Abstract
Background
Immune responses to lung associated self-antigens (SAgs) have been implicated in chronic lung allograft rejection. The goals of this study were to determine the prevalence of pre-existing antibodies (Abs) to the SAgs in pulmonary diseases and to determine the association between pre-existing Abs to SAgs and the development of primary graft dysfunction (PGD), donor specific antibodies (DSA) and chronic rejection.
Methods
Pre- and post-transplant sera were analyzed from 317 lung transplant (LTx) recipients between 2000–2011 with diagnosis of COPD (161), IPF (50), cystic fibrosis (CF) (55), and others (51). Samples were analyzed for Abs to SAgs by ELISA, DSA and cytokines by LUMINEX. The clinical diagnosis of PGD and BOS was based on ISHLT guidelines.
Results
Among LTx recipients the overall prevalence of Abs to SAgs was 22.71%, including 18% in COPD (p=0.033), 34% in IPF (p=0.0006), 29% in CF (p=0.0023) and 19.6% in other diagnoses (p=0.044). The incidence of PGD (88% vs 54%, p<0.05), DSA (70% vs 45%, p<0.01) and BOS (90% vs 38% (p<0.001) following LTx was significantly higher in patients with pre-Tx Abs to SAgs than without. Pro-inflammatory cytokines, IL-1β, IL-17 and IFN-γ, were elevated in patients who had pre-Tx Abs to SAgs along with a reduction in anti-inflammatory IL-10.
Conclusion
Patients with IPF and CF have the highest prevalence of Abs to SAgs. Patients with pre-existing Abs to SAgs are at increased risk for development of PGD, DSA and BOS. Strategies to remove pre-existing Abs to SAgs should be considered to improve lung allograft outcome.
Keywords: primary graft dysfunction, bronchiolitis obliterans syndrome, alloimmunity, autoimmunity, cytokines
Introduction
Immune responses to mismatched donor human leukocyte antigen (HLA) leading to the development of antibodies (Abs) to donor HLA (DSA) have been implicated in the development of chronic lung allograft rejection.1–3 Recently, it has also been proposed that the development of immune responses to tissue restricted self-antigens (SAgs) may play an important role in the development of chronic rejection and that there is cross talk between these immune responses to HLAs and SAgs.4, 5 Chronic rejection, clinically diagnosed as bronchiolitis obliterans syndrome (BOS) still remains a major hindrance to the long term survival of lung transplant (LTx) recipients.6 Collagen-V (Col-V) and Kα1 tubulin (Kα1T), are lung restricted SAgs, and immune responses towards these SAgs are considered to be important in the pathogenesis of BOS. 7–11 Studies in our laboratory have demonstrated that the development of Abs to SAgs precede the development of BOS.4, 12 We have also documented that the development of DSA correlates with and precedes, the development of BOS, suggesting a pathogenic role for the DSA.13 Therefore, it has been hypothesized that allo- and auto-immune responses are important insults leading to chronic rejection following LTx.14
Successful clearance of DSAs by Ab directed treatment with IVIG and/or rituximab was significantly associated with freedom from BOS and better survival than those who had persistent DSAs.15 However, a number of patients who cleared DSA and subsequently developed BOS were noted to have persistent Abs to SAgs. Earlier, in a preliminary analysis of 142 LTx patients we had reported that the presence of pre-transplant (Tx) Abs to SAgs increased the incidence of primary graft dysfunction (PGD), DSA and BOS.16 In this communication, using a larger cohort of 317 LTx patients from two different LTx centers we determined that there was a correlation between the incidence of these pre-Tx Abs and specific pulmonary disease states. Furthermore, our results confirmed our previous findings that patients with pre-Tx Abs to SAgs have an increased incidence of development of PGD, higher circulating proinflammatory cytokines and increased development of allo- and autoimmunity leading to chronic rejection.
Methods
Study Design and Patients
Pre- and post-Tx sera from LTxs performed between 2000–2011 (317; Washington University 296 and Cleveland Clinic 21) were analyzed for development of DSAs and Abs to SAgs. Of the total LTx recipients, 161 had chronic obstructive pulmonary disease (COPD), 50 had idiopathic pulmonary fibrosis (IPF), 55 had cystic fibrosis (CF), and 51 had other etiologies. Among these other etiologies: 7 had primary pulmonary hypertension, 9 sarcoidosis, 9 bronchiectasis not secondary to COPD, 8 lymphangioleiomyomatosis, and the remaining (<5 per disease) had eosinophilic granulomatosis, mixed connective tissue disease, or a combination of these 2 etiologies. All patients were included in the study following informed consent and with the approval of Washington University Human Studies Committee.
Detection of Abs to mismatched DSA
Before transplantation, all patients were screened once per 3 months for pre-formed Abs to HLA using the LABScreen single antigen assay (One Lambda Inc, CA). Because of fluctuations in mean fluorescence intensity (MFI) between positive controls, a “positive” DFA was defined to be a ratio of sample to positive-control MFI of 0.2 or higher.17 Donor lungs were accepted only if they passed a virtual crossmatch with all previously identified Abs. All recipients had a direct negative crossmatch using serum obtained on the day of surgery. After Tx, recipients were rescreened for the presence of DSAs using the LABScreen Single Antigen assay at the time of surveillance bronchoscopies (performed within 48 hours, 1 month, 3 months, 6 months and 1 year after Tx) or if there was clinical evidence of graft dysfunction
Detection of Abs to SAgs
Sera were tested for development of Abs to Col-I, Col-V and Kα1T by enzyme linked immunosorbent assay (ELISA) as detailed earlier.9, 18 In brief, 1µg/mL of Col-I, Col-V and Kα1T suspended in phosphate-buffered saline were coated onto an ELISA plate (Col-I and Col-V were obtained from, Chemicon/ Millipore, Billerica, MA, and recombinant Kα1T was expressed in our laboratory) and incubated overnight at 4°C. Diluted patient and normal sera were then added to these plates. Detection was done using anti-human IgG-HRP (1:10,000), developed using TMB substrate and read at 450 nm. A sample was considered positive if values were greater than the mean + 2 standard deviations (218±31 ng/mL for Kα1T, 125±23 ng/mL for Col-I and 160±28 ng/mL for Col-V) from normal age-matched sera (n=33, age 47.8±12.4, male=19 and female=14). Ab concentration was calculated using a standard curve from known concentrations of Kα1T, Col-I or Col-V Abs (Santa Cruz Biotechnology, CA).
Cytokine measurement
Serum levels of cytokines IL-1β, IL-10, IFN-γ and IL-17 were detected by LUMINEX (Invitrogen, Carlsbad, CA) following the manufacturer’s protocols. Briefly, multiplex beads were vortexed and sonicated for 30 seconds and 25 µL was added to each well and washed twice with wash buffer. The samples were diluted 1:2 with assay diluent and loaded onto a Millipore multiscreen 96-well plate. Serial dilutions of cytokine standards were added to the plate in parallel. The bead bound cytokines were detected by biotinylated anti-human multi-cytokine reporter and streptavidin-phycoerythrin detection. The plates were analyzed by Bio-Plex Luminex 100™ (Invitrogen, Carlsbad, CA) and cytokine concentrations were calculated using the Bio-Plex Manager 3.0 software with a five parameter curve-fitting algorithm applied for standard curve calculations.
Statistical Analysis
Continuous data were checked for normality using the Shapiro-Wilk test. Non-normal data were transformed with a log transformation. Type 1 error was controlled when performing multiple t tests using the Dunn-Sidak correction. Tabular data were compared using the Fisher’s exact test for 2×2 tables and X2 for 2×n tables. Relative risks and confidence intervals were calculated using the contingency tables. For more than two group comparisons and analyzing multiple dependent variables, multivariate analysis of variance was used. Univariate and multivariate Cox proportional hazard models were constructed to identify risk factors for BOS. Software used for the above analyses were GraphPad Prism 4 (GraphPad, La Jolla, CA), and SPSS 16.
Results
Increased prevalence of Abs to SAgs (Kα1T and Col-I and Col-V) in IPF, CF and COPD patients listed for LTx
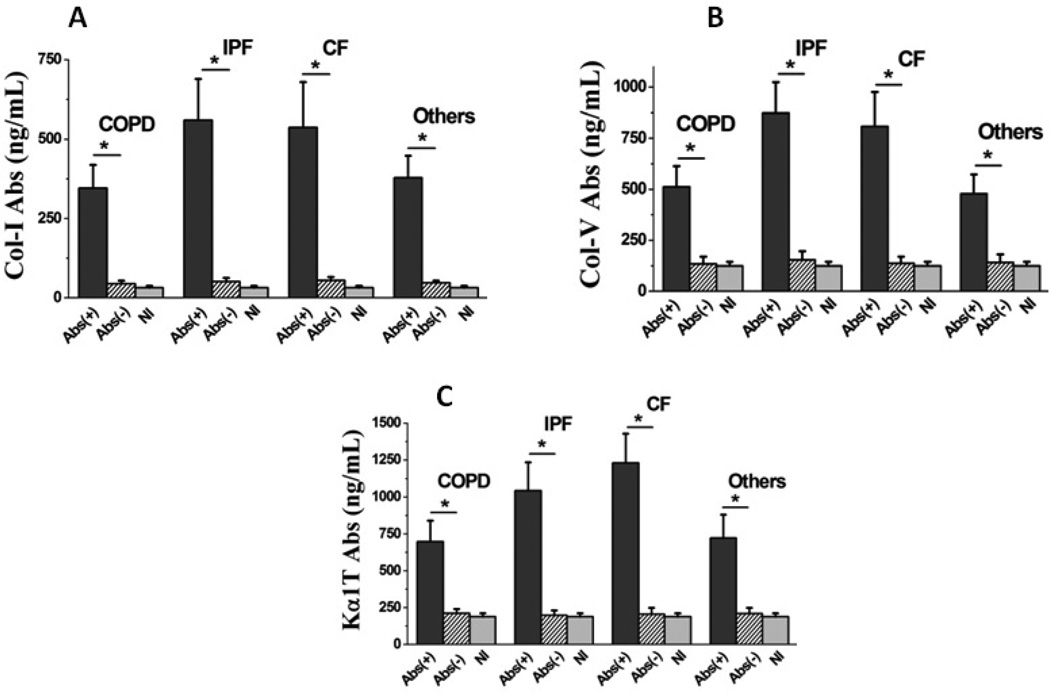
Previous studies have demonstrated a significant correlation between the development of Abs to SAgs and development of DSAs and BOS following human LTx.16 To determine the incidence of pre-existing Abs to SAgs in patients listed for LTx, we analyzed sera from 317 patients listed for LTx at 2 centers (Washington University and Cleveland Clinic) between 2000–2011. As shown in table 1, 29 out of 161 COPD (18.01%; p = 0.033), 17 out of 50 IPF (34%; p = 0.0006), 16 out of 55 CF (29.1%; p = 0.0023) and 10 out of 51 patients with other etiologies (19.6%; p = 0.044) were positive for Abs to lung associated SAgs, Col-I, Col-V and Kα1T. The statistical analysis were performed with comparison to the normal cohort of 33 subjects (table 1) with no lung diseases (17 male, 16 female; mean age: 49.3±13.6). The serum concentrations of Abs to SAgs were significantly elevated in all primary disease processes leading to LTx (Col-I (346±73 ng/mL in COPD; 561±129 ng/mL in IPF, 538±142 ng/mL in CF; 379±69 ng/mL in other etiologies; 32±6 ng/mL in normal), Col-V (513±101 ng/mL in COPD; 873±151 ng/mL in IPF, 809±167 ng/mL in CF; 479±94 ng/mL in other etiologies; 125±19 ng/mL in normal), and Kα1T (698±143 ng/mL in COPD; 1045±189 ng/mL in IPF, 1232±197 ng/mL in CF; 723±159 ng/mL in other etiologies; 190±23 ng/mL in normal), (figure 1).These results demonstrate that patients waiting for LTx often have pre-existing Abs to lung associated SAgs and that those with IPF and CF have the highest prevalence.
Table 1.
Incidence of Abs to lung restricted SAgs in various disease cohorts.
| Total | Pre-Tx Abs (+) | Odds Ratio |
CI | p-Value* | |
|---|---|---|---|---|---|
| COPD | 161 | 29 (18.01%) | 6.84 | 0.9 – 52.0 | 0.033 |
| IPF | 50 | 17 (34%) | 17 | 2.1 – 135.2 | 0.0006 |
| CF | 55 | 16 (29.1%) | 13.5 | 1.7 – 107.6 | 0.0023 |
| Others | 51 | 10 (19.6%) | 8.05 | 1.0 – 66.1 | 0.044 |
| Normal | 33 | 1 (2.94%) | n/a |
compared to the normal cohort.
Figure 1.
Serum concentration of Abs to lung restricted SAgs in various disease cohorts. Abs(+), represent the patients positive for pre-Tx Abs to SAgs; Abs(−), represent the patients negative for pre-Tx Abs to SAgs; and Nl, represent cohort of normal subjects. The statistical analysis was performed compared to the normal cohort. (*) represents statistically significant elevation of the concentration of serum Abs in Abs(+) cohort compared to the normal. There was no statistically elevated concentration of serum Abs in Abs(−) cohort compared to the normal, for any of the disease cohorts.
Pre-existing Abs to Kα1T, Col-V and Col-I increase the risk for development of PGD
We analyzed the incidence of PGD in patients with pre-Tx Abs to SAgs (grade II and III within 72 hrs following LTx). As shown in table 2, the presence of Abs to SAgs significantly increased the risk of PGD in those with COPD (82.76 vs 54.55%, p = 0.005), IPF (88.24 vs 42.42%, p = 0.0002), and CF (87.5 vs 48.72%, p = 0.008) with an odds ratio of 7 for the development of PGD. These results suggest a pathogenic role for Abs to lung associated SAgs in the development of PGD following LTx.
Table 2.
Incidence of PGD among various disease cohorts of LTx recipients with pre-Tx Abs to lung restricted SAgs.
| PGD $ | Odds Ratio |
CI | p- value* |
|||
|---|---|---|---|---|---|---|
| COPD | Pre-Tx Abs(+) | 29 | 24 (82.76%) | 4 | 1.4 – 11.1 | 0.005 |
| Pre-Tx Abs(−) | 132 | 72 (54.55%) | ||||
| total | 161 | 96 (59.63%) | ||||
| IPF | Pre-Tx Abs(+) | 17 | 15 (88.24%) | 10.2 | 2.0 – 51.9 | 0.002 |
| Pre-Tx Abs(−) | 33 | 14 (42.42%) | ||||
| total | 50 | 29 (58%) | ||||
| CF | Pre-Tx Abs(+) | 16 | 14 (87.5%) | 7.4 | 1.5 – 36.8 | 0.008 |
| Pre-Tx Abs(−) | 39 | 19 (48.72%) | ||||
| total | 55 | 33 (60%) | ||||
| Others | Pre-Tx Abs(+) | 10 | 7 (70%) | 1.4 | 0.3 – 6.0 | n/s |
| Pre-Tx Abs(−) | 41 | 26 (63.41%) | ||||
| total | 51 | 33 (64.7%) | ||||
= PGD2 & 3
= Compared between pre-Tx Abs(+) and pre-Tx Abs(−) cohorts.
Pre-existing Abs to Kα1T and Col-I and Col-V significantly increase de novo development of DSA
Alloimmunity, as defined by de novo development of DSAs, has been positively correlated with the development of BOS.1–3 Therefore, we analyzed the correlation between the presence of pre-Tx Abs to SAgs, the development of DSA and the incidence of PGD. As shown in table 3, the presence of pre-existing Abs to SAgs increased the incidence of DSA formation in COPD (62.07 vs 42.42%, p = 0.055), IPF (70.59 vs 48.48%, p = 0.112), and CF (62.5 vs 33.33%, p = 0.046) with an odds ratio of 2 for the development of PGD. These results suggest an association, albeit statistically insignificant, between Abs to lung associated SAgs and the development of DSAs following human LTx.
Table 3.
Incidence of DSA among various disease cohorts of LTx recipients with pre-Tx Abs to lung restricted SAgs.
| DSA | Odds ratio |
CI | p-value* | |||
|---|---|---|---|---|---|---|
| COPD | Pre-Tx Abs(+) | 29 | 18 (62.07%) | 2.2 | 1.0 – 5.1 | 0.055 (n/s) |
| Pre-Tx Abs(−) | 132 | 56 (42.42%) | ||||
| total | 161 | 74 (45.96%) | ||||
| IPF | Pre-Tx Abs(+) | 17 | 12 (70.59%) | 2.7 | 0.8 – 9.3 | 0.112 (n/s) |
| Pre-Tx Abs(−) | 33 | 17 (48.48%) | ||||
| total | 50 | 28 (56%) | ||||
| CF | Pre-Tx Abs(+) | 16 | 10 (62.5%) | 3.3 | 1.0 – 11.2 | 0.046 |
| Pre-Tx Abs(−) | 39 | 13 (33.33%) | ||||
| total | 55 | 23 (41.82%) | ||||
| Others | Pre-Tx Abs(+) | 10 | 5 (50%) | 1.9 | 0.5 – 7.8 | >0.8n/s |
| Pre-Tx Abs(−) | 41 | 14 (34.15%) | ||||
| total | 51 | 19 (37.25%) | ||||
= Compared between pre-Tx Abs(+) and pre-Tx Abs(−) cohorts.
Increased circulating proinflammatory cytokines in patients with pre-existing Abs to lung associated SAgs
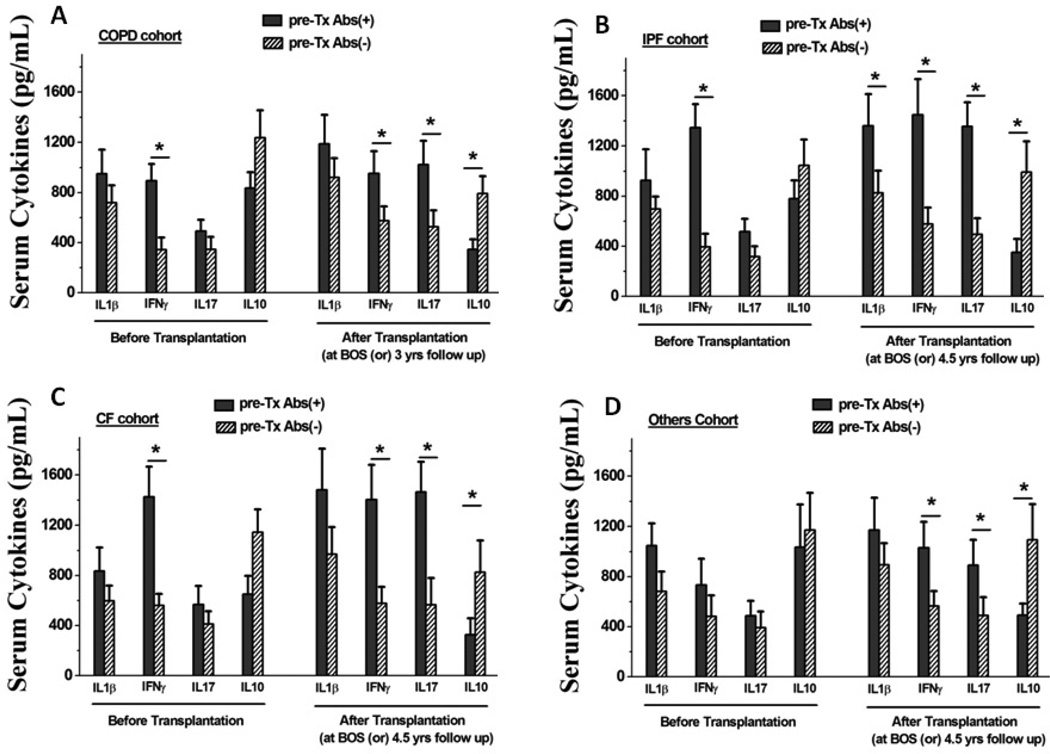
Pro-inflammatory (IL-1β, IFNγ and IL-17) and anti-inflammatory (IL-10) cytokines in the sera of patients with pre-Tx Abs to SAgs were determined using a sensitive Luminex method. As shown in figure 2A, patients with COPD, IPF and CF who had pre-Tx Abs to SAgs had higher circulating IFN-γ levels (figure 2A–C). It is of interest that following Tx these patients also demonstrated increased IL-17 levels with a concomitant decrease in IL-10 levels (figure 2A–D). These results demonstrated that the presence of pre-Tx Abs to SAgs may induce a SAg specific IL-17 response which can contribute to the development of autoimmune responses leading to poorer graft outcome following Tx.
Figure 2.
Serum concentration of cytokines among various disease cohorts of LTx recipients with pre-Tx Abs to lung restricted SAgs, prior to and 3 years following LTx. The pre-Tx Abs(+), represent the patients positive for pre-Tx Abs to SAgs; and the pre-Tx Abs(−), represent the patients negative for pre-Tx Abs to SAgs. The statistical analysis was performed between pre- Tx Abs(+) and pre-Tx Abs(−) cohort. (*) represents statistically significant elevation of the concentration of serum Abs in pre-Tx Abs(+) cohort compared to the pre-Tx Abs(−) cohort.
Pre-existing Abs to lung associated SAgs is a significant risk factor for the development of BOS following LTx
Development of allo and autoimmunity has been implicated as a major risk factor for the development of BOS.4 Therefore we analyzed the incidence of BOS in patients with pre-existing Abs to SAgs. As shown in table 4, the presence of pre-existing Abs to SAgs significantly increased the incidence of BOS in COPD (93.1 vs 41.67%, p < 0.001), IPF (88.24 vs 30.3%, p < 0.001), and CF (87.5 vs 35.9%, p < 0.001) LTx recipients with an odds ratio of 20. These results clearly demonstrate an important role for pre-existing Abs to lung associated SAgs in the development of BOS following human LTx.
Table 4.
Incidence of BOS among various disease cohorts of LTx recipients with pre-Tx Abs to lung restricted SAgs.
| BOS $ | Odds Ratio |
CI | p-value* | |||
|---|---|---|---|---|---|---|
| COPD | Pre-Tx Abs(+) | 29 | 27 (93.1%) | 18.9 | 4.3 – 82.8 | <0.001 |
| Pre-Tx Abs(−) | 132 | 55 (41.67%) | ||||
| total | 161 | 82 (50.93%) | ||||
| IPF | Pre-Tx Abs(+) | 17 | 16 (88.24%) | 36.8 | 4.3 –316.7 | <0.001 |
| Pre-Tx Abs(−) | 33 | 10 (30.3%) | ||||
| total | 50 | 26 (52%) | ||||
| CF | Pre-Tx Abs(+) | 16 | 14 (87.5%) | 12.5 | 2.5 – 63.1 | <0.001 |
| Pre-Tx Abs(−) | 39 | 14 (35.9%) | ||||
| total | 55 | 30 (54.55%) | ||||
| Others | Pre-Tx Abs(+) | 10 | 6 (60%) | 1.2 | 0.3 – 4.8 | n/s |
| Pre-Tx Abs(−) | 41 | 23 (56.1%) | ||||
| total | 51 | 29 (56.86%) | ||||
= BOS (clinical diagnosis)
= Compared between pre-Tx Abs(+) and pre-Tx Abs(−) cohorts.
Discussion
Studies from our laboratory, and others, have demonstrated that both humoral and cellular immunity to lung associated SAgs, Kα1T and Col-V can predispose patients to develop BOS.9, 11, 19, 20 In a previous communication from our center (n=142) we demonstrated that patients with pre-existing Abs to lung associated SAgs have an increased risk of developing PGD, DSAs and BOS.16 However, due to our limited sample size, we were unable to comment on the incidence of PGD, DSA formation and BOS development in specific disease states. Therefore, in our current study we analyzed a larger cohort of 317 patients specific from two LTx centers to determine the correlation between pre-Tx Abs, lung disease leading to Tx, and the development of PGD, DSAs and BOS, following LTx. Our results demonstrate that 18% of patients with COPD (29/161; p=0.033), 34% with IPF (17/50; p=0.0006), 29% with CF (16/55; p=0.0023) and 19.6% (10/51; p=0.044) with other diseases have pre-existing Abs to lung associated SAgs. These results are in agreement with reports demonstrating that various auto-Abs can be detected in higher rates in patients with certain lung diseases such as IPF and CF. For example, it has been shown that 40% of patients with IPF have Abs to the SAg, periplakin21, 22 and that the loss of peripheral regulatory T cells in patients with the same disease correlates with the development of Col-V specific Th17 and humoral responses.23 Similarly, studies in patients with CF have demonstrates increased levels of auto-Abs to ASCA and ANCA (43.7% and 40% respectively).24
As reported earlier, results from this study also demonstrated that patients who had pre- Tx Abs to SAgs had a significantly increased incidence of development of PGD (80 vs 45%, p<0.01), development of an alloimmune response (65 vs 40%, p<0.01) and increased risk for development of BOS (90 vs 40%, P<0.01). These findings strongly support our contention that either pre-existing Abs or de novo development of immune responses to lung associated SAgs increases the risk of developing BOS. Patients who developed Abs to SAgs also demonstrated significantly higher levels of circulating proinflammatory cytokines (figure 2) including increased IL-17 and loss of peripheral tolerance mediated by IL-10 (figure 2). We propose that this may be a contributing factor leading to autoimmunity and the development of BOS. Serial studies from our laboratory have shown that alloimmunity can predispose to development of immune responses to SAgs following human LTx.4 Therefore, we hypothesize that increased circulating proinflammatory cytokines leading to increased risk for development of alloimmunity can lead to the development of autoimmunity to lung associated SAgs. It is well accepted that Ab deposition can activate the complement cascade, which can then damage the epithelial cell surface by complement-mediated cytotoxicity. It has been demonstrated from lung biopsy specimens from patients with PGD following LTx, that those with C3d and C4d complement deposition have increased risk for the early development of BOS.25 However, the specificity of these Abs has not been identified. We propose that either Abs to mismatched HLA or lung associated SAgs can lead to complement activation and the resulting damage to the lung parenchyma. Preliminary studies from explanted lungs from patients who had pre-existing Abs to Kα1T or Col-V have demonstrated the presence of immunoglobulins and complement activation (C3d deposition) which support the notion that Abs to SAgs may have the ability to activate the complement cascade (unpublished data).
The pro-inflammatory milieu should also provide a conducive environment for the development of immune responses to SAgs and mismatched HLA Ags which can then directly activate epithelial and endothelial cells to increase stress proteins and growth factors.2 This can result in smooth muscle cell proliferation and fibrosis which are the hallmark lesions seen in BOS.26 There is evidence that ligation of SAgs by specific Abs can also result in inflammation that can augment the pro-fibrotic cascade leading to allograft failure.27 In a previous report, we demonstrated that exposure of the epithelial cell surface to Abs specific to Kα1T, an epithelial cell surface gap junction protein, can result in hypoxia inducible factor-1α mediated up-regulation of pro-fibrotic growth factors VEGF, HGF and TGF-β. 9, 27–29 These findings strongly suggest a direct pro-fibrotic role for some of these Abs to sAgs which can thus play a critical role in the development of chronic rejection.
Results presented in this manuscript also demonstrate that the presence of pre-Tx Abs to lung associated SAgs have an unfavorable correlation towards PGD and chronic lung allograft outcomes. We previously reported that LTx recipients who developed DSAs and received Ab directed therapy (ADRx) with IVIG and/or rituximab, but cleared only DSAs and not Abs to SAgs, had a significantly increased risk for the development of BOS.5 Furthermore, in patients who received ADRx, the incidence of BOS was significantly reduced by successful depletion of Abs to both DSAs and SAgs.5 Due to a strong correlation noted in our study between pre-existing Abs to SAgs and the development of DSAs following LTx, and in the light of the evidence suggesting that de novo development of DSAs is strongly associated with the development of BOS, we propose that ADRx with the goal of depleting pre-existing Abs to SAgs will be of benefit in reducing the incidence and severity of PGD, development of alloimmunity, and BOS.
Our study has several limitations which include lack of randomization and serial analysis. Furthermore, in spite of the inclusion of a larger number of patients (n=317) our sample size for individual etiologies still remains relatively small, leaving us unable to determine the risk of individual auto-Abss in specific diseases to leading to the formation of DSAs and BOS. The study size also did not permit us to analyze the relationship between these Abs and the risk of developing different grades of BOS. However, the analysis presented here does provide strong evidence for immune responses to lung associated SAgs contributing to the pathogenesis of BOS.
In conclusion, patients with all of the major etiologies leading to LTx have a significantly increased incidence of pre-existing Abs to SAgs. These patients are at increased risk for the development of PGD, DSAs and BOS. Therefore, strategies to remove pre-existing Abs to lung associated SAgs should be considered to improve lung allograft outcome. Following Tx, screening to detect Abs to SAgs coupled with intervention as soon as they are detected, has the potential to prevent the later development of immune responses to HLAs which we propose may prevent or significantly delay the development of chronic rejection.
Acknowledgments
Disclosure Statement
This work was supported by NIH HL056643 and the BJC Foundation (TM). The authors thank Ms. Billie Glasscock for her help in preparing this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflict of interest to disclose.
References
- 1.Sundaresan S, Mohanakumar T, Smith MA, et al. HLA-A locus mismatches and development of antibodies to HLA after lung transplantation correlate with the development of bronchiolitis obliterans syndrome. Transplantation. 1998;65:648–653. doi: 10.1097/00007890-199803150-00008. [DOI] [PubMed] [Google Scholar]
- 2.Smith MA, Sundaresan S, Mohanakumar T, et al. Effect of development of antibodies to HLA and cytomegalovirus mismatch on lung transplantation survival and development of bronchiolitis obliterans syndrome. J Thorac Cardiovasc Surg. 1998;116:812–820. doi: 10.1016/S0022-5223(98)00444-9. [DOI] [PubMed] [Google Scholar]
- 3.Palmer SM, Davis RD, Hadjiliadis D, et al. Development of an antibody specific to major histocompatibility antigens detectable by flow cytometry after lung transplant is associated with bronchiolitis obliterans syndrome. Transplantation. 2002;74:799–804. doi: 10.1097/00007890-200209270-00011. [DOI] [PubMed] [Google Scholar]
- 4.Saini D, Weber J, Ramachandran S, et al. Alloimmunity-induced autoimmunity as a potential mechanism in the pathogenesis of chronic rejection of human lung allografts. J Heart Lung Transplant. 2011;30:624–631. doi: 10.1016/j.healun.2011.01.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hachem RR, Tiriveedhi V, Patterson GA, Aloush A, Trulock EP, Mohanakumar T. Antibodies to K-alpha 1 Tubulin and Collagen V Are Associated With Chronic Rejection After Lung Transplantation. Am J Transplant. 2012;12:2164–2171. doi: 10.1111/j.1600-6143.2012.04079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trulock EP, Christie JD, Edwards LB, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fourth official adult lung and heart-lung transplantation report-2007. J Heart Lung Transplant. 2007;26:782–795. doi: 10.1016/j.healun.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Tiriveedhi V, Sarma N, Mohanakumar T. An important role for autoimmunity in the immunopathogenesis of chronic allograft rejection. Int J Immunogenet. 2012;39:373–380. doi: 10.1111/j.1744-313X.2012.01112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaramillo A, Smith MA, Phelan D, et al. Development of ELISA-detected anti-HLA antibodies precedes the development of bronchiolitis obliterans syndrome and correlates with progressive decline in pulmonary function after lung transplantation. Transplantation. 1999;67:1155–1161. doi: 10.1097/00007890-199904270-00012. [DOI] [PubMed] [Google Scholar]
- 9.Goers TA, Ramachandran S, Aloush A, Trulock E, Patterson GA, Mohanakumar T. De novo production of K-alpha1 tubulin-specific antibodies: role in chronic lung allograft rejection. J Immunol. 2008;180:4487–4494. doi: 10.4049/jimmunol.180.7.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bharat A, Fields RC, Steward N, Trulock EP, Patterson GA, Mohanakumar T. CD4+25+ regulatory T cells limit Th1-autoimmunity by inducing IL-10 producing T cells following human lung transplantation. Am J Transplant. 2006;6:1799–1808. doi: 10.1111/j.1600-6143.2006.01383.x. [DOI] [PubMed] [Google Scholar]
- 11.Burlingham WJ, Love RB, Jankowska-Gan E, et al. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117:3498–3506. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu KC, Jaramillo A, Mendeloff EN, et al. Concomitant allorecognition of mismatched donor HLA class I- and class II-derived peptides in pediatric lung transplant recipients with bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2003;22:35–43. doi: 10.1016/s1053-2498(02)00478-3. [DOI] [PubMed] [Google Scholar]
- 13.Jaramillo A, Smith MA, Phelan D, et al. Temporal relationship between the development of anti-HLA antibodies and the development of bronchiolitis obliterans syndrome after lung transplantation. Transplant Proc. 1999;31:185–186. doi: 10.1016/s0041-1345(98)01495-x. [DOI] [PubMed] [Google Scholar]
- 14.Kuo E, Maruyama T, Fernandez F, Mohanakumar T. Molecular mechanisms of chronic rejection following transplantation. Immunol Res. 2005;32:179–185. doi: 10.1385/IR:32:1-3:179. [DOI] [PubMed] [Google Scholar]
- 15.Hachem RR, Yusen RD, Meyers BF, et al. Anti-human leukocyte antigen antibodies and preemptive antibody-directed therapy after lung transplantation. J Heart Lung Transplant. 2010;29:973–980. doi: 10.1016/j.healun.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bharat A, Saini D, Steward N, et al. Antibodies to self-antigens predispose to primary lung allograft dysfunction and chronic rejection. Ann Thorac Surg. 2010;90:1094–1101. doi: 10.1016/j.athoracsur.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris GP, Phelan DL, Jendrisak MD, Mohanakumar T. Virtual crossmatch by identification of donor-specific anti-human leukocyte antigen antibodies by solid-phase immunoassay: a 30-month analysis in living donor kidney transplantation. Hum Immunol. 2010;71:268–273. doi: 10.1016/j.humimm.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Fukami N, Ramachandran S, Saini D, et al. Antibodies to MHC class I induce autoimmunity: role in the pathogenesis of chronic rejection. J Immunol. 2009;182:309–318. doi: 10.4049/jimmunol.182.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwata T, Philipovskiy A, Fisher AJ, et al. Anti-type V collagen humoral immunity in lung transplant primary graft dysfunction. J Immunol. 2008;181:5738–5747. doi: 10.4049/jimmunol.181.8.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bharat A, Fields RC, Trulock EP, Patterson GA, Mohanakumar T. Induction of IL-10 suppressors in lung transplant patients by CD4+25+ regulatory T cells through CTLA-4 signaling. J Immunol. 2006;177:5631–5638. doi: 10.4049/jimmunol.177.8.5631. [DOI] [PubMed] [Google Scholar]
- 21.Taille C, Grootenboer-Mignot S, Boursier C, et al. Identification of periplakin as a new target for autoreactivity in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;183:759–766. doi: 10.1164/rccm.201001-0076OC. [DOI] [PubMed] [Google Scholar]
- 22.Feghali-Bostwick CA, Wilkes DS. Autoimmunity in idiopathic pulmonary fibrosis: are circulating autoantibodies pathogenic or epiphenomena? Am J Respir Crit Care Med. 2011;183:692–693. doi: 10.1164/rccm.201010-1727ED. [DOI] [PubMed] [Google Scholar]
- 23.Kotsianidis I, Nakou E, Bouchliou I, et al. Global impairment of CD4+CD25+FOXP3+ regulatory T cells in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:1121–1130. doi: 10.1164/rccm.200812-1936OC. [DOI] [PubMed] [Google Scholar]
- 24.Lachenal F, Nkana K, Nove-Josserand R, Fabien N, Durieu I. Prevalence and clinical significance of auto-antibodies in adults with cystic fibrosis. Eur Respir J. 2009;34:1079–1085. doi: 10.1183/09031936.00006009. [DOI] [PubMed] [Google Scholar]
- 25.Westall GP, Snell GI, McLean C, Kotsimbos T, Williams T, Magro C. C3d and C4d deposition early after lung transplantation. J Heart Lung Transplant. 2008;27:722–728. doi: 10.1016/j.healun.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 26.Hachem RR, Trulock EP. Bronchiolitis obliterans syndrome: pathogenesis and management. Semin Thorac Cardiovasc Surg. 2004;16:350–355. doi: 10.1053/j.semtcvs.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Tiriveedhi V, Gelman AE, Mohanakumar T. HIF-1alpha signaling by airway epithelial cell K-alpha1-tubulin: Role in fibrosis and chronic rejection of human lung allografts. Cell Immunol. 2012;273:59–66. doi: 10.1016/j.cellimm.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tiriveedhi V, Angaswamy N, Weber J, Mohanakumar T. Lipid raft facilitated ligation of K-alpha1-tubulin by specific antibodies on epithelial cells: Role in pathogenesis of chronic rejection following human lung transplantation. Biochem Biophys Res Commun. 2010;399:251–255. doi: 10.1016/j.bbrc.2010.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tiriveedhi V, Gelman AE, Mohanakumar T. HIF-1alpha signaling by airway epithelial cell K-alpha1-tubulin: Role in fibrosis and chronic rejection of human lung allografts. Cell Immunol. doi: 10.1016/j.cellimm.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]