Abstract
The AMPA receptor is regulated by phosphorylation. Two major phosphorylation sites (S831 and S845) are located in the intracellular C-terminal tail of GluA1 subunits. The phosphorylation on these sites controls receptor expression and function and is subject to the regulation by psychostimulants. In this study, we further characterized the regulation of S831 and S845 phosphorylation by amphetamine (AMPH) in the adult rat striatum and medial prefrontal cortex (mPFC) in vivo. We focused on the specific fraction of GluA1/AMPA receptors enriched from synaptic and extrasynaptic membranes, using a pre-validated biochemical fractionation procedure. We found that acute AMPH administration elevated GluA1 S845 phosphorylation in the defined synaptic membrane from the striatum in a dose-dependent manner. AMPH also induced a comparable increase in S845 phosphorylation in the extrasynaptic fraction of striatal GluA1. Similar increases in S845 phosphorylation in both synaptic and extrasynaptic pools were observed in the mPFC. In contrast, S831 phosphorylation was not altered in synaptic and extrasynaptic GluA1 in striatal neurons and synaptic GluA1 in mPFC neurons in response to AMPH, although a moderate increase in S831 phosphorylation was seen in extrasynaptic GluA1 after an AMPH injection at a high dose. Total synaptic and extrasynaptic GluA1 expression remained stable in the two regions after AMPH administration. Our data demonstrate the differential sensitivity of S845 and S831 phosphorylation to dopamine stimulation. S845 is a primary site where phosphorylation of GluA1 is upregulated by AMPH in striatal and mPFC neurons at both synaptic and extrasynaptic compartments.
Keywords: Striatum, basal ganglia, striatum, prefrontal cortex, dopamine, glutamate, GluR1, excitatory amino acid, addiction
1. Introduction
The ionotropic AMPA receptor is broadly expressed in the mammalian brain. These receptors typically carry out function in a heterotetrameric complex assembled from four subunits (GluA1-4 or formerly GluR1-4) (Hollmann and Heinemann, 1994). Like other glutamate receptors, AMPA receptors are regulated by posttranslational phosphorylation (Wang et al., 2006; Mao et al., 2011). GluA1 is of particular interest due to its relatively long intracellular C-terminal tails and well-characterized phosphorylation by multiple protein kinases. Two serine residues (S831 and S845) in GluA1 C-termini are the major phosphorylation sites (Roche et al., 1996; Barria et al., 1997; Mammen et al., 1997; Serulle et al., 2007). The former (S831) is phosphorylated by protein kinase C (PKC) and Ca2+/calmodulin-dependent protein kinase II (CaMKII), whereas the latter (S845) by protein kinase A (PKA) and cGMP-dependent protein kinase II (cGKII). Altered phosphorylation levels at these sites modulate neurochemical and physiological properties of GluA1/AMPA receptors (Mao et al., 2011).
AMPA receptors are enriched in the striatum (Bernard et al., 1997; Kondo et al., 2000; Hu et al., 2004). In this region, GluA1/A2 receptors are the most common composition subtypes (Reimers et al., 2011) and are seen in all medium spiny projection neurons and most interneurons (Bernard et al., 1997). At the subsynaptic level, transmembrane AMPA receptors are concentrated on the postsynaptic membrane of asymmetric (excitatory) synapses, although extrasynaptic AMPA receptors are also present (Bernard et al., 1997; Ferrario et al., 2011b). The abundant distribution of AMPA receptors in striatal neurons supports their roles in experience-dependent synaptic and behavioral plasticity in response to stimulant exposure (Wolf and Ferrario, 2010).
Phosphorylation of AMPA receptors is a highly regulated event (Wang et al., 2006). The direct D1 dopamine receptor agonist SKF81297 or SKF38393 enhanced GluA1 phosphorylation at the PKA site (S845) although not at the PKC/CaMKII site (S831) in striatal or prefrontal cortical neurons (Price et al., 1999; Snyder et al., 2000; Chao et al., 2002b; Swayze et al., 2004). This enhancement was associated with an increase in surface-expressed AMPA receptors and AMPA receptor-mediated currents. Similarly, dopamine stimulation with an acute systemic injection of the psychostimulants (cocaine and methamphetamine) or a local injection of amphetamine (AMPH) into the nucleus accumbens (NAc) increased S845, but not S831, phosphorylation in the striatum in vivo (Snyder et al., 2000; Li et al., 2011). These data consistently identify S845 as a primary phosphorylation site sensitive to stimulant exposure. However, to date, little is known about the two specific pools of GluA1, i.e., GluA1 in synaptic versus extrasynaptic locations, in their S845 and S831 phosphorylation in response to stimulants.
We therefore conducted this study to determine the sensitivity of GluA1 enriched from distinct synaptic and extrasynaptic compartments to AMPH in terms of S831 and S845 phosphorylation. Using a pre-validated subsynaptic fractionation method, we isolated synaptic and extrasynaptic GluA1 proteins from the striatum and medial prefrontal cortex (mPFC) of adult rat brains. Changes in protein levels of phosphorylated GluA1 (pGluA1) at S831 (pS831) or S845 (pS845) were analyzed in the two regions following an acute injection of AMPH.
2. Materials and methods
2.1. Animals
Adult male Wistar rats weighing 275-350 g (Charles River, New York, NY) were individually housed in a controlled environment at a constant temperature of 23°C and humidity of 50 ± 10% with food and water available ad libitum. The animal room was on a 12-h/12-h light/dark cycle. Rats were allowed 5-7 days of habituation to the animal colony. All animal use and procedures were in strict accordance with the US National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee.
2.2. Systemic drug injection
Rats were treated with an intraperitoneal (i.p.) injection of d-amphetamine sulfate (Sigma-Aldrich, St. Louis, MO). AMPH was injected at three doses (0.5, 5, and 10 mg/kg). These doses were calculated as the salt and were chosen to include a dose of 5 mg/kg, an effective dose that caused typical motor stimulation and a marked increase in immediate early gene and opioid peptide gene expression in the rat striatum (Wang and McGinty, 1995). Age-matched rats received an acute injection of saline (1 ml/kg, i.p.) and served as controls. Rats were sacrificed 15 min after AMPH injection for the subsequent neurochemical analysis. We selected the time point of 15 min based on the finding that acute cocaine injection at 20 mg/kg increased GluA1-S845 phosphorylation in the mouse striatum at this time point (Snyder et al., 2000).
2.3. Fractionation of synaptic and extrasynaptic membranes
Enrichment of synaptic and extrasynaptic membranes was performed according to a method described previously (Mao et al., 2013). The method was developed based on the insolubility of the postsynaptic density and synaptic junctions in Triton X-100 (Davies et al., 2007; 2008; Goebel-Goody et al., 2009; Ferrario et al., 2011b). Briefly, rats were anesthetized with equithesin (5 ml/kg, i.p.) and decapitated. Brains were quickly removed and the striatum, including the dorsal caudate putamen and ventral nucleus accumbens, and mPFC, including the anterior cingulate, prelimbic, and infralimbic cortex, were dissected. These tissues were homogenized on ice in isotonic sucrose homogenization buffer containing 0.32 M sucrose, 10 mM HEPES, pH 7.4, 2 mM EDTA, and a protease inhibitor cocktail and a phosphatase inhibitor cocktail (Thermo Scientific, Rochester, NY) in a glass grinding vessel with a motor driven Teflon pestle (clearance = 0.125 mm) at 700 rpm (8 strokes). The homogenate was centrifuged at 760 g for 10 min at 4°C to generate the supernatant 1 (S1) and the pellet 1 (P1). P1 was resuspended, homogenized, and centrifuged at 760 g for 10 min at 4°C to generate the S1’ and P1’ fractions. The S1’ fraction was added to the S1 fraction. S1 was then centrifuged at 10,000 g for 30 min at 4°C to generate the supernatant 2 (S2) containing cytosol proteins and the pellet 2 (P2) containing crude synaptosomal plasma membranes. P2 was washed once and centrifuged at 10,000 g for 30 min at 4°C. A large portion of washed P2 was resuspended in the sucrose homogenization buffer containing Triton X-100 (0.5%, v/v) using a motorized pestle. The suspension was incubated (20 min) at 4°C, and centrifuged (20 min) at 32,000 g to yield the Triton X-100-insoluble pellet enriched with synaptic membranes and the Triton X-100-soluble supernatant enriched with extrasynaptic membranes. The extrasynaptic fraction was concentrated by acetone precipitation (−20° overnight and then centrifugation at 3,000 g). The concentrated extrasynaptic pellet and the synaptic fraction were solubilized in sucrose-Triton buffer containing 1% SDS, a protease inhibitor cocktail (Thermo Scientific), and a phosphatase inhibitor cocktail (Thermo Scientific). Protein concentrations were determined. Samples were stored at −80°C until use in Western blot.
2.4. Western blot analysis
As described previously (Mao et al., 2013), the equal amount of protein was separated on SDS NuPAGE Novex 4-12% gels (Invitrogen, Carsbad, CA). Proteins were transferred to the polyvinylidene fluoride membrane (Invitrogen) and blocked in a blocking buffer (5% nonfat dry milk in phosphate-buffered saline and 0.1% Tween 20) for 1 h. The blots were washed and incubated in the blocking buffer containing a primary antibody usually at 1:1000 overnight at 4°C. This was followed by 1 h incubation in a horseradish peroxidase-linked secondary antibody against rabbit (Santa Cruz) at 1:5000. Immunoblots were developed with the enhanced chemiluminescence reagents (ECL; Amersham Pharmacia Biotech, Piscataway, NJ). Kaleidoscope-prestained standards (Bio-Rad, Hercules, CA) and MagicMark XP Western protein standards (Invitrogen) were used for protein size determination. Immunoblots were measured using NIH gel analysis software. Primary antibodies used in this study include rabbit polyclonal antibodies against pGluA1-S831 (PhosphoSolutions, Aurora, CO), pGluA1-S845 (PhosphoSolutions), or C-terminus of GluA1 (Millipore).
2.5. Statistics
The results are presented as means ± S.E.M., and were evaluated using a one-way analysis of variance followed by a Bonferroni (Dunn) comparison of groups using least squares-adjusted means or two-tailed unpaired Student’s t-test. Probability levels of < 0.05 were considered statistically significant.
3. Results
3.1. Subsynaptic distribution of pGluA1 and GluA1 in the striatum
The efficiency of our fractionation method in enriching synaptic and extrasynaptic proteins has been demonstrated in details in our early report (Mao et al., 2013). In this study, this same method was used again to define the distribution pattern of total and phosphorylated GluA1 proteins in synaptic versus extrasynaptic compartments in striatal neurons. The entire striatum was dissected and used to determine basal expression of pS831, pS845, and GluA1 proteins in the two fractions. In synaptic fractions, robust pS831 expression was seen, while in extrasynaptic fractions, pS831 signals were minimal (Fig. 1). Strong pS845 signals were seen at synaptic sites (Fig. 1). Noticeably, the percentage of pS845 in extrasynaptic membranes over synaptic membranes is higher than that of pS831. GluA1 levels were predominant at synaptic sites relative to extrasynaptic sites (Fig. 1). Thus, under normal conditions, total and phosphorylated GluA1 proteins in striatal neurons are generally concentrated at synaptic sites, a distribution pattern similar to that detected in accumbal neurons (Ferrario et al., 2011b).
Figure 1. Normal subsynaptic distribution of pGluA1 and GluA1 in the rat striatum.
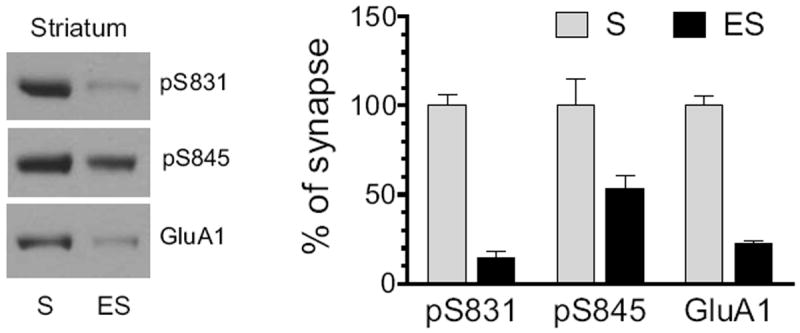
Distribution of pGluA1-S831, pGluA1-S845, and GluA1 was detected in synaptic and extrasynaptic membranes. Representative immunoblots are shown left to the quantified data. Proteins enriched from synaptic (S) and extrasynaptic (ES) membranes were loaded at the same amount (12 μg per lane). Data are presented as means ± S.E.M. (n = 3 per group).
3.2. Effects of AMPH on S831 phosphorylation in the striatum
We next assessed the impact of AMPH on S831 phosphorylation of GluA1 proteins enriched from synaptic and extrasynaptic membranes. To this end, we administered AMPH to rats at three different doses (0.5, 5, and 10 mg/kg, i.p.). We then sacrificed rats 15 min after drug injection. After striatal tissue was removed, we prepared synaptic and extrasynaptic membranes in addition to synaptosomal P2 membranes and detected changes in pS831 protein levels in these membrane fractions using Western blots. AMPH did not alter pS831 levels in synaptosomal fractions at all doses surveyed (Fig. 2A). Similarly, no significant change in pS831 signals in either synaptic or extrasynaptic fractions was observed in rats treated with AMPH relative to saline (Fig. 2B, synaptic fractions; Fig. 2C, extrasynaptic fractions). The total amount of GluA1 proteins remained stable in all three fractions of membranes after three doses of AMPH. These results indicate an insensitive nature of GluA1 phosphorylation at the S831 site in response to AMPH stimulation in striatal neurons.
Figure 2. Effects of AMPH on GluA1-S831 phosphorylation in the rat striatum.
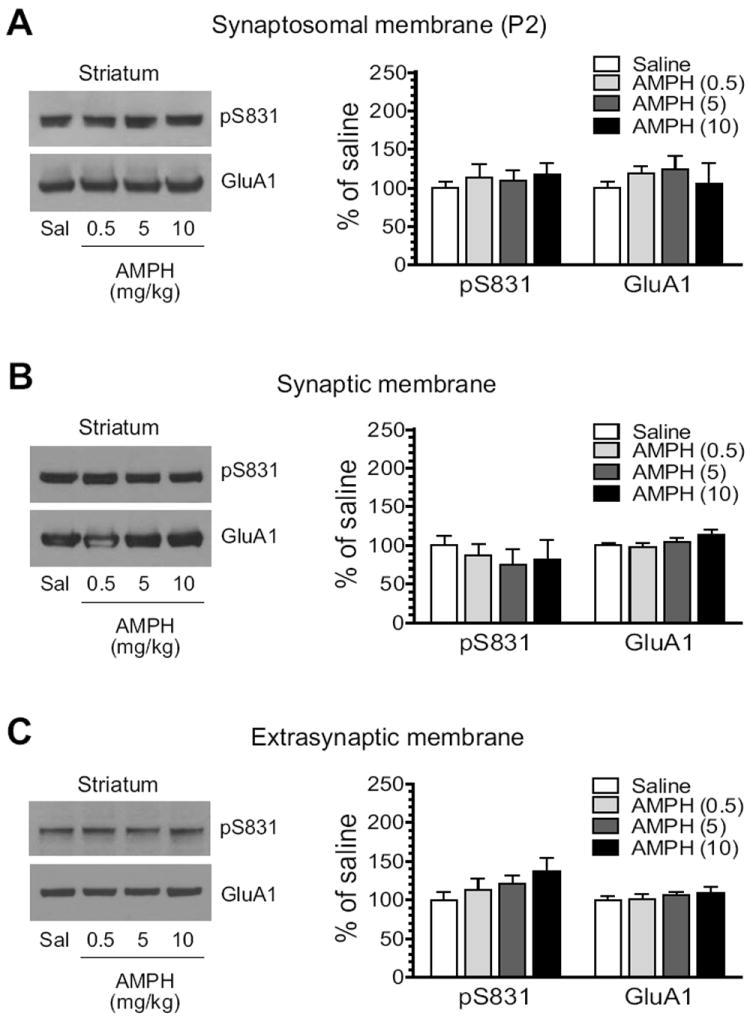
(A) Effects of AMPH on S831 phosphorylation in synaptosomal fractions. (B) Effects of AMPH on S831 phosphorylation in synaptic fractions. (C) Effects of AMPH on S831 phosphorylation in extrasynaptic fractions. Note that AMPH produced no significant change in S831 phosphorylation in all three membrane preparations. Representative immunoblots are shown left to the quantified data. Rats received a single dose of AMPH (0.5, 5, or 10 mg/kg, i.p.) and were sacrificed 15 min after drug injection for immunoblot analysis. Data are presented as means ± S.E.M. (n = 4 per group). *P < 0.05 versus saline.
3.3. Effects of AMPH on S845 phosphorylation in the striatum
S845 phosphorylation was then tested for its response to AMPH in the striatum. AMPH induced a dose-dependent increase in pS845 protein levels in synaptosomal membranes (Fig. 3A). Similarly, AMPH elevated pS845 in synaptic fractions. As shown in Fig. 3B, the stimulant at two higher doses (5 and 10 mg/kg) produced a significant increase in pS845 signals (5 mg/kg: 151.9 ± 5.6% of saline, P < 0.05; 10 mg/kg: 164.0 ± 11.9% of saline, P < 0.05). In extrasynaptic fractions, AMPH was still effective in stimulating S845 phosphorylation. At two higher doses, AMPH enhanced pS845 to 191.3 ± 29.2% of saline (P < 0.05; 5 mg/kg) and 222.5 ± 16.9% of saline (P < 0.05; 10 mg/kg) (Fig. 3C). These data demonstrate that GluA1 in the two subsynaptic compartments of striatal neurons is upregulated in its S845 phosphorylation in response to AMPH.
Figure 3. Effects of AMPH on GluA1-S845 phosphorylation in the rat striatum.
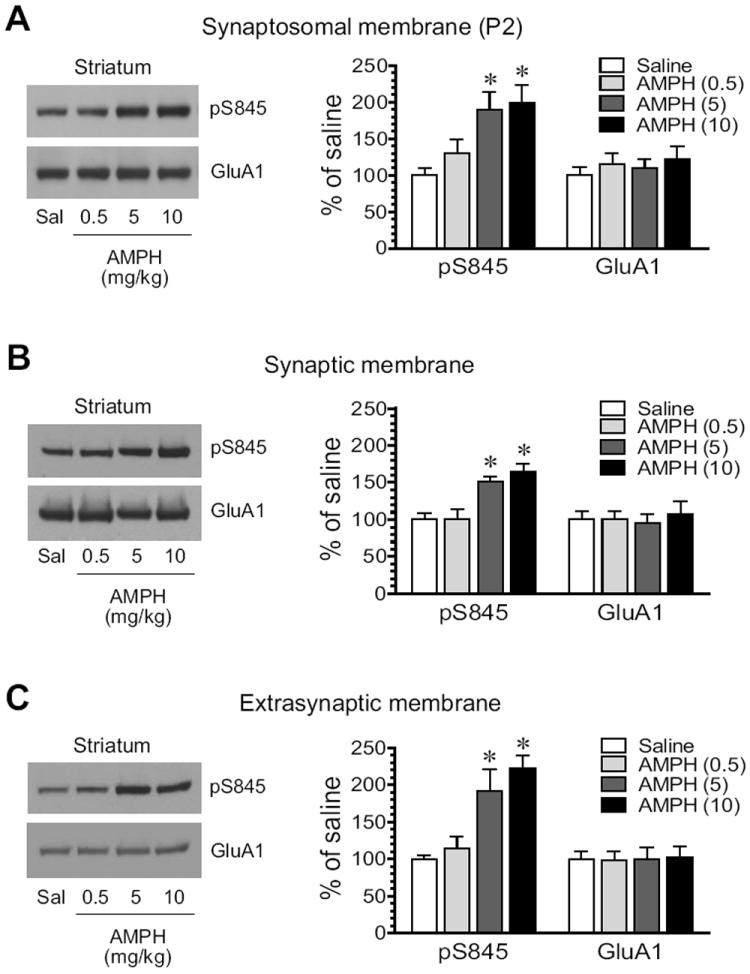
(A) Effects of AMPH on S845 phosphorylation in synaptosomal fractions. (B) Effects of AMPH on S845 phosphorylation in synaptic fractions. (C) Effects of AMPH on S845 phosphorylation in extrasynaptic fractions. Note that AMPH produced overall increases in pS845 proteins in all three membrane preparations. Representative immunoblots are shown left to the quantified data. Rats received a single dose of AMPH (0.5, 5, or 10 mg/kg, i.p.) and were sacrificed 15 min after drug injection for immunoblot analysis. Data are presented as means ± S.E.M. (n = 4 per group). * P < 0.05 versus saline.
3.4. Subsynaptic distribution of pGluA1 and GluA1 in the mPFC
The mPFC is another site in the mesocorticolimbic reward circuit critical for processing stimulant effects (Steketee, 2003; Van den Oever et al., 2010). We therefore expanded the present study to investigating responses of GluA1 phosphorylation to AMPH in this region. We first analyzed the basal level of phosphorylated GluA1 in synaptic and extrasynaptic pools of mPFC neurons. As shown in Fig. 4, protein levels of pS831 and pS845 were abundant in synaptic fractions. In contrast, these phosphorylated proteins were expressed at a low level in extrasynaptic fractions. Similar to this, GluA1 is distributed at a high and low level in synaptic and extrasynaptic fractions, respectively. Thus, the basal expression of total and phosphorylated GluA1 proteins in mPFC neurons is generally concentrated at synaptic sites.
Figure 4. Normal subsynaptic distribution of pGluA1 and GluA1 in the rat mPFC.
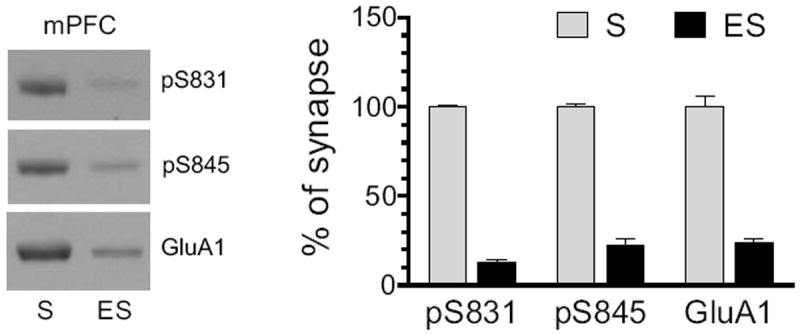
Distribution of pGluA1-S831, pGluA1-S845, and GluA1 was detected in synaptic and extrasynaptic membranes. Representative immunoblots are shown left to the quantified data. Proteins enriched from synaptic (S) and extrasynaptic (ES) membranes were loaded at the same amount (12 μg per lane). Data are presented as means ± S.E.M. (n = 3 per group).
3.5. Effects of AMPH on S831 phosphorylation in the mPFC
We next tested the effect of AMPH on GluA1 phosphorylation in the mPFC. No significant alteration of pS831 levels in synaptosomal membranes was seen in AMPH-treated rats compared to saline-treated rats (Fig. 5A). In synaptic membranes, a trend toward reduced expression of pS831 was shown following AMPH administration (Fig. 5B). At the highest dose of AMPH (10 mg/kg), pS831 was reduced to 79.4 ± 7.6% of saline, although it did not reach a statistically significant level (P = 0.14). In extrasynaptic fractions, in contrast to synaptic fractions, pS831 exhibited a graduate increase over escalating doses of AMPH (Fig. 5C). At 10 mg/kg, AMPH induced a significant increase in pS831 (187.5 ± 13.3 of saline, P < 0.05). At all doses, AMPH did not alter synaptic and extrasynaptic levels of GluA1 proteins. These data demonstrate differential responses of synaptic and extrasynaptic pools of GluA1 to AMPH in terms of S831 phosphorylation.
Figure 5. Effects of AMPH on GluA1-S831 phosphorylation in the rat mPFC.
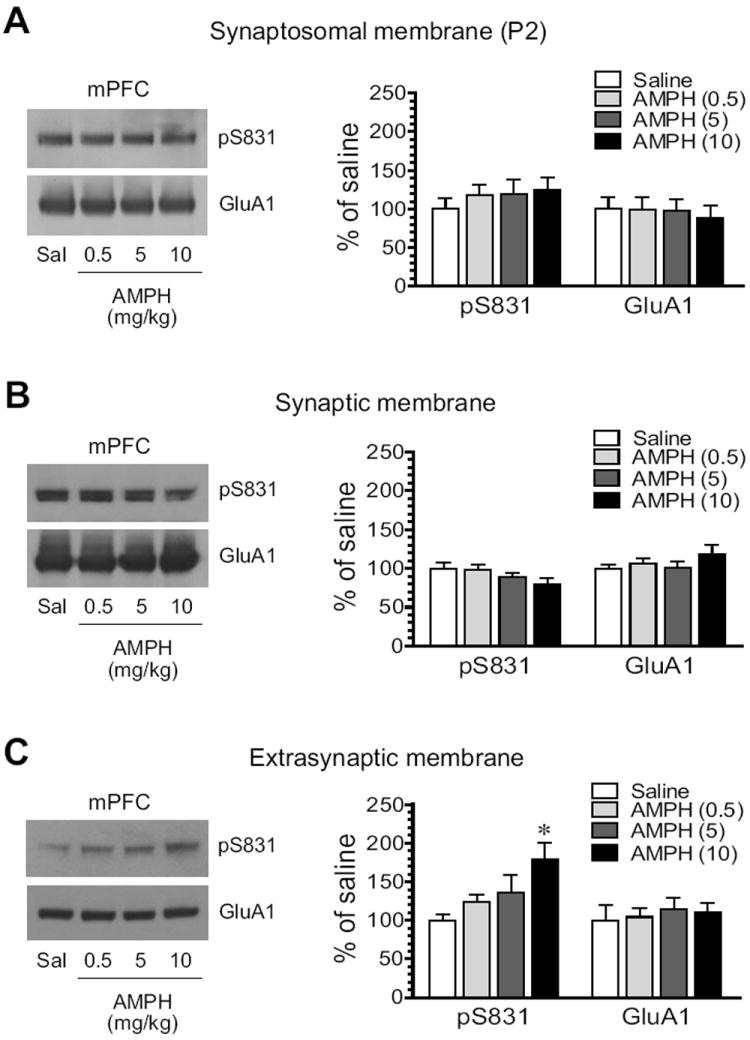
(A) Effects of AMPH on S831 phosphorylation in synaptosomal fractions. (B) Effects of AMPH on S831 phosphorylation in synaptic fractions. (C) Effects of AMPH on S831 phosphorylation in extrasynaptic fractions. Note that AMPH did not significantly alter pS831 protein levels in synaptosomal and synaptic fractions, while it increased pS831 in extrasynaptic fractions. Representative immunoblots are shown left to the quantified data. Rats received a single dose of AMPH (0.5, 5, or 10 mg/kg, i.p.) and were sacrificed 15 min after drug injection for immunoblot analysis. Data are presented as means ± S.E.M. (n = 4 per group). *P < 0.05 versus saline.
3.6. Effects of AMPH on S845 phosphorylation in the mPFC
S845 phosphorylation showed an overall positive response to AMPH in the mPFC, as seen in the striatum. A clear dose-dependent increase in pS845 protein levels occurred in synaptosomes extracted from AMPH-treated rats (Fig. 6A). Synaptic pS845 levels were also elevated in a dose-dependent manner (Fig. 6B). At the two higher doses, AMPH increased pS845 levels to 167.4 ± 13.7% of saline (5 mg/kg; P < 0.05) and 189.5 ± 23.4% (10 mg/kg, P < 0.05). In extrasynaptic fractions, we observed similar responses (Fig. 6C). Considerable increases in pS845 expression were seen following AMPH administration at 5 mg/kg (234.3 ± 8.5% of saline, P < 0.05) and 10 10 mg/kg (253.2 ± 14.7% of saline, P < 0.05). Thus, like what we observed in the striatum, S845 phosphorylation in both synaptic and extrasynaptic membranes of mPFC neurons is upregulated by AMPH.
Figure 6. Effects of AMPH on GluA1-S845 phosphorylation in the rat mPFC.
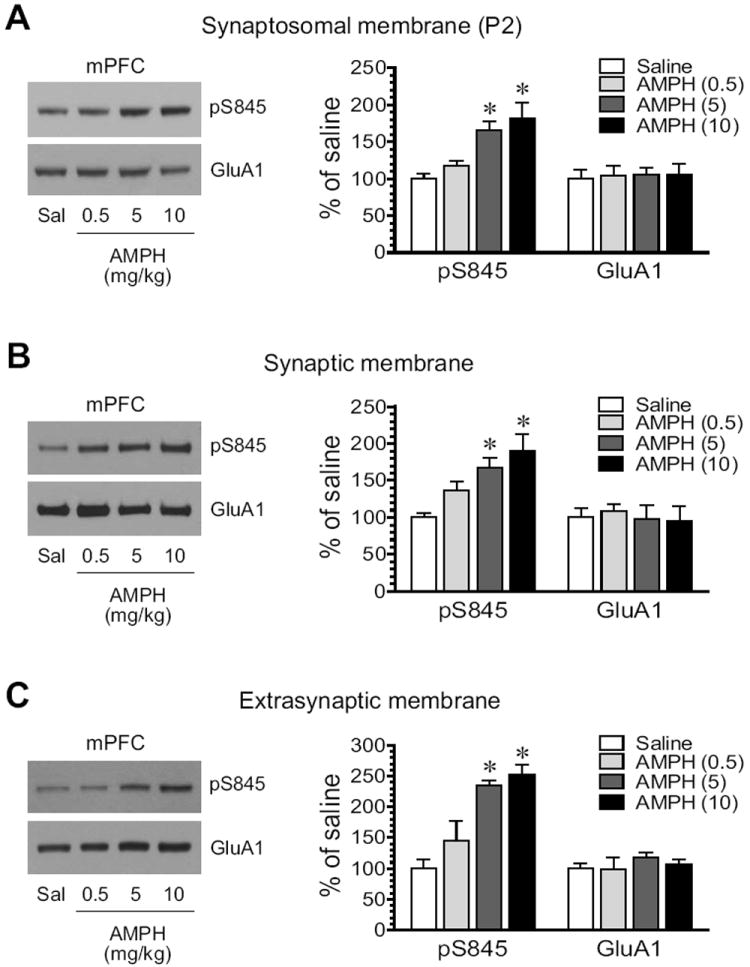
(A) Effects of AMPH on S845 phosphorylation in synaptosomal fractions. (B) Effects of AMPH on S845 phosphorylation in synaptic fractions. (C) Effects of AMPH on S845 phosphorylation in extrasynaptic fractions. Note that AMPH produced overall increases in pS845 proteins in all three membrane preparations. Representative immunoblots are shown left to the quantified data. Rats received a single dose of AMPH (0.5, 5, or 10 mg/kg, i.p.) and were sacrificed 15 min after drug injection for immunoblot analysis. Data are presented as means ± S.E.M. (n = 4 per group). * P < 0.05 versus saline.
4. Discussion
This study reflects an effort to expand the knowledge on specific subsynaptic pools of GluA1 and their phosphorylation in response to AMPH stimulation. Synaptic and extrasynaptic membranes were therefore enriched by a pre-validated method. It was found that total and phosphorylated GluA1 (pS831 and pS845) was largely concentrated at synaptic sites in striatal and cortical neurons. Acute AMPH administration increased S845 phosphorylation in both synaptic and extrasynaptic membranes in the striatum. This increase was dose-dependent and was not region-selective because the similar overall increase was also seen in the mPFC. However, S831 phosphorylation was differentially regulated. In the striatum, synaptic and extrasynaptic S831 phosphorylation was not altered. Only extrasynaptic S831 phosphorylation in the mPFC was significantly elevated by AMPH, whereas synaptic S831 phosphorylation showed a declining trend. These data confirm the sensitivity of S845 in synaptic and extrasynaptic locations in response to dopamine stimulation. In addition, mPFC S831 at the extrasynaptic site is vulnerable to AMPH.
Phosphorylation of AMPA receptors is an important posttranslational mechanism underlying the regulation of the receptor. Phosphorylation of AMPA receptors itself is also regulated by many extracellular and intracellular signals (Derkach et al., 1999; Banke et al., 2000). Dopamine stimulation with either direct or indirect agonists has been demonstrated to stimulate AMPA receptor phosphorylation (Wang et al., 2006). For instance, the direct D1 dopamine receptor agonist SKF81297 or SKF38393 enhanced GluA1 S845 although not S831 phosphorylation in striatal or cortical cultures or slices (Price et al., 1999; Snyder et al., 2000; Chao et al., 2002b; Swayze et al., 2004). Stimulants are another group of agents effective in stimulating S845 phosphorylation. An acute systemic injection of cocaine and methamphetamine or a local injection of AMPH into the NAc increased S845 but not S831 phosphorylation in the striatum in vivo (Snyder et al., 2000; Li et al., 2011). Chronic cocaine that caused behavioral sensitization enhanced pS845 but not pS831 levels in the NAc (Schierberl et al., 2011). Cocaine self-administration increased S845 phosphorylation in the NAc (Ferrario et al., 2011b) and S845 but not S831 phosphorylation in the ventral tegmental area (VTA) (Choi et al., 2011). These data identify the linkage of dopamine to GluA1 phosphorylation at S845. However, early studies have used the homogenate or synaptosome for immunoblot analysis. In this study, we expanded the study to using enriched synaptic and extrasynaptic membranes to detect GluA1 phosphorylation in defined subsynaptic microdomains. We found that AMPH induced a comparable increase in pS845 levels in synaptic and extrasynaptic fractions, indicating that S845 remains a sensitive site and is consistently upregulated by AMPH at both subsynaptic sites. In contrast, S831 phosphorylation was differentially regulated by AMPH, depending upon doses of AMPH, subsynaptic sites, and brain regions surveyed (see below).
While pS845 proteins were mainly expressed at synaptic sites, a fair amount of pS845 was present at extrasynaptic sites in striatal neurons (Ferrario et al., 2011b; this study). In the mPFC, pS845 expression at extrasynaptic sites was relatively less (this study). S845 is phosphorylated by PKA (Roche et al., 1996). Thus, activation of the D1/cAMP/PKA pathway was responsible for the dopamine-stimulated S845 phosphorylation in the striatum (Snyder et al., 2000; Chao et al., 2002b; Mangiavacchi et al., 2004). In addition, inhibition of protein phosphatase 1/2A contributes to the dopamine effect (Snyder et al., 2000). Enhanced phosphorylation at the PKA site (S845) is believed to drive surface trafficking of GluA1 (Chao et al., 2002a; Mangiavacchi et al., 2004; Man et al., 2007; Sun et al., 2008) likely onto extrasynaptic sites (Sun et al., 2005; Oh et al., 2006; Gao et al., 2006), and thereby facilitate insertion (or lateral diffusion from extrasynaptic locations) of GluA1/AMPA receptors to synapses (Esteban et al., 2003; Sun et al., 2005) and potentiate the peak current of AMPA receptor channels (Roche et al., 1996). Consistent with this notion, increased surface expression of GluA1 in the NAc was associated with an increase in surface and extrasynaptic pools of pS845 in rats self-administering cocaine (Ferrario et al., 2011a). Both GluA1 and pS845 were elevated in the VTA in cocaine self-administering rats. Overexpression of PKA-resistant GluA1 (S845A) in the VTA reduced cocaine self-administration (Choi et al., 2011).
Compared to S845, S831 is significantly less sensitive to stimulants as acute, chronic and self-administration of cocaine did not affect S831 phosphorylation in striatal or cortical neurons (see above). In this study, AMPH induced no significant change in S831 phosphorylation in either the synaptic or extrasynaptic pool in the striatum. However, cocaine challenge that elicited expression of behavioral sensitization elevated S831 phosphorylation in the NAc of chronic cocaine-pretreated mice (Schierberl et al., 2011). As a residue selectively phosphorylated by CaMKII in addition to PKC, CaMKII mediates the effect of cocaine on S831 phosphorylation and ultimately contributes to increased surface expression of GluA1 in the sensitized rats (Schierberl et al., 2011). In the mPFC, we observed an increase in extrasynaptic pS831 expression in AMPH-treated rats, which was accompanied by an apparent trend of a small decrease in synaptic pS831 (Fig. 5B). However, a high dose of AMPH (10 mg/kg) was required to produce these effects. The cause for the difference in pS831 responses to AMPH between the mPFC and the striatum is unclear. It can only be assumed that the difference in transmitter systems controlling activities of mPFC and striatal neurons may result in differential responses of pS831 in these regions to AMPH.
Acknowledgments
This work was supported by NIH grants R01DA10355 (JQW) and R01MH61469 (JQW). We thank Dr. Bing Xue for his technical assistance.
Footnotes
Conflict of interest: The authors declare that there are no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banke TG, Bowie D, Lee HK, Huganir RL, Schousboe A, Traynelis SF. Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J Neurosci. 2000;20:89–102. doi: 10.1523/JNEUROSCI.20-01-00089.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, Derkach V, Soderling T. Identification of the Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in the α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate-type glutamate receptor. J Biol Chem. 1997;272:32727–32730. doi: 10.1074/jbc.272.52.32727. [DOI] [PubMed] [Google Scholar]
- Bernard V, Somogyi P, Bolam JP. Cellular, subcellular, and subsynaptic distribution of AMPA-type glutamate receptor subunits in the neostriatum of the rats. J Neurosci. 1997;17:819–833. doi: 10.1523/JNEUROSCI.17-02-00819.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao SZ, Ariano MA, Peterson DA, Wolf ME. D1 dopamine receptor stimulation increases GluR1 phosphorylation in nucleus accumbens neurons. J Neurochem. 2002a;83:704–712. doi: 10.1046/j.1471-4159.2002.01164.x. [DOI] [PubMed] [Google Scholar]
- Chao SZ, Lu W, Lee HK, Huganir RL, Wolf ME. D(1) dopamine receptor stimulation increases GluR1 phosphorylation in postnatal nucleus accumbens cultures. J Neurochem. 2002b;81:984–992. doi: 10.1046/j.1471-4159.2002.00877.x. [DOI] [PubMed] [Google Scholar]
- Choi KH, Edwards S, Graham DL, Larson EB, Whisler KN, Simmons D, Friedman AK, Walsh JJ, Rahman Z, Monteggia LM, Eisch AJ, Neve RL, Nestler EJ, Han MH, Self DW. Reinforcement-treated regulation of AMPA glutamate receptor subunits in the ventral tegmental area enhances motivation for cocaine. J Neurosci. 2011;31:7927–7937. doi: 10.1523/JNEUROSCI.6014-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KD, Alvestad RM, Coultrap SJ, Browning MD. αCaMKII autophosphorylation levels differ depending on subcellular localization. Brain Res. 2007;1158:39–49. doi: 10.1016/j.brainres.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KD, Goebel-Goody SM, Coultrap SJ, Browning MD. Long-term synaptic depression that is associated with GluR1 dephosphorylation but not amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor internalization. J Biol Chem. 2008;283:33138–33146. doi: 10.1074/jbc.M803431200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkach V, Barria A, Soderling TR. Ca2+/calmodulin-kinase II enhances channel conductance of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proc Natl Acad Sci USA. 1999;96:3269–3274. doi: 10.1073/pnas.96.6.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estaban JA, Shi H, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci. 2003;6:136–143. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- Ferrario CR, Loweth JA, Milovanovic M, Ford KA, Galinanes GL, Heng LJ, Tseng KY, Wolf ME. Alterations in AMPA receptor subunits and TARPs in the rat nucleus accumbens related to the formation of Ca2+-permeable AMPA receptors during the incubation of cocaine craving. Neuropharmacology. 2011a;61:1141–1151. doi: 10.1016/j.neuropharm.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario C, Loweth JA, Milovanovic M, Wang X, Wolf ME. Distribution of AMPA receptor subunits and TARPs in synaptic and extrasynaptic membranes of the adult rat nucleus accumbens. Neurosci Lett. 2011b;490:180–184. doi: 10.1016/j.neulet.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Sun X, Wolf ME. Activation of D1 dopamine receptors increases surface expression of AMPA receptors and facilitates their synaptic incorporation in cultured hippocampal neurons. J Neurochem. 2006;98:1664–1677. doi: 10.1111/j.1471-4159.2006.03999.x. [DOI] [PubMed] [Google Scholar]
- Goebel-Goody SM, Davies KD, Alvestad Linger RM, Freund RK, Browning MD. Phospho-regulation of synaptic and extrasynaptic N-methyl-D-aspartate receptors in adult hippocampal slices. Neuroscience. 2009;158:1146–1159. doi: 10.1016/j.neuroscience.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Hu HJ, Chen LW, Yung KK, Chan YS. Differential expression of AMPA receptor subunits in substance P receptor-containing neurons of the caudate-putamen of rats. Neurosci Res. 2004;49:281–288. doi: 10.1016/j.neures.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Kondo M, Okabe S, Sumino R, Okado H. A high GluR1:GluR2 expression ratio is correlated with expression of Ca2+-binding proteins in rat forebrain neurons. Eur J Neurosci. 2000;12:2812–2822. doi: 10.1046/j.1460-9568.2000.00167.x. [DOI] [PubMed] [Google Scholar]
- Li D, Herrera S, Bubula N, Nikitina E, Palmer AA, Hanck DA, Loweth JA, Vezina P. Casein kinase 1 enables nucleus accumbens amphetamine-induced locomotion by regulating AMPA receptor phosphorylation. J Neurochem. 2011;118:237–247. doi: 10.1111/j.1471-4159.2011.07308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammen AL, Kameyama K, Roche KW, Huganir RL. Phosphorylation of the alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II. J Biol Chem. 1997;272:32528–32533. doi: 10.1074/jbc.272.51.32528. [DOI] [PubMed] [Google Scholar]
- Man HY, Sekine-Aizawa Y, Huganir RL. Regulation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. Proc Natl Acad Sci USA. 2007;104:3579–3584. doi: 10.1073/pnas.0611698104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiawacchi S, Wolf ME. D1 dopamine receptor stimulation increases the rate of AMPA receptor insertion onto the surface of cultured nucleus accumbens neurons through a pathway dependent on protein kinase A. J Neurochem. 2004;88:1261–1271. doi: 10.1046/j.1471-4159.2003.02248.x. [DOI] [PubMed] [Google Scholar]
- Mao LM, Guo ML, Jin DZ, Fibuch EE, Choe ES, Wang JQ. Posttranslational modification biology of glutamate receptors and drug addiction. Front Neuroanat. 2011;5:19. doi: 10.3389/fnana.2011.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao LM, Reusch JM, Fibuch EE, Liu Z, Wang JQ. Amphetamine increases phosphorylation of MAPK/ERK at synaptic sites in the rat striatum and medial prefrontal cortex. Brain Res. 2013;1494:101–108. doi: 10.1016/j.brainres.2012.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MC, Derkach VA, Guire ES, Soderling TR. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J Biol Chem. 2006;281:752–758. doi: 10.1074/jbc.M509677200. [DOI] [PubMed] [Google Scholar]
- Price CJ, Kim P, Raymond LA. D1 dopamine receptor-induced cyclic AMP-dependent protein kinase phosphorylation and potentiation of striatal glutamate receptors. J Neurochem. 1999;73:2441–2446. doi: 10.1046/j.1471-4159.1999.0732441.x. [DOI] [PubMed] [Google Scholar]
- Reimers JM, Milovanovic M, Wolf ME. Quantitative analysis of AMPA receptor subunit composition in addiction-related brain regions. Brain Res. 2011;1367:223–233. doi: 10.1016/j.brainres.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KW, O’Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- Schierberl K, Hao J, Tropea TF, Ra S, Giordano TP, Xu Q, Garraway SM, Hofmann F, Moosmang S, Striessnig J, Inturrisi CE, Rajadhyaksha AM. Cav1.2 L-type Ca2+ channels mediate cocaine-induced GluA1 trafficking in the nucleus accumbens, a long-term adaptation dependent on ventral tegmental area Ca(v)1.3 channels. J Neurosci. 2011;31:13562–13575. doi: 10.1523/JNEUROSCI.2315-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serulle Y, Zhang S, Ninan I, Puzzo D, McCarthy M, Khatri L, Arancio O, Ziff EB. A GluR1-cGKII interaction regulates AMPA receptor trafficking. Neuron. 2007;56:670–688. doi: 10.1016/j.neuron.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder GL, Allen PB, Fienberg AA, Valle CG, Huganir RL, Nairn AC, Greengard P. Regulation of phosphorylation of the GluR1 AMPA receptor in the neostriatum by dopamine and psychostimulants in vivo. J Neurosci. 2000;20:4480–4488. doi: 10.1523/JNEUROSCI.20-12-04480.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steketee JD. Neurotransmitter systems of the medial prefrontal cortex: potential role in sensitization to psychostimulants. Brain Res Rev. 2003;41:203–228. doi: 10.1016/s0165-0173(02)00233-3. [DOI] [PubMed] [Google Scholar]
- Sun X, Milovanovic M, Zhao Y, Wolf ME. Acute and chronic dopamine receptor stimulation modulates AMPA receptor trafficking in nucleus accumbens neurons cocultured with prefrontal cortex neurons. J Neurosci. 2008;28:4216–4230. doi: 10.1523/JNEUROSCI.0258-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Zhao Y, Wolf ME. Dopamine receptor stimulation modulates AMPA receptor synaptic insertion in prefrontal cortex neurons. J Neurosci. 2005;25:7342–7351. doi: 10.1523/JNEUROSCI.4603-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swayze RD, Lise MF, Levinson JN, Phillips A, El-Husseini A. Modulation of dopamine mediated phosphorylation of AMPA receptors by PSD-95 and AKAP79/150. Neuropharmacology. 2004;47:764–778. doi: 10.1016/j.neuropharm.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Van den Oever MC, Spijker S, Smit AB, De Vries TJ. Prefrontal cortex plasticity mechanisms in drug seeking and relapse. Neurosci Biobehav Rev. 2010;35:276–284. doi: 10.1016/j.neubiorev.2009.11.016. [DOI] [PubMed] [Google Scholar]
- Wang JQ, Liu X, Zhang G, Parelkar NK, Arora A, Haines M, Fibuch EE, Mao L. Phosphorylation of glutamate receptors: a potential mechanism for the regulation of receptor function and psychostimulant action. J Neurosci Res. 2006;84:1621–1629. doi: 10.1002/jnr.21050. [DOI] [PubMed] [Google Scholar]
- Wang JQ, McGinty JF. Dose-dependent alteration in zif/268 and preprodynorphin mRNA expression induced by amphetamine or methamphetamine in rat forebrain. J Pharmacol Exp Ther. 1995;273:909–917. [PubMed] [Google Scholar]
- Wolf ME, Ferrario CR. AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci Biobehav Rev. 2010;35:185–211. doi: 10.1016/j.neubiorev.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]


