Abstract
Background
We sought to characterize patient factors and regional variations associated with vitamin K antagonist (VKA) use in patients with heart failure (HF) and atrial fibrillation (AF) in areas outside the United States and Europe.
Methods
The ADHERE-International registry enrolled patients with decompensated HF from 10 Asia-Pacific and Latin American countries from December 2005 to January 2009. Rates of VKA use in patients with HF and either new onset AF or a history of AF were determined and compared according to CHADS2 scores. Multivariable logistic regression and hierarchical modeling with random effects for hospitals were used to determine clinical and regional factors associated with VKA use at discharge.
Results
Among 9706 admissions, there were 2358 (24.3%) with prior AF and 674 (6.9%) with new onset AF. The median age was 71 years (25th, 75th percentiles [59, 79]) for prior AF and 69 (57, 80) for new-onset AF patients. The overall rate of warfarin use at discharge was 39.5%. Warfarin use at discharge was 36.2% in patients with CHADS2 scores ≥2 versus 50.2% in patients with CHADS2 scores =1 (P<0.0001). VKA use was 36.4% in patients with hypertension, 28.1% in patients >75 years old, 34.8% in diabetics, and 44.4% in those with prior stroke/transient ischemic attack. After adjusting for patient characteristics, the highest and lowest rates of anticoagulation were in Australia (65.2%) and Taiwan (25.1%), respectively.
Conclusion
International use of guidelines-recommended anticoagulation in HF patients with AF varies significantly across countries and represents an important opportunity for improving quality of care.
Atrial fibrillation (AF) is the most common cardiac rhythm disturbance, affecting 2.3 million Americans and 4.5 million Europeans.1,2 The development of AF in patients with heart failure (HF) is common with an incidence ranging from 2–5% per year and a prevalence as high as 50% in patients with New York Heart Association (NYHA) functional class IV symptoms.3 These patients commonly have diminished quality of life and functional limitations.3,4 Furthermore, patients with AF and HF have an increased long-term risk of stroke5 leading to increased morbidity, mortality, and health care costs.6 As a result, the European Society of Cardiology (ESC) guidelines for the management of AF recommend that all HF patients with a history of paroxysmal or persistent AF and a CHADS2 score ≥2 be anticoagulated with a vitamin K antagonist (VKA).7 The 2005 American College of Cardiology/American Heart Association Clinical Performance Measures for Adults with Chronic Heart Failure included a performance measure for anticoagulant use at discharge in patients with chronic/recurrent AF and no contraindications.8 Therefore, VKA therapy in patients with AF and HF is also considered a measure of quality of care.
Despite these recommendations, the use of anticoagulation in HF patients with AF varies in clinical practice. In the United States, only two-thirds of patients with AF who were discharged from a HF hospitalization were discharged on anticoagulation and, paradoxically, the likelihood of anticoagulation was inversely related to the risk of stroke as assessed by CHADS2 score.9 Similarly, in Europe, one-third of patients with HF and AF were not anticoagulated with a VKA despite the lack of contraindications.10
Although the rates of anticoagulation and patient characteristics for AF among patients discharged from a HF hospitalization have been well characterized in the United States and Europe, much less is known about other areas of the world. The use of warfarin in AF patients from 44 countries (in Europe, North and South America, and Asia) with atherothrombotic disease has been reported to be 53%.11 Recent studies revealed the rate of warfarin use in AF patients in Japan to be as high as 87%,12 whereas the rate in Malaysia was only 16%.13 These regional differences warrant better characterization and attention.
As awareness of global cardiovascular health and the emergence of new agents for thromboembolic prophylaxis increase, it is important to understand how anticoagulation is used in all areas of the world. We sought to determine the clinical characteristics and regional variations in the use of warfarin in patients hospitalized with HF and AF in the ADHERE-International registry.
Methods
Data collection
Data were collected retrospectively through the ADHERE-International registry, which consists of approximately 70 hospitals from the Asia-Pacific region and Latin America (Australia, Hong Kong, Indonesia, Malaysia, Philippines, Singapore, Taiwan, Thailand, Brazil, and Mexico) ranging in size from 130 to 2908 hospital beds (mean=812). The registry includes adult inpatients with a principal admission diagnosis of acute decompensated HF (identified by International Classification of Disease-9/10 code). Each site received approval for the study by the local institutional human research ethics committee, and patient medical records were reviewed by trained research personnel from the individual hospitals. The data collection system did not require individual patient informed consent, and our study was in compliance with the Declaration of Helsinki. We analyzed data on 9706 admissions with a primary diagnosis of HF from December 2005 to January 2009 and characterized the clinical and regional variations in the use of warfarin in patients hospitalized with HF and AF.
Study population
Eligible patients were ≥18 years of age and hospitalized with a primary diagnosis of HF. History of AF was coded in the registry (with or without AF on admission), and new onset AF was defined as a patient with AF findings on the initial electrocardiogram without a documented history of AF.
Outcome measures
The primary outcome was warfarin use at discharge among eligible patients with a history of AF or new onset AF, without documented contraindications or intolerance. Patients who were discharged to hospice, left against medical advice, transferred out, or died were excluded. The study population for warfarin use comprised 2750 patients from 66 hospitals.
Statistical analysis
Patient sociodemographic factors, baseline characteristics, in-hospital procedures, and discharge quality of care measures were compared between patients with a history of AF, patients with new onset AF, and patients with no AF. Medians with 25th and 75th percentiles and percentages were used to describe the distribution of continuous and categorical variables, respectively. The 3 groups of patients were compared using the Pearson’s chi-square test for categorical variables and the Kruskal-Wallis test for continuous variables. Multivariable logistic regression analyses were performed to explore the patient characteristics independently associated with warfarin use at discharge. Candidate variables in the initial model included age, sex, chronic obstructive pulmonary disease, diabetes mellitus, dyslipidemia, hypertension, peripheral vascular disease, coronary artery disease, prior stroke, prior myocardial infarction, anemia, heart failure, renal insufficiency, implantable cardioverter defibrillator placement, pacemaker, dialysis, current smoker, presence of congestion on first X-ray, dyspnea, fatigue, rales, edema, heart rate, systolic blood pressure, respiratory rate, sodium, potassium, creatinine, hemoglobin, and blood urea nitrogen. The final model was selected based on statistical significance and clinical experience. Secondary analyses included warfarin use among eligible patients based on stroke risk according to CHADS2 score14 (congestive heart failure, hypertension, age >75 years, diabetes mellitus, and prior history of stroke/transient ischemic attack [TIA]) and whether differences exist in warfarin use between the regions. In addition, we ran a hierarchical model that treated hospitals as random effects to adjust the warfarin use for patient characteristics. We also calculated the country-specific adjusted VKA use rate using the observed rate divided by the expected rate ratio for each country. All P values were 2-sided, and a P value <0.05 was considered to be statistically significant. All analyses were performed using SAS software (Version 9.2, SAS Institute Inc., Cary, NC).
RESULTS
Baseline characteristics
Among 9706 admissions with a primary diagnosis of HF, 2358 (24.3%) patients had a prior history of AF, 674 (6.9%) had new onset AF, and 6674 (68.8%) had no AF. As shown in Table 1, the median age of patients with prior AF was 71 years (25th, 75th [59,79]), patients with new onset AF was 69 years (57, 80), and patients with no AF was 66 years (55, 75) (P<0.0001). Patients with prior AF were more likely to have a history of stroke/TIA (17.4% vs. 10.5% vs. 11.01%; P<0.0001). Patients with prior AF were also more likely to have peripheral vascular disease (7.5% vs. 2.8% vs. 5.7%; P<0.0001) and chronic renal insufficiency with a serum creatinine >2.0 (24.2% vs. 14.1% vs. 22.7%; P<0.0001).
Table 1.
Patient admission and in-hospital characteristics according to the onset of atrial fibrillation
| Characteristic | History of AF (n=2358) |
New onset AF (n=674) |
No AF (n=6674) |
P Value |
|---|---|---|---|---|
| Age, median (25th,75th), yrs | 71 (59, 79) | 69 (57, 80) | 66 (55, 75) | <0.0001 |
| Male, % | 55.3 | 55.0 | 59.4 | 0.0006 |
| Race/ethnicity, % | <0.0001 | |||
| White | 17.5 | 6.2 | 6.8 | |
| Black | 0.7 | 0.7 | 0.7 | |
| Asian | 45.5 | 58.0 | 47.0 | |
| Hispanic | 0.6 | 0.7 | 1.2 | |
| ATSI | 0.1 | 0.0 | 0.0 | |
| Chinese | 20.6 | 22.6 | 22.3 | |
| Malay | 8.5 | 5.9 | 10.7 | |
| Indian | 2.8 | 2.2 | 7.2 | |
| Eurasian | 0.2 | 0.3 | 0.3 | |
| Systolic blood pressure, median (25th, 75th), mm Hg | 129 (110, 149) | 131 (112, 155) | 135 (114, 159) | <0.0001 |
| Heart rate, median (25th, 75th), bpm | 89 (74, 109) | 97 (80, 120) | 90 (77, 103) | <0.0001 |
| LVEF, median (25th, 75th), % | 43 (29, 58) | 43 (29, 58) | 34 (24, 50) | <0.0001 |
| LVEF <40%, % | 39.0 | 37.7 | 51.4 | <0.0001 |
| History of, % | ||||
| Anemia | 49.8 | 42.9 | 51.2 | 0.0002 |
| Stroke/TIA | 17.4 | 10.5 | 11.0 | <0.0001 |
| Renal insufficiency–chronic, SCr>2.0 | 24.2 | 14.1 | 22.7 | <0.0001 |
| Dialysis | 1.1 | 0.7 | 1.3 | 0.3544 |
| Diabetes mellitus | 34.1 | 28.8 | 50.5 | <0.0001 |
| Hyperlipidemia | 38.6 | 27.3 | 45.5 | <0.0001 |
| Hypertension | 60.3 | 56.5 | 67.5 | <0.0001 |
| ICD | 2.5 | 0.3 | 1.7 | 0.0004 |
| Peripheral vascular disease | 7.5 | 2.8 | 5.7 | <0.0001 |
| Ischemic heart disease | 44.4 | 32.6 | 52.5 | <0.0001 |
| Pulmonary disease | 15.8 | 10.7 | 12.0 | <0.0001 |
| Smoking | 6.7 | 10.2 | 11.5 | <0.0001 |
| Previously listed for cardiac transplant | 3.0 | 0.5 | 0.9 | <0.0001 |
| Prior MI | 24.6 | 15.0 | 28.7 | <0.0001 |
| Initial laboratory data, median (25th, 75th) | ||||
| BNP, pg/mL | 944 (560, 2090) | 1661 (864, 2605) | 1644 (885, 3154) | <0.0001 |
| N-terminal pro-BNP, pg/mL | 3823 (1699, 10032) | 3481 (1741, 6050) | 4347 (1515, 9858) | 0.6771 |
| Blood urea nitrogen, mg/dL | 18 (10, 32) | 17 (10, 26) | 17 (10, 30) | 0.0770 |
| Creatinine, mg/dL | 1.3 (1.0, 1.8) | 1.2 (1.0, 1.6) | 1.3 (1.0, 1.9) | <0.0001 |
| Hemoglobin, g/dL | 12.4 (11.0, 13.8) | 12.8 (11.4, 14.1) | 12.4 (10.7, 14.0) | <0.0001 |
| Sodium, mEq/L | 138 (134, 140) | 137 (134, 140) | 138 (134, 140) | 0.2745 |
| In-hospital procedures, % | ||||
| Defibrillation | 2.0 | 2.6 | 1.3 | 0.0072 |
| CPR | 2.7 | 3.0 | 2.9 | 0.8385 |
| Defibrillation or CPR | 3.3 | 3.8 | 3.2 | 0.7491 |
| Cardiac catheterization | 6.3 | 6.7 | 9.2 | <0.0001 |
| Discharge QOC measures, % | ||||
| HF patients with LVEF assessed in hospital or plan post-discharge | 88.8 | 81.8 | 84.7 | <0.0001 |
| HF patients with LVSD discharged on ACE or ARB | 69.3 | 68.6 | 76.0 | <0.0001 |
| HF patients with smoking history discharge with smoking cessation | 17.1 | 19.8 | 25.8 | 0.0114 |
ACE indicates angiotensin-converting enzyme; AF, atrial fibrillation; ARB, angiotensin receptor blocker; ATSI, Aboriginal and Torres Strait Islanders; BNP, B-type natriuretic peptide; bpm, beats per minute; CPR, cardiopulmonary resuscitation; HF, heart failure; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; LVSD, left ventricular systolic dysfunction; MI, myocardial infarction; QOC, quality-of-care; TIA, transient ischemic attack.
Patients with new onset AF had higher heart rates (97 beats/minute [80, 120]) than those with prior AF (89 beats/minute [74, 109]) and those with no AF (90 beats/minute [77, 103]; P<0.0001), and were less likely to have a history of ischemic heart disease (32.6% vs. 44.4% vs. 52.5%; P<0.0001) or pulmonary disease (10.7% vs. 15.8% vs. 12.0%; P<0.0001). Patients with new onset AF had a lower incidence of left ventricular systolic dysfunction (37.7% vs. 39.0% vs. 51.4 %) defined as a left ventricular ejection fraction <40%.
Warfarin use at discharge
Among the 2750 HF admissions with any AF (prior history or new onset), the overall rate of warfarin use at discharge was 39.5%. There were 37 patients (1.3%) with contraindications to warfarin use who were not included in our analyses. Among eligible patients, the median use of warfarin therapy at discharge was 37.8% (20.0%, 55.2%) across hospitals, which was similar in the Asia-Pacific region (39.7%) and in Latin America (37.2%). In the univariate analysis, we found that patients who were older and had a history of anemia, diabetes mellitus, hypertension, and ischemic heart disease were less likely to be discharged on warfarin (Table 2). As shown in Table 3, our multivariable logistic regression model identified prior stroke and history of implantable cardioverter defibrillator and pacemaker implantation as factors independently associated with an increased risk of being discharged on warfarin. There was less warfarin use in older patients with a prior history of coronary artery disease and worsening renal function as measured by serum creatinine levels during this hospitalization. After adjusting for patient characteristics, the country with the highest warfarin use was Australia (65.2%). The country with both the lowest unadjusted (18.3%) and adjusted (25.1%) warfarin use was Taiwan (Table 4).
Table 2.
Univariate associations with discharge warfarin use among patients with heart failure and atrial fibrillation
| Characteristic | Warfarin (n=1087)* |
No Warfarin (n=1663)* |
P Value |
|---|---|---|---|
| Age, median (25th,75th), yrs | 66 (56, 76) | 73 (61, 81) | <0.0001 |
| Male, % | 57 | 54 | 0.1085 |
| Race/ethnicity, % | <0.0001 | ||
| White | 20.9 | 10.2 | |
| Black | 0.55 | 0.48 | |
| Asian | 46.1 | 49.6 | |
| Hispanic | 0.9 | 0.4 | |
| ATSI | 0.0 | 0.2 | |
| Chinese | 16.8 | 25.7 | |
| Malay | 9.0 | 7.4 | |
| Indian | 2.5 | 2.7 | |
| Eurasian | 0.2 | 0.3 | |
| Systolic blood pressure, median (25th,75th), mm Hg | 127 (109, 148) | 130 (112, 151) | <0.0001 |
| Heart rate, median (25th,75th), bpm | 89 (73, 110) | 90 (75, 110) | 0.2411 |
| LVEF, median (25th,75th), % | 41 (28, 57) | 44 (30, 58) | 0.2674 |
| LVEF < 40%, % | 40.2 | 37.3 | 0.6313 |
| History of, % | |||
| Anemia | 45.0 | 49.6 | 0.0153 |
| Stroke/TIA | 18.1 | 14.9 | 0.0227 |
| Renal insufficiency–chronic, SCr>2.0 | 20.9 | 21.4 | 0.7710 |
| Dialysis | 0.6 | 0.8 | 0.3818 |
| Diabetes mellitus | 29.2 | 35.7 | 0.0004 |
| Hyperlipidemia | 37.4 | 37.2 | 0.9456 |
| Hypertension | 54.9 | 62.8 | <0.0001 |
| ICD | 3.9 | 1.0 | <0.0001 |
| Peripheral vascular disease | 6.5 | 6.6 | 0.9812 |
| Ischemic heart disease | 38.0 | 44.5 | 0.0007 |
| Pulmonary disease | 13.0 | 16.2 | 0.0211 |
| Previously listed for cardiac transplant | 4.2 | 1.1 | <0.0001 |
| Prior MI | 20.7 | 23.4 | 0.0974 |
| Initial laboratory data, median (25th,75th) | |||
| BNP, pg/mL | 875 (542, 1768) | 1316 (691, 2519) | 0.0255 |
| N-terminal pro-BNP, pg/mL | 2705 (1327, 5970) | 4321 (1868, 11582) | 0.0141 |
| BUN, mg/dL | 16 (10, 25) | 18 (10, 30) | 0.0098 |
| Creatinine, mg/dL | 1.24 (1.0, 1.6) | 1.3 (1.0, 1.8) | 0.0045 |
| Hemoglobin, g/dL | 12.7 (11.3, 14) | 12.4 (11.0, 13.9) | 0.0011 |
| Sodium, mEq/L | 138 (134, 140) | 138 (134, 141) | 0.6842 |
| In-hospital procedures, % | |||
| Defibrillation | 0.74 | 1.38 | 0.1161 |
| CPR | 0.55 | 0.9 | 0.3026 |
| Defibrillation or CPR | 0.92 | 1.56 | 0.1467 |
| Cardiac catheterization | 7.64 | 5.41 | 0.0189 |
Eligible HF patients with history of or new-onset AF who did not have contraindications. A total of 282 patients were excluded due to missing values, transfer out, LAMA, hospice, or death.
ATSI indicates Aboriginal and Torres Strait Islanders; BNP, B-type natriuretic peptide; bpm, beats per minute; BUN, blood urea nitrogen; CPR, cardiopulmonary resuscitation; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; MI, myocardial infarction; TIA, transient ischemic attack.
Table 3.
Multivariate factors associated with warfarin use at discharge in patients with heart failure and atrial fibrillation
| Variable | OR | 95% CI | Chi-Square | P Value |
|---|---|---|---|---|
| Age (per 10 yr increase) | 0.78 | 0.71–0.86 | 25.0 | <0.0001 |
| CAD* | 0.81 | 0.68–0.97 | 5.3 | 0.0212 |
| Stroke* | 1.41 | 1.13–1.75 | 9.2 | 0.0025 |
| ICD* | 1.64 | 1.07–2.53 | 5.1 | 0.0237 |
| Pacemaker* | 1.47 | 1.12–1.93 | 7.8 | 0.0053 |
| Creatinine (per 0.1 unit increase)† | 0.98 | 0.97–1.00 | 7.0 | 0.0083 |
Past history.
From admission blood test.
CAD indicates coronary artery disease; CI, confidence interval; ICD, implantable cardioverter defibrillator; OR, odds ratio.
Table 4. Rates for warfarin use at discharge in patients with heart failure and atrial fibrillation.
| Country | Unadjusted Rate (%) |
Risk-Adjusted Rate* (%) |
OR (95% CI) |
P Value |
|---|---|---|---|---|
| Mexico | 60.0 | 48.6 | 2.34 (1.2–4.59) | 0.0132 |
| Australia | 59.4 | 65.2 | 3.74 (2.24–6.27) | <0.0001 |
| Hong Kong | 43.0 | 52.5 | 1.99 (1.28–3.10) | <0.0021 |
| Indonesia | 36.2 | 34.3 | 0.88 (0.37–2.06) | 0.76 |
| Malaysia | 50.8 | 52.9 | 2.08 (1.31–3.30) | 0.0018 |
| Philippines | 30.3 | 25.5 | 0.52 (0.33–0.84) | <0.0076 |
| Singapore | 30.2 | 35.8 | 1.01 (0.64–1.59) | 0.96 |
| Taiwan | 18.3 | 25.1 | 0.62 (0.36–1.04) | 0.0721 |
| Thailand | 49.4 | 49.4 | 1.93 (1.18–3.15) | 0.0086 |
| Brazil | 34.4 | 37.5 | Reference | -- |
Adjusted for age, coronary artery disease, pacemaker, and creatinine levels.
CI indicates confidence interval; OR, odds ratio.
Risk stratification and warfarin use
In order to examine the relationship between stroke risk factors and warfarin use, we analyzed warfarin use according to CHADS2 scores. There was significant risk-treatment mismatch between low-risk (CHADS2 score =1) and higher-risk (CHADS2 score ≥2) patients, with greater warfarin use in the low-risk patients (P value <0.0001 for trend). Figure 1 depicts the rates of warfarin use at discharge in low (=1) versus higher (≥2) CHADS2 scores by country. Among patients with HF as their only risk factor, 50.6% were discharged on warfarin compared with 38.5% of patients with a CHADS2 score of 6. The lowest rate of warfarin use was 30.5% in patients with a CHADS2 score of 4 (Figure 2). Furthermore, aspirin use was significantly greater in higher-risk patients (42.2%) compared with low-risk patients (29.8%). Finally, the anticoagulation rates were 36.4% in patients with a history of hypertension, 28.1% in patients >75 years of age, 34.8% in patients with diabetes mellitus, and 44.4% in patients with a history of stroke/TIA.
Figure 1.
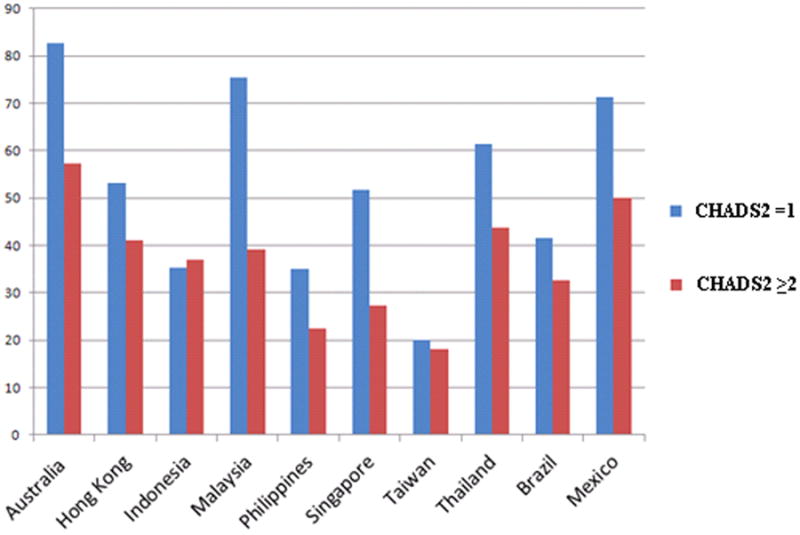
Warfarin use in low (=1) versus high (≥2) CHADS2 scores by country. Across all countries, HF patients with higher risk of stroke received less anticoagulation with warfarin than lower-risk patients. The only exception was Indonesia, where higher-risk patients received more warfarin.
Figure 2.
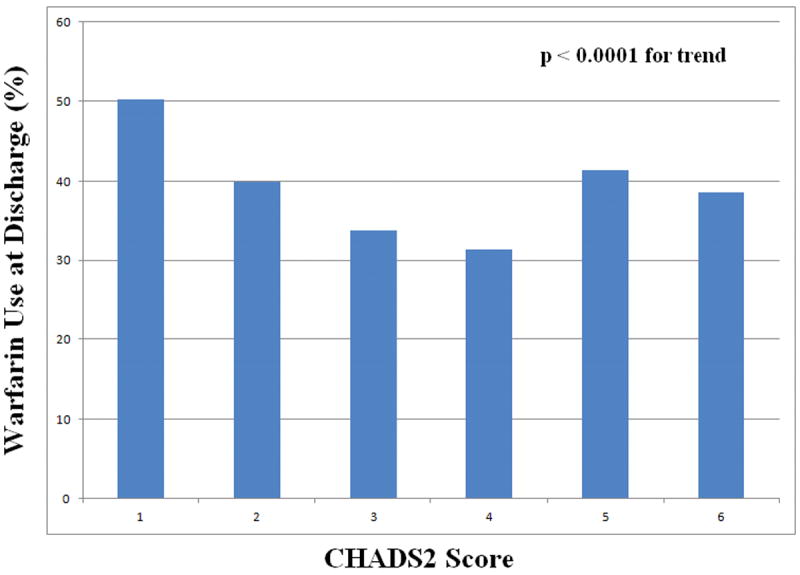
Warfarin use at discharge among HF patients according to CHADS2 score. Among eligible HF patients with AF, warfarin use declined with increasing stroke risk defined by CHADS2 score (P<0.0001 for trend).
DISCUSSION
By examining 9706 HF admissions in 10 countries across Latin America and the Asia-Pacific region, this is the largest study to investigate the use of anticoagulation outside the United States or Europe. There are 4 major findings from this international study of HF and AF. First, stroke prophylaxis is significantly underused in eligible patients with HF and AF. Second, there was a risk-treatment mismatch between the use of warfarin and stroke risk assessed by CHADS2 score. Patients with higher CHADS2 scores were less likely to receive anticoagulation than those with lower scores. Third, there were vast differences in the use of anticoagulation among the 10 countries participating in the registry. Finally, we identified several factors associated with the underuse of anticoagulation, including older age, prior history of coronary artery disease, and worsening renal function as measured by serum creatinine levels.
The ESC AF guidelines, the Asian Pacific Society of Cardiology, and the Latin-American Society of Cerebrovascular Diseases recommend that patients with a moderate-to-high risk of stroke be started on a VKA.7,15,16 Despite these recommendations, the risk-adjusted use of warfarin in the 10 countries included in our observations ranged from 25.1% to 65.5%, with the majority of countries having less than a 50% rate of use. The 2 countries with the lowest adjusted rates of anticoagulation were Taiwan and the Philippines with rates of 25.1% and 25.5%, respectively. The country with the highest adjusted rate was Australia with 65.2% warfarin use at discharge. These vast differences may reflect disparities in resources or access to care within these regions.
In the past 30 years Latin America has experienced significant demographic, epidemiologic, nutritional, and socioeconomic changes leading to an escalation in the prevalence of cardiovascular disease (CVD).17 In addition, most South American countries have a hybrid health care system of public and private medical coverage, which makes the level of coverage and access to medical resources obstacles for physicians who are managing chronic diseases.18 Likewise, CVD mortality in the Asia-Pacific region is on the rise due to poor adherence to recommended guidelines.16 This pattern is especially prominent in most migrant and mobile populations who have limited access to adequate health care.19 Therefore, the lack of access to medical resources and poor surveillance systems present obstacles to physicians prescribing and monitoring the use of VKAs. These disparities in the use of anticoagulation present an opportunity for improving quality of care.
Increasing the use of prophylactic anticoagulation in patients with HF and AF is essential to reducing the incidence and burden of stroke. The number of patients surviving a stroke is estimated to be 4.4 million in Southeast Asia and 9.1 million in the western Asia-Pacific region.16 Likewise, the prevalence of stroke has been found to be between 6% and 9% in Latin American patients older than 65 years.20 Because the majority of AF-related morbidity and mortality is due to stroke,21 it is important that all patients who meet the criteria be started on an effective anticoagulant. However, when deciding to initiate anticoagulation therapy, the risk of stroke must be carefully weighed against the risk of bleeding.
Interestingly, our findings indicated that patients with higher CHADS2 scores were less likely to be discharged on warfarin. We hypothesize that the paradoxical underuse of anticoagulation in patients with high CHADS2 scores might be explained by increased clinical concerns for bleeding, especially in older individuals. Moreover, worsening kidney function is a risk factor for not receiving warfarin at discharge. One possible explanation for this observation is that renal function is a surrogate for overall patient health. Consequently, physicians are less likely to prescribe anticoagulants for sicker patients (those with worsening renal function) for fear of bleeding. A survey study conducted in 2001 showed that when using VKAs, many physicians overestimate the risk of bleeding and underestimate the benefit from stroke prevention.22 Furthermore, many physicians also overestimated the benefit of aspirin in stroke prevention.22
In our study, we observed higher aspirin use in the higher-risk (CHADS2 score ≥2) patients compared with the low-risk (CHADS2 score =1) patients; this indicates that in higher-risk patients, physicians favored antiplatelet therapy. In fact, the effect of aspirin in preventing stroke in higher-risk patients is very modest when compared with the benefit of VKAs.23 Furthermore, the increased risk of bleeding associated with VKAs when compared with aspirin is offset by the stroke-reducing benefits of VKAs.23
A new bleeding risk score, HAS-BLED (Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or anemia, Labile INR, Elderly, and Drugs/alcohol), was developed using risk factors from a cohort study as well as bleeding risk factors from systematic reviews.24 In our cohort, warfarin use was decreased in older individuals with higher creatinine levels (2 components of the HAS-BLED model). Consequently, the patients at higher risk of bleeding are less likely to receive warfarin at discharge. Because age also contributes to the CHADS2 score, patients with higher risk of bleeding also have a higher risk of stroke. Therefore, the decrease in anticoagulation in patients with higher risk of stroke may be explained by increased physician concern for bleeding, especially in older individuals.
In addition, a physician managing a patient who experiences a major bleeding event is less likely to prescribe VKAs, whereas managing a patient who experiences a stroke event does not increase the use of VKAs.16 This phenomenon can be further complicated by the difficulty of maintaining a therapeutic international normalized ratio with VKAs. The use of VKAs requires frequent monitoring, drug adjustments, and significant diet changes. These challenges might help to explain why VKAs are underused by physicians who treat patients with limited access to health care resources in the developing world.
Novel oral anticoagulation agents that do not require strict monitoring, have better safety profiles, and have more predictable effects may dramatically change the approach to managing patients with AF. These novel agents, such as dabigatran and rivaroxaban, are more convenient to use than VKAs,25 are associated with lower rates of intracerebral hemorrhage, and have the potential to increase adherence to therapy in regions where access to care and resources are limited. Although these agents will have higher initial acquisition costs, their net economic impact on health care costs will require further analysis.
Study limitations
First, the data for this registry were collected retrospectively from medical chart review and are dependent on the accuracy of medical personnel and completeness of the medical records from the individual hospitals. Although contraindications and intolerances were recorded in the registry, a proportion of untreated patients classified as eligible for treatment may have had contraindications that were not documented. Second, the type and duration of AF was not documented in the registry. Third, for the purposes of our analysis, we combined new onset AF and history of AF, which can be a problem because they have different prognostic values, characteristics, outcomes, and indications for VKAs. Fourth, clinical outcomes, including stroke, mortality, and rehospitalization, were not included in the registry. Therefore, we were not able to explore the consequences of lack of anticoagulation in this patient population. Finally, the ADHERE-International registry may include hospitals with a higher likelihood of following evidence-based recommendations. Therefore, the true rates of anticoagulation use in these regions might be reduced.
CONCLUSIONS
Among the 10 countries in Latin America and the Asia-Pacific region included in our study, about one-third of patients hospitalized with HF had a history of AF or new onset AF on admission. Adherence to the ESC AF, Asian Pacific Society of Cardiology, and Latin-American Society of Cerebrovascular Diseases guidelines on warfarin use in eligible patients is less than optimal, with significant differences in warfarin use across the regions. There was a significant risk-treatment mismatch in anticoagulation rates, with patients with higher stroke risk being less likely to receive a VKA. The less than optimal use of VKAs in HF patients with AF in Latin America and the Asia-Pacific region represents an opportunity for improved quality of care.
Acknowledgments
Funding
This research was supported in part by Duke University’s Clinical and Translational Science Awards grant TL1 RR024126 from the National Center for Research Resources (NCRR)/National Institutes of Health (NIH). ADHERE-International was sponsored by Janssen-Cilag Asia Pacific.
References
- 1.Eagle KA, Cannom DS, Garcia DA. Management of atrial fibrillation: translating clinical trial data into clinical practice. Am J Med. 2011;124:4–14. doi: 10.1016/j.amjmed.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 2.Pamukcu B, Lane DA, Lip GY. The assessment of stroke and bleeding risk in atrial fibrillation: where are we now? Expert Rev Cardiovasc Ther. 2010;8:1703–10. doi: 10.1586/erc.10.153. [DOI] [PubMed] [Google Scholar]
- 3.Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol. 2003;91:2D–8D. doi: 10.1016/s0002-9149(02)03373-8. [DOI] [PubMed] [Google Scholar]
- 4.Pardaens K, Van Cleemput J, Vanhaecke J, et al. Atrial fibrillation is associated with a lower exercise capacity in male chronic heart failure patients. Heart. 1997;78:564–568. doi: 10.1136/hrt.78.6.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forleo GB, Santini L, Romeo F. Present concepts in management of atrial fibrillation: From drug therapy to ablation. World J Cardiol. 2009;1:11–22. doi: 10.4330/wjc.v1.i1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh M, Adigopula S, Patel P, et al. Recent advances in oral anticoagulation for atrial fibrillation. Ther Adv Cardiovasc Dis. 2010;4:395–407. doi: 10.1177/1753944710386844. [DOI] [PubMed] [Google Scholar]
- 7.Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the ESC. Europace. 2010;12:1360–420. doi: 10.1093/europace/euq350. [DOI] [PubMed] [Google Scholar]
- 8.Bonow RO, Bennett S, Casey DE, Jr, et al. ACC/AHA clinical performance measures for adults with chronic heart failure: A report of the ACC/AHA Task Force on Performance Measures. J Am Coll Cardiol. 2005;46:1144–78. doi: 10.1016/j.jacc.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Piccini JP, Hernandez AF, Zhao X, et al. Quality of care for atrial fibrillation among patients hospitalized for heart failure. J Am Coll Cardiol. 2009;54:1280–9. doi: 10.1016/j.jacc.2009.04.091. [DOI] [PubMed] [Google Scholar]
- 10.Nieuwlaat R, Eurlings LW, Cleland JG, et al. Atrial fibrillation and heart failure in cardiology practice: reciprocal impact and combined management from the perspective of atrial fibrillation: results of the Euro Heart Survey on atrial fibrillation. J Am Coll Cardiol. 2009;53:1690–8. doi: 10.1016/j.jacc.2009.01.055. [DOI] [PubMed] [Google Scholar]
- 11.Goto S, Bhatt DL, Rother J, et al. Prevalence, clinical profile, and cardiovascular outcomes of atrial fibrillation patients with atherothrombosis. Am Heart J. 2008;156:855–63. doi: 10.1016/j.ahj.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 12.Atarashi H, Inoue H, Okumura K, et al. Present status of anticoagulation treatment in Japanese patients with atrial fibrillation: A report from the J-RHYTHM registry. Circ J. 2011;75:1328–33. doi: 10.1253/circj.cj-10-1119. [DOI] [PubMed] [Google Scholar]
- 13.Freestone B, Rajaratnam R, Hussain N, et al. Admissions with atrial fibrillation in a multiracial population in Kuala Lumpur, Malaysia. Int J Cardiol. 2003;91:233–8. doi: 10.1016/s0167-5273(03)00031-7. [DOI] [PubMed] [Google Scholar]
- 14.Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–70. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 15.Alonso de Lecinana-Cases M, Perez RG, Diez-Tejedor E. recommendations for stroke treatment and prevention, 2004. Rev Neurol. 2004;39:465–86. [PubMed] [Google Scholar]
- 16.Goto S, Hankey G, Hills MT, et al. How can we avoid a stroke crisis in the Asia-Pacific region? [September 28, 2011]; http://www.stopafib.org/downloads/News333.pdf. Published May 2011.
- 17.Tejero ME. Cardiovascular disease in Latin American women. Nutr Metab Cardiovasc Dis. 2010;20:405–11. doi: 10.1016/j.numecd.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Lugon JR, Strogoff de Matos JP. Disparities in end-stage renal disease care in South America. Clin Nephrol. 2010;74(Suppl 1):S66–71. [PubMed] [Google Scholar]
- 19.United Nations Population Fund (UNFPA) Enhancing equity in access to health care in the asia-pacific region: Remediable inequities. New York, NY: UNFPA; 2007. [Google Scholar]
- 20.Ferri CP, Schoenborn C, Kalra L, et al. Prevalence of stroke and related burden among older people living in Latin America, India and China. J Neurol Neurosurg Psychiatry. 2011;82:1074–82. doi: 10.1136/jnnp.2010.234153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baruch L, Gage BF, Horrow J, et al. Can patients at elevated risk of stroke treated with anticoagulants be further risk stratified? Stroke. 2007;38:2459–63. doi: 10.1161/STROKEAHA.106.477133. [DOI] [PubMed] [Google Scholar]
- 22.Bungard TJ, Ghali WA, McAlister FA, et al. Physicians’ perceptions of the benefits and risks of warfarin for patients with nonvalvular atrial fibrillation. CMAJ. 2001;165:301–2. [PMC free article] [PubMed] [Google Scholar]
- 23.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: Antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–67. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 24.Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 25.Hart RG, Oczkowski WJ. What’s new in stroke? The top 10 studies of 2009-2011: Part II. Pol Arch Med Wewn. 2011;121:200–7. [PubMed] [Google Scholar]


