Abstract
BACKGROUND
IL28B polymorphisms have been associated with both treatment induced and spontaneous clearance of hepatitis C virus (HCV). We previously found that LDL cholesterol levels were higher in chronic hepatitis C (CHC) patients with the CC genotype at the rs12979860 polymorphism, located proximal to the IL28 gene. Here we analyzed the association of steatosis with IL28B genotype in treatment naïve patients with CHC.
METHODS
Two independent cohorts of 145 genotype 1 infected patients from an antifibrotic study and 180 genotype 1 patients from Duke were analyzed for presence and severity of steatosis in relation to the rs12979860 polymorphism at the IL28B locus. TaqMan assay based genotyping classified three groups CC, CT and TT.
RESULTS
CC genotype was associated with a lower prevalence of steatosis. In the antifibrotic study steatosis was found in 47.6% (50/105) of IL28B non-CC versus 22.5% (9/40; p=0.008) in CC patients. Similarly, steatosis was found in 67.4% (89/132) of non-CC patients compared to only 39.6% (19/48; p=0.001) of CC patients in the Duke cohort.
CONCLUSIONS
IL28B CC genotype is associated with less pronounced disturbances of lipid metabolism, as reflected both in serum lipoprotein levels and hepatic steatosis, in HCV infection.
Keywords: chronic hepatitis C, genetic, lipids, IL28B, steatosis
In a genome wide association study, SNPs in the IL28B region were found to be associated with treatment response to pegylated interferon and ribavirin in patients with hepatitis C virus (HCV infection [1], a finding which has subsequently been confirmed in multiple independent studies [2]. The SNP rs12979860 showed the strongest correlation with treatment response, and codes for either a T or a C leading to 3 different genotypes TT, CT, or CC. The CC genotype was the beneficial genotype associated with sustained response to peg-interferon plus ribavirin in hepatitis C.
The association between HCV infection and a reduction of lipid levels is well recognized. We previously studied the association of IL28B with different serum lipids, and found serum triglycerides to be lower and serum LDL cholesterol to be higher in IL28B CC patients [3], The effect on lipid levels in relation to IL28B genotype appeared to be stronger for HCV genotype 1 infection than for genotype 2 or 3 [3].
Here, we studied IL28B’s genotype in association with hepatic steatosis, another hallmark of CHC infection. Given the less pronounced effect on lowering cholesterol in IL28B CC compared to non CC genotypes, one might expect steatosis due to HCV infection to be lower in IL28B CC genotype patients compared to IL28B non-CC genotype patients with CHC. More importantly for steatosis, the finding of lower triglycerides in IL28B CC genotype patients with HCV, likewise argues for lower steatosis rates in these patients.
METHODS
Patient population
Patients for the current study were taken from two sources. One cohort of patients was from a recently completed multicenter Phase II clinical trial to assess the effectiveness of farglitazar, a PPAR γ agonist, as an antifibrotic agent among HCV-genotype 1 CHC patients who were non-responsive to prior interferon-based therapy [4]. Only 145 of the original 265 patients could be used for this study based on consent for IL28B genotyping (see table 1a), these patients did not show obvious differences to the original cohort [4].
Table 1.
Baseline characteristics for patients from the antifibritic study (A) and the Duke cohort (B)
| A | This cohort (n=145) | Original Farglitzar Study (n=265) | ||
|---|---|---|---|---|
| Original Placebo (n=88) | Original Farglitzar 0.5mg (n=89) | Original Farglitzar 0.5mg (n=88) | ||
| Age | 51.8 (± 6.8) | 52.1 (± 5.6) | 51.8 (± 7.3) | 51.4 (± 5.8) |
| Gende,r male sex | 90 (62.1%) | 53 (60%) | 53 (60%) | 59 (67%) |
| BMI | 28.3 (± 4.6) | 29.7 (± 5.5) | 27.9 (± 4.2) | 29.2 (± 6.0) |
| Race W, B, A, o | ||||
| White | 122 (84.1%) | 63 (72%) | 67 (76%) | 68 (77%) |
| Black | 12 (8.3%) | 12 (14%) | 14 (16%) | 9 (10%) |
| Asian | 4 (2.8) | 11 (13%) | 6 (7%) | 7 (8%) |
| other | 7 (4.8%) | 2 (2%) | 2 (2%) | 4 (2%) |
| Fibrosis score (Ishak) | ||||
| 2 | 50 (34.5%) | 35 (41%) | 31 (35%) | 32 (36%)* |
| 3 | 69 (47.6%) | 37 (43%) | 42 (48%) | 41 (47%) |
| 4 | 26 (19.9%) | 14 (16%) | 14 (17%) | 14 (16%)** |
| IL28B | ||||
| CC | 40 (27.6%) | |||
| CT | 77 (53.1%) | |||
| TT | 28 (19.3%) | |||
| B | This “Duke cohort”(n=145) | Larger “Duke lipids cohort” (n=634)[4] |
| Age | 50.3 (±6.8) | |
| Gende,r male sex | 125 (69.4%) | 62% |
| BMI | 29.3 (±5.5) | Unknown |
| Race W, B, A, o | ||
| White | 130 (72.2%) | 71% |
| Black | 45 (25%) | 29% |
| other | 5 (2.8%) | |
| Fibrosis score (MetavirIshak) | ||
| 0-1 | 54 (30%) | 2% |
| 2 | 61 (33.9%) | 39% |
| 3-4 | 65 (26.1%) | 33% |
| IL28B | ||
| CC | 48 (26.7%) | |
| CT | 98 (54.4%) | |
| TT | 34 (18.9%) |
Includes 2 patients withF1 fibrosis,
included 3 patients with F5 fibrosis
The second cohort was from the Duke Hepatology Clinical Research Database and Repository [5]. In the Duke cohort, liver biopsies were from a time-point prior to initiation of any Peg-Interferon-therapy and for this analysis only lipid levels determined in a sample prior to initiation of any interferon therapy were considered. Characteristics of lipid determination and characterization of viral status and genotype has been described earlier [3]. Only genotype 1 patients with a biopsy prior to treatment initiation and with available steatosis scoring were included (see table 1b).
Samples were collected under fasting within the antifibrotic study, while those from the Duke cohort were collected under non-fasting conditions.
We excluded patients co-infected with hepatitis B virus or HIV-1; and patients with poor quality DNA, defined as >10% failed genotypes in prior test-genotyping across a minimum of 10 single nucleotide polymorphisms (SNPs).
Hepatic steatosis was classified according to the Brunt classification: Absent or Grade 0 (<5% of hepatocytes affected), grade 1 (5-33% of hepatocytes affected), grade 2 (33-66% of hepatocytes affected); and grade 3 (>66% of hepatocytes affected) [6]. This study, the database and repository were approved by the Duke University Institutional Review Board. All patients provided written informed consent and the study was conducted in accordance with provisions of the Declaration of Helsinki and Good Clinical Practice guidelines.
IL28B Genotyping
The genomic region associated with HCV response in Ge et al.[1] contains several highly correlated SNPs around the IL28B gene. We selected the most strongly associated SNP, rs12979860, located upstream of this gene for genotyping in our cohort using the 5’ nuclease assay with allele specific TaqMan probes [7]. Based on this TaqMan assay, there are three possible results referred to as genotypes at this locus, and thus each sample was defined as CC, CT or TT (“IL-28B-type”). Genotyping was performed in the Duke Institute for Genome Sciences and Policy Genotyping Core. Genotyping calls were manually inspected and verified prior to release. Hardy-Weinberg Equilibrium was assessed in Caucasians and African Americans separately.
164 patients of the treatment naïve Duke cohort with genotype 1 HCV infection were also genotyped for IL18B rs8099917, where genotype TT was described as beneficial compared to GT or GG [2].
Statistical analysis
SPSS statistical software, Version 12.1 (SPSS Inc. Chicago, Illinois, USA) was used. Chi-square testing or Fisher’s exact test when appropriate was applied for testing the association of IL28B genotype with steatosis. T-test was used for testing difference in lipid levels in relation to steatosis and IL28B. Logistic regression analysis was performed to evaluate IL28B genotypes role for presence of steatosis when controlled for covariates. IL28B genotypes were coded to test a recessive model, comparing patients homozygous (CC) to those with one or no copies of the C allele (CT/TT).
We perfomed an exploratory analysis in the antifibrotic cohort and an independent confirmatory analyzes in the Duke Cohort. We also describe the data after controlling for gender, lipid levels, BMI, HCV viral load, race, and HCV genotype.
Finally, an exploratory assessment of the effect of steatosis and pre-treatment lipids compared to rs12979860 genotype as a predictor of response to PEG-IFN/RBV treatment was done using a multivariable logistic regression model controlling for age, gender, race, and fibrosis.
RESULTS
Association of IL28B genotype with Steatosis in the Antifibrotic Study
One hundred-forty-five patients (of 265 all HCV genotype-1 patients in the total antifibrotic study) were available for genotyping (121 were Caucasians, 12 African American and 12 various ethnic groups). Steatosis was significantly more frequent in IL28B non-CC vs. CC patients (47.6% [50/105] vs. 22.5% [9/40]; p=0.008), with no difference between IL28B CT and IL28B TT patients (48.1% [37/77] vs. 46.4% [13/28]). As there was no difference between IL28B CT and IL28B TT patients, we limited the analysis to IL28B CC versus non-CC patients. Severity of steatosis showed similar trend (p=0.02; see figure 1). Similar results were obtained if analysis was limited to Caucasian patients only (22.2% [8/36] vs. 47.1% [40/85], p=0.014).
Figure 1. Severity of Steatosis in relation to IL28B genotype (rs12979860).
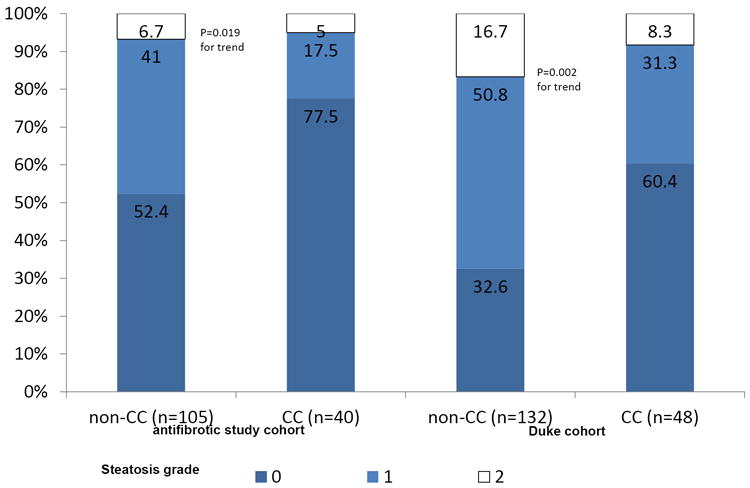
In comparing patients with hepatic steatosis to those without, univariate analysis showed that patients with steatosis were less likely to carry the IL28B CC genotype (15.5% versus 36%; see table 1), had higher BMI (29.5 to 27.5), were younger (50.6 years versus 52.6 years) and had higher triglyceride levels (110.9 mg/dl versus 95.0mg/dl; p=0.012; see table 1).
When all four statistically significant parameters from the univariate analysis (age, BMI, IL28B CC vs. non-CC and triglycerides) were analyzed in a logistic regression model, all except age remained independently associated with steatosis, where BMI and triglycerides were positively associated with steatosis while IL28B CC genotype was associated with less steatosis (see table 2a).
Table 2.
Comparison of patient with and without hepatic steatosis in antifibrotic cohort (A) and Duke Cohort (B)
| A | n/N | Steatosis n (%) | No steatosis n (%) | P value |
|---|---|---|---|---|
| Females | 59/86 | 22 (37.3%) | 33 (38.4%) | n.s. |
| Caucasians | 58/86 | 48 (82.8%) | 73 (84.9%) | n.s. |
| IL28B CC | 59/86 | 9 (15.3%) | 31 (36.0) | 0.008 |
| Age in years | 59/86 | 50.64 ± 6.8 | 52.62 ± 6.7 | 0.031 |
| Body Mass Index (BMI) | 59/86 | 29.5 ± 4.7 | 27.5 ± 4.3 | 0.01 |
| Metavir: Fibrosis | 59/86 | 1.90 ± 0.76 | 1.81± 0.70 | n.s. |
| LDL-C (mg/dL) | 53/73 | 89.9 ± 38.3 | 93.8 ± 25.8 | n.s. |
| Apo C3 (mg/dL) | 53/73 | 11.5 ± 3.4 | 10.5 ± 2.3 | n.s. |
| Apo B (mg/dL) | 53/73 | 73.3 ± 22.4 | 70.5 ± 15.8 | n.s. |
| Apo_A1_Ln | 53/73 | 139.8 ± 30.3 | 138.6 ± 27.1 | n.s. |
| Triglycerides (mg/dL) | 53/69 | 110.9 ± 45.5 | 95.0 ± 49.6 | 0.012 |
| B | n/N | Steatosis n (%) | No steatosis n (%N) | P value |
| Females | 66/107 | 32 (29.9%) | 20 (30.3%) | n.s. |
| Caucasians | 72/108 | 73 (67.6%) | 57 (79.2%) | n.s. |
| IL28B CC | 72/108 | 19 (17.6%) | 29 (40.3) | 0.001 |
| Age in years | 59/86 | 48.9 ± 6.8 | 46.1 ± 8.5 | 0.021 |
| Body Mass Index (BMI) | 55/98 | 30.3 ± 5.4 | 27.4 ± 5.3 | 0.002 |
| Metavir: Fibrosis | 71/108 | 2.50 ± 1.1 | 1.70 ± 1.1 | <0.001 |
| LDL-C (mg/dL) | 46/98 | 96.04 ± 30.1 | 105.7 ± 32.2 | n.s. 0.157 |
| HDL-C (mg/dL) | 51/94 | 45.2 ± 1.29 | 50.3 ± 15.8 | n.s. 0.061 |
| Apo C3 (mg/dL) | 47/98 | 9.0 ± 4.4 | 9.8 ± 4.0 | n.s. 0.066 |
| Apo B (mg/dL) | 47/98 | 76.1 ± 21.9 | 81.3 ± 25.8 | n.s. 0.453 |
| Apo_A1_Ln | 47/98 | 134.3 ± 26.1 | 143.2 ± 29.5 | 0.031 |
| Cholesterol (mg/dL) | 41/94 | 167.3 ± 37.4 | 179.1 ± 33.9 | n.s. 0.056 |
| Triglycerides (mg/dL) | 47/98 | 138.4 ± 70.6 | 135.1 ± 90.0 | n.s. |
n/N reflect with number of patients with steatosis (n) to without steatosis (N), not all data were available for all patients
Association of IL28B genotype with Steatosis in the Duke Database study
Genotype 1
As all patients from the antifibrotic study were HCV genotype 1, we similarly only analyzed the HCV genotype 1 infected patients within the Duke cohort. One-hundred and eighty treatment naïve patients with IL28B genotype from the Duke Database with genotype 1 HCV infection had information on steatosis in their liver biopsy prior to initiation of any antiviral therapy.
Steatosis was more frequent in the patients from the Duke cohort (60.0% [108/180; CI: 52.7 – 66.9%] versus 40.7% [59/145; CI: 33.0 – 48.8%] in the antifibrotic study cohort; p=0.001). Similar to the antifibrotic study cohort, the Duke cohort showed a significant difference between CC and non-CC patients (39.6% [19/48] vs. 67.4% [89/132]; p=0.001) in relation to presence of steatosis. In contrast but consistent with the antifibrotic cohort, no difference between CT patients and TT patients was seen (68.4%.[67/98] vs 64.7% [22/34]; n.s.,). Limiting the analysis to only the 130 Caucasian patients, gave similar results (36.6% [15/41] vs. 62.5% [58/89], p=0.004 or p=0.002 one sided), and no significant difference between CT and TT patients (66.28% [47/71] vs. 61.1% [11/18] n.s.).
In the Duke cohort, cholesterol levels were higher in the CC genotype patients with biopsy results on steatosis available than in the non-CC genotype patients (though not statistically, supplemental table 2b) similar to the combined cohort reported earlier [3], and as in the antifibrotic study cohort alone (see supplemental table 2a).
Duke patients with steatosis differed from those without steatosis in several parameters (see table 2b), of which only IL28B and BMI were concordant with the findings from the antifibrotic study (see table 2a). Age showed an opposite direction compared to the antifibrotic cohort, and triglyceride levels were not significantly different in the Duke cohort, most likely due to the non-fasting state of patients within this cohort. Apo A1 was significantly different between patients with steatosis in the Duke cohort only.
One-hundred and thirty seven patients had data on all 5 parameters. When these five parameters were entered into a binary logistic regression model, only BMI, fibrosis and IL28B were significantly associated with steatosis. IL28B was associated with less steatosis while BMI and fibrosis both were associated with steatosis. When Apolipoprotein B and age were excluded as not significant parameters, only IL28B (p=0.026) and fibrosis (p=0.001) remained significant (see table 3b)
Table 3.
Binary logistic regression analysis concerning presence of steatosis
| A) Multivariate analysis concerning the presence of steatosis in antifibrotic therapy cohort | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| unadjusted | adjusted | Adjusted excluding age | |||||||
| p-value | Odds ratio | 95.0% C.I. | p-value | Odds ratio | 95.0% C.I. | p-value | Odds ratio | 95.0% C.I. | |
| IL28B rs12979860 CC | 0.007 | 0.32 | 0.14 - 0.74 | 0.015 | 0.30 | 0.11 - 0.79 | 0.012 | 0.29 | 0.11 - 0.76 |
| BMI | 0.011 | 1.10 | 1.02 -1.19 | 0.045 | 1.10 | 1.00 -1.20 | 0.016 | 1.11 | 1.02 -1.22 |
| Triglycerides | 0.043 | 2.48 | 1.03 - 5.94 | 0.022 | 3.22 | 1.18 - 8.76 | 0.036 | 2.78 | 1.07 - 7.22 |
| age | 0.092 | 0.96 | 0.91 – 1.01 | 0.17 | 0.97 | 0.90 – 1.02 | |||
| B) Multivariate analysis concerning the presence of steatosis in Duke cohort | |||||||||
| unadjusted | adjusted | Adjusted* | |||||||
| p-value | Odds ratio | 95% C.I | p-value | Odds ratio | 95% C.I | p-value | Odds ratio | 95% C.I | |
| BMI | .004 | 1.12 | 1.04 - 1.22 | .047 | 1.096 | 1.00 - 1.20 | .069 | 1.09 | 0.99 - 1.19 |
| Apo_AI | .098 | 0.99 | 0.97 - 1.00 | .111 | 0.987 | 0.97 - 1.00 | |||
| Fibrosis | <0.001 | 2.23 | 1.47 - 3.37 | .001 | 2.179 | 1.37 - 3.47 | .001 | 2.15 | 1.40 - 3.32 |
| Age | .397 | 1.023 | 0.97 - 1.08 | .790 | 0.992 | 0.93 - 1.05 | |||
| IL28B rs12979860 CC | .0 08 | 0.33 | 0.15 - 0.75 | .041 | 0.383 | 0.15 - 0.96 | .026 | 0.38 | 0.15 - 0.95 |
When limited to the 99 Caucasian patients with data on triglycerides, only IL28B CC genotype was independently associated with steatosis (p=0.021).
adjusted with the significant parameters (BMI, Fibrosis and CC) included only
IL28B rs8099917 in relation to steatosis and its role on steatosis in comparison with 12979860
164 patients for the treatment naïve Duke cohort with genotype 1 were analyzed for rs8099917, where 97 patients had genotype TT, 59 had GT and 8 and GG. Steatosis was present in 45.4%, 32% and 40%, respectively (p=0.2). Likewise in Binary logistic regression, rs12979860 was relevant while rs8099917’s p=value was 0.137 in univariate analysis but 0.851 after inclusion of rs12979860, which was significant in univariate and multivariate analysis.
IL28B, steatosis and treatment response
54 genotype 1 patients underwent therapy with pegylated interferon and ribavirin. Rs12979860 CC genotype was associated with significantly increased odds ratio of response. Presence of steatosis showed a trend but was not significantly associated with failure to achieve SVR, The role of steatosis showed a stronger trend towards significance when IL28B genotype was included. Therefore the association of steatosis with reduced chance of SVR to pegylated interferon and ribavirin is not explained by IL28B genotype’s association with lower frequency of steatosis (see table 4).
Table 4.
Treatment response in relation to steatosis and IL28B CC
| unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
| p-value | Odds ratio | 95% C.I | p-value | Odds ratio | 95% C.I | |
| Steatosis | 0.126 | 0.40 | 0.07 - 1.40 | 0.056 | 0.10 | 0.01 - 1.06 |
| IL28B rs12979860 CC | <0.001 | 29.56 | 4.93 – 177.36 | 0.001 | 60.99 | 5.70 – 652.10 |
When limiting the analysis to Caucasian patients only, rs8099917 was significantly associated with steatosis as 39 of 60 (65%) non-TT patients had steatosis compared to 30 of 64 (46.9%) TT patients (p=0.048). However, when rs8099917 TT vs. non-TT and rs12979860 CC vs. non-CC were evaluated in a multivirate model, rs8099917 became irrelevant. However, all 46 rs12979860 CC patients tested for rs8099917 were also rs8099917 TT, while the remaining non-CC patients were either rs8099917 TT or non-TT.
DISCUSSION
When comparing IL28B rs12979860 and rs8099917 within the initial GWAS by Ge et al [1] rs8099917 was among the top three SNPs and even the top SNP within the Caucasians in relation to treatment response. In this study we found no significant association of rs8099917 with steatosis while we found a significant association with rs12979860. This would fit with two paper by the group of Sarrazin et al. who found a stronger role for rs2979860 vs. rs8099917 in a genotype 2/3 cohort [8], and in a genotype 1 cohort [9]. However, In Japan, no difference between rs8099917 and rs12979860 was reported [10]. Interestingly, in the current study of dominantly Caucasian patients, no significant association of rs8099917 with steatosis was observed, but an association with rs12979860.
We earlier demonstrated significant higher LDL-cholesterol and apolipoprotein B levels but lower triglyceride levels in CC patients with HCV compared to non-CC patients [3]. Hepatic steatosis is another frequent disturbance of lipid metabolism in patients with hepatitis C [11,12]. In the present study, we demonstrate that the presence of steatosis was lower in CC compared to non-CC patients in two independent HCV genotype 1 patient cohorts and a trend in a genotype 2/3 cohort. Though the relative small cohorts are a limitation, but in both cohorts the difference is significant, making a finding by chance unlikely. Furthermore, in 89 patients, who had received some form of therapy or where information regarding therapy was not available within the database showed similar results, with p-value of 0.03 for lower steatosis in CC vs. non-CC patients (data not shown). Importantly, this finding was independent from the lipid changes and provides further evidence that IL28B CC genotype is associated with less pronounced disturbances of lipid metabolism, as reflected both in serum lipoprotein levels and hepatic steatosis, in hepatitis C infection. Furthermore, these changes in lipid metabolism would be consistent with lower intrinsic interferon activity in IL28B CC patients, and explain the better response to treatment, as the difference of interferon activity from before to on treatment would be greater in the IL28B CC patients.
The higher serum lipid levels but lower steatosis in “IL28B” CC patients could be explained by more efficient export of lipids from hepatocytes in the setting of the beneficial CC genotype. But it furthermore, would fit with the hypothesis of higher intrahepatic interferon levels in presence on non-CC genotype. As discussed earlier [3], higher interferon expression is likely to lead to suppression of lipoprotein lipase, which would result in lower conversion of VLDL to LDL, and subsequent higher steatosis. Exogenous interferons have been shown to lower LDL cholesterol, and raise triglyceride in VLDL, concomitant with suppression of lipoprotein lipase in patients with known HCV infection [13,14,15], but also in individuals without known history of HCV [16].
Cholesterol synthesis and HCV-viral production, both, have been associated with microRNA-122 (miR-122) levels in the liver [17,18]. However, one would expect increased not decreased steatosis with elevated lipid synthesis especially in the context of miR-122 [19]. However, it is known that HCV-infection itself leads to decreases in serum cholesterol, still HCV infection is associated with steatosis. Thus, we hypothesize that HCV-infection might actually engage miR-122 for its life cycle and thereby decrease miR-122 availability for lipid production. We have, however, no samples available to prove this hypothesis that miR-122 is differently regulated, and thus cannot address this subject in our population. Though conflicting data have been presented, some studies have found steatosis to be associated with failure to achieve sustained viral response [20,21,22]. Our study was too small to reliably evaluate the independent role of steatosis. However, it appears that earlier reports on failure to achieve sustained virological response in the presence of steatosis might be partially attributable to non-CC genotype of IL28B.
In conclusion, IL28B CC is associated with absence of steatosis, which was independent from serum lipids.
Supplementary Material
Acknowledgments
We would like to thank all of the study participants who contributed their biospecimens and data to the Duke Hepatology Clinic Database and Biorepository and acknowledge Diane Uzarski, Crystal Cates, Chris Delionbach and Melissa Austin for continued maintenance of this valuable resource. We would also like to thank collaborators Dr. Arlene Hughes and Dr. Michael Mosteller at GlaxoSmithKline for facilitating the sharing of the farglitazar clinical trial data and providing valuable feedback on the manuscript. This study was funded in large part by a generous grant from the David H Murdock Institute for Business and Culture via the M.U.R.D.O.C.K. Study and NIH CTSA award 1 UL1 RR024128-01 to Duke University (Dr. Robert Califf, Principal Investigator). Drs. Thompson and Clark received funding support from the Duke Clinical Research Institute, a generous research gift from the Richard B. Boebel Family Fund, the National Health and Medical Research Council of Australia and the Gastroenterology Society of Australia.
List of Abbreviations
- LDL
low density lipoprotein
- HCV
hepatitis C virus
- VLDL
very low density lipoprotein
- HIV
human immunodeficiency virus
- PEG-IFN
pegylated interferon
- RBV
ribavirin
- SVR
sustained virologic response
- SNP
single nucleotide polymorphism
- Apo
apolipoprotein
Footnotes
There are no other conflicts to disclose.
References
- 1.Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 2.Balagopal A, Thomas DL. Thio CL.IL28B and the Control of Hepatitis C Virus Infection. Gastroenterology. 2010 Oct 13; doi: 10.1053/j.gastro.2010.10.004. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li JH, Lao XQ, Tillmann HL, Rowell J, Patel K, Thompson A, et al. Interferon lambda genotype and low serum LDL cholesterol levels in patients with chronic hepatitis C infection. Hepatology. 2010;51:1904–1911. doi: 10.1002/hep.23592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McHutchison J, Goodman Z, Patel K, Makhlouf H, Rodriguez-Torres M, Shiffman M, et al. Farglitazar Lacks Antifibrotic Activity in Patients With Chronic Hepatitis C Infection. Gastroenterology. 2010;138:1365–1373. 1373.e1–2. doi: 10.1053/j.gastro.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 5.White IR, Patel K, Symonds WT, Dev A, Griffin P, Tsokanas N, et al. Serum proteomic analysis focused on fibrosis in patients with hepatitis C virus infection. J Transl Med. 2007;5:33. doi: 10.1186/1479-5876-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunt E, Janney C, Di Bisceglie A, Neuschwander-Tetri B, Bacon B. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 7.Tillmann HL, Thompson AJ, Patel K, Wiese M, Tenckhoff H, Nischalke HD, et al. A polymorphism near IL28B is associated with spontaneous clearance of acute hepatitis C virus and jaundice. Gastroenterology. 2010;139:1586–1592. 1592.e1. doi: 10.1053/j.gastro.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Sarrazin C, Susser S, Doehring A, Lange CM, Müller T, Schlecker C, et al. Importance of IL28B gene polymorphisms in hepatitis C virus genotype 2 and 3 infected patients. J Hepatol. 2010 Sep 22; doi: 10.1016/j.jhep.2010.07.041. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 9.Doehring A, Hofmann WP, Schlecker C, Zeuzem S, Susser S, Geisslinger G, et al. Screening for IL28B gene variants identifies predictors of hepatitis C therapy success. Antivir Ther. 2010;15(8):1099–106. doi: 10.3851/IMP1689. [DOI] [PubMed] [Google Scholar]
- 10.Ochi H, Maekawa T, Abe H, Hayashida Y, Nakano R, Imamura M, et al. IL-28B predicts response to chronic hepatitis C therapy -fine-mapping and replication study in Asian populations. J Gen Virol. 2011 Jan 12; doi: 10.1099/vir.0.029124-0. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 11.Lefkowitch JH, Schiff ER, Davis GL, Perrillo RP, Lindsay K, Bodenheimer HC, Jr, et al. Pathological diagnosis of chronic hepatitis C: a multicenter comparative study with chronic hepatitis B. The Hepatitis Interventional Therapy Group. Gastroenterology. 1993;104:595–603. doi: 10.1016/0016-5085(93)90432-c. [DOI] [PubMed] [Google Scholar]
- 12.Bach N, Thung SN, Schaffner F. The histological features of chronic hepatitis C and autoimmune chronic hepatitis: a comparative analysis. Hepatology. 1992;15:572–577. doi: 10.1002/hep.1840150403. [DOI] [PubMed] [Google Scholar]
- 13.Ehnholm C, Aho K, Huttunen JK, Kostiainen E, Mattila K, Pakkarainen J, et al. Effect of interferon on plasma lipoproteins and on the activity of postheparin plasma lipases. Arteriosclerosis. 1982;2:68–73. doi: 10.1161/01.atv.2.1.68. [DOI] [PubMed] [Google Scholar]
- 14.Shinohara E, Yamashita S, Kihara S, Hirano K, Ishigami M, Arai T, Nozaki S, et al. Interferon alpha induces disorder of lipid metabolism by lowering postheparin lipases and cholesteryl ester transfer protein activities in patients with chronic hepatitis C. Hepatology. 1997;25:1502–1506. doi: 10.1002/hep.510250632. [DOI] [PubMed] [Google Scholar]
- 15.Andrade RJ, Garcia-Escano MD, Valdivielso P, Alcantara R, Sanchez-Chaparro MA, Gonzalez-Santos P. Effects of interferon-beta on plasma lipid and lipoprotein composition and post-heparin lipase activities in patients with chronic hepatitis C. Aliment Pharmacol Ther. 2000;14:929–935. doi: 10.1046/j.1365-2036.2000.00792.x. [DOI] [PubMed] [Google Scholar]
- 16.Schectman G, Kaul S, Mueller RA, Borden EC, Kissebah AH. The effect of interferon on the metabolism of LDLs. Arterioscler Thromb. 1992 Sep;12(9):1053–62. doi: 10.1161/01.atv.12.9.1053. [DOI] [PubMed] [Google Scholar]
- 17.Sarnow P, Normal KL. Modulation of hepatitis C virus RNA abundance and the isoprenoid biosynthesis pathway by microRNA miR-122 involves distinct mechanisms. J Virol. 2010;84:666–670. doi: 10.1128/JVI.01156-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lynn FC. Meta-regulation: microRNA regulation of glucose and lipid metabolism. Trends Endocrinol Metab. 2009;20:452–459. doi: 10.1016/j.tem.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006;3:87–98. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Kurosaki M, Matsunaga K, Hirayama I, Tanaka T, Sato M, Yasui Y, et al. A predictive model of response to peginterferon ribavirin in chronic hepatitis C using classification and regression tree analysis. Hepatol Res. 2010;40:251–260. doi: 10.1111/j.1872-034X.2009.00607.x. [DOI] [PubMed] [Google Scholar]
- 21.Petta S, Cammà C, Di Marco V, Cabibi D, Ciminnisi S, Caldarella R, et al. Time course of insulin resistance during antiviral therapy in non-diabetic, non-cirrhotic patients with genotype 1 HCV infection. Antivir Ther. 2009;14:631–639. [PubMed] [Google Scholar]
- 22.Nelson DR, Benhamou Y, Chuang WL, Lawitz EJ, Rodriguez-Torres M, Flisiak R, et al. Randomized Trial of albinterferon alfa-2b for the Treatment of Patients With Chronic Hepatitis C Virus Genotype 2 or 3. Gastroenterology. 2010;139:1267–1276. doi: 10.1053/j.gastro.2010.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


