Abstract
Recent studies have linked alcoholism with a dysfunctional neural reward system. Although several electrophysiological studies have explored reward processing in healthy individuals, such studies in alcohol‐dependent individuals are quite rare. The present study examines theta oscillations during reward processing in abstinent alcoholics. The electroencephalogram (EEG) was recorded in 38 abstinent alcoholics and 38 healthy controls as they performed a single outcome gambling task, which involved outcomes of either loss or gain of an amount (10 or 50¢) that was bet. Event‐related theta band (3.0–7.0 Hz) power following each outcome stimulus was computed using the S‐transform method. Theta power at the time window of the outcome‐related negativity (ORN) and positivity (ORP) (200–500 ms) was compared across groups and outcome conditions. Additionally, behavioral data of impulsivity and task performance were analyzed. The alcoholic group showed significantly decreased theta power during reward processing compared to controls. Current source density (CSD) maps of alcoholics revealed weaker and diffuse source activity for all conditions and weaker bilateral prefrontal sources during the Loss 50 condition when compared with controls who manifested stronger and focused midline sources. Furthermore, alcoholics exhibited increased impulsivity and risk‐taking on the behavioral measures. A strong association between reduced anterior theta power and impulsive task‐performance was observed. It is suggested that decreased power and weaker and diffuse CSD in alcoholics may be due to dysfunctional neural reward circuitry. The relationship among alcoholism, theta oscillations, reward processing, and impulsivity could offer clues to understand brain circuitries that mediate reward processing and inhibitory control Hum Brain Mapp, 2011. © 2011 Wiley‐Liss, Inc.
Keywords: alcoholism, event‐related oscillations, theta power, outcome‐related negativity, outcome‐related positivity, N2, P3, error‐related negativity, medial frontal negativity, gambling task, impulsivity, risk‐taking
INTRODUCTION
Alcohol dependence is characterized as a multifactorial disorder caused by biological, behavioral, and environmental factors. A variety of neurocognitive dysfunctions, as a result of impairments in several brain regions and/or neural circuitries, have been associated with alcoholism. In the recent years, alcoholism has often been linked with decreased volume of the brain reward system [Makris et al., 2008] and with decreased neural activity in reward circuitry [de Greck et al., 2009; Wrase et al., 2007]. In normal healthy individuals, neuroimaging methods have outlined the structures involved in the neural reward processing system or circuitry [Breiter and Rosen, 1999; Breiter et al., 2001; Delgado et al., 2000, 2003, 2005; Galvan et al., 2005; Hampton et al., 2007; Nieuwenhuis et al., 2005a; Yacubian et al., 2007; Xue et al., 2009]. Despite excellent spatial resolution, the neuroimaging methods suffer a major limitation of temporal resolution, which has been only complemented by electrophysiological methods such as electroencephalogram (EEG), event‐related potentials (ERPs), and event‐related oscillations (EROs). In addition to millisecond‐specific temporal resolution, these electrophysiological methods allow the possibility of exploring the functional brain dynamics during cognitive events. Therefore, several recent studies have used ERPs and EROs to understand the more subtle, progressive, and time‐domain‐specific neurocognitive changes during reward processing in gambling tasks that involved monetary loss and gain [Cohen et al., 2007; Gehring and Willoughby, 2002; Hajcak et al., 2005, 2006, 2007; Holroyd et al., 2004, 2006; Kamarajan et al., 2008, 2009; Luu et al., 2004; Marco‐Pallares et al., 2007; Mennes et al., 2008; Nieuwenhuis et al., 2004, 2005b; Toyomaki and Murohashi, 2005; Yeung et al., 2005; Yu and Zhou, 2006].
Abbreviations.
- BIS
Barratt impulsivity scale
- CSD
current source density
- EEG
electroencephalogram
- ERPs
event‐related potentials
- ERO
event‐related oscillations
- ERN
error‐related negativity
- MFN
medial frontal negativity
- ORN
outcome‐related negativity
- ORP
outcome‐related positivity
- RDS
reward deficiency syndrome
- SOG task
single outcome gambling task
- TRB scores
task‐related behavioral scores
- TFR
time‐frequency representation
In general, ERP studies using gambling tasks to examine outcome processing have identified two major components: (1) a negative going component around 200–250 ms and (2) a positive going component at about 300–500 ms [e.g., Gehring and Willoughby, 2002; Hajcak et al., 2005; Holroyd et al., 2004; Kamarajan et al., 2009; Nieuwenhuis et al., 2004]. In our earlier work on reward processing in healthy normals, we presented a rationale and arguments to label these component as “outcome‐related negativity (ORN)” and “outcome‐related positivity (ORP),” respectively [Kamarajan et al., 2009]. We have also reported that theta oscillations during the time window of ORN and ORP components (200–500 ms) represent the neurocognitive underpinning related to reward processing [Kamarajan et al., 2008].
Brain oscillations of different frequency bands have been shown to have specific functional significance [Basar, 1999a]; the event‐related θ‐band in particular has been shown to be related to a variety of behavioral, cognitive, and motivational or emotional aspects of human information processing, including reward processing [e.g., Basar, 1999b, 2000, 2001a, 2006; Basar‐Eroglu and Demiralp, 2001; Cohen et al., 2007; Kahana et al., 2001; Kamarajan et al., 2008; Klimesch, 1996, 1999; Klimesch et al., 1997a, b, 2001a, b, 2005, 2006; Knyazev, 2007; Luu et al., 2004; Raghavachari et al., 2001, 2006; Schacter, 1977; Trujillo and Allen, 2007]. Reward processing as unfolded during a gambling task involves a combination of behavioral, cognitive, motivational, and emotional states, which have been found to be mediated by brain oscillations in the theta band [Kamarajan et al., 2008]. Studies have demonstrated that the major ERP component of both error paradigms (i.e., ERN) and outcome paradigms (i.e., ORN) were predominantly composed of theta oscillations (e.g., Cohen et al., 2007; Gehring and Willoughby, 2004; Luu et al., 2003, 2004). More convincingly, by using independent component analysis, Makeig et al. [ 2002] identified that the largest independent contributors to the ERN were in the theta frequency range. However, it should be mentioned that only a few studies have analyzed theta oscillations during a gambling paradigm. For instance, Gehring and Willoughby [ 2004] found that frontally focused theta (4–7 Hz) activity was observed during the loss condition. Cohen et al. [ 2007] found that losses, compared to wins, were associated with enhanced power and phase coherence in the theta frequency band. Furthermore, Marco‐Pallares et al. [ 2008] reported that the ORN for loss was associated with increased theta power.
Several ERP studies during reward processing in healthy human subjects have been reported since early 1980s [e.g., Begleiter et al., 1983; Gehring and Willoughby, 2002; Hajcak et al., 2006; Homberg et al., 1980, 1981; Kamarajan et al., 2008, 2009; Otten et al., 1995; Ramsey and Finn, 1997; Yeung and Sanfey, 2004]. These studies have yielded a set of electrophysiological correlates/parameters of reward processing, and these parameters derived from normal individuals can be applied in and compared to clinical conditions, especially those relating to reward deficiency syndrome (RDS) [Blum et al., 2000]. Alcohol dependence has been viewed as a RDS [Bowirrat and Oscar‐Berman, 2005], and a few attempts have been already made to employ these electrophysiological methods to study reward processing in alcoholic individuals. Porjesz et al. [ 1987] reported that abstinent alcoholics showed a significant decrease in P3 amplitude in response to incentive stimuli in abstinent alcoholics. Fein and Chang [ 2008] reported smaller amplitude in feedback negativity in treatment‐naive alcoholics with a greater family history density of alcohol problems compared to controls. In a recent ERP study of reward processing in our laboratory, we have demonstrated amplitude reduction in ORN and ORP components of the ERPs in male alcoholics in comparison with healthy controls [Kamarajan et al., 2010]. However, oscillatory mechanisms of reward processing in alcoholics have not yet been explored, and the present study is the first to examine the event‐related theta activity during the time‐window of the ORN and ORP components. Although studies have already reported changes in theta oscillations during the performance of cognitive tasks in alcoholics [Kamarajan et al., 2004; Jones et al., 2006], offspring of alcoholics [Kamarajan et al., 2006; Rangaswamy et al., 2007], heavy drinkers [de Bruin et al., 2004], and alcohol‐administered to healthy individuals [Krause et al., 2002], no study has as yet examined theta activity during reward processing in alcoholics.
The overarching aim of the current study is to examine the oscillatory changes in theta activity during reward/outcome processing in abstinent male alcoholics as compared to healthy controls. Specifically, the study attempts to examine total theta activity (comprising both phase‐locked and nonphase‐locked activities) during reward processing in alcohol‐dependent individuals while they performed a gambling task. As both ORN and ORP components are predominantly composed of theta oscillations [e.g., Cohen et al., 2007; Gehring and Willoughby, 2004; Kamarajan et al., 2008; Luu et al., 2003, 2004], the time window for the analysis of theta activity will be analogous to the ORN and ORP activations as reflected in the time‐frequency representation (TFR; for details, see Kamarajan et al. [ 2008]). Furthermore, because our earlier ERP studies showed that current density of ORN and ORP components provided additional information such as sources and sinks of current flow and topography during reward processing [Kamarajan et al., 2008, 2009, 2010], we have examined the current source density (CSD) of theta oscillations in the present study. In addition, as alcoholics are reported to have increased impulsivity [e.g., Chen et al., 2007; Dom et al., 2007; Kamarajan et al., 2010], the current study attempts to analyze the behavioral measures of impulsivity and risk‐taking. Because our previous studies showed a significant correlation between ERP/ERO measures and behavioral measures of impulsivity and risk‐taking in normal subjects as well as in alcoholics [Kamarajan et al., 2008, 2009, 2010], we have further attempted to test the association between theta power and impulsivity measures in the present study. We hypothesized that alcoholics will show decreased theta power and significant changes in CSD activations, along with increased impulsivity and/or decision‐making. The findings of the present study may offer important clues regarding frontal lobe involvement in terms of event‐related theta activity, reward processing, and impulsivity in alcoholism and thus may help appraise some of the related models of alcoholism, such as RDS and neural disinhibition.
MATERIALS AND METHODS
Participants
The sample consisted of two groups of adult males: 38 abstinent alcoholics (age range = 24–46 years; mean = 38.41; SD = 6.38) and an equal number of healthy controls (age range = 18–35 years; mean = 21.26; SD = 3.27). The mean age of the alcoholics was significantly higher than that of the controls (t = 14.85; P < 0.001). Groups were matched for education (alcoholic: 12.11 ± 2.96 years; control: 12.49 ± 1.85 years; t = −0.66; P = 0.5118) and the score on Mini‐Mental State Examination [Folstein et al., 1975] (alcoholic: 27.65 ± 1.97; control: 27.82 ± 2.08; t = −0.357; P = 0.7222). Control subjects were recruited through newspaper advertisements and notices, while the abstinent alcoholics (DSM IV alcohol dependence) were recruited from in‐patient or out‐patient local hospitals/clinics in Brooklyn, NY. The alcoholic subjects were required to be abstinent from alcohol intake for at least 30 days, and those who were receiving treatment medication, such as antabuse and/or psychoactive drugs, were excluded from the study to control for the effect of drugs on EEG. All participants did not have any personal and/or family history of other major medical or psychiatric disorders, although alcoholics with drug abuse and other externalizing disorders have been included in the sample; alcoholics but not controls who had family history of alcoholism were also included in the study. Subjects who had positive findings (for their recent drug use within 48 h) in the urine screen and Breathalyzer test were excluded from the study. Furthermore, subjects with hearing or visual impairment, liver disease, or head injury were excluded from the study. The institutional review board of SUNY Downstate Medical Center at Brooklyn, NY, approved the experimental procedures and ethical guidelines of this study.
The Gambling Task
The gambling task used in the study, known as the single outcome gambling (SOG) task, is illustrated in Figure 1. At the start of the experiment, a choice stimulus (CS) with two numbers 10 (left box) and 50 (right box), with a monetary value in US cents, was displayed for 800 ms. The subject was instructed to select one number by pressing the left button for “10” or the right for “50.” The selected number, appeared 700 ms after the CS disappeared and lasted for 800 ms inside a green box (to indicate a gain) or a red box (to indicate a loss) and was thus designated the outcome stimulus (OS). Thus, there were four possible outcomes: namely, gain 50 (+50), loss 50 (−50); gain 10 (+10), and loss 10 (−10). The subject had to respond by selecting either 10 or 50¢ (US cents) within 1,500 ms of CS onset. If the subject did not respond/select within the specified time, the OS would not appear, and the next CS would appear as next trial. The intertrial interval was 3,000 ms throughout the experiment. There were 172 trials in this experiment. Although the event of loss (in red) and gain (in green) in the OS was maintained at equal probability, the order of appearance was pseudo‐randomized. The subjects were not aware of the probability or sequence of the task. The task was presented in two blocks, and each block lasted for 4 min; the procedure was identical in both blocks. At the end of each block, the net/overall loss or gain for the entire block (e.g., Gain $4.50) was displayed. The subject was instructed to press any button to start the next block.
Figure 1.
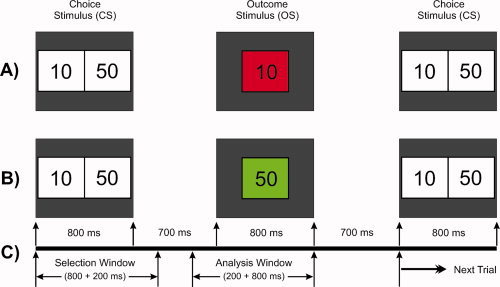
Schematic illustration of the gambling task used in this experiment. (A) A typical trial showing a loss of 10 in red box; (B) another trial having a gain of 50 in green box; and (C) the time duration for the task events: the selection window (1,000 ms), wherein the subject selects either of the numbers, and the analysis window (200‐ms prestimulus + 800‐ms poststimulus) represents the time segment that was used for the time‐frequency (S‐transform) analysis. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Measures of Impulsivity
There were two impulsivity measures used in the study: (1) Barratt impulsiveness scale, version 11 (BIS‐11) [Barratt, 1985; Patton et al., 1995], a self‐rated measure that assesses trait‐related impulsivity, and (2) task‐related behavioral (TRB) scores were derived from the performance of the gambling task. The behavioral scores that were derived from performance of the gambling task and from the impulsivity measures have been elaborated in our earlier studies on healthy controls [Kamarajan et al., 2008, 2009]. The BIS‐11 consists of thirty items, yielding a total score, and additional scores for three subcategories: motor impulsivity (acting without thinking), cognitive impulsivity (making decisions quickly), and nonplanning (lack of prior planning or of future orientation). The TRB scores were of two categories: (1) reaction time for the task conditions and responses and (2) selection frequency that represents the number of times a particular amount (10 or 50) was chosen following either the outcome of loss in the previous trial(s) or a losing or gaining trend in the previous 2, 3, and 4 trials. The gaining and losing trends were computed based on the resultant outcome of the cumulative account of the preceding outcomes. For example, if the previous three outcomes were −10, −10, and +50, then the trend was considered to be a gain (of 30¢), whereas if the previous three outcomes were +10, +10, and −50 then the trend would be considered as a loss (of 30¢). The entire list of the TRB scores can be found in our previous papers [Kamarajan et al., 2008, 2009].
EEG Data Acquisition and Signal Analysis
The subjects were comfortably seated in front of the computer monitor placed 1‐meter away. EEG activity was recorded on a Neuroscan system (Versions 4.1 and 4.3) using a 61‐channel electrode cap, which included 19 electrodes of the 10–20 International System and 42 additional electrodes (see Fig. 2). The electrodes were referenced to the tip of the nose, and the ground electrode was at the forehead (frontal midline). Eye movements were recorded using a supraorbital vertical lead and a horizontal lead on the external canthus of the left eye. Electrode impedance was maintained below 5 kΩ. The EEG signals were recorded continuously with a bandpass at 0.02–100 Hz and amplified 10,000 times using a set of amplifiers (Sensorium, Charlotte, VT). The data consisted of different sampling rates (256, 512, and 500 Hz) and were resampled at 256 Hz during the signal analysis for the sake of uniformity of signals.
Figure 2.
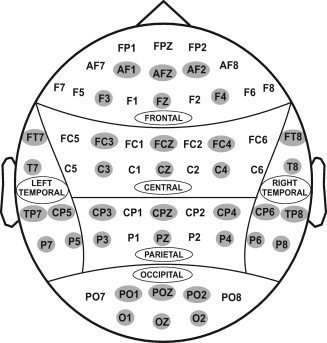
Sixty‐one electrodes as recorded from the surface of the scalp. For statistical analyses, only 36 electrodes (as highlighted) were selected to represent six electrodes in six regions of the brain viz., frontal, central, parietal, occipital, left‐temporal, and right‐temporal.
The EEG signals were decomposed using the S‐transform signal processing method. The methods used in this study have been explained in detail in our earlier paper [Kamarajan et al., 2008]. The S‐transform was introduced by Stockwell et al. [ 1996] and has been shown to produce reliable estimates of localized power of nonstationary evoked potential time series [Chu, 1996; Theophanis and Queen, 2000]. This method has been applied in several recent studies to analyze time‐frequency signals of event‐related oscillations [Jones et al., 2004, 2006; Kamarajan et al., 2006, 2008; Padmanabhapillai et al., 2006a, b; Rangaswamy et al., 2007]. The S‐transform is considered to be a generalization of the Gabor transform [Gabor, 1946] and an extension to the continuous wavelet transform. The S‐transform generates a TFR of a signal by integrating the signal at each time point with a series of windowed harmonics of various frequencies as follows:
where h (t) is the signal, f is frequency, τ is a translation parameter, the first exponential is the window function, and the second exponential is the harmonic function. The S‐transform TFR is computed by shifting the window function down the signal in time by τ across a range of frequencies. The window function is Gaussian with 1/f 2 variance and scales in width according to the examined frequency. This inverse dependence of the width of the Gaussian window with frequency provides the frequency‐dependent resolution. The amplitude envelope of the complex‐valued S‐transform TFR is calculated by taking the absolute value |ST(f, τ)|.
In the current study, event‐related total power (EROTOT) (which is a combination of both phase‐locked and non‐phase‐locked activity) was computed and analyzed across groups and conditions. Theta band (3–7 Hz) was further divided into θ1 or low theta (3–5 Hz) and θ2 or high theta (5–7 Hz) for further analysis. For each task condition, total power for each of the theta bands (3–7 Hz, 3–5 Hz, and 5–7 Hz) was acquired by the average of TFR data in individual trials of 1,000 ms (prestimulus 200 ms and poststimulus 800 ms). The filter setting to extract theta bands included a fifth order Chebyshev type I filter (two‐step cascade type) with ripple factor (ε) of 0.108 and ripple attenuation (R p) of 0.05. For the purpose of statistical analysis, mean theta power was extracted from the amplitude envelope within TFROI corresponding to 200–500 ms of poststimulus time window within which both N2 and P3 components of outcome trials had their peaks (see Kamarajan et al. [ 2008] for detail). The trials exceeding 100 μV were removed for artifacts. The minimum number of artifact‐free trials in all conditions for each subject was kept at 15 for the analysis.
CSD Mapping
The current source density (CSD) maps were constructed from the Laplacian transformed data as described by Wang and Begleiter [ 1999]. This method has been applied in our earlier studies [Kamarajan et al., 2005, 2009]. Because the recorded potential at each electrode represents the resultant contributions from several adjacent and distal sources, local sources cannot be clearly estimated [Nunez, 1981]. The CSD transform acts as a spatial filter and provides an estimate of the local radial current density [Hjorth, 1975; Nunez, 1981; Nunez and Pilgreen, 1991] and represents components of the primary neural activity in the scalp region [Hjorth, 1991]. In the present study, the CSD maps representing theta power sources (μV2/r 2, where r is the head radius in centimeters) were created and compared visually for both absolute values and Z‐scores for each of four outcomes (−50, −10, +50, and +10) and for control and alcoholic groups separately.
Statistical Analysis
Thirty‐six electrodes that represented six scalp regions were selected for the statistical analyses (see Fig. 2). The theta power was analyzed by performing a linear mixed model of the analysis of variance (ANOVA) using the Statistical Analysis System (SAS, version 9.2) (SAS Institute, NC 27513). The values with ±4 standard deviations were considered as outliers and removed from the data before the analysis. The covariance structure used in the model was “Compound Symmetry,” which has a constant variance and covariance. The model included five factors as fixed effects: Valence (loss, gain), Amount (50 and 10¢), Region (frontal, central, parietal, occipital, left‐temporal, and right‐temporal), and Electrode (6 electrodes) as within‐subjects factors, and Group (alcoholic, control) as a between‐subjects factor. One‐way ANOVA was used for the follow‐up analyses of pair‐wise comparisons between the (1) alcoholic versus control group, (2) loss versus gain condition, and (3) 50 versus 10¢. A Bonferroni correction procedure for multiple comparisons was used.
The sample characteristics and behavioral scores between groups were compared using independent sample t‐tests. The correlations between theta power variables and behavioral variables were computed as described in our earlier work [Kamarajan et al., 2009]. Initially, factor analysis, using principal component analysis with varimax rotation, was performed to reduce the theta variables (N = 108) as well as the TRB variables (N = 24) into a few specific factors. Then, Pearson bivariate correlations were performed to analyze the relationship between behavioral factors and theta power. Theta variables for the factor analysis comprised nine electrode sites (F3, Fz, F4, C3, Cz, C4, P3, Pz, and P4), four conditions (+50, +10, −50, and −10), and three theta frequencies (θ, θ1, and θ2). Only the factors that accounted for at least 10% of total variance were retained for further correlational analysis. However, factor analysis was not done on BIS scores as they were already factorized in the original work [Barratt, 1985; Patton et al., 1995].
RESULTS
Temporal and Spatial Characteristics of Theta Activity in Alcoholics and Controls
The waveforms of ERPs and all theta bands (θ, θ1, and θ2) during Loss 50 and Gain 50 conditions are shown in Figure 3 (Panels A and B). A partial phase‐alignment of the theta activity corresponding to the ORN (200–300 ms) and ORP (300–400 ms) components was observed. Alcoholics showed decreased amplitude in both broadband (θ) and subbands (θ1 and θ2) of theta activity, and this difference was more pronounced during the loss condition.
Figure 3.
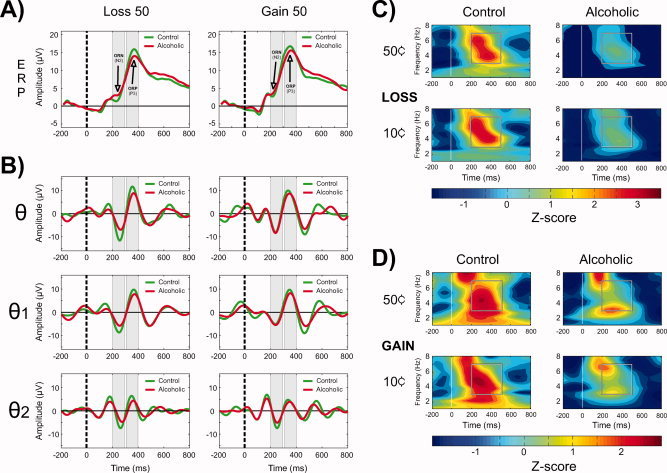
The grand‐averaged ERP waveforms are shown in Panel A. In Panel B, theta bands (rows 2, 3, and 4) during Loss 50 and Gain 50 conditions at FZ electrode are shown. The region of ORN and ORP peaks and the corresponding theta activity during the time window are shaded in gray color. There is a partial phase‐alignment of the theta activity corresponding to ORN (200–300 ms) and ORP (300–400 ms) components. Alcoholics showed decreased amplitude in both broadband (3–7 Hz) and subbands (3–5 and 5–7 Hz) of theta activity, and this difference was more pronounced during the loss condition. Time (in millisecond) is shown on the X‐axis, and the amplitude (in microvolt) is represented on the Y‐axis. The dashed vertical line (at 0 ms) represents the onset of an outcome stimulus. Panels C and D represent the time‐frequency (TF) plots during the loss condition at Fz electrode and the gain condition at Pz electrode in the alcoholic and control groups. The white line at 0 ms represents the onset of the outcome stimulus. The square box inside each plot marks the time‐frequency region of interest, namely the time interval of 200–500 ms across the theta frequency range 3–7 Hz for the analysis. The alcoholic group showed a significant reduction in theta power during each outcome condition. The color scale represents the theta power in terms of Z‐scores, which were computed from the overall data (representing all groups and conditions) and hence are comparable among the TF plots.
The TFRs for each condition and group are shown in Figure 3 (Panels C and D). Theta (3–7 Hz) power during 200–500 ms after the onset of an outcome stimulus was selected for the analysis. Although the alcoholics, when compared with controls, have reduced theta power during prestimulus baseline as well as poststimulus activation, the peak theta activation (in each group) as well as the group differences are more apparent and robust in the time window of 200–500 ms, which is the region of interest for the analysis of event‐related theta power. As shown in Figure 3, the alcoholic group showed markedly significant reductions in theta power (at 200–500 ms) during each outcome condition.
Topographic maps of theta power in alcoholic and control groups during loss and gain conditions are shown in Figure 4 (Panels A and B). Visual analysis of the topographic maps showed that the alcoholic group appeared to have decreased theta activity during each outcome condition. Statistical analyses of this observation are reported in the next section (see Fig. 5). High theta (θ2) power in alcoholics had an additional activity at occipital electrodes. In both groups, loss conditions had an anterior focus of theta activity while the gain conditions involved both anterior (primarily for θ2) and posterior (primarily for θ1) regions.
Figure 4.
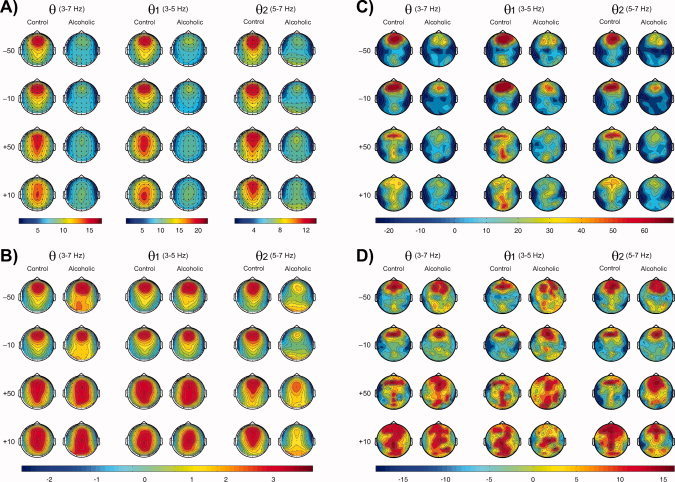
Topographic maps of theta power (in μV2) in alcoholic and control groups during loss and gain conditions are shown in Panels A and B. The top set of head maps (Panel A) represents the absolute power, and the bottom set (Panel B) represents the Z‐scored power. In both groups, loss conditions manifested anterior theta activity, while the gain conditions involved both the anterior region (primarily for θ2) and the posterior region (primarily for θ1). The alcoholic group showed significantly decreased theta activity during each outcome condition. High theta (θ2) power in alcoholics had additional activity at the occipital area. The Z‐score maps can be compared for the shape of the activation and not for the intensity as the Z‐scoring was computed for each headmap separately. The CSD maps (power/r 2, where r is the head radius in centimeters) between 200 and 500 ms during the outcomes (‐50, ‐10, +50, and +10) in the control, and alcoholic groups are shown in Panels C and D. The top set of topographic maps (Panel C) represents the Laplacian transformed (CSD) values of theta power, and the bottom set (Panel D) represents the Z‐scored values of CSD power. Controls showed a strong frontal focus particularly during loss conditions, while alcoholics showed weaker source activity compared to controls, especially at the frontal electrodes. During the ‐50 condition, while controls had a single and stronger midline prefrontal source, alcoholics showed bilateral and weaker prefrontal sources. The alcoholic group also showed more diffuse source activity compared to controls, especially during gain conditions. In both groups, loss conditions had predominant anterior sources, while gain conditions had both anterior and posterior sources. The Z‐scored maps can be compared only for the shape of the CSD activation and not for the intensity since the Z‐scoring was computed for each headmap separately.
Figure 5.
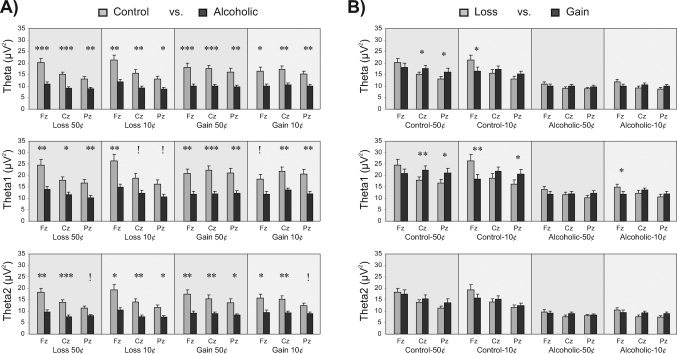
In Panel A, the bar graphs show the pair‐wise comparisons of mean theta power (in μV2) across groups (control vs. alcoholic group) during each outcome condition at three electrode sites (Fz, Cz, and Pz). Alcoholics show significantly decreased theta power during all outcomes. The asterisks (*) indicate the level of significance (*P < 0.05, **P < 0.01, and ***P < 0.001) after the Bonferroni correction for multiple comparisons, and the exclamation mark (!) indicates the loss of significance after the Bonferroni correction. In Panel B, the bar graphs show the pair‐wise comparisons of mean theta power (in μV2) across valence (loss vs. gain) during 50¢ and 10¢ conditions in control and alcoholic groups at three electrode sites (Fz, Cz, and Pz). In both control and alcoholic group, Loss was larger than Gain at Fz electrode, and Gain was larger than Loss at Cz and Pz electrodes. The comparisons (between loss and gain) were significant only in the control group for theta broadband and for low theta before Bonferroni adjustment. In the alcoholic group, although a similar pattern (anterior loss and posterior gain) is observed, the differences are not significant. None of the comparisons was significant after Bonferroni correction.
The CSD maps of theta power between 200 and 500 ms during each outcome in the control and alcoholic group are shown in Figure 4 (Panels C and D). Controls showed a strong anterior focus of theta activity, particularly during loss conditions, while alcoholics showed weaker source activity compared to controls, especially at the frontal electrodes. During the −50 condition, while controls had a single and stronger midline prefrontal source, alcoholics showed bilateral and weaker prefrontal sources. In addition, the alcoholic group showed more diffuse source activity compared to controls, especially during gain conditions. In both groups, loss conditions had predominant anterior sources while gain conditions had both anterior and posterior sources.
Theta Activity across Conditions and Groups in the Mixed Model
The statistical results of the mixed model ANOVA are shown in Table I, and the follow‐up analyses (in bar‐graphs) are shown in Figure 5. Although the main effect for Group was not significant, two‐way and three‐way interactions of Group with other factors (Valence, Amount, Region) were highly significant. Although the three‐way interaction of Group × Valence × Region was highly significant for all theta bands (θ, θ1, and θ2), the other important three‐way interaction Group × Amount × Region was not significant for any of the theta bands, suggesting that the effect of Valence (loss vs. gain) on theta power (variance) was greater than that for the Amount. Because alcoholics were significantly older than controls, age was included as a factor in the ANOVA model but not found to be significant. Specifically, age had neither a main effect (as Age and Age2 were not significant) nor an interactive effect with Group (as Age × Group and Age2 × Group were not significant) on theta power, indicating that age did not have significant bearing on theta power.
Table I.
ANOVA results with the F‐value, P‐value, and significance level for the main and interaction effects
| Theta full band (θ) | Low theta band (θ1) | High theta band (θ2) | ||||
|---|---|---|---|---|---|---|
| F | P | F | P | F | P | |
| Group | 0.61 | 0.4356 | 0.02 | 0.8972 | 1.47 | 0.2290 |
| Valence | 16.93 | <0.0001*** | 25.99 | <0.0001*** | 3.99 | 0.0494* |
| Amount | 1.92 | 0.1696 | 11.29 | 0.0012** | 0.40 | 0.5290 |
| Region | 533.48 | <0.0001*** | 415.72 | <0.0001*** | 434.54 | <0.0001*** |
| Group × valence | 28.23 | <0.0001*** | 33.15 | <0.0001*** | 15.68 | 0.0002*** |
| Group × amount | 7.15 | 0.0092** | 2.39 | 0.1267 | 5.63 | 0.0203* |
| Group × region | 126.65 | <0.0001*** | 49.37 | <0.0001*** | 141.82 | <0.0001*** |
| Valence × amount | 0.07 | 0.7851 | 0.00 | 0.9531 | 0.00 | 0.9833 |
| Valence × region | 28.87 | <0.0001*** | 46.13 | <0.0001*** | 8.94 | <0.0001*** |
| Amount × region | 0.72 | 0.6083 | 1.27 | 0.2754 | 0.11 | 0.9895 |
| Group × valence × amount | 7.16 | 0.0092** | 1.33 | 0.2524 | 13.60 | 0.0004*** |
| Group × valence × region | 8.36 | <0.0001*** | 7.54 | <0.0001*** | 5.91 | <0.0001*** |
| Group × amount × region | 2.23 | 0.0510ms | 0.53 | 0.7515 | 1.98 | 0.0809 ms |
| Valence × amount × region | 0.86 | 0.5061 | 2.07 | 0.0684 ms | 0.57 | 0.7226 |
| Electrode (region) | 31.57 | <0.0001*** | 22.90 | <0.0001*** | 24.67 | <0.0001*** |
| Age | 0.59 | 0.4452 | 0.02 | 0.8820 | 1.40 | 0.2415 |
| Age × group | 0.81 | 0.3708 | 0.05 | 0.8246 | 1.80 | 0.1837 |
| Age2 | 0.66 | 0.4198 | 0.04 | 0.8362 | 1.51 | 0.2237 |
| Age2 × group | 0.73 | 0.3969 | 0.03 | 0.8584 | 1.71 | 0.1949 |
The statistical significance is marked with asterisks.
P < 0.05;
**P < 0.01;
***P < 0.001; ms P < 0.10 (marginal significance).
Bar‐graphs of the follow‐up analysis involving the pair‐wise comparisons of mean theta power across groups (control vs. alcoholic group) during each outcome condition at three electrode sites (Fz, Cz, and Pz) are shown in Figure 5 (Panel A). Alcoholics showed significantly reduced theta power compared to controls (after Bonferroni correction) during all outcomes at one or more electrode sites. Comparisons across valence (loss vs. gain) (as shown in Fig. 5, Panel B) were significant only in the control group for theta broadband (3–7 Hz) and low theta band (3–5 Hz). However, in both groups, Loss was larger than Gain at the Fz electrode, and Gain was larger than Loss at Cz and Pz electrodes. None of these comparisons were significant after Bonferroni corrections. Furthermore, none of the comparisons between larger amount (50¢) and smaller amount (10¢) were significant either before or after the Bonferroni corrections, except for a single significance in low theta at Fz in the control group before Bonferroni adjustment.
Behavioral Data on Impulsivity and Risk‐Taking
Comparisons of the BIS and the selection frequency (representing the number of times a particular amount, either 50 or 10¢, is selected while performing the gambling task) between the control and alcoholic groups are shown in Figure 6. Alcoholics showed significantly higher impulsivity scores in motor, nonplanning, and total scores of the BIS (Fig. 6, Panel A). Furthermore, alcoholics selected the larger amount (50¢) more frequently and the smaller amount (10¢) less frequently in the face of losing trends (Fig. 6, Panel B). In other words, alcoholics showed more risk‐taking behavior compared to controls who “played safe” by selecting 10¢ more frequently during the losing trends. On the other hand, none of the scores of reaction time was significant between the groups.
Figure 6.
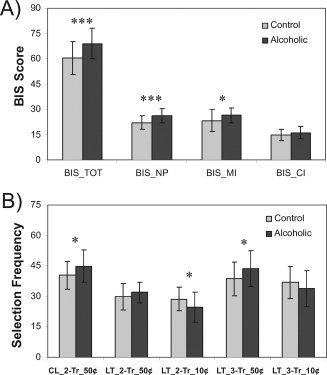
The behavioral data of BIS scores (Panel A) and selection frequency between control and alcoholic groups (Panel B). For the BIS, alcoholics showed significantly higher impulsivity in motor (MI), nonplanning (NP), and total scores (TOT). As for the selection frequency, alcoholics selected 50¢ (for betting) more frequently in the face of consecutive losses (CL) during previous two trials (2‐Tr) and following the losing trends (LT) during previous three trials (3‐Tr). Furthermore, alcoholics selected 10¢ less frequently during the losing trends. The error bar represents 1 SD.
The PCA method was used to extract specific factors from the variables of TRB scores and theta power separately. Factors converged at 25 and 50 iterations for TRB and theta variables, respectively. Factors that accounted for more than 10% of the total variance were retained (Table II). Total variance accounted for by TRB and theta factors were 81.16% and 59.64%, respectively. The four TRB factors were (1) all the RT variables, (2) selection frequency for 50¢ following losing trends (positive loadings) and for 10¢ following gaining trends (negative loadings), (3) number of times selecting 50 following gaining trends, and (4) number of times selecting 10 following gaining trends. The four theta factors were (1) θ, θ1, and θ2 power during +50 and +10 conditions at frontal and central leads, (2) θ, θ1, and θ2 power during −50 condition at parietal and central leads, (3) θ and θ2 power during −10 condition at frontal and central leads, and (4) θ, θ1, and θ2 power during +50 condition at parietal and central leads.
Table II.
Description of factors obtained from TRB variables and theta variables that accounted for more than 10% of total variance
| Factors | Eigen value | Accounted variance (in %) | Variables with positive loadings, (r), and [N] | Variables with negative loadings, (r), and [N] |
|---|---|---|---|---|
| TRB factor‐1 | 10.25 | 37.96 | All the RT variables (0.93 to 0.98) [10] | None |
| TRB factor‐2 | 5.60 | 20.73 | SF for 50 following losing trends/trials (0.93 to 0.85) [5] | SF for 10 following losing trends (−0.54 to −0.73) [3] |
| TRB factor‐3 | 3.33 | 12.33 | Number of times selecting 50 following gaining trends (0.85 to 0.91) [3] | None |
| TRB factor‐4 | 2.74 | 10.14 | Number of times selecting 10 following gaining trends (0.77 to 0.89) [3] | None |
| Theta factor‐1 | 23.91 | 22.13 | θ, θ1, and θ2 during +50 and +10 conditions at anterior areas: frontal (0.59 to 0.86) [18] and central leads (0.53 to 0.67) [15] | None |
| Theta factor‐2 | 13.78 | 12.76 | θ, θ1, and θ2 during –50 condition at posterior areas: parietal (0.56 to 0.85) [9] and central leads (0.55 to 0.84) [9] | None |
| Theta factor‐3 | 13.63 | 12.62 | θ and θ2 during –10 condition at anterior areas: frontal (0.68 to 0.81) [6] and central leads (0.66 to 0.79) [11] | None |
| Theta factor‐4 | 13.10 | 12.13 | θ, θ1, and θ2 during +50 condition at posterior areas: parietal (0.64 to 0.79) [9] and central leads (0.55 to 0.68) [8] | None |
SF, selection frequency or the number of times an amount (50¢ or 10¢) is chosen for betting.
For each factor, eigenvalue, percentage of variance accounted for, loading index (r), and the number of variables included (N) are listed. Only the factors that had significantly high positive (r ≥ +0.5) and negative (r ≤ –0.5) loadings have been selected.
Table III shows the correlation between theta factors and behavioral (TRB and BIS) factors. BIS factors did not show any correlations with factors of theta power. On the other hand, factor‐2 of the TRB variables (i.e., selection frequency following losses) had a significant negative correlation with factor‐1 of theta power (i.e., anterior theta power during gain conditions).
Table III.
Correlation between theta factors and impulsivity factors (BIS and TRB)
| Factors | Theta factor‐1 | Theta factor‐2 | Theta factor‐3 | Theta factor‐4 | ||||
|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | |
| BIS_total | 0.00 | 0.9872 | −0.10 | 0.4131 | −0.16 | 0.1817 | −0.18 | 0.1334 |
| BIS_NP | −0.05 | 0.6868 | 0.01 | 0.9086 | −0.12 | 0.3196 | −0.19 | 0.1111 |
| BIS_MI | 0.02 | 0.8553 | −0.21 | 0.0822 | −0.16 | 0.1914 | −0.21 | 0.0827 |
| BIS_CI | 0.02 | 0.8766 | 0.03 | 0.7997 | −0.05 | 0.6676 | 0.05 | 0.6774 |
| TRB factor‐1 | −0.12 | 0.3025 | −0.09 | 0.4598 | 0.05 | 0.6536 | 0.04 | 0.7263 |
| TRB factor‐2 | −0.27 | 0.0173* | 0.01 | 0.9433 | −0.01 | 0.9534 | −0.09 | 0.4231 |
| TRB factor‐3 | −0.07 | 0.5755 | 0.22 | 0.0591 | 0.01 | 0.9580 | 0.05 | 0.6776 |
| TRB factor‐4 | −0.15 | 0.1931 | −0.01 | 0.9294 | −0.05 | 0.6675 | −0.11 | 0.3629 |
Correlation coefficient (r) and the level of significance (P) before correcting for multiple testing are shown. The minus sign (–) indicates a negative correlation. Theta factor‐1 (anterior theta power during gain conditions) has a statistically significant negative correlation with TRB factor‐2 (selection frequency following losses).
P < 0.05.
Summary of Major Findings
-
1
Although a partial phase‐alignment corresponding to the peaks/troughs of ORN (200–300 ms) and ORP (300–400 ms) components was observed in the theta waveforms in both control and alcoholic groups, alcoholics showed decreased amplitude in both broadband and subbands of theta activity especially during Loss condition (Fig. 3, panels A and B).
-
2
Time‐frequency (TF) plots showed that the alcoholic group, compared to the control group, showed markedly decreased theta power (200–500 ms) during each outcome condition (Fig. 3, panels C and D).
-
3
It was obvious in the topographic maps that the alcoholic group had markedly reduced theta activity during each outcome condition. High theta (θ2) power in alcoholics had additional (and possibly intruding) occipital activity. In both groups, loss conditions had anteriorly focused theta activity while gain conditions involved both anterior maxima (primarily for θ2) as well as posterior maxima (primarily for θ1) (Fig. 4, panels A and B).
-
4
CSD activity showed drastically weaker source activity in alcoholics compared to controls, especially at the frontal electrodes. During the loss condition (−50), controls had a single and stronger midline prefrontal source, and alcoholics showed bilateral and weaker prefrontal sources. Furthermore, the alcoholic group showed more diffuse source activity compared to controls, especially during gain conditions. In both groups, loss conditions had predominant anterior sources, and gain conditions had both anterior and posterior sources (Fig. 4, panels C and D).
-
5
Mixed model ANOVA revealed that Group (as a factor) had significant two‐way and three‐way interactions with valence and/or amount, although the main effect for group was not significant (Table I).
-
6Follow‐up analyses using one‐way ANOVA showed
-
isignificantly reduced theta power in alcoholics compared to controls during all outcomes (Fig. 5, panel A)
-
iialthough the topographic pattern of “anterior focused loss” and “posterior focused gain” was observed in both control and alcoholic groups, the differences between loss and gain were significant only in the control group for broadband theta and low theta (θ1) power (before Bonferroni correction) (Fig. 5, panel B)
-
iiinone of the comparisons between amounts (50 vs. 10¢) with the same valence was significant (before adjusting for Bonferroni corrections) excepting a single comparison in the control group during the gain condition for low theta at Fz (not illustrated)
-
i
-
7
Comparison of behavioral data across groups suggested that (i) alcoholics had significantly higher impulsivity in motor, nonplanning, and total scores of BIS (Fig. 6, panel A) and (ii) alcoholics selected 50¢ (for betting) more frequently in the face of two consecutive losses and following the losing trends during the previous trials. Furthermore, alcoholics selected 10¢ less frequently during the losing trends (Fig. 6, panel B).
-
8
Factor analysis identified meaningful components of TRB variables and theta power variables (Table II). Theta factor‐1 (anterior theta power during gain conditions) had a significant negative correlation with TRB factor‐2 (selection frequency following losses). In other words, individuals with reduced anterior (predominantly frontal) theta power during gain conditions more frequently bet with 50¢ in the face of loss.
DISCUSSION
The present study examined reward/outcome processing in abstinent alcoholics and healthy controls in terms of event‐related theta activity during the time window of ORN and ORP. Because ORN and ORP components are primarily composed of theta oscillations [Cohen et al., 2007; Gehring and Willoughby, 2004; Kamarajan et al., 2008; Luu et al., 2003, 2004], the present study hypothesized that alcoholics, compared to controls, will show features of deficient theta activity. The findings of the present study have demonstrated that alcoholic individuals showed deficient theta activity compared to normal controls. Furthermore, theta activity was associated with impulsivity, and alcoholics showed more impulsivity and risk‐taking behavior. The major findings that are relevant to the goals of the present study are discussed in detail in the light of research studies in the scientific literature as below.
Theta Activity during Reward Processing in Alcoholics and Controls
The robust finding of the present study is that alcoholics, compared to controls, showed a significant reduction in theta activity, and this finding has been graphically illustrated (Figs. 3 and 4) as well as statistically demonstrated (see Fig. 5). The present study is the first ERO study to report reward processing dysfunction in alcoholics, although there have been a few ERP studies. Earlier, in a study from our laboratory, Porjesz et al. [ 1987] reported that alcoholics manifested lower P3 voltages to all visual stimuli regardless of incentive values (i.e., baseline and two monetary reward conditions). Fein and Chang [ 2008], using the Balloon Analogue Risk Task, which measures risk‐taking propensity, reported smaller ORN amplitudes in treatment‐naive alcoholics with a greater family history density of alcohol problems. In a recent study from our group using the gambling task used in the current study, we reported that alcoholics showed decreased amplitudes in ORN and ORP components [Kamarajan et al., 2010]. These findings strengthen the view that alcoholics may have a dysfunctional neural mechanism related to reward processing. This view is further supported by the recent imaging studies that reported both structural [Makris et al., 2008] and functional deficits [de Greck et al., 2009] in the neural reward system of alcohol‐dependent individuals.
Using other cognitive paradigms, studies have demonstrated event‐related theta changes related to alcohol use, viz., alcohol dependence, direct effect of alcohol intake, and regular alcohol use and social drinking. For example, event‐related theta power was found to be reduced in alcohol‐dependent individuals during response inhibition in a Go/NoGo task [Kamarajan et al., 2004] as well as during target detection in a visual oddball task [Jones et al., 2006]. Using an auditory memory task, Krause et al. [ 2002] reported that the administration of alcohol (i.e., the direct effect of alcohol intake) decreased the early‐appearing event‐related synchronization responses during auditory encoding and increased the later‐appearing event‐related desynchronization responses during retrieval in the theta band. Furthermore, during a mental‐rehearsal task, heavily drinking students (with regular use of alcohol) had more synchronization in the theta band than lightly drinking students (with social drinking) [de Bruin et al., 2004]. These studies may essentially support the view that theta activity may serve as an effective measure characterizing neurocognitive deficits in alcoholism.
It is important to note that there is strong evidence in the research literature for the view that a decrease (or desynchronization) of event‐related theta power during cognitive/affective processing suggests weaker/suppressed task processing, while increased theta activity (or synchronization), on the other hand, indicates more efficient processing. Several ERO studies performed during cognitive processing include the following: memory [e.g., Doppelmayr et al., 1998, 2000; Klimesch, 1996, 1999], working memory [e.g., Krause et al., 2000a; Raghavachari et al., 2001, 2006; Schmiedt et al., 2005], creative thinking [e.g., Razumnikova, 2007], intelligence [Doppelmayr et al., 2005], cognitive workload [e.g., Sammer et al., 2007], face perception [Basar et al., 2007], motor planning [Caplan et al., 2003], executive function [Gonzalez‐Hernandez et al., 2002], response inhibition/execution [e.g., Kamarajan et al., 2004, 2006], visual target discrimination [Karakas et al., 2000b; Jones et al., 2006; Rangaswamy et al., 2007], Stroop effect [Hanslmayr et al., 2008], emotion processing [Aftanas et al., 2003; Doppelmayr et al., 2002; Krause et al., 2000b], error processing [e.g., Trujillo and Allen, 2007; Luu et al., 2004], and outcome processing [Cohen et al., 2007; Gehring and Willoughby, 2004]. Because the phenomenon of decreased theta power has been observed in a wide variety of cognitive processes, this may indicate a generic deficiency in neurocognitive processing in alcoholics. On the other hand, significantly reduced theta power in alcoholics during reward processing may suggest a specific dysfunction in the neural reward processing mechanism of alcohol‐dependent individuals.
Although there have been several studies on event‐related theta, little is known about the specificity of theta activity to represent a cognitive phenomenon. Therefore, it could be debated whether the deficient theta activation (during a gambling task) represents a specific dysfunction in reward processing or a general deficiency in neurocognitive processing for performing any given cognitive task. In this regard, Yordanova et al. [ 2002, 2003] maintained that event‐related theta activity may reflect a general processing demand during stimulus evaluation by stating that because event‐related theta is “consistently observed across different modalities, a transient θ‐dominated state may reflect a processing stage that is obligatory for stimulus evaluation, during which interfering activations from other frequency networks are minimized.” However, on the other hand, several studies have attributed task‐specific cognitive functions to theta activation [e.g., Aftanas et al., 2002; Bastiaansen et al., 2002a, b; Hanslmayr et al., 2008; Jacobs et al., 2006; Krause et al., 2007; Luu et al., 2004; Marco‐Pallares et al., 2008; Raghavachari et al., 2001; Trujillo and Allen, 2007; Yordanova et al., 2004]. Although it seems unresolved that deficient theta power in alcoholics represents a general or task‐specific cognitive processing, it is reasonable to state that alcoholics may have both generic deficits in stimulus processing as shown in basic oddball tasks [e.g., Cohen et al., 1994; Jones et al., 2006; Porjesz and Begleiter, 1985, 1993, 2003, 2005] and domain‐specific deficits, such as inhibitory processing assessed by Go–NoGo tasks [Cohen et al., 1997; Fallgatter et al., 1997; Kamarajan et al., 2004, 2005]. Addressing a similar issue, an fMRI study by Pochon et al. [ 2002] found that monetary reward induced an increased activation in the areas already activated by working memory processing (i.e., a network including the dorsolateral prefrontal cortex and the lateral frontopolar areas) and additionally in the medial frontal pole. This study showed that although common neural structures subserve both cognitive load and reward processing, reward circuitry additionally involved specific brain areas. In summary, it may be suggested that decreased ERO theta power during reward processing could suggest a generic cognitive deficit as well as a specific deficiency for reward processing.
Topography of Theta Power and CSD
Topography of surface theta power and current source density (CSD) sources indicated markedly weaker theta activation during reward processing in alcoholics, especially at frontal areas (see Fig. 4). On the other hand, differences in the location and strength of CSD sources between controls and alcoholics suggested a possible dysfunction in the integrity of brain reward circuitry in alcoholic individuals. The diffuse source activations in all outcome conditions in alcoholics may be suggestive of additional or intruding nonspecific activations—either preexisting or reward‐induced activations—in the neural reward circuitry of alcoholics, in contrast to more focused or specific activations in controls. Prior ERP studies have reported similar differences in CSD activations in alcoholics during stimulus discrimination and during response inhibition [Cohen et al., 2002; Hada et al., 2000; Ji et al., 1999; Kamarajan et al., 2005; Rodriguez Holguin et al., 1999]. Furthermore, in a gambling paradigm, our earlier ERP study reported that alcoholics showed decreased current density activations at several brain regions [Kamarajan et al., 2010]. The current study is the first of its kind to report dysfunctional CSD activation of event‐related theta band in alcohol‐dependent individuals. On the other hand, perhaps worth mentioning is the unusual finding in the topography of theta power (see Fig. 4, panels A and B) in alcoholics that showed occipital activations in all outcome conditions during high theta activity, which was completely absent in controls. Because this activity was a contribution from high theta (5–7 Hz) band, it is possible that it is the result of “spill‐over” of low alpha activity (with occipital focus) in alcoholics, though the explanation requires further investigation. Another possibility is that the occipital activity (possibly closer to 7 Hz) could be interrupting slow alpha input from the (continuing) visual sensations during the visual feedback of an outcome stimulus, which lasts for 800 ms, while the theta activity measured is between 200 and 500 ms.
Overall, these topographic differences in alcoholics during reward processing may indicate a possible dysfunction in the neural reward circuitry. Prior findings, from both neuroimaging and electrophysiological studies, have reported dysfunctional neural reward systems in alcoholics. Many fMRI studies have identified the areas involved in reward processing in healthy individuals [e.g., Breiter and Rosen, 1999; Camara et al., 2008; Delgado et al., 2003, 2005; Knutson et al., 2000, 2001, 2003; Marco‐Pallares et al., 2007; McClure et al., 2004; Nieuwenhuis et al., 2005b], and a few imaging studies have documented the impairments in the key brain areas of reward circuitry in alcoholics [de Greck et al., 2009; Makris et al., 2008; Wrase et al., 2007]. Therefore, in the light of earlier reports on reward processing in normals as well as in alcoholics, decreased theta power and weaker and diffuse current density observed in alcoholics may suggest a dysfunctional reward circuitry, which might serve as a hallmark feature of alcoholism.
It should be noted that the findings and scope of the present study could potentially address the question of the role of frontal lobes in reward processing and in alcoholism. In this regard, the key findings of the present study that may serve as evidence for possible frontal lobe dysfunction in alcoholics during reward processing are (1) decreased theta power at frontal areas in alcoholics, (2) weaker and diffuse CSD source in frontal areas in alcoholics, and (3) anterior theta power (composed mainly of frontal electrodes) during gain conditions was correlated with risk‐taking behavior or behavioral impulsivity (assessed by the alcoholic group's higher selection frequency of 50¢ following losses). The role of frontal lobes in reward processing has been well documented. For example, neuroimaging studies have found that frontal lobes, especially the prefrontal areas including the medial frontal areas, play a critical role in the modulation of the reward circuitry [e.g., Bruguier et al., 2008; Fujiwara et al., 2009; Gilbert and Fiez, 2004; Knutson et al., 2000, 2001, 2003; Krawczyk et al., 2007; Nieuwenhuis et al., 2005b; Pochon et al., 2002; Weber et al., 2009; Yarkoni et al., 2005]. Furthermore, electrophysiologically, because the origin of event‐related theta activity itself is reported to be in the frontal lobes, decreased/dysfunctional theta system can be attributed to an impairment in frontal lobe functioning. According to Basar et al. [ 2001b], “frontal theta” is considered to be a major oscillation of the human frontal cortex and has a response‐controlling function. Several studies have reported the frontal origin of event‐related theta oscillations during several cognitive paradigms, as mentioned earlier. Our previous studies with this gambling paradigm found frontal activations (in cingulate cortex) during reward processing [Kamarajan et al., 2008, 2009] in healthy individuals and identified reduced frontal activations in alcoholic individuals [Kamarajan et al., 2010]. Additionally, reduced frontal activity (in terms of decreased current density activation) has been found in subjects with high impulsivity and alcoholism [Chen et al., 2007; Dom et al., 2007]. Reviewing the frontal lobe impairments in alcoholics, Moselhy et al. [ 2001] summarized that alcoholics had manifested frontal lobe dysfunctions at the neurophysiological, morphological, and neuropsychological levels. Therefore, it may be stated that the deficient theta activity during reward processing observed in alcoholics could be primarily due to the impaired frontal lobes, which in turn could have contributed to a variety of cognitive and executive deficits including the reward processing. It is also possible that other component parts of the brain reward circuitry could have contributed to these deficits, as this circuitry involves complex connections with several brain regions viz., medial prefrontal cortex, medial ventral striatum, medial ventral pallidum, septal complex, bed nucleus of stria terminalis, medial and lateral preoptic area, lateral and posterior hypothalamic areas, lateral habenula, posterior ventral tegmental area, midbrain raphe and rostromedial tegmental nuclei, medial and dorsal raphe nuclei, laterodorsal tegmental area, periaqueductal gray, and parabrachical nucleus [Ikemoto, 2010]. It may be summarized that although frontal lobes (especially prefrontal cortices) play a crucial role in addiction in general [Crews and Boettiger, 2009], vulnerability for developing and maintaining addiction involves multiple and complex neurocircuitries subserved by both cortical and subcortical regions at different stages of addiction [Koob and Volkow, 2010].
Impulsivity, Reward, Theta Oscillations, and Alcoholism
In the current study, there were three important findings that may interlink the domains of impulsivity, reward, theta oscillations and alcoholism: (1) alcoholics had significantly higher impulsivity in motor, nonplanning, and total scores of BIS (Fig. 6, panel A); (2) alcoholics selected 50¢ more frequently in the face of loss while controls selected 10¢ more frequently following losing trends (Fig. 6, panel B); and (3) anterior theta power during gain conditions (theta factor‐1) had a statistically significant negative correlation with selection frequency following losses (TRB factor‐2), suggesting that individuals with reduced anterior (predominantly of frontal) theta power during gain conditions selected 50¢ more frequently or 10¢ less frequently in the face of losses (Table III). The first finding links impulsivity and alcoholism as alcoholics had more trait impulsivity as assessed by BIS than that of controls. The second finding links alcoholism with risk‐taking behavior (or impulsive responding), because alcoholics betted with 50¢ more frequently in the face of losses as against controls who “played safe” by selecting 10¢ more frequently following losses or during a losing trend. The third finding, on the other hand, connects the frontal theta activity with risky/impulsive reward processing, that is, the lower the frontal theta power the more frequent the risky choice (i.e., betting with 50¢ in the face of loss). It should be noted that similar interlinks between variables such as impulsivity, reward‐related responses, alcoholism, and electrophysiological (ERP/ERO) measures have been previously reported from our laboratory [e.g., Chen et al., 2007; Kamarajan et al., 2008, 2009, 2010; Porjesz and Rangaswamy, 2007] and elsewhere [e.g., Bjork et al., 2004; Dom et al., 2006a, b, 2007; Finn et al., 1999; Justus et al., 2001; Mitchell et al., 2005; Nagoshi et al., 1991; Petry, 2001].
In our previous studies in healthy normals, it was found that impulsivity was associated with theta power [Kamarajan et al., 2008] as well as with ERP measures of reward processing [Kamarajan et al., 2009]. Similar to the findings of the present study, we demonstrated links among impulsivity, reward‐related behavior, alcoholism, and ERP measures in a sample of abstinent alcoholics and healthy controls [Kamarajan et al., 2010]. In terms of the significance of impulsivity, many studies have attempted to explain impulsivity in terms of its role in alcoholism and related disorders. Specifically, neurocognitive models of addiction disorders often implicate impulsivity as a major component. For example, according to Dom et al. [ 2007], impulsivity is a complex multidimensional construct and linked with the pathogenesis of addictive disorders. It has been proposed that the primary motivation circuitry involving cortical–striatal–thalamic–cortical loops were putatively involved in impulsivity, decision‐making, and the disorders of alcohol/drug addiction and pathological gambling [Chambers and Potenza, 2003; Chambers et al., 2003]. Goldstein and Volkow [ 2002] conceptualized alcohol/drug addiction as a syndrome of impaired response inhibition and salience attribution and summarized the involvement of the frontal–subcortical circuits in addiction disorders. Chen et al. [ 2007] reported that high impulsivity was associated with reduced frontal activation and alcoholism. Further, many researchers have considered impulsivity as the key vulnerability marker for substance‐use disorders, especially alcoholism [Verdejo‐Garcia et al., 2008, a review]. In addition, it has been suggested that the concepts of impulsivity, disinhibition, and risk propensity forms the vulnerability not only for substance use disorders but the entire rubric of disinhibitory or externalizing psychopathology [Iacono et al., 2008; Krueger et al., 2002]. Similar to our finding in alcoholics, Cantrell et al. [ 2008] suggested that decision‐making on the Iowa Gambling Task in those with alcohol dependence and related disinhibitory disorders may reflect an insensitivity to future consequences that is common to the covariance among these disorders but not unique to any one disorder. Fein et al. [ 2004] observed that long‐term abstinent alcoholics, compared to controls, had more externalizing symptoms, showed personality profiles associated with a proneness to social deviance, and made more disadvantageous decisions on a simulated gambling task. Furthermore, connecting the dimensions of electrophysiology, impulsivity, and markers of psychopathology, Hall et al. [2007] suggested that oscillatory correlates of cognitive control and/or impulsivity may assume a critical importance in identifying/establishing markers for the externalizing disorders associated with elevated impulsivity and disinhibition. Taken together, our current study has convincingly demonstrated the relationship between anterior theta response and impulsivity, and therefore we suggest that frontal theta oscillations can potentially serve as a useful marker for differentiating the alcoholics from the normal control and the high‐impulsive individuals from the low‐impulsive individuals.
Reward Deficiency in Alcoholism
The primary findings of the current study, viz., decreased theta power and weaker, diffuse CSD activations, have been interpreted to underlie dysfunctional brain reward circuitry. Because alcoholism has been considered to be a part of the RDS by many researchers [Blum et al., 2000; Bowirrat and Oscar‐Berman, 2005; Comings and Blum, 2000; de Greck et al., 2009; Diekhof et al., 2008; Makris et al., 2008; Wrase et al., 2007], it is worth exploring the validity of this possibility based on existing theories and findings as well as the findings of the present study. In our previous study on reward processing in alcoholics [Kamarajan et al., 2010], we explained the finding of reduced amplitude in ORN and ORP components as a possible dysfunction in the reward circuitry in alcoholics. However, we cautioned that the RDS model may not be sufficient to explain all the dimensions that may encompass the etiology of alcoholism and related disorders. Because alcoholics manifest neurocognitive disinhibition [Begleiter and Porjesz, 1999; Chen et al., 2007; Iacono et al., 2008; Kamarajan et al., 2004, 2005; Porjesz and Rangaswamy, 2007; Porjesz et al., 2005; Rangaswamy et al., 2007] as well as deficient reward processing [Blum et al., 2000; Bowirrat and Oscar‐Berman, 2005; de Greck et al., 2009; Diekhof et al., 2008; Makris et al., 2008; Porjesz et al., 1987; Wrase et al., 2007], our view is that alcoholism and other related disorders are the outcome of dysfunctions in both of these primary mechanisms (i.e., disinhibition and reward deficiency). Therefore, addictive disorders, inclusive of alcoholism, can be considered as a pathology of both reward processing and behavioral/cognitive control.
This proposition of a two‐dimensional approach to addiction has been supported by several researchers. For example, Diekhof et al. [ 2008], in their review, have outlined the neural mechanisms underlying reward processing and decision‐making processes in the healthy brain as well as pathophysiological alterations in the neural reward system observed in addictive and mood disorders. Integrating both dimensions as possible mechanism for addiction and drug‐seeking behavior, Schoenbaum et al. [ 2006] reasoned that addicted individuals commonly exhibit a decreased ability to control the desire to obtain drugs (i.e., inhibitory control), despite knowledge about the aversive consequences following drug intake or the low expectation of actual pleasure expected from the drug (i.e., decision‐making and reward consequences). Although explaining theories on addiction, Robinson and Berridge [ 2003] state that the compulsive character of drug seeking, the obvious lack of inhibitory control, and the lack of ability to avert/reduce risk can be due to pathologically amplified incentive salience of the drug. Incentive salience occurs when stimuli associated with drug‐taking behavior begin reinforcing themselves. When the drug becomes maximally salient at the expense of other available (naturally) rewarding stimuli, it can affect all stages of reward processing [cf. Diekhof et al., 2008]. Furthermore, Longe et al. [ 2009], in their fMRI data, observed functional connectivity between the lateral prefrontal cortex and the ventromedial prefrontal cortex during a high cognitive (memory) load context and during a highly motivational context, but not in the context of reward alone. Therefore, it is likely that although the reward processing deficiency observed in the current study may only partially explain the mechanism of alcohol addiction, reward processing as such may be an important dimension in alcohol/drug addiction. On the other hand, a multidimensional approach might help explain the multifactorial nature of alcoholism and related disorders. Nevertheless, it still remains a challenge as to how the deficiency in monetary reward processing is intricately related to drug‐seeking behavior. Addressing this specific issue, Wrase et al. [ 2007] reported in their interesting fMRI finding that detoxified alcoholics showed reduced activation of the ventral striatum during anticipation of monetary gain but showed increased ventral striatal activation in response to alcohol‐associated cues, suggesting that alcoholics craved for the pharmacological effects of alcohol to a greater extent than other conventional rewards such as monetary rewards. It is expected that similar studies in the future will shed more light on these complex issues.
CONCLUSIONS AND FUTURE DIRECTIONS
The current study has illustrated a possible deficiency in neural reward processing in detoxified, abstinent alcoholics in terms of decreased theta power, and weaker, diffuse CSD activations, and differences in topographic patterns, thus suggesting that power and topography of theta EROs during reward processing may serve as a marker for alcohol dependence. The model of reward deficiency only partially explains reward‐ and/or drug‐seeking behavior and therefore a multidimensional approach involving several factors such as reward processing, disinhibition, motivational context, drug salience, and reinforcement learning may help integrate the neurocognitive factors that cause, maintain, and perpetuate alcoholism and related disorders. It is further suggested that future studies should analyze these factors in offspring at high risk for developing alcoholism in order to further parse the state‐related from the trait‐related variables that may be involved in a predisposition. A major limitation of this study is that the mean age of the alcoholics was higher than that of the controls, although age did not significantly affect theta power across groups; the statistical analysis showed neither main effects of age nor interaction effects of age with group. However, it is suggested that future studies replicate the findings of the present study using an age‐matched control group.
Future studies are essential in order to determine additional information by using all the ERO measures (evoked/phase‐locked, induced/nonphase‐locked, and total power) along with ERP measures. Although the oscillations and ERP approaches each have merits of its own, both approaches are alternative or complementary methods to examine the same phenomena. Although the ERP method has been used to identify and explain the (sequence of) sensory and cognitive events (or components) in the time‐domain data of the averaged EEG activity, the ERO method presents both time and frequency information in evoked, induced, and total EEG activities during neurocognitive processing. A detailed comparison of both approaches have been discussed elsewhere by several authors [Andrew and Fein, 2010; Basar, 1980; Jansen et al., 2003; Jones et al., 2006; Karakas et al., 2000a, b; Klimesch et al., 2004; Makeig et al., 2002]. It would also be of interest to compare theta power across resting EEG, prestimulus baseline, and (poststimulus) event‐related oscillations in alcoholics and controls. Finally, future studies should focus on the application of gambling paradigms to a large spectrum of externalizing disorders, in order to understand the specific as well as common features among these disorders, which may help understand the neural and functional underpinnings of alcoholism and related disorders.
Acknowledgements
In memory of Dr. Henri Begleiter, founder and longtime mentor of the Neurodynamics Laboratory, we acknowledge with great admiration his seminal scientific contributions to the field. We are sincerely indebted to his charismatic leadership and luminous guidance, truly inspired by his scientific mission and vision, and highly motivated to carry forward the work he fondly cherished. We are grateful for the valuable technical assistance of Arthur Stimus, Carlene Haynes, Joyce Alonzia, Chamion Thomas, Tracy Crippen, Glenn Murawski, Eric Talbert, Patrick Harvey, Cindy Lipper, Gabriel Wurzel, Irina Kushnir, and Aleksandr Razran.
REFERENCES
- Aftanas LI, Varlamov AA, Pavlov SV, Makhnev VP, Reva NV ( 2002): Time‐dependent cortical asymmetries induced by emotional arousal: EEG analysis of event‐related synchronization and desynchronization in individually defined frequency bands. Int J Psychophysiol 44: 67–82. [DOI] [PubMed] [Google Scholar]
- Aftanas LI, Pavlov SV, Reva NV, Varlamov AA ( 2003): Trait anxiety impact on the EEG theta band power changes during appraisal of threatening and pleasant visual stimuli. Int J Psychophysiol 50: 205–212. [DOI] [PubMed] [Google Scholar]
- Andrew C, Fein G ( 2010): Event‐related oscillations versus event‐related potentials in a P300 task as biomarkers for alcoholism. Alcohol Clin Exp Res 34: 669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt ES ( 1985): Impulsiveness subtraits: Arousal and information processing In: Spence JT, Izard CE, editors. Motivation, Emotion and Personality. New York: Elsevier; p 137–146. [Google Scholar]
- Basar‐Eroglu C, Demiralp T ( 2001): Event‐related theta oscillations: An integrative and comparative approach in the human and animal brain. Int J Psychophysiol 39: 167–195. [DOI] [PubMed] [Google Scholar]
- Basar E ( 1980): EEG Brain Dynamics: Relation Between EEG and Brain Evoked Potentials. New York: Elsevier. [Google Scholar]
- Basar E ( 1999a): Brain Function and Oscillations, Vol. I: Principles and Approaches. Berlin: Springer Verlag. [Google Scholar]
- Basar E ( 1999b): Brain Function and Oscillations, Vol. II: Integrative Brain Function, Neurophysiology and Cognitive Processes. Berlin: Springer Verlag. [Google Scholar]
- Basar E, Basar‐Eroglu C, Karakas S, Schurmann M ( 2000): Brain oscillations in perception and memory. Int J Psychophysiol 35: 95–124. [DOI] [PubMed] [Google Scholar]
- Basar E, Basar‐Eroglu C, Karakas S, Schurmann M ( 2001a): Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int J Psychophysiol 39: 241–248. [DOI] [PubMed] [Google Scholar]
- Basar E, Schurmann M, Sakowitz O ( 2001b): The selectively distributjked theta system: Functions. Int J Psychophysiol 39: 197–212. [DOI] [PubMed] [Google Scholar]
- Basar E, Guntekin B, Oniz A ( 2006): Principles of oscillatory brain dynamics and a treatise of recognition of faces and facial expressions. Prog Brain Res 159: 43–62. [DOI] [PubMed] [Google Scholar]
- Basar E, Ozgoren M, Oniz A, Schmiedt C, Basar‐Eroglu C ( 2007): Brain oscillations differentiate the picture of one's own grandmother. Int J Psychophysiol 64: 81–90. [DOI] [PubMed] [Google Scholar]
- Bastiaansen MC, Posthuma D, Groot PF, de Geus EJ ( 2002a): Event‐related alpha and theta responses in a visuo‐spatial working memory task. Clin Neurophysiol 113: 1882–1893. [DOI] [PubMed] [Google Scholar]
- Bastiaansen MC, van Berkum JJ, Hagoort P ( 2002b): Event‐related theta power increases in the human EEG during online sentence processing. Neurosci Lett 323: 13–16. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B ( 1999): What is inherited in the predisposition toward alcoholism? A proposed model. Alcohol Clin Exp Res 23: 1125–1135. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Chou CL, Aunon JI ( 1983): P3 and stimulus incentive value. Psychophysiology 20: 95–101. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Hommer DW, Grant SJ, Danube C ( 2004): Impulsivity in abstinent alcohol‐dependent patients: Relation to control subjects and type 1‐/type 2‐like traits. Alcohol 34: 133–150. [DOI] [PubMed] [Google Scholar]
- Blum K, Braverman ER, Holder JM, Lubar JF, Monastra VJ, Miller D, Lubar JO, Chen TJ, Comings DE ( 2000): Reward deficiency syndrome: A biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J Psychoactive Drugs 32( Suppl i–iv): 1–112. [DOI] [PubMed] [Google Scholar]
- Bowirrat A, Oscar‐Berman M ( 2005): Relationship between dopaminergic neurotransmission, alcoholism, and Reward Deficiency Syndrome. Am J Med Genet B Neuropsychiatr Genet 132: 29–37. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Rosen BR ( 1999): Functional magnetic resonance imaging of brain reward circuitry in the human. Ann NY Acad Sci 877: 523–47. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P ( 2001): Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron 30: 619–639. [DOI] [PubMed] [Google Scholar]
- Bruguier A, Preuschoff K, Quartz S, Bossaerts P ( 2008): Investigating signal integration with canonical correlation analysis of fMRI brain activation data. Neuroimage 41: 35–44. [DOI] [PubMed] [Google Scholar]
- Camara E, Rodriguez‐Fornells A, Munte TF ( 2008): Functional connectivity of reward processing in the brain. Front Hum Neurosci 2: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell H, Finn PR, Rickert ME, Lucas J ( 2008): Decision making in alcohol dependence: Insensitivity to future consequences and comorbid disinhibitory psychopathology. Alcohol Clin Exp Res 32: 1398–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan JB, Madsen JR, Schulze‐Bonhage A, Aschenbrenner‐Scheibe R, Newman EL, Kahana MJ ( 2003): Human theta oscillations related to sensorimotor integration and spatial learning. J Neurosci 23: 4726–4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Potenza MN ( 2003): Neurodevelopment, impulsivity, and adolescent gambling. J Gambl Stud 19: 53–84. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN ( 2003): Developmental neurocircuitry of motivation in adolescence: A critical period of addiction vulnerability. Am J Psychiatry 160: 1041–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AC, Porjesz B, Rangaswamy M, Kamarajan C, Tang Y, Jones KA, Chorlian DB, Stimus AT, Begleiter H ( 2007): Reduced frontal lobe activity in subjects with high impulsivity and alcoholism. Alcohol Clin Exp Res 31: 156–165. [DOI] [PubMed] [Google Scholar]
- Chu PC ( 1996): The S‐transform for obtaining localized spectra. MTS J 29: 28–38. [Google Scholar]
- Cohen HL, Wang W, Porjesz B, Bauer L, Kuperman S, O'Connor SJ, Rohrbaugh J, Begleiter H ( 1994): Visual P300: An interlaboratory consistency study. Alcohol 11: 583–587. [DOI] [PubMed] [Google Scholar]
- Cohen HL, Porjesz B, Begleiter H, Wang W ( 1997): Neurophysiological correlates of response production and inhibition in alcoholics. Alcohol Clin Exp Res 21: 1398–1406. [PubMed] [Google Scholar]
- Cohen HL, Ji J, Chorlian DB, Begleiter H, Porjesz B ( 2002): Alcohol‐related ERP changes recorded from different modalities: A topographic analysis. Alcohol Clin Exp Res 26: 303–317. [PubMed] [Google Scholar]
- Cohen MX, Elger CE, Ranganath C ( 2007): Reward expectation modulates feedback‐related negativity and EEG spectra. Neuroimage 35: 968–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comings DE, Blum K ( 2000): Reward deficiency syndrome: Genetic aspects of behavioral disorders. Prog Brain Res 126: 325–341. [DOI] [PubMed] [Google Scholar]
- Crews FT, Boettiger CA ( 2009): Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Behav 93: 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin EA, Bijl S, Stam CJ, Bocker KB, Kenemans JL, Verbaten MN ( 2004): Abnormal EEG synchronisation in heavily drinking students. Clin Neurophysiol 115: 2048–2055. [DOI] [PubMed] [Google Scholar]
- de Greck M, Supady A, Thiemann R, Tempelmann C, Bogerts B, Forschner L, Ploetz KV, Northoff G ( 2009): Decreased neural activity in reward circuitry during personal reference in abstinent alcoholics—A fMRI study. Hum Brain Mapp 30: 1691–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA ( 2000): Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol 84: 3072–3077. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Locke HM, Stenger VA, Fiez JA ( 2003): Dorsal striatum responses to reward and punishment: Effects of valence and magnitude manipulations. Cogn Affect Behav Neurosci 3: 27–38. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Miller MM, Inati S, Phelps EA ( 2005): An fMRI study of reward‐related probability learning. Neuroimage 24: 862–873. [DOI] [PubMed] [Google Scholar]
- Diekhof EK, Falkai P, Gruber O ( 2008): Functional neuroimaging of reward processing and decision‐making: A review of aberrant motivational and affective processing in addiction and mood disorders. Brain Res Rev 59: 164–184. [DOI] [PubMed] [Google Scholar]
- Dom G, de Wilde B, Hulstijn W, van den Brink W, Sabbe B ( 2006a): Behavioural aspects of impulsivity in alcoholics with and without a cluster‐B personality disorder. Alcohol Alcohol 41: 412–420. [DOI] [PubMed] [Google Scholar]
- Dom G, D'Haene P, Hulstijn W, Sabbe B ( 2006b): Impulsivity in abstinent early‐ and late‐onset alcoholics: Differences in self‐report measures and a discounting task. Addiction 101: 50–59. [DOI] [PubMed] [Google Scholar]
- Dom G, De Wilde B, Hulstijn W, Sabbe B ( 2007): Dimensions of impulsive behaviour in abstinent alcoholics. Personal Indiv Differ 42: 465–476. [Google Scholar]
- Doppelmayr M, Klimesch W, Schwaiger J, Auinger P, Winkler T ( 1998): Theta synchronization in the human EEG and episodic retrieval. Neurosci Lett 257: 41–44. [DOI] [PubMed] [Google Scholar]
- Doppelmayr M, Klimesch W, Schwaiger J, Stadler W, Rohm D ( 2000): The time locked theta response reflects interindividual differences in human memory performance. Neurosci Lett 278: 141–144. [DOI] [PubMed] [Google Scholar]
- Doppelmayr M, Stadler W, Sauseng P, Rachbauer D, Klimesch W ( 2002): Gender‐related differences in theta bandpower changes of the EEG during the presentation of erotic and child related stimuli. Proceedings of the 12th annual conference emotions and the brain, Toronto, Canada.
- Doppelmayr M, Klimesch W, Sauseng P, Hodlmoser K, Stadler W, Hanslmayr S ( 2005): Intelligence related differences in EEG‐bandpower. Neurosci Lett 381: 309–313. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Brandeis D, Strik WK ( 1997): A robust assessment of the NoGo‐anteriorisation of P300 microstates in a cued Continuous Performance Test. Brain Topogr 9: 295–302. [DOI] [PubMed] [Google Scholar]
- Fein G, Chang M ( 2008): Smaller feedback ERN amplitudes during the BART are associated with a greater family history density of alcohol problems in treatment‐naive alcoholics. Drug Alcohol Depend 92: 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Klein L, Finn P ( 2004): Impairment on a simulated gambling task in long‐term abstinent alcoholics. Alcohol Clin Exp Res 28: 1487–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn PR, Justus A, Mazas C, Steinmetz JE ( 1999): Working memory, executive processes and the effects of alcohol on Go/No‐Go learning: Testing a model of behavioral regulation and impulsivity. Psychopharmacology (Berl) 146: 465–472. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR ( 1975): “Mini‐mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189–198. [DOI] [PubMed] [Google Scholar]
- Fujiwara J, Tobler PN, Taira M, Iijima T, Tsutsui KI ( 2009): Segregated and integrated coding of reward and punishment in the cingulate cortex. J Neurophysiol 101: 3284–3293. [DOI] [PubMed] [Google Scholar]
- Gabor D ( 1946): Theory of communications. J Inst Elec Eng 93: 429–457. [Google Scholar]
- Galvan A, Hare TA, Davidson M, Spicer J, Glover G, Casey BJ ( 2005): The role of ventral frontostriatal circuitry in reward‐based learning in humans. J Neurosci 25: 8650–8656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR ( 2002): The medial frontal cortex and the rapid processing of monetary gains and losses. Science 295: 2279–2282. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR ( 2004): Are all medial frontal negativities created equal? Toward a richer empirical basis for theories of action monitoring In: Ullsperger M, Falkenstein M, editors. Errors, Conflicts, and the Brain: Current Opinions on Performance Monitoring. Leipzig: Max Planck Institute of Cognitive Neuroscience. [Google Scholar]
- Gilbert AM, Fiez JA ( 2004): Integrating rewards and cognition in the frontal cortex. Cogn Affect Behav Neurosci 4: 540–552. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND ( 2002): Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry 159: 1642–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez‐Hernandez JA, Pita‐Alcorta C, Cedeno I, Bosch‐Bayard J, Galan‐Garcia L, Scherbaum WA, Figueredo‐Rodriguez P ( 2002): Wisconsin Card Sorting Test synchronizes the prefrontal, temporal and posterior association cortex in different frequency ranges and extensions. Hum Brain Mapp 17: 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hada M, Porjesz B, Begleiter H, Polich J ( 2000): Auditory P3a assessment of male alcoholics. Biol Psychiatry 48: 276–286. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Holroyd CB, Moser JS, Simons RF ( 2005): Brain potentials associated with expected and unexpected good and bad outcomes. Psychophysiology 42: 161–170. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Holroyd CB, Simons RF ( 2006): The feedback‐related negativity reflects the binary evaluation of good versus bad outcomes. Biol Psychol 71: 148–154. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Holroyd CB, Simons RF ( 2007): It's worse than you thought: The feedback negativity and violations of reward prediction in gambling tasks. Psychophysiology 44: 905–912. [DOI] [PubMed] [Google Scholar]
- Hampton AN, Adolphs R, Tyszka MJ, O'Doherty JP ( 2007): Contributions of the amygdala to reward expectancy and choice signals in human prefrontal cortex. Neuron 55: 545–555. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Pastotter B, Bauml KH, Gruber S, Wimber M, Klimesch W ( 2008): The electrophysiological dynamics of interference during the Stroop task. J Cogn Neurosci 20: 215–225. [DOI] [PubMed] [Google Scholar]
- Hjorth B ( 1975): An on‐line transformation of EEG scalp potentials into orthogonal source derivations. Electroencephalogr Clin Neurophysiol 39: 526–530. [DOI] [PubMed] [Google Scholar]
- Hjorth B ( 1991): Principles for transformation of scalp EEG from potential field into source distribution. J Clin Neurophysiol 8: 391–396. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Larsen JT, Cohen JD ( 2004): Context dependence of the event‐related brain potential associated with reward and punishment. Psychophysiology 41: 245–253. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Hajcak G, Larsen JT ( 2006): The good, the bad and the neutral: Electrophysiological responses to feedback stimuli. Brain Res 1105: 93–101. [DOI] [PubMed] [Google Scholar]
- Homberg V, Grunewald G, Grunewald‐Zuberbier E ( 1980): The incentive value of stimuli and the P300 component of cerebral evoked potentials. Prog Brain Res 54: 629–633. [DOI] [PubMed] [Google Scholar]
- Homberg V, Grunewald G, Grunewald‐Zuberbier E ( 1981): The variation of p300 amplitude in a money‐winning paradigm in children. Psychophysiology 18: 258–262. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M ( 2008): Behavioral disinhibition and the development of early‐onset addiction: Common and specific influences. Annu Rev Clin Psychol 4: 325–348. [DOI] [PubMed] [Google Scholar]
- Ikemoto S ( 2010): Brain reward circuitry beyond the mesolimbic dopamine system: A neurobiological theory. Neurosci Biobehav Rev 35: 129–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Hwang G, Curran T, Kahana MJ ( 2006): EEG oscillations and recognition memory: Theta correlates of memory retrieval and decision making. Neuroimage 32: 978–987. [DOI] [PubMed] [Google Scholar]
- Jansen BH, Agarwal G, Hegde A, Boutros NN ( 2003): Phase synchronization of the ongoing EEG and auditory EP generation. Clin Neurophysiol 114: 79–85. [DOI] [PubMed] [Google Scholar]
- Ji J, Porjesz B, Begleiter H ( 1999): Event‐related potential index of semantic mnemonic dysfunction in abstinent alcoholics. Biol Psychiatry 45: 494–507. [DOI] [PubMed] [Google Scholar]
- Jones KA, Porjesz B, Almasy L, Bierut L, Goate A, Wang JC, Dick DM, Hinrichs A, Kwon J, Rice JP, Rohrbaugh J, Stock H, Wu W, Bauer LO, Chorlian DB, Crowe RR, Edenberg HJ, Foroud T, Hesselbrock V, Kuperman S, Nurnberger J Jr, O'Connor SJ, Schuckit MA, Stimus AT, Tischfield JA, Reich T, Begleiter H ( 2004): Linkage and linkage disequilibrium of evoked EEG oscillations with CHRM2 receptor gene polymorphisms: Implications for human brain dynamics and cognition. Int J Psychophysiol 53: 75–90. [DOI] [PubMed] [Google Scholar]
- Jones KA, Porjesz B, Chorlian D, Rangaswamy M, Kamarajan C, Padmanabhapillai A, Stimus A, Begleiter H ( 2006): S‐transform time‐frequency analysis of P300 reveals deficits in individuals diagnosed with alcoholism. Clin Neurophysiol 117: 2128–2143. [DOI] [PubMed] [Google Scholar]
- Justus AN, Finn PR, Steinmetz JE ( 2001): P300, disinhibited personality, and early‐onset alcohol problems. Alcohol Clin Exp Res 25: 1457–1466. [DOI] [PubMed] [Google Scholar]
- Kahana MJ, Seelig D, Madsen JR ( 2001): Theta returns Curr Opin Neurobiol 11: 739–744. [DOI] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, Choi K, Chorlian DB, Padmanabhapillai A, Rangaswamy M, Stimus AT, Begleiter H ( 2004): The role of brain oscillations as functional correlates of cognitive systems: A study of frontal inhibitory control in alcoholism. Int J Psychophysiol 51: 155–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, Choi K, Chorlian DB, Padmanabhapillai A, Rangaswamy M, Stimus AT, Begleiter H ( 2005): Alcoholism is a disinhibitory disorder: Neurophysiological evidence from a Go/No‐Go task. Biol Psychol 69: 353–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones K, Chorlian D, Padmanabhapillai A, Rangaswamy M, Stimus A, Begleiter H ( 2006): Event‐related oscillations in offspring of alcoholics: Neurocognitive disinhibition as a risk for alcoholism. Biol Psychiatry 59: 625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Rangaswamy M, Chorlian DB, Manz N, Tang Y, Pandey AK, Roopesh BN, Stimus AT, Porjesz B ( 2008): Theta oscillations during the processing of monetary loss and gain: A perspective on gender and impulsivity. Brain Res 1235: 45–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Rangaswamy M, Tang Y, Chorlian DB, Padmanabhapillai A, Saunders R, Pandey AK, Roopesh BN, Manz N, Stimus AT, Begleiter H ( 2009): Brain signatures of monetary loss and gain: Outcome‐related potentials in a single outcome gambling task. Behav Brain Res 197: 62–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Rangaswamy M, Tang Y, Chorlian DB, Pandey AK, Roopesh BN, Manz N, Saunders R, Stimus AT, Porjesz B ( 2010): Dysfunctional reward processing in male alcoholics: An ERP study during a gambling task. J Psychiatr Res 44: 576–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakas S, Erzengin OU, Basar E ( 2000a): A new strategy involving multiple cognitive paradigms demonstrates that ERP components are determined by the superposition of oscillatory responses. Clin Neurophysiol 111: 1719–1732. [DOI] [PubMed] [Google Scholar]
- Karakas S, Erzengin OU, Basar E ( 2000b): The genesis of human event‐related responses explained through the theory of oscillatory neural assemblies. Neurosci Lett 285: 45–48. [DOI] [PubMed] [Google Scholar]
- Klimesch W ( 1996): Memory processes, brain oscillations and EEG synchronization. Int J Psychophysiol 24: 61–100. [DOI] [PubMed] [Google Scholar]
- Klimesch W ( 1999): EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res Brain Res Rev 29: 169–195. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Russegger H, Pachinger T ( 1996): Theta band power in the human scalp EEG and the encoding of new information. Neuroreport 7: 1235–1240. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Pachinger T, Ripper B ( 1997a): Brain oscillations and human memory: EEG correlates in the upper alpha and theta band. Neurosci Lett 238: 9–12. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Schimke H, Ripper B ( 1997b): Theta synchronization and alpha desynchronization in a memory task. Psychophysiology 34: 169–176. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Wimmer H, Schwaiger J, Rohm D, Gruber W, Hutzler F ( 2001a): Theta band power changes in normal and dyslexic children. Clin Neurophysiol 112: 1174–1185. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Yonelinas A, Kroll NE, Lazzara M, Rohm D, Gruber W ( 2001b): Theta synchronization during episodic retrieval: Neural correlates of conscious awareness. Brain Res Cogn Brain Res 12: 33–38. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Schack B, Schabus M, Doppelmayr M, Gruber W, Sauseng P ( 2004): Phase‐locked alpha and theta oscillations generate the P1‐N1 complex and are related to memory performance. Brain Res Cogn Brain Res 19: 302–316. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Schack B, Sauseng P ( 2005): The functional significance of theta and upper alpha oscillations. Exp Psychol 52: 99–108. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Hanslmayr S, Sauseng P, Gruber W, Brozinsky CJ, Kroll NE, Yonelinas AP, Doppelmayr M ( 2006): Oscillatory EEG correlates of episodic trace decay. Cereb Cortex 16: 280–290. [DOI] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D ( 2000): fMRI visualization of brain activity during a monetary incentive delay task. Neuroimage 12: 20–27. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D ( 2001): Dissociation of reward anticipation and outcome with event‐related fMRI. Neuroreport 12: 3683–3687. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D ( 2003): A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: Characterization with rapid event‐related fMRI. Neuroimage 18: 263–272. [DOI] [PubMed] [Google Scholar]
- Knyazev GG ( 2007): Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neurosci Biobehav Rev 31: 377–395. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND ( 2010): Neurocircuitry of addiction. Neuropsychopharmacology 35: 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause CM, Sillanmaki L, Koivisto M, Saarela C, Haggqvist A, Laine M, Hamalainen H ( 2000a): The effects of memory load on event‐related EEG desynchronization and synchronization. Clin Neurophysiol 111: 2071–2078. [DOI] [PubMed] [Google Scholar]
- Krause CM, Viemero V, Rosenqvist A, Sillanmaki L, Astrom T ( 2000b): Relative electroencephalographic desynchronization and synchronization in humans to emotional film content: An analysis of the 4‐6, 6‐8, 8‐10 and 10‐12 Hz frequency bands. Neurosci Lett 286: 9–12. [DOI] [PubMed] [Google Scholar]
- Krause CM, Aromaki A, Sillanmaki L, Astrom T, Alanko K, Salonen E, Peltola O ( 2002): Alcohol‐induced alterations in ERD/ERS during an auditory memory task. Alcohol 26: 145–153. [DOI] [PubMed] [Google Scholar]
- Krause CM, Pesonen M, Hamalainen H ( 2007): Brain oscillatory responses during the different stages of an auditory memory search task in children. Neuroreport 18: 213–216. [DOI] [PubMed] [Google Scholar]
- Krawczyk DC, Gazzaley A, D'Esposito M ( 2007): Reward modulation of prefrontal and visual association cortex during an incentive working memory task. Brain Res 1141: 168–177. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M ( 2002): Etiologic connections among substance dependence, antisocial behavior, and personality: Modeling the externalizing spectrum. J Abnorm Psychol 111: 411–424. [PubMed] [Google Scholar]
- Longe O, Senior C, Rippon G ( 2009): The lateral and ventromedial prefrontal cortex work as a dynamic integrated system: Evidence from FMRI connectivity analysis. J Cogn Neurosci 21: 141–154. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Derryberry D, Reed M, Poulsen C ( 2003): Electrophysiological responses to errors and feedback in the process of action regulation. Psychol Sci 14: 47–53. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Makeig S ( 2004): Frontal midline theta and the error‐related negativity: Neurophysiological mechanisms of action regulation. Clin Neurophysiol 115: 1821–1835. [DOI] [PubMed] [Google Scholar]
- Makeig S, Westerfield M, Jung TP, Enghoff S, Townsend J, Courchesne E, Sejnowski TJ ( 2002): Dynamic brain sources of visual evoked responses. Science 295: 690–694. [DOI] [PubMed] [Google Scholar]
- Makris N, Oscar‐Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, Marinkovic K, Breiter HC, Gasic GP, Harris GJ ( 2008): Decreased volume of the brain reward system in alcoholism. Biol Psychiatry 64: 192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco‐Pallares J, Muller SV, Munte TF ( 2007): Learning by doing: An fMRI study of feedback‐related brain activations. Neuroreport 18: 1423–1426. [DOI] [PubMed] [Google Scholar]
- Marco‐Pallares J, Cucurell D, Cunillera T, Garcia R, Andres‐Pueyo A, Munte TF, Rodriguez‐Fornells A ( 2008): Human oscillatory activity associated to reward processing in a gambling task. Neuropsychologia 46: 241–248. [DOI] [PubMed] [Google Scholar]
- McClure SM, York MK, Montague PR ( 2004): The neural substrates of reward processing in humans: The modern role of FMRI. Neuroscientist 10: 260–268. [DOI] [PubMed] [Google Scholar]
- Mennes M, Wouters H, van den Bergh B, Lagae L, Stiers P ( 2008): ERP correlates of complex human decision making in a gambling paradigm: Detection and resolution of conflict. Psychophysiology 45: 714–720. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Fields HL, D'Esposito M, Boettiger CA ( 2005): Impulsive responding in alcoholics. Alcohol Clin Exp Res 29: 2158–2169. [DOI] [PubMed] [Google Scholar]
- Moselhy HF, Georgiou G, Kahn A ( 2001): Frontal lobe changes in alcoholism: A review of the literature. Alcohol Alcohol 36: 357–368. [DOI] [PubMed] [Google Scholar]
- Nagoshi CT, Wilson JR, Rodriguez LA ( 1991): Impulsivity, sensation seeking, and behavioral and emotional responses to alcohol. Alcohol Clin Exp Res 15: 661–667. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, Holroyd CB, Schurger A, Cohen JD ( 2004): Sensitivity of electrophysiological activity from medial frontal cortex to utilitarian and performance feedback. Cereb Cortex 14: 741–747. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Heslenfeld DJ, von Geusau NJ, Mars RB, Holroyd CB, Yeung N ( 2005a): Activity in human reward‐sensitive brain areas is strongly context dependent. Neuroimage 25: 1302–1309. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Slagter HA, von Geusau NJ, Heslenfeld DJ, Holroyd CB ( 2005b): Knowing good from bad: Differential activation of human cortical areas by positive and negative outcomes. Eur J Neurosci 21: 3161–3168. [DOI] [PubMed] [Google Scholar]
- Nunez PL ( 1981): Electric Fields of the Brain: The Neurophysics of EEG. New York: Oxford University Press. [Google Scholar]
- Nunez PL, Pilgreen KL ( 1991): The spline‐Laplacian in clinical neurophysiology: A method to improve EEG spatial resolution. J Clin Neurophysiol 8: 397–413. [PubMed] [Google Scholar]
- Otten LJ, Gaillard AW, Wientjes CJ ( 1995): The relation between event‐related brain potential, heart rate, and blood pressure responses in an S1‐S2 paradigm. Biol Psychol 39: 81–102. [DOI] [PubMed] [Google Scholar]
- Padmanabhapillai A, Porjesz B, Ranganathan M, Jones KA, Chorlian DB, Tang Y, Kamarajan C, Rangaswamy M, Stimus A, Begleiter H ( 2006a): Suppression of early evoked gamma band response in male alcoholics during a visual oddball task. Int J Psychophysiol 60: 15–26. [DOI] [PubMed] [Google Scholar]
- Padmanabhapillai A, Tang Y, Ranganathan M, Rangaswamy M, Jones KA, Chorlian DB, Kamarajan C, Stimus A, Kuperman S, Rohrbaugh J, O'Connor SJ, Bauer LO, Schuckit MA, Begleiter H, Porjesz B ( 2006b): Evoked gamma band response in male adolescent subjects at high risk for alcoholism during a visual oddball task. Int J Psychophysiol 62: 262–271. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES ( 1995): Factor structure of the Barratt impulsiveness scale. J Clin Psychol 51: 768–774. [DOI] [PubMed] [Google Scholar]
- Petry NM ( 2001): Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology (Berl) 154: 243–250. [DOI] [PubMed] [Google Scholar]
- Pochon JB, Levy R, Fossati P, Lehericy S, Poline JB, Pillon B, Le Bihan D, Dubois B ( 2002): The neural system that bridges reward and cognition in humans: An fMRI study. Proc Natl Acad Sci USA 99: 5669–5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H ( 1985): Human brain electrophysiology and alcoholism In: Tarter RD, Van Thiel D, editors. Alcohol and the Brain. New York: Plenum; pp 139–182. [Google Scholar]
- Porjesz B, Begleiter H ( 1993): Neurophysiological factors associated with alcoholism In: Nixon SJ, Hunt WA, editors. NIAAA Research Monograph No. 22. Alcohol‐Induced Brain Damage. Rockville, MD: NIH Publications; pp 89–120. [Google Scholar]
- Porjesz B, Begleiter H ( 2003): Alcoholism and human electrophysiology. Alcohol Res Health 27: 153–160. [PMC free article] [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M ( 2007): Neurophysiological endophenotypes, CNS disinhibition, and risk for alcohol dependence and related disorders. Sci World J 7: 131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H, Bihari B, Kissin B ( 1987): Event‐related brain potentials to high incentive stimuli in abstinent alcoholics. Alcohol 4: 283–287. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M, Kamarajan C, Jones KA, Padmanabhapillai A, Begleiter H ( 2005): The utility of neurophysiological markers in the study of alcoholism. Clin Neurophysiol 116: 993–1018. [DOI] [PubMed] [Google Scholar]
- Raghavachari S, Kahana MJ, Rizzuto DS, Caplan JB, Kirschen MP, Bourgeois B, Madsen JR, Lisman JE ( 2001): Gating of human theta oscillations by a working memory task. J Neurosci 21: 3175–3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavachari S, Lisman JE, Tully M, Madsen JR, Bromfield EB, Kahana MJ ( 2006): Theta oscillations in human cortex during a working‐memory task: Evidence for local generators. J Neurophysiol 95: 1630–1638. [DOI] [PubMed] [Google Scholar]
- Ramsey SE, Finn PR ( 1997): P300 from men with a family history of alcoholism under different incentive conditions. J Stud Alcohol 58: 606–616. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Jones KA, Porjesz B, Chorlian DB, Padmanabhapillai A, Kamarajan C, Kuperman S, Rohrbaugh J, O'Connor SJ, Bauer LO, Schuckit MA, Begleiter H ( 2007): Delta and theta oscillations as risk markers in adolescent offspring of alcoholics. Int J Psychophysiol 63: 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razumnikova OM ( 2007): Creativity related cortex activity in the remote associates task. Brain Res Bull 73: 96–102. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC ( 2003): Addiction. Annu Rev Psychol 54: 25–53. [DOI] [PubMed] [Google Scholar]
- Rodriguez Holguin S, Porjesz B, Chorlian DB, Polich J, Begleiter H ( 1999): Visual P3a in male alcoholics and controls. Alcohol Clin Exp Res 23: 582–591. [PubMed] [Google Scholar]
- Sammer G, Blecker C, Gebhardt H, Bischoff M, Stark R, Morgen K, Vaitl D ( 2007): Relationship between regional hemodynamic activity and simultaneously recorded EEG‐theta associated with mental arithmetic‐induced workload. Hum Brain Mapp 28: 793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL ( 1977): EEG theta waves and psychological phenomena: A review and analysis. Biol Psychol 5: 47–82. [DOI] [PubMed] [Google Scholar]
- Schmiedt C, Brand A, Hildebrandt H, Basar‐Eroglu C ( 2005): Event‐related theta oscillations during working memory tasks in patients with schizophrenia and healthy controls. Brain Res Cogn Brain Res 25: 936–947. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA ( 2006): Orbitofrontal cortex, decision‐making and drug addiction. Trends Neurosci 29: 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell RG, Mansinha L, Lowe RP ( 1996): Localization of the complex spectrum: The S‐transform. IEEE Trans Sig Proc 44: 998–1001. [Google Scholar]
- Theophanis MR, Queen J ( 2000): Color display of the localized spectrum. Geophysics 65: 1330–1340. [Google Scholar]
- Toyomaki A, Murohashi H ( 2005): Discrepancy between feedback negativity and subjective evaluation in gambling. Neuroreport 16: 1865–1868. [DOI] [PubMed] [Google Scholar]
- Trujillo LT, Allen JJ ( 2007): Theta EEG dynamics of the error‐related negativity. Clin Neurophysiol 118: 645–668. [DOI] [PubMed] [Google Scholar]
- Verdejo‐Garcia A, Lawrence AJ, Clark L ( 2008): Impulsivity as a vulnerability marker for substance‐use disorders: Review of findings from high‐risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev 32: 777–810. [DOI] [PubMed] [Google Scholar]
- Wang K, Begleiter H ( 1999): Local polynomial estimate of surface Laplacian. Brain Topogr 12: 19–29. [DOI] [PubMed] [Google Scholar]
- Weber B, Rangel A, Wibral M, Falk A ( 2009): The medial prefrontal cortex exhibits money illusion. Proc Natl Acad Sci USA 106: 5025–5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, Wustenberg T, Bermpohl F, Kahnt T, Beck A, Strohle A, Juckel G, Knutson B, Heinz A ( 2007): Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage 35: 787–794. [DOI] [PubMed] [Google Scholar]
- Xue G, Lu Z, Levin IP, Weller JA, Li X, Bechara A ( 2009): Functional dissociations of risk and reward processing in the medial prefrontal cortex. Cereb Cortex 19: 1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacubian J, Sommer T, Schroeder K, Glascher J, Braus DF, Buchel C ( 2007): Subregions of the ventral striatum show preferential coding of reward magnitude and probability. Neuroimage 38: 557–563. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Braver TS, Gray JR, Green L ( 2005): Prefrontal brain activity predicts temporally extended decision‐making behavior. J Exp Anal Behav 84: 537–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung N, Sanfey AG ( 2004): Independent coding of reward magnitude and valence in the human brain. J Neurosci 24: 6258–6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung N, Holroyd CB, Cohen JD ( 2005): ERP correlates of feedback and reward processing in the presence and absence of response choice. Cereb Cortex 15: 535–544. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Kolev V, Rosso OA, Schurmann M, Sakowitz OW, Ozgoren M, Basar E ( 2002): Wavelet entropy analysis of event‐related potentials indicates modality‐independent theta dominance. J Neurosci Methods 117: 99–109. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Rosso OA, Kolev V ( 2003): A transient dominance of theta event‐related brain potential component characterizes stimulus processing in an auditory oddball task. Clin Neurophysiol 114: 529–540. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Falkenstein M, Hohnsbein J, Kolev V ( 2004): Parallel systems of error processing in the brain. Neuroimage 22: 590–602. [DOI] [PubMed] [Google Scholar]
- Yu R, Zhou X ( 2006): Brain potentials associated with outcome expectation and outcome evaluation. Neuroreport 17: 1649–1653. [DOI] [PubMed] [Google Scholar]


