 2012;89:E1353–E1363. doi: 10.1097/OPX.0b013e3182678df8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woods RL, Apfelbaum HL, Peli E. DLP-based dichoptic vision test system. J Biomed Optics. 2010;15:1–13. doi: 10.1117/1.3292015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossi PW, Kheyfets S, Reding MJ. Fresnel prisms improve visual perception in stroke patients with homonymous hemianopia or unilateral visual neglect. Neurology. 1990;40:1597–1599. doi: 10.1212/wnl.40.10.1597. [DOI] [PubMed] [Google Scholar]
- 19.Hoppe E, Perlin RR. The effectivity of Fresnel prisms for visual field enhancement. J Am Optom Assoc. 1993;64:46–53. [PubMed] [Google Scholar]
- 20.Jose RT, Smith AJ. Increasing peripheral field awareness with Fresnel prisms. Opt J Rev Optom. 1976;113:33–37. [Google Scholar]
- 21.Smith JL, Weiner IG, Lucero AJ. Hemianopic Fresnel prisms. J Clin Neuroophthalmol. 1982;2:19–22. [PubMed] [Google Scholar]
- 22.Young CA. Homonymous hemianopsia during pregnancy aided by reflecting prism. Arch Ophthalmol. 1929;2:560–565. [Google Scholar]
- 23.Wiener A. A preliminary report regarding a device to be used in lateral homonymous hemianopia. Arch Opthalmol. 1925;55 [Google Scholar]
- 24.Somani S, Brent MH, Markowitz SN. Visual field expansion in patients with retinitis pigmentosa. Can J Opthalmol. 2006;41:27–33. doi: 10.1016/S0008-4182(06)80062-1. [DOI] [PubMed] [Google Scholar]
- 25.Weiss NJ. An application of cemented prisms with severe field loss. Am J Optom Arch Am Acad Optom. 1972;49:261–264. doi: 10.1097/00006324-197203000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Lee AG, Perez AM. Improving awareness of peripheral visual field using sectorial prism. J Am Optom Assoc. 1999;70:624–628. [PubMed] [Google Scholar]
- 27.Bailey IL. Optometric Examination of the Older Adult. In: Rosenbloom AAJ, editor. Rosenbloom and Morgan's Vision and Aging. St. Louis: Butterworth-Heinemann; 2007. pp. 133–163. In. ed. [Google Scholar]
- 28.Bahill AT, Adler D, Stark L. Most naturally occurring human saccades have magnitudes of 15 degrees or less. Invest Ophthalmol. 1975;14:468–469. [PubMed] [Google Scholar]
- 29.Luo G, Vargas-Martin F, Peli E. The role of peripheral vision in saccade planning: learning from people with tunnel vision. J Vis. 2008;8:1–8. doi: 10.1167/8.14.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Good GW, Fogt N, Daum KM, Mitchell GL. Dynamic visual fields of one-eyed observers. Optometry. 2005;76:285–292. doi: 10.1016/s1529-1839(05)70311-0. [DOI] [PubMed] [Google Scholar]
- 31.Lovie-Kitchin J, Mainstone J, Robinson J, Brown B. What areas of the visual field are important for mobility in low vision patients? Clin Vis Sci. 1990;5:249–263. [Google Scholar]
- 32.Gottlieb DD, Freeman P, Williams M. Clinical research and statistical analysis of a visual field awareness system. J Am Optom Assoc. 1992;63:581–588. [PubMed] [Google Scholar]
- 33.Peli E. Field expansion for homonymous hemianopia by optically-induced peripheral exotropia. Optom Vis Sci. 2000;77:453–464. doi: 10.1097/00006324-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Bowers AR, Keeney K, Peli E. Community-based trial of a peripheral prism visual field expansion device for hemianopia. Arch Ophthalmol. 2008;126:657–664. doi: 10.1001/archopht.126.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blake R. A primer on binocular rivalry, including current controversies. Brain and Mind. 2001;2:5–38. [Google Scholar]
- 36.Giorgi RG, Woods RL, Peli E. Clinical and laboratory evaluation of peripheral prism glasses for hemianopia. Optom Vis Sci. 2009;86:492–502. doi: 10.1097/OPX.0b013e31819f9e4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Neill EC, Connell PP, O'Connor JC, Brady J, Reid I, Logan P. Prism therapy and visual rehabilitation in homonymous visual field loss. Optom Vis Sci. 2011;88:263–268. doi: 10.1097/OPX.0b013e318205a3b8. [DOI] [PubMed] [Google Scholar]
- 38.Bowers AR, Keeney K, Peli E. Randomized crossover clinical trial of real and sham peripheral prism glasses for hemianopia. JAMA Ophtalmol. doi: 10.1001/jamaophthalmol.2013.5636. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bowers AR, Tant M, Peli E. A pilot evaluation of on-road detection performance by drivers with hemianopia using oblique peripheral prisms. Stroke Res Treat. 2012;2012:10. doi: 10.1155/2012/176806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peli E. inventor; Peripheral field expansion device. US patent 7,374,284 B2. May 20, 2008. [Google Scholar]
- 41.Iorizzo DB, Riley ME, Hayhoe M, Huxlin KR. Differential impact of partial cortical blindness on gaze strategies when sitting and walking - an immersive virtual reality study. Vision Res. 2011;51:1173–1184. doi: 10.1016/j.visres.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vargas-Martin F, Garcia-Perez MA. Visual fields at the wheel. Optom Vis Sci. 2005;82:675–681. doi: 10.1097/01.opx.0000175624.34252.73. [DOI] [PubMed] [Google Scholar]
- 43.Cardenal MS, Hoban K, Toffel K. Novel low vision approach to bilateral superior altitudinal field loss. Optom Vis Sci. 2012;89(E-abstract 125908) [Google Scholar]
- 44.Woo GC, Mandelman T. Fresnel prism therapy for right hemianopia. Am J Optom Physiol Opt. 1983;60:739–743. doi: 10.1097/00006324-198308000-00012. [DOI] [PubMed] [Google Scholar]
- 45.van Waveren M, Jagle H, Besch D. Management of strabismus with hemianopic visual field defects. Graefe's Arch Clin Exp Ophthalmol. 2013;251:575–584. doi: 10.1007/s00417-012-2045-1. [DOI] [PubMed] [Google Scholar]
- 46.Perez AM, Jose RT. The use of Fresnel and ophthalmic prisms with persons with hemianopic visual field loss. J Vis Impair Blind. 2003;97:173–176. [Google Scholar]
- 47.Szlyk JP, Seiple W, Stelmack J, McMahon T. Use of prisms for navigation and driving in hemianopic patients. Ophthalmic Physiol Opt. 2005;25:128–135. doi: 10.1111/j.1475-1313.2004.00265.x. [DOI] [PubMed] [Google Scholar]