Abstract
Preemptive kidney transplantation is the optimal treatment for pediatric End Stage Renal Disease (ESRD) patients to avoid increased morbidity and mortality associated with dialysis. It is unknown how race/ethnicity and poverty influence preemptive transplant access in pediatric ESRD. We examined the incidence of living donor (LD) or deceased donor (DD) preemptive transplantation among all black, white, and Hispanic children (< 18 years) in the United States Renal Data System from 2000–2009. Adjusted risk ratios for preemptive transplant were calculated using multivariable-adjusted models and examined across health insurance and neighborhood poverty levels. Among 8,053 patients, 1117 (13.9%) received a preemptive transplant (66.9% from LD, 33.1% from DD). In multivariable analyses, there were significant racial/ethnic disparities in access to LD preemptive transplant where blacks were 66% (RR=0.34; 95% CI: 0.28–0.43) and Hispanics 52% (RR=0.48; 95% CI: 0.35–0.67) less likely to receive a LD preemptive transplant vs. whites. Blacks were 22% less likely to receive a DD preemptive transplant vs. whites (RR=0.78, 95% CI: 0.57–1.05) although results were not statistically significant. Future efforts to promote equity in preemptive transplant should address the critical issues of improving access to pre-ESRD nephrology care and overcoming barriers in living donation, including obstacles partially driven by poverty.
Keywords: children, United States Renal Data System, racial disparity, epidemiology, health policy, pediatric kidney transplantation, preemptive
Introduction
Among both deceased donor (DD) and living donor (LD) transplant recipients, the number of years on dialysis negatively correlates with patient and graft survival1,2. Renal transplant recipients have better survival and a higher quality of life than dialysis patients3. Preemptive renal transplantation, defined as transplantation prior to the initiation of dialysis, is the optimal treatment for patients with End Stage Renal Disease (ESRD)4,5 because it avoids the increased morbidity and mortality associated with dialysis, creation of surgical dialysis access and its complications, and the costs of dialysis6,7. Despite health care coverage for treatment of ESRD through Medicare, preemptive LD or DD kidney transplantation remains highly underutilized in the United States (U.S.); only 3.2% of incident ESRD patients < 70 years of age received a preemptive transplant in 20094,8.
In the pediatric ESRD population, where long-term outcomes take on added significance, the potential benefits of preemptive transplantation are multiplied8. Pediatric patients on chronic dialysis have markedly increased rates of cardiovascular disease, bone-mineral dysregulation and impaired cognitive function vs. those who are transplanted9–14. The influence of these comorbidities is demonstrated by the 95% 5-year patient survival rate of children who receive a preemptive transplant, compared to 75% for those who receive hemodialysis as their initial treatment15. Preemptive transplantation requires early recognition of kidney disease progression and thus necessitates early access to pre-ESRD nephrology care. In a study of 111 children in Austria, those with at least 12 months of pre-ESRD nephrology care were more than twice as likely to receive preemptive kidney transplant vs. those with less than one year of care16. Access to pre-ESRD nephrology care may be more challenging for minority children who more often lack insurance or a medical home17–20.
Racial disparities in preemptive transplantation have been reported in the adult ESRD population, where the odds of preemptive kidney transplantation among whites are more than twice as high than blacks and about 1.6 times higher than Hispanics21. Several small, single-center studies have reported racial/ethnic differences in children who present for preemptive kidney transplantation versus those who receive transplantation following dialysis22–24, but these disparities have not been examined nationally. We previously documented racial disparities in non-preemptive transplant access among pediatric ESRD patients, where black and Hispanic children in the U.S. have reduced access to DD kidney transplantation even after accounting for differences in SES25. Further, racial disparities in non-preemptive LD transplant exist, and following the implementation of the Share 35 allocation policy to preferentially allocate young (< 35 years) donor organs to young (< 18 years) recipients, the decline in non-preemptive LD transplants was more substantial among minority compared to white pediatric ESRD patients26. Whether racial disparities in access to pediatric preemptive transplant vary by donor source, DD vs. LD, is unknown. The goal of our study was to characterize racial/ethnic disparities in access to preemptive transplant among the national cohort of children with ESRD over the last decade (2000–2009), considering both donor source (LD vs. DD) and SES.
Concise Methods
Study Population and Data Sources
Incident, pediatric (age < 18 years) ESRD patients who entered the Medicare ESRD program between 2000–2009 from the United States Renal Data System (USRDS) were examined. Basic demographic data were obtained from the Centers for Medicare & Medicaid Services (CMS) Medical Evidence Form (CMS-2728), completed on all incident ESRD patients. Outcome data on transplantation were obtained from USRDS and are virtually 100% complete15. Data on neighborhood poverty were obtained from Census 2000 by patient ZIP Code.
There were 10,145 ESRD patients < 18 years of age who entered the Medicare ESRD program from January 1, 2000 through December 31, 2009. Due to small sample size, patients with race/ethnicity other than white non-Hispanic (white), white-Hispanic (Hispanic), or black non-Hispanic (black) were excluded (n=1247). Patients were excluded if missing a ZIP Code (n=17) or if ZIP Code could not be linked with census data (n=311). We excluded patients who had previously received a transplant (n=517). Thus, 8,053 patients were included in our analyses.
Study Variables
The primary outcome of interest was receipt of a LD or DD preemptive transplant (yes/no), defined as a transplant with no history of dialysis. Self-reported race/ethnicity was the primary effect of interest. Etiologic research attempting to isolate race/ethnicity as a cause of a health outcome has an ambiguous interpretation27, thus we attempted to estimate the effects of race/ethnicity as a contextual and social, rather than a biologic determinant28. Demographic and clinical covariates included patient age, sex, organ procurement organization (OPO) region, etiology of ESRD, and BMI. We examined pre-dialysis erythropoiesis-stimulating agent (ESA) use, hemoglobin ≥11 g/dL and serum albumin ≥ 3.5 g/dL at ESRD start as proxies for early access to nephrology care. We examined year of entry into the ESRD Medicare program to examine time trends over the decade. Prior research has established a role of contextual or neighborhood-level SES indicators above and beyond individual-level SES29–32. We estimated SES using health insurance as a proxy for individual SES and ZIP Code poverty at incident ESRD as a proxy for neighborhood SES using 2000 U.S. Census Bureau summary file 3 data on the proportion of individuals residing below the federal poverty level. Health insurance at incident ESRD was categorized as private (employer), public (Medicaid, Medicare, VA, or combination), other, or no health insurance. Rural Urban Commuting Area (RUCA) codes were obtained from the Community Health Status Indicators Project, linked with patients by ZIP Code, and considered a proxy for neighborhood-level access to care.
Statistical Analysis
Chi-square tests and t-tests (or non-parametric equivalents of the t-test) and exact statistics were used to examine differences between demographic and clinical characteristics of patients by race/ethnicity. To examine whether racial/ethnic differences exist in preemptive kidney transplantation, we calculated incident preemptive transplant count divided by total incident ESRD patients per calendar year separately by race/ethnicity. Incidence of preemptive transplantation was calculated separately by donor type (LD and DD). We derived 95% Confidence Intervals (CIs) for risks using binomial exact intervals.
To examine the multivariable-adjusted association of race/ethnicity and preemptive transplant, Risk Ratios (RR) and 95% CIs were calculated using multivariable log-binomial regression models accounting for potential correlation of observations within OPO Region through the use of generalized estimating equations that assumed an exchangeable (compound symmetric) covariance structure. To examine whether disparities in LD or DD preemptive transplantation varied by SES, we conducted stratified analyses examining the incidence of preemptive transplant by SES variables. Lastly, because pediatric priority allocation changed in 2005 with the implementation of Share 35 and there is evidence to support a reduction in racial disparities in access to transplantation for children after Share 35, we conducted an additional analysis stratifying by era: pre- vs. post-Share 35.
Secondary Analyses
Because one of the primary driving forces of preemptive transplant is to avoid dialysis exposure and dialysis associated morbidity, we compared the proportion of subjects within each racial/ethnic category by dialysis exposure time. We also wanted to examine whether access to preemptive LD transplant was more marked than previously reported disparities in LD transplant overall (preemptive and non-preemptive)26. We therefore examined the racial/ethnic composition of all patients who were transplanted, by donor source and preemptive vs. non-preemptive status.
Additionally, in 2005, the USRDS CMS-2728 form began to capture data on whether a patient was under the care of a nephrologist prior to ESRD. While our study period spanned 2000–2009 and thus > 50% of the study population did not have this information, we examined this among the 2005–2009 cohort.
Lastly, to ensure patients examined in analyses were eligible for transplant, we examined racial differences in the incidence of preemptive transplant among 1) waitlisted, and 2) transplanted patients only (i.e. excluded those not waitlisted).
Two-tailed p-values <0.05 were considered statistically significant in analyses. Missing covariate data, including those with missing albumin (12.9%), hemoglobin (6.3%), or other covariates (<3%) were handled by creating a missing category in multivariable analyses and conducting sensitivity analyses excluding missing data. All analyses were performed with SAS software (v9.2). The Emory University Institutional Review Board approved this study.
Results
Demographics of Pediatric ESRD Study Population by Race/Ethnicity
There were significant clinical and demographic differences by race/ethnicity at incident ESRD among the national, pediatric ESRD population. Blacks were older, and more likely to be overweight and have renal disease due to lupus nephritis or focal segmental glomerulonephritis compared with whites and Hispanics. Whites were more likely to have normal serum albumin levels and receive pre-dialysis ESA with less anemia compared with Hispanics and blacks. Minority patients were more likely to have public vs. private health insurance, live in urban vs. rural residential areas, and reside in high poverty neighborhoods compared to white patients (all p<0.0001) (Table 1).
Table 1.
Baseline Characteristics of Study Population at ESRD Start by Race/Ethnicity
| Total Study Population N=8,053 | White N = 3,890 (48.3%) | Hispanic N=2,220 (27.6%) | Black N=1,943 (24.1%) | P-value≠ | |
|---|---|---|---|---|---|
| Patient-Level Characteristics | |||||
| Age, Mean (SD), years | 10.4 ± 5.8 | 9.6 ± 6.0 | 10.8 ± 5.5 | 11.5 ± 5.5 | < 0.0001 |
| Age Category, N (%), yrs | < 0.0001 | ||||
| < 1 yrs | 901 (11.2%) | 552 (14.2%) | 189 (8.5%) | 160 (8.2%) | |
| 1–5 yrs | 1063 (13.2%) | 604 (15.5%) | 263 (11.9%) | 196 (10.1%) | |
| 6–10 yrs | 1295 (16.1%) | 651 (16.7%) | 384 (17.3%) | 260 (13.4%) | |
| 11–17 yrs | 4794 (59.5%) | 2083 (53.6%) | 1384 (62.3%) | 1327 (68.3%) | |
| Female, N (%) | 3497 (43.4%) | 1644 (42.3%) | 1017 (45.8%) | 836 (43.0%) | 0.0246 |
| Cause of ESRD, N (%) | < 0.0001 | ||||
| GN1 | 768 (9.5%) | 329 (8.5%) | 269 (12.1%) | 170 (8.8%) | |
| Secondary GN | 485 (6.0%) | 308 (7.9%) | 107 (4.8%) | 70 (3.6%) | |
| Cystic/hereditary | 3051 (37.9%) | 1753 (45.1%) | 756 (34.1%) | 542 (27.9%) | |
| FSGS2 | 1041 (12.9%) | 373 (9.6%) | 249 (11.2%) | 419 (21.6%) | |
| Lupus nephritis | 335 (4.2%) | 63 (1.6%) | 106 (4.8%) | 166 (8.5%) | |
| Other | 2373 (29.5%) | 1064 (27.4%) | 733 (33.0%) | 576 (29.6%) | |
| Organ Procurement Organization Region | < 0.0001 | ||||
| 1 | 247 (3.1%) | 166 (4.3%) | 41 (1.9%) | 40 (2.1%) | |
| 2 | 720 (8.9%) | 367 (9.4%) | 88 (4.0%) | 265 (13.6%) | |
| 3 | 1287 (16.0%) | 524 (13.5%) | 239 (10.8%) | 524 (27.0%) | |
| 4 | 885 (11.0%) | 289 (7.4%) | 418 (18.8%) | 178 (9.2%) | |
| 5 | 1547 (19.2%) | 463 (11.9%) | 966 (43.5%) | 118 (6.1%) | |
| 6 | 283 (3.5%) | 201 (5.2%) | 59 (2.7%) | 23 (1.2%) | |
| 7 | 655 (8.2%) | 391 (10.1%) | 138 (6.2%) | 126 (6.5%) | |
| 8 | 484 (6.0%) | 356 (9.2%) | 66 (3.0%) | 62 (3.2%) | |
| 9 | 471 (5.9%) | 209 (5.4%) | 112 (5.1%) | 150 (7.7%) | |
| 10 | 725 (9.0%) | 505 (13.0%) | 46 (2.1%) | 174 (9.0%) | |
| 11 | 749 (9.3%) | 419 (10.8%) | 47 (2.1%) | 283 (14.6%) | |
| Health Insurance Coverage | < 0.0001 | ||||
| Public | 3775 (46.9%) | 1394 (35.8%) | 1207 (54.4%) | 1174 (60.4%) | |
| Private | 2724 (33.8%) | 1849 (47.5%) | 404 (18.2%) | 471 (24.2%) | |
| Other | 1093 (13.6%) | 535 (13.8%) | 342 (15.4%) | 216 (11.1%) | |
| None | 461 (5.7%) | 112 (2.9%) | 267 (12.0%) | 82 (4.2%) | |
| Share 35 Policy Era 3 | 3481 (43.2%) | 1682 (43.2%) | 1083 (48.8%) | 716 (36.9%) | < 0.001 |
| Clinical and Laboratory Measures | |||||
| BMI > 85%4 | 808 (10.0%) | 313 (8.1%) | 202 (9.1%) | 293 (15.1%) | < 0.0001 |
| Albumin ≥ 3.5 g/dL | 3335 (41.4%) | 1739 (52.1%) | 948 (28.4%) | 648 (19.4%) | < 0.0001 |
| Hemoglobin ≥ 11 g/dL | 2292 (28.5%) | 1308 (33.6%) | 567 (25.5%) | 417 (21.5%) | < 0.0001 |
| Pre-dialysis ESA5 | 3420 (42.5%) | 1815 (46.7%) | 871 (39.2%) | 734 (37.8%) | < 0.0001 |
| Zip code-level characteristics for Patient Residence at ESRD Start | |||||
| Neighborhood Poverty (% zip below poverty) | < 0.0001 | ||||
| 0–4.9% | 1188 (14.8%) | 926 (23.8%) | 120 (5.4%) | 142 (7.3%) | |
| 5–9.9% | 2068 (25.7%) | 1311 (33.7%) | 390 (17.6%) | 367 (18.9%) | |
| 10–14.9% | 1588 (19.7%) | 812 (20.9%) | 419 (18.9%) | 357 (18.4%) | |
| 15–19.9% | 1175 (14.6%) | 437 (11.2%) | 432 (19.5%) | 306 (15.8%) | |
| > 20% | 2034 (25.3%) | 404 (10.4%) | 859 (38.7%) | 771 (39.7%) | |
| Residential Urban Commuting Area | < 0.0001 | ||||
| Urban | 6402 (79.5%) | 2797 (71.9%) | 1930 (86.9%) | 1675 (86.2%) | |
| Rural | 1651 (20.5%) | 1093 (28.1%) | 290 (13.1%) | 268 (13.8%) | |
Glomerulonephritis
Focal Segmental Glomerulosclerosis
Post-Sept. 2005 vs. Pre-Sept. 2005
Body Mass Index
Erythropoiesis-Stimulating Agent
p-values < 0.05 for each variable indicate that at least one variable level is significantly different across racial/ethnic groups.
Characteristics of Preemptive Kidney Transplant Recipients
Among 8,053 patients who registered in the ESRD Medicare program from 2000–2009, 1,117 patients (13.9%) had a start date of ESRD equivalent to their transplant date and no history of dialysis, including 66.9% (n=747) LD preemptive transplants (Table 2A) and 33.1% (n=370) DD preemptive transplants (Table 2B). Although only 48.3% of the incident pediatric ESRD population was white, they represented 71.8% of all preemptive transplant recipients. Hispanics and blacks represented only 17.3% and 10.9%, respectively, of the preemptive transplant population. Racial/ethnic differences were also evident in the type of preemptive transplant received, where a greater proportion of white preemptive transplant recipients had a LD preemptive transplant (74.7%) vs. Hispanics (47.7%) and blacks (45.9%) compared to a DD preemptive transplant (p<0.0001). Significant socioeconomic differences existed among those patients who were preemptively transplanted. While only 33.8% of the total study population had private health insurance, 59.3% of white LD preemptive transplant recipients had private insurance vs. 33.7% of Hispanics and 42.9% of blacks (p<0.0001) (Table 2A). Half (50.3%) of white DD preemptive transplant recipients had private insurance vs. only 11.9% of Hispanics and 16.7% of blacks (p<0.0001) (Table 2B).
Table 2A.
Demographic and Clinical Characteristics of Patients who Received a Living Donor Preemptive Kidney Transplant by Race/Ethnicity
| All Patients with LD Preemptive Transplant N=747 | Preemptively Transplanted with Living Donor, N (%) | P-value≠ | |||
|---|---|---|---|---|---|
| White N=599 (80.2%) | Hispanic N=92 (12.3%) | Black N=56 (7.5%) | |||
| Age, Mean (SD), yrs | 10.5 ± 5.0 | 10.6 ± 5.0 | 10.1 ± 4.7 | 9.1 ± 5.3 | 0.0361 |
| Age Category, N (%), yrs | 0.0402 | ||||
| 0–5 yrs | 161 (21.6%) | 124 (20.7%) | 19 (20.7%) | 18 (32.1%) | |
| 6–10 yrs | 166 (22.2%) | 130 (21.7%) | 22 (23.9%) | 14 (25.0%) | |
| 11–17 yrs | 420 (56.2%) | 345 (57.6%) | 51 (55.4%) | 24 (42.9%) | |
| Female, N (%) | 266 (35.6%) | 214 (35.7%) | 39 (42.4%) | 13 (23.2%) | 0.0608 |
| Cause of ESRD, N(%) | 0.3430 | ||||
| GN1 | 27 (3.6%) | 22 (3.7%) | 5 (5.4%) | 0 (0%) | |
| Secondary GN | 22 (3.0%) | 21 (3.5%) | 1 (1.1%) | 0 (0%) | |
| Cystic/hereditary | 463 (62.0%) | 382 (63.8%) | 41 (44.6%) | 40 (71.4%) | |
| FSGS2 | 30 (4.0%) | 15 (2.5%) | 10 (10.9%) | 5 (8.9%) | |
| Lupus nephritis | 2 (0.3%) | 0 (0%) | 1 (1.1%) | 1 (1.8%) | |
| Other | 203 (27.2%) | 159 (26.5%) | 34 (37.0%) | 10 (17.9%) | |
| Organ Procurement Organization Region | 0.0126 | ||||
| 1 | 32 (4.3%) | 29 (4.8%) | 1 (1.1%) | 2 (3.6%) | |
| 2 | 81 (10.8%) | 59 (9.9%) | 10 (10.9%) | 12 (21.4%) | |
| 3 | 111 (14.9%) | 90 (15.0%) | 6 (6.5%) | 15 (26.8%) | |
| 4 | 24 (3.2%) | 14 (2.3%) | 7 (7.6%) | 3 (5.4%) | |
| 5 | 105 (14.1%) | 67 (11.2%) | 36 (39.1%) | 2 (3.6%) | |
| 6 | 34 (4.6%) | 30 (5.0%) | 4 (4.4%) | 0 (0%) | |
| 7 | 104 (13.9%) | 92 (15.4%) | 11 (12.0%) | 1 (1.8%) | |
| 8 | 54 (7.2%) | 48 (8.0%) | 4 (4.4%) | 2 (3.6%) | |
| 9 | 39 (5.2%) | 23 (3.8%) | 11 (12.0%) | 5 (8.9%) | |
| 10 | 78 (12.4%) | 74 (12.4%) | 1 (1.1%) | 3 (5.4%) | |
| 11 | 85 (11.4%) | 73 (12.2%) | 1 (1.1%) | 11 (19.6%) | |
| Health Insurance Coverage | < 0.0001 | ||||
| Public | 205 (27.4%) | 134 (22.4%) | 47 (51.1%) | 24 (42.9%) | |
| Private | 410 (54.9%) | 355 (59.3%) | 31 (33.7%) | 24 (42.9%) | |
| Other | 119 (15.9%) | 101 (16.9%) | 10 (10.9%) | 8 (14.3%) | |
| None | 13 (1.7%) | 9 (1.5%) | 4 (4.4%) | 0 (0%) | |
| Clinical and Demographic Characteristics | |||||
| BMI < 85% 4 | 59 (7.9%) | 49 (8.2%) | 5 (5.4%) | 5 (8.9%) | 0.6329 |
| Albumin ≥ 3.5 g/dL | 582 (77.9%) | 474 (79.1%) | 72 (78.3%) | 36 (64.3%) | 0.0375 |
| Hemoglobin ≥ 11 g/dL | 424 (56.8%) | 347 (57.9%) | 49 (53.3%) | 28 (50.0%) | 0.3994 |
| Pre-dialysis ESA5 | 432 (57.8%) | 355 (59.3%) | 44 (47.8%) | 33 (58.9) | 0.1159 |
| Neighborhood Characteristics | |||||
| Neighborhood Poverty (% zip below poverty) | <0.0001 | ||||
| 0–4.9% | 207 (27.7%) | 189 (31.6%) | 10 (10.9%) | 8 (14.3%) | |
| 5–9.9% | 237 (31.7%) | 206 (34.4%) | 21 (22.8%) | 10 (17.9%) | |
| 10–14.9% | 133 (17.8%) | 107 (17.9%) | 14 (15.2%) | 12 (21.4%) | |
| 15–19.9% | 82 (11.0%) | 49 (8.2%) | 22 (23.9%) | 11 (19.6%) | |
| > 20% | 88 (11.8%) | 48 (8.0%) | 25 (27.2%) | 15 (26.8%) | |
| Residential Urban Commuting Area | 0.0026 | ||||
| Urban | 573 (76.7%) | 444 (74.1%) | 81 (88.0%) | 48 (85.7%) | |
| Rural | 174 (23.3%) | 155 (25.9%) | 11 (12.0%) | 8 (14.3%) | |
Glomerulonephritis
Focal Segmental Glomerulosclerosis
Post-Sept. 2005 vs. Pre-Sept. 2005
Body Mass Index
Erythropoiesis-Stimulating Agent
p-values < 0.05 for each variable indicate that at least one variable level is significantly different across racial/ethnic groups.
Table 2B.
Demographic and Clinical Characteristics of Patients who Received a Deceased Donor Preemptive Kidney Transplant by Race/Ethnicity
| All Patients with DD Preemptive Transplant N=370 | Preemptively Transplanted with Deceased Donor, N (%) | P-value≠ | |||
|---|---|---|---|---|---|
| White 203 (54.9%) | Hispanic 101 (27.3%) | Black 66 (17.8%) | |||
| Age, Mean (SD), yrs | 10.7 ± 5.0 | 11.1 ± 5.0 | 10.0 ± 5.1 | 10.5 ± 4.9 | 0.3957 |
| Age Category, N (%), yrs | 0.3906 | ||||
| 0–5 yrs | 75 (20.3%) | 38 (18.7%) | 25 (24.8%) | 12 (18.2%) | |
| 6–10 yrs | 84 (22.7%) | 40 (19.7%) | 25 (24.8%) | 19 (28.8%) | |
| 11–17 yrs | 211 (57.0%) | 125 (61.6%) | 51 (50.5%) | 35 (53.0%) | |
| Female, N (%) | 117 (31.6%) | 69 (34.0%) | 31 (30.7%) | 17 (25.8%) | 0.4456 |
| Cause of ESRD, N (%) | 0.0820 | ||||
| GN1 | 11 (3.0%) | 2 (1.0%) | 5 (5.0%) | 4 (6.1%) | |
| Secondary GN | 1 (0.3%) | 0 (0%) | 0 (0%) | 1 (1.5%) | |
| Cystic/hereditary | 222 (60.0%) | 141 (69.5%) | 51 (50.5%) | 30 (45.4%) | |
| FSGS2 | 23 (6.2%) | 10 (4.9%) | 5 (5.0%) | 8 (12.1%) | |
| Lupus nephritis | 1 (0.3%) | 0 (0%) | 1 (1.0%) | 0 (0%) | |
| Other | 112 (30.3%) | 50 (24.6%) | 39 (38.6%) | 23 (34.9%) | |
| Organ Procurement Organization Region | 0.6768 | ||||
| 1 | 11 (3.0%) | 6 (3.0%) | 2 (2.0%) | 3 (4.6%) | |
| 2 | 56 (15.1%) | 38 (18.7%) | 7 (6.9%) | 11 (16.7%) | |
| 3 | 42 (11.4%) | 18 (8.9%) | 13 (12.9%) | 11 (16.7%) | |
| 4 | 30 (8.1%) | 11 (5.4%) | 16 (15.8%) | 3 (4.6%) | |
| 5 | 74 (20.0%) | 25 (12.3%) | 45 (44.6%) | 4 (6.1%) | |
| 6 | 9 (2.4%) | 9 (4.4%) | 0 (0%) | 0 (0%) | |
| 7 | 33 (8.9%) | 22 (10.8%) | 4 (4.0%) | 7 (10.6%) | |
| 8 | 19 (5.1%) | 17 (8.4%) | 1 (1.0%) | 1 (1.5%) | |
| 9 | 30 (8.1%) | 13 (6.4%) | 9 (8.9%) | 8 (12.1%) | |
| 10 | 20 (5.4%) | 18 (8.9%) | 1 (1.0%) | 1 (1.5%) | |
| 11 | 46 (12.4%) | 26 (12.8%) | 3 (3.0%) | 17 (25.8%) | |
| Health Insurance Coverage | <0.0001 | ||||
| Public | 195 (52.7%) | 83 (40.9%) | 74 (73.3%) | 38 (57.6%) | |
| Private | 125 (33.8%) | 102 (50.3%) | 12 (11.9%) | 11 (16.7%) | |
| Other | 45 (12.2%) | 15 (7.4%) | 13 (12.9%) | 17 (25.8%) | |
| None | 5 (1.4%) | 3 (1.5%) | 2 (2.0%) | 0 (0%) | |
| Clinical and Demographic Characteristics | |||||
| BMI > 85% 4 | 30 (8.1%) | 14 (6.9%) | 13 (12.9%) | 3 (4.6%) | 0.1003 |
| Albumin ≥ 3.5 g/dL | 120 (32.4%) | 55 (27.1%) | 40 (39.6%) | 25 (37.9%) | 0.0522 |
| Hgb ≥ 11g/dL | 250 (67.6%) | 148 (72.9%) | 61 (60.4%) | 41 (62.1%) | 0.1205 |
| Pre-dialysis ESA5 | 187 (50.5%) | 108 (53.2%) | 47 (46.5%) | 32 (48.5%) | 0.5129 |
| Neighborhood Characteristics | |||||
| Neighborhood Poverty (% zip below poverty) | <0.0001 | ||||
| 0–4.9% | 57 (15.4%) | 47 (23.2%) | 7 (6.9%) | 3 (4.6%) | |
| 5–9.9% | 112 (30.3%) | 78 (38.4%) | 18 (17.8%) | 16 (24.2%) | |
| 10–14.9% | 64 (17.3%) | 35 (17.2%) | 17 (16.8%) | 12 (18.2%) | |
| 15–19.9% | 47 (12.7%) | 19 (9.4%) | 17 (16.8%) | 11 (16.7%) | |
| > 20% | 90 (24.3%) | 24 (11.8%) | 42 (41.6%) | 24 (36.4%) | |
| Residential Urban Commuting Area | 0.0057 | ||||
| Urban | 291 (78.7%) | 146 (71.9%) | 90 (89.1%) | 55 (83.3%) | |
| Rural | 79 (21.4%) | 57 (28.1%) | 11 (10.9%) | 11 (16.7%) | |
Glomerulonephritis
Focal Segmental Glomerulosclerosis
Post-Sept. 2005 vs. Pre-Sept. 2005
Body Mass Index
Erythropoiesis-Stimulating Agent
p-values < 0.05 for each variable indicate that at least one variable level is significantly different across racial/ethnic groups.
Trends in Preemptive Transplantation: 2000–2009
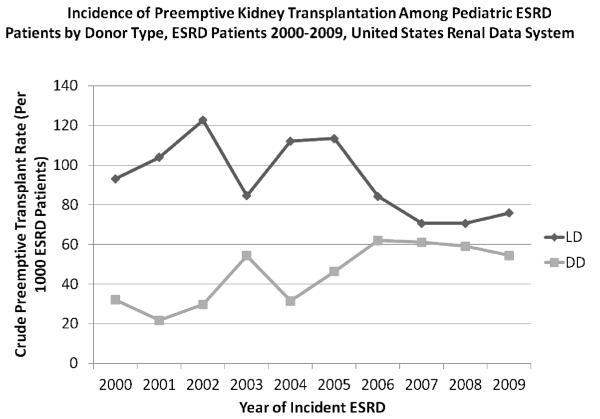
The incidence of preemptive transplantation varied significantly over the past decade, with a significant decrease in the incidence of LD preemptive transplant (p=0.0003) and a significant increase in DD preemptive transplant (p<0.0001) (Figure 1). These trends are consistent with the rise in the utilization of non-preemptive DD organs and the fall in non-preemptive LD transplantation among pediatric ESRD over the last decade26.
Figure 1.
The incidence of living donor (LD) and deceased donor (DD) preemptive transplantation among pediatric ESRD patients varied significantly over time, with a significant decrease in the incidence of LD preemptive transplant (p=0.0003) and a significant increase in DD preemptive transplant (p<0.0001) over the last decade (2000–2009).
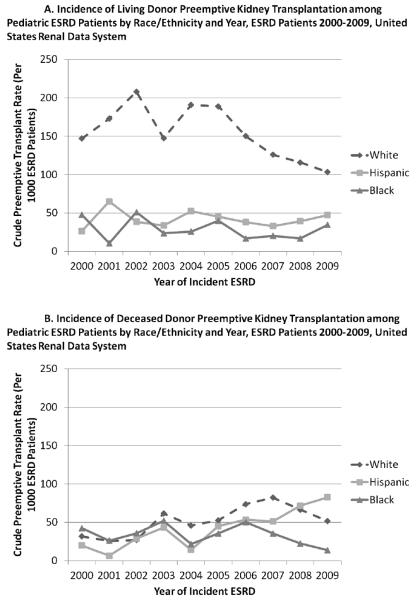
The average annual incidence of LD preemptive transplant was 154.0 per 1,000 ESRD patients among whites (95% CI: 142.8–165.7), 41.1 per 1,000 ESRD patients among Hispanics (95% CI: 33.5–50.6), and 28.8 per 1,000 ESRD patients among blacks (95% CI: 21.8–37.3). While the incidence of preemptive LD transplant has decreased among whites (p<0.0001), it has remained fairly stable among Hispanics (p=0.8546) and blacks (p=0.2800) (Figure 2A).
Figure 2.
The overall incidence of living donor (Panel A) preemptive transplant significantly declined among whites (p<0.0001), but remained stable among Hispanics (p=0.8546) and blacks (p=0.2800). The incidence of deceased donor (Panel B) preemptive transplant increased significantly among whites (p=0.0044) and Hispanics (p=0.0080), but not blacks (p=0.5815).
The average annual incidence of DD preemptive transplant was 52.2 per 1,000 ESRD patients among whites (95% CI: 45.4–59.6), 45.5 per 1,000 ESRD patients among Hispanics (95% CI: 37.2–55.0) and 34.0 per 1,000 ESRD patients among black patients (95% CI: 25.9–43.0). While the incidence of DD preemptive transplant increased significantly among whites (p=0.0044) and Hispanics (p=0.0080), it did not change among blacks (p=0.5815) (Figure 2B). Similarly, the racial/ethnic differences in preemptive transplant access by donor type are consistent with trends in non-preemptive LD and DD transplant access.26
Incidence of Preemptive Transplant Across SES Levels
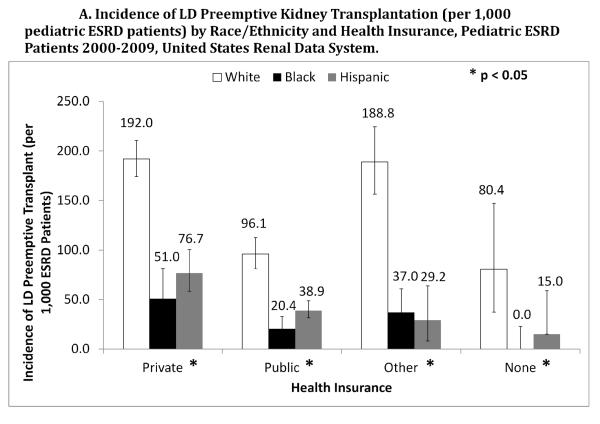
Among patients with private, public, and no health insurance, white pediatric patients had a higher incidence rate of LD (Figure 3A) and DD (Figure 3B) preemptive transplant compared to minorities (p<0.05). Similarly, white patients had a higher incidence of LD transplant across levels of neighborhood poverty, where even among wealthy (0–4.9% of zip code below poverty level) neighborhoods, the incidence rate of LD transplant was 204.1 per 1,000 ESRD patients for whites (95% CI: 178.6–231.5) vs. 83.3 per 1,000 ESRD patients for Hispanics (95% CI: 40.7–147.9) and 56.3 per 1000 ESRD patients for blacks (95% CI: 24.6–108.0) (p<0.0001).
Figure 3.
Across all types of health insurance coverage, white pediatric ESRD patients had a higher incidence rate of a living donor (LD) preemptive kidney transplant (Panel A) and a deceased donor (DD) preemptive kidney transplant (Panel B) compared to Hispanics and blacks.
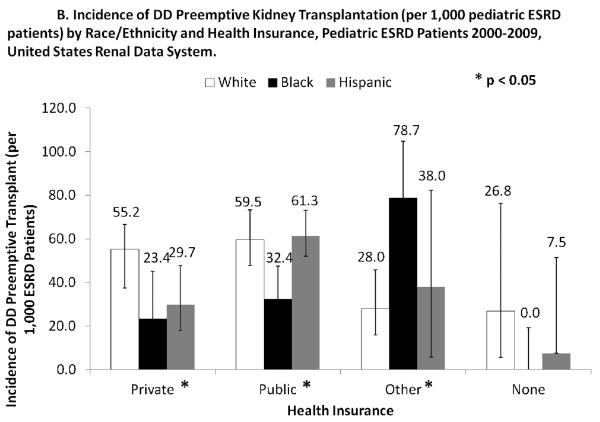
Neighborhood poverty was not significantly associated with DD preemptive transplant. However, access to DD preemptive transplant was higher among those with either private, public, or other insurance (45.9, 51.7, and 41.2/1000 ESRD patients, respectively), compared to those with no health insurance (10.8/1000 ESRD patients) (p<0.05). This access based on health insurance status was consistent across racial/ethnic groups (interaction between race/ethnicity and insurance not significant). For example, among patients who were privately insured, there were no racial/ethnic differences in access to DD preemptive transplantation. Similarly, access to DD preemptive transplant did not vary significantly across levels of neighborhood poverty (interaction between race/ethnicity and neighborhood poverty, p=0.2372).
Multivariable-adjusted Analyses
Crude and multivariable Risk Ratios for the effect of minority race/ethnicity on preemptive kidney transplantation by donor type are presented in Table 3. In crude analyses, both black (RR=0.18; 95% CI: 0.14–0.24) and Hispanic (RR=0.32; 95% CI: 0.24–0.43) pediatric ESRD patients had a lower relative incidence of LD preemptive transplant compared to whites. Disparities in access to LD preemptive transplant were nominally mitigated by adjustment for disease etiology (model 2) and further adjustment for demographic, clinical, socioeconomic and geographic factors reduced disparities (models 3 and 4) only modestly mitigated disparities. Factors associated with LD preemptive transplant included albumin >3.5 g/dL, hemoglobin > 11 g/dL, private or other health insurance status (vs. public), low (vs. high) neighborhood poverty, older age, and cystic/hereditary etiology of ESRD. Even in the fully adjusted model (model 4), the relative incidence of LD preemptive transplant was 66% lower among blacks compared to whites (RR=0.34; 95% CI: 0.28–0.43) and 52% lower among Hispanics compared to whites (RR=0.48; 95% CI: 0.35–0.67).
Table 3.
Effect of Minority Race/Ethnicity vs. White Race/Ethnicity on Access to Living or Deceased Donor Preemptive Kidney Transplantation
| Risk Ratios (RR) for Effect of Minority Race/Ethnicity vs. Whites on Preemptive Kidney Transplantation by Donor Type | ||
|---|---|---|
| Living Donor RR (95% CI) | Deceased Donor RR (95% CI) | |
| Black vs. White | ||
| Model 1 (Crude) | 0.18 (0.14–0.24) | 0.63 (0.47–0.85) |
| Model 2 (Etiology of ESRD) | 0.23 (0.18–0.30) | 0.76 (0.57–1.00) |
| Model 3 (Demographic + Clinical Factors) | 0.27 (0.21–0.35) | 0.80 (0.59–1.09) |
| Model 4 (Model 2 + SES + Geographic Factors) | 0.34 (0.28–0.43) | 0.78 (0.57–1.05) |
| Hispanic vs. White | ||
| Model 1 (Crude) | 0.32 (0.24–0.43) | 0.89 (0.73–1.09) |
| Model 2 (Etiology of ESRD) | 0.34 (0.24–0.48) | 0.96 (0.77–1.20) |
| Model 3 (Demographic + Clinical Factors) | 0.36 (0.26–0.49) | 0.98 (0.77–1.25) |
| Model 4 (Model 2 + SES + Geographic Factors) | 0.48 (0.35–0.67) | 1.03 (0.81–1.30) |
Model 2 adjusts for etiology of ESRD
Model 3 adjusts for etiology of ESRD, age, sex, BMI > 85%, hemoglobin ≥ 11 g/dL, Albumin < 3.5 g/dL, and pre-dialysis Erythropoiesis-Stimulating agent
Model 4 adjusts for all factors in model 3, plus OPO region, insurance status, zip code poverty level, and degree of urbanity of zip code residence.
In crude models, blacks had a 37% lower incidence of DD preemptive transplant vs. whites (RR=0.63; 95% CI: 0.47–0.85), but differences between Hispanics and whites were not statistically significant (RR=0.89; 95% CI: 0.73–1.09). Disparities in access to DD preemptive transplant for both blacks and Hispanics seemed largely driven by disease etiology (model 2), where adjusting for diagnosis at incident ESRD resulted in a reduction in the disparities to a statistically non-significant level. In addition, the inclusion of further demographic, clinical, socioeconomic and geographic factors did not substantially change the point estimates nor the confidence intervals. In fully-adjusted models (model 4), blacks had a 22% lower incidence of DD preemptive transplant compared to whites (RR=0.78; 95% CI: 0.57–1.05), although differences were not statistically significant. There were no racial/ethnic differences in access to DD preemptive transplant among Hispanics vs. whites in multivariable-adjusted models (RR=1.03; 95% CI: 0.81–1.30).
Stratified Analyses by Share 35 Era
Overall, the effect of race/ethnicity on preemptive transplant access did not significantly vary by Share 35 era (interaction p=0.1500 for DD and p=0.8151 for LD preemptive transplant access) in multivariable analyses (results not shown). However, we investigated trends in preemptive transplant access in age and sex-adjusted stratified analyses based on recent reports of an attenuation of disparities in access to non-preemptive transplant26. There were no significant black vs. white racial/ethnic disparities in access to preemptive DD transplant (RR=0.88; 95% CI: 0.63–1.23) pre-Share 35. However, following Share 35, there were significant black vs. white disparities in preemptive DD transplant access (RR=0.44; 95% CI: 0.26–0.77). Among Hispanics, the reverse was true - there were significant racial disparities in access to DD preemptive transplant prior to Share 35 (RR=0.55; 95% CI: 0.36–0.83) and there were no significant racial disparities following Share 35 (RR= 1.06; 95% CI: 0.82–1.36). These results suggest that although there were no statistically significant racial differences in preemptive DD transplant by era, access for blacks vs. whites appears to have declined following Share 35. Racial/ethnic disparities in access to LD preemptive transplant were consistent across both eras, where blacks and Hispanics were significantly less likely to receive a preemptive LD transplant.
Secondary Analyses
We compared dialysis exposure time in categories by race/ethnicity and donor source among patients transplanted. We found that both Hispanics and blacks comprised a substantially greater proportion of patients with greater than one year of dialysis exposure, regardless of donor source (42.4% Hispanics, 40.9% blacks vs. 27.4% whites for LD transplant recipients; 67.2% Hispanics, 63.5% blacks vs. 48.8% whites for DD transplant recipients) (Table 4). We also noted that among all transplant recipients (preemptive and non-preemptive), there were stark racial/ethnic differences in the proportion of patients who received a preemptive LD transplant (19.8% of LD transplants were preemptive for whites vs. 6% among Hispanics and 4.4% among blacks) (p<0.0001). Similarly, racial differences were observed among all DD transplant recipients, where the proportion of DD transplants that were preemptive were 16.5% among whites, 10.1% among Hispanics, and 7.0% among blacks (p<0.0001) (Table 5).
Table 4.
Proportion of Living or Deceased Donor Transplants by Race/Ethnicity and Time on Dialysis
| Living Donor Transplant Recipients (N=2,598) | ||||
|---|---|---|---|---|
| White, NH N=1763 | Hispanic N=522 | Black N=313 | P-value | |
| Preemptive transplant | 599 (33.9%) | 92 (17.6%) | 55 (17.6%) | <0.0001 |
| 0–3 months dialysis | 199 (11.3%) | 47 (9.0%) | 28 (9.0%) | |
| 3–6 months dialysis | 208 (11.8%) | 54 (10.3%) | 36 (11.5%) | |
| 6–12 months dialysis | 286 (16.2%) | 108 (20.7%) | 66 (21.1%) | |
| 1–2 years dialysis | 318 (18.0%) | 132 (25.3%) | 73 (23.3%) | |
| > 2 years on dialysis | 165 (9.4%) | 89 (17.1%) | 55 (17.6%) | |
| Deceased Donor Transplant Recipients (N=3,176) | ||||
|---|---|---|---|---|
| White, NH N=1232 | Hispanic N=1001 | Black N=943 | P-value | |
| Preemptive transplant | 203 (16.5%) | 101 (10.1%) | 66 (7.0%) | <0.0001 |
| 0–3 months dialysis | 104 (8.4%) | 38 (3.8%) | 30 (3.2%) | |
| 3–6 months dialysis | 109 (8.9%) | 46 (4.6%) | 66 (7.0%) | |
| 6–12 months dialysis | 214 (17.4%) | 144 (14.4%) | 154 (16.3%) | |
| 1–2 years dialysis | 306 (24.8%) | 317 (31.7%) | 276 (29.3%) | |
| > 2 years on dialysis | 296 (24.0%) | 355 (35.5%) | 351 (37.2%) | |
Table 5.
Proportion of Preemptive and Non-Preemptive DD and LD transplants by Racial/ethnic Group among Patients who Received a Kidney Transplant.
| Transplanted Population N=5,774 | White, NH N=2,995 | Hispanic N=1,523 | Black N=1,256 | P-value | |
|---|---|---|---|---|---|
| DD transplants | 3176 (55.0%) | 1232 (41.1%) | 1001 (65.7%) | 943 (75.1%) | <0.0001 |
| Preemptive | 377 (6.5%) | 209 (6.0%) | 101 (6.6%) | 67 (5.3%) | |
| Non-Preemptive | 2799 (48.5%) | 1023 (34.2%) | 900(59.1%) | 876 (69.8%) | |
| LD transplants | 2598 (45.0%) | 1763 (58.9%) | 522 (34.3%) | 313 (24.9%) | <0.0001 |
| Preemptive | 740 (12.8%) | 593 (19.8%) | 92 (6.0%) | 55 (4.4%) | |
| Non-Preemptive | 1858 (32.2%) | 1170 (39.1%) | 430 (28.2%) | 258 (20.5%) |
DD = Deceased Donor; LD = Living Donor
Among pediatric ESRD patients 2005–2009 who had information reported on receipt of pre-ESRD nephrology care, significant racial/ethnic and SES differences were observed. More than two-thirds (72.1%) of whites reported access to pre-ESRD nephrology care vs. 60.1% of Hispanics and 66.1% of blacks (p<0.0001). 71.3% of privately insured patients received pre-ESRD care vs. 69.2% of patients with public insurance and 33.0% of patients with no health insurance (p<0.0001). In crude log-binomial regression models, the incidence of LD preemptive transplantation was 24 times (RR=24.29; 95% CI: 10.05–58.73) and the incidence of DD preemptive transplantation was 10 times (RR=10.17; 95% CI: 5.03–20.57) higher among those who received pre-ESRD nephrology care compared to those who did not receive pre-ESRD nephrology care. In multivariable analyses restricted to patients from 2005–2009 only, adding pre-ESRD nephrology care to the multivariable-adjusted models attenuated but did not eliminate the racial disparity in access to LD preemptive kidney transplantation among Hispanics, but not black patients (results not shown).
To eliminate confounding by medical ineligibility, we repeated analyses among waitlisted (N=4,978) and transplanted (N=5,774) patients only and found consistent results (results not shown).
Discussion
This study is the first to examine the role of race/ethnicity and SES in access to preemptive kidney transplantation among a national cohort and the first study to closely examine differences in access to LD vs. DD preemptive transplant. In this comprehensive USRDS surveillance registry, only 13.9% of pediatric patients received a preemptive transplant over the decade-long study despite the many well-recognized increased risks to short- and long-term health associated with dialysis (vs. transplant) among children with ESRD. White patients made up less than half of the pediatric ESRD population, but comprised 71.8% of the preemptive kidney transplant population and received primarily LD transplants (75% LD vs. 25% DD) compared with black and Hispanic children, who received over 50% DD transplants. In adjusted analyses, racial disparities in access to preemptive DD transplant were largely explained by disease etiology. However, black ESRD patients still had a 22% lower incidence of DD preemptive transplant compared to whites in adjusted analyses, albeit differences were not statistically significant. However, when examining access to preemptive LD transplant, we noted that black patients had a 66% lower incidence and Hispanic patients a 52% lower relative incidence of LD preemptive transplant compared to whites, even after adjusting for demographic, clinical and socioeconomic factors.
These results are striking and require careful scrutiny to better understand why racial/ethnic differences in access to preemptive transplant should vary so dramatically by donor source. It is widely recognized in prior literature and also evident in our cohort that blacks and Hispanics experience ESRD more commonly due to systemic lupus erythematosus (SLE) and focal segmental glomerulosclerosis33,34. In both diagnoses, active disease may preclude preemptive transplantation. For example, active nephrotics may need nephrectomies preceding transplant and SLE patients may require time to “burn out” their disease or achieve disease quiescence. Thus, it is not entirely surprising that disease etiology drives racial differences in access to preemptive transplantation. The more perplexing finding is why disease etiology did not explain reduced access to LD preemptive transplant. In order to answer this question, we must consider how barriers to preemptive transplantation might compare and contrast by donor source.
In our analyses, independent risk factors for increased access to preemptive transplant that were consistent for both LD and DD preemptive transplant included higher albumin, higher hemoglobin and private insurance, all of which represent proxies for early access to care. Certainly, preemptive transplantation from any source necessitates early access to care. Providers must recognize the progression of renal disease and refer patients for transplantation in advance of the need for dialysis. Indeed, in our secondary analysis of patients with data on pre-ESRD nephrology care, we observed a substantially higher incidence of LD and DD preemptive transplantation among those who did vs. did not receive pre-ESRD nephrology care. Additionally, preemptive transplantation was associated with private insurance. In many centers, patients and families must have a financial plan in place to cover medical expenses and the costs of immunosuppressive medications as a condition of transplant eligibility. On the contrary, for uninsured patients who become Medicare-eligible when they start dialysis, dialysis opens the opportunity for public insurance eligibility and also potentially creates the ability to cover the expenses of transplantation (at least for three years post-transplant). Although the expansion of public insurance coverage for impoverished children through the Children's Health Insurance Program has increased healthcare utilization and reduced out-of-pocket healthcare spending for low income families35, it has not necessarily reduced household financial hardship. A recent study by Saloner et al suggests that public insurance access does not significantly improve other deeply seeded problems of poverty, such as food insecurity and housing problems36. Low-income families may be particularly vulnerable to the financial burdens of living donation as they are more likely to live paycheck to paycheck and lack substantial savings37.
One factor that was independently associated with increased access to preemptive LD transplant but not preemptive DD transplant was living in wealthier neighborhoods. Contextual-level poverty has long been recognized as an adverse social determinant of health38–40. Stress associated with living in a poor neighborhood has been consistently associated with poor individual health38, and may be a barrier in obtaining a LD for an impoverished parent of a child with ESRD. A higher prevalence of hypertension41, diabetes42, obesity43, and ESRD44 among minorities vs. white patients may preclude interested donors from eligibility45. Additionally, there may be greater social barriers among minorities living in poor neighborhoods. An impoverished parent eager to donate, particularly a single care provider, may be unable financially to miss work to complete a transplant evaluation or undergo transplant surgery. This parent may also be unable to pay for childcare during the evaluation or hospitalization. Impoverished neighborhoods are often associated with greater social fragmentation39 and thus there may be less social support to help the parent and child through the transplant process, including less availability of living donors. It should be noted that the addition of neighborhood-level poverty to multivariable analyses modestly attenuated but did not eliminate racial disparities in access to LD preemptive transplant. This may be due to the fact that the contextual factors associated with poverty were not well captured with our ZIP Code poverty measure. Alternatively, there may be other unrecognized confounders, or other SES measures, that may better explain these racial/ethnic differences in preemptive transplant access.
Previous studies have documented racial disparities in several of the required steps a patient or patient's family must navigate before getting a kidney transplant among children and adults25,46–49. The long-term effects of racial discrimination, both structurally and personally-mediated, intentional and unintentional, are well established as causal factors for racial/ethnic differences in health outcomes in the U.S. and may contribute to the observed racial/ethnic differences in access to preemptive LD transplantation50. For example, patient race/ethnicity may influence physicians' beliefs about a patient's behaviors and likelihood of treatment success, which may lead physicians to bias treatment preferences by race51. Structural-based inequities such as national allocation based on HLA-B matching52 and policies dictating immunusuppression drug regimen coverage53 may influence disparities in transplantation. An unintended consequence of the Share 35 policy that preferentially allocates young (< 35 years) DD organs to pediatric ESRD patients is the significant decline in preemptive LD transplants among all pediatric ESRD patients, with the greatest reduction among minorities26. This pattern is consistent with national trends for non-preemptive kidney transplants as well26. It may be that in the current era of Share 35, when waiting lists are shorter for children with incident ESRD, impoverished families may perceive that the struggles to attain a living donor may not be worth the effort. In addition, providers may communicate the benefits of priority allocation differentially if they know a family is under significant financial hardship and may incur tremendous burden and stress in seeking a living donor. These questions are unable to be answered with national data but deserve further inspection at a qualitative level.
There are several limitations to the current study. First, we did not have sufficient power to examine access to preemptive transplant among other races/ethnicities. Due to a small number of preemptive transplants among minorities, our confidence intervals around incidence estimates are wide, and thus while estimates suggest racial differences in access to preemptive DD, there may have been limited power to detect true differences (type II error). Second, we have no data on potential donor networks and the role of patient and family preferences in donation for each patient, so we are unable to assess patient access to eligible living donors. In adults, among African Americans, fear54 and lack of knowledge about the donation process55 play a role in reduced donation, however a recent study in pediatrics showed that family/patient preference was not a significant reason why a patient was not listed and there was no significant difference in the percentage of patients not listed for family choice by race56. Third, our measures of SES are measured at one point in time and are likely inadequate in measuring a patient's poverty status. Census tract neighborhood poverty is preferred57, but are unavailable in registry data. We used neighborhood-level poverty as our measure of community-level SES. We did not have data on personal income, educational attainment or net worth which are other important measures of SES. Thus, we may be underestimating the effects of poverty on access to LD preemptive transplant. There are likely other unmeasured factors that play a role in disparities in access to preemptive kidney transplantation, such as SES before ESRD start.
Our study findings suggest that interventions to improve racial parity in pediatric access to kidney transplantation must consider a focus on improving access to pre-ESRD nephrology care to improve access to preemptive transplantation. Further, it should be noted that, among the patients transplanted (LD and DD) in this cohort over 58.6% of Hispanic and 60.1% of black children spent greater than one year on dialysis preceding transplant (vs. 36.2% of whites). Policy initiatives such as U.S. healthcare reform which permit and promote better utilization of primary care services for children in the US will promote early kidney disease recognition and increase opportunities for planned transplantation in advance of the need for dialysis, particularly among minorities who are disproportionately represented among the uninsured and underinsured. Furthermore, once patients are referred for transplant evaluation, culturally-sensitive programs that improve patient and family education58 and help patients progress through transplant steps59 may be important to augment support for minority patients and families and reduce time on dialysis. The stark racial/ethnic differences we observed in access to LD preemptive kidney transplantation among pediatric ESRD patients need further exploration so that specific barriers to LD preemptive transplantation for minority children may be overcome. To truly attain racial parity in transplant access and outcomes in pediatric ESRD, a system must be implemented to optimize the receipt of the best quality kidneys for children, including optimizing opportunities for all children, regardless of race/ethnicity and SES, to receive the best-matched organs from living donors when feasible. Although Share 35 has improved access to DD transplantation for children, promoting access to LD transplantation for children is not currently recognized as a priority on a national level. While there is much debate about the ethics of incentivizing donors, identifying interventions that will overcome barriers to donation for motivated donors should not meet with such controversy.
Acknowledgements
The authors would like to acknowledge the assistance of the peer reviewers and editors of the AJT who thoughtfully read and commented on earlier drafts of this manuscript and added greatly to the final version. A portion of this work was presented at the American Society of Nephrology meeting in Philadelphia in November 2011 (Abstract # 22645). The interpretation and reporting of the data presented here are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government. R.E.P. was supported in part by grants from the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number ULl TR000454 and KL2TR000455 as well as 1R24MD008077-01 through the National Institute on Minority Health and Health Disparities. S.A. is supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases K23-DK083529-01A2. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- (ESRD)
End Stage Renal Disease
- (DD)
Deceased Donor
- (LD)
Living Donor
- (SES)
Socioeconomic Status
- (CMS)
Centers for Medicare & Medicaid Services
- (ESA)
Erythropoiesis-Stimulating Agent
- (OPO)
Organ Procurement Organization
- (RUCA)
Rural Urban Commuting Area
- (CI)
Confidence Interval
- (RI)
Risk Ratio
Footnotes
Disclosures
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Meier-Kriesche HU, Kaplan B. Waiting time on dialysis as the strongest modifiable risk factor for renal transplant outcomes: a paired donor kidney analysis. Transplantation. 2002 Nov 27;74(10):1377–1381. doi: 10.1097/00007890-200211270-00005. [DOI] [PubMed] [Google Scholar]
- 2.Meier-Kriesche HU, Port FK, Ojo AO, et al. Effect of waiting time on renal transplant outcome. Kidney Int. 2000 Sep;58(3):1311–1317. doi: 10.1046/j.1523-1755.2000.00287.x. [DOI] [PubMed] [Google Scholar]
- 3.Liem YS, Bosch JL, Arends LR, Heijenbrok-Kal MH, Hunink MG. Quality of life assessed with the Medical Outcomes Study Short Form 36-Item Health Survey of patients on renal replacement therapy: a systematic review and meta-analysis. Value Health. 2007 Sep-Oct;10(5):390–397. doi: 10.1111/j.1524-4733.2007.00193.x. [DOI] [PubMed] [Google Scholar]
- 4.Abecassis M, Bartlett ST, Collins AJ, et al. Kidney transplantation as primary therapy for end-stage renal disease: a National Kidney Foundation/Kidney Disease Outcomes Quality Initiative (NKF/KDOQITM) conference. Clin J Am Soc Nephrol. 2008 Mar;3(2):471–480. doi: 10.2215/CJN.05021107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mange KC, Weir MR. Preemptive renal transplantation: why not? Am J Transplant. 2003 Nov;3(11):1336–1340. doi: 10.1046/j.1600-6143.2003.00232.x. [DOI] [PubMed] [Google Scholar]
- 6.Liem YS, Bosch JL, Arends LR, Heijenbrok-Kal MH, Hunink MG. Quality of life assessed with the Medical Outcomes Study Short Form 36-Item Health Survey of patients on renal replacement therapy: a systematic review and meta-analysis. Value Health. 2007 Sep-Oct;10(5):390–397. doi: 10.1111/j.1524-4733.2007.00193.x. [DOI] [PubMed] [Google Scholar]
- 7.Kallab S, Bassil N, Esposito L, Cardeau-Desangles I, Rostaing L, Kamar N. Indications for and barriers to preemptive kidney transplantation: a review. Transplantation proceedings. 2010 Apr;42(3):782–784. doi: 10.1016/j.transproceed.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 8.Davis CL. Preemptive transplantation and the transplant first initiative. Curr Opin Nephrol Hypertens. Nov;19(6):592–597. doi: 10.1097/MNH.0b013e32833e04f5. [DOI] [PubMed] [Google Scholar]
- 9.Groothoff JW, Gruppen MP, Offringa M, et al. Mortality and causes of death of end-stage renal disease in children: a Dutch cohort study. Kidney Int. 2002 Feb;61(2):621–629. doi: 10.1046/j.1523-1755.2002.00156.x. [DOI] [PubMed] [Google Scholar]
- 10.Chavers BM, Solid CA, Daniels FX, et al. Hypertension in pediatric long-term hemodialysis patients in the United States. Clinical journal of the American Society of Nephrology : CJASN. 2009 Aug;4(8):1363–1369. doi: 10.2215/CJN.01440209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shamszad P, Slesnick TC, Smith EO, Taylor MD, Feig DI. Association between left ventricular mass index and cardiac function in pediatric dialysis patients. Pediatric nephrology. 2012 May;27(5):835–841. doi: 10.1007/s00467-011-2060-1. [DOI] [PubMed] [Google Scholar]
- 12.Arbeiter AK, Buscher R, Petersenn S, Hauffa BP, Mann K, Hoyer PF. Ghrelin and other appetite-regulating hormones in paediatric patients with chronic renal failure during dialysis and following kidney transplantation. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2009 Feb;24(2):643–646. doi: 10.1093/ndt/gfn529. [DOI] [PubMed] [Google Scholar]
- 13.Nissel R, Lindberg A, Mehls O, Haffner D. Factors predicting the near-final height in growth hormone-treated children and adolescents with chronic kidney disease. J Clin Endocrinol Metab. 2008 Apr;93(4):1359–1365. doi: 10.1210/jc.2007-2302. [DOI] [PubMed] [Google Scholar]
- 14.Rasbury WC, Fennell RS, 3rd, Fennell EB, Morris MK. Cognitive functioning in children with end stage renal disease pre- and post-dialysis session. Int J Pediatr Nephrol. 1986 Jan-Mar;7(1):45–50. [PubMed] [Google Scholar]
- 15.U S Renal Data System . USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2012. [Google Scholar]
- 16.Boehm M, Winkelmayer WC, Arbeiter K, Mueller T, Aufricht C. Late referral to paediatric renal failure service impairs access to pre-emptive kidney transplantation in children. Arch Dis Child. 2010 Aug;95(8):634–638. doi: 10.1136/adc.2009.174581. [DOI] [PubMed] [Google Scholar]
- 17.Conrey EJ, Seidu D, Ryan NJ, Chapman DS. Access to patient-centered medical home among Ohio's children with special health care needs. J Child Health Care. 2012 Dec 12; doi: 10.1177/1367493512456111. [DOI] [PubMed] [Google Scholar]
- 18.Chando S, Tiro JA, Harris TR, Kobrin S, Breen N. Effects of socioeconomic status and health care access on low levels of human papillomavirus vaccination among spanish-speaking hispanics in california. American journal of public health. 2013 Feb;103(2):270–272. doi: 10.2105/AJPH.2012.300920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher-Owens SA, Isong IA, Soobader MJ, et al. An examination of racial/ethnic disparities in children's oral health in the United States. J Public Health Dent. 2012 Sep 13; doi: 10.1111/j.1752-7325.2012.00367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.CDC Report: Early release of selected estimates based on data from the 2011 National Health Interview Survey. Lack of health insurance and type of coverage.
- 21.Kasiske BL, Snyder JJ, Matas AJ, Ellison MD, Gill JS, Kausz AT. Preemptive kidney transplantation: the advantage and the advantaged. Journal of the American Society of Nephrology : JASN. 2002 May;13(5):1358–1364. doi: 10.1097/01.asn.0000013295.11876.c9. [DOI] [PubMed] [Google Scholar]
- 22.Omoloja A, Stolfi A, Mitsnefes M. Racial differences in pediatric renal transplantation-24-year single center experience. J Natl Med Assoc. 2006 Feb;98(2):154–157. [PMC free article] [PubMed] [Google Scholar]
- 23.Vats AN, Donaldson L, Fine RN, Chavers BM. Pretransplant dialysis status and outcome of renal transplantation in North American children: a NAPRTCS Study. North American Pediatric Renal Transplant Cooperative Study. Transplantation. 2000 Apr 15;69(7):1414–1419. doi: 10.1097/00007890-200004150-00035. [DOI] [PubMed] [Google Scholar]
- 24.Butani L, Perez RV. Effect of pretransplant dialysis modality and duration on long-term outcomes of children receiving renal transplants. Transplantation. Feb 27;91(4):447–451. doi: 10.1097/TP.0b013e318204860b. [DOI] [PubMed] [Google Scholar]
- 25.Patzer RE, Amaral S, Klein M, et al. Racial disparities in pediatric access to kidney transplantation: does socioeconomic status play a role? American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012 Feb;12(2):369–378. doi: 10.1111/j.1600-6143.2011.03888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amaral S, Patzer RE, Kutner N, McClellan WM. Racial Disparities in Access to Pediatric Kidney Transplantation Since Share 35. Journal of American Society of Nephrology. 2012 doi: 10.1681/ASN.2011121145. [DOI] [PubMed] [Google Scholar]
- 27.Kaufman JS, Cooper RS. Commentary: considerations for use of racial/ethnic classification in etiologic research. American journal of epidemiology. 2001 Aug 15;154(4):291–298. doi: 10.1093/aje/154.4.291. [DOI] [PubMed] [Google Scholar]
- 28.Jones CP. Invited commentary: “race,” racism, and the practice of epidemiology. American journal of epidemiology. 2001 Aug 15;154(4):299–304. doi: 10.1093/aje/154.4.299. discussion 305–296. [DOI] [PubMed] [Google Scholar]
- 29.Patzer RE, Amaral S, Wasse H, Volkova N, Kleinbaum D, McClellan WM. Neighborhood poverty and racial disparities in kidney transplant waitlisting. J Am Soc Nephrol. 2009 Jun;20(6):1333–1340. doi: 10.1681/ASN.2008030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patzer RE, McClellan WM. Influence of race, ethnicity and socioeconomic status on kidney disease. Nat Rev Nephrol. 2012 Jun 26;8(9):533–541. doi: 10.1038/nrneph.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saunders MR, Cagney KA, Ross LF, Alexander GC. Neighborhood poverty, racial composition and renal transplant waitlist. Am J Transplant. 2010 Aug;10(8):1912–1917. doi: 10.1111/j.1600-6143.2010.03206.x. 2010. [DOI] [PubMed] [Google Scholar]
- 32.Hall YN, O'Hare AM, Young BA, Boyko EJ, Chertow GM. Neighborhood poverty and kidney transplantation among US Asians and Pacific Islanders with end-stage renal disease. Am J Transplant. 2008 Nov;8(11):2402–2409. doi: 10.1111/j.1600-6143.2008.02413.x. [DOI] [PubMed] [Google Scholar]
- 33.Hiraki LT, Lu B, Alexander SR, et al. End-stage renal disease due to lupus nephritis among children in the US, 1995–2006. Arthritis Rheum. 2011 Jul;63(7):1988–1997. doi: 10.1002/art.30350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boyer O, Moulder JK, Somers MJ. Focal and segmental glomerulosclerosis in children: a longitudinal assessment. Pediatric nephrology. 2007 Aug;22(8):1159–1166. doi: 10.1007/s00467-007-0493-3. [DOI] [PubMed] [Google Scholar]
- 35.Kenney G. The impacts of the State Children's Health Insurance Program on children who enroll: findings from ten states. Health Serv Res. 2007 Aug;42(4):1520–1543. doi: 10.1111/j.1475-6773.2007.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saloner B. Does Expanding Public Insurance Prevent Material Hardship for Families With Children? Med Care Res Rev. 2013 Jan 6; doi: 10.1177/1077558712470566. [DOI] [PubMed] [Google Scholar]
- 37.Moseley KL, Kershaw DB. African American and white disparities in pediatric kidney transplantation in the United States -- unfortunate or unjust? Camb Q Healthc Ethics. 2012 Jul;21(3):353–365. doi: 10.1017/S0963180112000072. [DOI] [PubMed] [Google Scholar]
- 38.Moskowitz D, Vittinghoff E, Schmidt L. Reconsidering the Effects of Poverty and Social Support on Health: A 5-Year Longitudinal Test of the Stress-Buffering Hypothesis. J Urban Health. 2012 Sep 5; doi: 10.1007/s11524-012-9757-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cattell V. Poor people, poor places, and poor health: the mediating role of social networks and social capital. Social Science & Medicine. 2001 May;52(10):1501–1516. doi: 10.1016/s0277-9536(00)00259-8. [DOI] [PubMed] [Google Scholar]
- 40.Gilman SE, Fitzmaurice GM, Bruce ML, et al. Economic Inequalities in the Effectiveness of a Primary Care Intervention for Depression and Suicidal Ideation. Epidemiology. 2013 Jan;24(1):14–22. doi: 10.1097/EDE.0b013e3182762403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA : the journal of the American Medical Association. 2003 Jul 9;290(2):199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 42.Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA : the journal of the American Medical Association. 2001 Sep 12;286(10):1195–1200. doi: 10.1001/jama.286.10.1195. [DOI] [PubMed] [Google Scholar]
- 43.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA : the journal of the American Medical Association. 2006 Apr 5;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 44.Choi AI, Rodriguez RA, Bacchetti P, Bertenthal D, Hernandez GT, O'Hare AM. White/black racial differences in risk of end-stage renal disease and death. The American journal of medicine. 2009 Jul;122(7):672–678. doi: 10.1016/j.amjmed.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shilling LM NM, Chavin KD, Hildebrand LG, Lunsford SL, Martin MS, Milton JE, et al. Healthcare Professionals' perceptions of the Barriers to Living Donor Kidney Transplantation among African Americans. Journal of The National Medical Association. 2006;98(6):834–840. [PMC free article] [PubMed] [Google Scholar]
- 46.Alexander GC, Sehgal AR. Barriers to cadaveric renal transplantation among blacks, women, and the poor. Jama. 1998 Oct 7;280(13):1148–1152. doi: 10.1001/jama.280.13.1148. [DOI] [PubMed] [Google Scholar]
- 47.Weng FL, Joffe MM, Feldman HI, Mange KC. Rates of completion of the medical evaluation for renal transplantation. Am J Kidney Dis. 2005 Oct;46(4):734–745. doi: 10.1053/j.ajkd.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 48.Epstein AM, Ayanian JZ, Keogh JH, et al. Racial disparities in access to renal transplantation--clinically appropriate or due to underuse or overuse? N Engl J Med. 2000 Nov 23;343(21):1537–1544. 1532. doi: 10.1056/NEJM200011233432106. preceding 1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patzer RE, Perryman JP, Schrager JD, et al. The role of race and poverty on steps to kidney transplantation in the southeastern United States. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012 Feb;12(2):358–368. doi: 10.1111/j.1600-6143.2011.03927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams DR, Neighbors HW, Jackson JS. Racial/ethnic discrimination and health: findings from community studies. American journal of public health. 2008 Sep;98(9 Suppl):S29–37. doi: 10.2105/ajph.98.supplement_1.s29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Ryn MaJB. The effect of patient race and socioeconomic status on physicians' perceptions of patients. Soc Sci Med. 2000;50(6):813–828. doi: 10.1016/s0277-9536(99)00338-x. [DOI] [PubMed] [Google Scholar]
- 52.Ashby VB, Port FK, Wolfe RA, et al. Transplanting kidneys without points for HLA-B matching: consequences of the policy change. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011 Aug;11(8):1712–1718. doi: 10.1111/j.1600-6143.2011.03606.x. [DOI] [PubMed] [Google Scholar]
- 53.Woodward RS, Page TF, Soares R, Schnitzler MA, Lentine KL, Brennan DC. Income-related disparities in kidney transplant graft failures are eliminated by Medicare's immunosuppression coverage. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2008 Dec;8(12):2636–2646. doi: 10.1111/j.1600-6143.2008.02422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jacob Arriola KR, Perryman JP, Doldren M. Moving beyond attitudinal barriers: understanding African Americans' support for organ and tissue donation. Journal of the National Medical Association. 2005 Mar;97(3):339–350. [PMC free article] [PubMed] [Google Scholar]
- 55.Jacob Arriola KR, Robinson DH, Perryman JP, Thompson N. Understanding the relationship between knowledge and African Americans' donation decision-making. Patient Educ Couns. 2008 Feb;70(2):242–250. doi: 10.1016/j.pec.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nguyen S, Martz K, Stablein D, Neu A. Wait list status of pediatric dialysis patients in North America. Pediatric transplantation. 2011 Jun;15(4):376–383. doi: 10.1111/j.1399-3046.2011.01495.x. [DOI] [PubMed] [Google Scholar]
- 57.Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Race/ethnicity, gender, and monitoring socioeconomic gradients in health: a comparison of area-based socioeconomic measures--the public health disparities geocoding project. Am J Public Health. 2003 Oct;93(10):1655–1671. doi: 10.2105/ajph.93.10.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patzer RE, Perryman JP, Pastan S, et al. Impact of a Patient Education Program on Disparities in Kidney Transplant Evaluation. Clinical journal of the American Society of Nephrology : CJASN. 2012 Feb 16; doi: 10.2215/CJN.10071011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Natale-Pereira A, Enard KR, Nevarez L, Jones LA. The role of patient navigators in eliminating health disparities. Cancer. 2011 Aug;117(15 Suppl):3543–3552. doi: 10.1002/cncr.26264. [DOI] [PMC free article] [PubMed] [Google Scholar]