Abstract
Purpose:
We determined the accuracy of the inferior > superior > nasal > temporal (ISNT) neuroretinal rim area rule and its variants in adult Asian populations, and evaluated whether disc area impacts its performance characteristics.
Methods:
Participants in the Singapore Malay Eye Study (SiMES) and Singapore Indian Eye Study (SINDI) underwent standardized ocular examinations, including optic disc imaging with the Heidelberg retinal tomograph (HRT). Glaucoma was defined using the ISGEO criteria. HRT rim areas in the superior, inferior, nasal, and temporal quadrants were quantified. We determined sensitivity, specificity, and positive (PPV) and negative (NPV) predictive values of violating the ISNT rule and 4 variants (I > S > T, I > S, I > T, and combined I > T and S > T). The influence of disc area was analyzed with multivariate marginal logistic regression.
Results:
There were 6112 participants (mean age: 57.6 ± 10.3 years). Glaucoma was present in 194 individuals (3.2%). Among 11,840 eyes, 232 (93.2%) of 249 glaucomatous eyes and 9768 (84.3%) of 11,591 nonglaucomatous eyes, violated the ISNT rule. The ISNT rule had highest sensitivity (93.5%), but lowest specificity (15.7%); I > T had highest specificity (98.2%), but low sensitivity (7.4%). For all variants, PPVs were low (2.1%–8.4%) and NPVs were high (97.9–99.1%). Larger disc area was associated with reduced specificity for the ISNT rule (P < 0.001), and reduced sensitivity (P = 0.01) and increased specificity for I > S > T (P < 0.05). PPV increased (P < 0.05) and NPV decreased (P < 0.001) with increasing disc area.
Conclusions:
The ISNT rule based on HRT has high sensitivity, and the I > T, S > T, and combined I > T and S > T variants have high specificity. Disc area influences sensitivity, specificity, PPV, and NPV of the ISNT rule and its variants.
Translational Relevance:
The high sensitivity of the ISNT rule, and high specificities of its variants, may have potential utility when used in combination with other HRT algorithms for glaucoma assessment.
Keywords: ISNT rule, HRT, glaucoma
Introduction
Accurate assessment of structural damage of the optic nerve head is key for the diagnosis of glaucoma. A cardinal clinical sign of glaucomatous optic neuropathy is thinning of the neuroretinal rim, with a regional preference for the superior and inferior poles of the optic disc.1,2 Jonas et al. first proposed the inferior > superior > nasal > temporal (ISNT) rule, which is used widely in clinical practice, as an aid to discriminate between glaucomatous and nonglaucomatous optic discs.3,4 The ISNT rule states that in normal eyes, the thickness of the neuroretinal rim along the cardinal meridians of the optic disc, that is the rim width, decreases in the order inferior (I) > superior (S) > nasal (N) > temporal (T),3 and that the neuroretinal rims in glaucomatous optic discs violate this quantitative relationship.4
The ability of the ISNT rule in screening for glaucoma in the population is not established. In a clinic-based Caucasian population, Harizman et al. found that the ISNT rule, evaluated in stereoscopic optic disc photographs, was violated 6 times more frequently in glaucomatous than in nonglaucomatous eyes.5 Progrebniak et al. reported, in a pediatric clinic-based population, that 81% and 27% of glaucomatous and nonglaucomatous eyes violated the ISNT rule, based on rim width measurements.6 However, several other clinic-based studies also raise concern that the ISNT rule may not be sufficiently valid to diagnose glaucoma.7–9
Despite the wide use of the ISNT rule in clinical practice, the application of the ISNT rule generally is conducted with ophthalmoscopy and, therefore, is a subjective clinical sign. The Heidelberg retinal tomograph (HRT), a confocal scanning laser ophthalmoscope, is able to provide objective and reproducible measurements of the optic nerve head10–15 and, thus, may have potential use for population-based glaucoma screening. In our study, we determined the diagnostic performance of the ISNT neuroretinal rim area rule, and variants of this rule, for glaucoma based on the HRT in an Asian population. In addition, disc size is known to influence the diagnostic performance of HRT classification algorithms, such as the Moorfields regression analysis (MRA), glaucoma probability score (GPS), and linear discriminant functions.16–19 Whether the performance of the ISNT rule on the HRT is influenced by the spectrum of optic disc sizes in the population is unclear. Therefore, a second objective of this study was to evaluate the impact of disc area on the performance of the ISNT rule and its variants.
Methods
Study Population
Subjects for our study were from participants in the Singapore Malay Eye Study (SiMES, 2004–2006) and the Singapore Indian Eye Study (SINDI, 2007–2009). The detailed methodology of the SiMES and SINDI studies have been reported previously.20–22 In brief, an age-stratified random sampling was used to select ethnic Malays and Indians 40 to 80 years of age living in Singapore during each study period. Both studies received ethical approval from the institutional review board of the Singapore Eye Research Institute. The study was conducted in accordance with the Declaration of Helsinki, and written informed consent was obtained from all participants.
Ophthalmic Examination
All participants underwent a standardized interview and ocular examination. A slit-lamp examination of the anterior segment was performed, and the van Herick technique was used to determine peripheral anterior chamber depth. Intraocular pressure (IOP) was measured with a Goldmann applanation tonometer before pupil dilation. Gonioscopy was performed with a Goldmann 2-mirror lens on participants with shallow anterior chambers (van Herick grade ≤2), glaucoma suspects (definition provided below), and 1 in 5 randomly selected participants not fulfilling the first 2 criteria. Dynamic indentation gonioscopy with a four-mirror Sussman lens was performed to identify peripheral anterior synechiae.
A dilated stereoscopic optic disc evaluation was performed with a +78 diopter (D) lens at ×16 magnification with a measuring graticule by trained ophthalmologists. The margins of the optic cup were defined stereoscopically as the point of maximal inflection of vessels crossing the neuroretinal rim; the vertical cup diameter was measured as the vertical distance between the points of maximal centrifugal extension of the cup between 11 and 1 o'clock, and 5 and 7 o'clock. Vertical cup-disc ratio (VCDR) was calculated.
Automated perimetry was performed with near refractive correction by study technicians (SITA 24-2 Fast program, Humphrey visual field analyzer II; Carl Zeiss Meditec, Dublin, CA) on all glaucoma-suspect participants, and 1 in 5 consecutive nonglaucoma-suspect participants before pupil dilation. The visual field test was repeated if the test reliability criteria were not satisfied (fixation losses >20%, false-positives >33%, or false negatives >33%), or if there was a visual field defect.
Definitions
Glaucoma was defined based on the International Society for Geographical and Epidemiological Ophthalmology (ISGEO) criteria.23 Category 1 cases were defined as a glaucomatous optic disc abnormality (VCDR or VCDR asymmetry ≥97.5 percentile, or neuroretinal rim width from 11 to 1 o' clock or 5 to 7 o'clock <0.1 VCDR) and a corresponding glaucomatous visual field defect. Category 2 cases were defined as a severely damaged optic disc (VCDR or VCDR asymmetry ≥99.5 percentile) in the absence of a visual field test. In diagnosing category 1 or 2 cases, there had to be no other explanation for the VCDR finding (e.g., marked anisometropia or dysplastic discs) or visual field defect (e.g., macular degeneration, retinal vascular or cerebrovascular disease). Category 3 cases were defined as blindness in individuals with no visual field or optic disc data (corrected visual acuity <3/60), or who had previous glaucoma filtration surgery, or an IOP ≥99.5 percentile. Data from the 1 in 10 participants with normal perimetry in both eyes on 2 separate occasions were used to establish the 97.5 and 99.5 percentiles for VCDR, VCDR asymmetry, and IOP in this population. The ISNT rule was entirely exclusive of the criteria.
A glaucomatous visual field defect was defined based on the Hodapp Anderson Parish criteria,24 that is if both the following conditions were met: (1) a cluster of 3 nonedge, contiguous points on the pattern deviation plot, not crossing the horizontal meridian with a probability of less than 5%, and (2) a glaucoma hemifield test outside normal limits. Final definition, adjudication, and classification of glaucoma cases were reviewed by a senior glaucomatologist (TA).
Optic Disc Imaging
All participants were imaged after pupil dilation with the Heidelberg Retina Tomograph II (HRT II), which is a confocal scanning laser ophthalmoscope using a 670 nm diode laser. The optic disc and peripapillary retina were scanned with the field of view set to 15 degrees. Magnification errors were corrected using subjects' refractive status and corneal curvature measurements. Supplemental cylindrical lenses were adapted for subjects with astigmatism ≥1.0 D. The HRT II optic nerve head scan protocol generates a mean topographic image with a resolution of 384 × 384 from 3 individual topographic images. After the baseline image was captured, the optic disc margin was defined manually by an ophthalmologist (SCL) masked to the subject's glaucoma diagnosis. This critical step was achieved with a series of 6 dots plotted around the margin of the optic disc on the reflectance image. The disc margin was defined as the inner edge of Elschnig's ring. The standard reference plane was defined at 50 μm posterior to the mean contour line height between 350° and 356° along the contour line, which demarcates the neuroretinal rim from the cup.
Sectorial neuroretinal rim area was measured and calculated with the HRT 3.0 software. Angles at the optic nerve head follow the convention: 0° (temporal), 90° (superior), 270° (inferior), regardless of whether a left or right eye is analyzed. The angles increase in a clockwise manner for right eyes and anticlockwise for left eyes. The optic nerve head is split into 6 sectors based on this convention: temporal (−45°–45°), superotemporal octant (45°–90°), inferotemporal octant (−90° to −45°), nasal quadrant (135°–225°), superonasal octant (90°–135°), and inferonasal octant (−135° to −90°).25 In our study, for comparison of neuroretinal rim sectorial areas, the superior neuroretinal rim was defined as the combination of the superonasal and superotemporal octants, while the inferior neuroretinal rim was defined as the combination of the inferonasal and inferotemporal octants (see Figure 1). Analyses of neuroretinal rim area were performed up to 3 decimal places, which is the limit of measurement of the HRT. For the analyses in this study, we included only eyes with good quality HRT images (i.e., SD >50 μm).
Figure 1. .
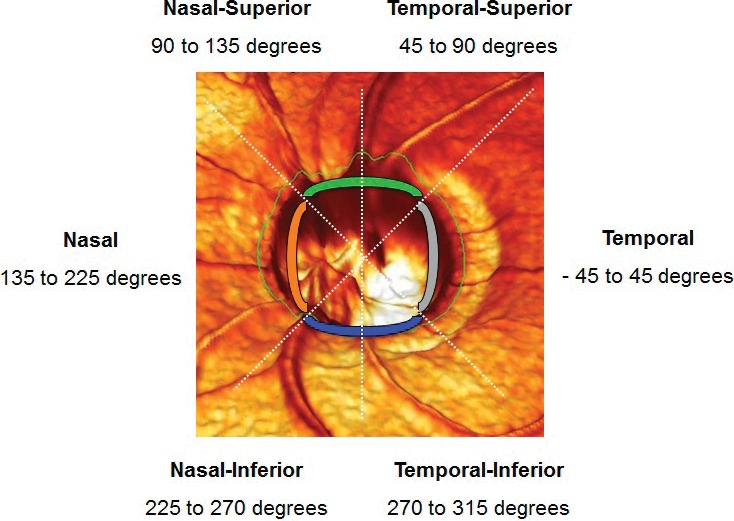
Schematic diagram of the optic nerve head captured and analyzed by the HRT showing temporal, nasal, superior, and inferior quadrants of the neuroretinal rim. The superior quadrant was based on combination of the superotemporal and superonasal octants, while the inferior quadrant was composed of by inferotemporal and inferonasal octants.
Statistical Analysis
Statistical analyses were performed using Stata 12.1 (Stata Corp., College Station, TX). Descriptive statistics of demographic and clinical characteristics were evaluated at the individual participant and eye levels.
The accuracy of the ISNT rule and 4 variants not involving the nasal rim (i.e., I > S > T; I > T; and S > T, I > T, and S > T) were evaluated. ISNT rule variants involving the nasal rim were excluded as the HRT measurements of the nasal rim (i.e., I > S > N, and I > N and S > N, I > N, and S > N) potentially may be inaccurate due to the more nasal site of the central retinal vessel trunk, which if included, would lead to inaccuracies in rim area measurements. The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated. The 95% confidence intervals (CIs) were calculated using clustered bootstrap with 100 resamplings.26 Subjects served as sampling unit to adjust for correlation between observations. The bootstrap analysis was implemented in R (R Development Core Team 2012).27
We analyzed the influence of disc area on the diagnostic accuracy of the ISNT rule and its variants using a generalized estimating equation (GEE) marginal logistic regression approach. This model is proposed by Leisenring et al.28 and Martus et al.,29 and adopted previously for evaluation of HRT diagnostic algorithms.15,30 According to the model, a binary variable for sensitivity was defined according to the results of the diagnostic test: 1 if the ISNT rule was violated and 0 otherwise. The general form for the model is as follows:
where S is the binary variable for sensitivity, D is the disease status of the eye, X is a covariate, and β is the regression coefficient of the model. The DX term is the interaction term between disease status and the covariate. For all models, only the interaction with disc area was included. To compute sensitivity, we evaluated the fitted model using D = 1. For specificity, we evaluated the fitted model using D = 0. PPV and NPV were modeled similarly by specifying the dependent variable to be the glaucoma status of the eye, and including the violation status of the ISNT rule as a covariate. By including disc area tertiles as covariates in the model, we computed sensitivity, specificity, PPV, and NPV separately for each tertile, while adjusting for potential confounders (age, sex, race, spherical equivalent, and IOP). A P value of < 0.05 was considered statistically significant.
Results
There were 3280 Malay and 3400 Indian participants in SiMES and SINDI, respectively. HRT II was performed on 6459 participants (3131 Malay, 3328 Indian). After exclusion of 126 participants who could not complete the test due to poor fixation or visual acuity, dense cataracts, or strabismus, there were 6333 participants (3056 Malay and 3277 Indian, total 12,666 eyes). Of these, we excluded further 801 eyes with unreliable HRT images, and 25 eyes with high myopia (i.e., spherical equivalent <−6 D) or congenital optic disc abnormalities. Thus, in our analysis, we included 11,840 eyes with acceptable HRT images.
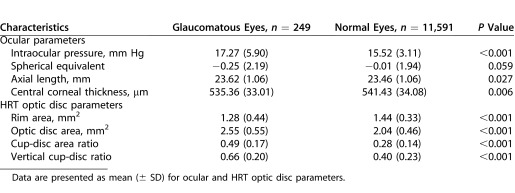
There were 194 (3.17%) individuals with glaucoma (249 eyes, 2.10%) and 5918 normal participants (11,591 eyes, 97.9%). Table 1 displays the baseline characteristics for the two groups. Compared to normal individuals, individuals with glaucoma were more likely to be older and Malay (both P < 0.001). Table 2 compares ocular characteristics between eyes with and without glaucoma. Eyes with glaucoma had higher IOP, longer axial length, and thinner central corneal thickness. There was no significant difference in spherical equivalent. Eyes with glaucoma had smaller rim area, and larger optic disc area, cup-disc area ratio, and VCDR (all P < 0.001).
Table 1. .
Demographic Characteristics of Glaucomatous and Nonglaucomatous Participants
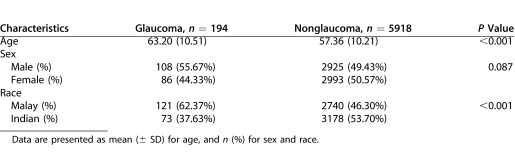
Table 2. .
Ocular and HRT Optic Disc Characteristics between Glaucomatous and Nonglaucomatous Eyes
Of the 11,840 study eyes, the ISNT rule was violated in 9768 (84.27%) of 11,591 eyes without glaucoma and 232 (93.17%) of 249 eyes with glaucoma. Table 3 summarizes the overall performance of the ISNT rule and the 4 variants excluding the nasal rim (i.e., I > S > T, combined I > T and S > T, I > T, and S > T). The ISNT rule had the highest sensitivity of 93.5%, specificity of 15.7%, PPV of 2.3%, and NPV of 99.1%. Sensitivities were lower for the I > S > T variant (44.4%) and lowest for the remaining 3 variants (i.e., combined I > T and S > T, I > T, and S > T, range 4.7%–10.3%). Compared to the ISNT rule, the specificity of the I > S > T variant was higher (55.9%), but the highest specificities were seen in the combined I > T and S > T, I > T, and S > T variants (range, 97.0%–98.2%). Regardless of the sensitivity or specificity values in the ISNT rule and the above variants, the PPVs were low (range, 2.1%–8.4%) and NPVs were high (range, 97.9%–99.1%). The PPV was highest for the I > T variant (8.4%) and NPV was highest for the ISNT rule (99.1%). Stratification by ethnicity showed largely similar results between Malays and Indians in sensitivity, specificity, PPV, and NPV for the ISNT rule and its variants, generally with 1% to 2% differences in point estimates. An exception was the I > S > T rule, where the sensitivity was 41.3% (95% CI 33.4,49.7) in Malays and 49.5% (95% CI 39.3,59.7) in Indians.
Table 3. .
Sensitivity, Specificity, PPV, and NPV of ISNT Rule and Variants for all Participants
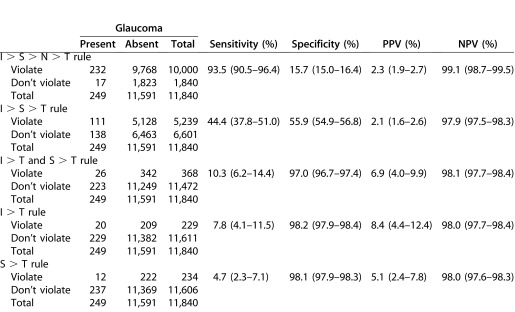
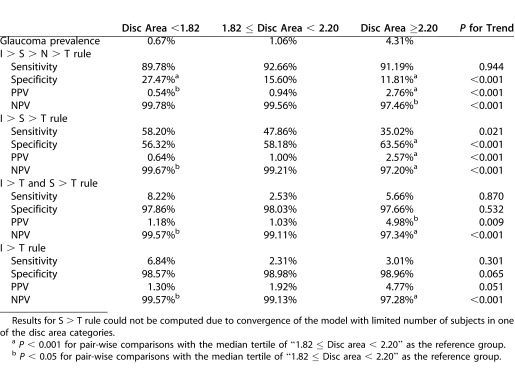
Table 4 shows the impact of disc area on glaucoma prevalence and trends in diagnostic accuracy of the ISNT rule and its variants. The S > T variant could not be computed due to convergence of the models with too few subjects in one of the disc area categories. Sensitivity generally was unaffected by disc area (P > 0.05), except for I > S > T, in which sensitivity decreased from 68.1% to 40.4% (P = 0.010). With increasing disc area, specificity decreased for the ISNT rule from 23.5% to 10.2% (P < 0.001), increased for the I > S > T rule from 53.0% to 59.6% (P < 0.001), but was unchanged for the combined I > T and S > T (P = 0.266), and I > T (P = 0.308) variants. There were statistically significant increases in PPV for the ISNT rule and all variants (P < 0.001); the increase in PPV for the ISNT rule and I > S > T variant was smaller (i.e., 0.8%–4.2%, and 0.9%–4.1%, respectively) than for the combined I > T and S > T, and I > T variants (1.7%–9.5%, 1.9%–10.0%, respectively). There were statistically significant decreases in the NPV for the ISNT rule and all variants (P < 0.001), but the magnitude of decrease was slight (between 99.7% and 95.9%). Sensitivity, specificity, PPV, and NPV estimates and trends, after adjustment for age, sex, ethnicity, IOP, and axial length in the GEE models (Table 5), were similar to the unadjusted values, with the exception that PPV for the I > T rule showed borderline significance (P = 0.051).
Table 4. .
Glaucoma Prevalence, and Sensitivity, Specificity, PPV, and NPV of ISNT Rule and Selected Variants (I > S > T, I > T, and S > T) Stratified by Disc Area
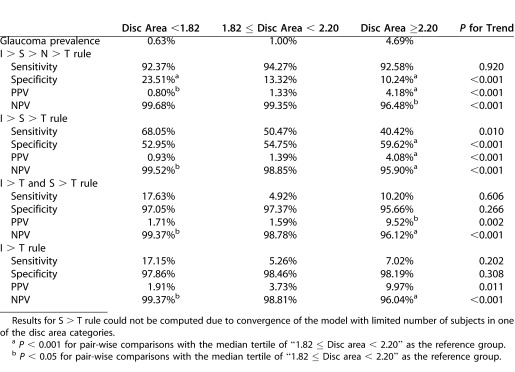
Table 5. .
Glaucoma Prevalence, and Sensitivity, Specificity, PPV, and NPV of ISNT Rule and Selected Variant (I > S > T, I > T, and S > T) Stratified by Disc Area, and Adjusted for Age, Sex, Ethnicity, IOP, and Axial Length
Discussion
Although several studies assessed the validity of the ISNT rule in select patient groups,5–9 to our knowledge this analysis provides the first population-based assessment of the performance of the ISNT rule, using objective neuroretinal rim area measurements by HRT.31,32 Unlike previous studies that largely analyzed sensitivities and specificities only, our study evaluated PPV and NPV performance indices, as PPV and NPV are influenced strongly by disease prevalence.30 This analysis indicated that the ISNT rule and its variants, determined based on the HRT, have limited value in population-based detection of glaucoma. No single algorithm had a combination of high sensitivity and specificity. In addition, due to the relatively low population prevalence of glaucoma, NPVs were high (97.9%–99.1%) and PPVs were low (2.1%–8.1%), indicating that a large number of individuals without glaucoma would be referred for further glaucoma evaluation based on the ISNT rule. We also showed that disc size influenced the performance of the ISNT rule and its variants. Larger disc area had mixed effects on sensitivity and specificity, and was associated with significant increases in PPV and decreases in NPV, independent of age, sex, ethnicity, axial length, and IOP.
Our study confirmed the high sensitivity of the ISNT rule with an earlier clinic-based report by Morgan et al. using manual planimetry,7 and further demonstrated that the NPVs for the ISNT rule and its other variants in a population setting are high (between 97.9% and 99.1%). In the earlier study,7 Morgan et al. found a high sensitivity of >90% for the ISNT rule, but a relatively more moderate negative likelihood ratio (LR) between 0.2 and 0.9, representing inability to exclude glaucoma with certainty. Our study concurred in that the sensitivity of the ISNT rule is high at 93.5%, but a key difference is that the NPV was also high at 99.1%, indicating reliability in excluding glaucoma at the population level. The high NPV of close to 100% is attributed to this being a population-based study with a low glaucoma prevalence, compared to the earlier clinic-based investigation. As expected, the specificity of the ISNT rule is considerably lower (15.7%), as sensitivity and specificity are negatively correlated, agreeing with findings by Morgan et al. (specificity 10%, positive LR 1.04).7 Importantly, the sensitivity of the ISNT rule in our study and the earlier study7 is higher than other current HRT diagnostic algorithms (i.e., MRA, GPS, and Mikelberg, Burk, and Bathija linear discriminant functions, which have reported sensitivities of <90% in clinic- and population-based studies).16,17,19,30,32,33 Thus, the high sensitivity and NPV of the ISNT rule per se revealed in our analysis indicated potential utility in population-based glaucoma evaluation.
Certain variants (I > T, S > T, and I > T and S > T) were notable for high specificities ranging between 97% and 98.2%; however, the PPVs for these algorithms were low (i.e., between 2.1% and 8.4%), which again reflected the low glaucoma prevalence. Morgan et al. also noted that, although the combined I > T and S > T variant had the highest specificity of 82% and highest positive LR of 3.37, the magnitude of the LR indicated that this variant per se could not confirm glaucoma reliably. These results have implications on the potentially high volume of unwarranted referrals for further evaluation of glaucoma, placing high demands on healthcare systems with limited resources. Nevertheless, because the specificities of selected variants (I > T, S > T, and S > T and S > T) are close to 100%, they have potential use to complement existing or novel HRT algorithms for glaucoma evaluation.
Disc area had mixed effects on sensitivity and specificity, but was linked with increased PPV, and decreased NPV. For the ISNT rule, larger disc area was associated with significantly reduced specificity from 23.5% to 10.3% (Table 4). This may be because larger discs, linked with higher cup-disc ratios, tend to violate the ISNT rule, and rim loss in small, crowded optic discs is more indicative of glaucoma. For the I > S > T rule, there was a significant decrease in sensitivity from 68.1% to 40.4%, and significant increase in specificity from 53% to 59.6%, with increasing disc area. Omission of the nasal rim, because of its proximity to the central retinal vessel trunk, is likely to change the sensitivity and specificity characteristics with regards to disc area. The link between disc area, and sensitivity and specificity also is well documented for other HRT glaucoma discrimination algorithms.16,19,30,32 Generally, with increasing optic disc size, PPV increased (from 0.8%–10%); this could be due to a higher glaucoma prevalence in eyes with larger optic discs (Table 4), giving a higher probability of identifying glaucomatous discs. There was a statistically significant decrease in NPV with disc area (ranging between 95.9% and 99.7%), which likely was due to the converse explanation, that is reduced likelihood of correctly identifying nonglaucomatous cases in eyes with larger discs.
Our study has several strengths. First, this was a large population-based evaluation involving more than 11,000 eyes. Previous clinic-based studies represent disease-enriched populations with higher glaucoma prevalence, and also comprised small numbers of individuals, frequently “supernormals.”5–9 Thus, this may skew estimates of test performance. Second, a segmental measure of neuroretinal rim area on the HRT is expected to provide more objective quantification of rim characteristics, than rim width measurements, which require precise determination of the cardinal meridia.8 Furthermore, a patient-friendly device allowing automated quantification of the neuroretinal rim, such as the HRT, is preferential to manual planimetry for population-based assessment, which is the cohort of interest in our study. Finally, we controlled for potential confounders, allowing confirmation that disc area independently influences the performance characteristics.
There are some limitations of this study. First, as mentioned earlier, HRT-measured nasal rim, as in the ISNT rule per se, may be inaccurate, as these measurements could have included the central retinal vessel trunk. The version of the ISNT rule analyzed used neuroretinal rim areas, rather than rim widths used in the traditional form of the rule; thus, differences may exist. Nevertheless, rim width measurements cannot be quantified on the HRT. In addition, we excluded severely distorted discs, for example myopic discs, as these are assessed inaccurately using HRT, but included all other discs, including tilted and torted discs, which may impose errors in rim contour assessment. However, the number of eyes with severely tilted or torted discs in our study was low. In clinical practice, such discs generally are poorly assessed by the HRT for glaucomatous change and, thus, excluding these from analysis would be appropriate.
In summary, to our knowledge this is the first study to use objective measurements of neuroretinal rim area on the HRT to evaluate the ISNT rule, demonstrating that these algorithms have limited use in population-based glaucoma screening. No single algorithm had a good combination of sensitivity and specificity, which limits the use of the ISNT rule as a stand-alone decision algorithm to identify glaucoma. Further work is required to identify alternative algorithms, or combination of algorithms leveraging on the high sensitivity of the ISNT rule, or high specificities of other rule variants (e.g., I > T and/or S > T), that would be optimal for use in glaucoma detection.
Acknowledgments
Disclosure: E.W. Chan, None; J. Liao, None; R. Wong, None; S.C. Loon, None; T. Aung, None; T.Y. Wong, None; C.-Y. Cheng, None
References
- 1.Hitchings RA, Spaeth GL. The optic disc in glaucoma. II. Correlation of the appearance of the optic disc with the visual field. Br J Ophthalmol. 1977;61:107–113. doi: 10.1136/bjo.61.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Airakisinen PJ, Drance SM. Neuroretinal rim area and retinal nerve fiber layer in glaucoma. Arch Ophthalmol. 1985;103:203–204. doi: 10.1001/archopht.1985.01050020055018. [DOI] [PubMed] [Google Scholar]
- 3.Jonas JB, Gusek GC, Naumann GO. Optic disc, cup and neuroretinal rim size, configuration and correlations in normal eyes. Invest Ophthalmol Vis Sci. 1988;29:1151–1158. [PubMed] [Google Scholar]
- 4.Jonas JB, Budde WM, Lang P. Neuroretinal rim width ratios in morphological glaucoma diagnosis. Br J Ophthalmol. 1998;82:1366–1371. doi: 10.1136/bjo.82.12.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harizman N, Oliveria C, Chiang A, et al. The ISNT rule and differentiation of normal from glaucomatous eyes. Arch Ophthalmol. 2006;124:1579–1583. doi: 10.1001/archopht.124.11.1579. [DOI] [PubMed] [Google Scholar]
- 6.Progrebniak AE, Wehrung B, Progrebniak KL, Shetty RK, Crawford P. Violation of the ISNT rule in nonglaucomatous pediatric optic disc cupping. Invest Ophthalmol Vis Sci. 2010;51:890–895. doi: 10.1167/iovs.09-3837. [DOI] [PubMed] [Google Scholar]
- 7.Morgan JE, Bourtsoukli I, Rajkumar KN, Ansari E, Cunliffe IA, North RV. The accuracy of the inferior>superior>nasal>temporal neuroretinal rim area rule for diagnosing glaucomatous optic disc damage. Ophthalmology. 2012;119:723–730. doi: 10.1016/j.ophtha.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Sihota R, Srinivasan G, Dada T, Gupta V, Ghate D, Sharma A. Is the ISNT rule violated in early primary open angle glaucoma – a scanning laser tomography study. Eye. 2008;22:819–824. doi: 10.1038/sj.eye.6702798. [DOI] [PubMed] [Google Scholar]
- 9.Hemamalini A, George R, Raju P, et al. Neural rim characteristics of healthy southern Indians: the Chennai Glaucoma Study. Invest Ophthalmol Vis Sci. 2008;49:3457–3464. doi: 10.1167/iovs.07-1210. [DOI] [PubMed] [Google Scholar]
- 10.Rohrschneider K, Burk RO, Kruse FE, Volcker HE. Reproducibility of the optic nerve head topography with a new laser tomographic scanning device. Ophthalmology. 1994;101:1044–1049. doi: 10.1016/s0161-6420(94)31220-6. [DOI] [PubMed] [Google Scholar]
- 11.Sharma P, Sample PA, Zangwill LM, Schuman JS. Diagnostic tools for glaucoma detection and management. Surv Ophthalmol. 2008;53((suppl)):S17–S32. doi: 10.1016/j.survophthal.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bathija R, Zangwill L, Berry CC, et al. Detection of early glaucomatous structural damage with confocal scanning laser tomography. J Glaucoma. 1998;7:121–127. [PubMed] [Google Scholar]
- 13.Zangwill LM, Bowd C, Berry CC, et al. Discriminating between normal and glaucomatous eyes using the Heidelberg retina tomography, GDx nerve fiber analyzer, and optical coherence tomography. Arch Ophthalmol. 2001;119:985–993. doi: 10.1001/archopht.119.7.985. [DOI] [PubMed] [Google Scholar]
- 14.Greaney MJ, Hoffman DC, Garway-Heath DF, et al. Comparison of optic nerve imaging methods to distinguish normal eyes from those with glaucoma. Invest Ophthalmol Vis Sci. 2002;43:140–145. [PubMed] [Google Scholar]
- 15.Zangwill LM, Jain S, Racette L, et al. The effect of disc size and severity of disease on the diagnostic accuracy of the Heidelberg retina tomography glaucoma probability score. Invest Ophthalmol Vis Sci. 2007;48:2653–2660. doi: 10.1167/iovs.06-1314. [DOI] [PubMed] [Google Scholar]
- 16.Oddone F, Centofanti M, Iester M, et al. Sector-based analysis with the Heidelberg Retinal Tomograph 3 across disc sizes and glaucoma stages. Ophthalmology. 2009;116:1106–1111. doi: 10.1016/j.ophtha.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 17.Ferreras A, Pajarin AB, Polo V, Larrosa JM, Pablo LE, Honrubia FM. Diagnostic ability of the Heidelberg Retina Tomograph 3 Classifications. Ophthalmology. 2007;114:1981–1987. doi: 10.1016/j.ophtha.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Iester M, Mikelberg FS, Drance SM. The effect of optic disc size on diagnostic precision with the Heidelberg retina tomography. Ophthalmology. 1997;104:545–548. doi: 10.1016/s0161-6420(97)30277-2. [DOI] [PubMed] [Google Scholar]
- 19.Zheng Y, Wong TY, Lamoureux EL, et al. Diagnostic ability of Heidelberg retina tomography in detecting glaucoma in a population setting. Ophthalmology. 2010;117:290–297. doi: 10.1016/j.ophtha.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 20.Foong AW, Saw SM, Loo JL, et al. Rational and methodology for a population-based study of eye disease in Malay people: the Singapore Malay Eye Study (SiMES) Ophthalmic Epidemiol. 2007;14:25–35. doi: 10.1080/09286580600878844. [DOI] [PubMed] [Google Scholar]
- 21.Shen SY, Wong TY, Foster PJ. The prevalence and types of glaucoma in Malay people: the Singapore Malay Eye Study. Invest Ophthalmol Vis Sci. 2008;49:3846–3851. doi: 10.1167/iovs.08-1759. [DOI] [PubMed] [Google Scholar]
- 22.Lavanya R, Jeganathan VS, Zheng Y, et al. Methodology of the Singapore Indian Chinese Cohort (SICC) Eye Study: quantifying ethic variations in the epidemiology of eye disease in Asians. Ophthalmic Epidemiol. 2009;16:325–336. doi: 10.3109/09286580903144738. [DOI] [PubMed] [Google Scholar]
- 23.Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86:238–242. doi: 10.1136/bjo.86.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodapp E, Parrish RU, Anderson DR. Classification of glaucomatous field loss by HFA. In: Hodapp E, et al., editors. , eds. Clinical Decisions in Glaucoma. St. Louis, MO: CV Mosby; 1993. pp. 52–61. In. [Google Scholar]
- 25.Heidelberg Retina Tomograph Glaucoma Module. Operating instructions. Software version 3.0. Heidelberg, Germany: Heidelberg Engineering;; 2005. [Google Scholar]
- 26.Rutter CM. Bootstrap estimation of diagnostic accuracy with patient-clustered data. AcadRadiol. 2000:7413–7419. doi: 10.1016/s1076-6332(00)80381-5. [DOI] [PubMed] [Google Scholar]
- 27.R Development Core Team. R: A language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. ISBN 3-900051-07-0. Available at: http://www.R-project.org. [Google Scholar]
- 28.Leisenring W, Pepe MS, Longton G. A marginal regression modelling framework for evaluating medical diagnostic tests. Stat Med. 1997;16:1263–1281. doi: 10.1002/(sici)1097-0258(19970615)16:11<1263::aid-sim550>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 29.Martus P, Stroux A, Junemann AM, et al. GEE approaches to marginal regression models for medical diagnostic tests. Stat Med. 2004;23:1377–1398. doi: 10.1002/sim.1745. [DOI] [PubMed] [Google Scholar]
- 30.Medeiros FA, Zangwill LM, Bowd C, et al. Influence of disease severity and optic disc size on the diagnostic performance of imaging instruments in glaucoma. Invest Ophthalmol Vis Sci. 2006;47:1008–1015. doi: 10.1167/iovs.05-1133. [DOI] [PubMed] [Google Scholar]
- 31.Shunmugam M, Azuara-blanco A. The quality of reporting of diagnostic accuracy studies in glaucoma using the Heidelberg Retina Tomograph. Invest Ophthalmol Vis Sci. 2006;47:2317–2323. doi: 10.1167/iovs.05-1250. [DOI] [PubMed] [Google Scholar]
- 32.Oddone F, Centofanti M, Rossetti L, et al. Exploring the Heidelberg retinal tomography 3 diagnostic accuracy across disc sizes and glaucoma stages. Ophthalmology. 2008;115:1358–1365. doi: 10.1016/j.ophtha.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Hawker MJ, Vernon SA, Ainsworth G. Specificity of the Heidelberg retina tomograph's diagnostic algorithms in a normal elderly population: the Bridlington Eye Assessment project. Ophthalmology. 2006;113:778–785. doi: 10.1016/j.ophtha.2005.10.068. [DOI] [PubMed] [Google Scholar]


