Abstract
Objective
Attention-deficit/hyperactivity disorder (ADHD) has been linked to deficits in the dopaminergic reward-processing circuitry, yet existing evidence is limited and the influence of genetic variation affecting dopamine signaling remains unknown. We investigated striatal responsivity to rewards in ADHD combined type (ADHD-CT) using functional magnetic resonance imaging (fMRI), and whether it is modulated by variation in the dopamine transporter gene (DAT1).
Method
We tested 29 male adolescents with ADHD-CT and 30 age-, handedness-and gender-matched healthy controls, selected for DAT110/6 haplotype dosage. Based on previous research, we focused our analysis on the ventral striatum and the caudate nucleus.
Results
Three main findings emerged: first, male adolescents with ADHD-CT did not differ from controls in terms of blood oxygen-level dependent (BOLD) fMRI response to reward-predicting cues (gain or loss-avoidance) in the ventral striatum. Second, male adolescents with ADHD-CT showed a relative increase, compared to controls, in the striatal BOLD response to successful outcomes. Third, DAT110/6 dosage differentially modulated neural activation to reward-predicting cues in the caudate nucleus in the ADHD-CT and control groups.
Conclusions
The findings challenge the idea of a deficit in anticipation-related activation in the ventral striatum in male adolescents with ADHD-CT, while suggesting that the processing of reward outcomes is dysfunctional, consistent with a recent neurobiological model of the disorder. Preliminary evidence suggests that polymorphic variations in genes affecting dopamine signaling need to be taken into consideration when investigating reward-related deficits in ADHD-CT.
Keywords: attention deficit hyperactivity disorder (ADHD), reward processing, ventral striatum, caudate nucleus, DAT1 (SLC6A3)
Introduction
Attention deficit hyperactivity disorder (ADHD) is a common childhood neuropsychiatric disorder characterized by developmentally inappropriate levels of inattention, hyperactivity and impulsivity. Deficits in executive functions and the underpinning neurocircuitry have been implicated in the pathophysiology of the disorder.1,2 Recent studies have further indicated deficits in the dopamine reward circuitry,3–5 as predicted by developmental neurobiological models of the disorder.6–8
To investigate reward circuitry functionality, functional magnetic resonance imaging (fMRI) studies have often used variants of the monetary incentive delay task (MID).9 This task involves two distinct phases: (a) an anticipation phase, where visual cues signal the potential to win or avoid losing money, and (b) an outcome phase, where participants receive feedback based on their performance. Research in non-human primates shows that rewards elicit the phasic release of dopamine from midbrain neurons to a wide network of regions, including the ventral striatum and caudate nucleus,10 which is captured as an increase in the functional magnetic resonance imaging (BOLD) signal (or activation) in fMRI studies.11–13 Over time, midbrain dopamine neurons respond to the predicting cues rather than the rewards themselves.10 Atypical brain function in MID-type tasks could be used as an index of reward-related dopamine signaling deficits. Such deficits have been associated with the development of ADHD behaviors in influential models of the disorder.6,8 Additionally, experimental data suggest an association between risky and impulsive behavior, which is characteristic of ADHD,14–16 and dopamine release or dopamine-dependent BOLD response to rewards in the striatum.11,17–20
Therefore, it is important to investigate the existence and understand the nature of reward-processing deficits in children and adolescents with ADHD. In adults with ADHD most studies report reduced activation in the ventral striatum (VS) during anticipation of monetary gains compared to controls,4,21,22 with one exception.23 Studies that investigated activation in the outcome phase reported increased activation in adults with ADHD (compared to controls) in the caudate nucleus and the orbitofrontal cortex4 or no differences.23 Yet the function of the dopaminergic mechanism and the role of polymorphic variation in dopamine genes vary with age,24,25 making extrapolation from adult studies difficult. To date, only a single study has focused on adolescents with ADHD (n=11), reporting decreased activation in the VS in the ADHD group (compared to controls) following cues predicting monetary gains, but not following cues predicting loss-avoidance, or in the outcome phase.3
The function of the reward circuitry can be modulated by functional genetic polymorphisms influencing dopamine neurotransmission in the striatum, directly (e.g. the dopamine transporter gene [DAT1]) or indirectly (e.g. the nitric oxide synthase gene [NOS1]).18,22,26 Neurochemical studies have demonstrated alterations in dopaminergic signaling in the striatum and the midbrain in ADHD, and linked such alterations with inattention symptoms and motivation problems in children and adults with ADHD.5,27–29 Such “baseline” deficits (i.e. not linked to any task) could result from genetic variations associated with the disorder, and molecular genetic studies have indeed linked risk for ADHD with polymorphic variants of dopamine genes.30
The stratification of samples by genotype can be used as a non-invasive way to investigate the effect of putative differences in dopaminergic neurotransmission on reward processing. In this study we focused on a haplotype of DAT1. DAT1 is mainly expressed in the striatum, with the highest density in the caudate nucleus.31 In the striatum the dopamine transporter (DAT) constitutes the main mechanism for terminating intrasynaptic dopamine activity.32 This haplotype consists of two polymorphic repeats that are in moderate to strong linkage disequilibrium:33 a variable-number-tandem-repeat (VNTR) in the 3′-untranslated (3′UTR) region, with the 9-repeat (9R) and 10-repeat (10R) alleles being the most frequent, and a VNTR at intron-8 that contains common 5-repeat (5R) and 6-repeat (6R) alleles. Both polymorphisms have been associated with ADHD.30,33 These polymorphisms have been separately linked with DAT density in the striatum, while recent evidence suggests that their joint consideration in haplotypes may provide more information than can be inferred from the analyses of single genetic markers.25 Similar to a previous study15, haplotype status was determined according to VNTR genotype status: homozygotes for the 10R and 6R alleles also possessed 2 copies of the DAT1 10-6 haplotype (DAT1 10-6 homozygotes). Carriers of at least one 9R allele would, by definition, possess <2 DAT1 10-6 copies, hence were DAT1 10-6 heterozygotes.
This study, using a well-characterized clinical sample of male adolescents with combined type ADHD (ADHD-CT) and matched controls, has two primary aims: First, to investigate if the BOLD response to incentive-predicting cues (for monetary gain or loss-avoidance), and the response to successful outcomes, differ in adolescents with ADHD-CT compared to controls in the VS and the caudate nucleus. Second, to provide an initial test of the hypothesis that genetic variation of DAT1 modulates striatal responsivity to incentive-predicting cues (which elicit phasic firing of midbrain dopamine neurons) in a diagnosis-specific manner. This hypothesis is based on two lines of evidence. First, there is a positive association between trait impulsivity or reward-related impulsivity and neural activation in the striatum following reward-predicting cues19,20,22 in healthy adult samples, and suggestion that the genetic variant that predicts increased reward-related activation in the striatum also predicts increased reward-related impulsivity.22 Second, previous data from the same sample showed that DAT110/6 homozygosity predicted reduced reward-related impulsivity in the ADHD-CT group, but increased impulsivity in the control group.15 Considering this evidence together, we hypothesized here that DAT110/6 homozygosity would be associated with decreased striatal responsivity to anticipated incentives in the ADHD-CT group and increased responsivity in the control group.
In this study, we measured neural activation to incentive-predicting cues and successful outcomes using the Motivated Incidental Learning Task (MILT)—a variant of the MID paradigm,34,35. Given that this exact variant has not been used before, a secondary but essential aim of this study was to confirm the validity of MILT as a measure of incentive cue-elicited activation. To this effect, we expect that, similar to the MID task, a whole brain analyses will yield a similar pattern of anticipatory activation of the reward circuitry and that activation in the VS in particular will increase with incentive magnitude.
Method
Participants
We recruited 29 Caucasian male adolescents with a clinical diagnosis of ADHD-CT and 30 age-, gender- and handedness-matched controls from a larger sample that participated in a previous study.36 The ADHD-CT group was part of the London subset of the International Multi-Centre ADHD Genetics (IMAGE) project.37 No co-morbid disorder was associated with either subgroup formed by the stratification of the ADHD sample by DAT110/6 dosage (2 copies, <2 copies; supplement 1 and Table S1, available online). Stimulant treatment (received by 72% of the ADHD-CT group) was discontinued at least 48 hours prior to testing (Table S2, available online). For details on inclusion and exclusion criteria for the IMAGE project and handedness, see supplement 1 (available online). The South London and Maudsley NHS Trust Research Ethics Committee approved the study and all participants, along with a parent/guardian provided written, informed consent.
Genetic analyses
Participants were selected to form four similar sized groups according to diagnostic status and the number of DAT1 10-6 copies (2 copies, <2 copies; Tables 1 and S3, available online). This stratification according to DAT1 10-6 dosage overlaps with and allows comparisons with the stratification according to either constituent genotype (namely, 10R homozygotes versus 9R carriers, which is most commonly used, or 6R homozygotes versus 5R carriers), while providing more information as it takes into account the joint information provided by both genotypes.25 Standard genotyping procedures were used to determine DAT110/6 status (see supplement 1, available online).
Table 1.
Clinical, experimental and demographic data for the attention-deficit/hyperactivity disorder combined type (ADHD-CT) and control participants.
| ADHD-CT (DAT110/6) |
Control (DAT110/6) |
|||||
|---|---|---|---|---|---|---|
| Mean (SD) | 2 copies (n=15) | <2 copies (n=14) | 2 copies (n=16) | <2 copies (n=14) | Significance Test | p |
| Age (years) | 15.60 (2.35) |
15.35 (2.06) |
15.35 (1.4) |
15.87 (2.06) |
Fgroup(1, 55)=0.06 Fhaplotype(1, 55)=0.07 Finteraction (1, 55)=0.54 |
.80 .79 .47 |
| IQ | 109.4 (12.8) |
101.07 (12.97) |
111.06 (9.9) |
115.5 (12.20) |
Fgroup(1, 55)=6.63 Fhaplotype(1, 55)=0.39 Finteraction (1, 55)=4.18 |
.013 .54 .046 |
| Conners' DSM-IV ADHD Inattention ratings (parent) |
65.67 (10.77) |
72.5 (8.23) |
46.53 (4.61) |
47.29 (6.35) |
Fgroup(1, 54)=115.56 Fhaplotype(1, 54)=3.38 Finteraction (1, 54)=2.17 |
<.001 .071 .15 |
| Conners' DSM-IV ADHD Hyperactivity/Impulsivity ratings (parent) |
77 (13.94) |
81.07 (12.80) |
47.4 (7.19) |
48.14 (6.13) |
Fgroup(1, 54)=126.14 Fhaplotype(1, 54)=0.75 Finteraction (1, 54)=0.36 |
<.001 .39 .55 |
| Conners' DSM-IV ADHD Total ratings (parent) |
72.6 (12.87) |
79.29 (9.98) |
46.53 (5.42) |
47.36 (6.03) |
Fgroup(1, 54)=146.16 Fhaplotype(1, 54)=2.45 Finteraction (1, 54)=1.49 |
<.001 .12 .23 |
| Mean response time, Gain trials (SE) | 609.24 (19.72) |
631.47 (20.42) |
595.01 (19.10) |
571.31 (20.42) |
Fgroup(1, 55)=3.49 Fhaplotype(1, 55)=0.00 Finteraction (1, 55)=1.33 |
.067 .97 .25 |
| Mean response time, Loss-avoidance trials (SE) | 621.31 (19.29) |
638.49 (19.97) |
604.33 (18.68) |
580.01 (19.97) |
Fgroup(1, 55)=3.75 Fhaplotype(1, 55)=0.03 Finteraction (1, 55)=1.13 |
.058 .86 .29 |
| Subjective ratings of excitement induced by gain-anticipation (£5 vs. £0) | 41.73 (17.06) |
39.54 (25.08) |
45.13 (22.76) |
49.57 (13.38) |
Fgroup(1, 54)=1.50 Fhaplotype(1, 54)=0.04 Finteraction (1, 54)=0.37 |
.23 .84 .55 |
| Subjective ratings of effort invested in large incentive trials (£5 vs. £0) | 40.47 (23.46) |
33.46 (29.28) |
42.20 (23.85) |
30.14 (17.51) |
Fgroup(1, 54)=0.01 Fhaplotype(1, 54)=2.12 Finteraction (1, 54)=0.15 |
.90 .15 .70 |
| Success rate (%) | 78.29 (0.48) |
77.59 (0.50) |
78.48 (0.47) |
78.48 (0.50) |
Fgroup(1, 55)=1.22 Fhaplotype(1, 55)=0.51 Finteraction (1, 55)=0.53 |
.27 .48 .47 |
| Total Earned (£) | 140 (3.14) |
140.93 (3.25) |
141.44 (3.04) |
135 (3.25) |
Fgroup(1, 54)=0.50 Fhaplotype(1, 54)=0.75 Finteraction (1, 54)=1.35 |
.48 .39 .25 |
Note: DAT1 = dopamine transporter gene; IQ = intelligence quotient; SE = standard error.
Motivated Incidental Learning Task (MILT)
In this task (Figure 1), one small or two large arrows represented incentives (£1/£5 respectively), with color denoting valence (green: win; red: avoid losing). A red/green rectangle represented trials involving no money (neutral). Trial set-up was as follows: incentive cue (1 sec), jittered anticipation delay (2–5 sec), picture (1.5 sec), requiring a fast and accurate semantic decision (living/non-living) about unambiguous stimuli (animals or inanimate artefacts), blank screen (0.5 sec), outcome notification (1.5 sec), jittered inter-trial interval (0.5–3 sec). An algorithm adjusted from trial to trial the upper time boundary for a valid response, maintaining a success rate of about 80%; the starting value was individually set based on the practice session, and the lower boundary was set to 80 msec. A separate aim was to investigate the effects of reinforcement context on episodic memory formation for target pictures (not reported here). Participants completed 160 trials (in pseudo-random order) in two 13.3 min sessions. There were 32 trials for each incentive valence and magnitude combination (±£1/£5, £0). All participants saw the same 160 colored pictures of living (50%) and non-living objects (see supplement 1, available online). Participants achieved a high rate (> 94%) of semantically correct responses. Only successful trials (where a semantically correct response was made within the acceptable time window) were included in the analyses reported here (see Table 2 for success rates by subgroup).
Figure 1.
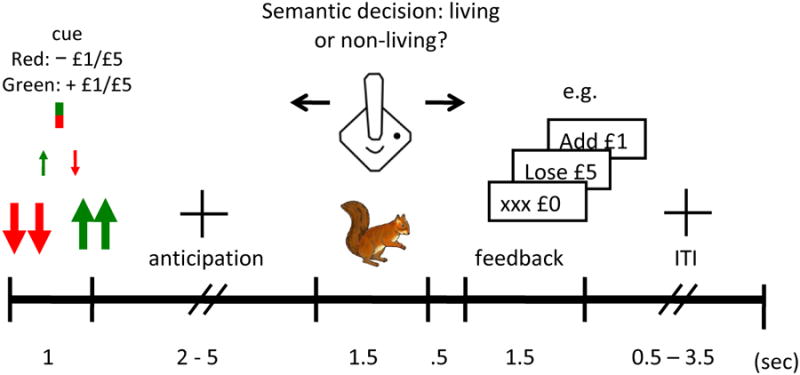
Motivated incidental learning task (MILT). Note: ITI = inter-trial interval.
Table 2.
Analyses of Covariance (ANCOVAs) investigating the effect of clinical group (attention-deficit/hyperactivity disorder [ADHD] combined type, control), dopamine transporter gene (DAT110/6) (2 copies, <2 copies), task phase (anticipation, outcome), incentive valence (gain, loss-avoidance) and incentive magnitude (small, large) on neural activation in the ventral striatum and the caudate nucleus head and body.
| Ventral striatum | Caudate nucleus body | Caudate nucleus head | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect | F | df | pb | η2p | p IQc | F | df | pb | η2p | p IQ3 | F | df | pb | η2p | p IQc |
| Clinical group | 0.52 | 1,54 | ns | .01 | ns | 0.97 | 1,54 | ns | .02 | ns | 1.18 | 1,54 | ns | .02 | ns |
| DAT110/6 | 0.87 | 1,54 | ns | .02 | ns | 0.44 | 1,54 | ns | .01 | ns | 0.04 | 1,54 | ns | .00 | ns |
| Group × DAT110/6 | 0.47 | 1,54 | ns | .01 | ns | 0.18 | 1,54 | ns | .00 | ns | 0.37 | 1,54 | ns | .01 | ns |
| Phasea | 39.95 | 1,54 | <.001 | .43 | <.001 | 18.41 | 1,54 | <.001 | .25 | <.001 | 23.87 | 1,54 | <.001 | .31 | <.001 |
| × Groupa | 0.03 | 1,54 | ns | .00 | ns | 1.53 | 1,54 | ns | .03 | ns | 0.29 | 1,54 | ns | .01 | ns |
| × DAT110/6a | 0.55 | 1,54 | ns | .01 | ns | 0.27 | 1,54 | ns | .01 | ns | 0.03 | 1,54 | ns | .00 | ns |
| × Group × DAT110/6a | 1.67 | 1,54 | ns | .03 | ns | 8.63 | 1,54 | .005 | .14 | .011 | 3.36 | 1,54 | .072 | .06 | ns |
| Valencea | 13.60 | 1,54 | .001 | .20 | <.001 | 2.09 | 1,54 | ns | .04 | ns | 5.05 | 1,54 | .029 | .09 | .027 |
| × Groupa | 0.10 | 1,54 | ns | .00 | ns | 3.51 | 1,54 | ns | .04 | ns | 0.31 | 1,54 | ns | .01 | ns |
| × DAT110/6a | 0.19 | 1,54 | ns | .00 | ns | 0.22 | 1,54 | ns | .00 | ns | 0.04 | 1,54 | ns | .00 | ns |
| × Group × DAT110/6a | 0.19 | 1,54 | ns | .00 | ns | 0.00 | 1,54 | ns | .00 | ns | 0.01 | 1,54 | ns | .00 | ns |
| Magnitudea | 12.84 | 1,54 | .001 | .19 | .001 | 7.22 | 1,54 | .010 | .12 | .010 | 7.85 | 1,54 | .007 | .13 | .007 |
| × Groupa | 4.07 | 1,54 | .049 | .07 | .058 | 2.05 | 1,54 | ns | .04 | ns | 2.68 | 1,54 | ns | .05 | .077 |
| × DAT110/6a | 0.20 | 1,54 | ns | .00 | ns | 0.16 | 1,54 | ns | .00 | ns | 0.03 | 1,54 | ns | .00 | ns |
| × Group × DAT110/6a | 0.03 | 1,54 | ns | .00 | ns | 0.04 | 1,54 | ns | .00 | ns | 0.02 | 1,54 | ns | .00 | ns |
| Phase × Valencea | 2.70 | 1,54 | ns | .05 | ns | 3.46 | 1,54 | .069 | .06 | .069 | 1.91 | 1,54 | ns | .03 | ns |
| × Groupa | 0.88 | 1,54 | ns | .02 | ns | 0.40 | 1,54 | ns | .01 | ns | 1.41 | 1,54 | ns | .03 | ns |
| × DAT110/6a | 1.12 | 1,54 | ns | .02 | ns | 0.30 | 1,54 | ns | .02 | ns | 0.60 | 1,54 | ns | .01 | ns |
| × Group × DAT110/6a | 0.00 | 1,54 | ns | .00 | ns | 0.39 | 1,54 | ns | .01 | ns | 0.00 | 1,54 | ns | .00 | ns |
| Phase × Magnitudea | 15.64 | 1,54 | <.001 | .23 | <.001 | 8.13 | 1,54 | .006 | .01 | .006 | 9.03 | 1,54 | .004 | .14 | .004 |
| × Groupa | 5.61 | 1,54 | .021 | .09 | .026 | 2.87 | 1,54 | .096 | .05 | ns | 6.38 | 1,54 | .015 | .11 | .022 |
| × DAT110/6a | 1.70 | 1,54 | ns | .03 | ns | 0.00 | 1,54 | ns | .00 | ns | 0.54 | 1,54 | ns | .01 | ns |
| × Group × DAT110/6a | 1.29 | 1,54 | ns | .02 | ns | 0.78 | 1,54 | ns | .01 | ns | 1.42 | 1,54 | ns | .03 | ns |
| Phase × Valence × | .0.08 | 1,54 | ns | .00 | ns | 1.65 | 1,54 | ns | .03 | ns | 0.41 | 1,54 | ns | .01 | ns |
| Magnitudea | |||||||||||||||
| × Groupa | 0.19 | 1,54 | ns | .01 | ns | 0.00 | 1,54 | ns | .00 | ns | 0.36 | 1,54 | ns | .01 | ns |
| × DAT110/6a | 0.44 | 1,54 | ns | .00 | ns | 0.50 | 1,54 | ns | .01 | ns | 0.46 | 1,54 | ns | .01 | ns |
| × Group × DAT110/6a | 0.64 | 1,54 | ns | .01 | ns | 0.08 | 1,54 | ns | .00 | ns | 0.14 | 1,54 | ns | .00 | ns |
Note: ns = non-significant.
With Greenhouse-Geisser correction.
p-values from ANCOVA, with age as a covariate.
p-values from ANCOVA, with age and intelligence quotient (IQ) as covariates (df=[1,53]). p-values <.10 are shown. p-values <.05 are displayed in bold.
MRI scanning was preceded by a rewarded 40-trial practice session in a mock scanner to familiarize participants with the scanning environment, train them on the task, teach them cue-reward associations, determine the initial values for the response time window and increase the salience of rewards. Participants were shown a box containing real money that they could earn and were informed that they would earn money in proportion to the amount they had accumulated playing the task (although all participants received £2.75 for the practice session and £7.50 for the main task). At the end of the scanning session, participants completed two visual-analog scales to indicate how exciting they found each incentive condition (“not exciting at all” to “extremely exciting”) or how much effort they exerted to get a fast and accurate response (“didn't bother much” to “tried very hard”). For technical reasons, visual analog scale data were only available for monetary gain trials.
Other measures
ADHD rating scales
ADHD symptoms were assessed using the 18 DSM-IV items from the long form of the revised Conners' Parent Rating Scales,38 obtained on the day that participants were scanned.
General intelligence
The vocabulary, similarities, picture completion and block design subtests of the Wechsler Intelligence scales for children 39 and adults 40 were used to estimate IQ at the time of the initial assessment (18–60 months before the current study; M=43.2, SD=9.36).
Sample and performance data analysis
We examined the effects of diagnosis and DAT110/6 dosage on a range of sample or task performance variables using a 2 (ADHD-CT, control) × 2 (DAT110/6 2 copies, <2 copies) analysis of variance (ANOVA; see Table 1). To investigate the effect of incentive magnitude on performance, we conducted a mixed 3 (£0, £1, £5) × 2 (ADHD-CT, control)
fMRI data: first-level analysis
MRI data were acquired on a General Electric SIGNA HD× 3.0T MR scanner (General Electric Medical Systems, Milwaukee, WI) and analyzed in SPM8 (www.fil.ion.ucl.ac.uk/spm) using the general linear model (see supplement 1, available online, for acquisition and pre-processing details). Event-specific regressors were convolved with the canonical hemodynamic response function to model the BOLD signal. We used 10 regressors marking events of interest: phase (anticipation or outcome onset), incentive magnitude (£0, £1 or £5) and valence (gain or loss-avoidance) in successful trials. Regressors for events of no interest were: unsuccessful/error trials and the first and second order movement parameters from the realignment procedure. A high-pass filter (128 sec) was applied to the data and first-order temporal autocorrelation was modeled. Weighted contrasts were used to test the effect of cue-elicited changes on BOLD signal (henceforth referred to as activation) during gain or loss-avoidance anticipation or outcome notification for each individual. For second level (group) analyses, the contrast images from the first-level analysis were used to calculate mean activation for each region of interest (ROI) using MarsBar (http://marsbar.sourceforge.net/),41 or to conduct whole brain analyses.
fMRI data: group analyses
In line with previous studies,3,4,21–23 our primary ROI was the VS. We further included separate ROIs for caudate nucleus head and body. The caudate nucleus is a core region of the dopamine reward-circuit,42 implicated in ADHD-control differences,4 and genetic variation in DAT1 is reported to affect its function and structure 26,43 (supplement 1, available online, describes anatomical ROI definition).
We calculated mean brain activation in each ROI, using neutral trials as the baseline, across task phase, incentive valence and magnitude. Data were averaged over left and right hemispheres, following a preliminary test showing no significant effect for brain hemisphere (or interaction with diagnostic group or DAT110/6). Both our primary aims were tested within an omnibus analysis of covariance (ANCOVA) model (fitted separately for each ROI), with clinical diagnosis (ADHD, control) and DAT110/6 dosage (2 copies, <2 copies) as the between-subjects factors, and task phase (anticipation, outcome), incentive valence (gain, loss-avoidance) and incentive magnitude (£1, £5) as the within-subjects factors (Table 2 presents the main effects, and the interactions of interest). Age was included as a covariate,42,44 and analyses were repeated covarying IQ (p-values reported separately). Significant interactions were followed-up using simple effects analyses. Complementary whole-brain analyses were conducted and are reported in supplemental material (available online).
To directly replicate and facilitate comparison with previous research in relation to our first aim,3 we ran a 2 (ADHD, control) × 2 (DAT110/6 2 copies, <2 copies) × 2 (£1, £5) ANCOVA, separately for gain and loss-avoidance anticipation and brain hemisphere, focusing on the VS (see Figure 2). This test was further used to confirm that anticipatory activation in the VS increased with incentive magnitude.34 The validity of the task was also confirmed by testing the BOLD response to incentive-predicting cues using whole-brain analyses and conducting one sample T-tests on the following contrast for each incentive valence: Anticipation±£5 > Anticipation£0, Inferences were conducted at the cluster-level using family-wise error correction (α=0.05); a voxel was considered for cluster-level analysis if p<.001 (see supplement 2, available online, for group comparisons). Finally, we present correlations between neural activation and ADHD symptom ratings in supplemental material (supplement 2; Tables S4 and S5, available online).
Figure 2.
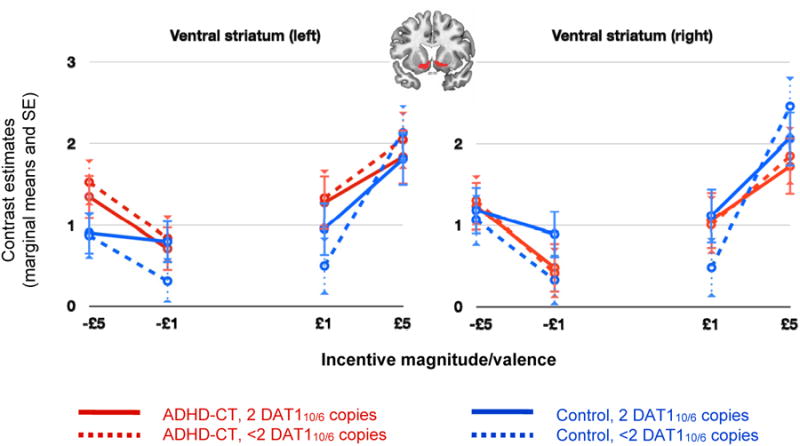
Ventral striatum (VS): response to incentive-predicting cues. Note: Cue-induced activation in the VS increased with incentive magnitude in gain (p < .001) and loss-avoidance (p = .006) trials. Main effects for diagnosis, dopamine transporter gene (DAT110/6) dosage or their interaction were not significant. ADHD-CT = attention deficit hyperactivity disorder combined type; DAT1 = dopamine transporter gene; SE = standard error.
Results
Does neural activation to incentive-predicting cues and successful outcomes differ between adolescents with ADHD-CT and controls?
While there were no significant main effects for diagnostic group, or interactions between diagnostic group, task phase and incentive valence, we observed significant interactions between diagnostic group, task phase and incentive magnitude in the VS and the caudate nucleus head (Table 2). We explored these interactions post-hoc using simple effects analyses. The interaction between diagnostic group and incentive magnitude was significant during the outcome presentation phase in the caudate nucleus head (F(1,54)=6.91, p=.011, η2p=.11; pIQ=.012) and the VS (F(1,54)=12.65, p<.001, η2p=.13; pIQ=.004), but not during the incentive anticipation phase (Fs<1.90, ns). Further post-hoc simple effects analyses to explore the interaction between diagnostic group and incentive magnitude showed that neural activation in the VS (F(1,54)=4.85, p=.032, η2p=.08; pIQ=.014) and the caudate nucleus head (F(1,54)=6.58, p=.013, η2p=.11; pIQ=.002) was higher in the ADHD-CT group, compared to controls, for large (£5), but not small (£1; Fs<1.00, ns) outcomes.
These results (Table 2) suggest the ADHD-CT and control groups did not differ in terms of neural activation to incentive-predicting cues in any ROI. To directly replicate and facilitate comparison with a previous publication3, we ran a 2 (ADHD, control) × 2 (DAT110/6 2 copies, <2 copies) × 2 (£1, £5) ANCOVA, separately for gain and loss-avoidance anticipation and brain hemisphere, focusing on the VS. We confirmed that the ADHD-CT and control groups did not differ in terms of neural activation to incentive cues in the VS, for either incentive valence or brain hemisphere, irrespective of incentive magnitude (or DAT110/6 haplotype; Fs<1.00, ns; Figure 2).
Does genetic variation in DAT110/6 modulate neural activation in response to incentive-predicting cues?
We observed a significant three-way interaction between diagnostic group, DAT110/6 and task phase. This interaction was explored post-hoc with separate ANCOVAs for each task phase using simple effects analysis. There was a significant two-way interaction between diagnosis and DAT110/6 in the incentive anticipation phase (F(1,54)=4.25, p=.044, η2p=.06; pIQ=.031), but not in the (successful) outcome presentation phase (F(1,54)=1.92, p=.17). Figure 3 shows that the interaction of diagnosis and DAT110/6 during incentive anticipation followed a crossover pattern, indicating that the effect of DAT110/6 dosage on neural activation to incentive-predicting cues differs between the two groups: in the ADHD-CT group, activation tended to decrease as DAT110/6 dosage increased, but in the control group the reverse was found.
Figure 3.
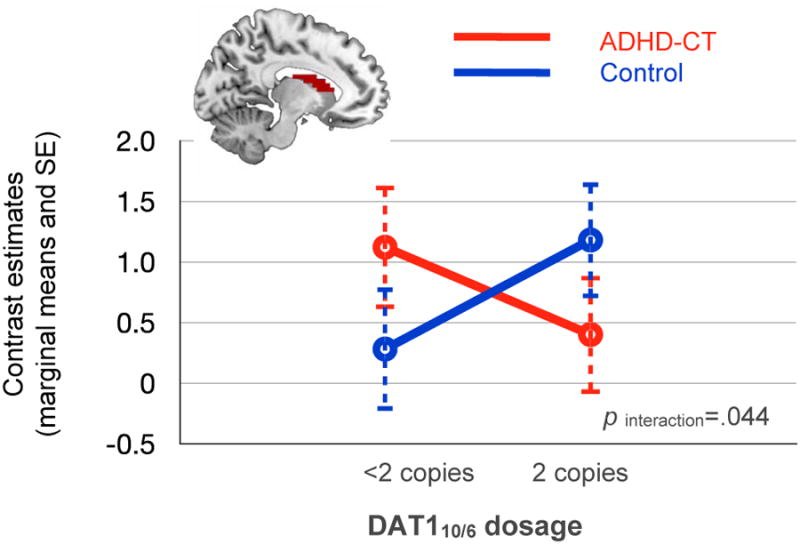
Caudate nucleus body: Blood oxygen level dependent (BOLD) response to incentive-predicting cues. Note: BOLD response to incentive-predicting cues in the caudate nucleus body, averaged over task valence (gain or loss-avoidance), incentive magnitude (£1 or £5) and brain hemisphere. ADHD-CT = attention deficit hyperactivity disorder combined type; DAT1 = dopamine transporter gene; SE = standard error.
The MILT task: behavior, reward-network activation and motivational effects
In both the ADHD-CT and control groups, the incentive-predicting cues activated the predicted network, as previously identified (see supplement 2, available online, for more details and group comparisons; Tables S6-S8 and Figure S1, available online). Consistent with previous reports,34 cue-elicited activation in the VS increased with the magnitude of the anticipated incentive (gain trials: F(1,55)=17.48, p<.001, η2p=.24; loss-avoidance: F(1,55)=8.17, p<.006, η2p=.13), irrespective of diagnosis or DAT110/6 dosage (Fs<1.81, ns; Figure 2). The increase in incentive magnitude also resulted in a linear decrease in response times for each incentive valence across groups (FGain(1,57)=138.52, P<.001; FLoss-avoidance(1,57)=57.37, p<.001), FsDiagnosis × magnitude <1, ns). The lack of significant group differences on indices of task performance indicated that all groups experienced similar outcomes and contributed a similar number of trials (Table 1).
Discussion
Three main findings emerged in this study of 29 male adolescents with ADHD-CT and 30 healthy controls. First, adolescents with ADHD-CT did not differ from controls in terms of cue-elicited (gain or loss-avoidance) neural activation in the VS. Second, adolescents with ADHD-CT showed a relative increase in the striatal BOLD response, compared to controls, following confirmation of a successful outcome. Third, DAT110/6 dosage modulated incentive cue-elicited activation in the caudate nucleus body differently in the ADHD-CT and control groups.
The lack of a deficit in cue-elicited activation in the VS for the ADHD-CT group (compared to the control group) cannot be explained by a general lack of task engagement. Both groups showed similar increases in anticipatory activation and decreases in response times with incentive magnitude, irrespective of incentive valence. The groups did not differ either in task performance measures. These effects confirm the validity of MILT as a measure of incentive cue-elicited neural activation, suggest that the motivational manipulation was effective and that the interpretation of potential group differences in brain activation is not confounded by performance differences.
To date, a single previous study has compared adolescents with ADHD to healthy controls using an MID paradigm3. Our findings, while in agreement with the reported lack of significant group differences in neural activation to loss-avoidance anticipation, apparently contradict the demonstration of reduced activation in the VS during gain anticipation.3 Yet the relatively small sample size in that study (n=11)3 and the inclusion of both genders and mixed ADHD subtypes (factors that have been associated with reward-related activation and impulsivity16,45) make direct comparisons difficult.
A further difference from previous studies regards the task itself. MILT differed from MID-type paradigms used in earlier ADHD research in two ways: first, the conditioned cues predicted successful outcomes with greater certainty (∼80%, compared to ∼66%3,4). The strength of the association between cue and outcome was matched between groups, as all groups demonstrated similar success rates. Research in non-human primates, confirmed in humans, indicates that differences the probability with which a cue predicts a reward produce a quantitative change in the engagement of dopaminergic systems.46,47 Thus, the increased certainty may even have sharpened the degree to which cues elicited anticipatory activity, while we would not expect that it makes a qualitative difference with regard to incentive-elicited activation. Nonetheless we cannot fully preclude different sensitivities to reward in ADHD and comparison groups between tasks using different reward rates based on the literature to date. Clearly, future studies should examine more explicitly the integration of absolute reward value and probability in ADHD. The second way in which MILT differed from previous applications in ADHD research (but see35 for a similar variant in cognitive neuroscience) was that, due to its nature as an incidental learning task (an aspect that is not discussed in this paper), the targets comprised drawings of living and non-living objects and required a semantic judgment (living or nonliving). Previous MID-variants have used targets requiring an element of cognitive processing,35 which is controlled for by using non-incentive trials that are matched on semantic processing as the comparison condition. Participants were pre-trained on this aspect of the task and achieved an extremely high (>94%) percentage of correct semantic decisions. Importantly, semantic decisions followed the anticipation phase. Moreover, the focus on ROIs directly relevant to reward processing reduces the possibility that our findings are biased by this aspect of the task.
The lack of a deficit in anticipatory activation was accompanied by increased neural activation in the striatum following confirmation of successful outcomes for the ADHD-CT group. Although in agreement with previous evidence from adults with ADHD,4 the single previous study that examined outcome-related activation in adolescents with ADHD had focused solely on the VS and did not find group differences.3 Yet, given that successful trials in MILT are more common (∼80%) compared to previous paradigms (∼66%), it is more difficult to directly compare findings across studies. The phasic firing of midbrain dopamine neurons is expected to transfer to reward-predicting cues,6,10 and therefore the higher the expected success rate the more complete this transfer would be expected to be. This is consistent with the lack of significant activation in response to successful outcomes in the control group in our study (see whole brain analyses in supplement 1, available online). Our finding of increased caudate nucleus activity in the ADHD-CT group is also consistent with a dysfunctional transfer of phasic dopamine release from the actual reward to its predicting stimulus. This may result in impaired appraisal of motivational outcomes and subsequent adaptation of behavior.6
Polymorphic variation in the DAT1 gene was found to modulate neural activation in response to incentive-predicting cues in the striatum in a diagnosis-specific manner. Specifically, DAT110/6 homozygosity was associated with decreased striatal responsivity to anticipated incentives in the ADHD-CT group and increased responsivity in the control group. The specificity of this finding for the caudate nucleus could be due to the relatively lower levels of the dopamine transporter in the ventral portion of the striatum.48 Moreover, apart from the VS, dorsal regions of the caudate also receive efferent phasic input from midbrain dopamine neurons in response to reward predicting cues. This finding confirms previous evidence from healthy volunteers that genetic variation in DAT1 modulates striatal reward-related activation.26,49,50 Furthermore, when our data here are considered together with our previous report using a hypothetical delay discounting task with the same participants,15 a pattern emerges, consistent with findings from other genes indirectly involved in dopamine signaling (NOS1), that the genetic variant that predicted increased reward-related activation in the striatum also predicted increased reward-related impulsivity.22 This pattern suggests a putative neural mechanism linking genetic variation in DAT1, striatal responsivity to rewards and behavioral impulsivity across diagnostic categories and indicates that the established inverted U-shape model of prefrontal cortical dopamine levels and function (and consequently neurocognitive performance), might also operate in the striatum.51-53
One limitation of our study is the modest sample size for genetic analyses. Our genetic findings should therefore be treated as preliminary and be interpreted with caution until further replication studies have been completed. While the use of an ADHD-CT sample that had a current or previous history of stimulant treatment is also a potential limitation, recent evidence showed that ventral striatal activation does not differ between medicated and medication-naïve adults with ADHD.22 The presence of possible comorbid oppositional-defiant disorder and conduct disorder in some patients may also limit the interpretation of our findings as reflecting ADHD-specific abnormalities in reward processing. While the effect sizes for those with and without comorbidities are similar, further research in larger groups assessed quantitatively against symptoms is clearly warranted.
In conclusion, our findings challenge the idea of a deficit in neural activation to incentive-predicting cues in the VS in male adolescents with ADHD-CT, while suggesting that the processing of reward outcomes is dysfunctional, which may impair learning and adaptive behavior.6 Furthermore, the results suggest that polymorphic variations in genes affecting dopamine signaling play a key role in regulating in neural activation to incentive-predicting cues and need to be taken into consideration when investigating deficits in disorders that may be characterized by genetically driven changes in the baseline function of the dopamine system. Future research needs to investigate the integrity of the different aspects of the reward-processing mechanism in ADHD and illuminate possible interactions with other genes affecting dopaminergic neurotransmission directly or indirectly (e.g. serotonergic) in the shaping of clinically relevant behavior.
Supplementary Material
Acknowledgments
This work was supported by UK Medical Research Council grant G03001896 (J.K.) and the National Institutes of Health (NIH) grants R01MH62873 and R01MH081803 (S.V.F.).
We thank Dr. Owen O'Daly, Mr. Jeff Dalton, and the research team members Kelly Harris and Chloe Booth, of King's College, London, for their contribution this project, and our participants and their families.
Footnotes
Supplemental material cited in this article is available online.
Disclosure: Dr. Mehta has received research funding from Eli Lilly and Co., and has served on the advisory board for Cambridge Cognition. Dr. Asherson has given sponsored talks and/or has served on the advisory boards for Shire, Janssen-Cilag, Eli Lilly and Co., Flynn Pharma, and Pfizer; all funds have been donated to the University research fund for attention deficit hyperactivity disorder (ADHD) studies. Dr. Faraone has received grant or research support from the National Institutes of Health (NIH) and Shire, and has received royalties from the Guilford Press and Oxford University Press. Dr. Faraone has also served as a consultant for, has served on advisory boards for, or has participated in continuing medical education programs sponsored by Shire, Otsuka, and Alcobra. Dr. Kuntsi has served on the speakers' bureau for Eli Lilly and Co.; all funds have been used for educational and research activities. Dr. Paloyelis acknowledges the direct benefit he received by attending the National Institutes of Health (NIH)–funded Neuroimaging Training Program, UCLA.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Paloyelis Y, Mehta MA, Kuntsi J, Asherson P. Functional MRI in ADHD: a systematic literature review. Expert Rev Neurother. 2007;7(10):1337–1356. doi: 10.1586/14737175.7.10.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biological psychiatry. 2005;57(11):1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Scheres A, Milham MP, Knutson B, Castellanos FX. Ventral striatal hyporesponsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2007;61(5):720–724. doi: 10.1016/j.biopsych.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 4.Strohle A, Stoy M, Wrase J, et al. Reward anticipation and outcomes in adult males with attention-deficit/hyperactivity disorder. Neuroimage. 2008;39(3):966–972. doi: 10.1016/j.neuroimage.2007.09.044. [DOI] [PubMed] [Google Scholar]
- 5.Volkow ND, Wang GJ, Kollins SH, et al. Evaluating dopamine reward pathway in ADHD: clinical implications. JAMA. 2009;302(10):1084–1091. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tripp G, Wickens JR. Research review: dopamine transfer deficit: a neurobiological theory of altered reinforcement mechanisms in ADHD. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2008;49(7):691–704. doi: 10.1111/j.1469-7610.2007.01851.x. [DOI] [PubMed] [Google Scholar]
- 7.Sonuga-Barke EJ. Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biological psychiatry. 2005;57(11):1231–1238. doi: 10.1016/j.biopsych.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Sagvolden T, Johansen EB, Aase H, Russell VA. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behav Brain Sci. 2005;28(3):397–419. doi: 10.1017/S0140525X05000075. discussion 419–368. [DOI] [PubMed] [Google Scholar]
- 9.Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12(17):3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- 10.Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36(2):241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- 11.Buckholtz JW, Treadway MT, Cowan RL, et al. Dopaminergic network differences in human impulsivity. Science. 2010;329:532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schott BH, Minuzzi L, Krebs RM, et al. Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. Journal of Neuroscience. 2008;28:14311–14319. doi: 10.1523/JNEUROSCI.2058-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knutson B, Gibbs SE. Linking nucleus accumbens dopamine and blood oxygenation. Psychopharmacology. 2007;191(3):813–822. doi: 10.1007/s00213-006-0686-7. [DOI] [PubMed] [Google Scholar]
- 14.Drechsler R, Rizzo P, Steinhausen HC. Decision-making on an explicit risk-taking task in preadolescents with attention-deficit/hyperactivity disorder. Journal of Neural Transmission. 2008;115(2):201–209. doi: 10.1007/s00702-007-0814-5. [DOI] [PubMed] [Google Scholar]
- 15.Paloyelis Y, Asherson P, Mehta MA, Faraone SV, Kuntsi J. DAT1 and COMT Effects on Delay Discounting and Trait Impulsivity in Male Adolescents with Attention Deficit/Hyperactivity Disorder and Healthy Controls. Neuropsychopharmacology. 2010;35:2414–2426. doi: 10.1038/npp.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scheres A, Tontsch C, Thoeny AL, Kaczkurkin A. Temporal Reward Discounting in Attention-Deficit/Hyperactivity Disorder: The Contribution of Symptom Domains, Reward Magnitude, and Session Length. Biological Psychiatry. 2010;67(7):641–648. doi: 10.1016/j.biopsych.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 17.Hariri AR, Brown SM, Williamson DE, Flory JD, de Wit H, Manuck SB. Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. Journal of Neuroscience. 2006;26(51):13213–13217. doi: 10.1523/JNEUROSCI.3446-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forbes EE, Brown SM, Kimak M, Ferrell RE, Manuck SB, Hariri AR. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Molecular Psychiatry. 2009;14:60–70. doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hahn T, Dresler T, Ehlis AC, et al. Neural response to reward anticipation is modulated by Gray's impulsivity. Neuroimage. 2009;46:1148–1153. doi: 10.1016/j.neuroimage.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 20.Simon JJ, Walther S, Fiebach CJ, et al. Neural reward processing is modulated by approach- and avoidance-related personality traits. Neuroimage. 2010;49:1868–1874. doi: 10.1016/j.neuroimage.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 21.Carmona S, Hoekzema E, Ramos-Quiroga JA, et al. Response inhibition and reward anticipation in medication-naive adults with attention-deficit/hyperactivity disorder: A within-subject case-control neuroimaging study. Hum Brain Mapp. doi: 10.1002/hbm.21368. published online ahead of print Aug 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoogman M, Aarts E, Zwiers M, et al. Nitric oxide synthase genotype modulation of impulsivity and ventral striatal activity in adult ADHD patients and healthy comparison subjects. Am J Psychiatry. 2011;168(10):1099–1106. doi: 10.1176/appi.ajp.2011.10101446. [DOI] [PubMed] [Google Scholar]
- 23.Stoy M, Schlagenhauf F, Schlochtermeier L, et al. Reward processing in male adults with childhood ADHD--a comparison between drug-naive and methylphenidate-treated subjects. Psychopharmacology. 2011 Jun;215(3):467–481. doi: 10.1007/s00213-011-2166-y. [DOI] [PubMed] [Google Scholar]
- 24.Dreher JC, Meyer-Lindenberg A, Kohn P, Berman KF. Age-related changes in midbrain dopaminergic regulation of the human reward system. Proceedings of the National Academy of Sciences of the United States of America; 2008; pp. 15106–15111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shumay E, Chen J, Fowler JS, Volkow ND. Genotype and Ancestry Modulate Brain's DAT Availability in Healthy Humans. PloS one. 2011;6(8):e22754. doi: 10.1371/journal.pone.0022754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dreher JC, Kohn P, Kolachana B, Weinberger DR, Berman KF. Variation in dopamine genes influences responsivity of the human reward system. Proceedings of the National Academy of Sciences of the United States of America; 2009; pp. 617–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volkow ND, Wang GJ, Newcorn JH, et al. Motivation deficit in ADHD is associated with dysfunction of the dopamine reward pathway. Molecular Psychiatry. 2011;16(11):1147–54. doi: 10.1038/mp.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ernst M, Zametkin AJ, Matochik JA, Jons PH, Cohen RM. DOPA decarboxylase activity in attention deficit hyperactivity disorder adults. A [fluorine-18]fluorodopa positron emission tomographic study. Journal of neuroscience. 1998;18(15):5901–5907. doi: 10.1523/JNEUROSCI.18-15-05901.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ernst M, Zametkin AJ, Matochik JA, Pascualvaca D, Jons PH, Cohen RM. High midbrain [18F]DOPA accumulation in children with attention deficit hyperactivity disorder. Am J Psychiatry. 156(8):1209–1215. doi: 10.1176/ajp.156.8.1209. [DOI] [PubMed] [Google Scholar]
- 30.Gizer IR, Ficks C, Waldman ID. Candidate gene studies of ADHD: a meta-analytic review. Human Genetics. 2009;126:51–90. doi: 10.1007/s00439-009-0694-x. [DOI] [PubMed] [Google Scholar]
- 31.Lewis DA, Melchitzky DS, Sesack SR, Whitehead RE, Auh S, Sampson A. Dopamine transporter immunoreactivity in monkey cerebral cortex: regional, laminar, and ultrastructural localization. J Comp Neurol. 2001;432(1):119–136. doi: 10.1002/cne.1092. [DOI] [PubMed] [Google Scholar]
- 32.Ciliax BJ, Drash GW, Staley JK, et al. Immunocytochemical localization of the dopamine transporter in human brain. Journal of Comparative Neurology. 1999;409(1):38–56. doi: 10.1002/(sici)1096-9861(19990621)409:1<38::aid-cne4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 33.Asherson P, Brookes K, Franke B, et al. Confirmation that a specific haplotype of the dopamine transporter gene is associated with combined-type ADHD. American Journal of Psychiatry. 2007;164(4):674–677. doi: 10.1176/ajp.2007.164.4.674. [DOI] [PubMed] [Google Scholar]
- 34.Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience. 2001(21):RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wittmann BC, Schott BH, Guderian S, Frey JU, Heinze HJ, Düzel E. Reward-related FMRI activation of dopaminergic midbrain is associated with enhanced hippocampus-dependent long-term memory formation. Neuron. 2005;45:459–467. doi: 10.1016/j.neuron.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 36.Kuntsi J, Wood AC, Rijsdijk F, et al. Separation of cognitive impairments in attention deficit hyperactivity disorder into two familial factors. Archives of General Psychiatry. 2010;67(11):1159–1167. doi: 10.1001/archgenpsychiatry.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuntsi J, Neale BM, Chen W, Faraone SV, Asherson P. The IMAGE project: methodological issues for the molecular genetic analysis of ADHD. Behav Brain Funct. 2006;2:27. doi: 10.1186/1744-9081-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conners CK, Sitarenios G, Parker JD, Epstein JN. The revised Conners' Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. Journal of Abnormal Child Psychology. 1998;26(4):257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- 39.Wechsler D. Wechsler Intelligence Scale for Children. 3rd. London: The Psychological Corporation; 1991. [Google Scholar]
- 40.Wechsler D. Wechsler Intelligence Scale for Adults. London: The Psychological Corporation; 1997. [Google Scholar]
- 41.Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox. Neuroimage. 2002;16(2) Abstract 497. [Google Scholar]
- 42.Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. Journal of Neuroscience. 2004;24(8):1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shook D, Brady C, Lee PS, et al. Effect of dopamine transporter genotype on caudate volume in childhood ADHD and controls. Am J Med Genet B Neuropsychiatr Genet. 156B(1):28–35. doi: 10.1002/ajmg.b.31132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Leijenhorst L, Moor BG, Op de Macks ZA, Rombouts SARB, Westenberg PM, Crone EA. Adolescent risky decision-making: neurocognitive development of reward and control regions. Neuroimage. 2010;51:345–355. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- 45.Forbes EE, Ryan ND, Phillips ML, et al. Healthy adolescents' neural response to reward: associations with puberty, positive affect, and depressive symptoms. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(2):162–172. doi: 10.1097/00004583-201002000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tobler PN, Fiorillo CD, Schultz W. Adaptive coding of reward value by dopamine neurons. Science. 2005;307(5715):1642–1645. doi: 10.1126/science.1105370. [DOI] [PubMed] [Google Scholar]
- 47.Abler B, Walter H, Erk S, Kammerer H, Spitzer M. Prediction error as a linear function of reward probability is coded in human nucleus accumbens. Neuroimage. 31(2):790–795. doi: 10.1016/j.neuroimage.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 48.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aarts E, Roelofs A, Franke B, et al. Striatal dopamine mediates the interface between motivational and cognitive control in humans: evidence from genetic imaging. Neuropsychopharmacology. 2010;35(9):1943–1951. doi: 10.1038/npp.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hahn T, Heinzel S, Dresler T, et al. Association between reward-related activation in the ventral striatum and trait reward sensitivity is moderated by dopamine transporter genotype. Human brain mapping. 32(10):1557–1565. doi: 10.1002/hbm.21127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cools R, Barker RA, Sahakian BJ, Robbins TW. Enhanced or impaired cognitive function in Parkinson's disease as a function of dopaminergic medication and task demands. Cerebral Cortex. 2001;11(12):1136–1143. doi: 10.1093/cercor/11.12.1136. [DOI] [PubMed] [Google Scholar]
- 52.Gamo NJ, Wang M, Arnsten AF. Methylphenidate and atomoxetine enhance prefrontal function through alpha2-adrenergic and dopamine D1 receptors. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(10):1011–1023. doi: 10.1016/j.jaac.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meyer-Lindenberg A, Kohn PD, Kolachana B, et al. Midbrain dopamine and prefrontal function in humans: interaction and modulation by COMT genotype. Nat Neurosci May. 2005;8(5):594–596. doi: 10.1038/nn1438. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


