Abstract
Decades of research have demonstrated strong links between social ties and health. Although considerable evidence has shown that social support can attenuate downstream physiological stress responses that are relevant to health, the neurocognitive mechanisms that translate perceptions of social ties into altered physiological responses are still not fully understood. This review integrates research from social and affective neuroscience to illuminate some of the neural mechanisms involved in social support processes, which may further our understanding of the ways in which social support influence health. This review focuses on two types of social support that have been shown to relate to health: receiving and giving social support. As the neural basis of receiving support, this article reviews the hypothesis that receiving support may benefit health through the activation of neural regions that respond to safety and inhibit threat-related neural and physiological responding. This article will then review neuroimaging studies in which subjects were primed with or received support during a negative experience as well as studies in which self-reports of perceived support were correlated with neural responses to a negative experience. As the neural basis of giving support, this article reviews the hypothesis that neural regions involved in maternal caregiving behavior may be critical for the health benefits of support-giving through the inhibition of threat-related neural and physiological responding. Neuroimaging studies in which subjects provided support to others or engaged in other related forms of prosocial behavior will then be reviewed. Implications of these findings for furthering our understanding of the relationships between social support and health are discussed.
Keywords: Social support, health, fMRI, social neuroscience, brain, neural
One of the most reliable findings in health psychology and social epidemiology is the strong relationship between social ties and both morbidity and mortality. Relative to socially isolated individuals, socially integrated individuals (those with more social ties) live longer (1–3), have better mental health outcomes (4), and show increased resistance to a variety of somatic diseases, including cardiovascular disease, cancer, and infectious diseases (5–7). In fact, some studies have shown that simply having at least one confidant (as opposed to none) significantly reduces the likelihood of a negative mental health outcome following a major life stressor (8). On the basis of these findings, it has been noted that a lack of social relationships constitutes a major risk factor for health, on par with other more `standard' risk factors such as high blood pressure, cigarette smoking, and obesity (2,3).
But what is it about social relationships that are critical for health? For the past several decades, scientists have taken a bottom-up approach to understanding the links between social ties and health by focusing their attention on the health-relevant physiological responses (autonomic, neuroendocrine, and immune responses) that are altered by social support. Indeed, considerable research has linked the perceived presence or absence of social support to altered activity of neural and endocrine systems that affect disease pathophysiology, such as the sympathetic nervous system (SNS) and the hypothalamus-pituitary-adrenal (HPA) axis (9,10). A growing body of research has also followed these dynamics downstream to chart their impact on disease-regulating biological processes such as immune cell gene expression (11–13) and inflammatory dynamics involved in metabolic disease, atherosclerosis, and tumor metastasis (6,14). Still, little is known about the upstream neurocognitive processes that translate the presence or absence of social support into these physiological responses that affect physical health.
This review aims to add to this foundational work by incorporating a top-down approach to understanding the relationship between social ties and health. To do this, this review integrates work from social and affective neuroscience to identify some of the neural mechanisms involved in social support processes, which may shed additional light on the ways in which social ties influence health. Specifically, this review will focus on the neural underpinnings of two types of social support processes that have been linked with health: 1) receiving or perceiving support from others and 2) giving support to others.
First, the behavioral literature that has highlighted the protective role of social support in the context of stress will be reviewed. This section will then outline a set of neural regions involved in responding to safety and threat and will suggest that receiving or perceiving social support may ultimately benefit health by activating safety-related neural regions and inhibiting threat-related neural regions, which may serve to reduce health-relevant physiological stress responding. Finally, this section will review studies in which participants received or were primed with social support during a negative experience as well as studies in which self-reported assessments of perceived support were correlated with neural responses to a stressful experience.
Next, the literature showing the health benefits of support-giving will be reviewed. This section will then outline a set of neural regions involved in maternal caregiving behavior in animals and will suggest that these regions may be critical for the health benefits of support giving, in part, because some of these regions act to inhibit threat-related responding. This section will then end by reviewing studies examining the neural substrates associated with giving support to others. These studies will include those in which participants provided support or help to someone (close other, stranger) and studies in which participants engaged in other forms of prosocial behavior.
Finally, this review will conclude by noting that the neural regions involved in processing the receipt and provision of social support are, generally speaking, part of a set of neural regions involved in processing reward. This review will then discuss the implications of this for understanding the neural bases of social support and will suggest that additional research is needed to further understand the reciprocal relationships between the reward-related neural regions involved in processing social support and the threat-related neural regions that appear to be attenuated by social support processes.
Receiving or Perceiving Social Support
Received/Perceived Social Support and Health
The study of the health consequences of social support was invigorated by a series of studies published in the late 1970s and early to mid 1980s showing that social integration (e.g., having more key social ties) was a prospective predictor of mortality (2). Across multiple studies, those who had more social ties were approximately 2 times less likely to die in a 9–13 year follow-up period (depending on the study) (1,15–18). These findings have, in the years since, led to an outpouring of studies aimed at indentifying the mechanisms underlying the health benefits of social support.
Although some work has indicated that social isolation may contribute to negative health outcomes (6,19–21), most of the work in this area has focused on the health benefits of `social support'—having or perceiving to have close others who can provide help or care, particularly during times of stress (10,22,23). The predominant hypothesis has been that receiving social support from others or perceiving that one has social support buffers the negative impact of stressors, thus reducing physiological stress responses that have implications for poor health (23–25). Indeed, this hypothesis has been supported by multiple experimental studies highlighting the protective effect that close others can have during times of stress (25,26).
Experimental animal research has demonstrated that aversive stimuli (e.g., shock) elicit less fear and stress when animals are tested in the presence of a companion than when they are tested alone (27–29), a phenomenon referred to as “social buffering.” For example, electric shock punishment was less effective in training rats that were tested in groups vs. alone (27), a rat's immobility due to electric shocks was reduced by the presence of a companion rat (28), and baby goats receiving electric shock displayed fewer emotional reactions when their mothers were present vs. absent (29). Similarly, in both monogamous prairie voles as well as non-human primates, stress responses (corticosterone/cortisol) to a novel environment were reduced when accompanied by a con-specific (30,31). Linking the presence of familiar others with health-related outcomes, the efficacy with which unexpected electric shock led to peptic ulcers in rats was shown to be due, in large part, to whether the rats were shocked in isolation (high ulcer rates) or in the presence of littermates (low ulcer rates) (32). Moreover, social crowding led to hypertension in mice, but only when mice were placed with strangers, not when they were placed with littermates (33).
Similar social buffering effects have been shown in humans as well. The presence of a friend or supportive companion during a stressor can attenuate cardiovascular reactivity (10,34–38) and cortisol responses (in males; 39,40). Similarly, the presence of a supportive companion (vs. stranger) has been shown to reduce self-reported pain unpleasantness in response to painful stimuli (41,42).
In addition, though not experimental, correlational studies have highlighted the benefits of social support on various health-relevant outcomes (25). For example, one study showed that 91% of pregnant women with high life stress and low social support had pregnancy-related complications, whereas only 33% of those with high life stress and high social support showed complications (43). In these correlational studies, a distinction is made between self-reports of received support (a measure of the specific supportive behaviors provided to the recipient) and perceived support (a measure of the perceptions of the general availability of and satisfaction with social support), with perceived support more strongly predicting health and well-being (44,45). However, it can be challenging to map these self-report measures onto experimental manipulations of social support. Even though self-reports of perceived and received support correlate only modestly (r=.35) (44), experiments that manipulate the presence versus absence of a supportive companion may increase this correlation in the moment. Thus, in the experimental neuroimaging studies reviewed here, the term `receiving' or `perceiving' social support will be used to describe features of the manipulation, rather than a self-report assessment of the subjective nature of the experience.
Together these studies suggest that having social support can be protective during times of stress and that this may occur through the buffering effect of social support on health-relevant physiological stress responses. In order to gain insight into the possible mechanisms linking perceptions of social support with attenuated stress reactivity, the next section turns to relevant findings from the fields of social and affective neuroscience.
Possible Neural Correlates of Received/Perceived Social Support
On the basis of considerable research showing that social support can attenuate stress-or threat-related responding, it seems likely that received or perceived social support will reduce neural activity in regions that respond to basic survival threats. However, how this threat-related activity might be reduced by social support remains more of a mystery.
One possible mechanism linking social support to attenuated threat-related neural activity is through neural regions that have been shown to be responsive to safety cues. Considerable research has shown that there are certain reward-related neural regions that: 1) are responsive to `safety', the relative absence (versus presence) of threat or the presence of stimuli known to be protective from threat and 2) reduce threat-related neural activity in response to detecting safety (46,47). Thus, it is possible that social support reduces threat-related neural and physiological responding through the activation of safety-related neural regions and the subsequent inhibition of threat-related neural regions. Indeed, this account maps nicely onto attachment theory which suggests that the attachment bond—first formed between child and caregiver—provides a sense of safety and a reduced sense of distress for the child who is not yet capable of taking care of him/herself (48). This sense of safety that comes from knowing that a caregiver is there or from receiving support from a loved one may serve as a kind of safety signal, letting the individual know that they are safe and leading to a reduction in threat- or distress-related processing.
On the basis of these proposed neural underpinnings of the stress-reducing effects of social support, the next section will review the neural regions that process threat or danger as well as those that are responsive to safety and reduce threat-related activity. This section will also review how these regions relate to downstream physiological stress responses (e.g., SNS, HPA) that may have health implications.
Threat-related neural regions
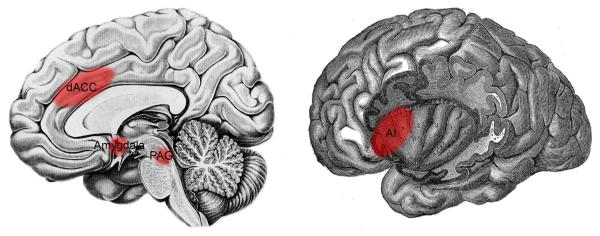
In studies of fear-related responding, it has been shown that the regions involved in detecting and responding to impending danger or threat include (but are not limited to) the amygdala, dorsal anterior cingulate cortex (dACC), anterior insula (AI), and periaqueductal gray (PAG) (Figure 1). The amygdala responds to innately threatening stimuli, such as impending pain or an approaching tarantula (49,50), and is also involved in fear conditioning—learning contingencies that predict aversive outcomes (46,51). The dACC, AI, and PAG, though most well-known for their roles in pain processing (52), respond similarly, showing increased activity to impending pain, an approaching threatening stimulus (e.g., spider, snake) (49,50,53,54), or during fear conditioning (51,55,56). Consistent with this, rodent studies demonstrate that the prelimbic cortex, homologous with the dACC and dorsal portion of the medial prefrontal cortex (DMPFC; BA 8/9) in humans, is involved in sustaining fear or threat responses (57), possibly through excitatory projections to the amygdala (56).
Figure 1.
Neural regions involved in detecting and responding to basic survival threats, include: the dorsal anterior cingulate cortex (dACC), amygdala, periaqueductal gray (PAG) (left), and anterior insula (AI) (right).
In response to detecting threat, most of these regions trigger downstream physiological stress response systems. The central nucleus of the amgydala controls the expression of threat-related changes in autonomic (SNS) and endocrine responses (cortisol) through projections to the hypothalamus and brainstem areas (46). Stimulating the central nucleus of the amgydala increases blood pressure in rats (58), and greater amgydala activity during fear acquisition in humans is associated with greater SNS activity (skin conductance response; SCR) to a conditioned stimulus (51). Conversely, lesions to the central nucleus of the amygdala can reduce SNS and HPA responses to conditioned stimuli (59,60). Similarly, stimulation of the dACC induces SNS responses, whereas lesions to the dACC reduce SNS responses (56,61). In fact, the amygdala and dACC may be particularly relevant for physiological stress responses that have negative health implications, as lesions to the dACC and amygdala were found to reduce inflammatory-related gastric pathology (62). With regard to the other regions, PAG activity can increase or decrease SNS responding depending on the type of stressor (e.g., escapable, inescapable) and the specific PAG column activated (63). The AI, on the other hand, while often associated with SNS activity, may be more involved in representing autonomic responses in conscious awareness than in directly generating these responses (64).
Safety-related neural regions
In addition to neural regions that respond to impending danger or threat, the brain is also equipped with certain neural regions that are responsive to safety, or the relative absence of threat, and that reduce threat-related neural activity in response. One of these regions, the ventromedial prefrontal cortex (VMPFC), is part of a larger neural circuit implicated in reward processing and appears to be involved more specifically in encoding the subjective value of stimuli (65). In the context of threat or stress, this region responds to the relative absence or reduction of threat or stress (66–68), which is more subjectively rewarding than the presence of threat or stress. However, from an experiential perspective, a reduction in perceived threat or stress seems less akin to `reward' and more akin to the experience of `relief' or `safety.' Hence, building on work showing that this reward-related region is also involved in responding to safety cues, the discussion of this region will be couched in terms of `safety' rather than `reward' per se (although both of these outcomes can be conceptualized as being rooted in greater subjective value).
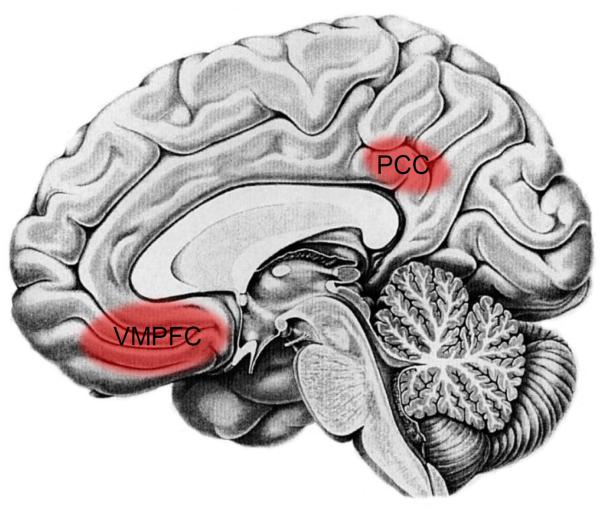
Along these lines, considerable research has implicated the VMPFC, a reward-related region, as well as the posterior cingulate cortex (PCC), in responding to cues that signal safety (46,47) (Figure 2). For example, moving a tarantula a safe distance away from a participant's foot is associated with increased VMPFC and PCC activity (50). Additionally, fear extinction, a form of `learned safety' in which a cue that previously predicted a negative outcome (e.g., shock) now predicts safety (e.g., no shock) also, activates these regions (46,50). Importantly, the VMPFC, in particular, reduces fear responding through inhibitory connections with the amygdala (69). Thus, stimulating the infralimbic cortex in rats—homologous to the VMPFC (BA 11) and subgenual anterior cingulate cortex (subACC; BA 25) in humans—diminishes fear responding to fear cues (69), and greater VMPFC activity is associated with reduced fear responding (SCRs) in humans (46,51). (These same inhibitory connections have not been observed for the PCC.) In addition, the VMPFC and PCC also appear to respond to safety in the context of pain and stress, activating to conditions of low (vs. high) pain or stress (66–68).
Figure 2.
Neural regions that are responsive to safety, include: the ventromedial prefrontal cortex (VMPFC) and posterior cingulate cortex (PCC).
Critical for the role that these regions may play in the threat-reducing effects of receiving social support, activity in these regions has been shown to correlate with reductions in autonomic and endocrine responding in humans. For example, VMPFC and PCC activity during mental or social stress correlates negatively with cardiovascular, cortisol, and threat-related neural responses (67,68,70–72). In addition, damage to the VMPFC increases feelings of threat and cortisol responses (for females) in response to social stress (73). Interestingly, greater activity in the VMPFC/subACC has been shown to be associated with increases in parasympathetic responding, which is associated with reduced cardiovascular arousal (74). Thus, activity in these safety-related regions may be involved in inhibiting sympathetic and promoting parasympathetic responses (75), which may be health-protective. Indeed, highlighting a causal role for these regions in inhibiting threat-related disease outcomes, lesioning the VMPFC or PCC in animals leads to increases in inflammatory-related gastric pathology (62).
Summary
In sum, research from social and affective neuroscience has highlighted a set of neural regions involved in processing threat and facilitating physiological stress responses as well as a separate set of neural regions involved in responding to safety and attenuating physiological stress responses. Given the importance of social support in producing a sense of safety and reducing distress, it is possible that social support attenuates psychological and physiological threat reactivity by activating safety-related neural regions and inhibiting threat-related neural and physiological responding. In the next section, findings from neuroimaging studies will be reviewed to examine the extent to which these regions are implicated in receiving or perceiving social support during stress.
Neuroimaging Studies of Received/Perceived Social Support
Several studies have now examined neural activity while individuals are either primed with or receive social support during stress. For example, two studies have examined neural activity while participants viewed pictures of social support figures (romantic relationship partners) while receiving physical pain (76,77). In one of these studies (76), viewing pictures of social support figures was meant to serve as a low-level form of social support—being reminded of a loved one who typically provides support (to be included, participants had to rate their partners as a significant source of support). Interestingly, in both studies, participants reported feeling significantly less pain when viewing their relationship partners (vs. strangers, acquaintances) while receiving pain. In addition, both studies showed increased activity in neural regions previously implicated in responding to safety and decreased activity in regions previously implicated in responding to threat and pain. Thus, Younger et al. (77) showed increased activity in the VMPFC and PCC, regions associated with responding to safety, when participants viewed their partners (vs. familiar acquaintances) during pain and decreased activity in the dACC and posterior insula. Moreover, greater pain relief was associated with decreased activity in the dACC and AI. Similarly, Eisenberger et al., (76) showed increased activity in the VMPFC when participants viewed their partners (vs. strangers) during pain and decreased activity in the dACC and AI. In this study, greater activity in the VMPFC was associated with greater perceived support from the partner as well as larger reductions in self-reported pain and pain-related dACC activity.
A similar study examined the receipt of social support in the context of awaiting a painful stimulus. In this study, married women were exposed to the threat of shock while holding their husband's hand, the hand of a male experimenter, or no hand (78). When participants held their husbands' hands (vs. strangers' hands or no hands), they reported lower levels of general unpleasantness. To investigate the types of neural activity altered by social support, the researchers first identified `threat-related' neural regions that were more active when participants awaited physical pain compared to when they knew they would not be receiving pain (during the no handholding condition). The researchers then showed that several of these regions were less active when participants held their partners' hands. Although this study showed reduced activity in task-defined threat-related regions, this study was not able to examine neural regions that were more active during the social support trials, because the analyses were restricted to neural regions that were more active during the threat of pain. However, on the basis of these findings, Coan and colleagues proposed the Social Baseline Theory (79), which posits that social proximity is the `baseline' human state and that deviations from this state lead to an increased need for cognitive resources and threat-related neural activity.
Two other studies examined the effect of social support during the stress of social exclusion. In one study, neural activity was assessed as participants received emotional support (e.g., “Sorry, I know it was unpleasant for you to be excluded”) during a social exclusion episode (80). Results demonstrated that participants showed increased activity in the VMPFC and PCC/precuneus and decreased activity in the insula during the emotional support (vs. exclusion) condition. Another study examined how neural responses to social exclusion were affected by the imagined presence of an attachment figure (81). Consistent with the stress-buffering effects of social support, participants reported less distress during exclusion when an attachment figure was imagined to be present. This effect was instantiated neurally by the hypothalamus; there was significantly less activity in the hypothalamus, a region implicated in stress-related responding, when participants imagined that their attachment figures were present during social exclusion compared to when they imagined that non-attachment figures were present during social exclusion.
Although the above-mentioned studies are the only ones that have used fMRI to assess neural activity while being primed with or actively receiving social support, a few other studies have examined how self-report measures of social support correlate with neural responding during threatening tasks (e.g., social exclusion). In one study, participants who interacted more frequently with supportive individuals on a daily basis (which may relate to greater perceived support) showed reduced activity in the dACC and PAG during an episode of social exclusion (72). In another study, participants who spent more time with friends during adolescence showed reduced activity in the dACC and AI during social exclusion (82). Finally, though not a direct measure of social support, participants low in attachment anxiety, who tend to feel more comfortable and secure in their closest relationships (which may relate to greater perceived support), showed reduced activity in the dACC and AI in response to social exclusion (83). Thus, in each of these studies, measures likely related to perceived social support correlated negatively with threat-related neural activity.
Finally, studies examining the neural underpinnings of thinking about close others have yielded activations similar to those observed in studies examining received social support. Subjects asked to think about close others vs. strangers showed greater activity in the VMPFC and PCC (84). Likewise, participants who were asked to make judgments about friends vs. strangers showed increased activity in the VMPFC and PCC (85). Although neither of these studies examined the stress-reducing aspects of thinking of close others, it is interesting that the pattern of activations were similar to those studies that have examined the benefits of being primed with or receiving social support during stress.
In sum, although there are relatively few studies examining the neural correlates of receiving or perceiving social support, some consistent findings have emerged. In most studies of social support, there is attenuated activity in threat-related neural regions. In addition, over half of the studies that have examined neural responses to primed or received social support have shown increased activity in regions responsive to safety. These regions also show activity in studies that simply require participants to think about close others. However, these regions are not typically observed in studies that examine correlations between self-reports of social support and neural responses to threat. It is possible that these safety-related regions are only recruited in response to the direct presentation of social support stimuli or that they are only observed when neural activity to the presence of social support is directly compared with its absence. It is also possible that there are other neurobiological mechanisms involved in the threat-reducing consequences of social support (79). Further work will be needed to better understand the mechanisms that lead to reduced threat-related activity among those who report higher levels of social support.
Giving Social Support
Giving Social Support and Health
Although it is commonly assumed that the benefits of social support come from the support that individuals receive or perceive from others, new research has begun to highlight the possible health benefits of giving support to others. Several studies have shown that support-giving is a strong predictor of reduced depressive symptoms and better mental health (86–89). In fact, one study demonstrated that those who provided more support, following the loss of a spouse, exhibited an accelerated decline in depressive symptoms (89). Support-giving also has implications for physiological responding and mortality. The tendency to give social support predicted lower ambulatory blood pressure and heart rate over a 24-hour period (87). Moreover, individuals who provided more support to others (90) or who engaged in more volunteer service (91,92) evidenced a reduced risk of mortality.
Although these findings are suggestive of a link between support-giving and health, they are limited by several factors. First, given the correlational nature of these studies, the relationship between support-giving and health may be driven, not by support-giving leading to better health, but by those who are in better health being better able to provide support. Additional experimental evidence is needed to further investigate whether support-giving can causally influence mental or physical health outcomes.
A second limitation of these findings is that, on the surface, they seem to contradict a separate literature highlighting the detrimental consequences of being a `caregiver' in the context of chronic illness (e.g., individuals who provide support to a spouse with Alzheimer's disease) (93). Numerous studies have shown that caregivers show an increased risk of physical health problems and mortality relative to non-caregivers (93,94). Although these findings have typically been interpreted as indicating that the act of providing care to others has negative health consequences, these studies have typically not disentangled the effects of providing care to others from those of watching the decline of an ailing loved one. Indeed, some data suggest that some of the negative effects of caregiving may stem from witnessing the decline and impending death of a loved one (95). In fact, a recent study demonstrated that individuals who provided more hours of care to a spouse had lower rates of mortality, whereas those whose spouses had poorer health had higher rates of mortality (96). Hence, while caregiving for an ailing loved one clearly has negative health implications, it is possible that this is not due specifically to the act of providing support or care to others, and thus it is important to examine the role of support-giving in the link between social relationships and health.
Possible Neural Correlates of Giving Social Support
To identify the neural substrates of support-giving, this review borrows from animal research on maternal caregiving behavior (which involves providing support and care to offspring), as certain forms of prosocial behavior, such as support-giving, have been hypothesized to rely on the neural and neuropeptide substrates of a mammalian caregiving system (97–99). Interestingly, work in this area has shown that neural regions involved in maternal caregiving behavior play a role in: 1) increasing activity in reward-related neural regions that promote maternal approach behaviors and 2) decreasing activity in threat-related regions in order to reduce avoidance responses to offspring and facilitate adaptive caregiving responses during times of stress (100). The following section reviews the neural regions implicated in maternal caregiving behavior and expands on the inhibitory relationship that these regions may have with threat-related neural and physiological responses.
It is important to note that while most of the work on caring for offspring comes from animal studies of female maternal behavior (as females are typically more involved in caring for offspring than males), humans who typically engage in biparental care may show fewer gender differences in the neural substrates and possible health benefits of support-giving. Indeed, many of the studies highlighting health benefits of support-giving show effects that remain significant after controlling for gender. Thus, although the research here will focus on maternal behavior in female animals, it is possible that the neural substrates of these behaviors are relevant to both genders in humans. Future work, however, will be needed to more directly test this hypothesis.
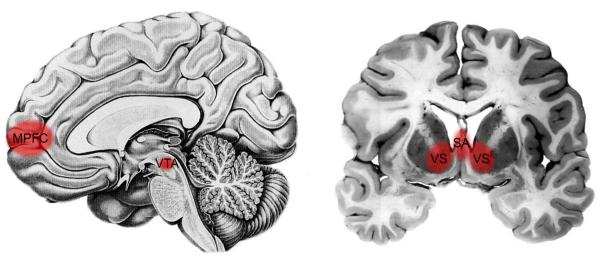
Animal research has shown that many of the regions that have been implicated in maternal caregiving behavior fall within the basal forebrain. These include the medial preoptic area (MPOA) in the rostral hypothalamus and the adjoining ventral bed nucleus of the stria terminals (vBST) as well as certain reward-related regions, such as the septal area (SA), the nucleus accumbens within the ventral striatum (VS), and the ventral tegmental area (VTA) (Figure 3; MPOA/vBST not shown).
Figure 3.
Neural regions involved in maternal caregiving behavior, include: the medial prefrontal cortex (MPFC), ventral tegmental area (VTA) (left), the ventral striatum (VS), and septal area (SA) (right).
In the rat, the MPOA/vBST plays a central role in maternal behavior (100), such that lesioning these regions disrupts maternal behavior (e.g., retrieving pups) (101,102), whereas hormonal stimulation of the MPOA/VBNST increases maternal responsiveness (100,103). According to animal models, the MPOA/vBST has inhibitory projections to threat-related regions, such as the anterior hypothalamic nucleus and the PAG, involved in withdrawal responses. Inhibiting activity in these regions is hypothesized to reduce a rat's natural avoidance response to pups in order to facilitate maternal approach behaviors (100).
The MPOA/vBST also has excitatory projections to reward-reward neural regions, such as the nucleus accumbens within the VS, the VTA, and the SA (100,104), which act to increase maternal responsivenss. The VS and VTA are part of a dopaminergic reward circuit and have been shown to activate to the anticipation of basic rewards such as food or money (105,106). The SA, though not part of this dopaminergic circuit, has been identified as a `pleasure center' on the basis of studies showing that both animals and humans will work to maintain electrical stimulation to this region (107,108). Consistent with the role of these regions in reward-seeking and reinforcement, these regions also underlie approach-related maternal behavior. Lesions to the VS, VTA, or SA dramatically disrupt maternal behavior, reducing approach and interaction with pups (109–112).
Interestingly, one of these regions, the SA, has also been shown to reduce threat-related responding, in part, through inhibitory connections with the amygdala (113,114). Hence, stimulating the SA decreases SNS activity, leading to cardiac decelerations and reductions in blood pressure (114–116), whereas lesioning the SA increases startle reactions, indicative of SNS activity (117). Indeed, it has been suggested that the SA may contribute to maternal behavior by reducing threat-related responding in order to facilitate responsive caregiving during times of stress (97,104). Although more research has focused on the role of reward-related regions in reinforcing maternal behavior (118), the threat-reducing effects of maternal caregiving may be a critical avenue to explore in elucidating the links between support-giving and health (90,97,119).
In addition to the regions described above, the medial prefrontal cortex (MPFC), which has extensive connections to the MPOA, VS, and VTA, also appears to play a role in maternal behavior (120). The MPFC appears to facilitate maternal behavior in more complex environments in which multiple stimuli compete for attention (120).
Although no studies to date have specifically examined the neural regions associated with maternal caregiving behavior in humans, some studies have examined neural activity while participants view infant pictures or hear infant cries, which may elicit caregiving motivations. These studies (though not reviewed systematically here because of the difficulty associated with identifying the specific experiential state evoked by these stimuli) generally show widespread activation that includes activity in the SA (121), VS (121–123), VTA (123), MPFC (121,123–126), as well as increased or decreased activity in the amgydala (124,126–128). Regions such as the MPOA/vBST are too small to reliably isolate with neuroimaging methods and thus are not typically reported.
Though not fully understood, the neural processes that facilitate the threat-reducing effects of maternal caregiving may be mediated, in part, by neuropeptides involved in social bonding, such as endogenous opioids and oxytocin (99,129). Linking these neuropeptides to caregiving-related neural regions, studies have shown that the VS, SA, and MPFC (as well as the amygdala) have high densities of opioid and/or oxytocin receptors (130–132). Moreover, opioids and oxytocin are known to reduce physiological stress responses. Opioids attenuate SNS and HPA activity, reduce conditioned fear responses, and enhance fear extinction (133). Opioids are also potent immunomodulators, inhibiting the production of proinflammatory cytokines (134). Likewise, oxtyocin reduces SNS and HPA responses and may do so, in part, through opioid-related activity (129). Future work will be needed to examine whether these caregiving-related neuropeptides contribute to the health benefits of support-giving.
Summary
In sum, animal models of maternal caregiving behavior provide useful information about the neural regions that may be implicated in support-giving in humans. Different regions within this network function to motivate approach-related maternal behavior as well as inhibit withdrawal or threat-related responding to facilitate adaptive caregiving during stress. In the next section, neuroimaging studies of support-giving will be reviewed in order to examine whether these neural regions underlie support-giving processes in humans.
Neuroimaging Studies of Giving Social Support
Though no neuroimaging studies have directly focused on the health benefits of support-giving, several studies have started to examine the psychological benefits of giving support to others. In the one study to examine the neural underpinnings of providing emotional support to a loved one, female participants were scanned as they provided support to their male partners who were in pain (97). On support-giving trials, each female participant was able to hold her partner's arm as he received painful electric shock (while standing just outside the scanner bore). Participants showed greater VS and SA activity while providing support to their partners (vs. control conditions), suggesting that some of the regions involved in maternal caregiving behavior in animals may underlie support-giving processes in humans. In addition, consistent with the role of the SA in inhibiting amygdala activity (113,114), those who showed greater SA activity during support-giving showed reduced bilateral amygdala activity. Although physiological responses were not measured here, reduced amygdala activation could have implications for reduced physiological threat responding (e.g., SNS responses) in the context of support-giving.
Another study examined the neural underpinnings of giving financial support to loved ones. White and Latino adolescents were scanned as they made decisions to contribute money to their family and/or themselves (135). Results demonstrated that Latino participants, who tend to value helping the family, showed greater activity in regions implicated in reward and caregiving (dorsal striatum (DS), VTA) when they provided costly donations to their family. Interestingly, for both white and Latino participants, those who derived more fulfillment from helping their family showed greater activity in regions implicated in reward and caregiving (VS, DS, VTA; 135) as well as greater functional coupling between the MPFC and VS (136) when making costly donations to the family. These findings suggest that the altruistic motivation to help family members may rely on some of the reward-related regions that underlie maternal caregiving behavior in animals (VS, VTA) as well as those that help sustain these responses under competing motivations (MPFC).
A few other studies have examined giving monetary support to charities. In one study, participants made a series of decisions regarding how they were willing to spend an endowment (98). For each trial, participants made yes or no decisions about various payoff types for themselves and a charity, including: 1) pure monetary reward (YOU: $+2 CHARITY: $0), 2) noncostly donations (YOU: $0 CHARITY: $+5), and 3) costly donations (YOU: $−2 CHARITY: $+5). Both costly and noncostly decisions to donate (vs. pure monetary reward) led to greater activity in the VS and SA. Moreover, greater activity in the VS/SA was associated with greater decisions to donate throughout the experiment. Similar results were demonstrated in a study looking at neural activity during voluntary giving to charity as well as mandatory giving for taxation (137). Here too, participants showed greater VS/SA activity for both mandatory (taxation) and voluntary (charity) forms of giving behavior, suggesting that regions involved in caregiving behavior may underlie these more abstract forms of giving behavior.
Moving from giving behavior to more general forms of prosocial behavior, several studies have examined neural responses to hypothetical scenarios that elicit prosocial feelings or motivations. Thus, imagining prosocial behavior toward a friend led to increased activity in the MPFC and VTA, and feeling more positively in response to this task was associated with greater SA activation (138). In another study, reading scenarios that elicit prosocial motivation led to increased activity in the VS and VTA (139). Moreover, in a related study of frontotemporal dementia patients, who tend to behave less prosocially, reduced activity in the SA and MPFC was directly related to feeling less prosocial motivation after reading these scenarios (140). Thus, several of the neural regions implicated in caregiving behavior appear to contribute to prosocial motivation.
In a related vein, another study attempted to disentangle affiliative motivation from positive emotional experience. Here, participants read hypothetical scenarios that varied in whether they were about close others (termed `affiliative') or strangers (termed `non-affiliative') as well as whether they were positive or negative (141). For example, participants read statements that were: 1) affiliative and positive (e.g., “You taught your son to ride a bike and he came to thank you with a hug”), 2) non-affiliative and positive (e.g., “You delivered a beautiful speech and the audience stood up to applaud you”), 3) affiliative and negative (e.g., “You were distracted and lost your young child in the park”), or non-affiliative and negative (e.g., “You were blamed for a problem that was not your fault and lost your job”). A direct comparison of affiliative vs. non-affiliative conditions revealed activation in the SA/MPOA/anterior hypothalamus and the MPFC that was not simply due to the valence of the stimuli. The authors suggested that their findings indicate that the SA/MPOA/anterior hypothalamic area may be uniquely engaged by affiliative experiences.
Finally, several studies have examined the neural underpinnings of empathy-induced prosocial behavior and have reliably shown the involvement of the MPFC in predicting these responses. Greater MPFC activity when viewing ingroup members in painful situations was associated with a greater willingness to donate time or money to these individuals (142). Greater MPFC activity in response to viewing another person experience social rejection was associated with greater prosocial behavior aimed at helping that person (143). Finally, greater MPFC and VS activity while empathizing with sad pictures was associated with a greater tendency to help friends in daily life (144). It is not yet clear why these studies converge on MPFC activity as a key predictor of prosocial behavior following empathy. Additional data on the neural substrates of prosocial behavior will be needed to further understand the complex role of the MPFC in caregiving-related behaviors.
In sum, although few studies have directly investigated the neural underpinnings of support-giving, those that have point to a fairly consistent pattern (in both males and females) of activity in reward-related regions known to contribute to maternal caregiving behavior in animals. In addition, widening the focus to include studies of prosocial behavior highlights similar patterns of neural activity. What remains to be examined is the role that these caregiving-related neural regions play in attenuating health-relevant physiological stress responses. Future work will be needed to examine whether increased activation in caregiving-related regions is associated with subsequent reductions in threat-related neural and physiological responses as well as whether these effects are similar in both males and females. This may be one important pathway whereby support-giving influences health.
Summary and Conclusions
Though it has long been demonstrated that social ties are strongly linked to health, the neural mechanisms that translate perceptions of social support into downstream health-relevant physiological responses are just beginning to be explored. In this review two possible pathways that might contribute to the link between social support and health were highlighted: receiving or perceiving social support from others and giving social support to others.
In response to receiving or perceiving social support, studies have consistently shown reductions in threat-related neural activity (e.g., dACC, AI, amgydala, PAG). In addition, studies that have specifically examined the threat-reducing effects of being primed with or receiving social support have also shown increased activity in regions that process safety (VMPFC, PCC). Given that animal models have shown inhibitory connections between the VMPFC and threat-related neural activity (69), it is possible that receiving or perceiving social support attenuates threat-related neural and physiological responses through the inhibitory action of the VMPFC. This may represent one pathway that links the receipt of social support with health. Other pathways should be explored as well (79).
Studies examining support-giving as well as more general forms of prosocial behavior have shown neural activity in regions implicated in maternal caregiving behavior, including parts of the basal forebrain (VS, VTA, SA) and the MPFC. On the basis of animal models showing that some of these neural regions have inhibitory connections with threat-related neural regions, it is possible that support-giving attenuates threat-related neural and physiological responses through the inhibitory action of caregiving-related neural regions. Indeed, animal work suggests that this serves to inhibit natural withdrawal responses to pups and facilitate responsive caregiving behavior towards offspring in the face of stress (100,104). Thus, caregiving-related reductions in threat-related responding may represent another pathway through which social support relates to health.
It is important to note that several of the neural regions that serve to inhibit threat-related responding (VMPFC, SA) also play a critical role in reward processing. Interestingly, this observation fits with early brain stimulation studies showing, not only that there are separate neural systems that mediate reward and punishment, but that these two systems are mutually inhibitory. Reward-related neural regions reduce sensitivity to pains and punishments, whereas punishment- or threat-related neural regions tend to reduce sensitivity to reward (145). For example, stimulating reward-related regions reduces fear behavior and pain sensitivity, whereas stimulating punishment/threat-related regions reduces the effect of stimulation in reward areas (146–148).
Behavioral studies reveal consistent findings. Rewarding stimuli, such as pleasant music or palatable food, can reduce pain or threat responding, whereas sustained pain can inhibit the experience of reward from morphine (149,150). Moreover, mu-opioids, which are known to increase the experience of reward also decrease the aversiveness of punishing stimuli (151). Thus, converging lines of evidence highlight a potentially inhibitory relationship between the neural systems that process reward and punishment.
The inhibitory relationship between the neural regions involved in reward vs. punishment may have important implications for health-relevant physiological responses. Although more work is needed in this area, it has been suggested that the reward system mediates parasympathetic function and is inhibitory with respect to autonomic and neuroendocrine responses, whereas the punishment/threat system facilitates sympathetic and neuroendocrine responses to stress (145). For example, stimulating the SA in animals appears to have parasympathetic-type effects, leading to reductions in heart rate and blood pressure (114–116). Likewise, the VMPFC has been shown to play a role in parasympathetic responding (74) and stimulating the VMPFC in rats can suppress cardiovascular responses to stress (152).
These findings lead to several new avenues of exploration for health research. For example, to the extent that caregiving-related neural regions contribute to reduced physiological stress responding, could other forms of behavior that use this caregiving system, such as volunteering or prosocial acts, also reduce health-relevant physiological responding? Might an intervention that manipulates prosocial behavior ultimately benefit physical health? Furthermore, to the extent that the VMPFC inhibits stress responding to social support, how might non-supportive relationships interfere with these inhibitory processes and does this affect other types of inhibitory processes, such as those involved in emotion regulation (47), which also rely on this neural region? Finally, how do early life experiences, particularly those involving exposure to harsh parenting and unstable attachment, fundamentally alter the functioning of these neural regions? And does this exposure to early life stress permanently change how an individual responds to various types of stressful experiences, which has implications for negative health outcomes?
In sum, neuroimaging research has just begun to examine the neural correlates of social support with the hope of uncovering additional clues regarding the mechanisms that link social support with health. On the basis of increasing evidence for the role of reward-related regions in social support processes as well as suggestive evidence for an inhibitory relationship between reward- and threat/punishment-related neural regions, future research would benefit from a more direct focus on the relationships between these reward-related neurobiological mechanisms and physiological stress responses. Although considerable research has focused on the negative effects of social stressors or a lack of social support on SNS and HPA pathways, much remains to be discovered about the possible reward-related pathways through which social support might independently regulate the physiological underpinnings of health. Focusing on the ways in which reward-related neurobiological processes attenuate health-relevant physiological responses may open up an important new area within health psychology that examines the ways in which various types of positive experience, including social support, can influence health.
Acknowledgments
I thank the members of the National Cancer Institute (NCI) Network on Biobehavioral Pathways in Cancer (Linda Alexander, Steve Cole, Susan Lutgendorf, Paige McDonald, Cheryse Sankar, Anil Sood, Giovanna Zappala) for supporting this review, Mona Moieni for assisting with the literature review, and Tristen Inagaki for discussion of some of the hypotheses put forth here. This project was funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This project was also supported by a grant from the National Institutes of Mental Health (NIMH R01MH091352) and a Young Investigator Award from the National Alliance for Research on Schizophrenia and Depression (NARSAD).
Abbreviations
- SNS
sympathetic nervous system
- HPA
hypothalamus-pituitary-adrenal
- dACC
dorsal anterior cingulate cortex
- AI
anterior insula
- PAG
periaqueductal gray
- DMPFC
dorsal portion of the medial prefrontal cortex
- VMPFC
ventromedial prefrontal cortex
- PCC
posterior cingulate cortex
- subACC
subgenual anterior cingulate cortex
- VS
ventral striatum
Footnotes
The author has no financial gain related to the outcome of this research, and there are no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berkman LF, Syme SL. Social networks, host resistance, and mortality: A nine-year follow-up study of Alameda county residents. Am J Epidemiol. 1979;109:186–203. doi: 10.1093/oxfordjournals.aje.a112674. [DOI] [PubMed] [Google Scholar]
- 2.House JS, Landis KR, Umberson D. Social relationships and Health. Sci. 1988;241:540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- 3.Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: a meta-analytic review. PLoS Med. 2010;7:e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seeman TE. Social ties and health: The benefits of social integration. Ann Epidemiol. 1996;6:442–451. doi: 10.1016/s1047-2797(96)00095-6. [DOI] [PubMed] [Google Scholar]
- 5.Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Prac Psych. 2008;5:466–475. doi: 10.1038/ncponc1134. [DOI] [PubMed] [Google Scholar]
- 6.Miller G, Chen E, Cole SW. Health psychology: Developing biologically plausible models linking the social world and physical health. Annu Rev Psychol. 2009;60:5.1–5.24. doi: 10.1146/annurev.psych.60.110707.163551. [DOI] [PubMed] [Google Scholar]
- 7.Uchino BN. Social support and health: A review of psychological processes potentially underlying links to disease outcomes. J Behav Med. 2006;29:377–387. doi: 10.1007/s10865-006-9056-5. [DOI] [PubMed] [Google Scholar]
- 8.Brown GW, Bhrolchain MN, Harris T. Social class and psychiatric disturbance among women in an urban population. Sociol. 1975;9:225–254. [Google Scholar]
- 9.Bosch JA, de Geus EJ, Carroll D, Goedhart AD, Anane LA, van Zanten JJ, Helmerhorst EJ, Edwards KM. A general enhancement of autonomic and cortisol responses during social evaluative threat. Psychosom Med. 2009;71:877–885. doi: 10.1097/PSY.0b013e3181baef05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uchino BN, Caccioppo JT, Kiecolt-Glaser JT. The relationship between social support and physiological processes: A review with emphasis on underlying mechanisms and implications for health. Psychol Bull. 1996;119:488–531. doi: 10.1037/0033-2909.119.3.488. [DOI] [PubMed] [Google Scholar]
- 11.Cole SW, Hawkley LC, Arevalo JM, Sung CY, Rose RM, Cacioppo JT. Social regulation of gene expression in human leukocytes. Genome Biol. 2007;8:R189. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole SW. Social regulation of human gene expression. Curr Dir Psychol Sci. 2009;18:132–137. doi: 10.1111/j.1467-8721.2009.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol. 2011;11:625–632. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finch CE. The biology of human longevity: Inflammation, nutrition, and aging in the evolution of life spans. Academic Press; Boston, MA: 2007. [Google Scholar]
- 15.House JS, Robbins C, Metzner HL. The association of social relationships and activities with mortality: Prospective evidence from the tecumesh community health study. Am J Epidemiol. 1982;116:123–140. doi: 10.1093/oxfordjournals.aje.a113387. [DOI] [PubMed] [Google Scholar]
- 16.Schoenbach VJ, Kaplan BH, Fredman L, Kleinbaum DG. Social ties and mortality in Evans County, Georgia. Am J Epidemiol. 1986;123:577–591. doi: 10.1093/oxfordjournals.aje.a114278. [DOI] [PubMed] [Google Scholar]
- 17.Welin L, Tibblin G, Svärdsudd K, Tibblin B, Ander-Peciva S, Larsson B, Wilhelmsen L. Prospective study of social influences on mortality: The study of men born in 1913 and 1923. The Lancet. 1985;325:915–918. doi: 10.1016/s0140-6736(85)91684-8. [DOI] [PubMed] [Google Scholar]
- 18.Orth-Gomer K, Johnson JV. Social network interaction and mortality: A six year follow-up study of a random sample of the Swedish population. J Chronic Dis. 1987;40:949–957. doi: 10.1016/0021-9681(87)90145-7. [DOI] [PubMed] [Google Scholar]
- 19.Eisenberger NI, Cole SW. Social neuroscience and health: Neurophysiological mechanisms linking social ties with physical health. Nat Neurosci. 2012;15:669–674. doi: 10.1038/nn.3086. [DOI] [PubMed] [Google Scholar]
- 20.Kiecolt-Glaser JK, Gouin J-P, Hantsoo L. Close relationship, inflammation, and health. Neurosci Biobehav Rev. 2010;35:33–38. doi: 10.1016/j.neubiorev.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller GE, Chen E, Sze J, Marin T, Arevalo JM, Doll R, Ma R, Cole SW. A genomic fingerprint of chronic stress in humans: Blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry. 2008;64:266–272. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen S, Syme SL. Issues in the study and application of social support. In: Cohen S, Syme SL, editors. Social Support and Health. Academic Press; San Francisco, CA: 1985. pp. 3–22. [Google Scholar]
- 23.Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychol Bull. 1985;98:310–357. [PubMed] [Google Scholar]
- 24.Cassel J. The contribution of the social environment to host resistance: The fourth Wade Hampton Frost Lecture. Am J Epidemiol. 1976;104:107–123. doi: 10.1093/oxfordjournals.aje.a112281. [DOI] [PubMed] [Google Scholar]
- 25.Cobb S. Social support as a moderator of life stresses. Psychosom Med. 1976;38:300–314. doi: 10.1097/00006842-197609000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Epley SW. Reduction of the behavioral effects of aversive stimulation by the presence of companions. Psychol Bull. 1974;81:271–283. doi: 10.1037/h0036389. [DOI] [PubMed] [Google Scholar]
- 27.Rasmussen EW. Social facilitation. Acta Psychol. 1939;4:275–291. [Google Scholar]
- 28.Davitz JR, Mason DJ. Socially facilitated reduction of a fear response in rats. J Comp Physiol Psychol. 1955;48:149–151. doi: 10.1037/h0046411. [DOI] [PubMed] [Google Scholar]
- 29.Liddell HS. Conditioning and emotions. Sci Am. 1954;190:48–57. [Google Scholar]
- 30.DeVries AC, DeVries MB, Taymans S, Carter CS. Modulation of pair bonding in female prairie voles (Microtus ochrogaster) by corticosterone. Prc Natl Acad Sci. 1995;92:7744–7748. doi: 10.1073/pnas.92.17.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology. 2003;28:910–918. doi: 10.1038/sj.npp.1300128. [DOI] [PubMed] [Google Scholar]
- 32.Conger JC, Sawrey W, Turrel ES. The role of social experience in the production of gastric ulcers in hooded rats placed in a conflict situation. J Abnorm Psychol. 1958;57:214–220. doi: 10.1037/h0041533. [DOI] [PubMed] [Google Scholar]
- 33.Henry JP, Meehan JP, Stephens PM. The use of psychosocial stimuli to induce prolonged hypertension in mice. Psychosom Med. 1967;29:408–432. doi: 10.1097/00006842-196709000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Amoroso DM, Walters RH. Effects of anxiety and socially mediated anxiety reduction on paired-associated learning. J Pers Soc Psychol. 1969;11:388–396. doi: 10.1037/h0027261. [DOI] [PubMed] [Google Scholar]
- 35.Gerin W, Pieper C, Levy R, Pickering T. Social support in social interactions: A moderator of cardiovascular reactivity. Psychosom Med. 1992;54:324–336. doi: 10.1097/00006842-199205000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Gerin W, Milner D, Chawla S, Pickering TG. Social support as a moderator of cardiovascular reactivity in women: A test of the direct effects and buffering hypotheses. Psychosom Med. 1995;57:16–22. doi: 10.1097/00006842-199501000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Kamarck TW, Manuck SB, Jennings JR. Social support reduces cardiovascularreactivity to psychological challenge: A laboratory model. Psychosom Med. 1990;52:42–58. doi: 10.1097/00006842-199001000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Kamarck TW, Annunziato B, Amateau LM. Affiliation moderates the effects of social threat on stress-related cardiovascular responses: Boundary conditions for a laboratory model of social support. Psychosom Med. 1995;57:183–194. doi: 10.1097/00006842-199503000-00011. [DOI] [PubMed] [Google Scholar]
- 39.Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- 40.Kirschbaum C, Klauer T, Hellhammer DH. Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychosom Med. 1995;57:23–31. doi: 10.1097/00006842-199501000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Brown JL, Sheffield D, Leary MR, Robinson ME. Social support and experimental pain. Psychosom Med. 2003;65:276–283. doi: 10.1097/01.psy.0000030388.62434.46. [DOI] [PubMed] [Google Scholar]
- 42.Master SL, Eisenberger NI, Taylor SE, Naliboff BD, Shirinyan D, Lieberman MD. A picture's worth. Psychol Sci. 2009;20:1316–1318. doi: 10.1111/j.1467-9280.2009.02444.x. [DOI] [PubMed] [Google Scholar]
- 43.Nuckolls KB, Cassel J, Kaplan BH. Psychological assets, life crisis and the prognosis of pregnancy. Am J Epidemiol. 1972;95:431–441. doi: 10.1093/oxfordjournals.aje.a121410. [DOI] [PubMed] [Google Scholar]
- 44.Haber MG, Cohen JL, Lucas T, Baltes BB. The relationship between self-reported received and perceived social support: A meta-analytic review. Am J Community Psychol. 2007;39:133–144. doi: 10.1007/s10464-007-9100-9. [DOI] [PubMed] [Google Scholar]
- 45.Reinhardt JP, Boerner K, Horowitz A. Good to have but not to use: Differential impact of perceived and received support on well-being. J Soc Pers Relationships. 2006;23:117–129. [Google Scholar]
- 46.Delgado MR, Olsson A, Phelps EA. Extending animal models of fear conditioning to humans. Biol Psychol. 2006;73:39–48. doi: 10.1016/j.biopsycho.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 47.Schiller D, Delgado MR. Overlapping neural systems mediating extinction, reversal, and regulation of fear. Trends Cogn Sci. 2010;14:268–276. doi: 10.1016/j.tics.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bowlby J. Attachment & Loss, Vol. I: Attachment. Basic Books; New York: 1969. [Google Scholar]
- 49.Mobbs D, Marchant JL, Hassabis D, Seymour B, Tan G, Gray M, Petrovic P, Dolan RJ, Frith CD. From threat to fear: The neural organization of defensive fear systems in humans. J Neurosci. 2009;29:12236–12243. doi: 10.1523/JNEUROSCI.2378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mobbs D, Yu R, Rowe JB, Eich H, FeldmanHall O, Dalgeish T. Neural activity associated with monitoring the oscillating threat value of a tarantula. Proc Nat Acad Sci USA. 2010;23:20582–20586. doi: 10.1073/pnas.1009076107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: Role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 52.Rainville P. Brain mechanisms of pain affect and pain modulation. Curr Opin Neurobiol. 2002;12:195–204. doi: 10.1016/s0959-4388(02)00313-6. [DOI] [PubMed] [Google Scholar]
- 53.Mobbs D, Petrovic P, Marchant JL, Hassabis D, Weiskopf N, Seymour B, Dolan RJ, Frith CD. When fear is near: Threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science. 2007;317:1079–1083. doi: 10.1126/science.1144298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nili U, Goldberg H, Weizman A, Dudai Y. Fear thou not: Activity of frontal and temporal circuits in moments of real-life courage. Neuron. 2010;66:949–962. doi: 10.1016/j.neuron.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 55.Büchel C, Dolan RJ. Classical fear conditioning in functional neuroimaging. Curr Opin Neurobiol. 2000;10:219–223. doi: 10.1016/s0959-4388(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 56.Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischi B, Rauch SL. A role for the human dorsal anterior cingulate cortex in fear expression. Biol Psychiatry. 2007;62:1191–1194. doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 57.Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci. 2009;29:8474–8482. doi: 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tellioğlu T, Aker R, Oktay S, Onat F. Effect of brain acetylcholine depletion on bicuculline-induced cardiovascular and locomotor reponses. International J Neurosci. 1997;89:143–152. doi: 10.3109/00207459708988470. [DOI] [PubMed] [Google Scholar]
- 59.Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J Neurosci. 2004;24:5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McCabe PM, Gentile CG, Markgraf CG, Teich AH, Schneiderman N. Ibotenic acid lesions in the amygdaloid central nucleus but not in the lateral subthalamic area prevent the acquisition of differential Pavlovian conditioning of bradycardia in rabbits. Brain Res. 1992;580:155–163. doi: 10.1016/0006-8993(92)90939-7. [DOI] [PubMed] [Google Scholar]
- 61.Critchley HD, Mathias CJ, Josephs O, O'Doherty J, Zanini S, Dewar BK, Cipolotti L, Shallice T, Dolan RJ. Human cingulate cortex and autonomic control: Converging neuroimaging and clinical evidence. Brain. 2003;126:2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- 62.Henke PG. The telencephalic limbic system and experimental gastric pathology: A review. Neurosci Biobehav Rev. 1982;6:381–390. doi: 10.1016/0149-7634(82)90047-1. [DOI] [PubMed] [Google Scholar]
- 63.Bandler R, Keay KA, Floyd N, Price J. Central circuits patterned autonomic activity during active vs. passive emotional coping. Brain Res Bulletin. 2000;53:95–104. doi: 10.1016/s0361-9230(00)00313-0. [DOI] [PubMed] [Google Scholar]
- 64.Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- 65.Levy DJ, Glimcher PW. The root of all value: A neural common currency for choice. Curr Opinion Neurobiology. 2012;22:1027–1038. doi: 10.1016/j.conb.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Atlas LY, Bolger N, Lindquist MA, Wager TD. Brain mediators of predictive cue effects on perceived pain. J Neurosci. 2010;30:12964–12977. doi: 10.1523/JNEUROSCI.0057-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pruessner JC, Dedovic K, Khalili-Mahani N, Engert V, Pruessner M, Buss C, Renwick R, Dagher A, Meaney MJ, Lupien S. Deactivation of the limbic system during acute psychosocial stress: Evidence from positron emission tomography and functional magnetic resonance imaging. Biol Psychiatry. 2008;63:234–240. doi: 10.1016/j.biopsych.2007.04.041. [DOI] [PubMed] [Google Scholar]
- 68.Wager TD, van Ast VA, Hughes BL, Davidson ML, Lindquist MA, Ochsner KN. Brain mediators of cardiovascular responses to social threat, Part II: Prefrontal-subcortical pathways and relationship with anxiety. NeuroImage. 2009;47:836–851. doi: 10.1016/j.neuroimage.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 70.Critchley HD, Corfield DR, Chandler MP, Mathias CJ, Dolan RJ. Cerebral correlates of autonomic cardiovascular arousal: A functional neuroimaging investigation in humans. J Physiol. 2000;5I:259–270. doi: 10.1111/j.1469-7793.2000.t01-1-00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gianaros PJ, van der Veen FM, Jennings JR. Regional cerebral blood flow correlates with heart period and high-frequency heart period variability during working-memory tasks: Implications for the cortical and subcortical regulation of cardiac autonomic activity. Psychophysiol. 2004;41:521–530. doi: 10.1111/1469-8986.2004.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eisenberger NI, Taylor SE, Gable SL, Hilmert CJ, Lieberman MD. Neural pathways link social support to attenuated neuroendocrine stress responses. NeuroImage. 2007;35:1601–1612. doi: 10.1016/j.neuroimage.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Buchanan TW, Driscoll D, Mowrer SM, Sollers JJ, 3rd, Thayer JF, Kirschbaum C, Tranel D. Medial prefrontal cortex damage affects physiological and psychological stress responses differently in men and women. Psychoneuroendocrinology. 2010;35:56–66. doi: 10.1016/j.psyneuen.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thayer JF, Ahs F, Fredrikson M, Sollers JJ, III, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev. 2012;36:747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 75.Porges SW. The polyvagal perspective. Biol Psychol. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eisenberger NI, Master SL, Inagaki TI, Taylor SE, Shirinyan D, Lieberman MD, Naliboff B. Attachment figures activate a safety signal-related neural region and reduce pain experience. Proc Nat Acad Sci USA. 2011;108:11721–11726. doi: 10.1073/pnas.1108239108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Younger J, Aron A, Parke S, Chatterjee N, Mackey S. Viewing pictures of a romantic partner reduces experimental pain: Involvement of neural reward systems. PLoS ONE. 2010;5:e133309. doi: 10.1371/journal.pone.0013309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Coan JA, Schaefer HS, Davidson RJ. Lending a hand: Social Regulation of the neural response to threat. Psychol Sci. 2006;17:1032–1039. doi: 10.1111/j.1467-9280.2006.01832.x. [DOI] [PubMed] [Google Scholar]
- 79.Beckes L, Coan JA. Social baseline theory: The role of social proximity in emotion and economy of action. Soc Pers Psychology Compass. 2011;5:976–988. [Google Scholar]
- 80.Onoda K, Okamoto Y, Nakashima K, Nittono H, Ura M, Yamawaki S. Decreased ventral anterior cingulate cortex activity is associated with reduced social pain during emotional support. Soc Neurosci. 2009;4:443–454. doi: 10.1080/17470910902955884. [DOI] [PubMed] [Google Scholar]
- 81.Karremans JC, Heslenfeld DJ, van Dillen LF, Van Lange PAM. Secure attachment partners attenuate neural responses to social exclusion: An fMRI investigation. Int J Psychophysiol. 2011;81:44–50. doi: 10.1016/j.ijpsycho.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 82.Masten CL, Telzer EH, Fuligni A, Lieberman MD, Eisenberger NI. Time spent with friends in adolescence relates to less neural sensitivity to later peer rejection. Soc Cogn Affect Neurosci. 2012;7:106–114. doi: 10.1093/scan/nsq098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.DeWall CN, Masten CL, Powell D, Schurtz DR, Eisenberger NI. Do neural responses to rejection depend on attachment style? An fMRI study. Soc Cogn Affect Neurosci. 2011;7:184–192. doi: 10.1093/scan/nsq107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maddock RJ, Garrett AS, Buonocore MH. Remembering familiar people: The posterior cingulate cortex and autobiographical memory retrieval. Neuroscience. 2001;104:667–676. doi: 10.1016/s0306-4522(01)00108-7. [DOI] [PubMed] [Google Scholar]
- 85.Krienen FM, Tu P-C, Buckner RL. Clan mentality: Evidence that the medial cortex responds to close others. J Neurosci. 2010;30:13906–13915. doi: 10.1523/JNEUROSCI.2180-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Krause N, Herzog AR, Baker E. Providing support to others and well-being in later life. J Gerontol. 1992;47:300–311. doi: 10.1093/geronj/47.5.p300. [DOI] [PubMed] [Google Scholar]
- 87.Piferi RL, Lawler KA. Social support and ambulatory blood pressure: An examination of both receiving and giving. Int J Psychophysiol. 2006;62:328–336. doi: 10.1016/j.ijpsycho.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 88.Schwartz C, Meisenhelder JB, Ma Y, Reed G. Altruistic social interest behaviors are associated with better mental health. Psychosom Med. 2003;65:778–785. doi: 10.1097/01.psy.0000079378.39062.d4. [DOI] [PubMed] [Google Scholar]
- 89.Brown SL, Brown RM, House JS, Smith DM. Coping with spousal loss: Potential buffering effects of self-reported helping behavior. Pers Soc Psychol Bull. 2008;34:849–861. doi: 10.1177/0146167208314972. [DOI] [PubMed] [Google Scholar]
- 90.Brown SL, Nesse RM, Vinokur AD, Smith DS. Providing social support may be more beneficial than receiving it: Results from a perspective study of mortality. Psychol Sci. 2003;14:320–327. doi: 10.1111/1467-9280.14461. [DOI] [PubMed] [Google Scholar]
- 91.Musick MA, Herzog AR, House JA. Volunteering and mortality among older adults: Findings from a national sample. J Gerontol. 1999;54B:S173–S180. doi: 10.1093/geronb/54b.3.s173. [DOI] [PubMed] [Google Scholar]
- 92.Oman D, Thoresen CE, McMahon K. Volunteerism and mortality among the community-dwelling elderly. J Health Psychol. 1999;4:301–316. doi: 10.1177/135910539900400301. [DOI] [PubMed] [Google Scholar]
- 93.Schulz R, Beach SR. Caregiving as a risk factor for mortality: The caregiver health effects study. J Am Med Assoc. 1999;22:15–19. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- 94.Vitaliano PP, Zhang J, Scanlan JM. Is caregiving hazardous to one's physical health? A meta-analysis. Psychol Bull. 2003;129:946–972. doi: 10.1037/0033-2909.129.6.946. [DOI] [PubMed] [Google Scholar]
- 95.Amirkhanyan AA, Wolf DA. Caregiver stress and noncaregiver stress: Exploring the pathways of psychiatric morbidity. Gerontologist. 2003;48:817–827. doi: 10.1093/geront/43.6.817. [DOI] [PubMed] [Google Scholar]
- 96.Brown SL, Smith DM, Schulz R, Kabeto MU, Ubel PA, Poulin M, Yi J, Kim C, Langa KM. Caregiving behavior is associated with decreased mortality risk. Psychol Sci. 2009;20:488–494. doi: 10.1111/j.1467-9280.2009.02323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Inagaki TK, Eisenberger NI. Neural correlates of giving support to a loved one. Psychosom Med. 2012;74:3–7. doi: 10.1097/PSY.0b013e3182359335. [DOI] [PubMed] [Google Scholar]
- 98.Moll J, Krueger F, Zahn R, Pardini M, de Oliveira-Souza R, Grafman J. Human fronto-mesolimbic networks guide decisions about charitable donation. Proc Nat Acad Sci USA. 2006;103:15623–15628. doi: 10.1073/pnas.0604475103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Panksepp J. Affective Neuroscience. Oxford University Press; New York: 1998. [Google Scholar]
- 100.Numan M. Hypothalamic neural circuits regulating maternal responsiveness toward infants. Behav Cogn Neurosci Rev. 2006;5:163–190. doi: 10.1177/1534582306288790. [DOI] [PubMed] [Google Scholar]
- 101.Numan M, Corodimas KP, Numan MJ, Factor EM, Piers WD. Axon-sparing lesions of the preoptic region and substantia innominata disrupt maternal behavior in rats. Behav Neurosci. 1988;102:381–396. doi: 10.1037//0735-7044.102.3.381. [DOI] [PubMed] [Google Scholar]
- 102.Numan M, Insel TR. The neurobiology of parental behavior. Springer-Verlag; New York: 2003. [Google Scholar]
- 103.Bridges RS, Robertson MC, Shiu RPC, Sturgis JD, Henriquez BM, Mann PE. Central lactogenic regulation of maternal behavior in rats: Steroid dependence, hormone specificity, and behavioral potencies of rat prolactin and rat placental lactogen. Endocrinol. 1997;138:756–763. doi: 10.1210/endo.138.2.4921. [DOI] [PubMed] [Google Scholar]
- 104.Stack EC, Balakrishnan R, Numan MJ, Numan M. A functional neuroanatomical investigation of the roles of medial preoptic area in neural circuits regulating maternal behavior. Behavioural Brain Res. 2002;131:17–36. doi: 10.1016/s0166-4328(01)00370-9. [DOI] [PubMed] [Google Scholar]
- 105.O'Doherty JP. Reward representations and reward-related learning in the human brain: Insights from neuroimaging. Curr Opin Neurobiol. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 106.Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Ann Rev Neurosci. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- 107.Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1965;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- 108.Bishop MP, Elder ST, Heath RG. Intracranial self-stimulation in man. Science. 1963;26:394–396. doi: 10.1126/science.140.3565.394. [DOI] [PubMed] [Google Scholar]
- 109.Hansen S. Maternal behavior of female rats with 6-OHDA lesions in the ventral striatum: Characterization of the pup retrieval deficit. Physiol Behav. 1994;55:615–620. doi: 10.1016/0031-9384(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 110.Numan M, Smith HG. Maternal behavior in rats: Evidence for the involvement of preoptic projections to the ventral tegmental area. Behav Neurosci. 1984;98:712–727. doi: 10.1037//0735-7044.98.4.712. [DOI] [PubMed] [Google Scholar]
- 111.Slotnick BM, Burton BJ. Maternal behavior of mice with cingulate cortical, amygdala, or septal lesions. J Comp Physiol Psychol. 1975;88:118–127. doi: 10.1037/h0076200. [DOI] [PubMed] [Google Scholar]
- 112.Flannelly KJ, Kemble ED, Blanchard DC, Blanchard RJ. Effects of septal-forebrain lesions on maternal aggression and maternal care. Behavioral and Neural Biology. 1986;45:17–30. doi: 10.1016/s0163-1047(86)80002-4. [DOI] [PubMed] [Google Scholar]
- 113.Melia KR, Sananes CB, Davis M. Lesions of the central nucleus of the amygdala block the excitatory effects of septal ablation on the acoustic startle reflex. Physiol Behav. 1991;51:175–180. doi: 10.1016/0031-9384(92)90220-v. [DOI] [PubMed] [Google Scholar]
- 114.Thomas E. Forebrain mechanisms in the relief of fear: The role of the lateral septum. Psychobiol. 1988;16:36–44. [Google Scholar]
- 115.Covian MR, Antunes-Rodrigues J, O'Flaherty JJ. Role of various brain structures on physiological functions. J Neurophysiol. 1964;27:394–407. doi: 10.1152/jn.1964.27.3.394. [DOI] [PubMed] [Google Scholar]
- 116.Malmo RB. Slowing down of heart rate after septal self-stimulation in rats. Science. 1961;133:1128–1130. doi: 10.1126/science.133.3459.1128. [DOI] [PubMed] [Google Scholar]
- 117.Brady JV, Nauta WJH. Subcortical mechanisms in emotional behavior: Affective changes following septal forebrain lesions in the albino rat. J Comp Physiol Psychol. 1953;46:339–346. doi: 10.1037/h0059531. [DOI] [PubMed] [Google Scholar]
- 118.Insel TR. A neurobiological basis of social attachment. Am J Psychiatry. 1997;154:726–735. doi: 10.1176/ajp.154.6.726. [DOI] [PubMed] [Google Scholar]
- 119.Taylor SE, Klein LC, Lewis BP, Gruenwald TL, Gurung AR, Updegraff JA. Biobehavioral responses to stress in females: Tend-and-befriend, not fight-or-flight. Psychol Rev. 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- 120.Pereira M, Morerell JI. Functional mapping of the neural circuitry of rat maternal motivation: effects of site-specific transient neural inactivation. J Neuroendochronol. 2011;23:1020–1035. doi: 10.1111/j.1365-2826.2011.02200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lorberbaum JP, Newman JD, Horwitz AR, Dubno JR, Lydiard RB, Hamner MB, Bohning DE, George MS. A potential role for thalamocingulate circuitry in human maternal behavior. Biol Psychiatry. 2002;51:431–445. doi: 10.1016/s0006-3223(01)01284-7. [DOI] [PubMed] [Google Scholar]
- 122.Glocker ML, Langleben DD, Ruparel K, Loughead JW, Valdez JN, Griffin MD, Sachser N, Gur RC. Baby schema modulates the brain reward system in nulliparous women. Proc Nat Acad Sci USA. 2009;106:9115–9119. doi: 10.1073/pnas.0811620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Strathearn L, Li J, Fonagy P, Montague PR. What's in a smile? Maternal brain responses to infant facial cues. Pediatrics. 2008;122:40–51. doi: 10.1542/peds.2007-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bartels A, Zeki S. The neural correlates of maternal and romantic love. NeuroImage. 2004;21:1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 125.Leibenluft E, Gobbini MI, Harrison T, Haxby JV. Mothers' neural activation in response to pictures of their children and other children. Biol Psychiatry. 2003;56:225–232. doi: 10.1016/j.biopsych.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 126.Seifritz E, Esposito F, Neuhoff JG, Lüthi A, Mustovic H, Dammann G, von Bardeleben U, Radue EW, Cirillo S, Tedeschi G, Di Salle F. Differential sex-independent amygdala response to infant crying and laughing in parents versus nonparents. Biol Psychiatry. 2003;54:1367–1375. doi: 10.1016/s0006-3223(03)00697-8. [DOI] [PubMed] [Google Scholar]
- 127.Ranote S, Elliott R, Abel KM, Mitchell R, Deakin JFW, Appleby L. The neural basis of maternal responsiveness to infants: An fMRI study. NeuroReport. 2004;15:1825–1829. doi: 10.1097/01.wnr.0000137078.64128.6a. [DOI] [PubMed] [Google Scholar]
- 128.Sander K, Frome Y, Scheich H. fMRI Activations of amygdala, cingulate cortex, and auditory cortex by infant laughing and crying. Hum Brain Mapp. 2007;28:1007–1022. doi: 10.1002/hbm.20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Uvnas-Moberg K. Oxytocin may mediate the benefits of positive social interaction and emotions. Psychoneuroendocrinol. 1998;23:819–835. doi: 10.1016/s0306-4530(98)00056-0. [DOI] [PubMed] [Google Scholar]
- 130.Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Nat Acad Sci USA. 1992;89:5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293:311–315. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]
- 132.Loup F, Tribollet E, Dubois-Dauphin M, Dreifuss JJ. Localization of high-affinity binding sites for oxytocin and vasopressin in the human brain: An autoradiographical study. Brain Res. 1991;555:220–232. doi: 10.1016/0006-8993(91)90345-v. [DOI] [PubMed] [Google Scholar]
- 133.Drolet G, Dumont EC, Gosselin I, Kinkead R, Laforest S, Trotter J-F. Role of endogenous opioid system in the regulation of the stress response. Progress in NeuroPsychopharmacology and Biol Psychiatry. 2001;25:729–741. doi: 10.1016/s0278-5846(01)00161-0. [DOI] [PubMed] [Google Scholar]
- 134.Bonnet M-P, Beloeil H, Benhamou D, Mazoit J-X, Asehnoune K. The mu-opioid receptor mediates morphine-induced tumor necrosis factor and interleukin-6 inhibition in toll-like receptor 2-stimulated monocytes. Anesth Analg. 2008;106:1142–1149. doi: 10.1213/ane.0b013e318165de89. [DOI] [PubMed] [Google Scholar]
- 135.Telzer EH, Masten CL, Berkman ET, Lieberman MD, Fuligni AJ. Gaining while giving: An fMRI study of the rewards of family assistance among White and Latino youth. Soc Neurosci. 2010;5:508–518. doi: 10.1080/17470911003687913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Telzer EH, Masten CL, Berkman ET, Lieberman MD, Fuligni AJ. Neural regions associated with self control and mentalizing are recruited during prosocial behaviors towards the family. NeuroImage. 2011;58:242–249. doi: 10.1016/j.neuroimage.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Harbaugh WT, Mayr U, Burghart DR. Neural responses to taxation and voluntary giving reveal motives for charitable donations. Science. 2007;316:1622–1625. doi: 10.1126/science.1140738. [DOI] [PubMed] [Google Scholar]
- 138.Zahn R, Moll J, Paiva M, Garrido G, Krueger F, Huey ED, Graftman J. The neural basis of human social values: Evidence from functional MRI. Cereb Cortex. 2009;19:276–283. doi: 10.1093/cercor/bhn080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Moll J, de Oliveira-Souza R, Garrido GJ, Bramati IE, Caparelli-Daquer EM, Paiva ML, Zahn R, Grafman J. The self as a moral agent: Linking the neural bases of social agency and moral sensitivity. Soc Neurosci. 2007;3–4:336–352. doi: 10.1080/17470910701392024. [DOI] [PubMed] [Google Scholar]
- 140.Moll J, Zahn R, de Oliveira-Souza R, Bramati IE, Krueger F, Tura B, Cavanagh AL, Grafman J. Impairment of prosocial sentiments is associated with frontopolar and septal damage in frontotemporal dementia. NeuroImage. 2011;54:1735–1742. doi: 10.1016/j.neuroimage.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Moll J, Bado P, de Oliveira-Souza R, Bramati IE, Lima DO, Paiva FF, Sato JR, Tovar-Moll F, Zahn R. A neural signature of affiliative emotion in the human septo-hypothalamic area. J Neurosci. 2012;32:12499–12505. doi: 10.1523/JNEUROSCI.6508-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mathur VA, Harada T, Lipke T, Chiao JY. Neural basis of extraordinary empathy and altruistic motivation. NeuroImage. 2010;51:1468–1475. doi: 10.1016/j.neuroimage.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 143.Masten CL, Morelli SA, Eisenberger NI. An fMRI investigation of empathy for `social pain' and subsequent prosocial behavior. NeuroImage. 2011;55:381–388. doi: 10.1016/j.neuroimage.2010.11.060. [DOI] [PubMed] [Google Scholar]
- 144.Rameson LT, Morelli SA, Lieberman MD. The neural correlates of empathy: Experience, automaticity, and prosocial behavior. J Cogn Neurosci. 2011;24:235–245. doi: 10.1162/jocn_a_00130. [DOI] [PubMed] [Google Scholar]
- 145.Bovard EW. The balance between negative and positive brain system activity. Perspect Biol Med. 1962;6:116–127. doi: 10.1353/pbm.1963.0009. [DOI] [PubMed] [Google Scholar]
- 146.Olds J. Differentiation of reward systems in the brain by self-stimulation technics. In: Ramey ER, O'Doherty DS, editors. Electrical Studies on the Unanesthetized Brain. Medical Division of Harper & Brothers; New York, NY: 1960. pp. 17–41. [Google Scholar]
- 147.Lilly JC. Learning motivated by subcortical stimulation: The “start” and the “stop” patterns of behavior. In: Ramey ER, O'Doherty DS, editors. Electrical Studies on the Unanesthetized Brain. Medical Division of Harper & Brothers; New York, NY: 1960. pp. 78–105. [Google Scholar]
- 148.Heath RG, Mickle WA. Evaluation of seven years' experience with depth electrode studies in human patients. In: Ramey ER, O'Doherty DS, editors. Electrical Studies on the Unanesthetized Brain. Medical Division of Harper & Brothers; New York, NY: 1960. pp. 214–247. [Google Scholar]
- 149.Leknes S, Tracey I. A common neurobiology for pain and pleasure. Nat Rev Neurosci. 2008;9:314–320. doi: 10.1038/nrn2333. [DOI] [PubMed] [Google Scholar]
- 150.Ulrich-Lai YM, Christiansen AM, Ostrander MM, Jones AA, Jones KR, Choi DC, Krause EG, Evanson NK, Furay AR, Davis JF, Solomon MB, deKloet AD, Tamashiro KL, Sakai RR, Seeley RJ, Woods JP, Herman JP. Pleasurable behaviors reduce stress via brain reward pathways. Proc Natl Acad Sci USA. 2010;107:20529–20534. doi: 10.1073/pnas.1007740107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Berridge KC. Pleasures of the brain. Brain Cogn. 2003;52:106–128. doi: 10.1016/s0278-2626(03)00014-9. [DOI] [PubMed] [Google Scholar]
- 152.Müller Ribeiro FC, Zaretsky DV, Zaretskaia MV, Santos RA, DiMicco JA, Fontes MAP. Contribution of infralimbic cortex in the cardiovascular response to acute stress. Am J Physiol Regul Integr Comp Physiol. 2012;303:639–650. doi: 10.1152/ajpregu.00573.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]