Abstract
Androgen and androgen receptor (AR) may play important roles in several skin related diseases, such as androgenetic alopecia and acne vulgaris. Current treatments for these androgen/AR-involved diseases, which target the synthesis of androgens or prevent its binding to AR, can cause significant adverse side effects. Based on the recent studies using AR knockout mice, it has been suggested that AR and androgens play distinct roles in the skin pathogenesis, and AR seems to be a better target than androgens for the treatment of these skin diseases. Here we review recent studies of androgen/AR roles in several skin-related disorders, including acne vulgaris, androgenetic alopecia, and hirsutism, as well as cutaneous wound healing.
Keywords: androgen, androgen receptor, wound healing, androgenetic alopecia, acne vulgaris, hirsutism
Introduction
The role of androgen/androgen receptor (AR) has been implicated in the skin physiology and pathogenesis based on the facts that AR and many androgenic steroidogenesis enzymes expressed in skin, and the presence of sexual dimorphism in the architecture and diseases of skin [35,55,117]. Recent progress using animal models and conditional AR knockout (ARKO) mice resulted in several important and interesting findings regarding how androgen/AR signals play roles in the skin-related diseases [61]. In this review, we will focus on the role of androgen/AR in several skin disorders, including acne vulgaris, androgenetic alopecia, hirsutism and cutaneous wound healing. The accumulated evidences and new findings suggest that AR, but not androgens, may become a potential target for more effective treatment of these androgen/AR-related skin disorders.
Androgen and androgen receptor
Androgens are well known to control the development and functions of the reproductive system in both male and female [56,114]. The major circulating androgen is testosterone, which is mainly produced by Leydig cells in the male testis; while adrenal glands are also capable of secreting testosterone, but at a much lesser degree. Testosterone can be metabolized by 5α-reductases into a more potent androgen, 5α-dihydrotestosterone (DHT). Both testosterone and DHT can bind to AR, but DHT has ten-fold higher affinity to AR compared to testosterone [16]. While skin is not the major source of androgen synthesis, in sebocytes, sweat glands, and dermal papilla cells, the circulating androgenic pro-hormones, dehydroepiandrosterone (DHEA) and androstenedione, can be converted into testosterone and DHT. These potent androgens subsequently regulate dermal physiology through intracrine or paracrine manners [117].
AR belongs to the nuclear receptor superfamily and contains a ligand-binding domain, a DNA-binding domain, and a N-terminal regulatory domain. Upon binding to its ligand (i.e. DHT or testosterone), AR translocates from the cytosol into the nucleus and serves as a transcriptional factor to regulate the expression of its target genes, such as prostate specific antigen (PSA). In addition to androgen-dependent activation, AR function can also be modulated by interaction with various coregulators through binding the AR N-terminal domain or ligand-binding domain. There have been more than 200 AR coregulators identified, including transcriptional factors (eg. ERα and AP-1), kinases (eg. ERK and MAK), chaperones (eg. HSP70 and HSP90), cytoskeletal proteins (eg. actin and filamin), and histone modifiers (eg. HDAC and SRC) [47,107], etc. Such complexity implicates AR serves as an important hub to converge and reflect multiple cellular functions and signals. The AR gene locates on the X chromosome; therefore, there is only one copy of AR gene in males, and its defect (naturally occurring mutant) in males results in testicular feminization syndrome (Tfm) [88].
As long ago as the 4th century BC, Aristotle saw a relationship between androgenetic alopecia and gender/sexual maturity. In 1942, Hamilton et al showed that prepubertal castration prevents sebum production and androgenetic alopecia. However, conditions were reversed with the addition of testosterone [45], suggesting that androgenetic alopecia is androgen-dependent. Indeed, it has been found that inherited 5α-reductase-II deficiency caused lower DHT levels and resulted in decreased facial and body hair [52]. The effects of androgens in the skin are mainly dependent on binding to AR. The lack of functional AR in skin prevents androgen action in skin appendages [118]. The AR gene has a polymorphism of glutamine repeats (polyQ) within exon 1, which when shortened may augment AR transactivation. It has been found that shorter polyQ polymorphisms in AR genes are more prevalent in people with androgenetic alopecia, hirsutism, and acne [27,86,94,112].
Due to the lack of a proper animal model, the detailed function of AR in the associated pathological roles remained difficult to elucidate until the conditional AR deficient mice became available [114]. Male ARKO mice were infertile with 80% smaller testes and lower testosterone levels than wild type males in addition to impaired prostate development [114]. It was also determined that a deficiency of AR in mice resulted in insulin resistance, a potential cause of type II diabetes [64]. The conditional ARKO strategy also plays a very important role in the research of the androgen/AR effects in skin-related diseases and will be discussed in the later sections of this review.
Androgen/AR in cutaneous wound healing
It has been suggested that sexual hormones are involved in cutaneous wound healing. Although there was no significant difference in the healing rate of young adults, the cutaneous wounds in elderly men heal more slower than those in elderly women; in addition, the serum testosterone level was negatively correlated to the healing rate in elderly men [3,31]. In the rodent models, AR has been detected in the keratinocytes, dermal fibroblasts, and infiltrating macrophages of healing wounds [3]. Castration of mice accelerates wound healing with attenuated inflammation, especially TNFα mRNA expression. Similarly, systemic flutamide (an antiandrogenic compound that can block the interaction between androgens and AR) treatment promotes wound closure as well [3]. Therefore, androgens seem to suppress cutaneous wound healing through binding to AR. In another study, Ashcroft and colleagues further suggested that DHT is important to suppress wound healing by modulating inflammatory responses [36]. Systemic inhibition of 5α-reductase enhances cutaneous wound healing in rats with less inflammatory cell infiltration, less interleukin-6 (IL-6) and TNFα expression, while TGFβ1-expressing cell number was increased in the wound sites. These findings imply that testosterone and DHT play distinct roles to suppress repair and DHT has more potent effects compared to testosterone [36]. Smad3 is downstream of TGFβ receptor signaling. Upon binding to its ligands, TGFβ receptor transduces signals to activate smad3 or smad2, which subsequently bind to smad4 and regulate gene expression [24]. Unlike in wild type (WT) mice, the effect of castration in enhancing wound healing is diminished in smad3 null mice, suggesting that androgen effects on wound healing suppression might be mediated by smad3 [4]. However in both castrated and ARKO mice, TGFβ1 levels were not significantly changed in wounds while healing rate was accelerated as compared to WT mice, indicating that this smad3 effect might be TGFβ1-independent [3,61]. It is noteworthy as well that cutaneous wound healing in smad3 null mice was already accelerated to the extent similar to that in castrated mice [5], so there might not be the further capacity for the effect of castration to be seen in the smad3 null mice. Castration of mice also accelerates collagen deposition in the healing wounds, and the concurrent increase of matrix metalloproteinase (MMP)-1 and MMP-13 suggests that androgen might suppress collagen deposition by modulating the expression and/or activation of collagenases [37].
Using conditional knockout strategy to study AR function in individual cell types, we found that, in general ARKO (total body ARKO) mice, cutaneous wound healing was accelerated with faster re-epithelialization, attenuated inflammation, and increased collagen deposition [61]. Interestingly, myeloid-specific ARKO mice showed a similar acceleration in wound healing, suggesting that AR in the inflammatory cells, especially macrophages, plays an important role to suppress wound repair. On the other hand, while AR in keratinocytes and fibroblasts was dispensable for regulating the overall wound healing rate, keratinocyte AR promoted but fibroblast AR suppressed re-epithelialization [61]. There is some controversy regarding the role of AR in keratinocyte functions. In vitro DHT treatment inhibited keratinocyte migration [38]; however, in vivo data showed that re-epithelialization was delayed in keratinocyte-specific ARKO mice without influencing keratinocyte proliferation, suggesting that keratinocyte AR might actually promote keratinocyte migration to hasten re-epithelialization [61]. Such controversy may be due to the fact that in vitro culture systems do not take into account the possible cross-talk among different cell types (such as fibroblasts and keratinocytes). In addition, the inhibitory effect on keratinocyte migration following in vitro DHT treatment only became obvious at higher doses (100 nM), which was higher than the physiological levels of DHT (1-10 nM). Therefore, the decrease in keratinocyte migration might be caused by cytotoxicity rather than through physiological DHT signaling.
TNFα, which is mainly expressed by the infiltrating macrophages, was reduced in the ARKO mice wounds compared to WT mice wounds, while local restoration of TNFα could reverse the acceleration of wound healing in ARKO mice, implicating that TNFα was critical in mediating AR function in wound healing suppression [61]. Further studies suggest that AR enhances local TNFα expression through multiple mechanisms, including increasing inflammatory monocyte population in the bone marrow and peripheral blood, promoting chemotaxis of monocytes by enhancing chemokine (C-C motif) receptor-2 (CCR2) expression, and enhancing TNFα expression in macrophages [61]. In addition to directly activating TNFα transcription [61], there are evidences showing that androgen/AR activates mTORC2, which subsequently activates Akt/SKG1 to phosphorylate FOXO1 and FOXO3a [30]. Upon phosphorylation, FOXO1/FOXO3a are released from the nucleus and degraded through the proteosome pathway [22]. Interestingly, dendritic cells deficient of FOXO3 showed increased TNFα and IL-6 production [22], implicating an indirect mechanism by which androgen/AR enhances TNFα production.
In terms of therapeutic purposes, blocking AR activation by using traditional antiandrogen (flutamide) or the AR degradation enhancer, 5-hydroxy-1, 7-bis (3,4-dimethoxyphenyl)-1,4,6-heptatrien-3-one (ASC-J9®) could suppress TNFα activity and hasten wound healing rate, suggesting the feasibility to promote healing by selectively targeting AR [3,61].
While AR plays a negative role in cutaneous wound healing, it might play an opposite role in mucosal wound healing, with men able to heal mucosal wounds faster than women. The possible explanation was the fundamental difference, including lower levels of neutrophils, macrophages, and their associated factors, in mucosal wounds compared to cutaneous wounds. Additionally, the mucosal epithelium has a quicker turnover rate, with more vascularization [28].
Androgen/AR in acne vulgaris
Acne is a disorder of the pilosebaceous unit in the face, neck, and upper trunk. It has been known that sebaceous glands are androgen target tissues. Castration could prevent sebum production, while testosterone replacement could reverse this condition [87]. AR has been detected in the epithelial cells of sebaceous glands by immunohistochemistry and biochemical binding assays [18].
In addition, sebaceous glands contain most of the steroidogenic enzymes for the conversion of DHEA/DHEAS (DHEA sulfate) into testosterone and DHT [15,32]. There are three isoforms of 5α-reductase, and their expression patterns vary across species and tissues [105]. Type I 5α-reductase mainly express in the sebocytes, keratinocytes, and dermal fibroblasts; type II 5α-reductase is mainly detected in the seminal vesicles, epididymis, prostate, and fibroblasts from adult genital skin, as well as the inner root sheath of the hair follicle; while the newly found type III 5α-reductase is detected in the prostate cancer and sebocyte cell lines [17,93]. In addition to its steroidogenic activity, type III 5α-reductase is critically involved in N-linked glycosylation [11]. Intriguingly, the sebum production rate in patients with the deficiency of type II 5α-reductase was similar to that of normal males [52], suggesting that DHT produced locally by type I 5α-reductase enhances sebum production. However in the clinical and in vitro studies, selective inhibitors for type I 5α-reductase did not significantly reduce sebum production and improve acne vulgaris [62,97], indicating that suppression of 5α-reductase alone might not be sufficient to improve acne. There are several possibilities to explain this. First, suppression of an individual type of 5α-reductase might not be sufficient to completely block DHT synthesis due to the redundancy between different types of 5α-reductase, and the sebaceous glands might be sensitive to even tiny amounts of DHT. Second, the newly found type III 5α-reductase might play a more important role in regulating sebum production. Third, in addition to the difference in their potency, testosterone and DHT do not act the same way on AR activation [36], suggesting that testosterone, rather than DHT, could be a more important regulator in sebum production.Additionally, the participation of AR coregulators might compensate for the deficit in DHT production.
Hormonal treatment is not usually the first option to treat female acne. However, some acne patients (30-80%) showed various degree of hyperandrogenemia [48,100]. Although there was no positive correlation between the severity of acne and markers of androgenecity [20], it is still possible to treat these patients by decreasing serum androgen levels or by inhibiting the action of androgens in the sebaceous glands. Flutamide is a nonsteroidal antiandrogen that inhibits the binding of DHT to AR but has no progestational effects [25]. The effects of flutamide on acne were observed mainly in patients with hirsutism and satisfactory results were observed for both conditions [95]. Although topical inocoterone treatment can reduce the size of sebaceous glands in the experimental animals [66], the clinical studies did not show significant effect to improve acne [66]. However, inocoterone indeed reduces the inflammatory acne lesions [66], likely due to suppression of AR function to dampen the inflammatory response [36,61]. Although in vitro studies with human SZ95 sebocytes and HaCaT keratinocytes, cyproterone acetate did not show significant effect on the testosterone-induced proliferation [97], but both topical and oral cyproterone acetate treatments improve acne lesions. Interestingly, the serum cyproterone level is much lower with topical treatment compared to oral treatment [42], suggesting that topical antiandrogen treatments of acne is practical and an important approach to consider, potentially with less risk of adverse effects compared to the systemic treatment [34]. Other AR blockers, e.g. spironolactone, also show certain levels of therapeutic effect on acne, but their applications are rather limited by significant side effects such as menstrual irregularity, birth defects, and hepatotoxicity [34]. Isotretinoin (13-cis-retinoic acid), a retinoid acid derivative, is a very effective sebum suppressive anti-acne agent. And there are evidences showing that Isotretinoin can modify AR signaling [8,9,72]. Human acnes are usually accompanied by excessive inflammation and infection [14]. Tetracycline is one of the antibiotics commonly used to treat acne. In addition to its antimicrobial activity, tetracycline can also inhibit inflammatory responses through regulating NFκB activation [81].
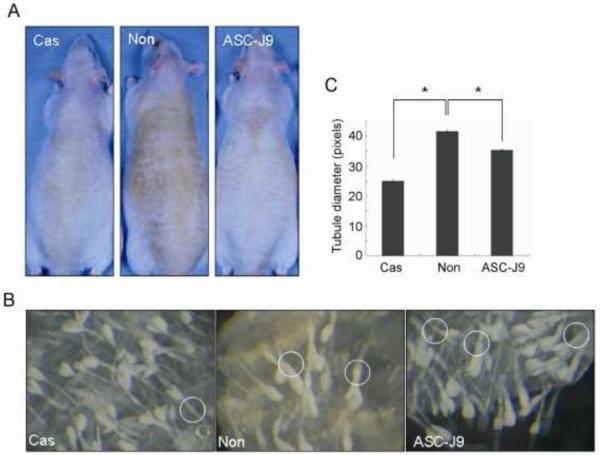
The characteristic of human acne is a multifaceted condition involving keratinization, androgens, sebum production, infections, and genetics. Evidences also showed the association between the western diet (especially diets with high glycemic load and high amounts of dairy proteins) and acne [21,68,71,101]. Unfortunately, none of the currently available animal models recapitulates all of these properties [76,83]. Among them, fuzzy rats are a mutant strain exhibiting androgen-dependent hyperplasia of sebaceous glands. Male fuzzy rats have increased secretion of sebum, and therefore, the dorsal skin shows a dark brownish seborrheic appearance. After castration, the seborrheic appearance of the skin becomes clean and whitish, the size of sebaceous glands decreases to female size, and sebum secretion is reduced as well (Fig. 1). Upon topical treatment with the AR antagonist (RU58841) or 5α-reductase inhibitors (finasteride and MK386) on the dorsal skin of male fuzzy rats, the inhibition of sebocytes proliferation, sebum production, and the size of sebaceous glands was observed, although with varying degrees of acne suppression [113]. The AR degradation enhancer, ASC-J9, has been shown effective on enhancing cutaneous wound healing following topical treatment [61], and the Phase II clinical studies of its topical application on acne vulgaris is on the way. Interestingly, the preliminary experiments on fuzzy rats show topical ASC-J9 treatment reduces sebum production and the size of sebaceous glands (Fig. 1). Another animal model for human acne uses Syrian golden hamsters, in which the growth of hamster flank organ is androgen dependent. After topically treating the animal with liposome-based 5α-reductase inhibitor (4-MA), the size of sebaceous gland is reduced and apoptosis is induced in flank organ [63]. The rhino mouse (hrrh/hrrh) is another mutant mouse strain of hairless mice to study the sebaceous gland activity related to human acneic comedones [83]. In spite of the advantages in handling the treatment conditions and genetic manipulations, it is noteworthy that animal models for acne treatments do not always mirror the effects in clinical studies [66].
Fig. 1.
ASC-J9® treatment alleviates seborrhea symptoms in male fuzzy rats. Starting from 28 days of age, male fuzzy rats were separated into 3 groups. One group was castrated (Cas), another was treated topically with ASC-J9® cream on the dorsal skin 5 days a week (ASC-J9®), and the other group was left untreated (Non). (A) Eight weeks after castration or ASC-J9® treatment, the dorsal skin was photographed to compare the seborrhea between groups. (B) The skin tissues were dissected and treated with 17 mM EDTA/PBS buffer for 2 hours at 37°C. Then the epidermis was separated from the dermis by forceps and fixed with 4% paraformaldehyde/PBS solution. The sebaceous glands were observed under a dissecting microscope at a magnification of 50X and photographed. Circles indicate the sebaceous ducts, and (C) the diameter of the ducts was measured using Image J software (NIH). Data are presented as mean ± S.E. *, p<0.01
The mechanisms by which androgen/AR regulate sebocyte activity in acne vulgaris are still unclear, and several possibilities have been suggested. For example, AR might enhance the activities of fibroblast growth factor receptor 2 (FGFR2), which has been shown critical in sebaceous gland development and homeostasis [69]. Second, AR might enhance lipogenesis in sebocytes through increasing the expression of sterol regulatory element binding proteins (SREBPs) [70]. Third, androgens might cross talk with insulin-like growth factor-1 (IGF-1) activities in regulating acne development [6,70]. IGF-1 has been shown able to induce SREBP-1 expression and lipogenesis in sebocytes [102,108]. In addition, IGF-1 can enhance AR activity through multitude mechanisms that will be discussed later in more details [70,74]. And fourth, androgens/AR have been suggested able to enhance inflammatory responses of macrophages and neutrophils [19,61]. As a result, androgen/AR might enhance not only the sebaceous gland activity, but also the inflammation that promotes acne formation and progression. Therefore, suppressing AR function by treating with antiandrogens alone, or in combination with antibiotics (i.e., to reduce bacterial infection) might be a potential therapeutic approach to treat acnes more effectively (Fig. 2). However, since most of these studies were carried out in animal models or in vitro, as mentioned above, it is necessary to further verify these mechanisms in clinical studies.
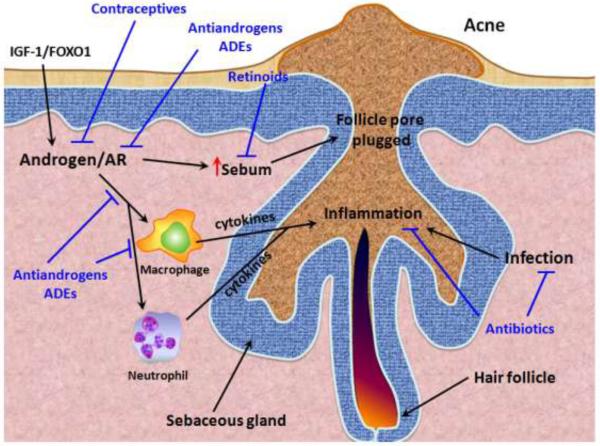
Fig. 2.
The role of androgens/AR in acne formation/progression. Acne formation in human results from excessive sebum formation and is usually accompanied by excessive inflammation and infections in the hair follicle. Macrophages and neutrophils are recruited to the inflamed follicles and secrete cytokines and other mediators to promote the inflammation and infection clearance. However the inflammatory response also damages the normal tissues in the follicles. AR can promote the inflammatory response mediated by macrophages and neutrophils, and androgens/AR can also directly promote sebum production that plugs the follicle pore. The IGF-1/FOXO1 pathway can enhance AR activity and subsequently promote sebum production. Current treatments for acne include antibiotics for infection clearance and therefore reducing the subsequent inflammation. Some antibiotics (eg. tetracycline) possess both antimicrobial and anti-inflammatory effects. Retinoid (and its derivatives, eg. isotretinoin) suppresses sebum production and promotes extrusion of the plugged material in the follicles. Antiandrogens reduce androgen levels, and AR degradation enhancers (ADEs) directly target AR to regulate the AR-mediated activities during acne formation/progression, such as reducing sebum production and suppressing the function of macrophages and neutrophils to dampen the inflammatory response.
IGF-1, the growth hormone of puberty, induces synthesis of androgens and enhances 5α-reductases activity in the skin [74]. In addition, IGF-1/PI3K/Akt activity stimulates phosphorylation of FOXO1, an AR co-repressor. Upon phosphorylation, FOXO1 translocates from the nucleus into the cytosol and releases its inhibition on AR transactivation [29,67,70]. In patients with Laron syndrome, who are deficient in IGF-1, the development of acne or hirsutism is absent, even though they have functional AR [6]. Higher FOXO proteins concentration is detected in the nucleus of these patients [43]. Once overdosed with IGF-1 treatment, Laron patients developed acne and hirsutism with hyperandrogenism [58,73].
It is noteworthy that AR is actually regulated by both androgens and AR coregulators, and the outcome of AR function is the convergence/balance of these signals [47]. In the case of acne regulation, AR activity, at least in part, is determined by androgens and insulin/IGF-1/FOXO signals. Both high glycemic-load diet and milk/dairy proteins increase the insulin/IGF-1 signaling, which in turn has substantial influence on AR activities. This might explain in part the effect of diet in acne pathogenesis [68].
Recent studies in prostate cancer cells showed that DHT could stimulate the mTORC2/Akt/FOXO1 pathway, which is important in promoting cell growth [30]. It merits further studies to examine if androgens also promote sebocyte growth through similar pathways.
Androgen/AR in androgenetic alopecia
Androgenetic alopecia (AGA), also known as “male pattern baldness”, is the most frequent type of hair loss in males [85]. This clinical condition affects ~50% of the Caucasian male population by middle age. By age 80, over 90% of Caucasian males are affected at various degrees. This pathophysiology is resultant from hair follicle miniaturization in the predisposed scalp. As in men, hair thinning and loss are commonly found in females. The prevalence of female pattern hair loss (FPHL) is ~12% among females at 20-29 years of age and ~50% for females over 80 years old [26]. The hallmark of FPHL, like that of AGA, is hair follicle miniaturization, manifested as a diffuse thinning (the so called “Christmas tree”) pattern in the mid-frontal scalp region. Although the relationship between FPHL etiology and androgen or AR is less clear, a subset of affected females displays hyperandrogenism; perturbations of the androgen/AR pathway could be an underlying cause for their suffering.
Androgens, which normally stimulate terminal hair production in many sites of the body (e.g. the beard and the axillary regions), exert an opposite effect to suppress hair growth on genetically predisposed frontal and vertex scalp [90]. In the hair follicle, the circulating male hormone, testosterone, is converted by 5α-reductase enzyme into DHT. The DHT level is increased in balding scalp and thus is the more relevant androgen for AGA pathogenesis. The role of AR in AGA disorder has been supported by: (i) no balding is seen in individuals lacking a functional AR (for example, in patients with androgen insensitivity syndrome) [41]; and (ii) AR expression in the scalp of AGA patients is site specific–elevated in the frontal and vertex regions but normal in the parietal and occipital regions [49]. The gene association studies highlighted the AR gene as the only risk gene confirmed to date for AGA phenotypes [51]. It has been accepted that an AR gene variant or variant(s) are the primary culprit responsible for AGA onset and development, likely through an aberrant expression of the AR protein in the scalp follicle. A recent gene association study found that men with Kennedy’s Disease (or spinal bulbar muscular atrophy), a mortal neuron disease caused by mutant AR gene, had significantly thicker hair than normal men. Kennedy’s Disease is associated with limited androgen sensitivity because of a dysfunctional AR, with longer polyQ repeats or polymorphism in their AR gene; therefore, this discovery suggests that active AR function is a risk factor for androgenetic alopecia [99]. However, balding may be inherited in a polygenic mode [59]. Genome-wide studies of phenotypic identical siblings have been initiated to study new susceptible genes for AGA and have discovered a link between this skin disorder and loci located at chromosomes 20p11 [92] and 3q26 [50]. It is possible that genes present in these loci increase the risk for AGA, although signaling pathways associated with this hair disorder have not been identified. Recent meta-analysis results identified a significant association between the G allele of the AR StuI polymorphism and the risk for AGA especially in the white populations, but no association was found between the CAG or GGC polymorphism and the risk for AGA [116].
In animal studies, spontaneous hair growth in mice is at least partly regulated by the AR activation pathway; gonadectomy induced a wave of hair regrowth in male mice [2] and the anagen phase of the second hair cycle was longer in ARKO mice than in their WT littermates [82].
The dermal papilla is considered to be the main site of androgen action in the hair follicles, because dermal papilla cells derived from androgen sensitive sites (e.g. beard and frontal scalp) contain low capacity, high affinity AR [91]. However, the molecular action of androgens on the dermal papilla remains largely unclear. The current hypothesis suggests that the androgen/AR complex alters the production of autocrine and paracrine regulatory factors in the dermal papilla to influence self growth or the growth of epithelial components of the follicle. Using co-culture techniques, androgens were found to stimulate the synthesis and secretion of TGFβ from the dermal papilla isolated from the bald scalp, and this peptide factor may be responsible for androgen-induced growth inhibition in co-cultured epithelial cells [53]. Other diffusible regulatory factors modulating papilla-epithelium interaction may exist, which likely include the Wnt (Wingless-type MMTV integration site family) proteins [57].
Minoxidil is the only topical therapy approved by the US Food and Drug Administration (FDA) to treat AGA. This drug is available as 2% (also, the only drug approved for FPHL) and 5% solution preparations, and a 5% foam formulation. Minoxidil, a peripheral vasodilator, stimulates the growth of follicle keratinocytes and prolongs the anagen phase [75]; it is recommended for individuals whose hairs have not been completely miniaturized. Topical twice per day dosing of Minoxidil could take 4-6 months to produce a therapeutic effect and the maximal effect is reached after approximately one year of use. In clinical trials, the 2% or 5% solution was found to arrest hair loss on the vertex and frontal scalp for a majority of patients, but stimulated mild to moderate hair regrowth in only 30%-45% of the recruits [85]. Minoxidil treatment, if effective, is a life-long therapy, because hair growth would stop and the retained hair would fall once the treatment is discontinued. The therapeutic target for Minoxidil in AGA is still elusive, while potassium channels and prostaglandins are among the suggested ones, there is yet conclusive evidence to prove this [75]. Recent studies suggested the increased prostaglandin D2 (PGD2) level in the hair follicles is one of the major causes for AGA [33]. The effect of Minoxidil in increasing Prostaglandin E2 (PGE2) [60] warrants further studies to determine if the treatment of Minoxidil reduces the production of PGD2, possibly due to competing for the common upstream substrates of PGD2 and PGE2. Given that prostaglandin D2 synthase (PTGDS), the key enzyme to generate PGD2, is one of the androgen-responsive genes [104,115], it might explain at least in part the mechanism behind androgen/AR effects in AGA.
Finasteride is a systemic medicine approved in 1997 by the FDA to treat AGA. This compound is an inhibitor of the type II 5α-reductase, which converts testosterone to DHT and is abundantly expressed in the scalp [54]. With the approved dose of 1 mg per day, finasteride stabilized hair loss in most subjects, but induced mild to moderate hair regrowth in only 37-61% of the sufferers. Improvement in hair density may not be perceived until after 6-12 months of treatment, and 2 years of use are required to achieve a maximal effect [85]. Finasteride, similar to minoxidil, is not curative. Research interest has been focused on the development of dual type I and type II 5α-reductase inhibitors, such as dutasteride [84], in hopes of finding a more satisfactory drug to replace finasteride.
Since currently available treatments for AGA are very limited and face the problems of a slow onset of beneficial effects, high individual variability in efficacy, and unwanted sexual side effects, it is important to find a novel approach to treat AGA. Based on the more updated understanding of AGA pathogenesis, AR may serve as a better target than DHT to tackle this hair loss disorder. Classic antiandrogens are undesirable, because they are ineffective through dermal dosing and their long-term systemic use raises the risks of gynaecomastia and impotence. A new approach to treat AGA safely and effectively is to develop a topical anti-AR therapy to specifically silence the AR protein in the scalp, but not elsewhere in the body.
Androgen/AR in hirsutism
Overproduction of androgens or increased sensitivity of hair follicles to androgens has been the common cause of hirsutism in females. There are two major paths for treating hirsutism. The first is cosmetic treatment, which includes plucking, shaving, waxing, and laser removal of unwanted hairs. The second is hormonal treatment that usually comes in forms of antiandrogens or AR blockers. While these antiandrogen treatments may have adverse side effects in men, the side effects for women who have hirsutism may be averted through a variety of ways, making these the preferred treatment methods. Because hair growth is a very slow cyclical process, it may take several months for drug effects to appear.
Spironolactone is an AR blocker that competes with androgens to bind with the AR without activating the AR’s transcriptional functions. However, there are several detrimental side effects to this drug, including menstrual irregularities, breast tenderness, fatigue, gastritis, and headaches. This may be an option for treating androgenetic alopecia, but is more often used to treat hirsutism, as most of the side effects may be evaded with the use of a contraceptive pill [12]. Studies have shown that spironolactone treatment of 100 mg/day reduces serum testosterone levels. The treatment with spironolactone is dose-dependent, but most physicians do not recommend taking more than 100 mg/day [65]. Whereas it may cause nausea and the other side effects stated above, the treatment also resulted in a significant reduction in hair growth [13].
Cyproterone acetate is another AR blocker that directly competes with androgens to bind to the AR. It is a progestin derivative, and therefore has antigonadotropic action and also blocks androgen secretion. Although it is internationally used as a drug for hirsutism treatment, it is not allowed in the United States because it increased the occurrence of liver tumors in rats [96], as well as the report of several cases of liver cancer in women who used cyproterone acetate [98].
Flutamide is also an AR blocker that may be used to treat hirsutism. Women taking flutamide 125 mg three times a day experience lower serum levels of androgens after a year. Treatment with flutamide also results in significant recovery from hirsutism conditions. Blocking the AR most likely resulted in reduced androgen levels, which in turn slowed hair growth [78]. However, while it is an effective treatment, there is also concern for liver toxicity which may occur after several months of continuous use [109].
Finasteride is currently used in clinics for male androgenetic alopecia. Studies have shown that finasteride treatment was as effective in reducing hirsutism as spironolactone or flutamide. This is most likely due to the ability of finasteride to block the conversion of testosterone to the much more potent DHT [80]. However, in another study, flutamide was shown more effective than finasteride in treating hirsute women [106]. The efficacy of finasteride as compared to flutamide needs further verification. Aside from the efficacy, there is concern about using finasteride during pregnancy as it may cause birth defects.
Eflornithine is a relatively new topical treatment that seems to have a very favorable effect in treating hirsutism. It is an irreversible inhibitor for ornithine decarboxylase, which is normally affected by androgens to regulate cell proliferation in the hair follicle. Because eflornithine blocks this enzyme, the reduced enzyme activity results in little or no hair growth. However, like most topical treatments, the effect is only temporary and the reduction of hair growth is reversed shortly after treatment is discontinued [79]. Eflornithine can also improve the efficiency of standard laser hair removal [46,110].
Chlormadinone acetate is a steroidal progestin with antiandrogen and antigonadotropic affects. There are also reports showed its beneficial effect for hirsutism treatment in women, although the detailed mechanism remains elusive [39,44].
Future direction and potential impact to the skin-related disorders
The involvement of androgen/AR in skin pathogenesis has been studied for decades; however, the molecular mechanisms of how androgen/AR participates in these skin disorders remain largely unknown. The establishment of the conditional ARKO mouse model could provide a useful tool to dissect AR function in the individual cells or tissue types in skin pathogenesis and lead to insights/mechanistic explanations concerning these diseases. For example, ARKO mice under the rhino mouse background could be used to test the effects of androgens/AR in the sebaceous gland activity related to acneic comedones [7]. ARKO mice under the B6CBAF1 background might be used for studying androgenetic alopecia [103]. More and more studies could be expected in the near future to reveal the underlying mechanisms. From the conventional point of view, androgens and AR rely on each other to execute their physiological functions; however, a new concept has been emerging lately which discriminate between the roles of androgens and AR [19,61,89]. Increasing evidence suggest that several other factors, such as estrogens, antiandrogens and kinases, are able to activate the AR function in the absence of testosterone and DHT. Even growth factor (eg. insulin/IGF-1) signaling can modulate AR activities. On the other hand, androgens also have AR-independent effects [77,89]. To take these “non classic” androgen and AR functions into consideration, conditional ARKO models will provide very useful tools to distinguish the roles of androgens from the roles of AR. In addition, using the Cre-loxP system to generate female ARKO mice, it is also feasible to study the AR functions in female mice.
Current treatments targeting androgens (such as in the patients with prostate cancer) employ either surgical or chemical castration and usually result in considerable side effects, such as impotence, loss of libido, osteoporosis, and fatigue [111]. Due to the profound adverse effects over the benefits for patients, these treatments are not to be used to treat skin disorders. Since the distinct roles of androgens and AR has been implicated, it is therefore very interesting and possible to develop better therapies that can specifically target AR (instead of androgens) or its downstream pathways to treat these disorders. Topical treatments with antiandrogens or AR degradation enhancers could be a better approach over systemic treatments to minimize the side effects, providing that these topical reagents can be efficiently delivered into the target cells and degraded before they can enter the circulation system at a significantly detrimental concentration. Indeed, an AR degradation enhancer has been demonstrated effective in the topical treatment of wound healing and acne with minimized adverse effects [61].
From the clinical point of view, advantages rise from using AR degradation enhancers or other small molecules to target AR in therapies. For example, in the clinical situation, impaired wound healing is prevalent in patients with diabetes mellitus, which is usually associated with prolonged and excessive inflammation in the wound tissues. In diabetic animal models, systemic anti-TNFα antibody treatment promotes cutaneous wound healing [40], suggesting that TNFα plays a critical role in impairing the healing of diabetic mice. Therefore, directly targeting AR might be a potentially better therapeutic approach for diabetic wounds, with the following advantages over TNFα neutralizing antibody treatment: First, although TNFα critically mediates the effects of AR on wound healing suppression, it does not encompass all functions of AR [61]. Therefore, directly targeting AR will promote wound healing on a more general scale than will targeting TNFα. Second, the cost of using synthetic small molecules is significantly less than the cost of using neutralizing antibodies. Finally, chemical-based therapeutics are more stable and flexible in terms of drug delivery than protein-based therapeutics.
Many skin diseases, such as acne and psoriasis, involve abnormal or excessive inflammatory responses. It has been demonstrated that AR in macrophages can promote inflammation, especially via up-regulation of TNFα expression, to suppress wound healing [3,61]. Therefore, it would be of great interest to examine if AR is also involved in the regulation of other (skin) diseases showing dysregulation of inflammatory responses [1,10].For example, in the autoinflammatory pyogenic arthritis, pyoderma gangrenosum and acne (PAPA) syndrome, the symptoms include acne at puberty (with increased androgen and IGF-1 signaling) as well as pyoderma gangrenosum, a chronic autoinflammatory problem with impaired wound healing. Treatment with a TNFα blocker could improve the pathology of PAPA syndrome [23]. It is therefore conceivable that reduction in AR activities might be beneficial for the disease. The ultimate goal is to attenuate the disease states of these androgen/AR-involved diseases more efficiently with minimized side effects.
Acknowledgement
We thank Karen Wolf for helping prepare the manuscript. This work was supported by George Whipple Professorship Endowment, NIH grant DK73414, and Department of Health Clinical Trial and Research Center of Excellence grant DOH99-TD-B-111-004 to China Medical University, Taichung, Taiwan.
Footnotes
Conflict of interest The authors declare no conflict of interest.
References
- 1.Aggarwal BB, Shishodia S, Takada Y, Jackson-Bernitsas D, Ahn KS, Sethi G, Ichikawa H. TNF blockade: an inflammatory issue. Ernst Schering Res Found Workshop. 2006;56:161–186. doi: 10.1007/3-540-37673-9_10. [DOI] [PubMed] [Google Scholar]
- 2.Arias NH, Houssay AB, Pieretti SA. Effects of cyproterone and tamoxifen upon the hair waves in mice. Acta Physiol Lat Am. 1982;32(4):261–266. [PubMed] [Google Scholar]
- 3.Ashcroft GS, Mills SJ. Androgen receptor-mediated inhibition of cutaneous wound healing. J Clin Invest. 2002;110(5):615–624. doi: 10.1172/JCI15704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashcroft GS, Mills SJ, Flanders KC, Lyakh LA, Anzano MA, Gilliver SC, Roberts AB. Role of Smad3 in the hormonal modulation of in vivo wound healing responses. Wound Repair Regen. 2003;11(6):468–473. doi: 10.1046/j.1524-475x.2003.11614.x. [DOI] [PubMed] [Google Scholar]
- 5.Ashcroft GS, Yang X, Glick AB, Weinstein M, Letterio JL, Mizel DE, Anzano M, Greenwell-Wild T, Wahl SM, Deng C, Roberts AB. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol. 1999;1(5):260–266. doi: 10.1038/12971. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Amitai D, Laron Z. Effect of insulin-like growth factor-1 deficiency or administration on the occurrence of acne. J Eur Acad Dermatol Venereol. 2011;25(8):950–954. doi: 10.1111/j.1468-3083.2010.03896.x. [DOI] [PubMed] [Google Scholar]
- 7.Bernerd F, Schweizer J, Demarchez M. Dermal cysts of the rhino mouse develop into unopened sebaceous glands. Arch Dermatol Res. 1996;288(10):586–595. doi: 10.1007/BF02505261. [DOI] [PubMed] [Google Scholar]
- 8.Boudou P, Chivot M, Vexiau P, Soliman H, Villette JM, Julien R, Belanger A, Fiet J. Evidence for decreased androgen 5 alpha-reduction in skin and liver of men with severe acne after 13-cis-retinoic acid treatment. J Clin Endocrinol Metab. 1994;78(5):1064–1069. doi: 10.1210/jcem.78.5.8175961. [DOI] [PubMed] [Google Scholar]
- 9.Boudou P, Soliman H, Chivot M, Villette JM, Vexiau P, Belanger A, Fiet J. Effect of oral isotretinoin treatment on skin androgen receptor levels in male acneic patients. J Clin Endocrinol Metab. 1995;80(4):1158–1161. doi: 10.1210/jcem.80.4.7714084. [DOI] [PubMed] [Google Scholar]
- 10.Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest. 2008;118(11):3537–3545. doi: 10.1172/JCI36389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cantagrel V, Lefeber DJ, Ng BG, Guan Z, Silhavy JL, Bielas SL, Lehle L, Hombauer H, Adamowicz M, Swiezewska E, De Brouwer AP, Blumel P, Sykut-Cegielska J, Houliston S, Swistun D, Ali BR, Dobyns WB, Babovic-Vuksanovic D, van Bokhoven H, Wevers RA, Raetz CR, Freeze HH, Morava E, Al-Gazali L, Gleeson JG. SRD5A3 is required for converting polyprenol to dolichol and is mutated in a congenital glycosylation disorder. Cell. 2010;142(2):203–217. doi: 10.1016/j.cell.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chapman MG, Dowsett M, Dewhurst CJ, Jeffcoate SL. Spironolactone in combination with an oral contraceptive: an alternative treatment for hirsutism. Br J Obstet Gynaecol. 1985;92(9):983–985. doi: 10.1111/j.1471-0528.1985.tb03081.x. [DOI] [PubMed] [Google Scholar]
- 13.Chapman MG, Katz M, Dowsett M, Hague W, Jeffcoate SL, Dewhurst CJ. Spironolactone in the treatment of hirsutism. Acta Obstet Gynecol Scand. 1986;65(4):349–350. doi: 10.3109/00016348609157358. [DOI] [PubMed] [Google Scholar]
- 14.Chen W, Obermayer-Pietsch B, Hong JB, Melnik B, Yamasaki O, Dessinioti C, Ju Q, Liakou A, Al-Khuzaei S, Katsambas A, Ring J, Zouboulis C. Acne-associated syndromes: models for better understanding of acne pathogenesis. J Eur Acad Dermatol Venereol. 2011;25(6):637–646. doi: 10.1111/j.1468-3083.2010.03937.x. [DOI] [PubMed] [Google Scholar]
- 15.Chen W, Thiboutot D, Zouboulis CC. Cutaneous androgen metabolism: basic research and clinical perspectives. J Invest Dermatol. 2002;119(5):992–1007. doi: 10.1046/j.1523-1747.2002.00613.x. [DOI] [PubMed] [Google Scholar]
- 16.Chen W, Zouboulis CC, Fritsch M, Blume-Peytavi U, Kodelja V, Goerdt S, Luu-The V, Orfanos CE. Evidence of heterogeneity and quantitative differences of the type 1 5alpha-reductase expression in cultured human skin cells--evidence of its presence in melanocytes. J Invest Dermatol. 1998;110(1):84–89. doi: 10.1046/j.1523-1747.1998.00080.x. [DOI] [PubMed] [Google Scholar]
- 17.Chen W, Zouboulis CC, Orfanos CE. The 5 alpha-reductase system and its inhibitors. Recent development and its perspective in treating androgen-dependent skin disorders. Dermatology. 1996;193(3):177–184. doi: 10.1159/000246242. [DOI] [PubMed] [Google Scholar]
- 18.Choudhry R, Hodgins MB, Van der Kwast TH, Brinkmann AO, Boersma WJ. Localization of androgen receptors in human skin by immunohistochemistry: implications for the hormonal regulation of hair growth, sebaceous glands and sweat glands. J Endocrinol. 1992;133(3):467–475. doi: 10.1677/joe.0.1330467. [DOI] [PubMed] [Google Scholar]
- 19.Chuang KH, Altuwaijri S, Li G, Lai JJ, Chu CY, Lai KP, Lin HY, Hsu JW, Keng P, Wu MC, Chang C. Neutropenia with impaired host defense against microbial infection in mice lacking androgen receptor. J Exp Med. 2009;206(5):1181–1199. doi: 10.1084/jem.20082521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cibula D, Hill M, Vohradnikova O, Kuzel D, Fanta M, Zivny J. The role of androgens in determining acne severity in adult women. Br J Dermatol. 2000;143(2):399–404. doi: 10.1046/j.1365-2133.2000.03669.x. [DOI] [PubMed] [Google Scholar]
- 21.Danby FW. Nutrition and acne. Clin Dermatol. 2010;28(6):598–604. doi: 10.1016/j.clindermatol.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 22.Dejean AS, Hedrick SM, Kerdiles YM. Highly specialized role of Forkhead box O transcription factors in the immune system. Antioxid Redox Signal. 2011;14(4):663–674. doi: 10.1089/ars.2010.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demidowich AP, Freeman AF, Kuhns DB, Aksentijevich I, Gallin JI, Turner ML, Kastner DL, Holland SM. Brief Report: Genotype, phenotype, and clinical course in five patients with PAPA syndrome (pyogenic sterile arthritis, pyoderma gangrenosum, and acne) Arthritis Rheum. 2012;64(6):2022–2027. doi: 10.1002/art.34332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425(6958):577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 25.Diamanti-Kandarakis E. Current aspects of antiandrogen therapy in women. Curr Pharm Des. 1999;5(9):707–723. [PubMed] [Google Scholar]
- 26.Dinh QQ, Sinclair R. Female pattern hair loss: current treatment concepts. Clin Interv Aging. 2007;2(2):189–199. [PMC free article] [PubMed] [Google Scholar]
- 27.Ellis JA, Stebbing M, Harrap SB. Polymorphism of the androgen receptor gene is associated with male pattern baldness. J Invest Dermatol. 2001;116(3):452–455. doi: 10.1046/j.1523-1747.2001.01261.x. [DOI] [PubMed] [Google Scholar]
- 28.Engeland CG, Bosch JA, Cacioppo JT, Marucha PT. Mucosal wound healing: the roles of age and sex. Arch Surg. 2006;141(12):1193–1197. doi: 10.1001/archsurg.141.12.1193. discussion 1198. [DOI] [PubMed] [Google Scholar]
- 29.Fan W, Yanase T, Morinaga H, Okabe T, Nomura M, Daitoku H, Fukamizu A, Kato S, Takayanagi R, Nawata H. Insulin-like growth factor 1/insulin signaling activates androgen signaling through direct interactions of Foxo1 with androgen receptor. J Biol Chem. 2007;282(10):7329–7338. doi: 10.1074/jbc.M610447200. [DOI] [PubMed] [Google Scholar]
- 30.Fang Z, Zhang T, Dizeyi N, Chen S, Wang H, Swanson KD, Cai C, Balk SP, Yuan X. Androgen Receptor Enhances p27 Degradation in Prostate Cancer Cells through Rapid and Selective TORC2 Activation. J Biol Chem. 2012;287(3):2090–2098. doi: 10.1074/jbc.M111.323303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fimmel S, Zouboulis CC. Influence of physiological androgen levels on wound healing and immune status in men. Aging Male. 2005;8(3-4):166–174. doi: 10.1080/13685530500233847. [DOI] [PubMed] [Google Scholar]
- 32.Fritsch M, Orfanos CE, Zouboulis CC. Sebocytes are the key regulators of androgen homeostasis in human skin. J Invest Dermatol. 2001;116(5):793–800. doi: 10.1046/j.1523-1747.2001.01312.x. [DOI] [PubMed] [Google Scholar]
- 33.Garza LA, Liu Y, Yang Z, Alagesan B, Lawson JA, Norberg SM, Loy DE, Zhao T, Blatt HB, Stanton DC, Carrasco L, Ahluwalia G, Fischer SM, FitzGerald GA, Cotsarelis G. Prostaglandin D2 Inhibits Hair Growth and Is Elevated in Bald Scalp of Men with Androgenetic Alopecia. Science Translational Medicine. 2012;4(126):126ra134. doi: 10.1126/scitranslmed.3003122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.George R, Clarke S, Thiboutot D. Hormonal therapy for acne. Semin Cutan Med Surg. 2008;27(3):188–196. doi: 10.1016/j.sder.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Gilliver SC, Ashcroft GS. Sex steroids and cutaneous wound healing: the contrasting influences of estrogens and androgens. Climacteric. 2007;10(4):276–288. doi: 10.1080/13697130701456630. [DOI] [PubMed] [Google Scholar]
- 36.Gilliver SC, Ashworth JJ, Mills SJ, Hardman MJ, Ashcroft GS. Androgens modulate the inflammatory response during acute wound healing. J Cell Sci. 2006;119(Pt 4):722–732. doi: 10.1242/jcs.02786. [DOI] [PubMed] [Google Scholar]
- 37.Gilliver SC, Ruckshanthi JP, Atkinson SJ, Ashcroft GS. Androgens influence expression of matrix proteins and proteolytic factors during cutaneous wound healing. Lab Invest. 2007;87(9):871–881. doi: 10.1038/labinvest.3700627. [DOI] [PubMed] [Google Scholar]
- 38.Gilliver SC, Ruckshanthi JP, Hardman MJ, Zeef LA, Ashcroft GS. 5alpha-dihydrotestosterone (DHT) retards wound closure by inhibiting re-epithelialization. J Pathol. 2009;217(1):73–82. doi: 10.1002/path.2444. [DOI] [PubMed] [Google Scholar]
- 39.Gomez Vazquez M, Navarra Amayuelas R, Lamarca M, Baquedano L, Romero Ruiz S, Vilar-Checa E, Iniesta MD. Ethinylestradiol/Chlormadinone acetate for use in dermatological disorders. Am J Clin Dermatol. 2011;12(Suppl 1):13–19. doi: 10.2165/1153875-S0-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goren I, Muller E, Schiefelbein D, Christen U, Pfeilschifter J, Muhl H, Frank S. Systemic anti-TNFalpha treatment restores diabetes-impaired skin repair in ob/ob mice by inactivation of macrophages. J Invest Dermatol. 2007;127(9):2259–2267. doi: 10.1038/sj.jid.5700842. [DOI] [PubMed] [Google Scholar]
- 41.Griffin JE, Wilson JD. The androgen resistance syndrome: 5α-reductase deficiency, testicular feminization, and related disorders. In: Scriver CR, Beaudet AL, Sly WS, D V, editors. The metabolic basis of inherited disease. McGraw-Hill; New York: 1989. pp. 1919–1944. [Google Scholar]
- 42.Gruber DM, Sator MO, Joura EA, Kokoschka EM, Heinze G, Huber JC. Topical cyproterone acetate treatment in women with acne: a placebo-controlled trial. Arch Dermatol. 1998;134(4):459–463. doi: 10.1001/archderm.134.4.459. [DOI] [PubMed] [Google Scholar]
- 43.Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng CW, Hwang D, Martin-Montalvo A, Saavedra J, Ingles S, de Cabo R, Cohen P, Longo VD. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med. 2011;3(70):70ra13. doi: 10.1126/scitranslmed.3001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guido M, Romualdi D, Campagna G, Ricciardi L, Bompiani A, Lanzone A. Ethinylestradiol-chlormadinone acetate combination for the treatment of hirsutism and hormonal alterations of normal-weight women with polycystic ovary syndrome: evaluation of the metabolic impact. Reprod Sci. 2010;17(8):767–775. doi: 10.1177/1933719110371515. [DOI] [PubMed] [Google Scholar]
- 45.Hamilton JB. Male Hormone stimulation is prerequisite and an incitant in common baldness. Am J Anat. 1942;71:451–480. [Google Scholar]
- 46.Hamzavi I, Tan E, Shapiro J, Lui H. A randomized bilateral vehicle-controlled study of eflornithine cream combined with laser treatment versus laser treatment alone for facial hirsutism in women. J Am Acad Dermatol. 2007;57(1):54–59. doi: 10.1016/j.jaad.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 47.Heemers HV, Tindall DJ. Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocr Rev. 2007;28(7):778–808. doi: 10.1210/er.2007-0019. [DOI] [PubMed] [Google Scholar]
- 48.Henze C, Hinney B, Wuttke W. Incidence of increased androgen levels in patients suffering from acne. Dermatology. 1998;196(1):53–54. doi: 10.1159/000017867. [DOI] [PubMed] [Google Scholar]
- 49.Hibberts NA, Howell AE, Randall VA. Balding hair follicle dermal papilla cells contain higher levels of androgen receptors than those from non-balding scalp. J Endocrinol. 1998;156(1):59–65. doi: 10.1677/joe.0.1560059. [DOI] [PubMed] [Google Scholar]
- 50.Hillmer AM, Flaquer A, Hanneken S, Eigelshoven S, Kortum AK, Brockschmidt FF, Golla A, Metzen C, Thiele H, Kolberg S, Reinartz R, Betz RC, Ruzicka T, Hennies HC, Kruse R, Nothen MM. Genome-wide scan and fine-mapping linkage study of androgenetic alopecia reveals a locus on chromosome 3q26. Am J Hum Genet. 2008;82(3):737–743. doi: 10.1016/j.ajhg.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hillmer AM, Hanneken S, Ritzmann S, Becker T, Freudenberg J, Brockschmidt FF, Flaquer A, Freudenberg-Hua Y, Jamra RA, Metzen C, Heyn U, Schweiger N, Betz RC, Blaumeiser B, Hampe J, Schreiber S, Schulze TG, Hennies HC, Schumacher J, Propping P, Ruzicka T, Cichon S, Wienker TF, Kruse R, Nothen MM. Genetic variation in the human androgen receptor gene is the major determinant of common early-onset androgenetic alopecia. Am J Hum Genet. 2005;77(1):140–148. doi: 10.1086/431425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Imperato-McGinley J, Gautier T, Cai LQ, Yee B, Epstein J, Pochi P. The androgen control of sebum production. Studies of subjects with dihydrotestosterone deficiency and complete androgen insensitivity. J Clin Endocrinol Metab. 1993;76(2):524–528. doi: 10.1210/jcem.76.2.8381804. [DOI] [PubMed] [Google Scholar]
- 53.Inui S, Itami S. Molecular basis of androgenetic alopecia: From androgen to paracrine mediators through dermal papilla. J Dermatol Sci. 2011;61(1):1–6. doi: 10.1016/j.jdermsci.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 54.Jenkins EP, Andersson S, Imperato-McGinley J, Wilson JD, Russell DW. Genetic and pharmacological evidence for more than one human steroid 5 alpha-reductase. J Clin Invest. 1992;89(1):293–300. doi: 10.1172/JCI115574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kanda N, Watanabe S. Regulatory roles of sex hormones in cutaneous biology and immunology. J Dermatol Sci. 2005;38(1):1–7. doi: 10.1016/j.jdermsci.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 56.Kimura S, Matsumoto T, Matsuyama R, Shiina H, Sato T, Takeyama K, Kato S. Androgen receptor function in folliculogenesis and its clinical implication in premature ovarian failure. Trends Endocrinol Metab. 2007;18(5):183–189. doi: 10.1016/j.tem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 57.Kitagawa T, Matsuda K, Inui S, Takenaka H, Katoh N, Itami S, Kishimoto S, Kawata M. Keratinocyte growth inhibition through the modification of Wnt signaling by androgen in balding dermal papilla cells. J Clin Endocrinol Metab. 2009;94(4):1288–1294. doi: 10.1210/jc.2008-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Klinger B, Anin S, Silbergeld A, Eshet R, Laron Z. Development of hyperandrogenism during treatment with insulin-like growth factor-I (IGF-I) in female patients with Laron syndrome. Clin Endocrinol (Oxf) 1998;48(1):81–87. doi: 10.1046/j.1365-2265.1998.00356.x. [DOI] [PubMed] [Google Scholar]
- 59.Kuster W, Happle R. The inheritance of common baldness: two B or not two B? J Am Acad Dermatol. 1984;11(5 Pt 1):921–926. doi: 10.1016/s0190-9622(84)80498-3. [DOI] [PubMed] [Google Scholar]
- 60.Kvedar JC, Baden HP, Levine L. Selective inhibition by minoxidil of prostacyclin production by cells in culture. Biochem Pharmacol. 1988;37(5):867–874. doi: 10.1016/0006-2952(88)90174-8. [DOI] [PubMed] [Google Scholar]
- 61.Lai JJ, Lai KP, Chuang KH, Chang P, Yu IC, Lin WJ, Chang C. Monocyte/macrophage androgen receptor suppresses cutaneous wound healing in mice by enhancing local TNF-alpha expression. J Clin Invest. 2009;119(12):3739–3751. doi: 10.1172/JCI39335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leyden J, Bergfeld W, Drake L, Dunlap F, Goldman MP, Gottlieb AB, Heffernan MP, Hickman JG, Hordinsky M, Jarrett M, Kang S, Lucky A, Peck G, Phillips T, Rapaport M, Roberts J, Savin R, Sawaya ME, Shalita A, Shavin J, Shaw JC, Stein L, Stewart D, Strauss J, Swinehart J, Swinyer L, Thiboutot D, Washenik K, Weinstein G, Whiting D, Pappas F, Sanchez M, Terranella L, Waldstreicher J. A systemic type I 5 alpha-reductase inhibitor is ineffective in the treatment of acne vulgaris. J Am Acad Dermatol. 2004;50(3):443–447. doi: 10.1016/j.jaad.2003.07.021. [DOI] [PubMed] [Google Scholar]
- 63.Li L, Tang L, Baranov E, Yang M, Amoh Y, Katsuoka K, Hoffman RM. Selective induction of apoptosis in the hamster flank sebaceous gland organ by a topical liposome 5-alpha-reductase inhibitor: a treatment strategy for acne. J Dermatol. 2010;37(2):156–162. doi: 10.1111/j.1346-8138.2009.00778.x. [DOI] [PubMed] [Google Scholar]
- 64.Lin HY, Xu Q, Yeh S, Wang RS, Sparks JD, Chang C. Insulin and leptin resistance with hyperleptinemia in mice lacking androgen receptor. Diabetes. 2005;54(6):1717–1725. doi: 10.2337/diabetes.54.6.1717. [DOI] [PubMed] [Google Scholar]
- 65.Lobo RA, Shoupe D, Serafini P, Brinton D, Horton R. The effects of two doses of spironolactone on serum androgens and anagen hair in hirsute women. Fertil Steril. 1985;43(2):200–205. doi: 10.1016/s0015-0282(16)48373-1. [DOI] [PubMed] [Google Scholar]
- 66.Lookingbill DP, Abrams BB, Ellis CN, Jegasothy BV, Lucky AW, Ortiz-Ferrer LC, Savin RC, Shupack JL, Stiller MJ, Zone JJ, et al. Inocoterone and acne. The effect of a topical antiandrogen: results of a multicenter clinical trial. Arch Dermatol. 1992;128(9):1197–1200. doi: 10.1001/archderm.128.9.1197. [DOI] [PubMed] [Google Scholar]
- 67.Ma Q, Fu W, Li P, Nicosia SV, Jenster G, Zhang X, Bai W. FoxO1 mediates PTEN suppression of androgen receptor N- and C-terminal interactions and coactivator recruitment. Mol Endocrinol. 2009;23(2):213–225. doi: 10.1210/me.2008-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Melnik B. Diet in Acne: Further Evidence for the Role of Nutrient Signalling in Acne Pathogenesis. Acta Derm Venereol. 2012;92(3):228–231. doi: 10.2340/00015555-1358. [DOI] [PubMed] [Google Scholar]
- 69.Melnik BC. Role of FGFR2-signaling in the pathogenesis of acne. Dermatoendocrinol. 2009;1(3):141–156. doi: 10.4161/derm.1.3.8474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Melnik BC. FoxO1 - the key for the pathogenesis and therapy of acne? J Dtsch Dermatol Ges. 2010;8(2):105–114. doi: 10.1111/j.1610-0387.2010.07344.x. [DOI] [PubMed] [Google Scholar]
- 71.Melnik BC. Evidence for acne-promoting effects of milk and other insulinotropic dairy products. Nestle Nutr Workshop Ser Pediatr Program. 2011;67:131–145. doi: 10.1159/000325580. [DOI] [PubMed] [Google Scholar]
- 72.Melnik BC. Isotretinoin and FoxO1: A scientific hypothesis. Dermatoendocrinol. 2011;3(3):141–165. doi: 10.4161/derm.3.3.15331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Melnik BC, John SM, Schmitz G. Over-stimulation of insulin/IGF-1 signaling by western diet may promote diseases of civilization: lessons learnt from laron syndrome. Nutr Metab (Lond) 2011;8:41. doi: 10.1186/1743-7075-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Melnik BC, Schmitz G. Role of insulin, insulin-like growth factor-1, hyperglycaemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009;18(10):833–841. doi: 10.1111/j.1600-0625.2009.00924.x. [DOI] [PubMed] [Google Scholar]
- 75.Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150(2):186–194. doi: 10.1111/j.1365-2133.2004.05785.x. [DOI] [PubMed] [Google Scholar]
- 76.Mirshahpanah P, Maibach HI. Models in acnegenesis. Cutan Ocul Toxicol. 2007;26(3):195–202. doi: 10.1080/15569520701502815. [DOI] [PubMed] [Google Scholar]
- 77.Miyamoto H, Yang Z, Chen YT, Ishiguro H, Uemura H, Kubota Y, Nagashima Y, Chang YJ, Hu YC, Tsai MY, Yeh S, Messing EM, Chang C. Promotion of bladder cancer development and progression by androgen receptor signals. J Natl Cancer Inst. 2007;99(7):558–568. doi: 10.1093/jnci/djk113. [DOI] [PubMed] [Google Scholar]
- 78.Moghetti P, Castello R, Negri C, Tosi F, Magnani CM, Fontanarosa MC, Armanini D, Muggeo M. Flutamide in the treatment of hirsutism: long-term clinical effects, endocrine changes, and androgen receptor behavior. Fertil Steril. 1995;64(3):511–517. doi: 10.1016/s0015-0282(16)57785-1. [DOI] [PubMed] [Google Scholar]
- 79.Moghetti P, Toscano V. Treatment of hirsutism and acne in hyperandrogenism. Best Pract Res Clin Endocrinol Metab. 2006;20(2):221–234. doi: 10.1016/j.beem.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 80.Moghetti P, Tosi F, Tosti A, Negri C, Misciali C, Perrone F, Caputo M, Muggeo M, Castello R. Comparison of spironolactone, flutamide, and finasteride efficacy in the treatment of hirsutism: a randomized, double blind, placebo-controlled trial. J Clin Endocrinol Metab. 2000;85(1):89–94. doi: 10.1210/jcem.85.1.6245. [DOI] [PubMed] [Google Scholar]
- 81.Monk E, Shalita A, Siegel DM. Clinical applications of non-antimicrobial tetracyclines in dermatology. Pharmacol Res. 2011;63(2):130–145. doi: 10.1016/j.phrs.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 82.Naito A, Sato T, Matsumoto T, Takeyama K, Yoshino T, Kato S, Ohdera M. Dihydrotestosterone inhibits murine hair growth via the androgen receptor. Br J Dermatol. 2008;159(2):300–305. doi: 10.1111/j.1365-2133.2008.08671.x. [DOI] [PubMed] [Google Scholar]
- 83.Nieves NJ, Ahrens JM, Plum LA, DeLuca HF, Clagett-Dame M. Identification of a unique subset of 2-methylene-19-nor analogs of vitamin D with comedolytic activity in the rhino mouse. J Invest Dermatol. 2010;130(10):2359–2367. doi: 10.1038/jid.2010.142. [DOI] [PubMed] [Google Scholar]
- 84.Olsen EA, Hordinsky M, Whiting D, Stough D, Hobbs S, Ellis ML, Wilson T, Rittmaster RS. The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. J Am Acad Dermatol. 2006;55(6):1014–1023. doi: 10.1016/j.jaad.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 85.Otberg N, Finner AM, Shapiro J. Androgenetic alopecia. Endocrinol Metab Clin North Am. 2007;36(2):379–398. doi: 10.1016/j.ecl.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 86.Pang Y, He CD, Liu Y, Wang KB, Xiao T, Wang YK, Zhu H, Wei B, Zhao N, Jiang Y, Wei HC, Chen HD. Combination of short CAG and GGN repeats in the androgen receptor gene is associated with acne risk in North East China. J Eur Acad Dermatol Venereol. 2008;22(12):1445–1451. doi: 10.1111/j.1468-3083.2008.02891.x. [DOI] [PubMed] [Google Scholar]
- 87.Pochi PE, Strauss JS. Endocrinologic control of the development and activity of the human sebaceous gland. J Invest Dermatol. 1974;62(3):191–201. doi: 10.1111/1523-1747.ep12676783. [DOI] [PubMed] [Google Scholar]
- 88.Quigley CA, De Bellis A, Marschke KB, el-Awady MK, Wilson EM, French FS. Androgen receptor defects: historical, clinical, and molecular perspectives. Endocr Rev. 1995;16(3):271–321. doi: 10.1210/edrv-16-3-271. [DOI] [PubMed] [Google Scholar]
- 89.Rahman F, Christian HC. Non-classical actions of testosterone: an update. Trends Endocrinol Metab. 2007;18(10):371–378. doi: 10.1016/j.tem.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 90.Randall VA, Thornton MJ, Hamada K, Redfern CP, Nutbrown M, Ebling FJ, Messenger AG. Androgens and the hair follicle. Cultured human dermal papilla cells as a model system. Ann N Y Acad Sci. 1991;642:355–375. [PubMed] [Google Scholar]
- 91.Randall VA, Thornton MJ, Messenger AG. Cultured dermal papilla cells from androgen-dependent human hair follicles (e.g. beard) contain more androgen receptors than those from non-balding areas of scalp. J Endocrinol. 1992;133(1):141–147. doi: 10.1677/joe.0.1330141. [DOI] [PubMed] [Google Scholar]
- 92.Richards JB, Yuan X, Geller F, Waterworth D, Bataille V, Glass D, Song K, Waeber G, Vollenweider P, Aben KK, Kiemeney LA, Walters B, Soranzo N, Thorsteinsdottir U, Kong A, Rafnar T, Deloukas P, Sulem P, Stefansson H, Stefansson K, Spector TD, Mooser V. Male-pattern baldness susceptibility locus at 20p11. Nat Genet. 2008;40(11):1282–1284. doi: 10.1038/ng.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Samson M, Labrie F, Zouboulis CC, Luu-The V. Biosynthesis of dihydrotestosterone by a pathway that does not require testosterone as an intermediate in the SZ95 sebaceous gland cell line. J Invest Dermatol. 2010;130(2):602–604. doi: 10.1038/jid.2009.225. [DOI] [PubMed] [Google Scholar]
- 94.Sawaya ME, Shalita AR. Androgen receptor polymorphisms (CAG repeat lengths) in androgenetic alopecia, hirsutism, and acne. J Cutan Med Surg. 1998;3(1):9–15. doi: 10.1177/120347549800300103. [DOI] [PubMed] [Google Scholar]
- 95.Schmidt JB. Other antiandrogens. Dermatology. 1998;196(1):153–157. doi: 10.1159/000017850. [DOI] [PubMed] [Google Scholar]
- 96.Schulte-Hermann R, Ohde G, Schuppler J, Timmermann-Trosiener I. Enhanced proliferation of putative preneoplastic cells in rat liver following treatment with the tumor promoters phenobarbital, hexachlorocyclohexane, steroid compounds, and nafenopin. Cancer Res. 1981;41(6):2556–2562. [PubMed] [Google Scholar]
- 97.Seiffert K, Seltmann H, Fritsch M, Zouboulis CC. Inhibition of 5alpha-reductase activity in SZ95 sebocytes and HaCaT keratinocytes in vitro. Horm Metab Res. 2007;39(2):141–148. doi: 10.1055/s-2007-961814. [DOI] [PubMed] [Google Scholar]
- 98.Shaw JC. Antiandrogen therapy in dermatology. Int J Dermatol. 1996;35(11):770–778. doi: 10.1111/j.1365-4362.1996.tb02970.x. [DOI] [PubMed] [Google Scholar]
- 99.Sinclair R, Greenland KJ, Egmond S, Hoedemaker C, Chapman A, Zajac JD. Men with Kennedy disease have a reduced risk of androgenetic alopecia. Br J Dermatol. 2007;157(2):290–294. doi: 10.1111/j.1365-2133.2007.08026.x. [DOI] [PubMed] [Google Scholar]
- 100.Slayden SM, Moran C, Sams WM, Jr., Boots LR, Azziz R. Hyperandrogenemia in patients presenting with acne. Fertil Steril. 2001;75(5):889–892. doi: 10.1016/s0015-0282(01)01701-0. [DOI] [PubMed] [Google Scholar]
- 101.Smith RN, Mann NJ, Braue A, Makelainen H, Varigos GA. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86(1):107–115. doi: 10.1093/ajcn/86.1.107. [DOI] [PubMed] [Google Scholar]
- 102.Smith TM, Gilliland K, Clawson GA, Thiboutot D. IGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinositide 3-kinase/Akt pathway. J Invest Dermatol. 2008;128(5):1286–1293. doi: 10.1038/sj.jid.5701155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sundberg JP, King LE, Bascom C. Animal models for male pattern (androgenetic) alopecia. Eur J Dermatol. 2001;11(4):321–325. [PubMed] [Google Scholar]
- 104.Treister NS, Richards SM, Suzuki T, Jensen RV, Sullivan DA. Influence of androgens on gene expression in the BALB/c mouse submandibular gland. J Dent Res. 2005;84(12):1187–1192. doi: 10.1177/154405910508401218. [DOI] [PubMed] [Google Scholar]
- 105.Uemura M, Tamura K, Chung S, Honma S, Okuyama A, Nakamura Y, Nakagawa H. Novel 5 alpha-steroid reductase (SRD5A3, type-3) is overexpressed in hormone-refractory prostate cancer. Cancer Sci. 2008;99(1):81–86. doi: 10.1111/j.1349-7006.2007.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Unluhizarci K, Ozel D, Tanriverdi F, Karaca Z, Kelestimur F. A comparison between finasteride, flutamide, and finasteride plus flutamide combination in the treatment of hirsutism. J Endocrinol Invest. 2009;32(1):37–40. doi: 10.1007/BF03345676. [DOI] [PubMed] [Google Scholar]
- 107.van de Wijngaart DJ, Dubbink HJ, van Royen ME, Trapman J, Jenster G. Androgen receptor coregulators: recruitment via the coactivator binding groove. Mol Cell Endocrinol. 2012;352(1-2):57–69. doi: 10.1016/j.mce.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 108.Vora S, Ovhal A, Jerajani H, Nair N, Chakrabortty A. Correlation of facial sebum to serum insulin-like growth factor-1 in patients with acne. Br J Dermatol. 2008;159(4):990–991. doi: 10.1111/j.1365-2133.2008.08764.x. [DOI] [PubMed] [Google Scholar]
- 109.Wysowski DK, Freiman JP, Tourtelot JB, Horton ML., 3rd Fatal and nonfatal hepatotoxicity associated with flutamide. Ann Intern Med. 1993;118(11):860–864. doi: 10.7326/0003-4819-118-11-199306010-00006. [DOI] [PubMed] [Google Scholar]
- 110.Xia Y, Cho S, Howard RS, Maggio KL. Topical eflornithine hydrochloride improves the effectiveness of standard laser hair removal for treating pseudofolliculitis barbae: A randomized, double-blinded, placebo-controlled trial. J Am Acad Dermatol. 2012 doi: 10.1016/j.jaad.2011.10.029. In press. [DOI] [PubMed] [Google Scholar]
- 111.Yang Z, Chang YJ, Yu IC, Yeh S, Wu CC, Miyamoto H, Merry DE, Sobue G, Chen LM, Chang SS, Chang C. ASC-J9 ameliorates spinal and bulbar muscular atrophy phenotype via degradation of androgen receptor. Nat Med. 2007;13(3):348–353. doi: 10.1038/nm1547. [DOI] [PubMed] [Google Scholar]
- 112.Yang Z, Yu H, Cheng B, Tang W, Dong Y, Xiao C, He L. Relationship between the CAG repeat polymorphism in the androgen receptor gene and acne in the Han ethnic group. Dermatology. 2009;218(4):302–306. doi: 10.1159/000202983. [DOI] [PubMed] [Google Scholar]
- 113.Ye F, Imamura K, Imanishi N, Rhodes L, Uno H. Effects of topical antiandrogen and 5-alpha-reductase inhibitors on sebaceous glands in male fuzzy rats. Skin Pharmacol. 1997;10(5-6):288–297. doi: 10.1159/000211517. [DOI] [PubMed] [Google Scholar]
- 114.Yeh S, Tsai MY, Xu Q, Mu XM, Lardy H, Huang KE, Lin H, Yeh SD, Altuwaijri S, Zhou X, Xing L, Boyce BF, Hung MC, Zhang S, Gan L, Chang C. Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proc Natl Acad Sci U S A. 2002;99(21):13498–13503. doi: 10.1073/pnas.212474399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhu H, Ma H, Ni H, Ma XH, Mills N, Yang ZM. Expression and regulation of lipocalin-type prostaglandin d synthase in rat testis and epididymis. Biol Reprod. 2004;70(4):1088–1095. doi: 10.1095/biolreprod.103.022079. [DOI] [PubMed] [Google Scholar]
- 116.Zhuo FL, Xu W, Wang L, Wu Y, Xu ZL, Zhao JY. Androgen receptor gene polymorphisms and risk for androgenetic alopecia: a meta-analysis. Clin Exp Dermatol. 2012;37(2):104–111. doi: 10.1111/j.1365-2230.2011.04186.x. [DOI] [PubMed] [Google Scholar]
- 117.Zouboulis CC, Chen WC, Thornton MJ, Qin K, Rosenfield R. Sexual hormones in human skin. Horm Metab Res. 2007;39(2):85–95. doi: 10.1055/s-2007-961807. [DOI] [PubMed] [Google Scholar]
- 118.Zouboulis CC, Degitz K. Androgen action on human skin -- from basic research to clinical significance. Exp Dermatol. 2004;13(Suppl 4):5–10. doi: 10.1111/j.1600-0625.2004.00255.x. Related articles recently published in Archives of Dermatological Research (selected by the journal’s editorial staff):
- 119.Lee WJ, Jung HD, Chi SG, Kim BS, Lee SJ, Kim dW, Kim MK, Kim JC. Effect of dihydrotestosterone on the upregulation of inflammatory cytokines in cultured sebocytes. Arch Dermatol Res. 2010;302:429–433. doi: 10.1007/s00403-009-1019-6. [DOI] [PubMed] [Google Scholar]
- 120.Su LH, Chen HH. Androgenetic alopecia in policemen: higher prevalence and different risk factors relative to the general population (KCIS no. 23) Arch Dermatol Res. 2011;303:753–761. doi: 10.1007/s00403-011-1173-5. [DOI] [PubMed] [Google Scholar]